Abstract
Interleukin15 (IL 15) is a proinflammatory cytokine with elevated concentrations in autoimmune diseases involving the periphery (e.g. rheumatoid arthritis) and CNS (e.g. multiple sclerosis). Its interactions with the blood-brain barrier (BBB) were studied in normal and lipopolysaccharide (LPS)-treated mice. 125I-IL15 remained intact for at least 10 min after i.v. injection and reached CNS parenchyma with regional differences between brain and spinal cord. Both in vivo and in situ brain perfusion of 125I-IL15 showed that its permeation of the BBB was non-saturable. LPS induced a significant increase of IL15 uptake by the brain and spinal cord, partly related to a higher general permeability of the BBB. The results suggest that the BBB is an interface for blood-borne IL15 to interact with the CNS in the basal state and during inflammation.
Keywords: blood-brain barrier, interleukin15, lipopolysaccharide, neuroinflammation, permeability
Interleukin-15 (IL15) is a unique proinflammatory cytokine in the 4 α-helix bundle family of cytokines. Mature IL15, a 14 kD protein relatively conserved across species (73% homology between human and murine IL15) contains two disulfide bonds and two N-glycosylation sites. It binds to three subunits of receptors: IL15Rα (high affinity and canonical receptor) as well as IL2Rβ and IL2Rγ which are shared with other members of this family. Activation of the receptor complex induces multiple signaling events, particularly Janus kinases and signal transducer and activator for transcriptions (Fehniger and Caligiuri 2001). IL15 is involved in immune responses and hematopoiesis, and it promotes the survival of natural killer cells and memory T cells independently of IL2. IL15 plays crucial roles in autoimmune diseases and is a target for the therapy of rheumatoid arthritis (McInnes et al. 1996, 1997; Baslund et al. 2005).
Although less studied in the CNS, IL15 can be produced by astrocytes, microglia, and neurons. Cultured astrocytes and microglial cells express IL15 as does the developing brain. IL15 receptors are also present during brain development (Hanisch et al. 1997; Kurowska et al. 2002). Cerebral IL15 can be up-regulated by the proinflammatory cytokines tumor necrosis factor α (TNFα), IL1β, and interferon γ (Lee et al. 1996; Hanisch et al. 1997; Satoh et al. 1998). IL15 is increased in serum and CSF of patients with active multiple sclerosis (Rentzos et al. 2006). Thus, IL15 plays an important role in neuroinflammation.
Beck et al. (2005) have shown in IL2 knockout mice that IL15 might be involved in hippocampal neurogenesis. This indicates a role for IL15 in brain development. Moreover, Kruger and colleagues showed that IL15 induces dose-dependent increases in non-rapid eye movement sleep in rabbits after i.c.v injection (Kubota et al. 2001).
The blood-brain barrier (BBB) is an exchange interface between circulating cytokines and the CNS. Circulating IL15, as seen in rheumatoid arthritis, may induce CNS changes. IL2 is a homologous cytokine that shares IL2Rβ and IL2Rγ with IL15, besides binding to its specific receptor IL2Rα. IL2 enters the brain in a non-saturable manner after i.v. injection (Waguespack et al. 1994) and exits the brain by efflux transport (Banks et al. 2004). Daclizumab, an antibody against IL2, has shown success in a recently completed phase II clinical trial of relapsing and remitting multiple sclerosis after monthly i.v. injection (Rose et al. 2007). This provides a basis to determine how IL15 interacts with the BBB and how it is changed during inflammatory challenges.
The cerebral microvascular endothelia provide the structural backbone for the BBB, joined by tight junctions, underlined by a continuous membrane, and reinforced by astrocytic endfeet, pericytes, and extracellular matrix. At the BBB, saturable transport systems have been identified for the proinflammatory cytokines TNF, leukemia inhibitory factor, several ILs, and interferons (Pan et al. 1997b, 1999, 2000). On the basis of the literature and the known interactions of IL15 with three subunits of receptors, this study addresses the following two questions: (i) does IL15 cross the BBB to enter the CNS from blood under basal conditions? and (ii) how does neuroinflammation induced by lipopolysaccharide (LPS) affect IL15 permeation across the BBB? The results should facilitate an understanding of the IL15 system at the BBB level and identify potential new therapeutic targets.
