Abstract
Purpose of review
Animal models will be critical for preclinical evaluations of novel HIV eradication and/or functional cure strategies in the setting of suppressive combination antiretroviral therapy (cART). Here, the strengths, limitations, and challenges of recent efforts to develop nonhuman primate (NHP) models of cART-mediated suppression for use in studies of persistent virus and curative approaches are discussed.
Recent findings
A number of combinations of NHP species and viruses that recapitulate key aspects of human HIV infection have been adapted for cART-mediated suppression studies. Different cART regimens implementing drugs targeting multiple different steps of the viral life cycle have provided varying levels virologic suppression, dependent in part upon the host species, virus, drug regimen and timing, and virologic monitoring assay sensitivity. New, increasingly sensitive virologic monitoring approaches for measurements of plasma viral RNA, cell- and tissue-associated viral RNA and DNA, and the replication-competent residual viral pool in the setting of cART in NHP models are being developed to allow for the assessment of persistent virus on cART and to evaluate the impact of viral induction/eradication strategies in vivo.
Summary
Given the vagaries of each specific virus and host species, and cART regimen, each model will require further development and analysis to determine their appropriate application for addressing specific experimental questions.
Keywords: nonhuman primate, antiretroviral therapy, SIV, cure
Introduction
Combination antiretroviral therapy (cART) has transformed human immunodeficiency virus (HIV) infection from a progressive, nearly uniformly fatal infection into a treatable chronic condition, but does not represent definitive treatment due to the persistence of residual virus despite prolonged, apparently effective suppressive treatment [1–4]. This fact, along with anecdotal evidence for a “functional cure” and possible eradication of HIV in a single person under very particular clinical circumstances has galvanized efforts to develop more definitive treatments [5**,6*]. However, the risk:benefit ratio for many of the approaches being proposed for viral eradication or functional cure is high, especially in relation to standard-of-care cART, providing strong impetus for evaluation in animal models prior to clinical testing. Non-human primate (NHP) models are established as powerful tools for studying HIV transmission, pathogenesis, immune responses, and vaccine and other prevention strategies strategies, but their use to examine suppressive cART, residual virus, and to evaluate potential approaches for achieving viral eradication or functional cure has been more limited. This review will focus on the state of the art and future directions of cART in NHP models of AIDS to address both the basic biology of residual virus in the face of suppressive cART, and to evaluate the safety and proof-of-concept activity of candidate approaches for targeting such residual virus.
NHP Species and Virus
Multiple different NHP species and primate lentiviruses have been used for models of HIV infection (Table 1). Each combination of NHP species and virus arguably constitutes a distinct model with different strengths and limitations, and investigators should select the model best suited to address the question of interest in a given study, within practical constraints. Pathogenic NHP models have primarily utilized Asian macaque species, specifically rhesus macaques (Macaca mulatta), pig-tailed macaques (Macaca nemestrina), and cynomolgus macaques (Macaca fascicularis). A variety of viruses, including different clonal and viral quasispecies (“swarm”) stocks of simian immunodeficiency viruses (SIV) and various chimeric simian-human immunodeficiency viruses (SHIVs), have been used in NHP cART studies. There has even been some initial development and limited evaluation of minimally chimeric HIV strains containing small SIV derived sequences intended to confer resistance to host restriction mechanisms that limit HIV replication in macaque cells [34,35,39].
Table 1.
Pathogenic Primate Lentivirus Infection Models Used in NHP cART Studies
| Macaque species/subspecies | Virus | Comments | Selected References |
|---|---|---|---|
| Macaca mulatta, Indian | SIVmac251 | Most widely used “swarm” virus infection, consistent high peak/chronic VLs# in majority of animals, early mucosal CD4 depletion, progressive peripheral CD4 depletion, cART suppression difficult | [7–11] |
| SIVmac239 | Most widely used clonal virus infection, consistent high peak/chronic VLs in majority of animals, early mucosal CD4 depletion, progressive peripheral CD4 depletion, cART suppression difficult | [12–14], Del Prete GQ, Lifson JD, unpublished data | |
| SIVsmE660 | Widely used “swarm” virus infection, high peak VLs, chronic viral loads high in animals with permissive TRIM5 genotype/controlled in restrictive TRIM5 genotypes, early mucosal CD4 depletion and progressive peripheral CD4 depletion in permissive TRIM5 genotypes, cART suppression currently under evaluation | Del Prete GQ, Lifson JD, unpublished data | |
| RT-SHIV239 | Most widely used RT-SHIV, high peak/variable chronic VLs, limited pathogenesis data, cART suppression achievable with NNRTI-containing 3 drug regimen | [15–18] | |
| SIVagm.sab92018 | Characterized by spontaneous control of infection in the absence of cART, persistent control of viremia, which rebounds upon CD8 depletion, proposed as potential model for interventions targeting residual virus | [19] | |
|
| |||
| Macaca mulatta, Chinese | SIVmac239 | More limited use than Indian Macaca mulatta, acute/chronic VLs lower than in SIVmac239/251 infected Indian rhesus, mucosal CD4 depletion less extensive, delayed peripheral CD4 depletion, cART suppression achievable with 2–3 drugs | [20*, 21] |
|
| |||
| Macaca nemestrina | SIV/17E-Fr | Limited use alone, macrophage-tropic CD4-independent virus, high peak VLs, spontaneous control/low chronic VLs, limited pathogenesis data, cART suppression achievable with 2–3 drugs | [22, 23] |
| SIV/17E-Fr + SIV/deltaB670 | Combination infection with clone (17E-Fr) and “swarm” (deltaB670) viruses, high peak/chronic VLs, highly accelererated disease with rapid peripheral CD4 depletion, near universal early CNS disease, cART suppression difficult, especially if started more than 2 wks post-infection | [24–28*, 29] | |
| RT-SHIVmne | Limited use, high peak VLs, variable chronic VLs, limited pathogenesis data, cART suppression achievable in subset of animals with NNRTI-containing 3 drug regimen | [30,31*–33] | |
| SHIV-1157ipd3N4 | Limited use, high peak VLs, intermediate chronic VLs, early mucosal CD4 depletion, progressive peripheral CD4 depletion, cART suppression currently under evaluation | Ruprecht RM, Hu SL, personal communication | |
| stHIV | Very Limited use, requires further development, low peak VLs, spontaneous control of chronic VLs, mucosal and peripheral CD4 spared, protection from infection with 3 drug PrEP## | [34, 35] | |
|
| |||
| Macaca fascicularis | SIVmac251 | Limited use, intermediate peak VLs, variable/low chronic VLs, limited pathogenesis data, progressive peripheral CD4 loss in some animals, cART suppression more easily achieved than in Indian Macaca mulatta | [36–38] |
VLs: Viral loads
PrEP: Pre-exposure prophylaxis
For investigations of cART suppression, residual virus remaining in the face of cART, and strategies targeting this residual virus, it is important to use a model that demonstrates robust, reproducible, authentic pathogenesis recapitulating key features of human HIV infection. Infections of Indian rhesus macaques with the SIVmac251 viral quasispecies or the genetically related SIVmac239 infectious molecular clone constitute the most extensively used and well characterized pathogenic NHP lentivirus infections, and have been shown to accurately model numerous aspects of human HIV-1 infection, including early and sustained depletion of mucosal CD4+ CCR5+ T cells, and systemic chronic immune activation, ultimately leading to AIDS-defining opportunistic infections and neoplasms within 1–2 years [reviewed in 40].
An alternative model of SIV infection, originally developed to model lentiviral central nervous system (CNS) disease, that has been adapted for NHP cART-suppression studies utilizes pig-tailed macaques inoculated with a mixture of the immunosuppressive SIV/DeltaB670 viral swarm and the CD4-independent, macrophage-tropic, neurovirulent infectious molecular clone, SIV/17E-Fr [26,41]. This model features an accelerated disease course with high viral loads, precipitous declines in CD4+ T cells in blood, and encephalitis within 3 months of infection in a majority of untreated animals [41]. Consistent infection of the brain in this model [41] may make it particularly well-suited for assessments of potential residual virus and ART sanctuaries in the CNS [26,29].
