Abstract
We examined the relationship of corpus callosum morphology and organization to hand preference and performance on a motor skill task in chimpanzees. Handedness was assessed using a complex tool use task that simulated termite fishing. Chimpanzees were initially allowed to perform the task wherein they could choose which hand to use (preference measure); then they were required to complete trials using each hand (performance measure). Two measures were used to assess the corpus callosum: midsagittal area obtained from in vivo magnetic resonance images and density of transcallosal connections as determined by fractional anisotropy values obtained from diffusion tensor imaging. We hypothesized that chimpanzees would perform better on their preferred hand compared to the non-preferred hand, and that strength of behavioral lateralization (rather the direction) on this task would be negatively correlated to regions of the corpus callosum involved in motor processing. Our results indicate that the preferred hand was the most adept hand. Performance asymmetries correlated with FA measures but not area measures of the CC.
Keywords: Corpus callosum, DTI, chimpanzee, handedness, tool use
Handedness can be defined using either preference or performance measures. In humans, hand preference is typically ascertained via handedness inventories such as the Edinburgh Handedness Inventory, where an individual states their preferences in hand use for various behaviors such as striking a match or using scissors (Beaton, 2003; Oldfield, 1971). Asymmetry in hand performance is determined from observations or recording of individual performance on tasks rather than a self-reported preference. Two common tasks measuring asymmetries in motor performance are the Annett and Purdue Peg board tests, though others tasks have been employed (e.g., (Solodkin, Hlustik, Noll, & Small, 2001)). Given that studies have shown hand preference and performance are correlated (though weakly), this has led to the position that the preferred hand is the most motorically adept hand (Annett, 1985). However, this is not necessarily the case: one may show a preference for using a given hand for a given task, but yet be more proficient with the opposite hand (Porac & Coren, 1981; Provins & Magliaro, 1993; Rigal, 1992), suggesting that performance and preference are somewhat dissociable motor processes. The relationship between hand preference and performance in humans is thus not completely understood.
In nonhuman primates, handedness is determined by subjects’ performance on various measures – wherein the subject can choose which hand to use in solving the task. In this manner, then, standard measures have determined handedness in nonhuman primates via preference. Several measures have been used to assess handedness in nonhuman primates, including simple reaching, tasks requiring bimanual coordination, and tool-use, just to mention a few. One conclusion is that there is little consistency of hand preference across various tasks, except for instances where solving different tasks requires similar motor actions (Hopkins & Pearson, 2000; Hopkins, Taglialatela, Leavens, Russell, & Schapiro, 2010; Lilak & Phillips, 2008). Furthermore, asymmetry of hand performance has not been shown to strongly correlate with hand preferences in chimpanzees, macaques or capuchin monkeys (Andrews & Rosenblum, 2001; Hopkins & Russell, 2004; Hopkins, Washburn, Berke, & Williams, 1992; Spinozzi, Truppa, & Lagana, 2004), though these studies used less complex measures such as grasping small food items for assessment of motor skill. Since task complexity influences manual performance in human and nonhuman primates (Bryden, Roy, Rohr, & Egilo, 2007; Fagot & Vauclair, 1991), this may explain the lack of strong correlations in these studies. Thus, employing a more complex task for assessment of hand performance might allow for relationships between preference and performance to be detected in nonhuman primates.
Recently, Hopkins et al. (2009) investigated the relationship between performance and preference for a tool use task in a sample of captive chimpanzees; a significant correlation between strength of hand preference and performance was found. When using the dominant hand for insertion of a stick into a dipping device, chimpanzees had shorter latencies compared to responses in which they used their non-dominant hand. However, the chimpanzees were not required to solve the task using each hand, thus the number of observations in hand use for the dominant and non-dominant were not balanced within and across subjects. Therefore, these findings may not convincingly represent the relationship between hand preference and performance, as the chimpanzees were allowed to determine which hand to use in the task.
The first aim of this study was to further assess the relationship between hand preference and performance asymmetries on a tool use task in chimpanzees. Chimpanzees were presented with a simulated termite fishing problem which required them to insert a stick into a small opening in a tube to retrieve a sticky, preferred food substance (such as mustard or yogurt). Chimpanzees initially completed trials wherein the apparatus was freely accessible and the individual could choose which hand to use. Their hand use on this task was used to assess their hand preferences. Subsequently, the chimpanzees were tested under conditions that required them to complete 30 discrete tool use trials with each hand and the latency to respond on each trial was recorded; this test provided a means to assess asymmetries in hand performance when controlling for differences in the number of response produced by each hand. We hypothesized that chimpanzees would perform significantly better with their preferred compared to non-preferred hand on this tool task.
The second aim of this investigation was to relate asymmetries in hand performance to organization of the corpus callosum, specifically the two subdivisions involved in motor processing. The corpus callosum is the major commissure in placental mammals, and connects homotopic and heterotopic cortical regions. In humans and chimpanzees, transcallosal connections between premotor and supplementary motor cortices (SMA) are contained within the anterior midbody, and transcallosal connections of the primary motor cortex (M1) pass in the medial midbody (Hofer & Frahm, 2006; Phillips & Hopkins, 2012). Hand morphology characteristics such as an opposable thumb and the ability to make precision grips allow for chimpanzees to engage in complex manipulation, such as using tools and holding food with one hand and peeling with the other hand. Corticospinal terminals in the ventral horn that innervate individual digits of the hand, and well-developed cortical processing regions for input from the hand further contribute to these abilities (Padberg et al., 2007).