Materials and methods
Animals
Adult male C57BL/6 (B6) mice 6–8 weeks old (Charles River, Wilmington, MA, USA) were used for the transport studies, immunohistochemistry, and microvessel analyses. The animal protocol was approved by the Institutional Animal Care and Use Committee. The mice were studied after anesthesia by i.p. injection of urethane. Carrier-free recombinant mouse IL15 (R & D Systems, Minneapolis, MN, USA) was labeled with 125I (Perkin Elmer, Waltham, MA, USA) by the iodogen method (Pierce, Rockford, IL, USA). Bovine serum albumin (BSA; Sigma, St Louis, MO, USA) was labeled with 131I by the chloramine-T (Sigma) method. The iodinated proteins were separated from free iodine by elution on Sephadex G-10 columns. The specific activity of 125I-IL15 was about 117 Ci/g, and that for 131I-albumin was about 45 Ci/g.
Degradation assays
To determine whether the radioactivity in blood and brain represented intact 125I-IL15, mice were killed 10 min after i.v. bolus injection of 125I-IL15 [2 μCi in 100 μL of lactated Ringer's (LR) solution] through the left jugular vein. The blood was collected into pre-chilled tubes for centrifugation to obtain serum. The brain was homogenized in 1 mL of phosphate buffered saline in the presence of complete protease inhibitor cocktail (Pierce) and centrifuged to obtain the supernatant. The processing controls involved addition of 125I-IL15 to the test tube for blood collection and to the homogenizer containing the brain from a naïve mouse. An aliquot of serum or brain supernatant was eluted on a polyacrylamide P10 column and 40 fractions were collected at 100 μL/fraction, as described previously (Tu et al. 2007). In addition, reversed phase HPLC was performed with a linear gradient of acetonitrile/0.1% trifluoroacetic acid from 0 to 100% over 30 min. This further confirmed that each peak of eluant from size-exclusion chromatography represented a single species of peptide (first peak) or 125I (second peak).
BBB transport
In the study examining the potential presence of a saturable transport system for IL15, two groups of mice were studied (n = 8 /group). The control group received radioactively labeled (radiolabeled) 125I-IL15 tracer only whereas the IL15 group received additional non-labeled IL15 (2 μg/mouse) as a co-injection. The unlabeled IL15, which was 117-fold in excess of 125I-IL15, was co-administered in the i.v. bolus injection.
In the study to determine the effects of LPS pre-treatment or potential inhibition of IL15 permeation by excess unlabeled IL15, three groups of mice were studied (n = 8/group). The control mice received normal saline (100 μL i.p.). The LPS group received LPS at 5 mg/kg i.p. in 100 μL of normal saline. These mice were studied 48 h later along with a third group that received 2 μg/mouse of unlabeled IL15 co-administered with the radiotracers.
Each mouse received a bolus of 125I-IL15 and 131I-albumin [30 000 cpm/μL in 100 μL of LR solution containing 1% BSA]. At 1, 2, 3, 5, 7, 10, 15, and 20 min, one mouse in each group was killed by decapitation immediately after blood was collected from the right carotid artery. The radioactivity of weighed brain, spinal cord, and 50 μL of serum was measured in a dual-channel (for 125I and 131I) γ-counter. Brain/serum and spinal cord/serum ratios of radioactivity were calculated [(cpm/g)/(cpm/μL) = μL/g] and plotted against the exposure time representing the theoretical steady-state value of the circulation time if the blood concentration of 125I-IL15 was constant. The exposure time was calculated as the integral of serum radioactivity over time divided by the experimental time (Kastin et al. 2001). The slope of the linear regression between CNS tissue/serum ratio and exposure time reflects the unidirectional influx rate, whereas the y-intercept represents the initial volume of distribution. The difference in the regression lines was compared with prism graphpad statistical software (GraphPad, San Diego, CA, USA).
Capillary depletion
To determine the compartmental distribution of 125I-IL15 in the brain, two groups of mice were injected with 1.5 μCi/mouse of 125I-IL15 in 100 μL of LR/BSA through the left jugular vein (n = 4/group). Blood was collected from the descending aorta at 10 min. One group received vascular washout with 25 mL of LR through the left cardiac ventricle after the descending aorta was blocked and outflow was created by severing the left atrium and bilateral jugular veins. The other group received no vascular washout but all other procedures were the same. The cerebral cortex of each mouse was dissected, weighed, and homogenized in a capillary buffer. The homogenate was mixed thoroughly with 26% dextran (Mol Wt: 64000–76000, Sigma) to yield a final concentration of dextran of about 16%. After centrifugation at 5400 g for 30 min at 4°C in a swing bucket rotor, the supernatant (including the lipid layer) and the pellet were carefully separated and the radioactivity was measured. The mean and SE of each fraction were calculated and statistical difference was determined by anova. The pellet/serum ratio of radioactivity in the group with washout represented capillary uptake of 125I-IL15. The supernatant/serum ratio of radioactivity in the same group was the parenchymal uptake. The mean difference of the total radioactivity between the groups with and without vascular washout represented the reversible vascular binding.