Non-nucleoside reverse transcriptase inhibitors (NNRTIs) are not effective against SIVs. While it is currently unclear how different cART regimens and their associated pharmacologic properties might affect the development and maintenance of a residual virus pool and its response to particular therapies, inactivity of NNRTIs against SIV limits cART regimen options for SIV/NHP models. Investigators have therefore engineered chimeric SHIVs, inserting a NNRTI sensitive HIV-1 RT gene into a SIVmac239 backbone (RT-SHIVmac239) or a SIVmne backbone (RT-SHIVmne) for use in rhesus or pig-tailed macaques, respectively [30,42–45]. Detailed pathogenesis data for RT-SHIV infections are somewhat limited, however RT-SHIVmac239 viral RNA (vRNA) has been detected at high levels in GALT cells of rhesus macaques [17] and RT-SHIV-infections of both pig-tailed and rhesus animals have led to AIDS-like clinical endpoints with declining CD4 T cell counts in blood [18,30,42].
Regardless of the model used, it is important to have a clearly established understanding of the relevant features of pathogenesis to provide interpretive context for results obtained with different interventions. As protective MHC alleles associated with an increased frequency of spontaneous host control of SIV infection have been described in some NHP models [46,47] similar to HLA-B27 and HLA-B57 in human HIV infection [48,49], it is important to account for this factor in designing and interpreting studies especially in seeking to meaningfully expand results from anecdotal observations in individual animals or small numbers of animals to protocols that can be robustly reproduced at the population level across larger experimental groups.
cART Drugs
Despite substantial relatedness between SIV and HIV, drugs developed to inhibit various aspects of HIV replication can have lower or, as in the case of NNRTIs, essentially no activity against the cognate SIV targets. It is therefore critical to establish the potency of antiretroviral drugs directly against the virus to be used in a NHP model, ideally determining a plasma-adjusted 95% inhibitory concentration (IC95) level. The recently introduced concept of instantaneous inhibitory potential [50**] may also help guide choice of drugs for different applications. As the pharmacology of different drugs can vary between humans and NHP, and even between different species of NHP, it is important to determine dosing regimens that achieve and maintain the intended inhibitory concentrations in vivo, with above threshold trough levels being particularly important for some drugs. Pharmacological monitoring is therefore critical for evaluating efficacy or lack of apparent efficacy. Drug penetration to key tissue sites of viral replication may also be important in achieving maximal cART suppression of viral replication [51] and NHP studies may have much to offer in addressing this question.
Confirmation of in vivo antiviral activity as monotherapy is helpful, and development of drug resistance mutations can provide evidence of in vivo selective pressure. The nucleoside reverse transcriptase inhibitor (NRTI) tenofovir (PMPA) (Table 2) was originally shown to prevent SIV infection in pre- and post-exposure prophylaxis in NHP [60,61] and this drug (and the related prodrug tenofovir disoproxil fumarate [TDF]) remains a core component of most cART regimens for NHP studies. Other NRTIs, such as emtricitabine (FTC) and lamivudine (3TC), have demonstrated reductions in plasma viremia in monotherapy studies in SIV-infected macaques, with subsequent selection for drug resistant viral variants [62]; interestingly, combining PMPA with FTC selects for reversion of FTC resistance [54]. Virologic suppression and selection for drug resistant viral variants in infected macaques have also been reported for some integrase strand transfer inhibitors (IN-STIs), such as Raltegravir (RAL) and L-870812 [9,58]. We have administered the protease inhibitor (PI) Darunavir (DRV) to SIV infected rhesus macaques in monotherapy experiments and observed a decline in plasma viremia and the outgrowth of drug resistant viral mutants (Del Prete GQ, Lifson JD, unpublished data). Other PIs, atazanavir (ATZ), saquinavir (SQV) [26,28*,29], lopinavir [55], and indinavir [37,38] have also been used effectively in cART-suppression studies in macaques. In addition to the drug classes discussed above, RT-SHIVs can also be inhibited by NNRTIs. Efavirenz (EFV) has effected virologic suppression in both RT-SHIVmac239 infected rhesus macaques and RT-SHIVmne infected pig-tailed macaques. Nevirapine (NVP), however, was ineffective in pig-tailed macaques, apparently due to pharmacologic/bioavailability issues [30] despite activity against RT-SHIV in vitro [43] and in cynomolgus macaques [57], emphasizing the importance of defining the activity of each ART regimen for a given host and virus model.
Table 2.
Antiretroviral Drugs used in NHP cART-Suppression Models
| Drug Class | Drug (Colloquial name) | Generic name | Trade name (if applicable) | Typical mode of administration* | Dosage range** | Comments/notes | Selected References |
|---|---|---|---|---|---|---|---|
| Reverse Transcriptase Inhibitor (RTI) | PMPA/TFV | Tenofovir | - | SubQ | 15–30 mg/kg/day | Mainstay component of cART regimens in NHP; Renal toxicity with overdosing | [52] |
| TDF | Tenofovir disoproxil fumarate | Viread | Oral | 5–22 mg/kg | Prodrug form of PMPA/TFV | [53] | |
| FTC | Emtricitabine | Emtriva | SubQ | 16–50 mg/kg/day | Mainstay component of cART regimens in NHP; Resistance mutations selected against when dosed with PMPA/TFV | [54] | |
| 3TC | Lamivudine | Epivir | SubQ | 5–8 mg/kg/day (QD or BID) | [37, 38, 55] | ||
| AZT | Azidothymidine/Zidovudine | Retrovir | SubQ | 4.5 mg/kg BID | [37, 38, 55] | ||
| ddI | Didanosine/Dideoxyinosine | Videx | Oral, I.V. | 5–10 mg/kg/day | Pancreatic toxicity with prolonged administration or overdosage | [7, 8, 56] | |
| d4T | Stavudine | Zerit | Oral | 1.2 mg/kg/day | [7, 8, 56] | ||
| EFV | Efavirenz | Sustiva | Oral | 60 mg/kg/day, 200 mg/day fixed | NNRTI, used with RT-SHIV | [15–17, 30] | |
| NVP | Nevirapine | Viramune | SubQ | 3–18 mg/kg/day | NNRTI, used with RT-SHIV | [57] | |
|
| |||||||
| Integrase Strand Transfer Inhibitor (IN-STI) | RAL | Raltegravir | Isentress | Oral | 50–100 mg/kg BID | [9, 11] | |
| L-‘812 | L-870812 | - | Oral | 10–40 mg/kg/day (QD or BID) | Unlicensed investigational IN-STI | [26, 28*, 29, 58] | |
| L-‘564 | L-900564 | - | Oral | 10 mg/kg BID | Unlicensed investigational IN-STI | Del Prete GQ, Lifson JD, Klatt NR, Brenchley JM, unpublished data | |
| DTG/’572 | Dolutegravir | - | SubQ | 2.5 mg/kg/day | Pre-clinical next generation IN-STI | Del Prete GQ, Lifson JD, unpublished data | |
|
| |||||||
| Protease Inhibitor (PI) | IDV | Indinavir | Crixivan | Oral | 60 mg/kg BID | [37, 38] | |
| LPV | Lopinavir | Kaletra (w/RTV) | Oral, SubQ | 200 mg/day, 45 mg/kg BID | [12, 55] | ||
| ATV | Atazanavir | Reyataz | Oral | 270 mg/kg BID | [26, 28*, 29] | ||
| SQV | Saquinavir | Fortovase | Oral | 205 mg/kg BID | No longer marketed | [26, 28*, 29] | |
| DRV | Darunavir | Prezista | Oral | 300–600 mg BID | Variable impact of RTV on plasma levels | [59*], Del Prete GQ, Lifson JD, unpublished data | |
| RTV | Ritonavir | Norvir | Oral | 20–200 mg/day (QD or BID) | Used as PRI booster; variable effectiveness, may be NHP species specific | [59*], Del Prete GQ, Lifson JD, unpublished data | |
|
| |||||||
| Entry Inhibitor | MRV | Maraviroc | Selzentry | Oral | 100 mg BID | [59*] | |
SubQ: Subcutaneous injection; I.V.: Intravenous injection
QD: Once daily; BID: Twice daily
NNRTI-containing three-drug regimens including EFV and two NRTIs (PMPA with FTC or 3TC) have demonstrated suppression of RT-SHIV replication to clinically relevant levels (i.e., below 50 vRNA copies/ml of plasma) [15,17,18,30]. Although individual antiretrovirals can effect declines in plasma viral loads and select drug resistant variants in SIV infected Indian rhesus macaques, durable reduction of SIVmac239 and SIVmac251 replication to clinically-relevant levels has proven more challenging (Fig. 1). Basic three-drug regimens in SIVmac239/251-infected Indian rhesus have often been insufficient to suppress plasma viremia to levels approaching those measured in well-suppressed HIV-1-infected humans, particularly in animals with higher pretreatment viral loads [7,11,14,59*], however cART regimens containing four or more drugs have been more promising (Fig. 1) [12,59*]. Similarly, in SIV/deltaB670-SIV/17E-Fr-infected pig-tailed macaques, a four drug cART regimen consisting of one NRTI (PMPA), one IN-STI (L-870912), and two PIs (SQV and ATZ), has resulted in plasma viremia declines to <100 vRNA copies/ml [26,28*,29].
