Abstract
Maternal n-3 and n-6 polyunsaturated fatty acid (PUFA) status may influence birth outcomes and child health. We assessed second trimester maternal diet with food frequency questionnaires (FFQs) (n = 1666), mid-pregnancy maternal erythrocyte PUFA concentrations (n = 1550), and umbilical cord plasma PUFA concentrations (n = 449). Mean (SD) maternal intake of total n-3 PUFA was 1.17 g/d (0.43), docosahexaenoic and eicosapentaenoic acids (DHA+EPA) 0.16 g/d (0.17), and total n-6 PUFA 12.25 g/d (3.25). Mean maternal erythrocyte and cord plasma PUFA concentrations were 7.0% and 5.2% (total n-3), 5.0% and 4.6% (DHA+EPA), and 27.9% and 31.4% (total n-6). Mid-pregnancy diet–blood and blood–blood correlations were strongest for DHA+EPA (r = 0.38 for diet with maternal blood, r = 0.34 for diet with cord blood, r = 0.36 for maternal blood with cord blood), and less strong for n-6 PUFA. The FFQ is a reliable measure of elongated PUFA intake, although inter-individual variation is present
Keywords: Pregnancy, Diet, n-3 fatty acids, n-6 fatty acids
1. Introduction
The fetus receives its fatty acid supply from the mother via active placental transport. Since polyunsaturated fatty acids (PUFAs) of the n-3 and n-6 families are essential nutrients, dietary fatty acid intake during pregnancy must be sufficient to meet the demands of both the mother and the developing fetus [1,2]. Maternal n-3 and n-6 PUFA status may influence birth outcomes and child health [3,4]. A recent international consensus statement recommended daily maternal intake of at least 200 mg of the long-chain n-3 PUFA docosahexaenoic acid (DHA) during pregnancy and lactation to support optimal infant growth and developmental outcomes [5]. Many pregnant women likely do not consume this amount [6–8]. Since n-6 PUFA may compete with n-3 PUFA for enzymes necessary for elongation and desaturation, the ratio of total or long-chain n-6:n-3 fatty acids might also be important for health outcomes [9,10].
Identification of the individual characteristics associated with nutrient intake patterns can help inform and target dietary recommendations for pregnant women. Several previous observational studies have examined the dietary fatty acid intake patterns of pregnant women [1,8,11,12]. To date, one Canadian study has focused on the maternal characteristics correlated with prenatal fish consumption [13]. No studies have specifically investigated maternal characteristics associated with prenatal intake of all n-3 and n-6 fatty acids.
It is important to compare dietary and blood measures of fatty acids, because many studies of maternal fatty acid intake and pregnancy or child outcomes do not have biomarker data. Additionally, it is possible that metabolism of fatty acids differs among individuals, based upon characteristics such as body size or race/ethnicity [14–16].
Available literature regarding associations of maternal PUFA intake with corresponding concentrations in maternal and/or cord blood is limited by small sample sizes and restricted populations (e.g., inner-city African-American women, women with gestational diabetes, non-US populations) [1,7,8,11,12, 16–19]. Only one small study of 30 Belgian women has included PUFA in maternal diet, maternal blood, and cord blood [1].
The purpose of the present study was to examine associations of maternal characteristics with dietary fatty acid intake, and to compare measures of PUFA status in maternal diet, maternal blood, and umbilical cord blood in a large cohort of pregnant women residing in the US.
2. Patients and methods
2.1. Study population
Study subjects were participants in Project Viva, a prospective observational cohort study of gestational diet, other prenatal exposures, pregnancy outcomes, and offspring health. We have previously described recruitment and retention procedures [20,21]. In brief, from 1999 to 2002 we recruited women at their first prenatal visit at one of eight obstetrical offices of an eastern Massachusetts multispecialty group practice. Women were eligible to participate if they presented for their initial clinical visit at <22 weeks of gestation, had a singleton pregnancy, did not plan to move away from the study area prior to delivery, and could complete study forms in English. All women provided informed consent, and all procedures were in accordance with the ethical standards for human experimentation established by the Declaration of Helsinki [22]. Institutional review boards of participating institutions approved the study.
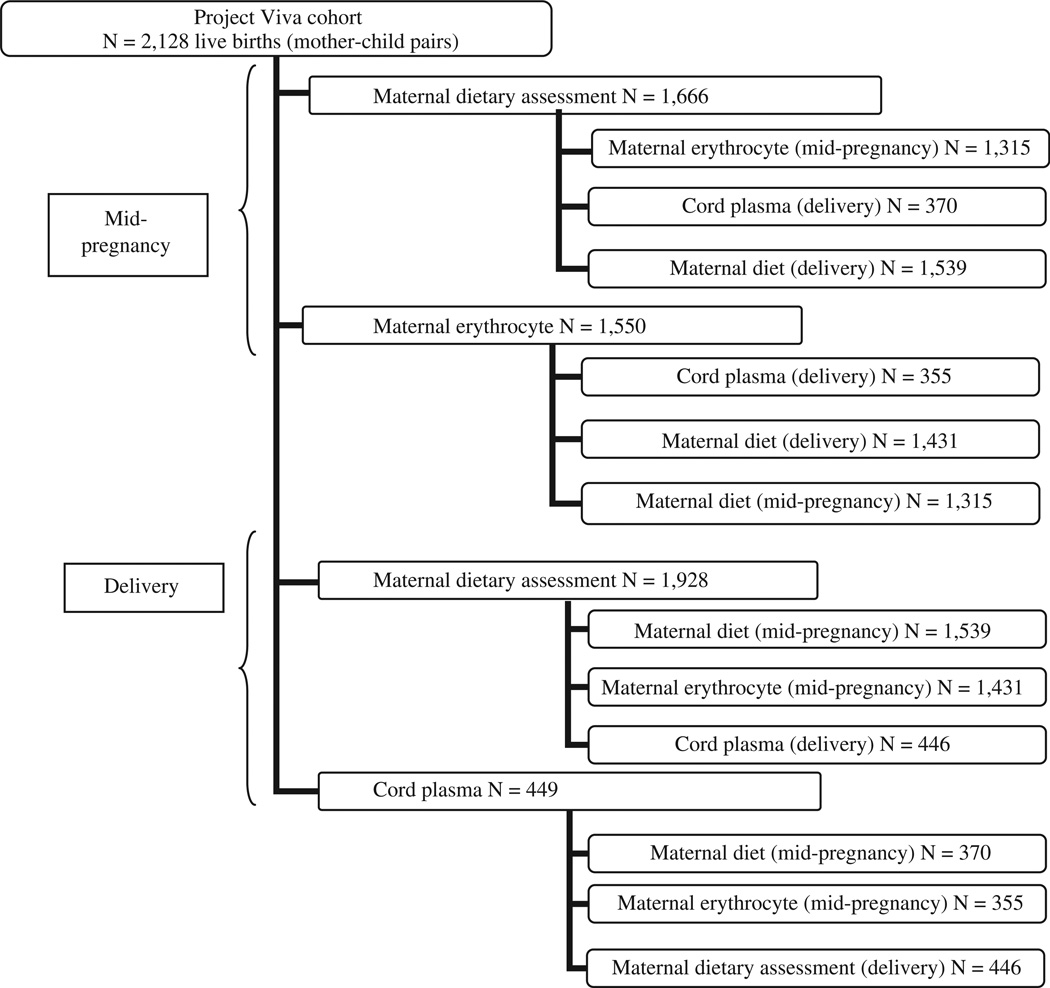
Of the original cohort of 2128 women with live births, we assessed dietary intake in mid-pregnancy among 1666 participants and in late pregnancy among 1928 participants. We analyzed the PUFA status of 1550 mid-pregnancy maternal blood samples and 449 cord blood samples from infants with gestational age at delivery ≥37 weeks. The number of subjects in the correlation analyses ranged from 355 (cord blood with maternal blood) to 1539 (mid-pregnancy diet with late pregnancy diet) (Fig. 1).
