Abstract
Objectives
New obturation biomaterials have been introduced over the past decade to improve the seal of the root canal system. However, it is not clear whether they have really produced a three-dimensional impervious seal that is important for reducing diseases associated with root canal treatment.
Methods
A review of the literature was performed to identify models that have been employed for evaluating the seal of the root canal system.
Results and Significance
In-vitro and in-vivo models are not totally adept at quantifying the seal of root canals obturated with classic materials. Thus, one has to resort to clinical outcomes to examine whether there are real benefits associated with the use of recently-introduced materials for obturating root canals. However, there is no facile answer because endodontic treatment outcomes are influenced by a host of other predictors that are more likely to take precedence over the influence of obturation materials. From the perspective of clinical performance, classic root filling materials have stood the test of time. Because many of the recently-introduced materials are so new, there is not enough evidence yet to support their ability to improve clinical performance. This emphasizes the need to translate anecdotal information into clinically relevant research data on new biomaterials.
Keywords: leakage models, root canal, root filling materials, sealability, treatment outcome
1. Introduction
There is increasing need for the medical and dental professions to base their clinical judgement on evidence-based knowledge [1,2]. The requisite for integrating biologically-based knowledge with the advent of new biomaterials designed to improve root canal treatment has been well conceded even before endodontics became a specialty [3]. Because diseases of the dental pulp are predominantly infectious in nature [4], development of new root filling materials should be targeted at improving the ability and efficacy for endodontists to eliminate infections and prevent recontamination [5,6]. As early as 1925, Dr. Walter Hess and his student Dr. Ernst Zürcher showed that root canal systems have exceedingly complex morphologies that may not be amendable to complete shaping and cleaning [7]. Contemporary non-destructive imaging such as micro-computed tomography further reinforced this notion from a three-dimensional (3-D) perspective [8,9]. Between then and now, knowledge of root canal infections has also improved. Apical periodontitis, as it is now known, is not caused by fluid stagnation within unfilled canal spaces [10]. Rather, it is an inflammatory reaction of the periapical tissues and an immunological response of the host defense system to microbes that have infected the root canal system [11,12]. Successful root canal treatment, therefore, depends critically on controlling pulp space infection [13–15]. Root canal infection, like many other infections, is predominantly a biofilm-induced disease [16–18]. The association between bacterial biofilms and apical periodontitis has been confirmed by more recent histobacteriological studies [19,20].
Success in root canal treatment was founded upon the triad of thorough canal débridement, effective disinfection and obturation of the canal space [21]. Historically, a significant share of this triad had been allocated to obturation of the canal space. Obturation of the canal space to the working length [22] has been depicted as the most critical component of root canal treatment for sealing and isolating the canal space from irritants that remain after appropriate shaping and cleaning, and for eliminating subsequent leakage from the periradicular tissues or oral cavity into the filled canal space [23,24]. Results from these early works are supported by recent outcome studies that highlight the contribution of root filling quality to the success of primary and secondary root canal treatment [25–28]. Nevertheless, contemporary research attests that shaping and cleaning are strategically more significant than obturation of the canal space for eliminating root canal infections [29]. Apical periodontitis has been shown to coronal seal [30]. It is indubitable that a high quality coronal seal is heal in teeth with unfilled canals following meticulous shaping and cleaning, and placement of a important for endodontic success [31]. However, insisting that healing reliably occurs in the presence of a defective root filling is to have created a very narrow view [32] on the important roles played by canal obturation in preserving the environment created by shaping and cleaning and preventing microbial reinfection of the canal space [33], both of which are essential for securing long-term periradicular heath.
New obturation materials have been introduced into the endodontic market over the last decade. Some of these are modifications of materials developed for restorative dentistry. Others may be considered as new breeds, embracing radically new concepts that have not been employed before in the field of endodontics. These seemingly unrelated new developments share a bona fide feature: their promotions are accompanied by impressive case reports. It is beyond doubt that those clinical cases are performed by outstanding clinicians who, in every way, have demonstrated their “conscientious adherence to high (professional) ideals” [34]. Those cases are demonstrations of the results of excellent shaping and cleaning, and perhaps of sound obturation techniques. On further reflection, do they really demonstrate the superiority of one particular new filling material? The present review highlights and analyzes how the quality of seal is determined for the classic root canal obturation materials. This will provide the background for clinicians to determine, based on the best available evidence to date, whether the recently-introduced root canal obturation materials have improved the seal of the root canal system. Search of the literature on the seal of the root canal system was accomplished via the MEDINE (Ovid) database on in vitro and in vivo studies published between 1981 and 2013, using key words “root filling” OR “obturation” OR “leakage” OR “seal”. Four journals (Journal of Endodontics, International Endodontic Journal, Oral Surgery Oral Medicine Oral Pathology Oral Radiology and Endodontology, and Endodontics and Dental Traumatology) and the bibliography of all relevant articles and review articles were also manually searched.
2. The Classics (Traditional Gutta-percha and Sealer)
Historically, gutta-percha, the trans-isomer of polyisoprene, has been the material of choice as a solid, inert core filling material for root canal obturation, beginning with Bowman's introduction of the material into endodontics in 1867 [35]. Contemporary gutta-percha–based root filling materials utilizes approximately 20% of the raw polymer, with the remaining composition consisting of zinc oxide, wax/resin and metal sulphates [36,37]. Different gutta-percha obturation techniques have been employed, including single-cone technique, solvent softening techniques, cold or warm lateral compaction, warm vertical compaction, continuous wave compaction, thermoplastic injection techniques, thermomechanical compaction techniques, as well as core-carrier techniques [38,39]. Of notable mention is that the single-cone obturation technique, developed in the 1980s for standardization of endodontic instruments and filling points [40], has been revived with the introduction of some contemporary filling techniques [41].
Although gutta-percha is not the ideal filling material for root canals, it has satisfied most of the criteria for an ideal root filling material. In particular, gutta-percha exhibits minimal toxicity, allergenicity and tissue irritability when it is retained within the canal space [42]. Gutta-percha is predominantly non-resorbable and is well tolerated in cases of inadvertent overextension into the periradicular tissues [43], when the overextended material is present in bulk instead of fine particles [44]. Gutta-percha is degradable by bacteria species associated with the Nocardia genus [45]. However, such species have not been identified as endodontic pathogens, despite the vast diversity of microbial flora reported in untreated teeth and root-filled teeth with apical periodontitis [46–49]. Although gutta-percha retrieved from retreatment cases had been reported to degrade chemically over time via a slow oxidative process [50], there was no associated loss of texture or disappearance of the material from the obturated canal space. Moreover, those root fillings are defective by default as the internal milieu of the canal space should be predominantly anaerobic if the latter is appropriately sealed.
A shortcoming of gutta-percha-based root filling materials is their lack of adhesiveness to canal wall dentin. Because of this limitation, a sealer or cement has to be used with gutta-percha to attain a fluid-tight seal, and to fill the space between the canal wall dentin and the obturating material interface. Sealers also fill voids and irregularities in the root canal, lateral and accessory canals, and spaces between gutta-percha points used in lateral condensation techniques. Different types of sealers are currently available, including zinc oxide eugenol and noneugenol sealers, calcium hydroxide sealers, glass ionomer sealers, epoxy resin–based sealers, silicone sealers, medicated sealers, and the more recently introduced methacrylate-resin-based sealers and calcium silicate-based sealers. Penetration of sealers and even gutta-percha into dentinal tubules has been demonstrated when the canal wall smear layer is removed [51–53]. The extent of penetration of root canal sealers into dentinal tubules [54–56] has often been utilized for demonstrating the improvement of sealability to root canals during the launch of new root filling materials and techniques. However, studies have shown that there is no correlation between sealer penetration into dentinal tubules and sealability in non-bonded [57] or bonded [58] root fillings. Along the same line of thought, a small puff of sealer extending through the apical ramifications [22] or lateral canals [59–61] has been considered by some clinicians to be indicative of an optimally-obturated canal space with a well-sealed apical constriction or lateral portal of exit. The need to force filling materials into these regions to enhance treatment outcome is not supported by a recent histopathological study [62]. In that study, pulp tissues within those ramifications were not removed by contemporary chemomechanical preparation techniques. Forcing materials into those ramifications in teeth with vital pulps resulted in tissue damage and inflammation. The radiographic appearance of filling materials within those ramifications, while visibly appealing, could not guarantee optimal sealing or disinfection. In large ramifications of necrotic pulps where microbes could reach sufficient quantity to cause or maintain disease, the authors opined that alternative strategies that enable thorough disinfection of those regions should be sought to enhance treatment outcome.
Over the last couple of decades, endodontics has benefited from ground breaking advancements in imaging capabilities, better magnification, more accurate working length determination technology, more efficient canal shaping armamentarium, more advanced canal irrigation solutions, and radical changes in tissue dissolution and disinfectant delivery techniques. All of these improvements have contributed to enhanced obturation of the canal space. However, even in countries with highly-developed economies and advanced technological infrastructures, the majority of teeth that require non-surgical root canal treatment are obturated today with the same classic material that was introduced more than 160 years ago, using classical filling techniques that were established in the 1940s and 1950s, with a relatively high and predictable degree of success [25–28]. Despite the paucity of high-level, evidence-based studies [63,64], randomized control clinical trials remain the gold standard in evaluating treatment outcomes. Nevertheless, they suffer from the limitation of not being able to discern whether failure is caused by the material or by the operator. Considering that root canal treatment in teeth without previous histories of apical periodontitis has over 90% success, it is logical to assume that the use of classic obturation materials, from which the majority of those studies were based, should result in an excellent seal of the canal space. Paradoxically, regardless of the technique or delivery system employed for these classic materials, the literature is replete with in-vitro studies indicating that they do not routinely provide an impervious seal of the root canal system. Thus, it is appropriate to briefly review how leakage is evaluated for these classic endodontic obturation materials. Specifically, it is important to understand how clinically relevant the currently available laboratory methods are in evaluating the seal of filled root canals.
2.1 In–vitro leakage models
Because of the prevailing thinking of opinion leaders from academia in the early 1990s that incomplete filling of the root canal is the major factor in endodontic failure [23,24], different laboratory-based models have been designed to evaluate the sealability of endodontic filling materials and techniques, either in the form of leakage from the root apex (apical leakage) or the coronal aspect of the root canal filling (coronal leakage). Many of these models measure the penetration of a tracer along the root canal filling, including dyes, radio-isotopes, bacteria and lipopolysaccharides. Other models have been employed apart from the tracer penetration/extraction techniques. Fluorometry measures the fluorescence emitted by a fluorescent tracer around the root fillings. The electrochemical technique measures the passage of an electrical current through electrolyte-filled voids along the root filling. The fluid transport method evaluates the passage of water or saline across through-and-through voids along the root filling. Capillary flow porometry measures the displacement of a wetting liquid from pores that extend along the entire root filling by applying a gas at increasing pressure. The glucose penetration test quantifies the amount of glucose that can be forced under hydrostatic pressure applied from the coronal aspect of the root filling using an enzyme detection method. The results from all in-vitro leakage models unanimously show that no canal obturation material/technique can achieve or maintain a long-term seal under the most ideal conditions in extracted teeth.
The major limitations of in-vitro leakage studies are their lack of reproducibility, small sample size and inadequate statistical power, absence of standardization and absence of correlation among different leakage models [65–68]. There are also limitations associated with individual models, particularly those involving dye leakage evaluation [69–73]. Even the most logical model for examining reinfection of root-treated teeth, the microbial leakage model, suffers from severe criticism in that most of the studies published to date lack an appropriate control that takes into account the leakage between the root surface cementum and the sticky wax coating [74,75]. This model also does not distinguish between sealability of the root filling from the antimicrobial effects of the obturating material [76]. For example, an in-vitro bacteria leakage model was used to confirm the seal of obturated root canals by the absence of turbidity in the culture broth-containing lower reservoir, after 120 days of testing against the ingress of Enterococcus faecalis, a bacteria species frequently identified from canals with recalcitrant periradicular lesions. However, subsequent histologic analysis of demineralized sections prepared from the corresponding teeth demonstrated abundant bacteria within the obturated root canals and dentinal tubules [77]. The results of that study re-emphasize the need to utilize histology to validate the routes of microbial leakage in the classical two-chamber microbial leakage model to avoid generation of erroneous results. Another “apparently relevant” leakage model, the glucose leakage model, which is supposed to measure the availability of nutrients to bacteria, has been found to produce inaccurate results due to reaction of glucose with some root canal sealers [78].
Although in-vitro leakage models have been adopted by manufacturers for initial screening of new materials and techniques for canal obturation and to justify their superiority for clinical applications, these models have limited clinical relevance. Dr. Thomas Pitt Ford was among the first in 1983 to alert the specialty that the value of in-vitro leakage testing has been overemphasized [79], after he found no correlation between apical leakage of root-filled teeth immersed in eosin dye and the tissue response of roots of dogs' teeth filled with the same materials. Similarly, Oliver and Abbott observed that treatment outcome could not be predicted from the results of dye leakage studies, after they identified no statistical significant difference in apical dye leakage between clinically successful and unsuccessful human teeth that had been root-filled for at least 6 months prior to extraction [80]. In that study, the methylene blue dye penetrated 99.5% of the specimens under the effect of a vacuum. The authors concluded that the presence of dye in the canal is a poor indicator of whether the obturation material or technique will result in a successful treatment outcome. More recently, Susini et al. also concluded that evaluation of apical leakage using the dye extraction method has no predictive value for the development of periradicular lesions [81]. In that study, the authors immersed extracted human teeth that had been root-treated clinically for at least 4 years into methylene blue dye and then extracted the dye with 65% nitric acid. They reported no statistically significant difference in the amount of extracted dye in teeth that exhibited an apical radiolucency and those without an apical radiolucency.
Although the use of methylene blue dye has been criticized in a subsequent study for its futility as a tracer for dye penetration studies due to its chemical reaction with some sealers [73], the endodontic academia at large is skeptical of the clinical implications of currently available in-vitro sealability models, and the absence of evidence associated with studies utilizing these models. As an illustration, Shanahan and Duncan recently published a review paper on root canals obturated by a recently-introduced root filling material that has been introduced into the market as a replacement for gutta-percha [82]. The authors identified 46 leakage studies, of which 16 studies (34.5%) demonstrated that the recently-introduced root filling material was statistically superior to gutta-percha in preventing apical or coronal leakage. Nineteen out of the 46 studies (41.3%), on the other hand, demonstrated no statistical difference between the two materials, while 11 studies (23.9%) demonstrated gutta-percha was statistically superior to the recently-introduced material in preventing apical or coronal leakage! This exemplifies the chasmic discrepancies in leakage study findings that do not permit one to support or refute the value of nearly any endodontic material. This has led the Journal of Endodontics [83], and subsequently the International Endodontic Journal [84], to publish position statements in not accepting future studies that are related to comparative appraisals of root filling materials or techniques using in-vitro leakage models alone.