Figure 1. Suppression of plasma viremia with cART in SIVmac239-infected Indian rhesus macaques.
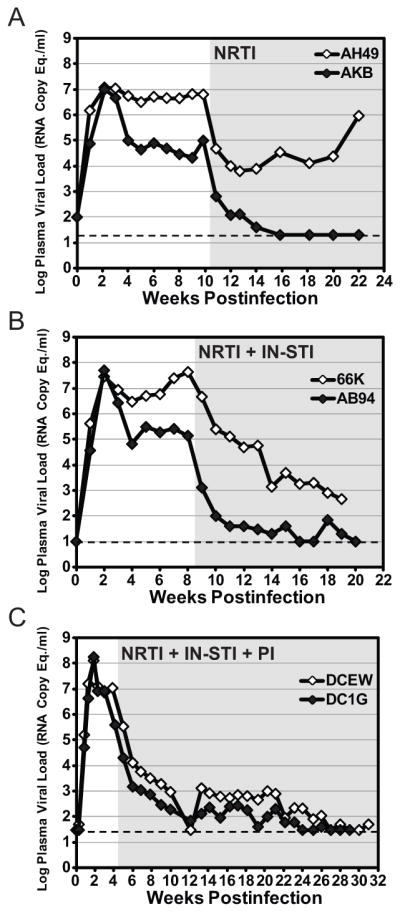
Each panel shows SIV RNA plasma viral load profiles for representative animals from a different experiment in a series of studies, with samples obtained prior to and during treatment with cART. Each cohort received a different cART regimen: A) two NRTIs (PMPA, FTC) starting at 10 weeks postinfection, B) two NRTIs (PMPA, FTC) plus two IN-STIs (L-‘564, L-‘812) starting at 8 weeks postinfection, C) two NRTIs (PMPA, FTC) plus two IN-STIs (L-‘564, L-‘812) plus a boosted PI (DRV boosted with RTV) starting at 4 weeks postinfection. As shown, it is possible to suppress viral replication in some animals with lower pretreatment viral loads (≤105 RNA copy Equivalents/ml) to clinically relevant levels (<50 RNA copy Eq./ml) with as few as two drugs from one inhibitor class, while a greater number of drugs spanning multiple classes, such as the five drug, three class regimen shown in (C), are typically required to achieve clinically relevant virologic suppression in animals with higher pretreatment viral loads (~106–108 RNA copy Eq./ml). PMPA was administered at 20 mg/kg/day SubQ, FTC at 40 mg/kg/day SubQ, L-‘564 at 10 mg/kg BID oral, L-‘812 at 20 mg/kg BID oral, DRV at 600 mg BID, and RTV at 100 mg BID. The gray shaded area of each plot represents the on-treatment phase of the study. The limit of quantification for the plasma viral load assay used for each cohort is indicated by a dashed line (A, 20 copy Eq./ml; B, 10 copy Eq./ml; C, 30 copy Eq./ml).
Drug toxicity is a general concern, particularly as protocols in NHP models can involve dosing at levels higher than typical clinical doses. Renal toxicity with either acute or chronic overdosage of PMPA has been demonstrated but can be avoided with appropriate dosing [52,63]. While the NRTIs didanosine (ddI) and stavudine (d4T) have demonstrated anti-SIV activity, a significant proportion of macaques receiving this regimen have developed diabetes after >25 weeks of dosing, likely due to ddI toxicity [8,56]. Investigators should always be aware of the possibility of drug:drug interactions that could influence drug levels and either efficacy or toxicity when evaluating new drug combinations, as was seen when a ddI-containing cART regiment was combined with administration of the IDO inhibitor D-1mT and the CTLA-4 blocking antibody MDX-010, resulting in fatal pancreatitis in all treated animals [56].
In addition to the particular drugs administered (Table 2), the timing and duration of treatment is critical for any experimental cART regimen. For many NHP cART-suppression studies, therapy is initiated relatively early in infection [15,17,18,26,28*–30] due to practical considerations as well as concerns over the potential development and archiving of multiple drug resistant variants during prolonged high level viral replication. Indeed, a recent RT-SHIVmne EFV monotherapy study using an ultrasensitive allele-specific PCR method demonstrated that EFV drug resistance mutations existed at low frequencies within the circulating virus population at 13 weeks postinfection, prior to the initiation of therapy [31*]. Early cART initiation has been shown to impact the magnitude of later, off-treatment viremia rebound, however, suggesting that cART timing may influence the seeding and establishment of the latent viral reservoir and/or the development of antiviral immune responses [37,61,64–68], similar to findings for humans treated during acute infection [69–71*]. It remains possible that initiating therapy later in infection might benefit from the additive impact of adaptive immune responses, and several studies have demonstrated good virologic suppression when cART was initiated later in infection [8,9,14,59*]. Further studies are required within each model system to determine the effects of the timing of ART initiation, including with regard to modeling clinically relevant phenomena.
Virological monitoring
Key to the use of NHP models for these studies is the availability of assays for virological monitoring to assess the efficacy of cART and the impact of interventions on residual virus. The most prominent methods used are quantitative PCR/RT-PCR (qPCR/qRT-PCR) methods, typically employing real time assay approaches [72,73]. Although standard clinical plasma viral load assays used for HIV-1-infected humans have a 50 vRNA copy/ml quantification limit, studies using more sensitive measures have found that the majority of cART-suppressed HIV-1 infected patients have measurable residual viremia below this level [74–78]. A comparable degree of suppression may thus be important for modeling cART-suppressed patients and would also provide low baseline viral loads, which may be critical for evaluating the effect of therapies designed to impact rare and limited numbers of target cells [79**]. Ultrasensitive assays have been developed for measuring plasma vRNA in NHPs, utilizing real time qRT-PCR assays with larger volumes of source specimen to achieve sensitivities approaching ultrasensitive assays for plasma HIV RNA [15,28*].