In situ brain perfusion
Two groups of mice were studied after anesthesia by i.p. injection of urethane (n = 7/group). The control group received 125I-IL15 (1000 cpm/μL) along with the vascular marker Evan's blue albumin (2% solution). The experimental group also received 200-fold excess of unlabeled IL15 by co-administration. The reagents were dissolved in modified Zlokovic's buffer as follows (g/L): NaCl 7.19, KCl 0.3, CaCl2 0.28, NaHCO3 2.1, KH2PO4 0.16, MgCl2·6H2O 0.37, and d-glucose 0.99 (pH 7.4). Albumin (0.5%) was added after oxygenation of the solution for 1 h to reduce the potential adsorption of IL15 to the plastic tubing. The solution was delivered by perfusion pump at a constant rate of 2 mL/min. Pre-perfusion for 2 min was used to remove blood components from the cerebral vasculature. After 5 min of perfusion of the radioactive solution, a post-perfusion for 2 min was applied to remove the residual radioactive solution. The brain and perfusate were sampled for measurement of radioactivity, and the brain/perfusate ratio of 125I-IL15 was calculated. Statistical analysis of the difference between the control and the experimental group with excess unlabeled IL15 was performed by anova.
Results
IL15 remained intact in blood after i.v. delivery and entered the brain intact
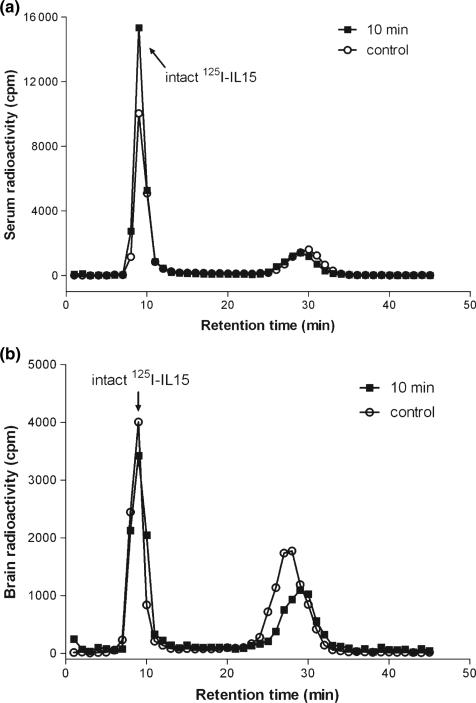
The stability of 125I-IL15 in blood and brain was determined by size-exclusion chromatography and further verified by HPLC. Analysis of serum samples showed that most of the circulating IL15 remained intact 10 min after i.v. injection. At this time, about 95% of the radioactivity recovered from brain represented intact IL15 corresponding to that of the processing control. Thus, IL15 was extremely stable; the dissociation of free iodine occurred as a result of external processing rather than in vivo degradation (Fig. 1a and b).
Fig. 1.
(a) Size-exclusion chromatography of serum radioactivity 10 min after i.v. injection of 125I-IL15. The control involved collection of blood into a test tube containing the 125I-IL15 to assess the extent of ex vivo degradation. In both samples, intact 125I-IL15 represented more than 95% of the total radioactivity, the rest being free 125I. (b) Size-exclusion chromatography of radioactivity in brain homogenate 10 min after i.v. injection of 125I-IL15. The control involved homogenization of an untreated brain in a homogenizer containing 125I-IL15. Intact 125I-IL15 accounted for 95% of the total radioactivity in these samples. IL, interleukin.
Pharmacokinetics of IL15 permeation across the BBB compared with albumin
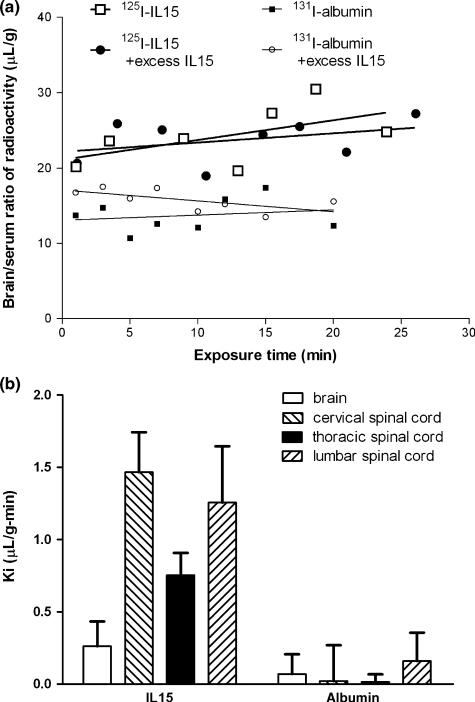
By comparison with the vascular permeability marker albumin, which showed minimal uptake by the CNS, IL15 had significant influx into the brain (Fig. 2a). At 20 min after i.v. injection, the extraction of 125I-IL15 was about 0.37%/g of brain. There was even greater influx into the cervical, thoracic, and lumbar segments of the spinal cord (Fig. 2b). In the brain, excess unlabeled IL15 failed to reduce the unidirectional influx rate (Ki) for 125I-IL15. This indicates the lack of saturability of the BBB permeation of circulating IL15 in normal mice. Excess unlabeled IL15 appeared to cause a slight elevation of the volume of distribution of 131I-albumin in the brain, particularly during the first few minutes after i.v. bolus delivery. This might indicate redistribution of cerebral perfusion and vascular volume, although it is not known whether IL15 has vasoactive effects. The difference between the groups with and without excess IL15 was not significantly different. In the spinal cord, the influx rate of IL15 was lower in the thoracic region than in the cervical and lumber spinal cord, regional differences probably related to heterogeneity of the general permeability of the BBB as we have described in studies with the inert tracers sucrose and albumin (Pan et al. 1996, 1997a).