Callosal connections between M1 are sparse (Gould, Cusick, Pons, & Kaas, 1986; Rouiller et al., 1994) whereas connections between the SMA are dense (Rouiller et al., 1994). The SMA therefore is a more bilaterally organized system which may indicate this cortical area contributes more to bimanual coordination. Thus, we hypothesized that the anterior midbody (premotor and SMA callosal areas) would have a greater density of transcallosal connections in individuals who show more bimanual activity of the hands (less lateralization) compared to those who showed greater lateralization. As the medial midbody (M1) is associated with less interhemispheric connectivity, highly lateralized individuals were expected to have less dense transcallosal connections. We thus were interested in the relationship between callosal organization and strength of lateralization, rather than right-handedness or left-handedness per se.
To measure density of transcallosal connections we obtained diffusion tensor images (DTI) from each chimpanzee. DTI provides for the in vivo study of white matter microstructure through measurement of the random diffusion of water molecules (Le Bihan, 1995). In white matter, water diffusion is anisotropic, with water diffusion greater along white matter fibers that are parallel rather than perpendicular to these fibers (Basser, 1995; Basser, Mattiello, & Le Bihan, 1994; Basser & Pierpaoli, 1996, 1998). Diffusion anisotropy measures the difference between these two directions of water diffusion. One of the most commonly reported measures of this is fractional anisotropy (FA), the normalized standard deviation of the diffusivities (Basser & Pierpaoli, 1996). FA values range from 0 to a theoretical maximum of 1; white matter FA values that are high indicate fast diffusivity along the fibers. Based on this assumption, we hypothesized that strongly lateralized individuals were expected to have lower FA in the medial midbody (reflecting even fewer transcallosal connections), and lower FA in anterior midbody (as this region shows dense interhemispheric connectivity) than more weakly lateralized individuals.
Method
Subjects
For the behavioral component of the study, there were 59 subjects including 40 females and 19 males ranging in age from 10 to 51 years of age. All of the chimpanzees resided at the Yerkes National Primate Research Center of Emory University.
MRI
Noninvasive in vivo MRI scans were acquired from 55 chimpanzees that participated in the behavioral component of the study; DTI scans were also acquired from 31 of these individuals (male n = 17; female n = 14). All the chimpanzees were housed at the Yerkes National Primate Research Center (YNPRC). Animals were anesthetized for this procedure and the collection of the brain images was coordinated with each subject’s annual physical exam. Anesthesia was used only for the purpose of restraint and to keep the subject immobilized during their physical exam and collection of the brain images. Subjects remained anaesthetized throughout the imaging procedure and respiration rate, heart rate, and oxygen consumption were continually monitored by a veterinarian.
Subjects were initially immobilized using ketamine (10 mg/kg), and subsequently anesthetized with propofol (40 – 60 mg (kg/h)) following standard procedures at the YNPRC. Subjects were then transported to the MRI facility. Subjects remained anesthetized for the duration of the scan as well as transport time to and from the imaging facility. Subjects were scanned on a Siemens 3.0 T Trio at the YNPRC. T1-weighted images were acquired using a 3D gradient echo sequence (pulse repetition = 2300 ms, echo time = 4.4 ms, number of signals averaged = 3, matrix size = 320×320, with .6 mm isotropic resolution). We acquired two sets of whole brain diffusion-weighted data with a single-shot EPI sequence with a b value of 1000 s/mm2 with 64 diffusion directions; plus one image without diffusion weighting (b value of 0 s/mm2). DTI data were acquired transaxially (FOV = 243×243) using 42 contiguous slices with no gap that covered the entire brain with resolution of 1.9 X 1.9 X 1.9 mm. Averages of two sets of diffusion-weighted data were collected per subject with phase-encoding directions of opposite polarity (left – right) to correct for susceptibility distortion. Acquisition time for both the MRI and DTI scans was approximately 1 hour. After completing the DTI and MRI procedures the subjects were temporarily housed in a single cage for 6 – 12 hours, to allow for the effects of anesthesia to wear off, after which they were returned to their home cage and social group.