2.2 In-vivo canine models
Most of the materials for root canal obturation may be classified as class II medical devices according to the U. S. Food and Drug Administration [85]. Class II devices are defined as those which are considered as having “moderate risk” in terms of their risk to the patients and how the risks are mitigated. Class II materials are tested for toxicity and physical properties, not clinical performance. There is no functional test that goes into classification and quality control requirements for these materials. Nevertheless, from a research perspective, the lack of a standardized, clinically relevant in-vitro methodology that permits comparative evaluation of functional efficacy has prompted research scientists to develop animal models for comparing the functional efficacy of classic obturation materials in creating an impervious seal of the root canal system.
In endodontics, the most relevant animal model adopted for such a purpose is the in-vivo canine model. Two versions of the in-vivo model have been employed. The first model was designed to examine the effect of root filling materials in preventing post-treatment periradicular infections via a coronal route [79,86–93]. In this version, dogs were anesthetized using general anesthesia and their premolars were shaped, cleaned and obturated with different materials/techniques, with appropriate positive and negative controls. After canal obturation and temporization, the pulp chamber and access cavity of those teeth were restored. In other studies, the access cavities were subsequently re-accessed and left exposed to the oral environment, or inoculated with dog's plaque and re-sealed. The dogs were humanely sacrificed after a determined period of time and tissue blocks containing the root-filled teeth were fixed, demineralized, laboratory-processed, sectioned and stained to examine the extent of periapical inflammation as well as soft and hard tissue responses, and, occasionally, the presence of bacteria within the intraradicular dentin.
The second version of the in-vivo canine model was designed to evaluate the effects of classic endodontic filling materials on the healing of pre-existing apical periodontitis [94–98]. The experiments were similar to the first version, with the exception that the root canals were first exposed to the oral environment to induce the formation of periapical lesions over time [99] prior to shaping, cleaning and obturation of the canal spaces. The examination criteria were similar to the first version, but with the addition of assessment of the lesion size at designated time periods.
While it is undeniable that the results generated by in-vivo animal models are more clinically relevant than those produced by in-vitro leakage models, it must be pointed out that data derived from animal studies do not represent the result of the seal of the root canal system per se. Rather, those results are the combined effects of: a) the sealability of the root filling materials; b) tissue responses to the short- or long-term cytotoxicity of the materials [100,101]; c) antimicrobial properties of the materials [76]; d) innate antimicrobial and immune-regulatory responses by host defense peptides (defensins and cathelicidins) [102]; and e) the effect of dentinal tubule occlusion on microbial contamination of the root canal space [75]. It has previously been reported that tubular sclerosis (i.e. sclerotic radicular dentin or transparent root dentin [103]) impedes dye penetration into the dentin of instrumented root canals [104]. The more recent work by Rechenberg et al. [75] further attests that leakage of microbes through filled root canals occurs through dentinal tubules and their anastomoses instead of along the interface between the root filling material and the canal wall. When leakage occurs, bacteria permeate the tubular aspects of root-filled teeth while sclerotic dentin remains bacteria-tight. This phenomenon should be applicable to both in-vitro and in-vivo models. Thus, in comparing the “sealability” of different materials using an in-vivo animal model, it is unclear if one is evaluating the real sealability or the variability of sclerotic root dentin in different teeth or animals.
One can see that both in-vitro and in-vivo models are not totally reliable in quantifying the seal of root canals obturated with classic materials. Although in-vitro leakage models are not useful as predictors of treatment outcomes, it should be emphasized that they still play an important role as a first simple screening test prior to the commercialization of a new material. The purpose of an initial screening test is to ensure that the new material meets the standards established by pre-existing techniques prior to its application on animals or patients. Until there is a better initial screening test, all in-vitro leakage models described in the previous section still have validity. This is because the clinical validity of the in vivo canine model is questionable in terms of the different apical anatomy in the root apices of dog's roots, compared with the roots present in human teeth. Moreover, it is questionable whether sacrificing dogs for the sake of examining the sealability of root canal fillings is really justifiable. Nevertheless, the succeeding question to be answered is whether the new obturation materials/techniques recently introduced into the market are capable of improving the seal already achieved by classic materials/techniques of the root canal system.
3. Recently-introduced Materials
Commercial presentations and glossy advertising have never been more prominent when it comes to the introduction of new dental products into a highly competitive market. Controversies and disputes have emanated, dividing practitioners into philosophical cults [105]. It is not the intent of this review to associate brand names with any individual product or category of products. Rather, they will be discussed in terms of their purported functions or generic compositions. Accordingly, the new commercially-available root filling materials which claim betterment of their seal of the root canal system may be classified by function into those that:
Adhere to canal wall dentin and root filling materials to eliminate interfacial gaps;
Attempt to self-seal gaps by setting or hygroscopic expansion;
Enhance flow and adaptation of the root filling material to canal walls; and
Utilize bioactive reaction products to salvage a compromised seal.
3.1 Adhere to canal wall dentin and root filling materials to eliminate interfacial gaps
This category is by far the most extensively studied. As envisioned by Dr. John Ingle in 1995 [106], one of the recent trends in endodontics has been the development of obturating materials that are capable of bonding to canal wall dentin in order to eliminate interfacial gaps coronally and apically [107]. Dentin adhesive technology has been adapted from restorative dentistry and applied to obturating materials. As far as adhesion to intraradicular dentin is concerned, the same mechanism of hybrid layer formation that has been reported for bonding to crown dentin is recapitulated in intraradicular dentin with the use of etch-and-rinse adhesives [108,109], or methacrylate resin-based sealers that utilize a separate self-etching primer component or self-adhesive sealers that incorporate acidic resin monomers [110]. Likewise, glass ionomer cements which chemically bond to dentin via ion exchange and formation of an intermediate layer along the dentin-material interface [111] have been adopted to produce glass ionomer root canal sealers [112].
In the early-2000s, there was a surge of enthusiasm among manufacturers of endodontic obturation materials to produce, via adhesive mechanisms, a continuum of bonded interfaces from dentin to sealer to the core obturation material, and from the coronal to apical aspects of the canal space, including the canal fins, cul-de-sacs and isthmuses. This hypothetical concept results in creating a monoblock in the root canal [113] which, if successful, should completely eliminate interfacial gaps, produce perfect coronal and apical seal, and prevent post-treatment re-infection of the canal space [114]. As gutta-percha does not adhere well to root canal sealers, one way to achieve this goal is to chemically bind or mechanically impregnate a coating over the surface of gutta-percha cones so that the chemistry and surface energy of the coating is akin to the sealer. Examples of this strategy includes resin-coated gutta-percha with functional methacrylate groups for use with a hydrophilic self-priming methacrylate resin-based sealer system [115], and bioactive glass power-impregnated gutta-percha cones for use with a glass ionomer-based sealer [116] or a premixed calcium silicate-based sealer [117]. Theoretically, this type of intracanal bonding strategy produces a tertiary monoblock with three adhesive interfaces: dentin-sealer, sealer-coating and coating-gutta-percha [113]. As demonstrated by finite element analysis modeling, the magnitude of stress created within the root canal during force application increases with the number of interfaces, from 1.67 to 8.33 MPa, in hypothetical monoblocks created by the sealers [118]. There are manufacturing difficulties in creating a uniform coating over the entire surface of a gutta-percha cone. It is not infrequent to see denuded gutta-percha with incomplete resin coating (Figures 1A and 1B) or non-uniform distribution of glass particles (Figures 1C and 1D). It is known that an oxygen inhibition layer is not required for immediate bonding of fresh resins that cure by a free radical polymerization mechanism, due to the presence of active free radicals within the material [119]. However, bonding does not occur once the free radicals are depleted [120]. Thus, it is hard to envisage how adhesion of methacrylate resin-based sealers to non-sticky, commercially-packaged resin-coated gutta-percha can occur even if a continuous resin coating exists.
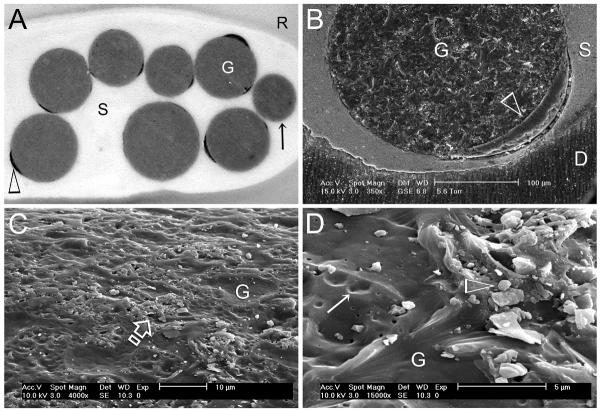
Figure 1.
A. Stereoscopic microscope image of incomplete coating (open arrowhead) of resin-coated gutta-percha points (G) that are used with a hydrophilic self-priming methacrylate resin-based root canal sealer (S). Some obturation points are devoid of resin coating (arrow). B. Environmental scanning electron microscopy (ESEM) image of a partially resin-coated gutta-percha point (G), taken at 95% relative humidity, showing a gap between the resin coating (open arrowhead) and sealer; D: root dentin. C. SEM of the surface of a bioactive glass particle-coated gutta-percha point (G) that is used with a glass ionomer-based root canal sealer. Distribution of the glass particles is limited to some areas only (open arrow). D. High magnification of Figure 1C. Area on the left is devoid of glass particles (open arrowhead). Areas previously occupied by glass particles (open arrowhead) are denoted by depressions on the gutta-percha surface (arrow).
Another strategy to achieve an intracanal monoblock is to incorporate a cross-linkable difunctional methacrylate into an alternative thermoplastic polymer to render the root filling material bondable to self-etch or self-adhesive root canal sealers. This strategy was brought to fruition by the introduction of a biodegradable, polycaprolactone-based thermoplastic root filling material that is currently marketed as a replacement for gutta-percha [121]. Ideally, this type of intracanal bonding strategy produces a secondary monoblock with two adhesive interfaces: dentin-sealer and sealer-root filling material [113]. However, the dimethacrylate is not miscible with the polycaprolactone in the commercialized version of the material, and phase separates into discontinuous droplets within the bulk of the filling material (Figure 2). As there is no catalyst and co-initiator in the root filling material, polymerization of the surface droplets can only occur if they are exposed on the surface of the root filling material to enable diffusion of free radicals from the resin sealer. Unfortunately, the dimethacrylate resin droplets are usually covered by the polycaprolactone polymer and are only exposed after laboratory differential etching of the polycaprolactone. This probably explains why adhesion of a methacrylate resin sealer to this root filling material is low [122]. In a recent review on this root filling material, the authors concluded that the material cannot presently be considered an evidence-based alternative to gutta-percha, the current gold standard for root filling material [82]. Nevertheless, because the system requires no changes in the techniques of canal preparation or obturation, it provides an alternative for patients suffering from natural rubber allergy, who, despite reassurance of the lack of cross-reactivity between natural rubber and gutta-percha [123,124], are apprehensive in having their root canals obturated by gutta-percha.
Figure 2.
A. SEM image of the heat-pressed surface of thermoplastic polycaprolactone-based root filling material (C) showing phase separation of the methacrylate resin component (arrow) from the polycaprolactone. F: fillers. Bar = 5 μm. B. SEM image of the polycaprolactone-based root filling material after surface etching of the polycaprolactone root filling material with 0.1 N NaOH revealing exposed, partially-coalesced methacrylate resin droplets (arrow). Open arrowhead: unexposed fillers. Bar = 20 μm.
An important issue that jeopardizes the seal of root canals that are filled with these relatively rapid-polymerizing, resin sealer systems is their extended setting shrinkage behavior [125], and the inability of the long narrow root canals to cope with the relief of polymerization shrinkage stresses created by these systems on the bonded interfaces (i.e. dentin, resin-coating or bondable core material) [126–128]. This is particularly so when manufacturers recommend light-curing through the canal orifices to create an immediate coronal seal. Although there may be partial relief of shrinkage stresses as the resin sealer flows into patent dentinal tubules, gaps are still formed along the interfaces despite the presence of long resin tags in the hydrophilic self-priming methacrylate resin sealer system [58]. Similar results can be seen in root canals obturated with the polycaprolactone-based filling material and its accompanying self-etching resin sealer, either by destructive or non-destructive evaluation methods. Using micro-computed tomography, intracanal voids were found to be significantly fewer in canals that are obturated with gutta-percha and accompanying sealers [129]. Using confocal laser scanning microscopy (CLSM), gap distribution was significantly higher, and gap thickness were significantly larger in canals that were obturated with the polyacaprolactone-based filling material and its accompanying sealers, when compared to roots obturated with gutta-percha and an epoxy resin-based sealer (Figure 3) [130]. Another CLSM study came to the same conclusion that obturation of root canals with gutta-percha using non-bonding techniques resulted in significantly more gap-free regions, when compared with the use of the polycaprolactone-based material and its accompanying self-etching sealer [131]. Unlike leakage, interfacial gaps are clinically relevant because as little as 1% shrinkage of root canal sealers can result in gaps that are large enough for bacteria penetration [132]. Although epoxy resin also shrinks during polymerization [133], commercially available epoxy resin-based root canal sealers set slowly enough to permit the shrinkage stresses to be relieved through resin flow. Subsequent water sorption from incompletely desiccated dentinal tubules further contributes to relief of residual shrinkage stresses via hygroscopic expansion [132,134].
Figure 3.
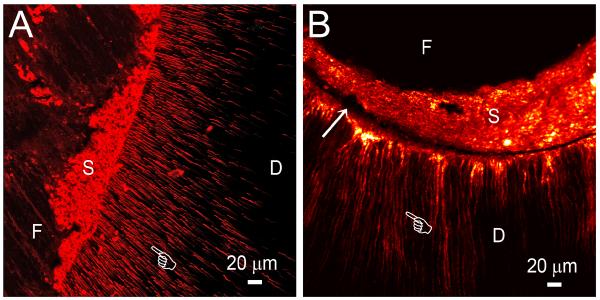
A. Subsurface confocal laser scanning microscopy of a gap-free region of a root canal that is obturated by a root filling material and sealer. B. Subsurface confocal laser scanning microscopy of a gap-containing region (arrow) of a root canal that is obturated by a root filling material and sealer. In both specimens, penetration of the root canal sealer into the dentinal tubules is evident (pointer), irrespective of the presence or absence of gaps. F: root filling material; S: root canal sealer; D: root canal dentin.