Fig. 2.
(a) Influx of 125I-IL15 from blood to brain after i.v. injection. The volume of distribution of 125I-IL15 was significantly (p < 0.001) higher than that of the co-administered 131I-albumin, which served as a paracellular permeability control (n = 8/group). Inclusion of excess unlabeled IL15 had no significant effect on the influx of 125I-IL15 or 131I-albumin. (b) The influx rate of 125I-IL15 and 131I-albumin in brain and spinal cord regions after i.v. injection (n = 8/group). The y-axis represents the means of the influx rate (Ki) with their standard errors. The cervical and lumbar spinal cord appeared to have a higher influx rate than the brain and thoracic spinal cord. IL, interleukin.
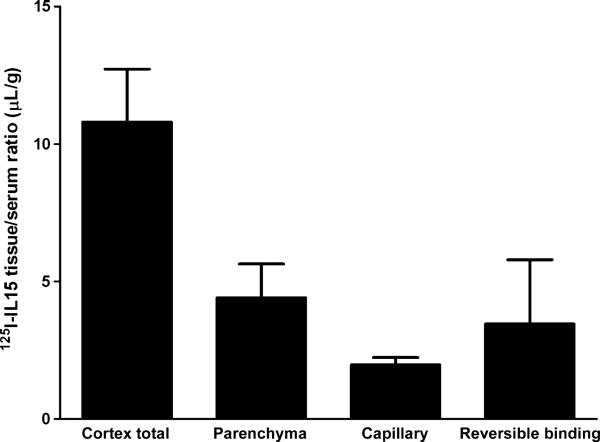
Compartmental distribution of IL15 in cerebral cortex
The uptake of IL15 by the cerebral cortex consisted of three compartments: vascular (reversible binding of IL15 to the luminal surface of the endothelial cells), endocytosis (IL15 tightly bound or trapped within the endothelial cells), and brain parenchyma after complete exocytosis. At 10 min after i.v. bolus delivery of IL15, the parenchymal uptake of IL15 was nearly twice that trapped in the capillaries (Fig. 3).
Fig. 3.
Compartmental distribution of 125I-IL15 was determined 10 min after i.v. injection. Parenchymal uptake accounted for almost 50% of the radioactivity in the brain, though the three fractions (parenchymal uptake, capillary uptake, and reversible vascular binding) that compose the total brain radioactivity did not show significant differences from each other (n = 4/group). IL, interleukin.