Behavioral Performance
Apparatus
The tool use device used in this study was the same one as used by Hopkins et al. (2009). A threaded poly-vinyl-chloride (PVC) base was affixed to the subjects’ home enclosures at multiple locations approximately 60 to 80 cm above the ground or floor. In the initial test of hand preference, the device was positioned in the center of the housing enclosure equidistant from the two lateral walls. For the test of hand performance, the devices were positioned on the far left or right ends of their outdoor home cage, near the walls, thereby constraining the chimpanzees ability to position themselves directly in front of the device in order to use their preferred hand. This was done so as to “force” or encourage the chimpanzees to use either the left or right hand depending on the position of the device, relative to the subject. The motor and cognitive requirements of termite fishing were simulated using threaded PVC pipes (approx. 4 cm in diameter and approx. 20 cm in length) attached to the bases. The pipes were fitted with a disc in one end with a 7 mm hole cut out to greatly reduce the size of the opening available for tool insertion, thus increasing the motor demands of the task. The other end was closed with a removable screw on cap. The pipes were filled with a preferred food substance that would adhere to the tool, such as mustard, yogurt, syrup, or applesauce, before being screwed into the bases. The chimpanzees were provided with flexible, thin ‘lollipop’ sticks (approx. 11 cm in length and 4 mm in diameter, like those used to make large lollipops) made out of tightly rolled, thin paper. The animals use the lollipop sticks to dip into the small hole of the pipe and retrieve the food (See Figure 1).
Figure 1.
Photograph of a chimpanzee using a lollipop stick as a tool to retrieve food from the simulated termite fishing device.
For hand preference, we recorded 50 responses from each subject. Each time the chimpanzees inserted the stick into the hole of the device, we recorded their hand use as right or left. During performance testing, we recorded the duration to successfully insert the stick into the tube. Each chimpanzee received 30 trials with the left and the right hand and the order of testing was counterbalanced across subjects and days. On each trial, latency was measured from the time the subject initiated an attempt to insert the tool with one hand and ended when the chimpanzee successfully inserted the tool. The latencies were averaged across the 30 trials for each subject and hand.
Lateralization in hand preference and hand performance was calculated following the formula: [HI=(R – L) / R + L)]. For hand preference (HI_Pref), R and L represented the frequency in left and right hand use. For the performance measure (HI_Perf), R and L represented the mean dipping latencies of the right and left hands, respectively. For HI_Pref, positive scores indicated right hand preference and negative values indicated left hand preferences. For the HI_Perf scores, positive values indicated better performance by the left hand whereas negative values indicated better performance by the right hand. The absolute values of the HI_Pref and HI_Perf scores indicated the degree of lateralization in motor preference and performance; higher HI scores reflect greater lateralization in motor preference and performance while values close to 0 would indicate no bias.
Image quantification
Image preprocessing steps included realignment, correction for head motion and eddy current distortion, and removal of non-brain tissue and were carried out with FSL tools (FMRIB Software Library; www.fmrib.ox.ac.uk/fsl). Area measurements of the corpus callosum (CC) and callosal subdivisions were taken from the midsagittal slice of the MRI using Analyze 10.0, a MRI analysis software program (Analyze Direct, Overland Park, KS, USA). The length of the CC was traced to obtain the entire CC area. Rather than use the standard geometric partitioning scheme, which subdivides the CC into 5 regions ((Witelson, 1989), the CC was subdivided into five regions based upon the transcallosal projections into specific cortical areas. Phillips and Hopkins (2012) used tractography to identify in chimpanzees the transcallosal projections into specific cortical regions and subdivided the CC based upon these projections. Tractography was carried out using Analyze MR Diffusion Tensor Imaging based on fiber assignment by continuous tracking (FACT) algorithm (Jiang, van Zijl, Kim, Pearlson, & Mori, 2006) with a fractional anisotropy threshold of 0.2 for initial seeding and stopping and a principal eigenvector angle stopping threshold of 60°. Landmarks used to define the projections into the cortex were the arcuate sulcus (for prefrontal cortex); the arcuate sulcus and central sulcus (for premotor, supplementary motor, and motor cortices); postcentral sulcus and parietooccipital sulcus (parietal cortex); lateral fissure (temporal cortex); and the inferior occipital sulcus and the parietooccipital sulcus (occipital cortex). The CC was then partitioned into five regions based upon fiber projections into specific cortical regions as follows: Region I, the most rostral region, into the prefrontal cortex; region II into premotor and supplementary motor cortices; region III into motor cortex; region IV into sensory cortex; and region V into parietal, temporal and occipital lobes. We were particularly interested in regions II and III in this investigation. Region II, with projections to the premotor and SMA cortices is analogous to the anterior midbody. Region III with projections to M1 is analogous to the medial midbody. To statistically adjust the CC area measures for total brain volume, we followed a recommendation by Smith (2005) wherein the square root of each CC subdivision area was divided by the cube root of total brain volume for each subject to bring all measures into the same geometric dimensionality.
To determine FA values for each callosal subdivision from those subjects who had a DTI scan, each subject’s MRI image was initially spatially registered to their respective DTI image using 3D voxel registration with a linear transformation using Analyze 10.0 (Analyze Direct, Overland Park, KS, USA). FA of each callosal region was measured in the midsagittal and two CC sections 1 mm lateral to the midsagittal using the above-defined callosal regions to quantify the measure of diffusion anisotropy (see Figure 2). Obtained values were then averaged for each subject for each callosal region.