A recent human study reported an increase in cell-associated vRNA in resting CD4 T cells from blood following a single dose of the histone deacetylase inhibitor Vorinostat, intended to induce expression of latent proviruses in cART-suppressed subjects [79**]. Assays for measuring cell-associated vRNA and viral DNA (vDNA), normalized to parameters reflecting the number of cell equivalents analyzed, have been developed for NHP models, including assays to assess different viral transcript classes, and are being used for similar kinds of studies. Such assays are also being applied to NHP biopsy and necropsy tissues. In cART-suppressed RT-SHIVmac239 infected rhesus macaques, cell-associated vRNA was detectable only in lymphoid and gastrointestinal tissues, while vDNA could be detected in a wide range of tissues, including lymphoid, gastrointestinal, and reproductive tissues as well as sporadic, limited detection in neurological tissues [18]. Intriguingly, vRNA was below the limit of detection in brain samples from ART-treated SIV/deltaB670-SIV/17E-Fr-infected pig-tailed macaques, however vDNA levels in this compartment were comparable between ART-treated and untreated animals, reflecting the biology of this system [24,29]. Variability in nucleic acid yield and purity can make such analyses challenging, requiring internal controls to assess possible inhibition of PCR or RT-PCR reactions. In addition, the variable content of potential viral target cells in different tissues can complicate interpretation of results, so qPCR/qRT-PCR results are ideally complemented by flow cytometry analysis to assess the frequency of potential target cells in analyzed tissues. Alternatively, analyses can be performed on FACS sorted specimens enriched for potential target cells, although selective loss of cell populations in the processing and analysis of samples can complicate this approach.
While real time qPCR/qRT-PCR assays typically provide a nominal copy number of target template per reaction, based on interpolation into an external standard curve, such approaches are limited by the amount of nucleic acid input per reaction that can be assayed before reaction inhibition affects results. With assays approaching or at the theoretical maximum per reaction sensitivity, the only way to further increase practical sensitivity in determining viral nucleic acid copies/cell equivalents analyzed is to increase the effective number of cells surveyed. As this cannot be achieved by increasing per reaction nucleic acid input, methods have been developed that employ the use of nested PCR or RT-PCR amplification in many replicate reactions, allowing estimation of the frequency of cells harboring amplifiable nucleic acid templates based on Poisson distribution calculations [73]. For direct evaluation of viral nucleic acids in fixed tissue sections, improved in situ hybridization (ISH) techniques using viral lineage specific probes [80] or novel branched probe signal amplification approaches [81] offer improved sensitivity over traditional ISH methods.
While the above methods allow quantification of viral nucleic acids, they do not address the replication competence of the sequences detected, a key distinction since it is well documented that a substantial fraction of viral DNA sequences in chronic infection represent replication defective variants [reviewed in 82]. Accordingly, various virus rescue culture methods have been employed to assess the frequency of replication competent viruses [2,3,22,26]. While such assays, typically referred to as IUPM (infectious units per million cells) assays, certainly provide at least a minimum estimate of the frequency of cells harboring replication competent viruses potentially capable of giving rise to recrudescent infection when cART is stopped, such assays have been expensive, time and labor intensive, and relatively insensitive with a limited dynamic range, restricting their use. Efforts to date to address these issues with simplified versions of these assays have only partially addressed the problems [83]. An alternative approach, intermediate between measurement of viral nucleic acids and assessment of replication competent virus, is to use shorter term in vitro cultures of stimulated cell populations with a supernatant RT-PCR readout for virus production (Del Prete GQ, Lifson JD, unpublished data).
While all of these in vitro analyses have their merits, for studies of residual virus in cART suppressed animals and evaluation of strategies targeting this residual virus, the gold standard remains diagnostic withdrawal of cART to assess viral rebound, with lack of such rebound on cART withdrawal constituting de facto evidence of a “functional cure”. Absent such clear cut qualitative results (no measurable off cART viral rebound), demonstrable impact on the extent or kinetics of off cART viral rebound may provide evidence of a partial beneficial effect of intervention, but the natural history of these parameters following cART cessation in the absence of other interventions is insufficiently characterized in most models to allow interpretable results, particularly when such results can be dramatically influenced by experimental parameters such as the timing of cART initiation and its duration [37,61,64–68]. Sequence analysis of the rebound viremia, coupled with tissue based sequence analysis, may provide insights into the sources of recrudescent virus on cART withdrawal. The use of NHP models allows an additional in vivo functional readout to assess potential viral eradication that is not available to clinical investigators—adoptive transfer of large numbers of cells from a putatively cured animal to a naïve host. Transfer of cells from cART suppressed animals rapidly results in robust infection of the recipients (Picker LJ, personal communication), providing a baseline against which to interpret results of transfer of cells from putatively cured animals.
Conclusions
Given the imperative for development of more definitive treatment approaches for HIV infection, NHP models will continue to play a key role in this important research area. However, to be effective, this will entail much more extensive model development and characterization than has occurred to date. Even with optimal cART regimens and assays, there may be clinically relevant questions that cannot be effectively modeled in NHP systems. It has been suggested that dynamic virologic and immunologic changes can continue through the first three years on cART in HIV infected patients, while in current practice cART administration in NHP is limited to less than two years. Nevertheless, NHP models have much to offer the field in addressing key questions. Especially for evaluating interventions targeting residual virus it will be important to characterize what fraction of potential target cells are impacted by the intervention (fractional hit), what is the fate of the impacted cells (induction of virus expression, vial cytolysis, apoptosis, immune clearance, cell proliferation?) and what is the duration of the effects. Studies to date suggest that multimodal treatments will likely be required to meaningfully impact residual virus, including novel therapeutic vaccination approaches with vectors that induce novel forms of persistent immune surveillance [73,84,85]. NHP models will play a key role in the evaluation of such approaches. In conducting this work it will be important to resist the seductive appeal of premature standardization of the field around a particular NHP model, until the strengths and limitations of different models for different aspects of this research are more fully understood.
Key Points.
Multiple NHP models are available for studies of suppressive cART, and strategies for targeting this virus to achieve functional cure or virus eradication; each has its own strengths and limitations.
NHP models will play a key role in the evaluation of residual virus remaining in the face of suppressive cART, and strategies for targeting this virus to achieve functional cure or virus eradication.
In vivo levels and activity of antiretroviral drugs, as dosed, should be determined empirically to allow interpretation of cART suppression studies in NHP models.
Premature standardization of NHP models for studies of suppressive cART, and strategies for targeting this virus to achieve functional cure or virus eradication should be avoided, until the different models are better developed and characterized to understand their properties, advantages, and limitations.
Acknowledgments
This work was supported with federal funds from the National Cancer Institute, National Institutes of Health under contract HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
The authors thank the Specimen Support Core, the Quantitative Molecular Diagnostics Core, and the Cellular Immunity Core of the AIDS and Cancer Virus Program, and the Laboratory Animal Sciences Program of SAIC-Frederick, Inc, Frederick National Laboratory for Cancer Research for expert support, as well as Drs. Paul Luciw, Janice Clements, Christine Zink, Robert Siliciano, David Margolis, Louis Picker, Mike McCune, Steve Deeks, Romas Geleziunas, Joe Hesselgesser and Daria Hazuda for helpful discussions.