Fig. 1.
Project Viva participant PUFA measures. The upper boxes present the number of participants for whom each measure of fatty acid status was assessed. The lower boxes present the number of subjects in each correlation analysis of the relationships between measures of fatty acid intake and blood concentrations.
2.2. Dietary assessment
At a mean of 29 weeks, participants completed a semiquantitative food frequency questionnaire (FFQ) modified for use in pregnancy from a well-validated instrument used in several large cohorts [23,24] and previously calibrated against erythrocyte levels of fatty acids [25]. The FFQ quantified average frequency of consumption of >140 specified foods and beverages, including alcohol, during the preceding 3 months. Response options varied by food item. The FFQ also collected information about vitamin and supplement use, including two questions on frequency of use of “cod liver oil” and “fish oil (omega-3 fatty acids).”
Participants provided additional information on diet during the month before delivery in a brief dietary questionnaire completed in the hospital following delivery. The nine questions queried frequency of consumption of major dietary contributors to n-3 and trans fatty acid intake (four groupings of fish; poultry; beef, pork, or lamb; margarine, baked products, and deep fried foods). Compared with the longer mid-pregnancy FFQ, the brief delivery questionnaire did not specify portion sizes, had different response options, and did not allow assessment of intake of total n-3 PUFA, the parent n-3 fatty acid alpha linolenic acid (ALA), or of n-6 fatty acids. Late pregnancy trans fatty acid intake was not assessed for the first 293 participants.
We calculated nutrient intakes from the mid-pregnancy FFQ by multiplying a weight assigned to the frequency of consumption of each food item from the FFQ by the nutrient composition for the portion size specified. For intake in the month prior to delivery, we multiplied a weight assigned to the frequency of consumption of each food unit from the FFQ by an average nutrient composition for the food item or items. For each time point we used the sum of contributions to intake across all foods to generate total intake of a variety of nutrients for each subject [26]. Nutrient estimates were derived from the Harvard nutrient composition database, which is based on US Department of Agriculture publications and is continually supplemented by other published sources and personal communications from laboratories and manufacturers [27–29]. We energy adjusted the estimates of micronutrient intake from the mid-pregnancy FFQ using the nutrient residuals method [30].
2.3. Blood collection and fatty acid assays
We collected maternal blood from participants at the same time as the routine non-fasting mid-pregnancy clinical blood draw. Samples were not collected from women whose clinical visit did not overlap with the second study visit or who were otherwise unable to provide a sample. Whole blood samples were collected in tubes containing the anticoagulant ethylenediaminetetraacetic acid (EDTA), and refrigerated immediately. Within 24 h, tubes were centrifuged at 2000 rpm at 4 °C for 10 min and plasma and white cells were removed. The red blood cell samples then underwent two saline washes and were stored in liquid nitrogen freezers (80 °C) until use.
We collected blood from the umbilical vein following delivery from infants who were delivered on a weekday at one of the two study hospital sites. Whole blood samples were collected in tubes containing EDTA, refrigerated, and within 24 h centrifuged at 2000 rpm at 4 °C for 10 min. Plasma aliquots were stored in liquid nitrogen until assay. We did not retain erythrocytes from the umbilical cord blood.
After a single freeze/thaw, fatty acids in maternal erythrocytes and umbilical cord plasma were quantitated by gas-liquid chromatography [31]. The method identified up to 57 individual fatty acids, including 5 or 6 types of n-3 fatty acids (docosantrienoic acid was not identified in maternal erythrocytes) and 7 types of n-6 fatty acids. Peak retention times and area percentages of total fatty acids were identified by injecting known standards (NuCheck Prep, Elysium, Minn) and analyzed with the Agilent Technologies ChemStation A.08.03 software. Fatty acid concentrations are reported as area percentages of total fatty acids measured.
2.4. Maternal characteristics
We obtained information on maternal demographic characteristics, medical history, and lifestyle habits using mailed questionnaires and in-person interviews during study visits. We examined maternal characteristics found to be associated with fish consumption in previous studies [13,21]. We categorized characteristics as follows: age at study enrollment (14–24, 25–34, ≥35 years), race/ethnicity (non-Hispanic white, black, Hispanic, asian, and other race), number of previous live births (0, 1, or more), marital status (married or co-habiting, not married or co-habiting), education (<college graduate, college graduate, or more), tobacco use (never smoked, quit before pregnancy, smoked during pregnancy), total physical activity during mid-pregnancy (<2.5, ≥2.5 h/week), annual household income at study enrollment (≤$40,000, >$40,000), and alcohol use during mid-pregnancy (0 drinks/week, >0 drinks/week). We calculated pre-pregnancy body mass index (BMI) using self-reported height and pre-pregnancy weight and classified women as normal weight (BMI<25 kg/m2), overweight (BMI 25 to <30 kg/m2), or obese (BMI≥30 kg/m2). We calculated gestational weight gain as the difference between the last weight prior to delivery recorded in medical records and self-reported prepregnancy weight. We used Institute of Medicine guidelines for adequacy of weight gain (inadequate, adequate, excessive) [32]. We calculated an overall diet quality index (Alternate Healthy Eating Index-Pregnancy) using a 90-point scale based on intake of vegetables, fruit, ratio of white to red meat, fiber, trans fat, ratio of polyunsaturated to saturated fatty acids, and folate, calcium, and iron from foods [33], and classified women into quartiles of diet quality score (29–53, >53–60, >60–68, >68–89).
2.5. Statistical analyses
We calculated means and standard deviations (SDs) for measures of dietary and blood fatty acids. We examined the independent relationships of maternal characteristics with dietary intake of fatty acids by performing multivariate analyses using linear regression.