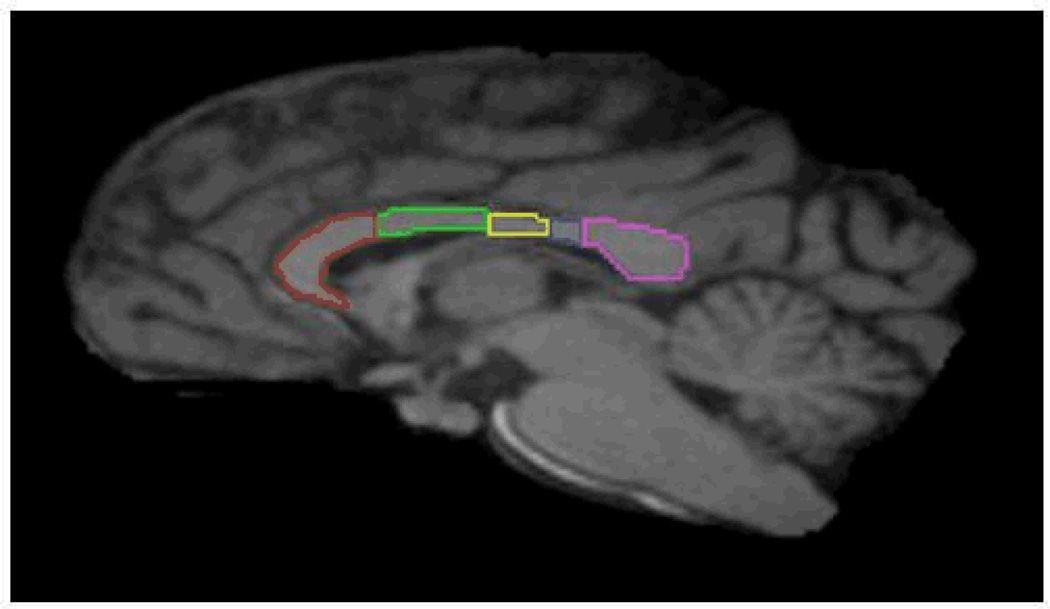
Figure 2.
Midsagittal section illustrating the subdivisions of the corpus callosum. Region I (red) = prefrontal cortex; Region II (green) = premotor and supplementary motor cortices; Region III (yellow) = motor cortex; Region IV (blue) = sensory cortex; Region V (violet) = parietal, temporal and occipital cortices.
Results
Hand preference and performance
The hand preference and performance results are shown in Table 1. Overall, no significant bias in hand preference was found for the tool use task t(58)= −1.60, p > .05, though the trend was toward left handedness (Mean HI = −0.12). An independent samples t-test failed to reveal sex differences in the HI_Pref measure, though the males were much more left hand biased (Mean HI = −.274) compared to the females (Mean HI = −.050). Forty-eight chimpanzees completed all trials for assessment of hand performance. No significant group-level performance asymmetries were found (Mean HI_Perf = .005, SEM = .03; one sample t-test, t(45) = −0.18, p = 0.86).
Table 1.
Mean HI values for hand preference and hand performance for male and female chimpanzees
| Subject | Sex | HI_Preference | HI_Performance |
|---|---|---|---|
| Subject 1 | F | 0.29 | 0.33 |
| Subject 2 | F | 0.04 | 0.25 |
| Subject 3 | F | 0.56 | −0.23 |
| Subject 4 | M | 0.26 | −0.29 |
| Subject 5 | M | 0.32 | −0.25 |
| Subject 6 | F | −0.62 | 0.15 |
| Subject 7 | F | −0.64 | −0.03 |
| Subject 8 | F | 0.16 | 0.04 |
| Subject 9 | F | −0.84 | −0.25 |
| Subject 10 | F | −0.7 | −0.21 |
| Subject 11 | M | −0.82 | −0.04 |
| Subject 12 | M | −0.79 | - |
| Subject 13 | F | 0.88 | −0.27 |
| Subject 14 | F | −0.35 | −0.06 |
| Subject 15 | F | 0.39 | −0.14 |
| Subject 16 | F | −0.24 | 0.28 |
| Subject 17 | M | −0.45 | - |
| Subject 18 | M | −0.82 | 0.42 |
| Subject 19 | M | −0.1 | - |
| Subject 20 | F | −0.21 | 0.1 |
| Subject 21 | F | −0.42 | −0.24 |
| Subject 22 | M | 0.67 | −0.16 |
| Subject 23 | F | 0.86 | −0.14 |
| Subject 24 | F | 0.04 | −0.16 |
| Subject 25 | F | 0.57 | 0.02 |
| Subject 26 | M | −0.28 | 0.03 |
| Subject 27 | M | 0.54 | - |
| Subject 28 | F | −0.6 | −0.12 |
| Subject 29 | F | −0.32 | −0.46 |
| Subject 30 | F | 0.69 | - |
| Subject 31 | F | −0.36 | −0.15 |
| Subject 32 | M | −0.42 | - |
| Subject 33 | M | −0.2 | - |
| Subject 34 | F | −0.96 | 0.24 |
| Subject 35 | M | −0.12 | 0.28 |
| Subject 36 | F | 0.44 | −0.13 |
| Subject 37 | F | 0.28 | −0.19 |
| Subject 38 | F | 0.58 | - |
| Subject 39 | F | −0.46 | −0.07 |
| Subject 40 | F | −0.65 | −0.03 |
| Subject 41 | F | 0.91 | 0.05 |
| Subject 42 | M | −0.76 | - |
| Subject 43 | M | −0.68 | 0.07 |
| Subject 44 | F | 0.79 | −0.14 |
| Subject 45 | F | −0.88 | - |
| Subject 46 | F | −0.08 | 0.13 |
| Subject 47 | F | −0.83 | 0.32 |
| Subject 48 | F | 0.68 | - |
| Subject 49 | M | −0.12 | 0.48 |
| Subject 50 | F | −0.68 | 0.25 |
| Subject 51 | M | −1 | 0.01 |
| Subject 52 | F | −0.2 | −0.05 |
| Subject 53 | F | 0.5 | 0.24 |
| Subject 54 | F | −0.19 | 0.28 |
| Subject 55 | F | 0.09 | 0.01 |
| Subject 56 | F | 0.12 | −0.05 |
| Subject 57 | F | −0.71 | −0.05 |
| Subject 58 | M | 0.63 | 0.1 |
| Subject 59 | M | −0.67 | −0.05 |
Note: A “-“ indicates that the subject did not complete the performance trials.
