Abstract
Background & Aims
Rifaximin is used to treat patients with functional gastrointestinal disorders, but little is known about its therapeutic mechanism. We propose that rifaximin modulates the ileal bacterial community, reduces subclinical inflammation of the intestinal mucosa, and improves gut barrier function to reduce visceral hypersensitivity.
Methods
We induced visceral hyperalgesia in rats, via chronic water avoidance or repeat restraint stressors, and investigated whether rifaximin altered the gut microbiota, prevented intestinal inflammation, and improved gut barrier function. Quantitative polymerase chain reaction and 454 pyrosequencing were used to analyze bacterial 16S rRNA in ileal contents from the rats. Reverse transcription, immunoblot, and histologic analyses were used to evaluate levels of cytokines, the tight junction protein occludin, and mucosal inflammation, respectively. Intestinal permeability and rectal sensitivity were measured.
Results
Water avoidance and repeat restraint stress each led to visceral hyperalgesia, accompanied by mucosal inflammation and impaired mucosal barrier function. Oral rifaximin altered the composition of bacterial communities in the ileum (Lactobacillus species became the most abundant) and prevented mucosal inflammation, impairment to intestinal barrier function, and visceral hyperalgesia in response to chronic stress. Neomycin also changed the composition of the ileal bacterial community (Proteobacteria became the most abundant species). Neomycin did not prevent intestinal inflammation or induction of visceral hyperalgesia induced by water avoidance stress.
Conclusions
Rifaximin alters the bacterial population in the ileum of rats, leading to a relative abundance of Lactobacillus. These changes prevent intestinal abnormalities and visceral hyperalgesia in response to chronic psychological stress.
Keywords: antibiotic, intestine, irritable bowel syndrome, gut flora
Introduction
Rifaximin is a nonsystemic, broad-spectrum antibiotic that, in vitro, acts against Gram-positive, Gram-negative, and anaerobic bacteria. Approved by the Food and Drug Administration for traveler's diarrhea and minimal hepatic encephalopathy, rifaximin also appears beneficial in treating irritable bowel syndrome (IBS), effectively improving global symptoms and bloating.1-3 Although the precise mechanism responsible for its ability to alleviate IBS symptoms remains incompletely defined, a plausible mechanism may be the modulation of gut bacterial communities after rifaximin treatment.
Low-grade mucosal inflammation and abnormal intestinal permeability are common in functional bowel disorders,4-7 intestinal bacterial overgrowth8,9 or following gastrointestinal infections.10 These changes may contribute to both the pathogenesis and the symptom generation of these conditions.4,11
Chronic psychological stress contributes significantly to symptoms of functional bowel disorders,12,13 and may predispose patients to gastrointestinal infection.13,14 Chronic psychological stress may also induce visceral hyperalgesia.15,16 In animal models of visceral hypersensitivity, psychological stress appears to alter gut microbiota and gut barrier function.17-19 It is conceivable that rifaximin alters gut bacterial communities, thus preventing intestinal inflammation and impaired permeability induced by chronic stress.
Recognizing the lack of suitable IBS animal models, we chose two animal models of visceral hyperalgesia, induction by chronic water avoidance stress (WAS) or by repeat restraint stress (RS), to determine if rifaximin alters the gut microbiota, prevents subclinical intestinal inflammation, improves gut barrier function and reduces visceral hyperalgesia.
Materials and Methods
Animals
Experiments were performed on adult male Wistar rats (200–225 g). Animals were housed in plastic cages, 3 per cage, and maintained on a 12 h light:12 h dark cycle. Surgical preparations involved anesthetization with a xylazine/ketamine mixture. All procedures were performed in accordance with National Institutes of Health guidelines and were approved by the University Committee on Use and Care of Animals at the University of Michigan.
Chronic WAS protocol
Chronic exposure of adult rats to WAS was conducted as described previously.16 Animals were placed on a block in the middle of a Plexiglas tank filled with sterile water (25°C) to 1 cm below the platform height. They were maintained on the block for 1 h daily for 10 consecutive days. The sham WAS rats were placed similarly in a tank, without water, for 1 h daily for 10 days. In separate studies, rats were treated with oral gavage of rifaximin (150 mg/kg), neomycin (150 mg/kg), or water, twice daily, 6 h apart (AM and PM), for 10 consecutive days. The rats were then submitted to daily sessions of WAS or sham WAS 3 h after each AM gavage, for 10 days. Specific rifaximin and neomycin doses were based on published reports.20,21,28
Repeat RS protocol
Repeat RS of adult rats was conducted as described previously.22 Rats were submitted to a 120-min restraint in a plastic cylinder daily for 7 consecutive days. The control group remained in their cages, undisturbed, throughout the experiment. In separate studies, rats were treated with rifaximin (150 mg/kg) twice daily for 7 consecutive days, as described for the WAS group. Further details in Supplementary Methods.
Microbial DNA isolation
Microbial genomic DNA was isolated from the distal ileal contents of each rat using a modified protocol of the Qiagen DNeasy Blood & Tissue kit. Further details in Supplementary Methods.
Quantitative PCR of 16S rRNA gene
Quantitative PCR (qPCR36) was used to quantify the total number of 16S rRNA gene copies/gram in our samples. Reactions were performed on a Lightcycler 480 (Roche) with universal 16S rRNA gene primers. Further details in Supplementary Methods.
454 Pyrosequencing and data analysis
The University of Michigan Microbiome Core processed samples using the Roche 454 GS FLX Titanium platform. V3–V5 hypervariable regions of the 16S rRNA gene were targeted. Primer sets corresponded to 357F and 929R.
Operational taxonomic units, phylotype assignments, and diversity measurements
The open-source, platform-independent, community-supported software, mothur (http://www.mothur.org) was used to bin 16S rRNA gene sequences into operational taxonomic units (OTUs) and phylotypes following the Schloss standard operating procedure (SOP) (http://www.mothur.org/wiki/Schloss_SOP).23 Further details in Supplementary Methods.
Quantitative or semiquantitative RT-PCR for inflammatory cytokines and tight junction protein
Total RNA was extracted from samples of distal ileum using TRIzol reagent (Invitrogen, Carlsbad, CA). cDNA was synthesized using a reverse transcription kit (Promega, Madison, WI). Quantitative PCR of inflammatory cytokines and GAPDH and semiquantitative PCR of the tight junction protein occludin were performed on tissue-specific cDNA. Further details and primer sequences described in Supplementary Methods.
Western blot analysis
Proteins extracted from distal ileal tissues were analyzed on Ready Gel Tris-HCl (Bio-Rad, Hercules, CA). Proteins were transferred to PVDF membranes, probed with rabbit anti-occludin antibody (Zymed Laboratories, San Francisco, CA) and then with peroxidase-conjugated goat anti-rabbit IgG. Further details in Supplementary Methods.