We calculated Spearman correlation coefficients to estimate the relationships between measures of fatty acid intake and blood concentrations. We explored whether the relationship between dietary intake and biomarkers differed by race/ethnicity or by prepregnancy BMI by separately calculating Spearman correlation coefficients for whites and blacks and for three pre-pregnancy BMI categories (BMI<25, 25 to <30, ≥30 kg/m2).
We used SAS version 9.1 (SAS Institute, Cary, NC) for all analyses.
3. Results
Among the 1666 mothers with information on mid-pregnancy diet, mean maternal age at study enrollment was 32.3 years (standard deviation 4.8). Most women were white (72.6%), married (93.5%), and well educated (70.6% had at least a college degree). About half (49.2%) were nulliparous. More than one-third of women were overweight or obese prior to pregnancy, and half had excessive weight gain during pregnancy (Table 1).
Table 1.
Multivariate-adjusted associations of maternal characteristics with mid-pregnancy dietary intakes of selected fatty acids among 1666 participants in Project Viva.a
| Overall |
Maternal dietary intake during mid-pregnancy |
||||||
|---|---|---|---|---|---|---|---|
| Total n-3 PUFA(g/d) | DHA+EPA (g/d) | Fish servings/week | Total n-6 PUFA (g/d) | n-6:n-3 ratio | trans fatty acids (g/d) | ||
| 1.17 (0.43)b | 0.16 (0.17) | 1.62 (1.49) | 12.25 (3.25) | 11.07 (2.61) | 2.20 (0.71) | ||
| Age at enrollment (year) | |||||||
| 14–24 | 110 (6.6)c | −0.10 (−0.22, 0.02)d | −0.08 (−0.13, −0.04) | −0.49 (−0.87, −0.10) | −0.19 (−1.09, 0.71) | 0.72 (−0.02, 1.45) | 0.11 (−0.04, 0.27) |
| 25–34 | 1072 (64.4) | −0.07 (−0.12, −0.02) | −0.03 (−0.05, −0.01) | −0.07 (−0.23, 0.09) | −0.21 (−0.59, 0.16) | 0.44 (0.13, 0.74) | −0.02 (−0.08, 0.04) |
| ≥ 35 | 484 (29.1) | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| Race/ethnicity | |||||||
| Asian | 97 (5.9) | −0.02 (−0.12, 0.07) | 0.11 (0.07, 0.15) | 0.45 (0.13, 0.76) | −0.59 (−1.33, 0.14) | 0.01 (−0.59, 0.61) | −0.13 (−0.25, −0.01) |
| Black | 205 (12.4) | 0.07 (−0.01, 0.14) | 0.07 (0.04, 0.10) | 0.43 (0.18, 0.68) | −0.29 (−0.88, 0.30) | −1.11 (−1.59, −0.63) | −0.18 (−0.27, −0.08) |
| Hispanic | 92 (5.6) | −0.10 (−0.20, 0.00) | 0.02 (−0.02, 0.05) | 0.34 (0.02, 0.67) | −1.15 (−1.90, −0.40) | −0.24 (−0.85, 0.37) | −0.06 (−0.18, 0.07) |
| Other race | 61 (3.7) | 0.07 (−0.05, 0.20) | 0.14 (0.09, 0.19) | 0.37 (−0.04, 0.78) | −0.72 (−1.67, 0.23) | −0.88 (−1.65, −0.10) | −0.13 (−0.29, 0.03) |
| Non-Hispanic White | 1203 (72.6) | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| Previous live births | |||||||
| 0 | 820 (49.2) | −0.05 (−0.10, 0.00) | −0.01 (−0.02, 0.01) | −0.21 (−0.36, −0.07) | −0.13 (−0.48, 0.21) | 0.46 (0.18, 0.75) | −0.01 (−0.07, 0.05) |
| 1 or more | 846 (50.8) | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| Marital status | |||||||
| Not married or co-habiting | 107 (6.5) | 0.06 (−0.05, 0.17) | −0.01 (−0.05, 0.03) | 0.10 (−0.26, 0.46) | 0.13 (−0.71, 0.97) | −0.47 (−1.15, 0.21) | 0.05 (−0.09, 0.19) |
| Married or co-habiting | 1550 (93.5) | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| Education | |||||||
| < College | 488 (29.4) | −0.03 (−0.08, 0.03) | −0.03 (−0.05, −0.01) | −0.23 (−0.42, −0.05) | −0.07 (−0.49, 0.35) | 0.14 (−0.20, 0.48) | −0.05 (−0.12, 0.02) |
| ≥ College graduate | 1170 (70.6) | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| Pre-pregnancy BMI (kg/m2) | |||||||
| < 25 | 1085 (65.4) | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| 25 to < 30 | 354 (21.3) | −0.03 (−0.09, 0.03) | −0.01 (−0.03, 0.01) | 0.01 (−0.17, 0.20) | −0.02 (−0.44, 0.41) | 0.18 (−0.16, 0.53) | 0.07 (−0.01, 0.14) |
| ≥ 30 | 221 (13.3) | 0.10 (0.03, 0.17) | 0.01 (−0.01, 0.04) | 0.20 (−0.02, 0.42) | 0.87 (0.35, 1.38) | −0.08 (−0.49, 0.34) | 0.14 (0.06, 0.23) |
| Gestational weight gain based on IOM guidelines | |||||||
| Inadequate | 233 (14.1) | 0.00 (−0.07, 0.07) | 0.01 (−0.01, 0.04) | 0.16 (−0.06, 0.38) | −0.16 (−0.69, 0.36) | −0.20 (−0.63, 0.22) | −0.05 (−0.14, 0.03) |
| Adequate | 590 (35.8) | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| Excessive | 825 (50.1) | 0.03 (−0.01, 0.08) | 0.00 (−0.02, 0.02) | 0.02 (−0.14, 0.18) | 0.00 (−0.37, 0.38) | −0.32 (−0.62, −0.02) | 0.02 (−0.04, 0.09) |
| Tobacco use | |||||||
| Never smoked | 1090 (67.2) | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| Quit before pregnancy | 344 (21.2) | 0.04 (−0.01, 0.10) | 0.01 (−0.01, 0.03) | 0.09 (−0.09, 0.27) | 0.36 (−0.05, 0.77) | −0.17 (−0.50, 0.17) | 0.02 (−0.05, 0.09) |
| Smoked during pregnancy | 188 (11.6) | 0.06 (−0.02, 0.13) | 0.01 (−0.02, 0.03) | 0.29 (0.05, 0.53) | 0.37 (−0.19, 0.93) | −0.16 (−0.62, 0.30) | 0.10 (0.00, 0.19) |
| Mid–pregnancy physical activity (h/week) | |||||||
| < 2.5 | 341 (21.3) | −0.03 (−0.09, 0.02) | −0.02 (−0.04, 0.00) | −0.23 (−0.40, −0.05) | −0.06 (−0.46, 0.35) | 0.27 (−0.06, 0.60) | −0.06 (−0.13, 0.01) |
| ≥ 2.5 | 1257 (78.7) | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| Annual household income ($) | |||||||