We next compared the HI_PERF scores as function of sex and hand preference of the chimpanzees. For this analysis, chimpanzees with positive HI_Pref scores were classified as right-handed (n = 21) and apes with negative HI_Pref scores were classified as left handed (n = 27). We then compared the HI_Perf scores as a function of sex and hand preference groups using an analysis of variance. A significant main effect for handedness group was found F(1, 44)=6.162, p < .02. Right-handed chimpanzees performed better with their right hand (Mean HI_Perf = −.091). In contrast, left-handed chimpanzees performed significant better with their left hand (Mean HI_Perf = .081).
Relating hand preference and performance to organization of the CC
In this next set of analyses, we correlated the direction and strength of the HI_Pref and HI_Perf scores with both the CC area measures and the FA values. For direction in hand preference and performance, no significant associations were found with the CC area measures. Furthermore, no significant associations were found with the CC area measures and strength of asymmetry for HI_Pref and HI_Perf. In short, neither direction nor strength of hand preference or performance correlated with the CC area measures.
For the FA values, strength of hand preference was positively correlated with the genu (Region I; r = .37, p < .02; see Figure 3). More lateralized subjects had higher FA of this region. For the hand performance measures, we did not detect significant correlations between strength of lateralization and FA values in the callosal regions connecting M1 or SMA (Regions II and III). However, a significant correlation was found between strength of lateralization and FA of Region IV (r = 0.59, p = 0.003; see Figure 4), the callosal region associated with primary sensory transcallosal fibers. More strongly lateralized individuals had greater FA of this region. The results of these correlational analyses are presented in Table 2.
Figure 3.
Figure 3a: Strength of hand preference for tool use showed a positive correlation with fractional anisotropy (FA) of Region I. Figure 3b: Strength of hand performance for tool use showed a positive correlation with FA of Region IV.
Table 2.
Correlations of strength of hand preference and hand performance to subdivisions of the corpus callosum. RI = region I; RII = region II; RIII = region III; RIV = region IV; RV = region V.
| Corpus Callosum Area Measure | Corpus Callosum FA Value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| RI | RII | RIII | RIV | RV | RI | RII | RIII | RIV | RV | |
| Direction of hand preference | −.012 | .041 | .101 | .207 | −.169 | .210 | .136 | .089 | .053 | .075 |
| Direction of hand performance | −.289 | −.211 | −.168 | −.272 | −.115 | .011 | .218 | .109 | .297 | .165 |
| Strength of hand preference | .102 | .015 | .179 | −.091 | .014 | .369* | .208 | −.115 | −.223 | −.083 |
| Strength of hand performance | .062 | −.150 | −.155 | .084 | −.039 | .262 | .111 | .249 | .591** | .364 |
p < 0.02
p = 0.001
Note: Some might consider it appropriate to perform a Bonferroni correction. If such procedures were used, then only the correlation between strength of hand preference and FA of region III would be significant.
We also correlated preference and performance measures with another quantitative measure, apparent diffusion coefficient (ADC). ADC reports the magnitude of diffusion of water molecules; low ADC values indicate high organization of cortical white matter tissue. No significant associations were found between hand preference or hand performance and ADC of any callosal subdivision.
Discussion
Hand preference and hand performance on a complex tool use task were significantly associated in chimpanzees. Chimpanzees that preferred to use their right hand performed better when using the right hand; chimpanzees that preferred to use their left hand in solving the task performed better when using the left hand. As our study required subjects to complete trials using each hand during the performance trials (not simply the dominant hand), the results obtained support the position that, at least in chimpanzees, the preferred hand for tool use is the most adept hand in performing that task and the most parsimonious explanation for this observation is a practice effect. Previous investigations relating hand preference and performance in nonhuman primates have failed to demonstrate strong relationships (Andrews & Rosenblum, 2001; Hopkins & Russell, 2004; Hopkins et al., 2009; Spinozzi et al., 2004). However, these studies are limited in two ways: simpler measures of handedness were used (grasping small food items) and most did not acquire performance measures from each hand systematically. The present study is a methodological improvement from previous work as we addressed both of these concerns. Hand performance assessment was based on quantifying the latency for dipping by each hand. Thus, we specifically incorporated performance differences in motor output.