Histology and immunohistochemistry
Measurement of gut permeability in vivo
In vivo permeability measurement was modified from previously described methods based on the gut permeability to 4-kDa fluorescent dextran.24 Further details in Supplementary Methods.
Visceromotor response to colorectal distention
The protocol for measuring visceromotor response (VMR) to colorectal distention (CRD) has been previously described.15,16 See Supplementary Methods.
Statistical analyses
EMG amplitudes, represented by calculating the area under the curve, were normalized as a percentage of baseline response for the highest pressure (60 mm Hg) for each rat. The effects of stress and/or pharmacologic treatment on the VMR to CRD was analyzed by comparing post-stress or post-treatment measurements with baseline values at each distention pressure using 2-way repeated-measures ANOVA, followed by Bonferroni post-test comparisons. To examine data for other studies, differences between groups were compared using 1-way ANOVA followed by a Tukey post hoc test or Student t test if only two groups were applied. Results are expressed as means ± SEM. P < .05 was considered statistically significant.
Results
Chronic WAS and repeat RS induce visceral hyperalgesia
Both control and WAS rats showed pressure-dependent increases in visceromotor response (VMR) to colorectal distention (CRD) on days 0 and 11 after 10 days WAS or sham WAS. On day 11, chronic WAS induced greater increases in VMR to CRD compared to control. The increase was significantly larger compared to sham WAS at 40 mm Hg (ΔEMG response after WAS over baseline: 56.5 ± 13.8 vs. -3.0 ± 7.6 after sham WAS over baseline; P < .05), and 60 mm Hg (ΔEMG response after WAS over baseline: 65.3 ± 9.6 vs. 1.0 ± 12.2 after sham WAS over baseline; 2-way repeated-measures ANOVA/Bonferroni post-test, P < .05) (Figure 1A). Similarly, repeat RS for 7 days also induced greater increases in VMR to CRD at 40 and 60 mmHg (Figure 1B).
Figure 1.
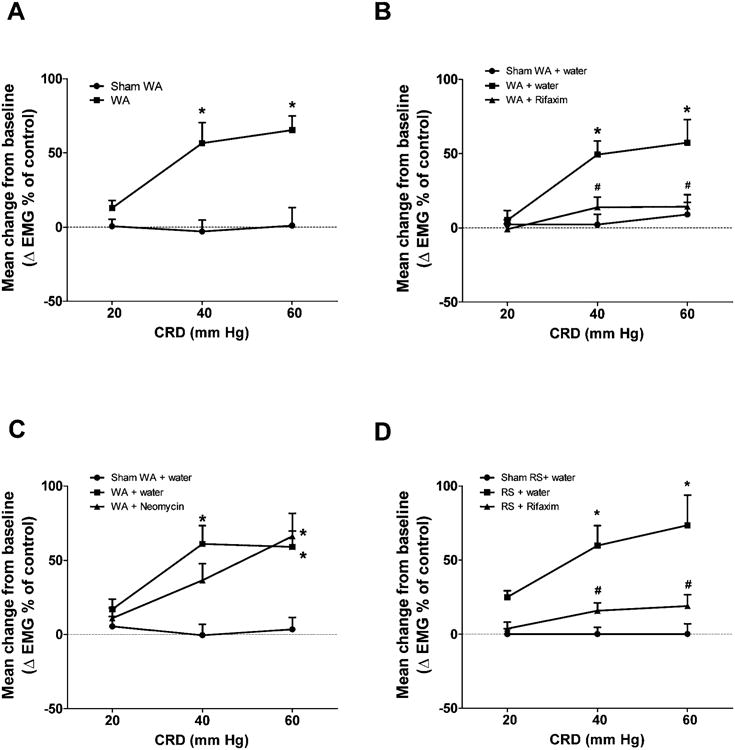
Effect of chronic stress and antibiotics on VMR to CRD. (A) EMG amplitude expressed as mean change from baseline in rats subjected to chronic WAS or sham WAS. (B) EMG amplitude expressed as mean change from baseline after chronic treatment with vehicle (water) or rifaximin (150 mg/kg, twice daily, oral gavage) in rats previously subjected to chronic WAS or sham WAS. (C) EMG amplitude expressed as mean change from baseline after chronic treatment with vehicle or neomycin (150 mg/kg, twice daily, oral gavage) in rats previously subjected to chronic WAS or sham WAS. n = 8–9. *P < .05, compared with the sham WAS, or sham WAS + water gavage controls; #P < .05, compared with WAS + water gavage rats. (D) EMG amplitude expressed as mean change from baseline after chronic treatment with vehicle (water) or rifaximin (150 mg/kg, twice daily, oral gavage) in rats previously subjected to repeat RS or sham RS. n = 6. *P < .05, compared with sham RS + water gavage controls; #P < .05, compared with RS + water gavage rats.
Chronic WAS alters bacterial community composition and diversity
We used 454 pyrosequencing of the 16S rRNA gene to determine if WAS could significantly reshape the gut bacterial community identified in the luminal contents of the terminal ileum. The three major phyla in the ileal community were Bacteroidetes, Firmicutes, and Proteobacteria, with Firmicutes being most abundant. WAS did not alter the bacterial community composition at the phylum level; however, there was an obvious reduction of several less-abundant family groups in WAS rats compared to controls (Figure 2A). Family-level classification showed that the more abundant family groups Peptostreptococcaceae, Lactobacillaceae, and unclassified Clostridiales were similar between the two cohorts. Less-abundant family groups, presented as the “tail” of the rank abundance distribution in controls, were markedly diminished in WAS rats.
Figure 2.
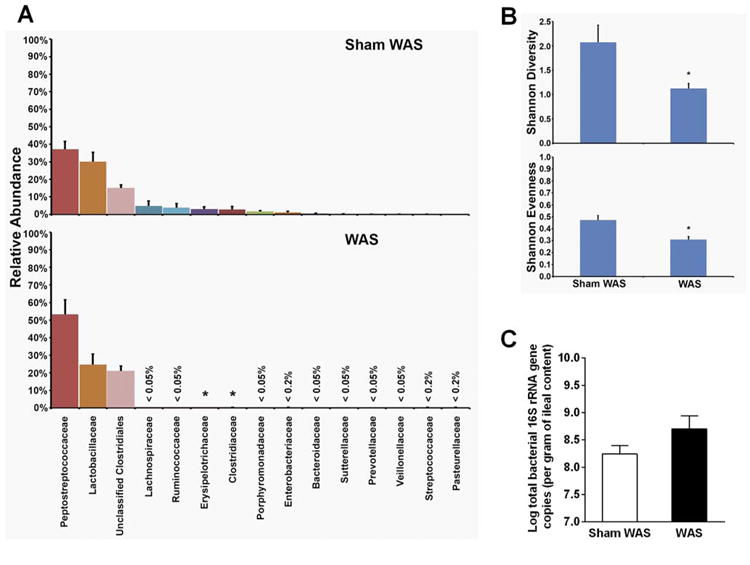
Comparison of bacterial community composition and diversity, and total bacterial load in the ileal contents of rats previously subjected to WAS or sham WAS. (A) Phylotypes classified to the level of family in order of rank abundance as a percentage of the total community. (B) Diversity (Shannon diversity index and Shannon evenness) of bacterial communities. (C) Realtime qPCR analysis of total bacterial 16s rRNA gene copies (total bacterial load). n = 9. For all figures, *significantly different than sham WAS (P < .05).