| ≤ 40,000 | 184 (11.9) | −0.01 (−0.09, 0.07) | 0.02 (−0.01, 0.05) | 0.02 (−0.24, 0.28) | −0.35 (−0.95, 0.26) | 0.05 (−0.44, 0.54) | −0.08 (−0.18, 0.02) |
| > 40,000 | 1367 (88.1) | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| Mid-pregnancy alcohol use (drinks/week) | |||||||
| > 0 | 218 (13.1) | 0.03 (−0.04, 0.10) | 0.05 (0.02, 0.07) | 0.27 (0.06, 0.49) | −0.18 (−0.68, 0.32) | −0.52 (−0.93, −0.11) | 0.01 (−0.08, 0.09) |
| 0 | 1448 (86.9) | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| Mid-pregnancy Alternate Healthy Eating Index-Pregnancy score | |||||||
| 29–53 | 409 (24.6) | 0.13 (0.06, 0.19) | −0.05 (−0.08,−0.03) | −0.91 (−1.12,−0.70) | 1.17 (0.67, 1.66) | −0.30 (−0.70, 0.11) | n/a |
| > 53–60 | 395 (23.7) | 0.09 (0.03, 0.15) | −0.04 (−0.07,−0.02) | −0.60 (−0.80,−0.39) | 0.61 (0.14, 1.09) | −0.41 (−0.80, −0.03) | n/a |
| > 60–68 | 449 (27.0) | 0.05 (−0.01, 0.11) | −0.03 (−0.06,−0.01) | −0.49 (−0.69,−0.30) | 0.45 (−0.01, 0.90) | −0.08 (−0.45, 0.29) | n/a |
| > 68–89 | 413 (24.8) | Ref. | Ref. | Ref. | Ref. | Ref. | n/a |
PUFA, polyunsaturated fatty acids; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid.
Estimates of effect were simultaneously adjusted for all other characteristics in the table. In multivariable analysis, we excluded 211 participants with missing covariate data.
In the first row, value inside the bracket gives SD and the value outside the bracket gives the mean.
In this column, value inside the bracket gives percentage and the value outside the bracket gives N. (Numbers may not add to total due to missing information.)
If the entry has the format a(b,c), a gives the value of β and (b,c) gives the 95% CI.
In multivariate regression analyses, we observed associations of several maternal sociodemographic characteristics and health behaviors with energy-adjusted intake of fatty acids in midpregnancy (Table 1). Compared to white women, black women had higher fish intake (β = 0.43 servings/week; 95% confidence interval [CI] 0.18, 0.68) and a lower ratio of n-6:n-3 PUFA (β = −1.11 g/d; 95% CI −1.59, −0.63). Higher diet quality score, higher education, and more physical activity were also associated with higher fish intake (Table 1). Compared to women with normal pre-pregnancy BMI, women who were obese prior to pregnancy had higher intakes of total n-3 (β = 0.10 g/d; 95% CI 0.03, 0.17) and total n-6 PUFA (β = 0.87 g/d; 95% CI 0.35, 1.38) and of trans fatty acids (β = 0.14 g/d; 95% CI 0.06, 0.23).
In Table 2 we present the means and ranges of daily dietary intake and fatty acid concentrations of selected fatty acids. The relative concentrations (percentage of total fatty acids) of most fatty acids were similar in maternal erythrocytes and cord plasma. The n-3 concentration in cord plasma was lower than the respective concentration in mid-pregnancy maternal erythrocytes, while the n-6 concentration was higher.
Table 2.
Fatty acid status among Project Viva participants, measured through maternal dietary intake during mid-pregnancy and during the month before delivery and through fatty acid composition of mid-pregnancy maternal erythrocyte phospholipids and of umbilical cord plasma lipids.
| Fatty acid | Maternal dietary intake (g/d) during mid-pregnancy (N = 1666) |
Relative concentration (% of total fatty acids) of mid-pregnancy maternal erythrocyte phospholipids (N = 1550) |
Maternal dietary intake during the month before delivery (g/d) (N = 1,928)a |
Relative concentration (% of total fatty acids) of umbilical cord plasma lipids (N = 449)b |
||||
|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Range (min, max) | Mean (SD) | Range (min, max) | Mean (SD) | Range (min, max) | Mean (SD) | Range (min, max) | |
| Total n-3 PUFA | 1.17 (0.43) | 0.33, 3.99 | 6.96 (2.27) | 0.33, 14.23 | – | – | 5.15 (1.39) | 2.33, 11.56 |
| ALA | 0.99 (0.40) | 0.27, 3.92 | 0.13 (0.06) | 0.00, 0.69 | – | – | 0.17 (0.07) | 0.04, 0.54 |
| DHA | 0.11 (0.09) | 0.00, 1.08 | 4.74 (1.68) | 0.00, 9.38 | 0.08 (0.09) | 0.00, 1.09 | 4.20 (1.07) | 1.97, 9.06 |
| EPA | 0.06 (0.08) | 0.00, 1.63 | 0.30 (0.17) | 0.00, 2.12 | 0.04 (0.06) | 0.00, 0.63 | 0.34 (0.35) | 0.00, 1.85 |
| DHA+EPA | 0.16 (0.17) | 0.00, 2.72 | 5.04 (1.81) | 0.00, 11.00 | 0.12 (0.14) | 0.00, 1.72 | 4.60 (1.28) | 2.01, 10.20 |
| Total n-6 PUFA | 12.25 (3.25) | 4.52, 35.36 | 27.91 (5.39) | 2.51, 34.13 | – | – | 31.35 (2.65) | 21.41, 39.44 |
| AA | 0.09 (0.03) | 0.00, 0.29 | 13.09 (3.30) | 0.00, 17.20 | – | – | 15.53 (2.11) | 9.20, 21.82 |
| LA | 12.14 (3.24) | 4.42, 35.24 | 9.00 (1.49) | 1.35, 13.97 | – | – | 11.07 (1.61) | 7.08, 17.31 |
| n-6:n-3 ratio | 11.07 (2.61) | 2.99, 29.73 | 4.71 (2.80) | 1.55, 34.47 | – | – | 6.52 (1.63) | 2.68, 11.57 |
| AA:DHA+EPA ratio | 12.32 (60.43) | 0.04, 784.64 | 9.79 (269.97) | 0.00, 10,631.68 | 3.62 (0.96) | 1.51, 7.36 | ||
| trans fatty acids | 2.20 (0.71) | 0.37, 7.53 | 2.13 (0.62) | 0.51, 5.61 | 0.65 (0.44) | 0.01, 3.92 | 1.49 (0.49) | 0.58, 6.35 |
PUFA, polyunsaturated fatty acids; ALA, alpha-linolenic acid; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; AA, arachidonic acid; LA, linoleic acid; SD, standard deviation.