LPS selectively facilitated IL15 permeation across the BBB
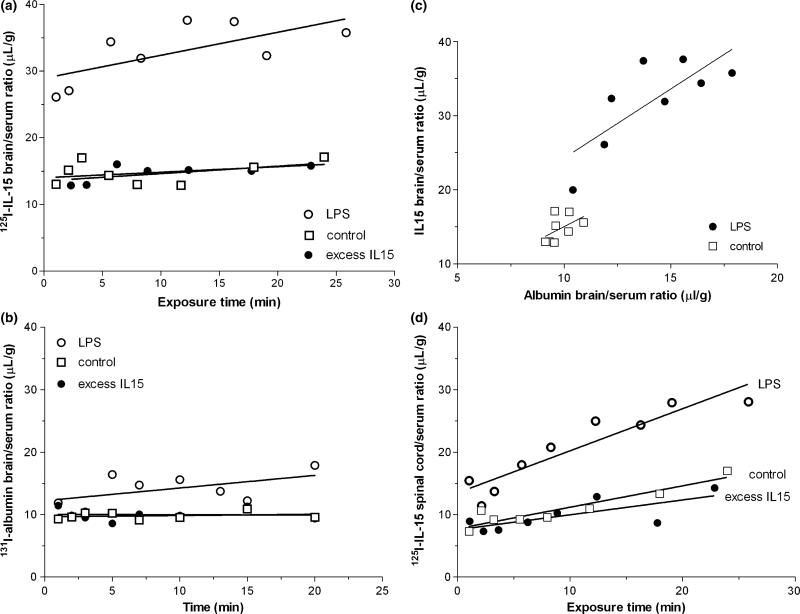
In groups of mice treated with LPS (5 mg/kg) 48 h earlier, there was a significantly greater volume of distribution of IL15 than in the control group (p < 0.001). This contrasts with the lack of effect of excess unlabeled IL15, a third group in the study (Fig. 4a). The elevation of the volume of distribution (y-values) of 125I-IL15 was extremely robust, unlike the change of influx rate (slope of the regression) induced by LPS. Overall, LPS enhanced the BBB capacity for the permeation of IL15, but did not cause a significant increase in the permeation of albumin (Fig. 4b). However, when the IL15 brain/serum ratio was plotted against the albumin brain/serum ratio at each time point, the pattern of lack of linear correlation in the control group was changed. LPS treatment induced a significant correlation between the uptake of IL15 and albumin, indicating that the increased IL15 uptake after LPS treatment was mainly attributed to a higher vascular space or BBB permeability (Fig. 4c).
Fig. 4.
(a) The effect of lipopolysaccharide (LPS) on the uptake of 125I-IL15 by brain after administration 48 h earlier. The volume of distribution of 125I-IL15 in the LPS group was significantly higher (p < 0.001) than the control. By contrast, unlabeled IL15 (117-fold excess) co-treatment had no effect on the influx of 125I-IL15 (n = 8/group). (b) The influx rate of 131I-albumin from blood to brain was unchanged by LPS treatment, but the volume of distribution showed a significant increase (p < 0.001), whereas co-administered excess unlabeled IL15 had no effect (n = 8 /group). (c) Relationship between the brain uptake of IL15 and albumin at each time point. In the control group, there was no significant correlation (r = 0.5, p > 0.05). In the LPS group, there was a significant correlation between the permeation of IL15 and albumin (r = 0.8, p < 0.05). (d) The influx rate of 125I-IL15 in the thoracic spinal cord after LPS was significantly greater than in the NS-treated control group (p < 0.05). Co-injection of excess unlabeled IL15 did not affect this influx of 125I-IL15. IL, interleukin; NS, normal saline.
As in the brain, IL15 influx in the spinal cord was also significantly increased by LPS pre-treatment (p < 0.005 for the overall slope) but unchanged by the excess unlabeled IL15 included in the transport assay (Fig. 4d). The results suggest that LPS pre-treatment was a stronger stimulus than the administered dose of excess unlabeled IL15 (2 μg/mouse co-injection) in increasing the permeation of 125I-IL15 across the blood-spinal cord barrier.
In situ brain perfusion showing the lack of saturability of IL15 uptake by the brain
As the permeation of blood-borne IL15 may be affected by the presence of binding proteins in the circulation, we performed in situ brain perfusion in blood-free physiological buffer. The brain uptake of 125I-IL15 (6 μL/g at 5 min in the control group) was about 50% higher than that of albumin, but lower than that of leptin and brain-derived neurotrophic factor seen in our previous studies (Pan et al. 1998, 2008). Co-administration of 200-fold excess of unlabeled IL15 did not cause a significant change in the brain uptake of 125I-IL15 (Fig. 5). The results further support the results from the multiple-time regression studies (above) that permeation of IL15 in the basal state is not mediated by a saturable transport system.
Fig. 5.
Brain uptake of 125I-IL15 after in situ brain perfusion for 5 min at a rate of 2 mL/min. The data shown represent the net uptake after vascular washout. The control group received 125I-IL15 whereas the experimental group also received 200-fold excess of unlabeled IL15 by co-administration. No significant difference was seen between the groups (n = 7/group). IL, interleukin.
Discussion
Interleukin15 production increases during inflammation and autoimmune diseases involving peripheral organs. As cytokines are often involved in sickness behavior and neuroendocrine changes, circulating IL15 may affect CNS functions by interacting with the BBB in at least two ways: direct permeation from blood to the CNS or activation of cerebral microvascular endothelial cells to produce secondary mediators. In this study, we mainly addressed the pharmacokinetics of IL15 permeation across the BBB and determined its potential regulation by a strong inflammatory stimulus, LPS, a component of the wall of gram-negative bacteria.