Shannon diversity and Shannon evenness were used to assess within-community diversity (α-diversity). The α-diversity of bacterial communities in the distal ileum of WAS rats was significantly lower compared to controls (Figure 2B). qPCR of total bacterial 16S rRNA gene in the effluent samples showed no significant difference in the total bacterial load in the ileal contents from WAS rats and controls (Figure 2C). Hence, our studies demonstrated that WAS led to dysbiosis characterized by loss of diversity and reduction of less-abundant family groups in the bacterial community of the distal ileum.
Chronic WAS and repeat RS increase mucosal inflammation and gut permeability
We examined gene expression levels of six inflammatory cytokines involved with mucosal inflammation in distal ileal tissues: IL-17, IL-6, IL-10, TNF-α, IFN-γ, and IL-1β. qRT-PCR measured a significant increase in IL-17, IL-6, and TNF-α mRNA levels in WAS and RS rats (Fig 3A and C) compared to controls (Student t test, P < .05). No significant difference was measured in IL-10, IFN-γ, and IL-1β expression in WAS rats (Figure 3A). In contrast, IL10 mRNA levels decreased slightly and IFN-γ and IL-1β mRNA levels were unchanged in repeat RS rats (Figure 3C). Histological analysis revealed an increased number of neutrophils and mononuclear cells in the lamina propria of the distal ileum of WAS rats compared to controls, suggesting low-grade mucosal inflammation after exposure to chronic WA stress (Supplementary Table 1). In vivo assessment of gut permeability revealed a significant increase in plasma fluorescein-conjugated dextran in WAS and repeat RS rats (Student t test, P < .05), indicating impairment of intestinal barrier function after chronic stress (Figure 5A and B).
Figure 3.
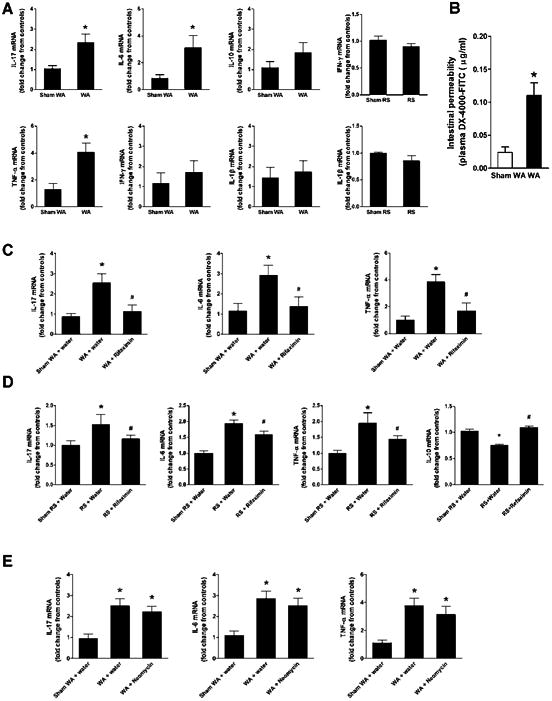
Effect of chronic stress and antibiotics on inflammatory cytokine expression in ileal tissue and gut permeability. (A, B, C, D) Cytokine mRNA levels measured with real-time qRT-PCR. Data represent fold change in target mRNA levels relative to expression in control samples after normalization to GAPDH. (A) IL-17, IL-6, IL-10, TNF-α, IFN-γ, and IL-1β mRNA levels in rats previously subjected to chronic WAS or sham WAS. (B) IL-17, IL-6, and TNF-α mRNA levels after chronic treatment with vehicle (water) or rifaximin (150 mg/kg, twice daily, oral gavage) in rats previously subjected to chronic WAS or sham WAS. (C) IL-17, IL-6, TNF-α, IL-10, IFN-γ, and IL-1β mRNA levels in rats previously subjected to repeat RS or sham RS. Note: rifaximin (150 mg/kg twice daily) significantly reduced the increase in IL-17, IL-6 and TNF-α mRNA evoked by chronic stress. Rifaximin also normalized the reduced IL-10 mRNA levels observed in repeat RS rats. (D) IL-17, IL-6, and TNF-α mRNA levels after chronic treatment with vehicle or neomycin (150 mg/kg, twice daily, oral gavage) in rats previously subjected to chronic WAS or sham WAS. n = 8-9. *P < .05, compared with sham WAS or sham RS. P < .05, compared with WAS or RS + water gavage).
Figure 5.
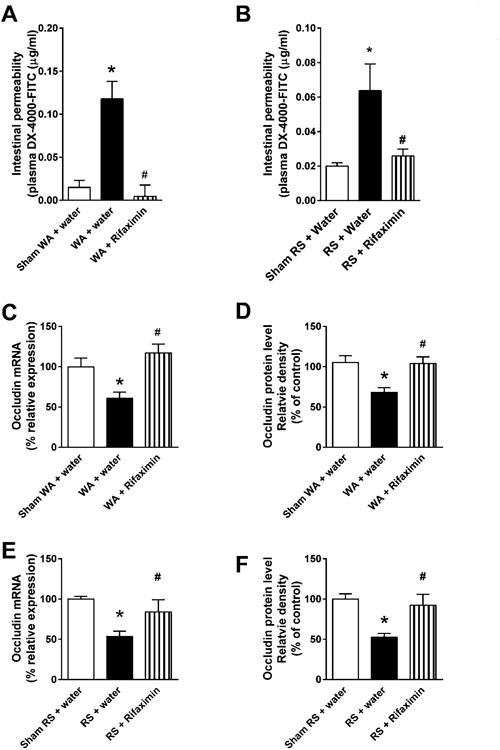
Effect of chronic treatment with rifaximin (150 mg/kg, twice daily, oral gavage) on gut permeability and ileal tight junction protein expression. (A, B) Gut permeability measured by appearance of FITC-labeled dextran in plasma. n = 8. *P < .05, compared with sham WAS + water gavage controls or sham RS + water gavage controls; #P < .05, compared with WAS + water gavage rats or RS + water gavage rats. (C) Densitometric quantification of occludin mRNA normalized to GAPDH band intensity. n = 8. *P < .05, compared with sham WAS + water gavage controls; #P < .05, compared with WAS + water gavage rats. (D) Densitometric quantification of occludin protein normalized to GAPDH band intensity. n = 6. *P < .05, compared with sham WAS + water gavage controls; #P < .05, compared with WAS + water gavage rats. (E) Densitometric quantification of occludin mRNA normalized to GAPDH band intensity, n = 6. *P < .05, compared with sham RS + water gavage controls; #P < .05, compared with RS + water gavage rats. (F) Densitometric quantification of occludin protein normalized to GAPDH band intensity. n = 6. *P < .05, compared with sham RS + water gavage controls; #P < .05, compared with RS + water gavage.