Another route of interfacial void formation is the potential biodegradation of the core filling material itself. There are no published reports indicating that this phenomenon occurs in gutta-percha to the extent that permits bacteria penetration. However, there are continuing anecdotal discussions that such a phenomenon occurs in polycaprolactone-based root filling materials when there is a breach of the coronal seal, so that bacteria can penetrate the root filling and propagate. Development of apical periodontitis has been noted in teeth obturated with polycaprolactone-based material, in which there was no evidence of pre-operative periodontitis [135], with occasional disappearance of the filling material (Figure 4, a–d). A more common observation in cases that require re-treatment, is the loss of structural integrity of the polycaprolactone-based root filling material “from the rigid state to a pliable, almost gelatinous form” [135]. Also, black material was sometimes identified during retreatment, in canals previously treated with this filling material. The color change from pink to black was clarified by the manufacturer to be due to bismuth sulphide, a reaction product between bismuth oxychloride, the radiopacifier, and proteins derived from leaked body fluids. Contrary to what the manufacturer claimed, a similar color change was identified after exposure of the polycaprolactone-based root filling material to bacteria present in root canal infections [136]. This potential degradation process requires several years before it can be detected in a radiograph. A possible cause of such a phenomenon is enzymatic degradation of the polycaprolactone component of the root filling material [137,138] by remnant microbes from infected root canals. Under starvation, these bacteria are capable of producing inducible enzymes to use polycaprolactone as an alternative source of nutrients [139]. The polycaprolactone-based obturation concept was originally marketed as a better product than gutta-percha for preventing coronal leakage [140]. Results of the recent endodontic microbiota-related biodegradation studies [135,136] suggest that should coronal leakage occur, root canals obturated with the polycaprolactone-based material are more likely to leak than those obturated with gutta-percha. Further work should be performed to clarify this potential biodegradation phenomenon. It would be interesting also to see if the seal of the root canal system can be improved when polycaprolactone is replaced by polybutylene [141].
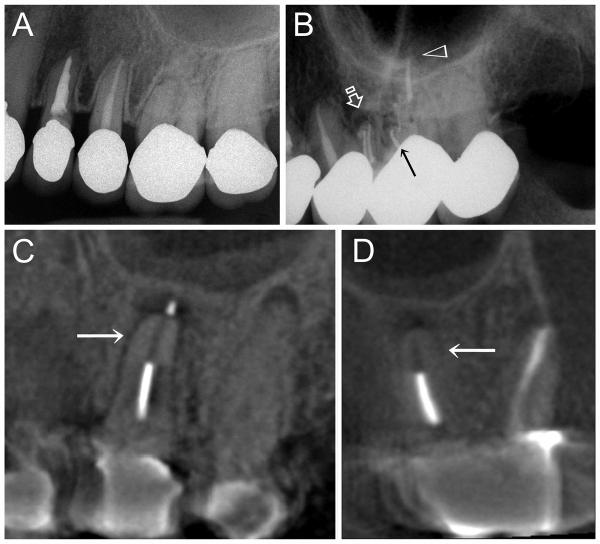
Figure 4.
A. Pre-operative periapical radiograph of the upper left first molar (second tooth from the right). Although the tooth was non-vital, there was no periapical radiolucency associated with the palatal root. B. Post-operative periapical radiograph of the upper left first molar after root canal treatment and obturation with a polycaprolactone-based root filling material and accompanied resin-based sealer. Four radiopaque filled canals can be identified: 2 canals in the mesiobuccal root (open arrow), one canal in the distobuccal root (arrow) and one canal in the palatal root (open arrowhead). C. Three-year post-treatment cone beam computer tomography image (sagittal projection across the palatal root) of the same root-treated upper left first molar showing partial disappearance of the polycaprolactone root filling material (arrow) and the development of a periapical lesion at the root tip of the palatal root. D. Three-year post-treatment cone beam computer tomography image of the same tooth taken along an oblique coronal projection, revealing the palatal root and the distobuccal root. Disappearance of the polycaprolactone root filling material from the apical third of the root canal (arrow) is also evident from this angulation.
3.2 Attempt to self-seal gaps by setting or hygroscopic expansion
Expansion of obturation materials to improve the seal of root-filled canals has been reported even for classic obturation materials. Gutta-percha expands in the presence of eugenol [142,143] which may help to reduce gaps within canals obturated with zinc oxide-eugenol sealer-filled canals caused by the release of eugenol from the set sealer [144], shrinkage [132, 145] or dissolution of sealer over time [146]. Apart from eugenol-induced expansion, closure of interfacial gaps in gutta-percha-filled root canals may also occur by slow, hygroscopic expansion of gutta-percha [147], due to sorption of incompletely removed moisture present within the canal space [148].
Some of the recently-introduced materials also utilize expansion of the sealer or obturation points as a mechanism to compensate for or self-seal gaps inside the canal space. The first is a polydimethylsiloxane-based sealer than contains gutta-percha particles. This sealer has been reported to expand by less than 1% (0.44–0.76% at 37°C) after setting [125,149]. Although in-vitro leakage studies are common, they are of limited value, as discussed in Section 2. The second expansion method is a cold lateral condensation technique that utilizes single or multiple cones of a new water-expandable obturation material together with an epoxy resin-based or a calcium silicate-based sealer [150]. This new obturation point employs technology derived from water-expandable polymers utilized in contact lens [151], and is designed to radially expand as it absorbs water that is resident in the instrumented canal space and dentinal tubules. The manufacturer claims that the lateral expansion occurs non-isotropically, with the expansion relying on the extent to which the hydrophilic polymer is pre-stressed (i.e. expansion will be reduced when it is in contact with the canal wall) [152]. This non-uniform expansion is said to enhance the sealability of root canal fillings by pushing the sealer into close contact with the canal wall during the initial setting of the sealer. The obturation point consists of an inner radiopaque nylon core and an outer radiopaque polymer coating of a cross-linked hydrophilic copolymer of acrylonitrile and vinylpyrrolidone [150]. A recent study showed that the expansion of the new hydrophilic obturation points is complete within 20 minutes, a time period in which most root canal sealers still exhibit flow during setting [153]. The magnitude of linear or volumetric setting expansion was not included in that study; based on the data presented, linear expansion is estimated to be in the range of 11.5–14%. This high value undoubtedly exceeds the standard set by the American National Standards Institute, which states that the mean linear expansion of the sealer shall not exceed 0.1% [154]. However, results from that study [153] were obtained by direct immersion of obturation points directly in water. The amount of available water for causing lateral expansion was much more than what would be expected from residual moisture in intraradicular dentin, after the root canal was blot-dried with paper points or from naturally occurring intra-radicular moisture. Moreover, no sealer was employed for coating, which might have impeded diffusion of water into the obturation points.
To date, very limited information is available on this novel obturating point. Further work should be performed to identify whether over-swelling into the gaps may generate high enough positive root strains, or produce intraradicular microcracks that slowly propagate with time during intraoral functioning to result in vertical root fracture [155,156]. It cannot be overemphasized that while crack initiation may be rapid, fatigue crack growth in dentin caused by the generation of initial flaws is a slow process and may take years to result in catastrophic failure [157,158]. Although there have been anecdotal reports of short-term clinical success in teeth filled with these water-induced expandable non-gutta-percha obturation points, the results cannot be acceptable without long-term clinical validation.
3.3 Enhance flow and adaptation of the root filling material to canal walls
Coating metal cores such as gold wire, silver points and endodontic files with heat-softened gutta-percha for three-dimensional obturation of the canal space has long existed before the commercialization of different prefabricated gutta-percha core-carrier systems that are claimed to enhance adaptation of the gutta-percha to the canal wall, and flow of the guttapercha into lateral canals. Due to the difficulties encountered in retreatment and in the preparation of post spaces, the original metal carriers were subsequently replaced by plastic obturators [159,160]. Although the core-carrier technique has been regarded as the only genuine warm gutta-percha technique for adaptation to the apical third of the canal space, voids could not be completely eliminated when canals obturated with this technique were examined using impression casts (Figures 5A and 5B) or 3-D non-destructive investigation techniques [161,162]. As discussed in Section 1, the need for gutta-percha to flow into the dentinal tubules (Figure 5C and 5D) or lateral canals has also been challenged [62]. In terms of clinical outcome, results from two separate retrospective studies were unanimous in that there was no significant difference in the success rate achieved using the core-carrier technique or the cold lateral condensation technique for primary root canal treatment [163,164].
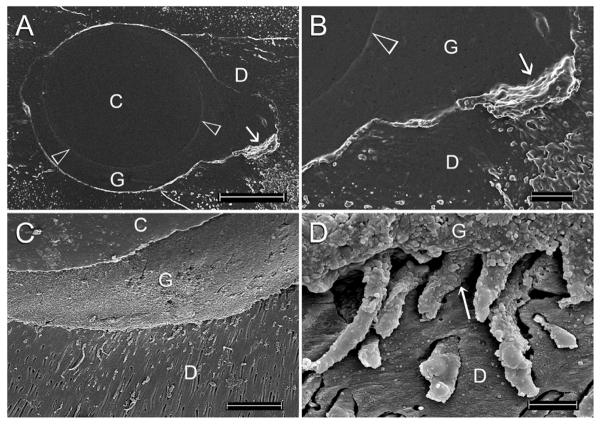
Figure 5.
A. SEM of a polyvinylsiloxane impression negative replica of a section of a single-rooted canal that was obturated using a gutta-percha-based core-carrier technique. The gap-free interface between the plastic core (C) and the gutta-percha (G) can be seen (open arrowheads). A gap is evident within the canal fin (arrow). D: root dentin. Bar = 200 μm. B. High magnification of Figure 5A showing the aforementioned gap as a protruded piece of impression material (arrow) between the gutta-percha (G) and root dentin (D). Open arrowhead: interface between the obturator core and gutta-percha. Bar = 50 μm. C. SEM image obtained by direct observation of a single-rooted canal obturated using a gutta-percha-based core-carrier technique. Extension of the root filling material and sealer into patent dentinal tubules can be seen. C: plastic core; G: gutta-percha; D: root dentin. Bar = 200 μm. D. High magnification of Figure 5C showing extension of highly granular gutta-percha (G) into the dentinal tubular orifices (arrow). The rest of the tags that occupied the dentinal tubules is contributed by the root canal sealer. D: root dentin. Bar = 5 μm.
There are two recent modifications to the classic core-carrier systems. The first modification involves replacement of the gutta-percha carrier with a coating of the polycaprolactone-based filling material described in Section 3.1 [165], which is chemically united with a resin-based core. Removal of this modified obturator during retreatment may be achieved by mechanical means or softening with chloroform as the resin-based core is soluble in this organic solvent. The second modification involves replacing the Vectra or polysulphone plastic carriers in a classic gutta-percha-based system with cross-linked thermoset gutta-percha that does not melt by heat used in an obturator oven, and is insoluble in common organic solvents employed for root canal retreatment [166]. Removal of this modified obturator during retreatment is achieved by mechanical trephining through the core. To date, there are no peer-reviewed studies on the clinical efficacy of this new core-carrier system.
3.4 Utilize bioactive reaction products to salvage a compromised seal
A new category of root canal sealers based on mineral trioxide aggregate (MTA) has recently been commercially available. These sealers are an outgrowth of the popular MTA materials, which are based on tricalcium silicate, a hydraulic (water-setting) powder used for various surgical and vital pulp therapy treatments [167]. This type of root canal sealer is attractive because of its hydrophilicity [168] and bioactivity that has been reported for MTA-type materials [169,170]. Calcium silicate-based sealers include some of same hydraulic compounds found in Portland cement, primarily tricalcium silicate and dicalcium silicate powder [168]. They set by reaction with water and form a highly alkaline (pH ~ 12) cement composed of a rigid matrix of calcium silicate hydrates and calcium hydroxide [171]. These hydrates form on the surface of the original calcium silicate particles and hydration gradually penetrates inward. When these sealers set, the dimensional change is less than 0.1% expansion, which helps in creating a barrier that is important for sealing the canal space [172]. For the tricalcium silicate particles to contribute to sealing, they must become hydrated by water from the sealer liquid or in the root canal.
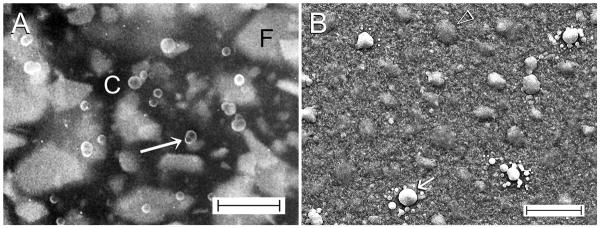
Although some calcium silicate-based sealers do not contain phosphate, they react with phosphate ions derived from tissue fluids to form needle-shaped carbonated apatite crystallites (Figure 6) [169]. Others reported formation of an interfacial layer between MTA and dentin that stems from a similar apatite formation reaction [162,173]. Theoretically, when obturated root canals come into contact with tissue fluids in the apical region, or when there is ingress of tissue fluids via a defective coronal restoration, these intracanal bioactive reactions may contribute to salvaging a compromised seal.
Figure 6.
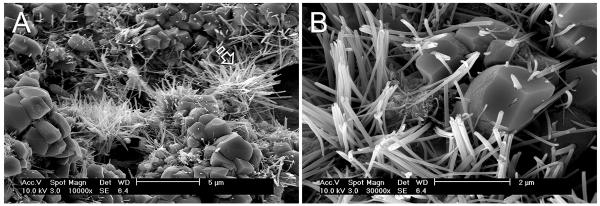
A. SEM image of the surface of a bioactive calcium silicate-based root canal sealer showing the presence of apatite crystalline clusters (open arrow) after exposure to a phosphate ion-containing simulated body fluid for 24 hours. B. High magnification of Figure 6A showing the needle-shaped apatite crystallites, some of which were sprouting from the calcium silicate filler particles.
Four calcium silicate-based sealers are currently commercially available [174–177]. To date, no peer-reviewed papers have been published to establish the benefits of incorporate tricalcium silicate powders in sealers in clinical studies. All studies are based on in-vitro testing and in-vivo animal models on tissue responses after implantation of set sealers in subcutaneous tissues.