First, we showed that IL15 was stable in the circulation and that intact blood-borne IL15 could be detected in brain homogenates at 10 min after i.v. delivery. This indicates that IL15 from peripheral sources can interact with the CNS, probably through the BBB. However, the permeation of IL15 across the normal BBB was relatively slow in comparison with many other cytokines and neurotrophins that enter the BBB by specific transport systems (Pan and Kastin 2003a,b). The amount of brain uptake was about 0.37%/g of brain. This concentration, however is higher than what would be expected from the binding affinity of IL15 to its receptors. Thus, the permeated IL15 can exert physiological effects after reaching its targets in the CNS.
The regional difference of IL15 permeation was consistent with that of albumin, a marker of vascular permeability, although several cytokines with saturable transport (TNF and interferon α) also show similar regional differences. Overall, these results suggest the lack of specific transport of IL15 across the BBB. Nonetheless, about half of the IL15 in the brain entered brain parenchyma at 10 min, the rest remaining in the cerebral vasculature. These portions of IL15 may be sufficient to affect CNS and cerebral vascular function.
In the multiple-time regression analysis after i.v. administration to normal mice, excess unlabeled IL15 failed to modulate the influx of 125I-IL15. In situ brain perfusion was performed to rule out the possible interference of serum binding proteins such as the soluble IL15Rα receptor that might have masked the presence of a saturable transport system. Soluble IL15Rα binds to IL15 to antagonize its effects (Smith et al. 2000; Ruckert et al. 2005); such high affinity binding could prevent free IL15 from being available for BBB transport. In the blood-free perfusion buffer, however, we still did not detect the self-inhibition of 125I-IL15 uptake by the brain that would be indicative of a saturable and specific transport system. Cytokine signal modification at the BBB level also has been shown by interactions between TNF and leukemia inhibitory factor (Yu et al. 2007b,c).
We further determined how neuroinflammation regulates IL15 permeation across the BBB. LPS challenge by i.p. injection was used to stimulate the production of proinflammatory cytokines including IL15. A duration of 48 h was chosen between the LPS challenge and BBB permeability assay to ensure maximal induction of cytokine receptors at the BBB as suggested by previous studies (Xaio et al. 2001; Yu et al. 2007a).
Neuroinflammation induced by LPS was associated with a significant increase of IL15 uptake by the brain and spinal cord. There was a correlation of IL15 and albumin uptake in the LPS-treated mice. This indicates that increased IL15 permeation was related to the higher general permeability of the BBB. Despite the abundant reports that LPS induces sickness behavior, it has not been previously shown whether IL15 is a crucial cytokine mediator. Although the existing mouse models with IL15 knockout (Kennedy et al. 2000) and receptor knockout mice (Cao et al. 1995; Lodolce et al. 1998) show developmental abnormality in the immune system, it is hopeful that generation of tissue-specific conditional knockout mice will eventually provide novel information for the role of the IL15 system in normal CNS functions and the response to inflammation.
In summary, IL15 can cross the BBB to reach the brain and spinal cord in intact form, but its permeation was not saturable. LPS enhanced the CNS uptake of blood-borne IL15, and this was associated with increased BBB permeability to the vascular marker albumin. The availability of blood-borne IL15 to the CNS in the basal state and after LPS challenge suggests that IL15 participates in neuroinflammation.
Acknowledgement
Grant support was provided by NIH (NS45751, NS46528, and DK54880). Editorial assistance was provided by Ms Loula Burton.