References
- 1.Chun TW, Finzi D, Margolick J, Chadwick K, Schwartz D, Siliciano RF. In vivo fate of HIV-1-infected T cells: quantitative analysis of the transition to stable latency. Nat Med. 1995;1:1284–1290. doi: 10.1038/nm1295-1284. [DOI] [PubMed] [Google Scholar]
- 2.Chun TW, Carruth L, Finzi D, Shen X, DiGiuseppe JA, Taylor H, Hermankova M, Chadwick K, Margolick J, Quinn TC, et al. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature. 1997;387:183–188. doi: 10.1038/387183a0. [DOI] [PubMed] [Google Scholar]
- 3.Finzi D, Blankson J, Siliciano JD, Margolick JB, Chadwick K, Pierson T, Smith K, Lisziewicz J, Lori F, Flexner C, et al. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat Med. 1999;5:512–517. doi: 10.1038/8394. [DOI] [PubMed] [Google Scholar]
- 4.Wong JK, Hezareh M, Gunthard HF, Havlir DV, Ignacio CC, Spina CA, Richman DD. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science. 1997;278:1291–1295. doi: 10.1126/science.278.5341.1291. [DOI] [PubMed] [Google Scholar]
- **5.Hutter G, Nowak D, Mossner M, Ganepola S, Mussig A, Allers K, Schneider T, Hofmann J, Kucherer C, Blau O, et al. Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. N Engl J Med. 2009;360:692–698. doi: 10.1056/NEJMoa0802905. Seminal case report of Timothy Brown, the “Berlin Patient”, in whom functional cure and potential eradication of HIV infection was achieved by a complex series of intensive treatments, including multiple allogeneic stem cell transplants using CCR5 32 donor cells. [DOI] [PubMed] [Google Scholar]
- *6.Richman DD, Margolis DM, Delaney M, Greene WC, Hazuda D, Pomerantz RJ. The challenge of finding a cure for HIV infection. Science. 2009;323:1304–1307. doi: 10.1126/science.1165706. A manifesto for the HIV functional cure/virus eradication agenda, containing both helpful background information and policy recommendations. [DOI] [PubMed] [Google Scholar]
- 7.Demberg T, Brocca-Cofano E, Xiao P, Venzon D, Vargas-Inchaustegui D, Lee EM, Kalisz I, Kalyanaraman VS, Dipasquale J, McKinnon K, et al. Dynamics of memory B-cell populations in blood, lymph nodes, and bone marrow during antiretroviral therapy and envelope boosting in simian immunodeficiency virus SIVmac251-infected rhesus macaques. J Virol. 2012;86:12591–12604. doi: 10.1128/JVI.00298-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dunham RM, Gordon SN, Vaccari M, Piatak M, Huang Y, Deeks SG, Lifson J, Franchini G, McCune JM. Preclinical Evaluation of HIV Eradication Strategies in the Simian Immunodeficiency Virus-Infected Rhesus Macaque: A Pilot Study Testing Inhibition of Indoleamine 2,3-Dioxygenase. AIDS Res Hum Retroviruses. 2012 doi: 10.1089/aid.2012.0162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lewis MG, Norelli S, Collins M, Barreca ML, Iraci N, Chirullo B, Yalley-Ogunro J, Greenhouse J, Titti F, Garaci E, et al. Response of a simian immunodeficiency virus (SIVmac251) to raltegravir: a basis for a new treatment for simian AIDS and an animal model for studying lentiviral persistence during antiretroviral therapy. Retrovirology. 2010;7:21. doi: 10.1186/1742-4690-7-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lori F, Lewis MG, Xu J, Varga G, Zinn DE, Jr, Crabbs C, Wagner W, Greenhouse J, Silvera P, Yalley-Ogunro J, et al. Control of SIV rebound through structured treatment interruptions during early infection. Science. 2000;290:1591–1593. doi: 10.1126/science.290.5496.1591. [DOI] [PubMed] [Google Scholar]
- 11.Lugli E, Mueller YM, Lewis MG, Villinger F, Katsikis PD, Roederer M. IL-15 delays suppression and fails to promote immune reconstitution in virally suppressed chronically SIV-infected macaques. Blood. 2011;118:2520–2529. doi: 10.1182/blood-2011-05-351155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Horiike M, Iwami S, Kodama M, Sato A, Watanabe Y, Yasui M, Ishida Y, Kobayashi T, Miura T, Igarashi T. Lymph nodes harbor viral reservoirs that cause rebound of plasma viremia in SIV-infected macaques upon cessation of combined antiretroviral therapy. Virology. 2012;423:107–118. doi: 10.1016/j.virol.2011.11.024. [DOI] [PubMed] [Google Scholar]
- 13.Leone A, Rohankhedkar M, Okoye A, Legasse A, Axthelm MK, Villinger F, Piatak M, Jr, Lifson JD, Assouline B, Morre M, et al. Increased CD4+ T cell levels during IL-7 administration of antiretroviral therapy-treated simian immunodeficiency virus-positive macaques are not dependent on strong proliferative responses. J Immunol. 2010;185:1650–1659. doi: 10.4049/jimmunol.0902626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.zur Megede J, Sanders-Beer B, Silvera P, Golightly D, Bowlsbey A, Hebblewaite D, Sites D, Nieves-Duran L, Srivastava R, Otten GR, et al. A therapeutic SIV DNA vaccine elicits T-cell immune responses, but no sustained control of viremia in SIVmac239-infected rhesus macaques. AIDS Res Hum Retroviruses. 2008;24:1103–1116. doi: 10.1089/aid.2008.0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deere JD, Higgins J, Cannavo E, Villalobos A, Adamson L, Fromentin E, Schinazi RF, Luciw PA, North TW. Viral decay kinetics in the highly active antiretroviral therapy-treated rhesus macaque model of AIDS. PLoS One. 2010;5:e11640. doi: 10.1371/journal.pone.0011640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hofman MJ, Higgins J, Matthews TB, Pedersen NC, Tan C, Schinazi RF, North TW. Efavirenz therapy in rhesus macaques infected with a chimera of simian immunodeficiency virus containing reverse transcriptase from human immunodeficiency virus type 1. Antimicrob Agents Chemother. 2004;48:3483–3490. doi: 10.1128/AAC.48.9.3483-3490.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.North TW, Van Rompay KK, Higgins J, Matthews TB, Wadford DA, Pedersen NC, Schinazi RF. Suppression of virus load by highly active antiretroviral therapy in rhesus macaques infected with a recombinant simian immunodeficiency virus containing reverse transcriptase from human immunodeficiency virus type 1. J Virol. 2005;79:7349–7354. doi: 10.1128/JVI.79.12.7349-7354.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.North TW, Higgins J, Deere JD, Hayes TL, Villalobos A, Adamson L, Shacklett BL, Schinazi RF, Luciw PA. Viral sanctuaries during highly active antiretroviral therapy in a nonhuman primate model for AIDS. J Virol. 2010;84:2913–2922. doi: 10.1128/JVI.02356-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pandrea I, Gaufin T, Gautam R, Kristoff J, Mandell D, Montefiori D, Keele BF, Ribeiro RM, Veazey RS, Apetrei C. Functional cure of SIVagm infection in rhesus macaques results in complete recovery of CD4+ T cells and is reverted by CD8+ cell depletion. PLoS Pathog. 2011;7:e1002170. doi: 10.1371/journal.ppat.1002170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *20.Jasny E, Geer S, Frank I, Vagenas P, Aravantinou M, Salazar AM, Lifson JD, Piatak M, Jr, Gettie A, Blanchard JL, et al. Characterization of peripheral and mucosal immune responses in rhesus macaques on long-term tenofovir and emtricitabine combination antiretroviral therapy. J Acquir Immune Defic Syndr. 2012;61:425–435. doi: 10.1097/QAI.0b013e318266be53. Impressive suppression of plasma viremia demonstrated in a subset of SIVmac239 infected Chinese-origin rhesus macaques using an ART regimen containing only PMPA/FTC highlights the association between pretreatment/untreated viral loads and ease of cART-mediated suppression. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vagenas P, Aravantinou M, Williams VG, Jasny E, Piatak M, Jr, Lifson JD, Salazar AM, Blanchard JL, Gettie A, Robbiani M. A tonsillar PolyICLC/AT-2 SIV therapeutic vaccine maintains low viremia following antiretroviral therapy cessation. PLoS One. 2010;5:e12891. doi: 10.1371/journal.pone.0012891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shen A, Zink MC, Mankowski JL, Chadwick K, Margolick JB, Carruth LM, Li M, Clements JE, Siliciano RF. Resting CD4+ T lymphocytes but not thymocytes provide a latent viral reservoir in a simian immunodeficiency virus-Macaca nemestrina model of human immunodeficiency virus type 1-infected patients on highly active antiretroviral therapy. J Virol. 