4. In-vivo animal studies involving the recently-introduced materials
All published studies were conducted on root fillings using methacrylate resin-based sealers. Two of the studies were performed using the hydrophilic self-priming methacrylate resin-based sealer described in Section 3.1. The first study compared the sealability of the hydrophilic self-priming methacrylate resin-based sealer and two other sealers, when root canals of dog's premolars were obturated with gutta-percha and one of three sealers after post-space preparation [178]. That study was essentially a dye leakage study as the teeth were extracted after exposure to the oral environment and immersed in India ink, and thus the results had limited value. The second study compared tissue reactions of the hydrophilic self-priming methacrylate resin-based sealer in root fillings that were prepared short of, or beyond the apical foramen-like apical communication in dogs' teeth [179]. The conclusion that “limiting the sealer short of the communication resulted in a better treatment outcome” was not different from what is already known from earlier clinical studies using classic root canal sealers [24].
The rest of these in-vivo canine studies were related to the use of the polycaprolactone-based root filling material and its proprietary bondable methacrylate resin root canal sealers. All of these studies utilized the first model version (Section 2.2) to compare the seal of root fillings in preventing post-treatment periradicular reinfections via a coronal route. Histopathological data generated by these in-vivo studies were as conflicting as leakage results derived from in-vitro models [82]. Whereas earlier studies indicated that dogs' teeth obturated with the polycaprolactone-based material using different obturation techniques produced more favorable periapical tissue responses than gutta-percha filling techniques [180–182], more recent studies indicated no difference between the two root filling materials [183–185].
As discussed in Section 2.2, tissue responses are not equivalent to the seal of the root canal system. Other factors, not necessarily related to the seal, could have contributed to the in-vivo responses. When teeth were left exposed to the oral environment to induce bacteria leakage, differences in antimicrobial activity between the classic and the recently-introduced root canal sealers could have accounted for the difference in tissue responses [186]. Another issue that has not been considered in most in vivo canine studies is the role played by over-instrumentation or overfilling in the tissue response, in particular between what may be classified as “no inflammation” versus “mild inflammation”. For example, in the study by Shipper et al. [180], 82% of the dog's teeth that were obturated with gutta-percha and an epoxy resin-based sealer exhibited had mild periapical inflammation and 18% had no inflammation. By contrast, 19% of the dog's teeth that were obturated with the polycaprolactone-based root filling material and its accompanying self-etching sealer exhibited mild periapical inflammation and 81% had no inflammation. Without stratifying those teeth into under-filled, fully-filled or over-filled, it is dubious if differences between those two root filling materials are meaningful. Conversely, when the dogs' teeth were stratified and allocated to the two root filling material groups with approximately equal numbers in the study performed by Brasil et al. [184], there were no significant differences in both the extent and the intensity of inflammatory reaction in teeth obturated with either root filling material.
5. Clinical studies: classic vs recently-introduced materials
The outcome of root canal treatment depends on a multitude of factors and not simply on materials or techniques of obturation [187]. While specific filling materials have been ardently promoted, there is little, if any, clinical data that supports the superiority of one material over another. In the past, treatment outcome studies have rarely vigorously examined the contribution of obturation materials to clinical success. Even for obturation techniques (vertical vs lateral condensation), their contribution to treatment outcome is weak and of questionable significance, and then only in cases with preoperative apical periodontitis [26,188].
Traditionally, non-surgical root canal treatment has enjoyed an excellent track record. Epidemiological studies based on insurance records indicated that 92.1% of these treatments remained functional after a 3.5 year period [189]. Using the same concept of functionality as a less stringent method for assessing treatment outcome, other authors showed that 97% of cases remained functional in the oral cavity after an 8-year period of primary root canal treatment. [190], while 89% of the teeth were retained in the oral cavity after endodontic retreatment [191]. For meta-analyses of treatment outcomes, Friedman reported that 90–97% of the cases remained functional. When a more stringent method of assessment based on definitive radiographic healing was used for examination, 88–97% of the cases without apical periodontitis were successful, while 74–91% of the cases with apical periodontitis were successful [192]. A similar trend was also reported by Ng et al. in that the presence of pre-operative apical periodontitis reduces the success of primary root canal treatment [193]. Using functionality as a less stringent method of assessment, successful outcomes were identified in 87–93% of the cases without apical periodontitis, and 76–87% of the cases with apical periodontitis. When definitive healing was employed as a more stringent criterion for success, 73–92% of the cases without apical periodontitis were successful, while 61–78% of the cases with apical periodontitis were successful. These results are also supported by those derived from a prospective cohort study by Ricucci et al. [194]. Using definitive healing as the yardstick for assessment, successful outcome was observed in 89.5% of the cases without apical periodontitis and 82.7% of the cases with apical periodontitis. Taken together, results from these classical studies predicate that the future of new obturation material lies in its potential to improve specifically the healing of apical periodontitis, and not simply improving the overall outcome of root canal treatment. As all treatment outcome studies rely on the use of radiographs in quantifying success [195], a confounding variable that affects the quality of future clinical studies for the recently-introduced materials is their ability to demonstrate healing of apical periodontitis from a 3-D perspective [196,197]. For comparisons among materials or techniques, however, conventional 2-D radiography of low sensitivity, but high specificity, may still be valuable.
For the different categories of recently-introduced obturation materials described in Section 3, clinical studies could be identified for only those materials that promote adhesion to canal walls. Between 2004 and 2012, Zmener and Pameijer conducted a longitudinal series of retrospective cohort studies on the clinical performance of root canals obturated with gutta-percha and the hydrophilic self-priming methacrylate resin-based sealer, using 2-D radiographic and clinical evaluation criteria. Although the success data were initially categorized into cases with or without apical periodontitis, with an overall success rate of 91.3% at 14–24 months [198], only cumulative probabilities of success were reported at 5-year recall (86.3%) [199], 8-year recall (86.5%) [200] and 10-year recall (92.1%) [201]. These studies generated valuable information on the clinical performance of a particular obturation material for marketing to general practitioners who desire a simpler obturation technique. Nevertheless, there was no comparison with existing materials or techniques. The authors concluded that the hydrophilic self-priming methacrylate resin-based sealer has similar performance as conventional root canal sealers reported in the literature. The corollary to that statement is that the use of a self-priming, bondable resin-based sealer does not improve the clinical performance over the use of classic, non-bondable root canal sealers.
There are four additional clinical studies that reported the use of the bondable polycaprolactone-based root filling material in conjunction with its proprietary self-etching sealers. The first study is a prospective case series study that examined 21 cases using 2-D radiographic and clinical assessment criteria at 1-year recall, but without a gutta-percha comparison group. Results were reported qualitatively and no conclusion can be reached apart from anecdotal impressions [202]. The second study by Conner et al. [203] is a retrospective case series study conducted using radiographic and “clinical impression of healing” quantification procedures, without a gutta-percha comparison group. This study reported that 89.4% of cases were healed or healing after at least one year of root canal treatment. The authors concluded that root canals obturated with the recently-introduced material has a similar healing rate as gutta-percha obturated canals reported in the literature. The corollary to that statement is that the use of the polycaprolactone-based root filling material does not improve the clinical performance over the use of gutta-percha.
The third study by Cotton et al. [204] is a retrospective cohort study that compared the clinical outcome of root canals obturated with gutta-percha and a zinc oxide eugenol-based sealer with those obturated with the polycaprolactone-based root filling material and its accompanying self-etching methacrylate resin-based sealer. The study was conducted using 2-D radiographic and clinical evaluation criteria, with a recall period between 12–25 months. Similar to previous studies, the presence of a preoperative periradicular lesion was found to be statistically significant in predicting treatment outcome. However, there was no statistically significant difference between the two obturation materials when outcomes were evaluated by combining teeth with or without periradicular lesions. Even when those cases were stratified by examination of only teeth with pre-operative periradicular lesions, there was still no statistically significant difference in treatment outcome between the two obturation materials.
By far, the most convincing study was the one performed by Tehrany [205] at the University of North Carolina at Chapel Hill. This retrospective case series study examined periapical healing of teeth with asymptomatic apical periodontitis only, after root canal treatment with gutta-percha or the polycaprolactone-based root filling material. Radiographic and clinical outcomes of root canal treatments completed by undergraduate dental students under direct supervision of an endodontic faculty member for a minimum of 12 months were evaluated separately. Teeth filled with gutta-percha or the polycaprolactone-based root filling material did not differ statistically in their radiographic outcome (81.1% versus 78.4%, respectively), when the cases were evaluated using 2-D periapical radiographs. Likewise, teeth obturated with gutta-percha or the polycaprolactone-based root filling material did not differ statistically in their clinical success (90.1% versus 89.6%, respectively), which was defined as absence of all clinical signs and symptoms associated with persistent inflammation or infection.
6. Conclusion
Over the past decade, there has been an exceptionally fine harvest of novel root canal obturation materials that are designed to improve the seal of the root canal system. Without doubt, sealing of root canals three-dimensionally contributes to the success of root canal treatment. However, the quest for a clinically relevant model for evaluating the seal of the root canal system has evolved along a topsy-turvy path of intellectual discourse to become an intangible philosophical ideal. As the genre of currently available in-vitro and in-vivo models is not totally suitable for quantifying the seal of root canals obturated with classic materials, one has to resort to clinical outcomes to infer if there are patient-related benefits associated with the use of the recently-introduced materials for obturating root canals. Yet, there is no facile answer; essays on endodontic treatment outcome are confounded by a host of other predictors that are more likely to take precedence over the influence of obturation materials [28].
From the perspective of clinical performance, classic root filling materials have substantiated what Aristotle expressed as `historical truth' [206] and have stood the test of time. Because many of the recently-introduced materials are so new, there is not enough evidence yet to support their ability to improve clinical performance. For those recently-introduced materials they may be comparable to, but do not exceed outcomes already achieved by the use of classic biomaterials.
As elegantly expressed by Dr. John Whitworth [38], improvements in technological possibilities in root canal obturation may have met needs other than healing more disease: “As no single obturation technique or material can claim superior evidence of a better seal, decisions may be based on factors such as speed, simplicity, economics or `how it feels in my hands'. For some, there may be other issues at stake, such as the desire to keep `up to date,' to demonstrate `mastery,' to keep ahead of referring colleagues, or to enliven the working day by `the thrill of the fill'”. Within that agenda, the choice of the practitioner may be pragmatically justified based on a merger of individual preference, experience and practice circumstance, or simply, as partisan to the encomium delivered by a master storyteller. While such a stance may appear errant to a health care profession that has consistently envisioned the importance to base clinical judgement on evidence-based knowledge, it emphasizes the need to transl ate anecdotal opinions into analyzable research data to provide stronger evidence to support the launch of new biomaterials for improving patient care. It is often the lack of relevance of the currently available testing techniques, rather than the willingness of the manufacturers to perform pre-clinical testing, that limits the empirical rationale for adopting or rejecting new endodontic materials. Consequently, anecdotal evidence is what has been adopted as critique of new endodontic materials. It makes sense to get oneself acquainted with one or two commonly used techniques and materials, respecting the principles of good root canal treatment, in order to create consistent, radiographically well-adapting, homogeneous root canal filling at the right length. It is important to realize that no filling material or technique can compensate for inadequate asepsis and disinfection procedures.