Abbreviations used
- BBB
blood-brain barrier
- BSA
bovine serum albumin
- IL
interleukin
- LPS
lipopolysaccharide
- LR
lactated Ringer
- TNF
tumor necrosis factor α
References
- Banks WA, Niehoff ML, Zalcman SS. Permeability of the mouse blood-brain barrier to murine interleukin-2: predominance of a saturable efflux system. Brain Behav. Immun. 2004;18:434–442. doi: 10.1016/j.bbi.2003.09.013. [DOI] [PubMed] [Google Scholar]
- Baslund B, Tvede N, Danneskiold-Samsoe B, et al. Targeting interleukin-15 in patients with rheumatoid arthritis: a proof-of-concept study. Arthritis Rheum. 2005;52:2686–2692. doi: 10.1002/art.21249. [DOI] [PubMed] [Google Scholar]
- Beck RD, Jr, Wasserfall C, Ha GK, Cushman JD, Huang Z, Atkinson MA, Petitto JM. Changes in hippocampal IL-15, related cytokines, and neurogenesis in IL-2 deficient mice. Brain Res. 2005;1041:223–230. doi: 10.1016/j.brainres.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Cao X, Shores EW, Hu-Li J, et al. Defective lymphoid development in mice lacking expression of the common cytokine receptor gamma chain. Immunity. 1995;2:223–238. doi: 10.1016/1074-7613(95)90047-0. [DOI] [PubMed] [Google Scholar]
- Fehniger TA, Caligiuri MA. Interleukin 15: biology and relevance to human disease. Blood. 2001;97:14–32. doi: 10.1182/blood.v97.1.14. [DOI] [PubMed] [Google Scholar]
- Hanisch U, Lyons S, Prinz M, Nolte C, Weber J, Kettenmann H, Kirchhoff F. Mouse brain microglia express interleukin-15 and its multimeric receptor complex functionally coupled to Janus kinase activity. J. Biol. Chem. 1997;272:28853–28860. doi: 10.1074/jbc.272.46.28853. [DOI] [PubMed] [Google Scholar]
- Kastin AJ, Akerstrom V, Pan W. Validity of multiple-time regression analysis in measurement of tritiated and iodinated leptin crossing the blood-brain barrier: meaningful controls. Peptides. 2001;22:2127–2136. doi: 10.1016/s0196-9781(01)00569-1. [DOI] [PubMed] [Google Scholar]
- Kennedy MK, Glaccum M, Brown SN, et al. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J. Exp. Med. 2000;191:771–780. doi: 10.1084/jem.191.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota T, Brown RA, Fang J, Krueger JM. Interleukin-15 and interleukin-2 enhance non-REM sleep in rabbits. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001;281:R1004–R1012. doi: 10.1152/ajpregu.2001.281.3.R1004. [DOI] [PubMed] [Google Scholar]
- Kurowska M, Rudnicka W, Maslinska D, Maslinski W. Expression of IL-15 and IL-15 receptor isoforms in select structures of human fetal brain. Ann. N Y Acad. Sci. 2002;966:441–445. doi: 10.1111/j.1749-6632.2002.tb04245.x. [DOI] [PubMed] [Google Scholar]
- Lee YB, Satoh J, Walker DG, Kim SU. Interleukin-15 gene expression in human astrocytes and microglia in culture. NeuroReport. 1996;7:1062–1066. doi: 10.1097/00001756-199604100-00022. [DOI] [PubMed] [Google Scholar]
- Lodolce JP, Boone DL, Chai S, Swain RE, Dassopoulos T, Trettin S, Ma A. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity. 1998;9:669–676. doi: 10.1016/s1074-7613(00)80664-0. [DOI] [PubMed] [Google Scholar]
- McInnes IB, al-Mughales J, Field M, Leung BP, Huang FP, Dixon R, Sturrock RD, Wilkinson PC, Liew FY. The role of interleukin-15 in T-cell migration and activation in rheumatoid arthritis. Nat. Med. 1996;2:175–182. doi: 10.1038/nm0296-175. [DOI] [PubMed] [Google Scholar]
- McInnes I, Leung B, Sturrock R, Field M, Liew F. Inter-leukin-15 mediates T cell-dependent regulation of tumor necrosis factor-alpha production in rheumatoid arthritis. Nat. Med. 1997;3:189–195. doi: 10.1038/nm0297-189. [DOI] [PubMed] [Google Scholar]
- Pan W, Kastin AJ. Interactions of cytokines with the blood-brain barrier: implications for feeding. Curr. Pharm. Des. 2003a;9:827–831. doi: 10.2174/1381612033455332. [DOI] [PubMed] [Google Scholar]
- Pan W, Kastin AJ. Transport of cytokines and neurotrophins across the BBB and their regulation after spinal cord injury. In: Shanker H, Westman J, editors. Blood-Spinal Cord and Brain Barriers in Health and Disease. Elsevier, Academic Press; San Diego, CA, USA: 2003b. pp. 395–407. [Google Scholar]