While no significant population-level hand preference was found for the dipping tool use task, a trend toward left-handedness was detected, with males displaying more left-handedness than females. This result confirms similar findings reported for both captive (Hopkins et al. 2009) and wild chimpanzees for termite fishing (Lonsdorf & Hopkins, 2005). We did not detect sex differences in performance. However, other studies have reported sex differences in performance in tool using tasks, with males performing less successfully than females (Hopkins et al., 2009) and showing slower acquisition of tool use (Lonsdorf & Hopkins, 2005).
No significant associations were found between hand preference or performance and the CC when considering midsagittal area alone. Phillips and Hopkins (2010) reported no significant association between handedness on a bimanual coordinated task and development of the CC in chimpanzees. However, other studies investigating hand preference and organization of the CC in chimpanzees reported that left handed chimpanzees had significantly smaller midsagittal area of the CC than right handed chimpanzees (Dunham & Hopkins, 2006; Hopkins, Dunham, Cantalupo, & Taglialatela, 2007). One reason for the differing results between these studies may be due to different measures of hand preference employed. In the present study, a tool use task was used while in previous studies, hand preference was determined via tasks of simple reaching, bimanual feeding, manual gesture, and a test requiring coordinated bimanual actions. A second reason for these different results involves methodologies employed. Discrepancies exist in the literature regarding callosal size and degree of lateralization in humans, with positive (Habib et al., 1991), negative (Luders et al., 2010) or no correlations (Moffat, Hampson, & Lee, 1998) found between callosal morphology and degree of lateralization. Differences in measurement of callosal size, sample size, and whether (or not) the callosal area is adjusted for brain size all likely contribute to the inconsistency of results. In the present study we adjusted the CC area for total brain volume in accordance with Smith (2005), as did Dunham and Hopkins (2006) and Hopkins et al (2007). However, in the present study the partitioning of the CC was based on DTI tractography (Phillips & Hopkins, 2012), whereas the previous studies employed a geometrically based method (Witelson, 1989).
When considering the organization of the CC as informed by DTI, we found significant associations with hand preference and performance. Strength of hand preference correlated positively with FA of Region I, the region of the CC that provides interhemispheric connection across the prefrontal cortex. Assuming callosal size correlates positively with the amount of information transferred between the two hemispheres, these data suggest that more strongly lateralized chimpanzees (regardless of direction of hand preference) have greater interhemispheric communication in this region. An unexpected result was the association between strength of performance asymmetry and FA of Region IV. More sensory cross-callosal communication, as reflected by greater FA, was found in strongly lateralized individuals. These results also suggest that more strongly lateralized individuals have greater axon density in posterior regions of the CC which connect temporal, parietal and occipital cortices.
Contrary to our hypothesis, we did not detect correlations between hand performance or preference measures and regions of the CC associated with M1. One possible explanation for this is that handedness mediates the expression of other functional or anatomical asymmetries and their interhemispheric connectivity at different points within the CC. It has previously been reported that asymmetries in the planum temporale (PT) and parietal operculum (PO) differ significantly between right- and left-handed tool using chimpanzees (Gilissen & Hopkins, 2012). Asymmetries in the PT and PO have been linked to variation in the size of the CC in the isthmus and splenium in human brains (Foundas, Leonard, & Hanna-Pladdy, 2002; Foundas, Leonard, & Heilman, 1995; Steinmetz, 1996). Thus, the association reported here between handedness and FA values in the posterior CC regions may reflect anatomical differences between the PT and/or PO in this cohort of chimpanzees. Another explanation for the lack of a correlation between these factors could lie in that we did not isolate and measure the region of the CC containing only SMA and M1 fibers. We undoubtedly recorded FA from transcallosal fibers connecting other cortical areas. Thus our measures of FA were influenced by these other fibers. The use of a different analysis technique, such as tract-based spatial statistics (TBSS), may provide a more sensitive measure of these isolated callosal projections, and thus may lead to different conclusions. TBSS uses the white matter tract skeleton for spatial normalization and is specifically useful for aligning FA images from multiple subjects, whereas the seed mask method (as used in the present study) uses the native image. In our study we used tractography to create the seed mask for each subject. If we used tractography to create a standard space seed, then FA measures would be dependent upon the accuracy of alignment of each subject to the standard space. We avoided this problem by using each subject’s tractography results to determine the parcellation of the corpus callosum.
In conclusion, hand preference and hand performance on a dipping tool use task are correlated in chimpanzees; the preferred hand is the most adept hand. Furthermore, performance asymmetries correlated with FA measures but not area measures of the CC. The midsagittal area of the CC is frequently used as a marker of hemispheric specialization (Josse, Seghier, Kherif, & Price, 2008; Witelson & Goldsmith, 1991); however, midsagittal area alone may not provide the complete picture of the relationship of CC organization such as FA.
Acknowledgements
This work was supported by the National Institute of Neurological Disorders and Stroke, grants NS070717 (to KAP), NS42867 and NS73134 (to WDH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders And Stroke or the National Institutes of Health.