Acknowledgments
This work was supported by grants R01 DE015306-06 from the National Institute of Dental & Craniofacial Research (PI: David H Pashley) and National Key Basic Research Program of China grant 2012CB526704 (PI: Jihua Chen). The authors thank Mrs. Michelle Barnes and Mrs. Marie Churchville for their secretarial support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
RFERENCES
- [1].Sathorn C, Parashos P. Questions and answers in evidence-based patient care. British Dental Journal. 2007;203:309–19. doi: 10.1038/bdj.2007.834. [DOI] [PubMed] [Google Scholar]
- [2].Shafiei L, Shahravan A. The level of evidence in two leading endodontic journals. Iranian Endodontic Journal. 2013;8:18–21. [PMC free article] [PubMed] [Google Scholar]
- [3].Naidorf IJ. Inflammation and infection of pulp and periapical tissues. Oral Surgery, Oral Medicine, and Oral Pathology. 1972;34:486–97. doi: 10.1016/0030-4220(72)90328-3. [DOI] [PubMed] [Google Scholar]
- [4].Kakehashi S, Stanley HR, Fitzgerald RJ. The effects of surgical exposures of dental pulps in germfree and conventional laboratory rats. Oral Surgery, Oral Medicine, and Oral Pathology. 1965;20:340–9. doi: 10.1016/0030-4220(65)90166-0. [DOI] [PubMed] [Google Scholar]
- [5].Byström A, Happonen RP, Sjögren U, Sundqvist G. Healing of periapical lesions of pulpless teeth after endodontic treatment with controlled asepsis. Endodontics & Dental Traumatology. 1987;3:58–63. doi: 10.1111/j.1600-9657.1987.tb00543.x. [DOI] [PubMed] [Google Scholar]
- [6].Lin LM, Rosenberg PA, Lin J. Do procedural errors cause endodontic treatment failure? Journal of the American Dental Association. 2005;136:187–93. doi: 10.14219/jada.archive.2005.0140. [DOI] [PubMed] [Google Scholar]
- [7].Hess W, Zürcher E. The Anatomy of the Root-Canals of the Teeth of the Permanent Dentition and the Anatomy of the Root Canals of the Deciduous Dentition and the First Permanent Molars. William Wood and Co; New York: 1925. http://babel.hathitrust.org/cgi/pt?id=mdp.39015023388500;view=2up;seq=14. [Google Scholar]
- [8].Dowker SE, Davis GR, Elliott JC. X-ray microtomography: nondestructive three-dimensional imaging for in vitro endodontic studies. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology & Endodontics. 1997;83:510–6. doi: 10.1016/s1079-2104(97)90155-4. [DOI] [PubMed] [Google Scholar]
- [9].Peters OA, Laib A, Rüegsegger P, Barbakow F. Three-dimensional analysis of root canal geometry by high-resolution computed tomography. Journal of Dental Research. 2000;79:1405–9. doi: 10.1177/00220345000790060901. [DOI] [PubMed] [Google Scholar]
- [10].Rickert UG, Dixon CM. The controlling of root surgery. 8th International Dental Congress Meeting.1931. pp. 15–22. [Google Scholar]
- [11].Torabinejad M, Eby WC, Naidorf IJ. Inflammatory and immunological aspects of the pathogenesis of human periapical lesions. Journal of Endodontics. 1985;11:479–88. doi: 10.1016/S0099-2399(85)80221-1. [DOI] [PubMed] [Google Scholar]
- [12].Stashenko P, Teles R, D'Souza R. Periapical inflammatory responses and their modulation. Critical Reviews in Oral Biology and Medicine. 1998;9:498–521. doi: 10.1177/10454411980090040701. [DOI] [PubMed] [Google Scholar]
- [13].Nair PN, Henry S, Cano V, Vera J. Microbial status of apical root canal system of human mandibular first molars with primary apical periodontitis after “one-visit” endodontic treatment. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology & Endodontics. 2005;99:231–52. doi: 10.1016/j.tripleo.2004.10.005. [DOI] [PubMed] [Google Scholar]
- [14].Wu MK, Dummer PM, Wesselink PR. Consequences of and strategies to deal with residual post-treatment root canal infection. International Endodontic Journal. 2006;39:343–56. doi: 10.1111/j.1365-2591.2006.01092.x. [DOI] [PubMed] [Google Scholar]
- [15].Nair PN. On the causes of persistent apical periodontitis: a review. International Endodontic Journal. 2006;39:249–81. doi: 10.1111/j.1365-2591.2006.01099.x. [DOI] [PubMed] [Google Scholar]
- [16].Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999;21:1318–22. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- [17].Carr GB, Schwartz RS, Schaudinn C, Gorur A, Costerton JW. Ultrastructural examination of failed molar retreatment with secondary apical periodontitis: an examination of endodontic biofilms in an endodontic retreatment failure. Journal of Endodontics. 2009;35:1303–9. doi: 10.1016/j.joen.2009.05.035. [DOI] [PubMed] [Google Scholar]
- [18].Schaudinn C, Carr G, Gorur A, Jaramillo D, Costerton JW, Webster P. Imaging of endodontic biofilms by combined microscopy (FISH/cLSM - SEM) Journal of Microscopy. 2009;235:124–7. doi: 10.1111/j.1365-2818.2009.03201.x. [DOI] [PubMed] [Google Scholar]
- [19].Ricucci D, Siqueira JF., Jr Biofilms and apical periodontitis: study of prevalence and association with clinical and histopathologic findings. Journal of Endodontics. 2010;36:1277–88. doi: 10.1016/j.joen.2010.04.007. [DOI] [PubMed] [Google Scholar]
- [20].Vera J, Siqueira JF, Jr, Ricucci D, Loghin S, Fernández N, Flores B, Cruz AG. One- versus two-visit endodontic treatment of teeth with apical periodontitis: a histobacteriologic study. Journal of Endodontics. 2012;38:1040–52. doi: 10.1016/j.joen.2012.04.010. [DOI] [PubMed] [Google Scholar]
- [21].Schilder H. Vertical compaction of warm gutta-percha. In: Gerstein H, editor. Techniques in Clinical Endodontics. WB Saunders; Philadelphia, PA: 1983. pp. 76–98. [Google Scholar]
- [22].Schilder H. Filling root canals in three dimensions. Dental Clinics of North America. 1967;11:723–44. [PubMed] [Google Scholar]
- [23].Ingle JI, Beveridge EE, Glick DH, Weichman JA. Endodontic success & failure: the Washington Study. Chapter 1: Modern Endodontic Therapy. In: Ingle JL, Bakland LK, editors. Endodontics. 4th ed Williams & Wilkins; Baltimore, MD: 1994. pp. 21–45. [Google Scholar]
- [24].Sjögren U, Hägglund B, Sundqvist G, Wing K. Factors affecting the long-term results of endodontic treatment. Journal of Endodontics. 1990;16:498–504. doi: 10.1016/S0099-2399(07)80180-4. [DOI] [PubMed] [Google Scholar]
- [25].Ng YL, Mann V, Rahbaran S, Lewsey J, Gulabivala K. Outcome of primary root canal treatment: systematic review of the literature - Part 2. Influence of clinical factors. International Endodontic Journal. 2008;41:6–31. doi: 10.1111/j.1365-2591.2007.01323.x. [DOI] [PubMed] [Google Scholar]
- [26].de Chevigny C, Dao TT, Basrani BR, Marquis V, Farzaneh M, Abitbol S, Friedman S. Treatment outcome in endodontics: the Toronto study - phase 4: initial treatment. Journal of Endodontics. 2008;34:258–63. doi: 10.1016/j.joen.2007.10.017. [DOI] [PubMed] [Google Scholar]
- [27].de Chevigny C, Dao TT, Basrani BR, Marquis V, Farzaneh M, Abitbol S, Friedman S. Treatment outcome in endodontics: the Toronto study--phases 3 and 4: orthograde retreatment. Journal of Endodontics. 2008;34:131–7. doi: 10.1016/j.joen.2007.11.003. [DOI] [PubMed] [Google Scholar]
- [28].Ng YL, Mann V, Gulabivala K. A prospective study of the factors affecting outcomes of nonsurgical root canal treatment: part 1: periapical health. International Endodontic Journal. 2011;44:583–609. doi: 10.1111/j.1365-2591.2011.01872.x. [DOI] [PubMed] [Google Scholar]
- [29].Haapasalo M, Endal U, Zandi H, Coil JM. Eradication of endodontic infection by instrumentation and irrigation solutions. Endodontic Topics. 2005;10:77–102. [Google Scholar]
- [30].Sabeti MA, Nekofar M, Motahhary P, Ghandi M, Simon JH. Healing of apical periodontitis after endodontic treatment with and without obturation in dogs. Journal of Endodontics. 2006;32:628–33. doi: 10.1016/j.joen.2005.12.014. [DOI] [PubMed] [Google Scholar]
- [31].Gillen BM, Looney SW, Gu LS, Loushine BA, Weller RN, Loushine RJ, et al. Impact of the quality of coronal restoration versus the quality of root canal fillings on success of root canal treatment: a systematic review and meta-analysis. Journal of Endodontics. 2011;37:895–902. doi: 10.1016/j.joen.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Bergenholtz G, Spångberg L. Controversies in endodontics. Critical Reviews in Oral Biology and Medicine. 2004;15:99–114. doi: 10.1177/154411130401500204. [DOI] [PubMed] [Google Scholar]
- [33].Ricucci D, Lin LM, Spångberg LS. Wound healing of apical tissues after root canal therapy: a long-term clinical, radiographic, and histopathologic observation study. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology & Endodontics. 2009;108:609–21. doi: 10.1016/j.tripleo.2009.05.028. [DOI] [PubMed] [Google Scholar]
- [34].Hatton EH. Changes produced in the pulp and periapical regions, and their relationship to pulp-canal treatment and to systemic disease. Dental Cosmos. 1924;66:1183. [Google Scholar]
- [35].Anthony LP, Grossman LT. A brief history of root canal therapy in the United States. Journal of the American Dental Association. 1945;32:43–50. [Google Scholar]
- [36].Friedman CE, Sandrik JL, Heuer MA, Rapp GW. Composition and physical properties of gutta-percha endodontic filling materials. Journal of Endodontics. 1977;3:304–8. doi: 10.1016/S0099-2399(77)80035-6. [DOI] [PubMed] [Google Scholar]
- [37].Gurgel-Filho ED, Andrade Feitosa JP, Teixeira FB, Monteiro de Paula RC, Araújo Silva JB, Souza-Filho FJ. Chemical and X-ray analyses of five brands of dental gutta-percha cone. International Endodontic Journal. 2003;36:302–7. doi: 10.1046/j.1365-2591.2003.00653.x. [DOI] [PubMed] [Google Scholar]
- [38].Whitworth J. Methods of filling root canals: principles and practices. Endodontic Topics. 2005;12:2–24. [Google Scholar]
- [39].Wesselink PR. The filling of the root canal system. Nederlands Tijdschrift voor Tandheelkunde. 2005;12:471–7. Article in Dutch. [PubMed] [Google Scholar]
- [40].Ingle J. A standardized endodontic technique utilizing newly designed instruments and filling materials. Oral Surgery, Oral Medicine, and Oral Pathology. 1961;14:83–91. doi: 10.1016/0030-4220(61)90477-7. [DOI] [PubMed] [Google Scholar]
- [41].Pereira AC, Nishiyama CK, Pintro LDC. Single-cone obturation technique: a literature review. Revista Sul-Brasileira de Odontologia. 2012;9:442–7. [Google Scholar]
- [42].Ørstavik D. Materials used for root canal obturation: technical, biological and clinical testing. Endodontic Topics. 2005;12:25. [Google Scholar]
- [43].Khabbaz MG, Papadopoulos PD. Deposition of calcified tissue around an overextended gutta-percha cone: case report. International Endodontic Journal. 1999;32:232–5. doi: 10.1046/j.1365-2591.1999.00209.x. [DOI] [PubMed] [Google Scholar]
- [44].Sjögren U, Sundqvist G, Nair PN. Tissue reaction to gutta-percha particles of various sizes when implanted subcutaneously in guinea pigs. European Journal of Oral Sciences. 1995;103:313–21. doi: 10.1111/j.1600-0722.1995.tb00032.x. [DOI] [PubMed] [Google Scholar]
- [45].Warneke S, Arenskötter M, Tenberge KB, Steinbüchel A. Bacterial degradation of poly(trans-1,4-isoprene) (gutta percha) Microbiology. 2007;153(Pt 2):347–56. doi: 10.1099/mic.0.2006/000109-0. [DOI] [PubMed] [Google Scholar]
- [46].Rôças IN, Siqueira JF., Jr Identification of bacteria enduring endodontic treatment procedures by a combined reverse transcriptase-polymerase chain reaction and reverse-capture checkerboard approach. Journal of Endodontics. 2010;36:45–52. doi: 10.1016/j.joen.2009.10.022. [DOI] [PubMed] [Google Scholar]
- [47].Saber MH, Schwarzberg K, Alonaizan FA, Kelley ST, Sedghizadeh PP, Furlan M, et al. Bacterial flora of dental periradicular lesions analyzed by the 454-pyrosequencing technology. Journal of Endodontics. 2012;38:1484–8. doi: 10.1016/j.joen.2012.06.037. [DOI] [PubMed] [Google Scholar]
- [48].Anderson AC, Hellwig E, Vespermann R, Wittmer A, Schmid M, Karygianni L, et al. Comprehensive analysis of secondary dental root canal infections: a combination of culture and culture-independent approaches reveals new insights. PLoS One. 2012;7:e49576. doi: 10.1371/journal.pone.0049576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Li X, Zhu XF, Zhang CF, Cathro P, Seneviratne CJ, Shen S. Endodontic bacteria from primary and persistent endodontic lesions in Chinese patients as identified by cloning and 16S ribosomal DNA gene sequencing. Chinese Medical Journal (English) 2013;126:634–9. [PubMed] [Google Scholar]
- [50].Maniglia-Ferreira C, Silva JB, Jr, de Paula RC, Feitosa JP, Zaia AA, Ferraz CC, et al. Degradation of trans-polyisoprene over time following the analysis of root fillings removed during conventional retreatment. International Endodontic Journal. 2007;40:25–30. doi: 10.1111/j.1365-2591.2006.01172.x. [DOI] [PubMed] [Google Scholar]
- [51].Gutmann JL. Adaptation of injected thermoplasticized gutta-percha in the absence of the dentinal smear layer. International Endodontic Journal. 1993;26:87–92. doi: 10.1111/j.1365-2591.1993.tb00548.x. [DOI] [PubMed] [Google Scholar]
- [52].Gençoğlu N, Samani S, Günday M. Dentinal wall adaptation of thermoplasticized gutta-percha in the absence or presence of smear layer: a scanning electron microscopic study. Journal of Endodontics. 1993;19:558–62. doi: 10.1016/S0099-2399(06)81286-0. [DOI] [PubMed] [Google Scholar]