Rifaximin modulates bacterial load and bacterial community composition
Chronic rifaximin treatment in WAS rats significantly decreased the total bacterial number of 16S copies from 109.6. copies/g ileal content in controls to 108.8 copies/g in WAS rats, representing an 84% reduction in total bacterial load (1-way ANOVA/Bonferroni post-test, P < .05) (Figure 4A). At the phylum level, the bacterial community composition was unchanged (Figure 4B). However, at the family level, changes were obvious (Figure 4B). Most striking was the change in abundance of Lactobacillaceae (identified as Lactobacillus spp.) in WAS rats after rifaximin treatment. The abundance of Lactobacillus in sham WAS and WAS rats after rifaximin treatment increased significantly from 30% and 25%, respectively, to 87% (Kruskal–Wallis, all pairwise comparisons, P < .05) (Figure 4C). The relative abundance of Clostridiaceae, Erysipelotrichaceae, and Peptostreptococcaceae (CEP) was significantly less (Figure 4C). The α-diversity decreased significantly from 1.5 ± 0.2 in sham WAS to 0.4 ± 0.2 in the WAS + rifaximin group (Tukey post hoc test, all pairwise comparisons, P < .05). This loss of α-diversity after rifaximin treatment may be attributed to the significant increase in the relative abundance of Lactobaccillus and the decrease in the more abundant CEP groups. In a replicate experiment, a significant increase in Lactobacillus was observed in WAS + rifaximin groups (Supplementary Figure 1). Three-dimensional nonmetric multidimensional scaling (NMDS) plots, created using èYC distances, revealed that the community structure of the WAS group was significantly different from that of the sham WAS group (AMOVA, P < .05) (Figure 4D). The WAS + rifaximin community structures were significantly different from those of the sham WAS and WAS groups (AMOVA, all pairwise comparisons P < .05) (Figure 4D).
Figure 4.
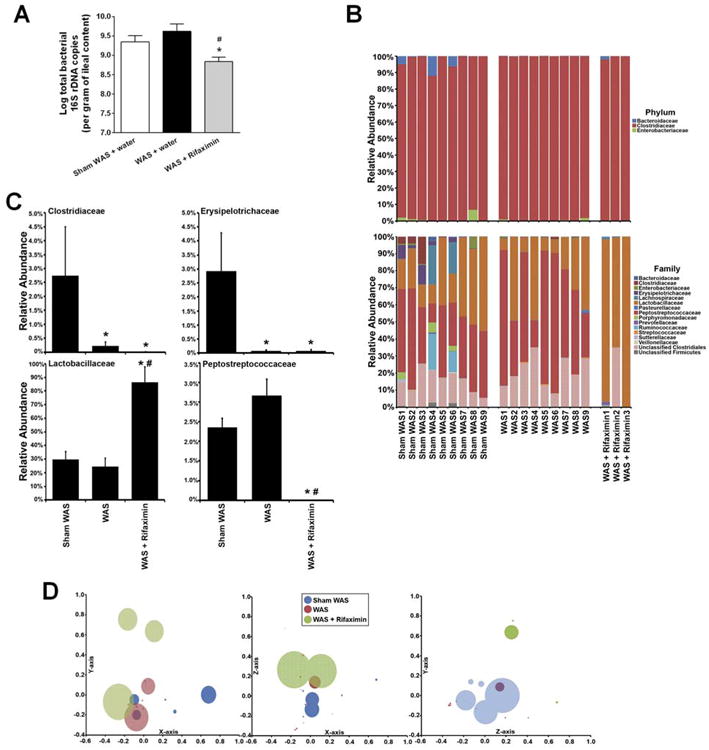
Effect of chronic treatment with rifaximin (150 mg/kg, twice daily, oral gavage) on total bacterial load and bacterial community composition in ileal contents. (A) Total bacterial 16s rRNA gene copies. n = 8. *P < .05, compared with sham WAS + water gavage controls; P < .05, compared with WAS + water gavage rats. (B) Variation in bacterial community composition in the terminal ileum at phylum and family levels. (C) Relative abundance of selected phylotypes, identified at family level. n = 9 in sham WAS and WAS groups, n = 3 in WAS + rifaximin group. * significantly different than sham WAS; significantly different than WAS (Kruskal–Wallis with Conover–Inman post hoc test for multiple comparisons, P < .05). (D) Bacterial community composition analyzed with NMDS plots using a θYC distance matrix of OTU-based data. Circles represent distinct bacterial communities identified in luminal contents of terminal ileum of individual rats. Circles that appear larger are closer in the axis not represented and circles that are smaller are further away in the axis not represented; some circles have been made transparent. For this NMDS plot, stress = 0.09, r2 = 0.97.
Rifaximin prevents stress-induced mucosal inflammation, intestinal barrier impairment, and visceral hyperalgesia
Oral gavage of rifaximin prevented increased IL-17, IL-6, and TNF-α mRNA levels in rats subjected to chronic WAS or repeat RS (1-way ANOVA/Bonferroni post-test, P < .05, vs the WAS + water gavage rats or RS + water gavage rats) (Figure 3B and C). Rifaximin also normalized IL-10 mRNA levels in the repeat RS group (P < 0.05, Fig 3D). Chronic rifaximin treatment significantly attenuated mucosal infiltration of neutrophils and mononuclear cells (Supplementary Table 2).
In separate studies, rifaximin treatment prevented the increase of gut permeability to fluorescein-conjugated dextran induced by chronic stress or repeat RS (1-way ANOVA/Bonferroni post-test, P < .05, vs WAS + water gavage rats or RS + water gavage rats) (Figure 5A and B). qRT-PCR and Western blot analysis of the tight junction protein occludin, a key marker of tight junction integrity,25 in distal ileal tissues showed a significant decrease in gene and protein expression levels after chronic WAS or repeat RS compared to sham WAS or sham RS (1-way ANOVA/Bonferroni post-test, P < .05) (Figure 5C–F). Oral gavage of rifaximin prevented the reduction of occludin mRNA and protein induced by both forms of stress (Figure 5C–F). These results suggest that modulation of ileal bacterial communities by rifaximin prevents impairment of intestinal barrier function induced by chronic stress.