References
- Andrews MA, Rosenblum LA. New methodology applied to bonnet macaques (Macaca radiata) to address some contradictory evidene on manual asymmetries in Old World monkeys. Journal of Comparative Psychology. 2001;115(4):418–422. doi: 10.1037/0735-7036.115.4.418. [DOI] [PubMed] [Google Scholar]
- Annett M. Left, Right, Hand and Brain: The Right Shift Theory. London: Lawrence Erlbaum Associates; 1985. [Google Scholar]
- Basser PJ. Inferring microstructural features and the physiological state of tissues from diffusion-weighted images. NMR in Biomedicine. 1995;7–8:583–591. doi: 10.1002/nbm.1940080707. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Mattiello J, Le Bihan D. MR diffusion tensor spectroscopy and imaging. Biophysics Journal. 1994;66:259–267. doi: 10.1016/S0006-3495(94)80775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. Journal of Magnetic Resonance Series B. 1996;111:209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Pierpaoli C. A simplified method to measure the diffusion tensor from seven MR images. Magn Reson Med. 1998;39:928–934. doi: 10.1002/mrm.1910390610. [DOI] [PubMed] [Google Scholar]
- Beaton AA. The nature and determination of handedness. In: Hugdahl K, Davidson RJ, editors. The asymmetrical brain. Cambridge, MA: MIT Press; 2003. [Google Scholar]
- Bryden PJ, Roy EA, Rohr LE, Egilo S. Task demands affect manual asymmetries in pegboard performance. Laterality. 2007;12(4):365–377. doi: 10.1080/13576500701356244. [DOI] [PubMed] [Google Scholar]
- Dunham LA, Hopkins WD. Sex and handedness effects on corpus callosum morphology in chimpanzees (Pan troglodytes) Behavioral Neuroscience. 2006;120:1025–1032. doi: 10.1037/0735-7044.120.5.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagot J, Vauclair J. Manual laterality in nonhuman primates: A distinction between handedness and manual specialization. Psychological Bulletin. 1991;109(1):76–89. doi: 10.1037/0033-2909.109.1.76. [DOI] [PubMed] [Google Scholar]
- Foundas AL, Leonard CM, Hanna-Pladdy B. Variability in the anatomy of the planum temporale and posterior ascending ramus: do right- and left handers differ? Brain and Language. 2002;83:403–424. doi: 10.1016/s0093-934x(02)00509-6. [DOI] [PubMed] [Google Scholar]
- Foundas AL, Leonard CM, Heilman KM. Morphological cerebral asymmetries and handedess: the pars triangularis and lanum temporale. Archives of Neurology. 1995;52:501–508. doi: 10.1001/archneur.1995.00540290091023. [DOI] [PubMed] [Google Scholar]
- Gilissen EP, Hopkins WD. Asymmetries of the parietal operculum in chimpanzees (Pan troglodytes) in relation to handedness for tool use. Cerebral Cortex. 2012 doi: 10.1093/cercor/bhs029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould HJ, Cusick CG, Pons TP, Kaas JH. The relationship of the corpus callosum connections to electrical simulation maps of motor, supplementary motor and the frontal eye fields in owl monkeys. Journal of Comparative Neurology. 1986;247:297–325. doi: 10.1002/cne.902470303. [DOI] [PubMed] [Google Scholar]
- Habib MK, Gayraud D, Oliva A, Regis J, Salamon G, Khalil R. Effects of handedness and sex on the morphology of the corpus callosum: a study with brain magnetic resonance imaging. Brain and Cognition. 1991;16:41–61. doi: 10.1016/0278-2626(91)90084-l. [DOI] [PubMed] [Google Scholar]
- Hofer S, Frahm J. Topography of the human corpus callosum revisited - Comprehensive fiber tractography using diffusion tensor magnetic resonance imaging. NeuroImage. 2006;32:989–994. doi: 10.1016/j.neuroimage.2006.05.044. [DOI] [PubMed] [Google Scholar]
- Hopkins WD, Dunham L, Cantalupo C, Taglialatela JP. The association between handedness, brain asymmetries, and corpus callosum size in chimpanzees (Pan troglodytes) Cerebral Cortex. 2007;17(8):1757–1765. doi: 10.1093/cercor/bhl086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Pearson K. Chimpanzee (Pan troglodytes) handedness: variability across multiple measures of hand use. Journal of Comparative Psychology. 2000;114:126–135. doi: 10.1037/0735-7036.114.2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Russell JL. Further evidence of a right hand advantage in motor skill by chimpanees (Pan troglodytes) Neuropsychologia. 2004;42(7):990–996. doi: 10.1016/j.neuropsychologia.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Hopkins WD, Russell JL, Schaeffer JA, Gardner M, Schapiro SJ. Handedness for tool use in captive chimpanzees (Pan troglodytes): Sex differences, performance, heritability and comparion to the wild. Behaviour. 2009;146:1463–1483. doi: 10.1163/156853909X441005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Taglialatela J, Leavens DA, Russell JL, Schapiro SJ. Behavioral and brain asymmetries in chimpanzees. In: Lonsdorf EV, Ross SR, Matsuzawa T, editors. The mind of the chimpanzee. Chicago University of Chicago Press; 2010. pp. 60–74. [Google Scholar]