- Pan W, Banks WA, Kennedy MK, Gutierrez EG, Kastin AJ. Differential permeability of the BBB in acute EAE: enhanced transport of TNF-a. Am. J. Physiol. 1996;271:E636–E642. doi: 10.1152/ajpendo.1996.271.4.E636. [DOI] [PubMed] [Google Scholar]
- Pan W, Banks WA, Kastin AJ. Blood-brain barrier permeability to ebiratide and TNF in acute spinal cord injury. Exp. Neurol. 1997a;146:367–373. doi: 10.1006/exnr.1997.6533. [DOI] [PubMed] [Google Scholar]
- Pan W, Banks WA, Kastin AJ. Permeability of the blood-brain and blood-spinal cord barriers to interferons. J. Neuroimmunol. 1997b;76:105–111. doi: 10.1016/s0165-5728(97)00034-9. [DOI] [PubMed] [Google Scholar]
- Pan W, Banks WA, Fasold MB, Bluth J, Kastin AJ. Transport of brain-derived neurotrophic factor across the blood-brain barrier. Neuropharmcol. 1998;37:1553–1561. doi: 10.1016/s0028-3908(98)00141-5. [DOI] [PubMed] [Google Scholar]
- Pan W, Kastin AJ, Bell RL, Olson RD. Upregulation of tumor necrosis factor a transport across the blood-brain barrier after acute compressive spinal cord injury. J. Neurosci. 1999;19:3649–3655. doi: 10.1523/JNEUROSCI.19-09-03649.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan W, Kastin AJ, Brennan JM. Saturable entry of leukemia inhibitory factor from blood to the central nervous system. J. Neuroimmunol. 2000;106:172–180. doi: 10.1016/s0165-5728(00)00241-1. [DOI] [PubMed] [Google Scholar]
- Pan W, Barron M, Hsuchou H, Tu H, Kastin AJ. Increased leptin permeation across the blood-brain barrier after chronic alcohol ingestion. Int. J. Neuropsychopharm. 2008;33:859–866. doi: 10.1038/sj.npp.1301452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rentzos M, Cambouri C, Rombos A, Nikolaou C, Anagnostouli M, Tsoutsou A, Dimitrakopoulos A, Triantafyllou N, Vassilopoulos D. IL-15 is elevated in serum and cerebrospinal fluid of patients with multiple sclerosis. J. Neurol. Sci. 2006;241:25–29. doi: 10.1016/j.jns.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Rose JW, Burns JB, Bjorklund J, Klein J, Watt HE, Carlson NG. Daclizumab phase II trial in relapsing and remitting multiple sclerosis: MRI and clinical results. Neurology. 2007;69:785–789. doi: 10.1212/01.wnl.0000267662.41734.1f. [DOI] [PubMed] [Google Scholar]
- Ruckert R, Brandt K, Braun A, Hoymann HG, Herz U, Budagian V, Durkop H, Renz H, Bulfone-Paus S. Blocking IL-15 prevents the induction of allergen-specific T cells and allergic inflammation in vivo. J. Immunol. 2005;174:5507–5515. doi: 10.4049/jimmunol.174.9.5507. [DOI] [PubMed] [Google Scholar]
- Satoh J, Kurohara K, Yukitake M, Kuroda Y. Interleukin-15, a T-cell growth factor, is expressed in human neural cell lines and tissues. J. Neurol. Sci. 1998;155:170–177. doi: 10.1016/s0022-510x(97)00310-9. [DOI] [PubMed] [Google Scholar]
- Smith XG, Bolton EM, Ruchatz H, Wei X, Liew FY, Bradley JA. Selective blockade of IL-15 by soluble IL-15 receptor alpha-chain enhances cardiac allograft survival. J. Immunol. 2000;165:3444–3450. doi: 10.4049/jimmunol.165.6.3444. [DOI] [PubMed] [Google Scholar]
- Tu H, Pan W, Feucht L, Kastin AJ. Convergent trafficking pattern of leptin after endocytosis mediated by ObRa – ObRd. J. Cell. Physiol. 2007;212:215–222. doi: 10.1002/jcp.21020. [DOI] [PubMed] [Google Scholar]
- Waguespack PL, Banks WA, Kastin AJ. Interleukin-2 does not cross the blood-brain barrier by a saturable transport system. Brain Res. Bull. 1994;34:103–109. doi: 10.1016/0361-9230(94)90005-1. [DOI] [PubMed] [Google Scholar]
- Xaio H, Banks WA, Niehoff ML, Morley JE. Effect of LPS on the permeability of the blood-brain barrier to insulin. Brain Res. 2001;896:36–42. doi: 10.1016/s0006-8993(00)03247-9. [DOI] [PubMed] [Google Scholar]
- Yu C, Kastin AJ, Ding Y, Pan W. Gamma glutamyl transpeptidase is a dynamic indicator of endothelial response to stroke. Exp. Neurol. 2007a;203:116–122. doi: 10.1016/j.expneurol.2006.07.023. [DOI] [PubMed] [Google Scholar]
- Yu C, Kastin AJ, Pan W. TNF reduces LIF endocytosis despite increasing NFkappaB-mediated gp130 expression. J. Cell. Physiol. 2007b;213:161–166. doi: 10.1002/jcp.21105. [DOI] [PubMed] [Google Scholar]
- Yu C, Kastin AJ, Tu H, Pan W. Opposing effects of proteasomes and lysosomes on LIFR: modulation by TNF. J. Mol. Neurosci. 2007c;32:80–89. doi: 10.1007/s12031-007-0017-4. [DOI] [PubMed] [Google Scholar]