The food frequency questionnaire used in the post-delivery interview differed from the questionnaire used in mid-pregnancy; not all sources of fatty acids were captured. N = 1635 for intake of trans fatty acids.
N = 367 for total n-3 PUFA, EPA, DHA+EPA, n-6:n-3 ratio, AA:DHA+EPA ratio.
In Table 3 we report correlations among the concentrations of fatty acids in maternal diet, maternal erythrocytes, and cord plasma. The Spearman correlation r for mid-pregnancy diet and erythrocytes was 0.38 for the sum of the elongated n-3 fatty acids DHA and eicosapentaenoic acid (EPA) and 0.19 for the ratio of n-6:n-3 PUFA (both p<0.0001). Spearman correlation coefficients between mid-pregnancy diet and concentration of total cord plasma phospholipids were 0.36 for DHA+EPA and 0.29 for n-6:n-3 (both p<0.0001). Fatty acid levels in the two biomarkers were correlated (e.g., r = 0.33 for DHA+EPA; r = 0.44 for n-6:n-3 PUFA ratio; both p<0.0001) about as strongly as dietary measures were correlated with blood levels. DHA+EPA intake in late pregnancy was correlated with the two biomarkers (r = 0.18 with maternal erythrocyte; r = 0.21 with cord plasma; both p<0.0001). Estimated intake of DHA+EPA in mid-pregnancy was highly correlated with fish consumption during the same period (r = 0.83, p<0.0001). Maternal intake of the parent n-3 PUFA ALA was not correlated with either maternal (r = 0.04, p = 0.20) or cord blood (r = −0.01, p = 0.91); however, the biomarkers were moderately correlated (r = 0.22, p<0.0001). Intake of the parent n-6 PUFA linoleic acid (LA) was moderately correlated with maternal and cord blood (r = 0.20, r = 0.16, respectively; p<0.0001, p = 0.002, respectively), and the biomarkers were similarly correlated (r = 0.15, p = 0.006).
Table 3.
Spearman correlation coefficients (r) for associations among fatty acid measures.
| Fatty acid measurement | Mid-pregnancy maternal erythrocyte phospholipids (% of total fatty acids) (N = 1550) |
Maternal dietary intake during the month before delivery (g/d) (N = 1928)a |
Umbilical cord plasma lipids (% of total fatty acids) (N = 449)b |
|||
|---|---|---|---|---|---|---|
| r | p-value | r | p-value | r | p-value | |
| Maternal dietary intake during mid-pregnancy (g/d) (N = 1666) | ||||||
| Total n-3 PUFA | 0.03 | 0.21 | – | – | 0.12 | 0.04 |
| ALA | 0.04 | 0.20 | – | – | −0.01 | 0.91 |
| DHA | 0.36 | <0.0001 | 0.36 | <0.0001 | 0.31 | <0.0001 |
| EPA | 0.34 | <0.0001 | 0.36 | <0.0001 | 0.36 | <0.0001 |
| DHA+EPA | 0.38 | <0.0001 | 0.35 | <0.0001 | 0.36 | <0.0001 |
| Total n–6 PUFA | 0.14 | <0.0001 | – | – | 0.12 | 0.02 |
| AA | 0.01 | 0.70 | – | – | 0.01 | 0.89 |
| LA | 0.20 | <0.0001 | – | – | 0.16 | 0.002 |
| n-6:n-3 ratio | 0.19 | <0.0001 | – | – | 0.29 | <0.0001 |
| AA:DHA+EPA ratio | 0.46 | <0.0001 | – | – | 0.40 | <0.0001 |
| trans fatty acids | 0.30 | <0.0001 | 0.37 | <0.0001 | 0.08 | 0.14 |
| Mid-pregnancy maternal erythrocyte phospholipids (% of total fatty acids) (N = 1550) | ||||||
| Total n-3 PUFA | – | – | 0.33 | <0.0001 | ||
| ALA | – | – | 0.22 | <0.0001 | ||
| DHA | 0.17 | <0.0001 | 0.31 | <0.0001 | ||
| EPA | 0.19 | <0.0001 | 0.21 | 0.0003 | ||
| DHA+EPA | 0.18 | <0.0001 | 0.33 | <0.0001 | ||
| Total n-6 PUFA | – | – | 0.16 | 0.002 | ||
| AA | – | – | 0.19 | 0.0003 | ||
| LA | – | – | 0.15 | 0.006 | ||
| n-6:n-3 ratio | – | – | 0.44 | <0.0001 | ||
| AA:DHA+EPA ratio | – | – | 0.45 | <0.0001 | ||
| trans fatty acids | 0.10 | 0.0005 | 0.22 | <0.0001 | ||
| Maternal dietary intake during the month before delivery (g/d) (N = 1928)a | ||||||
| DHA | 0.22 | <0.0001 | ||||
| EPA | 0.17 | 0.001 | ||||
| DHA+EPA | 0.21 | <0.0001 | ||||
| trans fatty acids | −0.05 | 0.37 | ||||
PUFA, polyunsaturated fatty acids; ALA, alpha-linolenic acid; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; AA, arachidonic acid; LA, linoleic acid.
N = 1635 for intake of trans fatty acids.
N = 367 for total n-3 PUFA, EPA, DHA+EPA, n-6:n-3 ratio, AA:DHA+EPA ratio.
The correlations between the two dietary measures of n-3 PUFA were similar among women who were overweight or obese prior to pregnancy compared to those with normal pre-pregnancy weight (e.g., DHA+EPA r = 0.38 for obese women, r = 0.41 for overweight women, r = 0.32 for normal weight women, Supplemental Table 1). For n-6 PUFA, the strength of the correlation between dietary intake and the biomarkers was weaker among obese women, and in several cases was in the opposite direction as observed among normal weight or overweight women. For example, mid-pregnancy intake of total n-6 was correlated with maternal and cord blood among normal weight and overweight women (e.g., normal weight: r = 0.19, p<0.0001; r = 0.13, p = 0.04; maternal and cord blood, respectively), but not among obese women (r = −0.10, p = 0.20; r = 0.15, p = 0.32; maternal and cord blood, respectively). The strength of the correlation between biomarkers was similar for obese women compared to the other BMI groups (e.g., total n-3 PUFA r = 0.30 among normal weight women, r = 0.48 among overweight women, and r = 0.21 among obese women). We have included the results of analyses stratified by maternal BMI in Supplemental Table 1.