- [53].Pallarés A, Faus V, Glickman GN. The adaptation of mechanically softened gutta-percha to the canal walls in the presence or absence of smear layer: a scanning electron microscopic study. International Endodontic Journal. 1995;28:266–9. doi: 10.1111/j.1365-2591.1995.tb00312.x. [DOI] [PubMed] [Google Scholar]
- [54].Patel DV, Sherriff M, Ford TR, Watson TF, Mannocci F. The penetration of RealSeal primer and Tubliseal into root canal dentinal tubules: a confocal microscopic study. International Endodontic Journal. 2007;40:67–71. doi: 10.1111/j.1365-2591.2006.01184.x. [DOI] [PubMed] [Google Scholar]
- [55].Chandra SS, Shankar P, Indira R. Depth of penetration of four resin sealers into radicular dentinal tubules: a confocal microscopic study. Journal of Endodontics. 2012;38:1412–6. doi: 10.1016/j.joen.2012.05.017. [DOI] [PubMed] [Google Scholar]
- [56].Chadha R, Taneja S, Kumar M, Gupta S. An in vitro comparative evaluation of depth of tubular penetration of three resin-based root canal sealers. Journal of Conservative Dentistry. 2012;15:18–21. doi: 10.4103/0972-0707.92600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].De-Deus G, Brandão MC, Leal F, Reis C, Souza EM, Luna AS, et al. Lack of correlation between sealer penetration into dentinal tubules and sealability in nonbonded root fillings. International Endodontic Journal. 2012;45:642–51. doi: 10.1111/j.1365-2591.2012.02023.x. [DOI] [PubMed] [Google Scholar]
- [58].Bergmans L, Moisiadis P, De Munck J, Van Meerbeek B, Lambrechts P. Effect of polymerization shrinkage on the sealing capacity of resin fillers for endodontic use. Journal of Adhesive Dentistry. 2005;7:321–9. [PubMed] [Google Scholar]
- [59].Reader CM, Himel VT, Germain LP, Hoen MM. Effect of three obturation techniques on the filling of lateral canals and the main canal. Journal of Endodontics. 1993;19:404–8. doi: 10.1016/S0099-2399(06)81505-0. [DOI] [PubMed] [Google Scholar]
- [60].Wolcott J, Himel VT, Powell W, Penney J. Effect of two obturation techniques on the filling of lateral canals and the main canal. Journal of Endodontics. 1997;23:632–5. doi: 10.1016/S0099-2399(97)80176-8. [DOI] [PubMed] [Google Scholar]
- [61].Venturi M, Di Lenarda R, Breschi L. An ex vivo comparison of three different gutta-percha cones when compacted at different temperatures: rheological considerations in relation to the filling of lateral canals. International Endodontic Journal. 2006;39:648–56. doi: 10.1111/j.1365-2591.2006.01133.x. [DOI] [PubMed] [Google Scholar]
- [62].Ricucci D, Siqueira JF., Jr Fate of the tissue in lateral canals and apical ramifications in response to pathologic conditions and treatment procedures. Journal of Endodontics. 2010;36:1–15. doi: 10.1016/j.joen.2009.09.038. [DOI] [PubMed] [Google Scholar]
- [63].Torabinejad M, Kutsenko D, Machnick TK, Ismail A, Newton CW. Levels of evidence for the outcome of nonsurgical endodontic treatment. Journal of Endodontics. 2005;31:637–46. doi: 10.1097/01.don.0000153593.64951.14. [DOI] [PubMed] [Google Scholar]
- [64].Paik S, Sechrist C, Torabinejad M. Levels of evidence for the outcome of endodontic retreatment. Journal of Endodontics. 2004;30:745–50. doi: 10.1097/01.don.0000137636.93933.51. [DOI] [PubMed] [Google Scholar]
- [65].Wu MK, Wesselink PR. Endodontic leakage studies reconsidered. Part I. Methodology, application and relevance. International Endodontic Journal. 1993;26:37–43. doi: 10.1111/j.1365-2591.1993.tb00540.x. [DOI] [PubMed] [Google Scholar]
- [66].Schuurs AH, Wu MK, Wesselink PR. Duivenvoorden HJ. Endodontic leakage studies reconsidered. Part II. Statistical aspects. International Endodontic Journal. 1993;26:44–52. doi: 10.1111/j.1365-2591.1993.tb00541.x. [DOI] [PubMed] [Google Scholar]
- [67].AliGhamdi A, Wennberg A. Testing of sealing ability of endodontic filling materials. Endodontics & Dent Traumatology. 1994;10:249–55. doi: 10.1111/j.1600-9657.1994.tb00079.x. [DOI] [PubMed] [Google Scholar]
- [68].Lucena C, Lopez JM, Pulgar R, Abalos C, Valderrama MJ. Potential errors and misuse of statistics in studies on leakage in endodontics. International Endodontic Journal. 2013;46:323–31. doi: 10.1111/j.1365-2591.2012.02118.x. [DOI] [PubMed] [Google Scholar]
- [69].Goldman M, Simmonds S, Rush R. The usefulness of dye-penetration studies reexamined. Oral Surgery, Oral Medicine, and Oral Pathology. 1989;67:327–32. doi: 10.1016/0030-4220(89)90365-4. [DOI] [PubMed] [Google Scholar]
- [70].Spångberg LS, Acierno TG, Yongbum Cha B. Influence of entrapped air on the accuracy of leakage studies using dye penetration methods. Journal of Endodontics. 1989;15:548–51. doi: 10.1016/s0099-2399(89)80199-2. [DOI] [PubMed] [Google Scholar]
- [71].Kersten HW, Moorer WR. Particles and molecules in endodontic leakage. International Endodontic Journal. 1989;22:118–24. doi: 10.1111/j.1365-2591.1989.tb00909.x. [DOI] [PubMed] [Google Scholar]
- [72].Camps J, Pashley D. Reliability of the dye penetration studies. Journal of Endodontics. 2003;29:592–4. doi: 10.1097/00004770-200309000-00012. [DOI] [PubMed] [Google Scholar]
- [73].Souza EM, Pappen FG, Shemesh H, Bonanato-Estrela C, Bonetti-Filho I. Reliability of assessing dye penetration along root canal fillings using methylene blue. Australian Endodontic Journal. 2009;35:158–63. doi: 10.1111/j.1747-4477.2009.00161.x. [DOI] [PubMed] [Google Scholar]
- [74].Rechenberg DK, De-Deus G, Zehnder M. Potential systematic error in laboratory experiments on microbial leakage through filled root canals: review of published articles. International Endodontic Journal. 2011;44:183–94. doi: 10.1111/j.1365-2591.2010.01821.x. [DOI] [PubMed] [Google Scholar]
- [75].Rechenberg DK, Thurnheer T, Zehnder M. Potential systematic error in laboratory experiments on microbial leakage through filled root canals: an experimental study. International Endodontic Journal. 2011;44:827–35. doi: 10.1111/j.1365-2591.2011.01888.x. [DOI] [PubMed] [Google Scholar]
- [76].Heyder M, Kranz S, Völpel A, Pfister W, Watts DC, Jandt KD, et al. Antibacterial effect of different root canal sealers on three bacterial species. Dental Materials. 2013;29:542–9. doi: 10.1016/j.dental.2013.02.007. [DOI] [PubMed] [Google Scholar]
- [77].Brosco VH, Bernardineli N, Torres SA, Consolaro A, Bramante CM, de Moraes IG, et al. Bacterial leakage in obturated root canals-part 2: a comparative histologic and microbiologic analyses. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology & Endodontics. 2010;109:788–94. doi: 10.1016/j.tripleo.2009.11.036. [DOI] [PubMed] [Google Scholar]
- [78].Shemesh H, Souza EM, Wu MK, Wesselink PR. Glucose reactivity with filling materials as a limitation for using the glucose leakage model. International Endodontic Journal. 2008;41:869–72. doi: 10.1111/j.1365-2591.2008.01440.x. [DOI] [PubMed] [Google Scholar]
- [79].Pitt Ford TR. Relation between seal of root fillings and tissue response. Oral Surgery, Oral Medicine, and Oral Pathology. 1983;55:291–4. doi: 10.1016/0030-4220(83)90330-4. [DOI] [PubMed] [Google Scholar]
- [80].Oliver CM, Abbott PV. Correlation between clinical success and apical dye penetration. International Endodontic Journal. 2001;34:637–44. doi: 10.1046/j.1365-2591.2001.00442.x. [DOI] [PubMed] [Google Scholar]
- [81].Susini G, Pommel L, About I, Camps J. Lack of correlation between ex vivo apical dye penetration and presence of apical radiolucencies. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology & Endodontics. 2006;102:e19–23. doi: 10.1016/j.tripleo.2006.03.015. [DOI] [PubMed] [Google Scholar]
- [82].Shanahan DJ, Duncan HF. Root canal filling using Resilon: a review. British Dental Journal. 2011;211:81–8. doi: 10.1038/sj.bdj.2011.573. [DOI] [PubMed] [Google Scholar]
- [83].Editorial Board of the Journal of Endodontics Wanted: a base of evidence. Journal of Endodontics. 2007;33:1401–2. doi: 10.1016/j.joen.2007.09.004. [DOI] [PubMed] [Google Scholar]
- [84].De-Deus G. Research that matters - root canal filling and leakage studies. International Endodontic Journal. 2012;45:1063–4. doi: 10.1111/j.1365-2591.2012.02104.x. [DOI] [PubMed] [Google Scholar]
- [85].Buch B. FDA medical device approval: things you didn't learn in medical school or residency. American Journal of Orthopedics. 2007;36:407–12. [PubMed] [Google Scholar]
- [86].Friedman S, Torneck CD, Komorowski R, Ouzounian Z, Syrtash P, Kaufman A. In vivo model for assessing the functional efficacy of endodontic filling materials and techniques. Journal of Endodontics. 1997;23:557–61. doi: 10.1016/S0099-2399(06)81120-9. [DOI] [PubMed] [Google Scholar]
- [87].Leonardo MR, Almeida WA, da Silva LA, Utrilla LS. Histological evaluation of the response of apical tissues to glass ionomer and zinc oxide-eugenol based sealers in dog teeth after root canal treatment. Endodontic & Dental Traumatology. 1998;14:257–61. doi: 10.1111/j.1600-9657.1998.tb00849.x. [DOI] [PubMed] [Google Scholar]
- [88].Leonardo MR, da Silva LA, Almeida WA, Utrilla LS. Tissue response to an epoxy resin-based root canal sealer. Endodontic & Dental Traumatology. 1999;15:28–32. doi: 10.1111/j.1600-9657.1999.tb00745.x. [DOI] [PubMed] [Google Scholar]
- [89].Holland R, de Souza V, Nery MJ, Otoboni Filho JA, Bernabé PF, Dezan Júnior E. Reaction of dogs' teeth to root canal filling with mineral trioxide aggregate or a glass ionomer sealer. Journal of Endodontics. 1999;25:728–30. doi: 10.1016/s0099-2399(99)80118-6. [DOI] [PubMed] [Google Scholar]
- [90].Friedman S, Komorowski R, Maillet W, Klimaite R, Nguyen HQ, Torneck CD. In vivo resistance of coronally induced bacterial ingress by an experimental glass ionomer cement root canal sealer. Journal of Endodontics. 2000;26:1–5. doi: 10.1097/00004770-200001000-00001. [DOI] [PubMed] [Google Scholar]
- [91].Barbosa HG, Holland R, de Souza V, Dezan EJ, Bernabé PF, Otoboni JA, et al. Healing process of dog teeth after post space preparation and exposition of the filling material to the oral environment. Brazilian Dental Journal. 2003;14:103–8. doi: 10.1590/s0103-64402003000200006. [DOI] [PubMed] [Google Scholar]
- [92].Holland R, Sant'Anna Júnior A, Souza Vd, Dezan Junior E, Otoboni Filho JA, Bernabé PF, et al. Influence of apical patency and filling material on healing process of dogs' teeth with vital pulp after root canal therapy. Brazilian Dental Journal. 2005;16:9–16. doi: 10.1590/s0103-64402005000100002. [DOI] [PubMed] [Google Scholar]
- [93].Leonardo MR, Flores DS, de Paula E, Silva FW, de Toledo Leonardo R, da Silva LA. A comparison study of periapical repair in dogs' teeth using RoekoSeal and AH plus root canal sealers: a histopathological evaluation. Journal of Endodontics. 2008;34:822–5. doi: 10.1016/j.joen.2008.03.029. [DOI] [PubMed] [Google Scholar]
- [94].Tanomaru Filho M, Leonardo MR, Silva LA, Utrilla LS. Effect of different root canal sealers on periapical repair of teeth with chronic periradicular periodontitis. International Endodontic Journal. 1998;31:85–9. doi: 10.1046/j.1365-2591.1998.00134.x. [DOI] [PubMed] [Google Scholar]
- [95].Grecca FS, Leonardo MR, da Silva LA, Tanomaru Filho M, Borges MA. Radiographic evaluation of periradicular repair after endodontic treatment of dog's teeth with induced periradicular periodontitis. Journal of Endodontics. 2001;27:610–2. doi: 10.1097/00004770-200110000-00002. [DOI] [PubMed] [Google Scholar]
- [96].Berbert FL, Leonardo MR, Silva LA, Tanomaru Filho M, Bramante CM. Influence of root canal dressings and sealers on repair of apical periodontitis after endodontic treatment. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology & Endodontics. 2002;93:184–9. doi: 10.1067/moe.2002.117803. [DOI] [PubMed] [Google Scholar]
- [97].Leonardo MR, Salgado AA, da Silva LA, Tanomaru Filho M. Apical and periapical repair of dogs' teeth with periapical lesions after endodontic treatment with different root canal sealers. Pesquisa Odontológica Brasileira. 2003;17:69–74. doi: 10.1590/s1517-74912003000100013. [DOI] [PubMed] [Google Scholar]
- [98].Tanomaru JM, Leonardo MR, Silva LA, Poliseli-Neto A, Tanomaru-Filho M. Histopathological evaluation of different methods of experimental induction of periapical periodontitis. Brazilian Dental Journal. 2008;19:238–44. doi: 10.1590/s0103-64402008000300012. [DOI] [PubMed] [Google Scholar]
- [99].Leonardo MR, Almeida WA, Ito IY, da Silva LA. Radiographic and microbiologic evaluation of posttreatment apical and periapical repair of root canals of dogs' teeth with experimentally induced chronic lesion. Oral Surgery, Oral Medicine, and Oral Pathology. 1994;78:232–8. doi: 10.1016/0030-4220(94)90153-8. [DOI] [PubMed] [Google Scholar]
- [100].Pascon EA, Leonardo MR, Safavi K, Langeland K. Tissue reaction to endodontic materials: methods, criteria, assessment, and observations. Oral Surgery, Oral Medicine, and Oral Pathology. 1991;72:222–37. doi: 10.1016/0030-4220(91)90168-c. [DOI] [PubMed] [Google Scholar]
- [101].Leonardo MR, Flores DS, de Paula E, Silva FW, de Toledo Leonardo R, da Silva LA. A comparison study of periapical repair in dogs' teeth using RoekoSeal and AH plus root canal sealers: a histopathological evaluation. Journal of Endodotics. 2008;34:822–5. doi: 10.1016/j.joen.2008.03.029. [DOI] [PubMed] [Google Scholar]
- [102].Steinstraesser L, Kraneburg U, Jacobsen F, Al-Benna S. Host defense peptides and their antimicrobial-immunomodulatory duality. Immunobiology. 2011;216:322–33. doi: 10.1016/j.imbio.2010.07.003. [DOI] [PubMed] [Google Scholar]