2003;77:4938–4949. doi: 10.1128/JVI.77.8.4938-4949.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shen A, Yang HC, Zhou Y, Chase AJ, Boyer JD, Zhang H, Margolick JB, Zink MC, Clements JE, Siliciano RF. Novel pathway for induction of latent virus from resting CD4(+) T cells in the simian immunodeficiency virus/macaque model of human immunodeficiency virus type 1 latency. J Virol. 2007;81:1660–1670. doi: 10.1128/JVI.01396-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clements JE, Li M, Gama L, Bullock B, Carruth LM, Mankowski JL, Zink MC. The central nervous system is a viral reservoir in simian immunodeficiency virus--infected macaques on combined antiretroviral therapy: a model for human immunodeficiency virus patients on highly active antiretroviral therapy. J Neurovirol. 2005;11:180–189. doi: 10.1080/13550280590922748-1. [DOI] [PubMed] [Google Scholar]
- 25.Clements JE, Gama L, Graham DR, Mankowski JL, Zink MC. A simian immunodeficiency virus macaque model of highly active antiretroviral treatment: viral latency in the periphery and the central nervous system. Curr Opin HIV AIDS. 2011;6:37–42. doi: 10.1097/COH.0b013e3283412413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dinoso JB, Rabi SA, Blankson JN, Gama L, Mankowski JL, Siliciano RF, Zink MC, Clements JE. A simian immunodeficiency virus-infected macaque model to study viral reservoirs that persist during highly active antiretroviral therapy. J Virol. 2009;83:9247–9257. doi: 10.1128/JVI.00840-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Graham DR, Gama L, Queen SE, Li M, Brice AK, Kelly KM, Mankowski JL, Clements JE, Zink MC. Initiation of HAART during acute simian immunodeficiency virus infection rapidly controls virus replication in the CNS by enhancing immune activity and preserving protective immune responses. J Neurovirol. 2011;17:120–130. doi: 10.1007/s13365-010-0005-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *28.Queen SE, Mears BM, Kelly KM, Dorsey JL, Liao Z, Dinoso JB, Gama L, Adams RJ, Zink MC, Clements JE, et al. Replication-competent simian immunodeficiency virus (SIV) Gag escape mutations archived in latent reservoirs during antiretroviral treatment of SIV-infected macaques. J Virol. 2011;85:9167–9175. doi: 10.1128/JVI.00366-11. Demonstrates apparent ongoing viral evolution even during decay of plasma viremia due to initiation of antiretroviral drug treatment, a cautionary note about the evolutionary capacity of retroviruses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zink MC, Brice AK, Kelly KM, Queen SE, Gama L, Li M, Adams RJ, Bartizal C, Varrone J, Rabi SA, et al. Simian immunodeficiency virus-infected macaques treated with highly active antiretroviral therapy have reduced central nervous system viral replication and inflammation but persistence of viral DNA. J Infect Dis. 2010;202:161–170. doi: 10.1086/653213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ambrose Z, Palmer S, Boltz VF, Kearney M, Larsen K, Polacino P, Flanary L, Oswald K, Piatak M, Jr, Smedley J, et al. Suppression of viremia and evolution of human immunodeficiency virus type 1 drug resistance in a macaque model for antiretroviral therapy. J Virol. 2007;81:12145–12155. doi: 10.1128/JVI.01301-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *31.Boltz VF, Ambrose Z, Kearney MF, Shao W, Kewalramani VN, Maldarelli F, Mellors JW, Coffin JM. Ultrasensitive allele-specific PCR reveals rare preexisting drug-resistant variants and a large replicating virus population in macaques infected with a simian immunodeficiency virus containing human immunodeficiency virus reverse transcriptase. J Virol. 2012;86:12525–12530. doi: 10.1128/JVI.01963-12. Detailed analysis demonstrates presence of drug resistance mutations, pre-existing at low levels in RT-SHIVmne-infected animals, even prior to ART. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kearney M, Spindler J, Shao W, Maldarelli F, Palmer S, Hu SL, Lifson JD, KewalRamani VN, Mellors JW, Coffin JM, et al. Genetic diversity of simian immunodeficiency virus encoding HIV-1 reverse transcriptase persists in macaques despite antiretroviral therapy. J Virol. 2011;85:1067–1076. doi: 10.1128/JVI.01701-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shao W, Kearney M, Maldarelli F, Mellors JW, Stephens RM, Lifson JD, KewalRamani VN, Ambrose Z, Coffin JM, Palmer SE. RT-SHIV subpopulation dynamics in infected macaques during anti-HIV therapy. Retrovirology. 2009;6:101. doi: 10.1186/1742-4690-6-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hatziioannou T, Ambrose Z, Chung NP, Piatak M, Jr, Yuan F, Trubey CM, Coalter V, Kiser R, Schneider D, Smedley J, et al. A macaque model of HIV-1 infection. Proc Natl Acad Sci U S A. 2009;106:4425–4429. doi: 10.1073/pnas.0812587106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thippeshappa R, Polacino P, Yu Kimata MT, Siwak EB, Anderson D, Wang W, Sherwood L, Arora R, Wen M, Zhou P, et al. Vif substitution enables persistent infection of pigtailed macaques by human immunodeficiency virus type 1. J Virol. 2011;85:3767–3779. doi: 10.1128/JVI.02438-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Benlhassan-Chahour K, Penit C, Dioszeghy V, Vasseur F, Janvier G, Riviere Y, Dereuddre-Bosquet N, Dormont D, Le Grand R, Vaslin B. Kinetics of lymphocyte proliferation during primary immune response in macaques infected with pathogenic simian immunodeficiency virus SIVmac251: preliminary report of the effect of early antiviral therapy. J Virol. 2003;77:12479–12493. doi: 10.1128/JVI.77.23.12479-12493.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bourry O, Mannioui A, Sellier P, Roucairol C, Durand-Gasselin L, Dereuddre-Bosquet N, Benech H, Roques P, Le Grand R. Effect of a short-term HAART on SIV load in macaque tissues is dependent on time of initiation and antiviral diffusion. Retrovirology. 2010;7:78. doi: 10.1186/1742-4690-7-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moreau M, Le Tortorec A, Deleage C, Brown C, Denis H, Satie AP, Bourry O, Deureuddre-Bosquet N, Roques P, Le Grand R, et al. Impact of short-term HAART initiated during the chronic stage or shortly post-exposure on SIV infection of male genital organs. PLoS One. 2012;7:e37348. doi: 10.1371/journal.pone.0037348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuroishi A, Saito A, Shingai Y, Shioda T, Nomaguchi M, Adachi A, Akari H, Nakayama EE. Modification of a loop sequence between alpha-helices 6 and 7 of virus capsid (CA) protein in a human immunodeficiency virus type 1 (HIV-1) derivative that has simian immunodeficiency virus (SIVmac239) vif and CA alpha-helices 4 and 5 loop improves replication in cynomolgus monkey cells. Retrovirology. 2009;6:70. doi: 10.1186/1742-4690-6-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Whetter LE, Ojukwu IC, Novembre FJ, Dewhurst S. Pathogenesis of simian immunodeficiency virus infection. J Gen Virol. 1999;80 ( Pt 7):1557–1568. doi: 10.1099/0022-1317-80-7-1557. [DOI] [PubMed] [Google Scholar]