The correlations between the two dietary measures were stronger for n-3 PUFA among the 1203 white women compared to the 205 black women (e.g., DHA r = 0.39 for white women, r = 0.23 for black women; 1122 white women and 184 black women had data on both dietary measures). In contrast to the overall population and to white participants alone, the correlations between mid-pregnancy intake of elongated n-3 and total n-6 PUFA and the respective cord blood measures were weaker among black participants, although the direction of the associations were similar. For example, the correlation between maternal mid-pregnancy intake of EPA and cord blood levels was 0.38 among white participants and 0.14 among black participants. Similarly, the correlations between the biomarkers of n-6 PUFA were weaker among black participants. We have included the results of analyses stratified by maternal race/ethnicity in Supplemental Table 2.
4. Discussion and conclusions
In this study, we determined prenatal fatty acid intake in a cohort of US pregnant women using food frequency questionnaires and we quantified blood levels of fatty acids in maternal erythrocyte and umbilical cord plasma phospholipids. In multivariate analyses, higher prenatal intake of n-3 fatty acids was associated with black race, pre-pregnancy obesity, and higher diet quality score. Higher intake of n-6 fatty acids was associated with pre-pregnancy obesity and lower diet quality score. Dietary intake of DHA, EPA, and the parent n-6 PUFA LA, but not of the parent n-3 PUFA ALA, were moderately correlated with maternal and umbilical cord blood phospholipid status. Maternal and infant biomarkers were correlated with each other.
Dietary intakes of fatty acids in this cohort were similar to intakes observed in several other populations in the US, Canada, Belgium, and the UK [1,6,7,11,34], thus this population is comparable to other women. Among women in Project Viva, mean DHA intake in mid-pregnancy was 105 mg/d, about half the recommended daily intake [5], suggesting many women were consuming an amount insufficient for optimal child growth and development. Reported intake in late pregnancy, the time of the most rapid fetal brain uptake, was even lower (78 mg/d). The n-6:n-3 ratio of 11.07 was higher than the ratio reported in some other North American studies [6,7], but was similar to the ratio reported in a US national survey of food intake conducted in 1994–1996 [35]. The high n-6:n-3 ratio found in modern diets could contribute to adverse health outcomes such as asthma and cardiovascular disease [36,37]. Thus, the present results are useful because they provide novel information about the relative intakes of n-6 and n-3 fatty acids in a large cohort of pregnant women in the US.
Maternal characteristics associated with higher dietary n-3 PUFA intake included older age, non-white race/ethnicity, higher education, and pre-pregnancy obesity. Higher dietary n-6:n-3 ratio was associated with younger maternal age, nulliparity, and non-Hispanic white race/ethnicity. Less frequent fish consumption was associated with younger age, white race, lower diet quality, and lower levels of physical activity. These results were comparable to those found in a recent study of sociodemographic and lifestyle correlates of fish consumption among a pregnant Canadian cohort [13]. The findings may help inform the design of future studies examining prenatal intake of n-3 and n-6 fatty acids in relation to health outcomes, as these same maternal factors may be independently associated with outcomes of interest such as pregnancy complications and fetal and postnatal growth.
Directly quantified maternal mid-pregnancy blood fatty acid levels were similar to those observed in other populations [6,15,18,38]. The fatty acid levels measured in cord plasma were also similar to those measured in Dutch [19,38] and Belgian [1] infants.
Dietary intake of fatty acids was directly correlated with respective fatty acid concentrations in maternal blood and cord plasma phospholipids for most PUFA, indicating the mid-pregnancy FFQ and the brief dietary questionnaire administered following delivery are valid measures of prenatal PUFA status. Notable exceptions included weak correlations between diet and blood levels for the parent n-3 (ALA) and n-6 (LA) PUFA. The weak correlation of total n-3 PUFA in maternal diet compared with maternal blood may reflect differences in the metabolism of the parent n-3 compared to the elongated n-3 PUFA; ALA storage in adipose tissue, not blood; or incomplete recall of dietary sources of ALA. Fetal uptake of maternal PUFAs may account in part for the moderate correlations we observed between maternal dietary intake and umbilical cord blood levels.
The correlations between the two biomarkers were modest. Few studies have reported correlations of intake and blood or of two blood markers, and only one study has evaluated all three measures of PUFA status. De Vriese et al. [1] examined the relationship of dietary fat intake during the first or second trimester with the fatty acid composition of maternal and umbilical cord plasma phospholipids in a sample of 30 pregnant Belgian women. In that study, average intake of elongated PUFA (DHA+EPA) was 0.46 g/d, higher than the mean of 0.16 g/d in the present study. The correlations between PUFA concentrations in diet and umbilical cord blood were strongest for EPA (r = 0.59) and for total n-6 PUFA (r = 0.38), similar to the present study. However, several other correlations were weak and some were in the opposite direction as observed in the Viva cohort. Thus, the present results are useful because they provide additional information about the relationships between dietary and blood measures of fatty acid status among a relatively large population of women with modest intake of elongated n-3 PUFA. Further, the analysis of several measures of PUFA status offers additional information on the inter-relationships between measures.
The few published studies of diet during pregnancy have demonstrated substantial racial/ethnic differences in overall dietary habits [39], but to our knowledge no studies have examined differences in the associations between dietary intake of fatty acids and respective biomarkers by racial/ethnic group. We observed stronger correlations between PUFA measures among white participants compared with black participants. Nevertheless, the correlations between intake and maternal blood PUFA concentration among black participants were stronger than those reported in the one previous study of PUFA status among African-American women [8], in which dietary intake of n-6 PUFA and of ALA was higher than observed in the present study but PUFA concentrations in maternal blood were similar. Additional studies are necessary to confirm the racial/ethnic differences in the strength of the correlations between measures of fatty acid status observed in the present study.
Obesity may affect the metabolism of fatty acids during pregnancy [15,16]. The correlation between dietary intake of fatty acids and respective maternal and infant PUFA status measured in blood was weaker among the obese women in this cohort compared with normal weight and overweight women. These results may reflect differences in metabolism among obese women compared with other women, or could indicate dietary intake is not as suitable a measure of prenatal PUFA status among this group.
Strengths of this study include a large sample, prospective collection of dietary intake, and measurement of a number of maternal characteristics potentially associated with fatty acid intake. We used a detailed, validated FFQ and analyzed both maternal and infant biomarkers of fatty acid intake.
Results should be interpreted with some caution based on the study limitations. Dietary intake was assessed using a self-reported measurement tool, a method that may under- or overestimate true intake. For example, it is unlikely that any individuals actually consumed 0 mg/d of AA. However, the FFQ is a very useful instrument for ranking dietary intake, as we have used it here. Other unmeasured maternal characteristics may be important correlates of fatty acid intake. The Project Viva cohort is a relatively affluent and well-educated population compared with the overall population of pregnant women, and study subjects are all insured. These characteristics may limit generalizability of our results to other populations. Correlations between the biomarkers might have been stronger if we had erythrocyte levels of fatty acids in both maternal and umbilical cord blood, but unfortunately we did not retain erythrocytes from the umbilical cord blood.