- [103].Paqué F, Luder HU, Sener B, Zehnder M. Tubular sclerosis rather than the smear layer impedes dye penetration into the dentine of endodontically instrumented root canals. International Endodontic Journal. 2006;39:18–25. doi: 10.1111/j.1365-2591.2005.01042.x. [DOI] [PubMed] [Google Scholar]
- [104].Kinney JH, Nalla RK, Pople JA, Breunig TM, Ritchie RO. Age-related transparent root dentin: mineral concentration, crystallite size, and mechanical properties. Biomaterials. 2005;26:3363–76. doi: 10.1016/j.biomaterials.2004.09.004. [DOI] [PubMed] [Google Scholar]
- [105].Christensen GJ. I have had enough! Journal of Esthetic and Restorative Dentistry. 2004;16:83–6. doi: 10.1111/j.1708-8240.2004.tb00011.x. [DOI] [PubMed] [Google Scholar]
- [106].Ingle JI. A new paradigm for filling and sealing root canals. Compendium of Continuing Education in Dentistry. 1995;16:306, 308, 310, 322. [PubMed] [Google Scholar]
- [107].Schwartz RS. Adhesive dentistry and endodontics. Part 2: bonding in the root canal system-the promise and the problems: a review. Journal of Endodontics. 2006;32:1125–34. doi: 10.1016/j.joen.2006.08.003. [DOI] [PubMed] [Google Scholar]
- [108].Leonard JE, Gutmann JL, Guo IY. Apical and coronal seal of roots obturated with a dentine bonding agent and resin. International Endodontic Journal. 1996;29:76–83. doi: 10.1111/j.1365-2591.1996.tb01165.x. [DOI] [PubMed] [Google Scholar]
- [109].Andrade-Júnior CV, Kawagoe ST, Almeida JF, Gomes BP, Zaia AA, Ferraz CC. Bond strength to radicular dentin and sealing ability of AH Plus in combination with a bonding agent. Acta Odontologica Scandinavica. 2013;71:1200–5. doi: 10.3109/00016357.2012.757363. [DOI] [PubMed] [Google Scholar]
- [110].Kim YK, Grandini S, Ames JM, Gu LS, Kim SK, Pashley DH, et al. Critical review on methacrylate resin-based root canal sealers. Journal of Endodontics. 2010;36:383–99. doi: 10.1016/j.joen.2009.10.023. [DOI] [PubMed] [Google Scholar]
- [111].Sennou HE, Lebugle AA, Grégoire GL. X-ray photoelectron spectroscopy study of the dentin-glass ionomer cement interface. Dental Materials. 1999;15:229–37. doi: 10.1016/s0109-5641(99)00036-6. [DOI] [PubMed] [Google Scholar]
- [112].Mohammadi Z, Shalavi S. Clinical applications of glass ionomers in endodontics: a review. International Dental Journal. 2012;62:244–50. doi: 10.1111/j.1875-595x.2012.00125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Tay FR, Pashley DH. Monoblocks in root canals: a hypothetical or a tangible goal. Journal of Endodontics. 2007;33:391–8. doi: 10.1016/j.joen.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Teixeira FB, Teixeira EC, Thompson J, Leinfelder KF, Trope M. Dentinal bonding reaches the root canal system. Journal of Esthetic and Restorative Dentistry. 2004;16:348–54. doi: 10.1111/j.1708-8240.2004.tb00066.x. [DOI] [PubMed] [Google Scholar]
- [115].Tay FR, Loushine RJ, Monticelli F, Weller RN, Breschi L, Ferrari M, et al. Effectiveness of resin-coated gutta-percha cones and a dual-cured, hydrophilic methacrylate resin-based sealer in obturating root canals. Journal of Endodontics. 2005;31:659–64. doi: 10.1097/01.don.0000171942.69081.53. [DOI] [PubMed] [Google Scholar]
- [116].Monticelli F, Sword J, Martin RL, Schuster GS, Weller RN, Ferrari M, et al. Sealing properties of two contemporary single-cone obturation systems. International Endodontic Journal. 2007;40:374–85. doi: 10.1111/j.1365-2591.2007.01231.x. [DOI] [PubMed] [Google Scholar]
- [117].Ghoneim AG, Lutfy RA, Sabet NE, Fayyad DM. Resistance to fracture of roots obturated with novel canal-filling systems. Journal of Endodontics. 2011;37:1590–2. doi: 10.1016/j.joen.2011.08.008. [DOI] [PubMed] [Google Scholar]
- [118].Belli S, Eraslan O, Eskitascioglu G, Karbhari V. Monoblocks in root canals: a finite elemental stress analysis study. International Endodontic Journal. 2011;44:817–26. doi: 10.1111/j.1365-2591.2011.01885.x. [DOI] [PubMed] [Google Scholar]
- [119].Shawkat ES, Shortall AC, Addison O, Palin WM. Oxygen inhibition and incremental layer bond strengths of resin composites. Dental Materials. 2009;25:1338–46. doi: 10.1016/j.dental.2009.06.003. [DOI] [PubMed] [Google Scholar]
- [120].Dall'Oca S, Papacchini F, Goracci C, Cury AH, Suh BI, Tay FR, et al. Effect of oxygen inhibition on composite repair strength over time. Journal of Biomedical Materials Research. Part B, Applied Biomaterials. 2007;81:493–8. doi: 10.1002/jbm.b.30689. [DOI] [PubMed] [Google Scholar]
- [121].Jia WT, Trope M, Alpert B. Patent number. Vol. 7. United States Patent Office; 2007. Dental filling material; p. 211,136. [Google Scholar]
- [122].Tay FR, Hiraishi N, Pashley DH, Loushine RJ, Weller RN, Gillespie WT, et al. Bondability of Resilon to a methacrylate-based root canal sealer. Journal of Endodontics. 2006;32:133–7. doi: 10.1016/j.joen.2005.10.026. [DOI] [PubMed] [Google Scholar]
- [123].Hamann C, Rodgers PA, Alenius H, Halsey JF, Sullivan K. Cross-reactivity between gutta-percha and natural rubber latex: assumptions vs. reality. Journal of the American Dental Association. 2002;133:1357–67. doi: 10.14219/jada.archive.2002.0051. [DOI] [PubMed] [Google Scholar]
- [124].Kang PB, Vogt K, Gruninger SE, Marshall M, Siew C, Meyer DM. The immuno cross-reactivity of gutta percha points. Dental Materials. 2007;23:380–4. doi: 10.1016/j.dental.2006.02.006. [DOI] [PubMed] [Google Scholar]
- [125].Hammad M, Qualtrough A, Silikas N. Extended setting shrinkage behavior of endodontic sealers. Journal of Endodontics. 2008;34:90–3. doi: 10.1016/j.joen.2007.10.014. [DOI] [PubMed] [Google Scholar]
- [126].Bouillaguet S, Troesch S, Wataha JC, Krejci I, Meyer JM, Pashley DH. Microtensile bond strength between adhesive cements and root canal dentin. Dental Materials. 2003;19:199–205. doi: 10.1016/s0109-5641(02)00030-1. [DOI] [PubMed] [Google Scholar]
- [127].Tay FR, Loushine RJ, Lambrechts P, Weller RN, Pashley DH. Geometric factors affecting dentin bonding in root canals: a theoretical modeling approach. Journal of Endodontics. 2005;31:584–9. doi: 10.1097/01.don.0000168891.23486.de. [DOI] [PubMed] [Google Scholar]
- [128].Bouillaguet S, Bertossa B, Krejci I, Wataha JC, Tay FR, Pashley DH. Alternative adhesive strategies to optimize bonding to radicular dentin. Journal of Endodontics. 2007;33:1227–30. doi: 10.1016/j.joen.2007.05.016. [DOI] [PubMed] [Google Scholar]
- [129].Hammad M, Qualtrough A, Silikas N. Evaluation of root canal obturation: a three-dimensional in vitro study. Journal of Endodontics. 2009;35:541–4. doi: 10.1016/j.joen.2008.12.021. [DOI] [PubMed] [Google Scholar]
- [130].De-Deus G, Reis C, Di Giorgi K, Brandão MC, Audi C, Fidel RA. Interfacial adaptation of the Epiphany self-adhesive sealer to root dentin. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology & Endodontics. 2011;111:381–6. doi: 10.1016/j.tripleo.2010.08.020. [DOI] [PubMed] [Google Scholar]
- [131].Cavenago BC, Duarte MA, Ordinola-Zapata R, Marciano MA, Carpio-Perochena AE, Bramante CM. Interfacial adaptation of an epoxy-resin sealer and a self-etch sealer to root canal dentin using the System B or the single cone technique. Brazilian Dental Journal. 2012;23:205–11. doi: 10.1590/s0103-64402012000300004. [DOI] [PubMed] [Google Scholar]
- [132].Ørstavik D, Nordahl I, Tibballs JE. Dimensional change following setting of root canal sealer materials. Dental Materials. 2001;17:512–9. doi: 10.1016/s0109-5641(01)00011-2. [DOI] [PubMed] [Google Scholar]
- [133].Zarrelli M, Skordos AA, Patridge IK. Investigation of cure induced shrinkage in unreinforced epoxy resin. Plastics, Rubber and Composites Processing and Applications. 2002;31:377–84. [Google Scholar]
- [134].Carvalho-Junior JR, Correr-Sobrinho L, Correr AB, Sinhoreti MA, Consani S, Sousa-Neto MD. Solubility and dimensional change after setting of root canal sealers: a proposal for smaller dimensions of test samples. Journal of Endodontics. 2007;33:1110–6. doi: 10.1016/j.joen.2007.06.004. [DOI] [PubMed] [Google Scholar]
- [135].Whatley JJ. Masters thesis. Department of Endodontics, Indiana University School of Dentistry; 2012. An in-vitro evaluation of the biodegradability of Resilon by the microbiota of the infected root canal utilizing an agar disc diffusion assay. https://scholarworks.iupui.edu/bitstream/handle/1805/3093/Thesis%20of%20Jenny.pdf?sequenc e=1. [Google Scholar]
- [136].Rexford AM. Masters thesis. Department of Endodontics, Indiana University School of Dentistry; 2012. Biodegradability of Resilon, a resin-based root canal obturating material, by typical endodontic pathogens. https://scholarworks.iupui.edu/bitstream/handle/1805/3179/Thesis%20of%20Dr%20Ashleigh%2 0Rexford%20.pdf%20file%20for%20upload%281%29.pdf?sequence=1. [Google Scholar]
- [137].Tay FR, Pashley DH, Yiu CK, Yau JY, Mak YF, Loushine RJ, et al. Susceptibility of a polycaprolactone-based root canal filling material to degradation. II. Gravimetric evaluation of enzymatic hydrolysis. Journal of Endodontics. 2005;31:737–41. doi: 10.1097/01.don.0000155225.40794.79. [DOI] [PubMed] [Google Scholar]
- [138].Tay FR, Pashley DH, Loushine RJ, Kuttler S, García-Godoy F, King NM, Ferrari M. Susceptibility of a polycaprolactone-based root canal filling material to degradation. Evidence of biodegradation from a simulated field test. American Journal of Dentistry. 2007;20:365–9. [PubMed] [Google Scholar]
- [139].Jacob F, Monod J. Genetic regulatory mechanism in the synthesis of proteins. Journal of Molecular Biology. 1961;3:318–56. doi: 10.1016/s0022-2836(61)80072-7. [DOI] [PubMed] [Google Scholar]
- [140].Teixeira FB. Ideal obturation using synthetic root-filling systems: coronal sealing and fracture resistance. Practical Procedures & Aesthetic Dentistry. 2006;18:S7–11. [PubMed] [Google Scholar]
- [141].Jia WT. Patent number. Vol. 8. United States Patent Office; 2012. Dental material ad methods of use; p. 113,836. [Google Scholar]
- [142].Michaud RA, Burgess J, Barfield RD, Cakir D, McNeal SF, Eleazer PD. Volumetric expansion of gutta-percha in contact with eugenol. Journal of Endodontics. 2008;34:1528–32. doi: 10.1016/j.joen.2008.08.025. [DOI] [PubMed] [Google Scholar]
- [143].Chandrasekhar V, Morishetty PK, Metla SL, Raju RV. Expansion of gutta-percha in contact with various concentrations of zinc oxide-eugenol sealer: a three-dimensional volumetric study. Journal of Endodontics. 2011;37:697–700. doi: 10.1016/j.joen.2011.02.014. [DOI] [PubMed] [Google Scholar]
- [144].Meryon SD, Johnson SG, Smith AJ. Eugenol release and the cytotoxicity of different zinc oxide-eugenol combination. Journal of Dentistry. 1988;16:66–70. doi: 10.1016/0300-5712(88)90053-x. [DOI] [PubMed] [Google Scholar]
- [145].Bandyopadhyay S. A study of the volumetric setting shrinkage of some dental materials. Journal of Biomedical Materials Research. 1982;16:135–44. doi: 10.1002/jbm.820160206. [DOI] [PubMed] [Google Scholar]
- [146].Ørstavik D. Weight loss of endodontic sealers, cements and pastes in water. Scandinavian Journal of Dental Research. 1983;91:316–9. [PubMed] [Google Scholar]
- [147].Wu MK, Fan B, Wesselink PR. Diminished leakage along root canals filled with gutta-percha without sealer over time: a laboratory study. International Endodontic Journal. 2000;33:121–5. doi: 10.1046/j.1365-2591.2000.00274.x. [DOI] [PubMed] [Google Scholar]
- [148].Nagas E, Uyanik MO, Eymirli A, Cehreli ZC, Vallittu PK, Lassila LV, et al. Dentin moisture conditions affect the adhesion of root canal sealers. Journal of Endodontics. 2012;38:240–4. doi: 10.1016/j.joen.2011.09.027. [DOI] [PubMed] [Google Scholar]
- [149].Flores DS, Rached FJ, Jr, Versiani MA, Guedes DF, Sousa-Neto MD, Pécora JD. Evaluation of physicochemical properties of four root canal sealers. International Endodontic Journal. 2011;44:126–35. doi: 10.1111/j.1365-2591.2010.01815.x. [DOI] [PubMed] [Google Scholar]
- [150].Eid AA, Nikonov SY, Looney SW, Didato A, Niu LN, Levin MD, et al. In vitro biocompatibility evaluation of a root canal filling material that expands on water sorption. Journal of Endodontics. 2013;39:883–8. doi: 10.1016/j.joen.2013.03.003. [DOI] [PubMed] [Google Scholar]
- [151].Highgate DJ, Frankland JD. Patent number. Vol. 4. United States Patent Office; 1986. Deformable polymeric compositions; p. 565,722. [Google Scholar]
- [152].Highgate DJ, Lloyd JA. Patent number. Vol. 7. United States Patent Office; 2007. Expandable/contractable composition for surgical or dental use; p. 210,935. [Google Scholar]
- [153].Didato A, Eid AA, Levin MD, Khan S, Tay FR, Rueggeberg FA. Time-based lateral hygroscopic expansion of a water-expandable endodontic obturation point. Journal of Dentistry. 2013;41:796–801. doi: 10.1016/j.jdent.2013.06.012. [DOI] [PubMed] [Google Scholar]
- [154].American National Standards Institute/American Dental Association . Specification number 57 for Endodontic Sealing Materials (ADA57-2000) ADA Publishing; Chicago, USA: 2000. [Google Scholar]
- [155].Shemesh H, van Soest G, Wu MK, Wesselink PR. Diagnosis of vertical root fractures with optical coherence tomography. Journal of Endodontics. 2008;34:739–42. doi: 10.1016/j.joen.2008.03.013. [DOI] [PubMed] [Google Scholar]