We next assessed the preventive effect of rifaximin on the development of visceral hyperalgesia in chronic WAS and repeat RS rats. Repeated oral gavage of rifaximin significantly attenuated the increased VMR to CRD induced by both forms of stress at 40 and 60 mm Hg (P < .05) (Figure 1B and D).
Effect of neomycin on ileal bacterial communities
To determine if the effects of rifaximin on stress-induced intestinal abnormalities and visceral hyperalgesia are antibiotic specific, we compared the effects of rifaximin with those of neomycin.
Repeated oral administration of neomycin to WAS rats significantly reduced the total bacterial number of 16S copies, from 109.5 to 108.4 copies/g ileal content, (P < .05, 1-way ANOVA/Bonferroni post-test) (Figure 6A). However, changes in bacterial communities, distinct from those observed with rifaximin, were measured in the WAS + neomycin group (Figure 6B). The abundance of Proteobacteria increased significantly after neomycin treatment vs sham WAS and WAS rats (Kruskal–Wallis, all pairwise comparisons, P < .05). This increase in Proteobacteria was attributed to significant changes in the relative abundance of Enterobacteriaceae (Figure 6B–C) and Enterococcaceae (Figure 6B). Enterococcaceae was not detected in sham WAS and WAS groups. However, after neomycin treatment, Enterococcaceae had a mean abundance of 4.7 ± 1.9; accompanied by a significant decrease in the abundance of Corynebacteriaceae, Erysipelotrichaceae, Peptostreptococcaceae, and Staphylococcaceae (Figure 6C). Neomycin treatment did not significantly change the relative abundance of lactobacilli compared to WAS (19.5 ± 6.2 vs 26.5 ± 3.9, NS). The α-diversity significantly increased in the WAS + neomycin (2.1 ± 0.14) vs WAS (1.3 ± 0.05) (Tukey post hoc test, all pairwise comparisons, P < .05). This observed increase in α-diversity was caused by loss of Firmicutes and increase in Proteobacteria, which facilitated community diversification. NMDS plots, using θYC distances, revealed that sham WAS and WAS community structures were significantly different from that of the WAS + neomycin group (AMOVA, all pairwise comparisons, P < .05) (Figure 6D).
Figure 6.
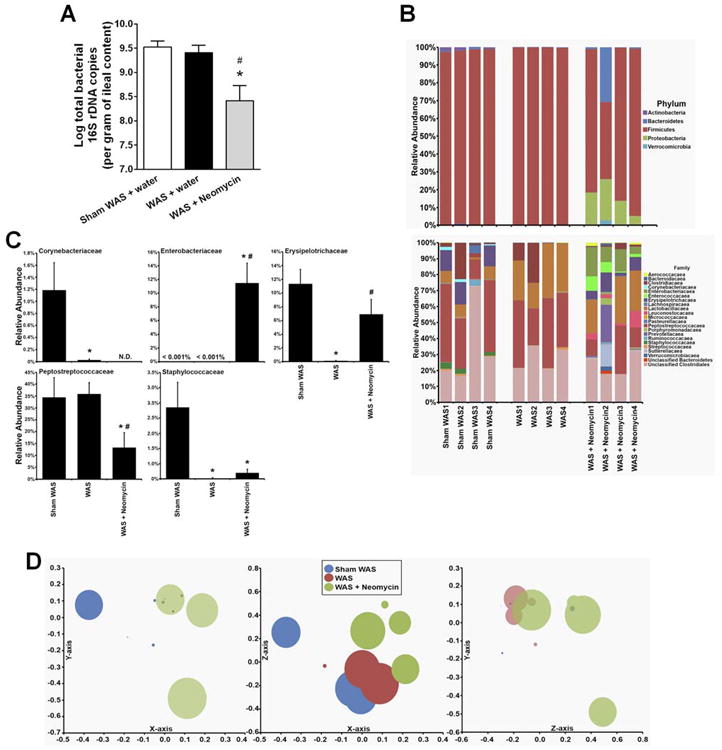
Effect of chronic neomycin treatment (150 mg/kg, twice daily, oral gavage) on total bacterial load and bacterial community composition in ileal contents. (A) Total bacterial 16s rRNA gene copies. *P < .05, compared with sham WAS + water gavage controls; #P < .05, compared with WAS + water gavage rats. (B) Variation in bacterial community composition in the terminal ileum at phylum and family levels. (C) Relative abundance of selected phylotypes, identified at the family level. *significantly different than sham WAS; #significantly different than WAS (Kruskal–Wallis with Conover–Inman post hoc test for multiple comparisons, P < .05). (D) Bacterial community composition analyzed with NMDS plots using a θYC distance matrix of OTU-based data. For this NMDS plot, stress = 0.06, r2 = 0.98. n = 4. N.D. = not detected.
Effect of neomycin on stress-induced mucosal inflammation, impaired intestinal barrier function, and visceral hyperalgesia
Neomycin did not prevent the increase of IL-17, IL-6, and TNF-α mRNA levels in rats subjected to chronic WAS (Figure 3D), nor mucosal infiltration of neutrophils and mononuclear cells (Supplementary Table 3). Also, in vivo measurement of gut permeability, qRT-PCR, and Western blotting of the tight junction protein occludin indicated that neomycin did not prevent intestinal barrier dysfunction induced by WAS (Figure 7). In contrast to rifaximin, repeated oral gavage of neomycin was ineffective in preventing the stress-induced increase in VMR to CRD s (Figure 1C).
Figure 7.
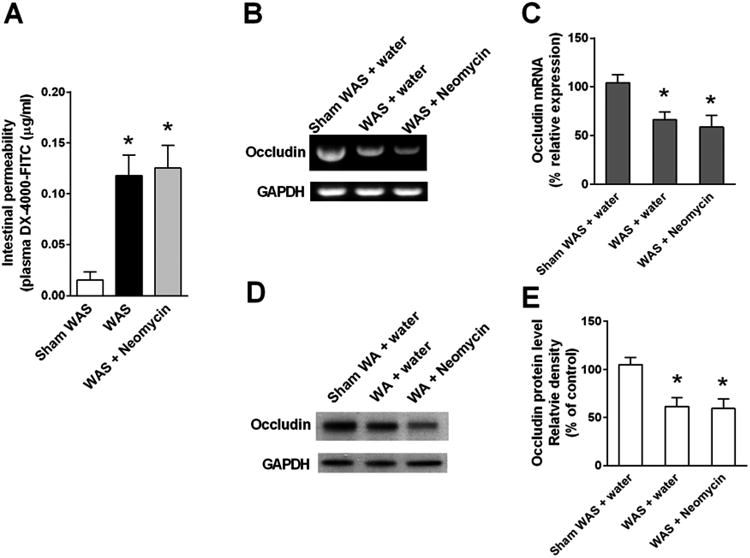
Effect of chronic treatment with neomycin (150 mg/kg, twice daily, oral gavage) on gut permeability and ileal tight junction protein expression. (A) Gut permeability measured by appearance of FITC-labeled dextran in plasma. n = 8. (B) Representative gel images of semiquantitative RT-PCR of tight junction protein occludin mRNA in ileal tissue. (C) Densitometric quantification of occludin mRNA normalized to GAPDH band intensity. n = 6. (D) Representative immunoblots show occludin expression in ileal tissue. (E) Densitometric quantification of occludin protein normalized to GAPDH band intensity. n = 6. For all groups, *P < .05, compared with sham WAS + water gavage controls.