In conclusion, dietary intake of fatty acids during mid-pregnancy in this large cohort of pregnant women was similar to intake observed in other populations. Dietary intake of DHA was well below the recommended level, and the total n-6:n-3 ratio was over 10:1. Correlations between the dietary measurement tool and biomarkers suggest the FFQ is a reliable measure of elongated n-3 and n-6 fatty acid intake, although inter-individual variation in reporting or in metabolism is still present. Future research is warranted to increase understanding of the factors that contribute to prenatal dietary intake of PUFA and other nutrients. We have previously studied associations of maternal dietary intake of fatty acids with fetal growth, gestation length, gestatinal diabetes, and preeclampsia [21,40,41]. The present results will inform future investigations of the association of prenatal n-3 and n-6 PUFA exposure with pregnancy outcomes and infant and child health.
Supplementary Material
Acknowledgements
We thank the participants and staff of Project Viva.
Footnotes
Sources of support: This project was supported by grants from the National Institutes of Health (HD44807, HD34568, HD68041), Mead Johnson Nutritionals (Evansville, Indiana), and by Harvard Medical School and the Harvard Pilgrim Health Care Foundation.
Conflict of interest
None declared.
Appendix A. Supporting Information
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.plefa.2009.02.007.
References
- 1.De Vriese SR, Matthys C, De Henauw S, De Backer G, Dhont M, Christophe AB. Maternal and umbilical fatty acid status in relation to maternal diet. Prostaglandins Leukot. Essent. Fatty Acids. 2002;67(6):389–396. doi: 10.1054/plef.2002.0446. [DOI] [PubMed] [Google Scholar]
- 2.Haggarty P. Placental regulation of fatty acid delivery and its effect on fetal growth—a review. Placenta. 2002;23(Suppl. A):S28–S38. doi: 10.1053/plac.2002.0791. [DOI] [PubMed] [Google Scholar]
- 3.Jensen CL. Effects of n-3 fatty acids during pregnancy and lactation. Am. J. Clin. Nutr. 2006;83(Suppl. 6):1452S–1457S. doi: 10.1093/ajcn/83.6.1452S. [DOI] [PubMed] [Google Scholar]
- 4.Szajewska H, Horvath A, Koletzko B. Effect of n-3 long-chain polyunsaturated fatty acid supplementation of women with low-risk pregnancies on pregnancy outcomes and growth measures at birth: a meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2006;83(6):1337–1344. doi: 10.1093/ajcn/83.6.1337. [DOI] [PubMed] [Google Scholar]
- 5.Koletzko B, Cetin I, Brenna JT. Dietary fat intakes for pregnant and lactating women. Br. J. Nutr. 2007;98(5):873–877. doi: 10.1017/S0007114507764747. [DOI] [PubMed] [Google Scholar]
- 6.Denomme J, Stark KD, Holub BJ. Directly quantitated dietary (n-3) fatty acid intakes of pregnant Canadian women are lower than current dietary recommendations. J. Nutr. 2005;135(2):206–211. doi: 10.1093/jn/135.2.206. [DOI] [PubMed] [Google Scholar]
- 7.Loosemore ED, Judge MP, Lammi-Keefe CJ. Dietary intake of essential and long-chain polyunsaturated fatty acids in pregnancy. Lipids. 2004;39(5):421–424. doi: 10.1007/s11745-004-1246-y. [DOI] [PubMed] [Google Scholar]
- 8.Stark KD, Beblo S, Murthy M, et al. Comparison of bloodstream fatty acid composition from African-American women at gestation, delivery, and postpartum. J. Lipid Res. 2005;46(3):516–525. doi: 10.1194/jlr.M400394-JLR200. [DOI] [PubMed] [Google Scholar]
- 9.Reece MS, McGregor JA, Allen KG, Harris MA. Maternal and perinatal long-chain fatty acids: possible roles in preterm birth. Am. J. Obstet. Gynecol. 1997;176(4):907–914. doi: 10.1016/s0002-9378(97)70620-3. [DOI] [PubMed] [Google Scholar]
- 10.Velzing-Aarts FV, van der Klis FR, van der Dijs FP, Muskiet FA. Umbilical vessels of preeclamptic women have low contents of both n-3 and n-6 long-chain polyunsaturated fatty acids. Am. J. Clin. Nutr. 1999;69(2):293–298. doi: 10.1093/ajcn/69.2.293. [DOI] [PubMed] [Google Scholar]
- 11.Innis SM, Elias SL. Intakes of essential n-6 and n-3 polyunsaturated fatty acids among pregnant Canadian women. Am. J. Clin. Nutr. 2003;77(2):473–478. doi: 10.1093/ajcn/77.2.473. [DOI] [PubMed] [Google Scholar]
- 12.Olsen SF, Hansen HS, Sandstrom B, Jensen B. Erythrocyte levels compared with reported dietary intake of marine n-3 fatty acids in pregnant women. Br. J. Nutr. 1995;73(3):387–395. doi: 10.1079/bjn19950041. [DOI] [PubMed] [Google Scholar]
- 13.Sontrop JM, Campbell MK, Evers SE, Speechley KN, Avison WR. Fish consumption among pregnant women in London, Ontario: associations with socio-demographic and health and lifestyle factors. Can. J. Public Health. 2007;98(5):389–394. doi: 10.1007/BF03405425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koletzko B, Agostoni C, Carlson SE, et al. Long chain polyunsaturated fatty acids (LC-PUFA) and perinatal development. Acta Paediatr. 2001;90(4):460–464. [PubMed] [Google Scholar]
- 15.Stewart F, Rodie VA, Ramsay JE, Greer IA, Freeman DJ, Meyer BJ. Longitudinal assessment of erythrocyte fatty acid composition throughout pregnancy and post partum. Lipids. 2007;42(4):335–344. doi: 10.1007/s11745-006-3005-5. [DOI] [PubMed] [Google Scholar]
- 16.Wijendran V, Bendel RB, Couch SC, et al. Maternal plasma phospholipid polyunsaturated fatty acids in pregnancy with and without gestational diabetes mellitus: relations with maternal factors. Am. J. Clin. Nutr. 1999;70(1):53–61. doi: 10.1093/ajcn/70.1.53. [DOI] [PubMed] [Google Scholar]
- 17.Matorras R, Ruiz JI, Perteagudo L, et al. Longitudinal study of fatty acids in plasma and erythrocyte phospholipids during pregnancy. J. Perinat. Med. 2001;29(4):293–297. doi: 10.1515/JPM.2001.042. [DOI] [PubMed] [Google Scholar]