- [156].Chai H, Tamse A. Fracture mechanics analysis of vertical root fracture from condensation of gutta-percha. Journal of Biomechanics. 2012;45:1673–8. doi: 10.1016/j.jbiomech.2012.03.022. [DOI] [PubMed] [Google Scholar]
- [157].Ivancik J, Majd H, Bajaj D, Romberg E, Arola D. Contributions of aging to the fatigue crack growth resistance of human dentin. Acta Biomaterialia. 2012;8:2737–46. doi: 10.1016/j.actbio.2012.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Ivancik J, Arola DD. The importance of microstructural variations on the fracture toughness of human dentin. Biomaterials. 2013;34:864–74. doi: 10.1016/j.biomaterials.2012.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Johnson WB. A new gutta-percha technique. Journal of Endodontics. 1978;4:184–8. doi: 10.1016/S0099-2399(78)80173-3. [DOI] [PubMed] [Google Scholar]
- [160].Gutmann JL, Saunders WP, Saunders EM, Nguyen L. A assessment of the plastic Thermafil obturation technique. Part 1. Radiographic evaluation of adaptation and placement. International Endodontic Journal. 1993;26:173–8. [PubMed] [Google Scholar]
- [161].Somma F, Cretella G, Carotenuto M, Pecci R, Bedini R, De Biasi M, et al. Quality of thermoplasticized and single point root fillings assessed by micro-computed tomography. International Endodontic Journal. 2011;44:362–9. doi: 10.1111/j.1365-2591.2010.01840.x. [DOI] [PubMed] [Google Scholar]
- [162].Gandolfi MG, Parrilli AP, Fini M, Prati C, Dummer PM. 3D micro-CT analysis of the interface voids associated with Thermafil root fillings used with AH Plus or a flowable MTA sealer. International Endodontic Journal. 2013;46:253–63. doi: 10.1111/j.1365-2591.2012.02124.x. [DOI] [PubMed] [Google Scholar]
- [163].Chu CH, Lo EC, Cheung GS. Outcome of root canal treatment using Thermafil and cold lateral condensation filling techniques. International Endodontic Journal. 2005;38:179–85. doi: 10.1111/j.1365-2591.2004.00929.x. [DOI] [PubMed] [Google Scholar]
- [164].Hale R, Gatti R, Glickman GN, Opperman LA. Comparative analysis of carrier-based obturation and lateral compaction: a retrospective clinical outcomes study. International Journal of Dentistry. 2012;2012:954675. doi: 10.1155/2012/954675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Heeren TJ, Levitan ME. Effect of canal preparation on fill length in straight root canals obturated with RealSeal 1 and Thermafil Plus. Journal of Endodontics. 2012;38:1380–2. doi: 10.1016/j.joen.2012.06.021. [DOI] [PubMed] [Google Scholar]
- [166].Beasley RT, Williamson AE, Justman BC, Qian F. Time required to remove Guttacore, Thermafil Plus, and thermoplasticized gutta-percha from moderately curved root canals with ProTaper files. Journal of Endodontics. 2013;39:125–8. doi: 10.1016/j.joen.2012.10.014. [DOI] [PubMed] [Google Scholar]
- [167].Parirokh M, Torabinejad M. Mineral trioxide aggregate: a comprehensive literature review--Part III: Clinical applications, drawbacks, and mechanism of action. Journal of Endodontics. 2010;36:400–13. doi: 10.1016/j.joen.2009.09.009. [DOI] [PubMed] [Google Scholar]
- [168].Camilleri J, Pitt Ford TR. Mineral trioxide aggregate: a review of the constituents and biological properties of the material. International Endodontic Journal. 2006;39:747–54. doi: 10.1111/j.1365-2591.2006.01135.x. [DOI] [PubMed] [Google Scholar]
- [169].Tay FR, Pashley DH, Rueggeberg FA, Loushine RJ, Weller RN. Calcium phosphate phase transformation produced by the interaction of the Portland cement component of white mineral trioxide aggregate with a phosphate-containing fluid. Journal of Endodontics. 2007;33:1347–51. doi: 10.1016/j.joen.2007.07.008. [DOI] [PubMed] [Google Scholar]
- [170].Gandolfi MG, Taddei P, Tinti A, Prati C. Apatite-forming ability (bioactivity) of ProRoot MTA. International Endodontic Journal. 2010;43:917–20. doi: 10.1111/j.1365-2591.2010.01768.x. [DOI] [PubMed] [Google Scholar]
- [171].Darvell BW, Wu RC. “MTA”-an Hydraulic Silicate Cement: review update and setting reaction. Dental Materials. 2011;27:407–22. doi: 10.1016/j.dental.2011.02.001. [DOI] [PubMed] [Google Scholar]
- [172].Camilleri J. Evaluation of selected properties of mineral trioxide aggregate sealer cement. Journal of Endodontics. 2009;35:1412–7. doi: 10.1016/j.joen.2009.07.008. [DOI] [PubMed] [Google Scholar]
- [173].Sarkar NK, Caicedo R, Ritwik P, Moiseyeva R, Kawashima I. Physicochemical basis of the biologic properties of mineral trioxide aggregate. Journal of Endodontics. 2005;31:97–100. doi: 10.1097/01.don.0000133155.04468.41. [DOI] [PubMed] [Google Scholar]
- [174].Loushine BA, Bryan TE, Looney SW, Gillen BM, Loushine RJ, Weller RN, et al. Setting properties and cytotoxicity evaluation of a premixed bioceramic root canal sealer. Journal of Endodontics. 2011;37:673–7. doi: 10.1016/j.joen.2011.01.003. [DOI] [PubMed] [Google Scholar]
- [175].Borges RP, Sousa-Neto MD, Versiani MA, Rached-Júnior FA, De-Deus G, Miranda CE, et al. Changes in the surface of four calcium silicate-containing endodontic materials and an epoxy resin-based sealer after a solubility test. International Endodontic Journal. 2012;45:419–28. doi: 10.1111/j.1365-2591.2011.01992.x. [DOI] [PubMed] [Google Scholar]
- [176].Gomes-Filho JE, Moreira JV, Watanabe S, Lodi CS, Cintra LT, Dezan E, Junior, et al. Sealability of MTA and calcium hydroxide containing sealers. Journal of Applied Oral Sciences. 2012;20:347–51. doi: 10.1590/S1678-77572012000300009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Camilleri J, Formosa L, Damidot D. The setting characteristics of MTA Plus in different environmental conditions. International Endodontic Journal. 2013;46:831–40. doi: 10.1111/iej.12068. [DOI] [PubMed] [Google Scholar]
- [178].Pereira Cda C, de Oliveira EP, Gomes MS, Della-Bona A, Vanni JR, Kopper PM, et al. Comparative in vivo analysis of the sealing ability of three endodontic sealers in dog teeth after post-space preparation. Australian Endodontic Journal. 2007;33:101–6. doi: 10.1111/j.1747-4477.2007.00069.x. [DOI] [PubMed] [Google Scholar]
- [179].Suzuki P, de Souza V, Holland R, Murata SS, Gomes-Filho JE, Dezan E, Junior, et al. Tissue reaction of the EndoREZ in root canal fillings short of or beyond an apical foramenlike communication. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology & Endodontics. 2010;109:e94–9. doi: 10.1016/j.tripleo.2009.12.047. [DOI] [PubMed] [Google Scholar]
- [180].Shipper G, Teixeira FB, Arnold RR, Trope M. Periapical inflammation after coronal microbial inoculation of dog roots filled with gutta-percha or Resilon. Journal of Endodontics. 2005;31:91–6. doi: 10.1097/01.don.0000140569.33867.bf. [DOI] [PubMed] [Google Scholar]
- [181].Leonardo MR, Barnett F, Debelian GJ, de Pontes Lima RK, Bezerra da Silva LA. Root canal adhesive filling in dogs' teeth with or without coronal restoration: a histopathological evaluation. Journal of Endodontics. 2007;33:1299–303. doi: 10.1016/j.joen.2007.07.037. [DOI] [PubMed] [Google Scholar]
- [182].Duggan D, Arnold RR, Teixeira FB, Caplan DJ, Tawil P. Periapical inflammation and bacterial penetration after coronal inoculation of dog roots filled with RealSeal 1 or Thermafil. Journal of Endodontics. 2009;35:852–7. doi: 10.1016/j.joen.2009.03.050. [DOI] [PubMed] [Google Scholar]
- [183].Tanomaru-Filho M, Tanomaru JM, Leonardo MR, da Silva LA. Periapical repair after root canal filling with different root canal sealers. Brazilian Dental Journal. 2009;20:389–95. doi: 10.1590/s0103-64402009000500006. [DOI] [PubMed] [Google Scholar]
- [184].Brasil DS, Soares JA, Horta MC, Ferreira CL, Nunes E, Chaves GG, et al. Periapical repair in dog teeth: root canal adhesive filling by using the Resilon System. Journal of Endodontics. 2010;36:482–8. doi: 10.1016/j.joen.2009.11.020. [DOI] [PubMed] [Google Scholar]
- [185].Aminozarbian MG, Barati M, Razavi SM, Ghateh AR, Feizianfard M. A histopathological evaluation of periradicular tissues after root canal filling with gutta-percha/AH26 or Resilon/Epiphany in dogs' teeth. Research Journal of Biological Sciences. 2011;6:108–13. [Google Scholar]
- [186].Nawal RR, Parande M, Sehgal R, Naik A, Rao NR. A comparative evaluation of antimicrobial efficacy and flow properties for Epiphany, Guttaflow and AH-Plus sealer. International Endodontic Journal. 2011;44:307–13. doi: 10.1111/j.1365-2591.2010.01829.x. [DOI] [PubMed] [Google Scholar]
- [187].Kirkevang LS, Hørsted-Bindslev P. Technical aspects of treatment in relation to treatment outcome. Endodontic Topics. 2002;2:89–102. [Google Scholar]
- [188].Aqrabawi JA. Outcome of endodontic treatment of teeth filled using lateral condensation versus vertical compaction (Schilder's technique) The Journal of Contemporary Dental Practice. 2006;7:17–24. [PubMed] [Google Scholar]
- [189].Lazarski MP, Walker WA, 3rd, Flores CM, Schindler WG, Hargreaves KM. Epidemiological evaluation of the outcomes of nonsurgical root canal treatment in a large cohort of insured dental patients. Journal of Endodontics. 2001;27:791–6. doi: 10.1097/00004770-200112000-00021. [DOI] [PubMed] [Google Scholar]
- [190].Salehrabi R, Rotstein I. Endodontic treatment outcomes in a large patient population in the USA: an epidemiological study. Journal of Endodontics. 2004;30:846–50. doi: 10.1097/01.don.0000145031.04236.ca. [DOI] [PubMed] [Google Scholar]
- [191].Salehrabi R, Rotstein I. Epidemiologic evaluation of the outcomes of orthograde endodontic retreatment. Journal of Endodontics. 2010;36:790–2. doi: 10.1016/j.joen.2010.02.009. [DOI] [PubMed] [Google Scholar]
- [192].Friedman S. Expected outcomes in the prevention and treatment of apical periodontitis. In: Ørstavik D, Pitt Ford T, editors. Essential endodontology. Blackwell Munksgaard Ltd; Oxford, UK: 2008. pp. 408–69. [Google Scholar]
- [193].Ng YL, Mann V, Rahbaran S, Lewsey J, Gulabivala K. Outcome of primary root canal treatment: systematic review of the literature - part 1. Effects of study characteristics on probability of success. International Endodontic Journal. 2007;40:921–39. doi: 10.1111/j.1365-2591.2007.01322.x. [DOI] [PubMed] [Google Scholar]
- [194].Ricucci D, Russo J, Rutberg M, Burleson JA, Spångberg LS. A prospective cohort study of endodontic treatments of 1,369 root canals: results after 5 years. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology & Endodontics. 2011;112:825–42. doi: 10.1016/j.tripleo.2011.08.003. [DOI] [PubMed] [Google Scholar]
- [195].Ørstavik D, Kerekes K, Eriksen HM. The periapical index: a scoring system for radiographic assessment of apical periodontitis. Endodontics & Dental Traumatology. 1986;2:20–34. doi: 10.1111/j.1600-9657.1986.tb00119.x. [DOI] [PubMed] [Google Scholar]
- [196].Wu MK, Shemesh H, Wesselink PR. Limitations of previously published systematic reviews evaluating the outcome of endodontic treatment. International Endodontic Journal. 2009;42:656–66. doi: 10.1111/j.1365-2591.2009.01600.x. [DOI] [PubMed] [Google Scholar]
- [197].Patel S, Wilson R, Dawood A, Foschi F, Mannocci F. The detection of periapical pathosis using digital periapical radiography and cone beam computed tomography - part 2: a 1-year post-treatment follow-up. International Endodontic Journal. 2012;45:711–23. doi: 10.1111/j.1365-2591.2012.02076.x. [DOI] [PubMed] [Google Scholar]
- [198].Zmener O, Pameijer CH. Clinical and radiographic evaluation of a resin-based root canal sealer. American Journal of Dentistry. 2004;17:19–22. [PubMed] [Google Scholar]
- [199].Zmener O, Pameijer CH. Clinical and radiographical evaluation of a resin-based root canal sealer: a 5-year follow-up. Journal of Endodontics. 2007;33:676–9. doi: 10.1016/j.joen.2007.03.009. [DOI] [PubMed] [Google Scholar]
- [200].Zmener O, Pameijer CH. Clinical and radiographic evaluation of a resin-based root canal sealer: an eight-year update. Journal of Endodontics. 2010;36:1311–4. doi: 10.1016/j.joen.2010.04.020. [DOI] [PubMed] [Google Scholar]
- [201].Zmener O, Pameijer CH. Clinical and radiographic evaluation of a resin-based root canal sealer: 10-year recall data. International Journal of Dentistry. 2012;2012:763248. doi: 10.1155/2012/763248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Pawińska M, Kierklo A, Marczuk-Kolada G. New technology in endodontics--the Resilon-Epiphany system for obturation of root canals. Advances in Medical Sciences. 2006;51(Suppl 1):154–7. [PubMed] [Google Scholar]
- [203].Conner DA, Caplan DJ, Teixeira FB, Trope M. Clinical outcome of teeth treated endodontically with a nonstandardized protocol and root filled with Resilon. Journal of Endodontics. 2007;33:1290–2. doi: 10.1016/j.joen.2007.07.003. [DOI] [PubMed] [Google Scholar]
- [204].Cotton TP, Schindler WG, Schwartz SA, Watson WR, Hargreaves KM. A retrospective study comparing clinical outcomes after obturation with Resilon/Epiphany or Gutta-Percha/Kerr sealer. Journal of Endodontics. 2008;34:789–97. doi: 10.1016/j.joen.2008.03.018. [DOI] [PubMed] [Google Scholar]
- [205].Tehrany AM. Masters thesis. Department of Endodontics, School of Dentistry, University of North Carolina; Chapel Hill: 2009. Outcome study of gutta-percha and Resilon filled root canals: A radiographic and clinical analysis. http://dc.lib.unc.edu/cdm/ref/collection/etd/id/2837. [Google Scholar]
- [206].Translated with an introduction by H. Lawson-Tancred. Penguin; 1998. Aristotle's Metaphysics (Book II) [Google Scholar]