Discussion
We used two rat models of chronic stress to study the effects of rifaximin on gut microbiota and mucosal inflammation. Repeated exposure to WAS for 10 days or RS for 7 days induced mucosal inflammation characterized by an increase in cytokines gene expression and a marked increase in gut permeability. An accompanying reduction in gene and protein expression of the tight junction protein occludin indicated impairment of tight junction integrity. Behavioral pain studies showed marked enhancement of CRD-induced VMR in rats subjected to both forms of stress, similar to previously reported findings.16,22
Preclinical15-19 and clinical studies4-7 link visceral hyperalgesia to enhanced gut permeability. A cause–effect relationship between mucosal barrier alterations and visceral hypersensitivity has been described in a rodent model in which chemical blockade of enhanced stress-induced paracellular permeability was accompanied by reduced sensitivity to colonic distention.26 We have shown that small bowel inflammation evoked by chronic stress releases cytokines, such as PGE2, which activates the HCN2 channel in the dorsal root ganglia innervating the lower gut. This may contribute to rectal hypersensitivity.27,28
Psychological stressors have been reported to alter bacterial community structure. Two studies, both using 454 pyrosequencing, describe the effects of social stress and prolonged restraint on murine bacterial communities.19,29 The stress of prolonged restraint significantly changed the bacterial community structure, reduced species richness and diversity, and facilitated host susceptibility to a bacterial pathogen.19 Social stress also significantly altered bacterial community composition and decreased species richness and diversity.29 We showed that chronic stress caused a significant loss of several bacterial family groups and decreased community diversity and evenness. These changes in bacterial community composition may contribute to mucosal barrier impairment and inflammation.
Research indicates that commensal bacteria influence the expression of host genes whose products regulate mucosal barrier function, nutrient absorption, xenobiotic metabolism, and angiogenesis.30 Disruption of the delicate and mutually beneficial relationship between the intestinal microbiota and host results in dysbiosis,31-34 which may contribute to mucosal inflammation, barrier dysfunction, and visceral hypersensitivity.35
We showed that oral administration of rifaximin modulated gut bacterial communities by significantly reducing the ileal bacterial load and altering the composition of bacterial community in the distal ileum. Rifaximin also prevented mucosal inflammation, barrier impairment, and visceral hyperalgesia in chronically stressed rats. This supports our hypothesis that the development of visceral hyperalgesia is likely, at least in part, mediated by aberrant microbial interactions resulting in mucosal inflammation and barrier impairment after exposure to chronic stress.
We identified specific changes in ileal bacterial communities characterized by a dominance of lactobacilli after rifaximin treatment, which may explain the anti-inflammatory effect of rifaximin on the intestinal mucosa. Exposure of rats to the probiotics L. helveticus and L. rhamnosus has been shown to prevent the stress-induced increase in adherence as well as the translocation of commensal bacteria.36 Another study reported that L. paracasei significantly restored normal mucosal permeability and reduced visceral pain in stressed rats.17 In our study, rifaximin treatment led to a dominance of lactobacilli and normalization of IL-6 and TNF-α mRNA levels in the distal ileum of WAS rats. Inflammatory cell infiltration in the lamina propria was also reduced; an effect likely mediated by Lactobacillus, considering the reported Lactobacillus-induced downregulation of proinflammatory cytokines IL-6 and TNF-α in Crohn's disease.37,38
To show that the beneficial actions of rifaximin are unrelated to the nonspecific effects of a reduced ileal bacterial load, we studied the effects of neomycin, another poorly absorbed antibiotic used to treat hepatic encephalopathy and small bowel bacterial overgrowth. Oral rifaximin and neomycin in WAS rats significantly reduced the ileal bacterial content and altered the composition of the bacterial community. In neomycin-treated WAS rats the increase in the relative abundance of Proteobacteria was attributed to Enterobacteriaceae and Enterococcaceae, in contrast to the dominance of Lactobacillaceae after rifaximin treatment. Further, neomycin did not prevent mucosal inflammation and gut barrier impairment. Note that neomycin with bacitracin has been shown to alter the microbiota in mice, substantially reducing the lactobacilli; the resulting subclinical mucosal inflammation and visceral hyperalgesia were attenuated by treatment with L. paracasei.39
Although rifaximin appears to alter ileal bacterial communities, leading to a relative abundance of Lactobacillus that may prevent mucosal abnormalities and visceral hyperalgesia induced by chronic stress, a direct immunomodulatory effect must be considered.40 Rifaximin's anti-inflammatory effect depends on its ability to activate the pregnane X receptor (PXR). PXR ligand-binding domains are highly divergent across species.41 For example, human and rat PXRs share only 76% amino acid identity in their ligand-binding domains. PXR activation profiles also differ significantly across species. Rifampicin, a well-known human PXR ligand and a macrolide with strong structural similarity to rifaximin, activates human and rabbit PXRs but has virtually no activity on mouse or rat PXRs.41 Taken together, these characteristics suggest that the effects of rifaximin observed in our stressed rat models are not likely caused by “direct” anti-inflammatory action on the mucosa through rat PXR activation. We also considered that gastrointestinal motility may alter the composition of gut microbiota. Although it is a macrolide, rifaximin does not affect rat gastric or intestinal motility.20 Thus, the changes in bacterial counts and composition following rifaximin treatment are not likely caused by altered gastrointestinal motility.
Several clinical trials have evaluated the efficacy and safety of rifaximin in IBS treatment.1,3,42,43 Meta-analysis has shown rifaximin to be more efficacious than placebo in improving global symptoms and bloating in IBS.2 Rifaximin offers modest therapeutic gain (∼10%), similar to other available IBS therapies. It is tempting to speculate that these beneficial effects are related to the increased prevalence of lactobacilli induced by rifaximin. However, one must recognize that rodent models have limited predictive value for IBS treatment in humans, as illustrated by extensive research describing positive pharmacological benefits for rodents that never translated into benefits for humans with IBS. Furthermore, our experimental design does not necessarily imply that rifaximin treatment is beneficial for the symptomatic patient but rather, rifaximin prophylaxis may be beneficial in preventing a stress-induced flare.
In conclusion, rifaximin reduces ileal bacterial load andalters bacterial communities with a dominance of lactobacilli in rats subjected to chronic stress and these results are followed by amelioration of mucosal inflammation and normalization of visceral hypersensitivity. This may explain the benefits of rifaximin in the treatment of various functional gastrointestinal disorders.