- Hopkins WD, Washburn DA, Berke L, Williams M. Behavioral asymmetries of psychomotor performance in rhesus monkeys (Macaca mulatta): a dissociation between hand preference and skill. Journal of Comparative Psychology. 1992;106(4):392–397. doi: 10.1037/0735-7036.106.4.392. [DOI] [PubMed] [Google Scholar]
- Jiang H, van Zijl PC, Kim J, Pearlson GD, Mori S. DtiStudio: resource program for diffusion tensor computation and fiber bundle tracking. Computer Methods and Programs in Biomedicine. 2006;81:106–116. doi: 10.1016/j.cmpb.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Josse G, Seghier ML, Kherif F, Price CJ. Explaining function with anatomy: language lateralization and corpus callosum size. The Journal of Neuroscience. 2008;28:14132–14139. doi: 10.1523/JNEUROSCI.4383-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bihan D. Diffusion and perfusion MRI. New York: Raven; 1995. [Google Scholar]
- Lilak AL, Phillips KA. Consistency of hand preference across low-level and high-level tasks in capuchin monkeys (Cebus apella) American Journal of Primatology. 2008;70:254–260. doi: 10.1002/ajp.20485. [DOI] [PubMed] [Google Scholar]
- Lonsdorf EV, Hopkins WD. Wild chimpanzees show population level handedness for tool use. Proceedings of the National Academy of Sciences. 2005;102:12634–12638. doi: 10.1073/pnas.0505806102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luders E, Cherbuin N, Thompson PM, Gutman B, Anstey KJ, Sachdev P, Toga AW. When more is less: Associations between corpus callosum size and handedness lateralization. Neuroimage. 2010;52:43–49. doi: 10.1016/j.neuroimage.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffat SD, Hampson E, Lee DH. Morphology of the planum temporale and corpus callosum in left handes with evidence of left and right hemisphere speech representation. Brain. 1998;121:2369–2379. doi: 10.1093/brain/121.12.2369. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh Inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Padberg J, Franca JG, DCooke DF, Soares JGM, Rosa MGP, Fiorani M, Jr, Krubitzer L. Parallel evolution of cortical areas involved in skilled hand use. J Neurosci. 2007;27:10106–10115. doi: 10.1523/JNEUROSCI.2632-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips KA, Hopkins WD. Topography of the chimpanzee corpus callosum. PLoS ONE. 2012;7(2):e31941. doi: 10.1371/journal.pone.0031941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porac C, Coren S. Lateral preferences and human behavior. New York: Springer; 1981. [Google Scholar]
- Provins KA, Magliaro The measurement of handedness by preference and performance tests. Brain and Cognition. 1993;22(2):171–181. doi: 10.1006/brcg.1993.1032. [DOI] [PubMed] [Google Scholar]
- Rigal RA. Which handedness: preference or performance? Perceptual and Motor Skills. 1992;75(851–866) doi: 10.2466/pms.1992.75.3.851. [DOI] [PubMed] [Google Scholar]
- Rouiller EM, Babalian A, Kazennikov O, Moret V, Yu X-H, Wiesendanger M. Transcallosal connections of the distal forelimb representations of the primary and supplementary motor cortical areas in macaque monkeys. Experimental Brain Research. 1994;102:227–243. doi: 10.1007/BF00227511. [DOI] [PubMed] [Google Scholar]
- Smith RJ. Relative size versus controlling for size: interpretation of ratios in research on sexual dimorphism in the human corpus callosum. Current Anthropology. 2005;46:249–273. [Google Scholar]
- Solodkin A, Hlustik P, Noll DC, Small SL. Lateralization of motor circuits and handedness during finger movements. Euorpean Journal of Neurology. 2001;8:425–434. doi: 10.1046/j.1468-1331.2001.00242.x. [DOI] [PubMed] [Google Scholar]
- Spinozzi G, Truppa V, Lagana T. Grasping behavior in tufted capuchin monkeys (Cebus apella): Grip types and manual laterality for picking up a small food item. American Journal of Physical Anthropology. 2004;125:30–41. doi: 10.1002/ajpa.10362. [DOI] [PubMed] [Google Scholar]
- Steinmetz H. Structure, function and cerebral asymmetry: in vivo morphometry of the planum temporale. Neuroscience and Biobehavioral Reviews. 1996;20:587–591. doi: 10.1016/0149-7634(95)00071-2. [DOI] [PubMed] [Google Scholar]
- Witelson SF. Hand and sex differences in the isthmus and genu of the human corpus callosum. Brain. 1989;112:799–835. doi: 10.1093/brain/112.3.799. [DOI] [PubMed] [Google Scholar]
- Witelson SF, Goldsmith CH. The relationship of hand preference to anatomy of the corpus callosum in men. Brain Research. 1991;545(1–2):175–182. doi: 10.1016/0006-8993(91)91284-8. [DOI] [PubMed] [Google Scholar]