- 18.Parra MS, Schnaas L, Meydani M, Perroni E, Martinez S, Romieu I. Erythrocyte cell membrane phospholipid levels compared against reported dietary intakes of polyunsaturated fatty acids in pregnant Mexican women. Public Health Nutr. 2002;5(6A):931–937. doi: 10.1079/PHN2002381. [DOI] [PubMed] [Google Scholar]
- 19.Rump P, Hornstra G. The n-3 and n-6 polyunsaturated fatty acid composition of plasma phospholipids in pregnant women and their infants. relationship with maternal linoleic acid intake. Clin. Chem. Lab. Med. 2002;40(1):32–39. doi: 10.1515/CCLM.2002.007. [DOI] [PubMed] [Google Scholar]
- 20.Gillman MW, Rich-Edwards JW, Rifas-Shiman SL, Lieberman ES, Kleinman KP, Lipshultz SE. Maternal age and other predictors of newborn blood pressure. J. Pediatr. 2004;144(2):240–245. doi: 10.1016/j.jpeds.2003.10.064. [DOI] [PubMed] [Google Scholar]
- 21.Oken E, Kleinman KP, Olsen SF, Rich-Edwards JW, Gillman MW. Associations of seafood and elongated n-3 fatty acid intake with fetal growth and length of gestation: results from a US pregnancy cohort. Am. J. Epidemiol. 2004;160(8):774–783. doi: 10.1093/aje/kwh282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.World Medical Association declaration of Helsinki. Recommendations guiding physicians in biomedical research involving human subjects. JAMA. 1997;277(11):925–926. [PubMed] [Google Scholar]
- 23.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am. J. Epidemiol. 1992;135(10):1114–1126. doi: 10.1093/oxfordjournals.aje.a116211. [DOI] [PubMed] [Google Scholar]
- 24.Willett WC, Sampson L, Stampfer MJ, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am. J. Epidemiol. 1985;122(1):51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- 25.Fawzi WW, Rifas-Shiman SL, Rich-Edwards JW, Willett WC, Gillman MW. Calibration of a semi-quantitative food frequency questionnaire in early pregnancy. Ann. Epidemiol. 2004;14(10):754–762. doi: 10.1016/j.annepidem.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 26.Rifas-Shiman SL, Willett WC, Lobb R, Kotch J, Dart C, Gillman MW. PrimeScreen, a brief dietary screening tool: reproducibility and comparability with both a longer food frequency questionnaire and biomarkers. Public Health Nutr. 2001;4(2):249–254. doi: 10.1079/phn200061. [DOI] [PubMed] [Google Scholar]
- 27.Hu FB, Bronner L, Willett WC, et al. Fish and omega-3 fatty acid intake and risk of coronary heart disease in women. JAMA. 2002;287(14):1815–1821. doi: 10.1001/jama.287.14.1815. [DOI] [PubMed] [Google Scholar]
- 28.Iso H, Rexrode KM, Stampfer MJ, et al. Intake of fish and omega-3 fatty acids and risk of stroke in women. JAMA. 2001;285(3):304–312. doi: 10.1001/jama.285.3.304. [DOI] [PubMed] [Google Scholar]
- 29.US Department of Agriculture. Agricultural Research Service. USDA Nutrient Database for Standard Reference. Release 13, 1999, cited; Available from: < http://www.ars.usda.gov/main/site_main.htm?modecode=12354500S>.
- 30.Willett W. Nutritional Epidemiology. second ed. New York: Oxford University Press; 1998. p. 514. [Google Scholar]
- 31.Baylin A, Kim MK, Donovan-Palmer A, et al. Fasting whole blood as a biomarker of essential fatty acid intake in epidemiologic studies: comparison with adipose tissue and plasma. Am. J. Epidemiol. 2005;162(4):373–381. doi: 10.1093/aje/kwi213. [DOI] [PubMed] [Google Scholar]
- 32.Institute of Medicine. Nutrition During Pregnancy. vol. I. Washington, DC: National Academy Press; 1990. p. 428. [Google Scholar]
- 33.Rifas-Shiman SL, Rich-Edwards JW, Kleinman KP, Oken E, Gillman MW. Predictors of dietary quality during the 1st trimester of pregnancy. Pediatr. Res. 2005;58(5):1120–1121. [Google Scholar]
- 34.Thomas B, Ghebremeskel K, Lowy C, Crawford M, Offley-Shore B. Nutrient intake of women with and without gestational diabetes with a specific focus on fatty acids. Nutrition. 2006;22(3):230–236. doi: 10.1016/j.nut.2005.07.017. [DOI] [PubMed] [Google Scholar]
- 35.Benisek D, Shabert J, Skornik R. Dietary intake of polyunsaturated fatty acids by pregnant or lactating women in the United States. Obstet. Gynecol. 2000;95(4 (Suppl.)):77S–78S. [Google Scholar]
- 36.Cordain L, Eaton SB, Miller JB, Mann N, Hill K. The paradoxical nature of hunter-gatherer diets. Eur. J. Clin. Nutr. 2002;56(Suppl. 1):S42–S52. doi: 10.1038/sj.ejcn.1601353. [DOI] [PubMed] [Google Scholar]
- 37.Simopoulos AP. Evolutionary aspects of diet, the omega-6/omega-3 ratio and genetic variation: nutritional implications for chronic diseases. Biomed. Pharmacother. 2006;60(9):502–507. doi: 10.1016/j.biopha.2006.07.080. [DOI] [PubMed] [Google Scholar]
- 38.Vlaardingerbroek H, Hornstra G. Essential fatty acids in erythrocyte phospholipids during pregnancy and at delivery in mothers and their neonates: comparison with plasma phospholipids. Prostaglandins Leukot. Essent. Fatty Acids. 2004;71(6):363–374. doi: 10.1016/j.plefa.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 39.Siega-Riz AM, Bodnar LM, Savitz DA. What are pregnant women eating? Nutrient and food group differences by race. Am. J. Obstet. Gynecol. 2002;186(3):480–486. doi: 10.1067/mob.2002.121078. [DOI] [PubMed] [Google Scholar]
- 40.Oken E, Ning Y, Rifas-Shiman SL, Rich-Edwards JW, Olsen SF, Gillman MW. Diet during pregnancy and risk of preeclampsia or gestational hypertension. Ann. Epidemiol. 2007;17(9):663–668. doi: 10.1016/j.annepidem.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Radesky JS, Oken E, Rifas-Shiman SL, Kleinman KP, Rich-Edwards JW, Gillman MW. Diet during early pregnancy and development of gestational diabetes. Paediatr. Perinat. Epidemiol. 2008;22(1):47–59. doi: 10.1111/j.1365-3016.2007.00899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.