Supplementary Material
Acknowledgments
Grant support: The studies were supported by the National Institutes of Health grants R01DK058913 and P30DK34933
Abbreviations
- AMOVA
analysis of molecular variation
- ANOVA
analysis of variance
- CRD
colorectal distention
- EMG
electromyographic
- IBS
irritable bowel syndrome
- IL
interleukin
- INF-γ
interferon-γ
- NMDS
nonmetric multidimensional scaling
- OTU
operational taxonomic unit
- RF
rifaximin
- RT-PCR
reverse-transcription–polymerase chain reaction
- TNF-α
tumor necrosis factor-α
- VMR
visceromotor response
- WAS
water avoidance stress
Footnotes
Disclosures: The authors declare no conflicts of interest.
Author contributions:
Study concept and design: Dabo Xu and Chung Owyang
Acquisition of data: Dabo Xu, Jun Gao, Merritt Gillilland III, Xiaoyin Wu, Il Song, and John Kao
Analysis and interpretation of data: Dabo Xu, Jun Gao, Merritt Gillilland III
Critical revision of manuscript for important intellectual content: Chung Owyang
Statistical analysis: Dabo Xu, Jun Gao, Merritt Gillilland III
Obtained funding: Chung Owyang
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pimentel M, Lembo A, Chey WD, et al. Rifaximin therapy for patients with irritable bowel syndrome without constipation. N Engl J Med. 2011;364:22–32. doi: 10.1056/NEJMoa1004409. [DOI] [PubMed] [Google Scholar]
- 2.Menees SB, Maneerattannaporn M, Kim HM, et al. The efficacy and safety of rifaximin for the irritable bowel syndrome: a systematic review and meta-analysis. Am J Gastroenterol. 2012;107:28–35. doi: 10.1038/ajg.2011.355. quiz 36. [DOI] [PubMed] [Google Scholar]
- 3.Pimentel M, Park S, Mirocha J, et al. The effect of a nonabsorbed oral antibiotic (rifaximin) on the symptoms of the irritable bowel syndrome: a randomized trial. Ann Intern Med. 2006;145:557–563. doi: 10.7326/0003-4819-145-8-200610170-00004. [DOI] [PubMed] [Google Scholar]
- 4.Ford AC, Talley NJ. Mucosal inflammation as a potential etiological factor in irritable bowel syndrome: a systematic review. J Gastroenterol. 2011;46:421–431. doi: 10.1007/s00535-011-0379-9. [DOI] [PubMed] [Google Scholar]
- 5.Dunlop SP, Hebden J, Campbell E, et al. Abnormal intestinal permeability in subgroups of diarrhea-predominant irritable bowel syndromes. Am J Gastroenterol. 2006;101:1288–1294. doi: 10.1111/j.1572-0241.2006.00672.x. [DOI] [PubMed] [Google Scholar]
- 6.Zhou Q, Zhang B, Verne GN. Intestinal membrane permeability and hypersensitivity in the irritable bowel syndrome. Pain. 2009;146:41–46. doi: 10.1016/j.pain.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Piche T, Barbara G, Aubert P, et al. Impaired intestinal barrier integrity in the colon of patients with irritable bowel syndrome: involvement of soluble mediators. Gut. 2009;58:196–201. doi: 10.1136/gut.2007.140806. [DOI] [PubMed] [Google Scholar]
- 8.Lauritano EC, Valenza V, Sparano L, et al. Small intestinal bacterial overgrowth and intestinal permeability. Scand J Gastroenterol. 2010;45:1131–1132. doi: 10.3109/00365521.2010.485325. [DOI] [PubMed] [Google Scholar]
- 9.Camilleri M, Madsen K, Spiller R, et al. Intestinal barrier function in health and gastrointestinal disease. Neurogastroenterol Motil. 2012;24:503–512. doi: 10.1111/j.1365-2982.2012.01921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marshall JK, Thabane M, Garg AX, et al. Intestinal permeability in patients with irritable bowel syndrome after a waterborne outbreak of acute gastroenteritis in Walkerton, Ontario. Aliment Pharmacol Ther. 2004;20:1317–1322. doi: 10.1111/j.1365-2036.2004.02284.x. [DOI] [PubMed] [Google Scholar]
- 11.Nobaek S, Johansson ML, Molin G, et al. Alteration of intestinal microflora is associated with reduction in abdominal bloating and pain in patients with irritable bowel syndrome. Am J Gastroenterol. 2000;95:1231–1238. doi: 10.1111/j.1572-0241.2000.02015.x. [DOI] [PubMed] [Google Scholar]
- 12.Bennett EJ, Tennant CC, Piesse C, et al. Level of chronic life stress predicts clinical outcome in irritable bowel syndrome. Gut. 1998:256–261. doi: 10.1136/gut.43.2.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gwee KA, Leong YL, Graham C, et al. The role of psychological and biological factors in postinfective gut dysfunction. Gut. 1999;44:400–406. doi: 10.1136/gut.44.3.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elenkov IJ, Chrousos GP. Stress system—organization, physiology and immunoregulation. Neuroimmunomodulation. 2006;13:257–267. doi: 10.1159/000104853. [DOI] [PubMed] [Google Scholar]
- 15.Coutinho SV, Plotsky PM, Sablad M, et al. Neonatal maternal separation alters stress-induced responses to viscerosomatic nociceptive stimuli in rat. Am J Physiol Gastrointest Liver Physiol. 2002;282:G307–16. doi: 10.1152/ajpgi.00240.2001. [DOI] [PubMed] [Google Scholar]
- 16.Bradesi S, Schwetz I, Ennes HS, et al. Repeated exposure to water avoidance stress in rats: a new model for sustained visceral hyperalgesia. Am J Physiol Gastrointest Liver Physiol. 2005;289:G42–53. doi: 10.1152/ajpgi.00500.2004. [DOI] [PubMed] [Google Scholar]
- 17.Eutamene H, Lamine F, Chabo C, et al. Synergy between Lactobacillus paracasei and its bacterial products to counteract stress-induced gut permeability and sensitivity increase in rats. J Nutr. 2007;137:1901–1907. doi: 10.1093/jn/137.8.1901. [DOI] [PubMed] [Google Scholar]
- 18.O'Mahony SM, Marchesi JR, Scully P, et al. Early life stress alters behavior, immunity, and microbiota in rats: implications for irritable bowel syndrome and psychiatric illnesses. Biol Psychiatry. 2009;65:263–267. doi: 10.1016/j.biopsych.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 19.Bailey MT, Dowd SE, Parry NM, et al. Stressor exposure disrupts commensal microbial populations in the intestines and leads to increased colonization by Citrobacter rodentium. Infect Immunity. 2010;78:1509–1519. doi: 10.1128/IAI.00862-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scarpignato C, Pelosini I. Experimental and clinical pharmacology of rifaximin, a gastrointestinal selective antibiotic. Digestion. 2006;73(Suppl 1):13–27. doi: 10.1159/000089776. [DOI] [PubMed] [Google Scholar]