- 41.Zink MC, Suryanarayana K, Mankowski JL, Shen A, Piatak M, Jr, Spelman JP, Carter DL, Adams RJ, Lifson JD, Clements JE. High viral load in the cerebrospinal fluid and brain correlates with severity of simian immunodeficiency virus encephalitis. J Virol. 1999;73:10480–10488. doi: 10.1128/jvi.73.12.10480-10488.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Uberla K, Stahl-Hennig C, Bottiger D, Matz-Rensing K, Kaup FJ, Li J, Haseltine WA, Fleckenstein B, Hunsmann G, Oberg B, et al. Animal model for the therapy of acquired immunodeficiency syndrome with reverse transcriptase inhibitors. Proc Natl Acad Sci U S A. 1995;92:8210–8214. doi: 10.1073/pnas.92.18.8210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ambrose Z, Boltz V, Palmer S, Coffin JM, Hughes SH, Kewalramani VN. In vitro characterization of a simian immunodeficiency virus-human immunodeficiency virus (HIV) chimera expressing HIV type 1 reverse transcriptase to study antiviral resistance in pigtail macaques. J Virol. 2004;78:13553–13561. doi: 10.1128/JVI.78.24.13553-13561.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Balzarini J, De Clercq E, Uberla K. SIV/HIV-1 hybrid virus expressing the reverse transcriptase gene of HIV-1 remains sensitive to HIV-1-specific reverse transcriptase inhibitors after passage in rhesus macaques. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;15:1–4. doi: 10.1097/00042560-199705010-00001. [DOI] [PubMed] [Google Scholar]
- 45.Balzarini J, Weeger M, Camarasa MJ, De Clercq E, Uberla K. Sensitivity/resistance profile of a simian immunodeficiency virus containing the reverse transcriptase gene of human immunodeficiency virus type 1 (HIV-1) toward the HIV-1-specific non-nucleoside reverse transcriptase inhibitors. Biochem Biophys Res Commun. 1995;211:850–856. doi: 10.1006/bbrc.1995.1890. [DOI] [PubMed] [Google Scholar]
- 46.O’Connor DH, Mothe BR, Weinfurter JT, Fuenger S, Rehrauer WM, Jing P, Rudersdorf RR, Liebl ME, Krebs K, Vasquez J, et al. Major histocompatibility complex class I alleles associated with slow simian immunodeficiency virus disease progression bind epitopes recognized by dominant acute-phase cytotoxic-T-lymphocyte responses. J Virol. 2003;77:9029–9040. doi: 10.1128/JVI.77.16.9029-9040.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Loffredo JT, Friedrich TC, Leon EJ, Stephany JJ, Rodrigues DS, Spencer SP, Bean AT, Beal DR, Burwitz BJ, Rudersdorf RA, et al. CD8+ T cells from SIV elite controller macaques recognize Mamu-B*08-bound epitopes and select for widespread viral variation. PLoS One. 2007;2:e1152. doi: 10.1371/journal.pone.0001152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kaslow RA, Carrington M, Apple R, Park L, Munoz A, Saah AJ, Goedert JJ, Winkler C, O’Brien SJ, Rinaldo C, et al. Influence of combinations of human major histocompatibility complex genes on the course of HIV-1 infection. Nat Med. 1996;2:405–411. doi: 10.1038/nm0496-405. [DOI] [PubMed] [Google Scholar]
- 49.Hendel H, Caillat-Zucman S, Lebuanec H, Carrington M, O’Brien S, Andrieu JM, Schachter F, Zagury D, Rappaport J, Winkler C, et al. New class I and II HLA alleles strongly associated with opposite patterns of progression to AIDS. J Immunol. 1999;162:6942–6946. [PubMed] [Google Scholar]
- **50.Deng K, Zink MC, Clements JE, Siliciano RF. A quantitative measurement of antiviral activity of anti-human immunodeficiency virus type 1 drugs against simian immunodeficiency virus infection: dose-response curve slope strongly influences class-specific inhibitory potential. J Virol. 2012;86:11368–11372. doi: 10.1128/JVI.01563-12. Assessment of the potency of different antiretroviral drug classes against SIV in macaque cells by determining instantaneous inhibitory potentials, a novel approach to evaluating drug potency. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cohen J. Tissue Says Blood Is Misleading, Confusing HIV Cure Efforts. Science. 2011;334:1614. doi: 10.1126/science.334.6063.1614. [DOI] [PubMed] [Google Scholar]
- 52.Van Rompay KK, Durand-Gasselin L, Brignolo LL, Ray AS, Abel K, Cihlar T, Spinner A, Jerome C, Moore J, Kearney BP, et al. Chronic administration of tenofovir to rhesus macaques from infancy through adulthood and pregnancy: summary of pharmacokinetics and biological and virological effects. Antimicrob Agents Chemother. 2008;52:3144–3160. doi: 10.1128/AAC.00350-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Radzio J, Aung W, Holder A, Martin A, Sweeney E, Mitchell J, Bachman S, Pau CP, Heneine W, Garcia-Lerma JG. Prevention of vaginal SHIV transmission in macaques by a coitally-dependent Truvada regimen. PLoS One. 2012;7:e50632. doi: 10.1371/journal.pone.0050632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Murry JP, Higgins J, Matthews TB, Huang VY, Van Rompay KK, Pedersen NC, North TW. Reversion of the M184V mutation in simian immunodeficiency virus reverse transcriptase is selected by tenofovir, even in the presence of lamivudine. J Virol. 2003;77:1120–1130. doi: 10.1128/JVI.77.2.1120-1130.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brandin E, Thorstensson R, Bonhoeffer S, Albert J. Rapid viral decay in simian immunodeficiency virus-infected macaques receiving quadruple antiretroviral therapy. J Virol. 2006;80:9861–9864. doi: 10.1128/JVI.00394-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vaccari M, Boasso A, Fenizia C, Fuchs D, Hryniewicz A, Morgan T, Weiss D, Doster MN, Heraud JM, Shearer GM, et al. Fatal pancreatitis in simian immunodeficiency virus SIV(mac251)-infected macaques treated with 2′,3′-dideoxyinosine and stavudine following cytotoxic-T-lymphocyte-associated antigen 4 and indoleamine 2,3-dioxygenase blockade. J Virol. 2012;86:108–113. doi: 10.1128/JVI.05609-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zuber B, Bottiger D, Benthin R, ten Haaft P, Heeney J, Wahren B, Oberg B. An in vivo model for HIV resistance development. AIDS Res Hum Retroviruses. 2001;17:631–635. doi: 10.1089/088922201300119734. [DOI] [PubMed] [Google Scholar]
- 58.Hazuda DJ, Young SD, Guare JP, Anthony NJ, Gomez RP, Wai JS, Vacca JP, Handt L, Motzel SL, Klein HJ, et al. Integrase inhibitors and cellular immunity suppress retroviral replication in rhesus macaques. Science. 2004;305:528–532. doi: 10.1126/science.1098632. [DOI] [PubMed] [Google Scholar]
- *59.Shytaj IL, Norelli S, Chirullo B, Della Corte A, Collins M, Yalley-Ogunro J, Greenhouse J, Iraci N, Acosta EP, Barreca ML, et al. A highly intensified ART regimen induces long-term viral suppression and restriction of the viral reservoir in a simian AIDS model. PLoS Pathog. 2012;8:e1002774. doi: 10.1371/journal.ppat.1002774. Intriguing and provocative results in small numbers of animals with an intensive cART regimen including maraviroc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tsai CC, Follis KE, Sabo A, Beck TW, Grant RF, Bischofberger N, Benveniste RE, Black R. Prevention of SIV infection in macaques by (R)-9-(2-phosphonylmethoxypropyl)adenine. Science. 1995;270:1197–1199. doi: 10.1126/science.270.5239.1197. [DOI] [PubMed] [Google Scholar]
- 61.Tsai CC, Emau P, Follis KE, Beck TW, Benveniste RE, Bischofberger N, Lifson JD, Morton WR. Effectiveness of postinoculation (R)-9-(2-phosphonylmethoxypropyl) adenine treatment for prevention of persistent simian immunodeficiency virus SIVmne infection depends critically on timing of initiation and duration of treatment. J Virol. 1998;72:4265–4273. doi: 10.1128/jvi.72.5.4265-4273.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Van Rompay KK, Matthews TB, Higgins J, Canfield DR, Tarara RP, Wainberg MA, Schinazi RF, Pedersen NC, North TW. Virulence and reduced fitness of simian immunodeficiency virus with the M184V mutation in reverse transcriptase. J Virol. 2002;76:6083–6092. doi: 10.1128/JVI.76.12.6083-6092.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sanders-Beer BE, Spano YY, Golighty D, Lara A, Hebblewaite D, Nieves-Duran L, Rhodes L, Mansfield KG. Clinical monitoring and correlates of nephropathy in SIV-infected macaques during high-dose antiretroviral therapy. AIDS Res Ther. 2011;8:3. doi: 10.1186/1742-6405-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lifson JD, Piatak M, Jr, Cline AN, Rossio JL, Purcell J, Pandrea I, Bischofberger N, Blanchard J, Veazey RS. Transient early post-inoculation anti-retroviral treatment facilitates controlled infection with sparing of CD4+ T cells in gut-associated lymphoid tissues in SIVmac239-infected rhesus macaques, but not resistance to rechallenge. J Med Primatol. 2003;32:201–210. doi: 10.1034/j.1600-0684.2003.00026.x. [DOI] [PubMed] [Google Scholar]