- 21.Jahraus CD, Schemera B, Rynders P, et al. Rifaximin diminishes neutropenia following potentially lethal whole-body radiation. Exp Biol Med (Maywood) 2010;235:900–905. doi: 10.1258/ebm.2010.009333. [DOI] [PubMed] [Google Scholar]
- 22.Zelena D, Mergl Z, Foldes A, et al. Role of hypothalamic inputs in maintaining pituitary-adrenal responsiveness in repeated restraint. Am J Physiol Endocrin Metab. 2003;285:E1110–E1117. doi: 10.1152/ajpendo.00219.2003. [DOI] [PubMed] [Google Scholar]
- 23.Schloss PD, Westcott SL, Ryabin T, et al. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol. 2009;75:7537–7541. doi: 10.1128/AEM.01541-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Q, Fang CH, Hasselgren PO. Intestinal permeability is reduced and IL-10 levels are increased in septic IL-6 knockout mice. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1013–R1023. doi: 10.1152/ajpregu.2001.281.3.R1013. [DOI] [PubMed] [Google Scholar]
- 25.Brun P, Castagliuolo I, Di Leo V, et al. Increased intestinal permeability in obese mice: new evidence in the pathogenesis of nonalcoholic steatohepatitis. Am J Physiol Gastrointest Liver Physiol. 2007;292:G518–G525. doi: 10.1152/ajpgi.00024.2006. [DOI] [PubMed] [Google Scholar]
- 26.Ait-Belgnaoui A, Bradesi S, Fioramonti J, et al. Acute stress-induced hypersensitivity to colonic distension depends upon increase in paracellular permeability: role of myosin light chain kinase. Pain. 2005;113:141–147. doi: 10.1016/j.pain.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 27.Grabauskas G, Xu D, Wu X, et al. HCN2 ion channel in dorsal root ganglion neurons is responsible for visceral hypersensitivity in chronically stressed rat. Gastroenterology. 2012;142(Suppl 1):A–238. [Google Scholar]
- 28.Grabauskas G, Xu D, Song I, et al. PGE2which activates HCN2 ion channel in dorsal root ganglia mediates visceral hypersensitivity in chronically stressed rats. Gastroenterology. 2013;144(Suppl 1):S-133–S-134. [Google Scholar]
- 29.Bailey MT, Dowd SE, Galley JD, et al. Exposure to a social stressor alters the structure of the intestinal microbiota: implications for stressor-induced immunomodulation. Brain Behav Immunity. 2011;25:397–407. doi: 10.1016/j.bbi.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hooper LV, Wong MH, Thelin A, et al. Molecular analysis of commensal host-microbial relationships in the intestine. Science. 2001;291:881–884. doi: 10.1126/science.291.5505.881. [DOI] [PubMed] [Google Scholar]
- 31.Rajilic-Stojanovic M, Biagi E, Heilig HG, et al. Global and deep molecular analysis of microbiota signatures in fecal samples from patients with irritable bowel syndrome. Gastroenterology. 2011;141:1792–1801. doi: 10.1053/j.gastro.2011.07.043. [DOI] [PubMed] [Google Scholar]
- 32.Malinen E, Rinttila T, Kajander K, et al. Analysis of the fecal microbiota of irritable bowel syndrome patients and healthy controls with real-time PCR. Am J Gastroenterol. 2005;100:373–382. doi: 10.1111/j.1572-0241.2005.40312.x. [DOI] [PubMed] [Google Scholar]
- 33.Kassinen A, Krogius-Kurikka L, Makivuokko H, et al. The fecal microbiota of irritable bowel syndrome patients differs significantly from that of healthy subjects. Gastroenterology. 2007;133:24–33. doi: 10.1053/j.gastro.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 34.Jeffery IB, O'Toole PW, Ohman L, et al. An irritable bowel syndrome subtype defined by species-specific alterations in faecal microbiota. Gut. 2012;61:997–1006. doi: 10.1136/gutjnl-2011-301501. [DOI] [PubMed] [Google Scholar]
- 35.Collins SM, Bercik P. The relationship between intestinal microbiota and the central nervous system in normal gastrointestinal function and disease. Gastroenterology. 2009;136:2003–2014. doi: 10.1053/j.gastro.2009.01.075. [DOI] [PubMed] [Google Scholar]
- 36.Zareie M, Johnson-Henry K, Jury J, et al. Probiotics prevent bacterial translocation and improve intestinal barrier function in rats following chronic psychological stress. Gut. 2006;55:1553–1560. doi: 10.1136/gut.2005.080739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Borruel N, Carol M, Casellas F, et al. Increased mucosal tumour necrosis factor alpha production in Crohn's disease can be downregulated ex vivo by probiotic bacteria. Gut. 2002;51:659–664. doi: 10.1136/gut.51.5.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Llopis M, Antolin M, Carol M, et al. Lactobacillus casei downregulates commensals' inflammatory signals in Crohn's disease mucosa. Inflamm Bowel Dis. 2009;15:275–283. doi: 10.1002/ibd.20736. [DOI] [PubMed] [Google Scholar]
- 39.Verdú EF, Bercik P, Verma-Gandhu M, et al. Specific probiotic therapy attenuates antibiotic induced visceral hypersensitivity in mice. Gut. 2006;55:182–190. doi: 10.1136/gut.2005.066100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mencarelli A, Renga B, Palladino G, et al. Inhibition of NF-Kb by a PXR-dependent pathway mediates counter-regulatory activities of rifaximin on innate immunity in intestinal epithelial cells. Eur J Pharmacol. 2011;668:317–324. doi: 10.1016/j.ejphar.2011.06.058. [DOI] [PubMed] [Google Scholar]
- 41.Jones SA, Moore LB, Shenk JL, et al. The pregnane X receptor: a promiscuous xenobiotic receptor that has diverged during evolution. Mol Endocrinol. 2000;14:27–39. doi: 10.1210/mend.14.1.0409. [DOI] [PubMed] [Google Scholar]
- 42.Sharara AI, Aoun E, Abdul-Baki H, et al. A randomized double-blind placebo-controlled trial of rifaximin in patients with abdominal bloating and flatulence. Am J Gastroenterol. 2006;101:326–333. doi: 10.1111/j.1572-0241.2006.00458.x. [DOI] [PubMed] [Google Scholar]
- 43.Lembo A, Zakko SF, Ferreira NL, et al. Rifaximin for the treatment of diarrhea-associated irritable bowel syndrome: short-term treatment leading to long-term sustained response. Gastroenterology. 2008;134(Suppl 1):A–545. abstract T1390. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