- 65.Hel Z, Venzon D, Poudyal M, Tsai WP, Giuliani L, Woodward R, Chougnet C, Shearer G, Altman JD, Watkins D, et al. Viremia control following antiretroviral treatment and therapeutic immunization during primary SIV251 infection of macaques. Nat Med. 2000;6:1140–1146. doi: 10.1038/80481. [DOI] [PubMed] [Google Scholar]
- 66.Sellier P, Mannioui A, Bourry O, Dereuddre-Bosquet N, Delache B, Brochard P, Calvo J, Prevot S, Roques P. Antiretroviral treatment start-time during primary SIV(mac) infection in macaques exerts a different impact on early viral replication and dissemination. PLoS One. 2010;5:e10570. doi: 10.1371/journal.pone.0010570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kader M, Hassan WM, Eberly M, Piatak M, Lifson JD, Roederer M, Mattapallil JJ. Antiretroviral therapy prior to acute viral replication preserves CD4 T cells in the periphery but not in rectal mucosa during acute simian immunodeficiency virus infection. J Virol. 2008;82:11467–11471. doi: 10.1128/JVI.01143-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lifson JD, Rossio JL, Arnaout R, Li L, Parks TL, Schneider DK, Kiser RF, Coalter VJ, Walsh G, Imming RJ, et al. Containment of simian immunodeficiency virus infection: cellular immune responses and protection from rechallenge following transient postinoculation antiretroviral treatment. J Virol. 2000;74:2584–2593. doi: 10.1128/jvi.74.6.2584-2593.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.von Wyl V, Gianella S, Fischer M, Niederoest B, Kuster H, Battegay M, Bernasconi E, Cavassini M, Rauch A, Hirschel B, et al. Early antiretroviral therapy during primary HIV-1 infection results in a transient reduction of the viral setpoint upon treatment interruption. PLoS One. 2011;6:e27463. doi: 10.1371/journal.pone.0027463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Steingrover R, Pogany K, Fernandez Garcia E, Jurriaans S, Brinkman K, Schuitemaker H, Miedema F, Lange JM, Prins JM. HIV-1 viral rebound dynamics after a single treatment interruption depends on time of initiation of highly active antiretroviral therapy. Aids. 2008;22:1583–1588. doi: 10.1097/QAD.0b013e328305bd77. [DOI] [PubMed] [Google Scholar]
- *71.Hamlyn E, Ewings FM, Porter K, Cooper DA, Tambussi G, Schechter M, Pedersen C, Okulicz JF, McClure M, Babiker A, et al. Plasma HIV viral rebound following protocol-indicated cessation of ART commenced in primary and chronic HIV infection. PLoS One. 2012;7:e43754. doi: 10.1371/journal.pone.0043754. Evaluation of the magnitude and kinetics of off-treatment virologic rebound in HIV-infected humans that initiated therapy during early or chronic HIV infection, illustrating the potential impact of cART timing on the establishment and maintenance of the residual virus pool. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cline AN, Bess JW, Piatak M, Jr, Lifson JD. Highly sensitive SIV plasma viral load assay: practical considerations, realistic performance expectations, and application to reverse engineering of vaccines for AIDS. J Med Primatol. 2005;34:303–312. doi: 10.1111/j.1600-0684.2005.00128.x. [DOI] [PubMed] [Google Scholar]
- 73.Hansen SG, Ford JC, Lewis MS, Ventura AB, Hughes CM, Coyne-Johnson L, Whizin N, Oswald K, Shoemaker R, Swanson T, et al. Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature. 2011;473:523–527. doi: 10.1038/nature10003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Maldarelli F, Palmer S, King MS, Wiegand A, Polis MA, Mican J, Kovacs JA, Davey RT, Rock-Kress D, Dewar R, et al. ART suppresses plasma HIV-1 RNA to a stable set point predicted by pretherapy viremia. PLoS Pathog. 2007;3:e46. doi: 10.1371/journal.ppat.0030046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dornadula G, Zhang H, VanUitert B, Stern J, Livornese L, Jr, Ingerman MJ, Witek J, Kedanis RJ, Natkin J, DeSimone J, et al. Residual HIV-1 RNA in blood plasma of patients taking suppressive highly active antiretroviral therapy. Jama. 1999;282:1627–1632. doi: 10.1001/jama.282.17.1627. [DOI] [PubMed] [Google Scholar]
- 76.Fischer M, Gunthard HF, Opravil M, Joos B, Huber W, Bisset LR, Ott P, Boni J, Weber R, Cone RW. Residual HIV-RNA levels persist for up to 2. 5 years in peripheral blood mononuclear cells of patients on potent antiretroviral therapy. AIDS Res Hum Retroviruses. 2000;16:1135–1140. doi: 10.1089/088922200414974. [DOI] [PubMed] [Google Scholar]
- 77.Palmer S, Wiegand AP, Maldarelli F, Bazmi H, Mican JM, Polis M, Dewar RL, Planta A, Liu S, Metcalf JA, et al. New real-time reverse transcriptase-initiated PCR assay with single-copy sensitivity for human immunodeficiency virus type 1 RNA in plasma. J Clin Microbiol. 2003;41:4531–4536. doi: 10.1128/JCM.41.10.4531-4536.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Palmer S, Maldarelli F, Wiegand A, Bernstein B, Hanna GJ, Brun SC, Kempf DJ, Mellors JW, Coffin JM, King MS. Low-level viremia persists for at least 7 years in patients on suppressive antiretroviral therapy. Proc Natl Acad Sci U S A. 2008;105:3879–3884. doi: 10.1073/pnas.0800050105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **79.Archin NM, Liberty AL, Kashuba AD, Choudhary SK, Kuruc JD, Crooks AM, Parker DC, Anderson EM, Kearney MF, Strain MC, et al. Administration of vorinostat disrupts HIV-1 latency in patients on antiretroviral therapy. Nature. 2012;487:482–485. doi: 10.1038/nature11286. Demonstrates induction of HIV RNA in ex vivo analysis of resting CD4 cells from cART suppressed patients receiving single doses of the HDACi vorinostat. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Brenchley JM, Vinton C, Tabb B, Hao XP, Connick E, Paiardini M, Lifson JD, Silvestri G, Estes JD. Differential infection patterns of CD4+ T cells and lymphoid tissue viral burden distinguish progressive and nonprogressive lentiviral infections. Blood. 2012;120:4172–4181. doi: 10.1182/blood-2012-06-437608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang F, Flanagan J, Su N, Wang LC, Bui S, Nielson A, Wu X, Vo HT, Ma XJ, Luo Y. RNAscope: a novel in situ RNA analysis platform for formalin-fixed, paraffin-embedded tissues. J Mol Diagn. 2012;14:22–29. doi: 10.1016/j.jmoldx.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Eisele E, Siliciano RF. Redefining the viral reservoirs that prevent HIV-1 eradication. Immunity. 2012;37:377–388. doi: 10.1016/j.immuni.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Siliciano JD, Siliciano RF. Enhanced culture assay for detection and quantitation of latently infected, resting CD4+ T-cells carrying replication-competent virus in HIV-1-infected individuals. Methods Mol Biol. 2005;304:3–15. doi: 10.1385/1-59259-907-9:003. [DOI] [PubMed] [Google Scholar]
- 84.Hansen SG, Vieville C, Whizin N, Coyne-Johnson L, Siess DC, Drummond DD, Legasse AW, Axthelm MK, Oswald K, Trubey CM, et al. Effector memory T cell responses are associated with protection of rhesus monkeys from mucosal simian immunodeficiency virus challenge. Nat Med. 2009;15:293–299. doi: 10.1038/nm.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Picker LJ, Hansen SG, Lifson JD. New paradigms for HIV/AIDS vaccine development. Annu Rev Med. 2012;63:95–111. doi: 10.1146/annurev-med-042010-085643. [DOI] [PMC free article] [PubMed] [Google Scholar]


