Abstract
The blood-brain barrier (BBB) provides a vast interface for cytokines to affect CNS function. The BBB is a target for therapeutic intervention. It is essential, therefore, to understand how cytokines interact with each other at the level of the BBB and how secondary signals modulate CNS functions beyond the BBB. The interactions between cytokines and lipids, however, have not been fully addressed at the level of the BBB. Here, we summarize current understanding of the localization of cytokine receptors and transporters in specific membrane microdomains, particularly lipid rafts, on the luminal (apical) surface of the microvascular endothelial cells composing the BBB. We then illustrate the clinical context of cytokine effects on the BBB by neuroendocrine regulation and amplification of inflammatory signals. Two unusual aspects discussed are signaling crosstalk by different classes of cytokines and genetic regulation of drug efflux transporters. We also introduce a novel area of focus on how cytokines may act through nuclear hormone receptors to modulate efflux transporters and other targets. A specific example discussed is the ATP-binding cassette transporter-1 (ABCA-1) that regulates lipid metabolism. Overall, cytokine signaling at the level of the BBB is a crucial feature of the dynamic regulation that can rapidly change BBB function and affect brain health and disease.
Keywords: Cytokines, blood-brain barrier, TNF, IL-15, leptin, adipokines, NFκB, STAT3, promoter analysis, ABCA-1, P-gp
1. INTRODUCTION: THE BBB FOR CYTOKINES AND RELATED PEPTIDES
The blood-brain barrier (BBB) is an extensive three-dimensional interface between the brain and the blood vessels carrying material from the rest of the body for selective exchange of information. The BBB is affected by essentially all neurological diseases, and it in turn modulates disease processes. It is also a delivery interface for therapeutic molecules. The structural backbone of the BBB is a monolayer of microvascular endothelial cells that are joined by tight junctions and underlined by a continuous basement membrane. The endothelia are polarized, and they have more mitochondria and peptidase activity but fewer endocytic vesicles than their counterparts in non-BBB structures The BBB is reinforced by astrocytic endfeet, pericytes, microglia, and extracellular matrix. It protects the brain from harmful toxins by acting as a physical, enzymatic, and transport barrier between the vasculature and CNS parenchyma. The presence of high resistance tight junction proteins, which seal adjacent endothelial cells, results in physical restrictions [1-4]. Abundant degradative enzymes and scarce endocytic vesicles accelerate the biotransformation and clearance of substances that cannot cross the BBB, thereby forming a chemical barrier [5]. In the absence of a specific transport system, the physicochemical properties most important in determining the permeability of a substance across the BBB include its lipophilicity, conformation, and charge.
Several classes of influx and efflux transporters located on the luminal and abluminal sides of the brain endothelia regulate the transport of both endogenous and exogenous molecules in and out of brain parenchyma. Some of the essential nutrients and small proteins enter through influx transporters. This may involve receptor-mediated transport or facilitated entry by carrier proteins, while others may be barred outside the BBB or reach the CNS by simple diffusion [6,7]. Because of the presence of efflux transporters at the BBB endothelia, several endogenous compounds and drugs, including anticancer, antiviral, antibacterial, antiepileptic, and analgesic substances, or drugs used to treat neurodegenerative disease, have limited CNS uptake [8-10].
The BBB is a regulatory interface in response to cytokines. Functioning one way, the BBB can selectively transport several cytokines. This includes interleukin (IL)-1α and IL-1β [11-14], IL-1 receptor antagonist (IL-1ra) [15], IL-6 [16], tumor necrosis factor-α (TNF) [17-21], leukemia inhibitory factor (LIF) [22-24], cilliary neurotrophic factor [25], and many adipokines (see reviews [26-28]). The transported cytokines play important roles in the physiological response to inflammation and neuroregeneration [29-31]. Functioning another way, the BBB restricts some cytokines from crossing the BBB. Transforming growth factor (TGF)-α accumulates in the cerebral vasculature [32], and TGF-β1 does not enter the brain [33]. By contrast, epidermal growth factor (EGF), which may compete with TGF-α for receptor binding, rapidly crosses the BBB [34]. Moreover, many cytokines and peptides are degraded before reaching CNS parenchyma. A lack of permeation, however, does not prevent cytokines from affecting cerebral vascular functions, including modification of tight junction structures and endothelial signaling [26,35].
Many cytokines are produced both within the CNS and in the periphery. BBB transport assays for both influx and efflux pathways usually show directionality of cytokine transport [36-38]. This indicates polarized distribution of transporters, which may or may not be their receptors. The structure of the ligand appears to play a role in different steps of endocytosis and trafficking [39-43]. Regional differences in metabolic activity, particularly the abundance of degrading enzymes, also determine the availability of a peptide to the CNS [44].
In the Transwell culture system of primary cerebral microvessel endothelial cells, lipopolysaccharide (LPS) induces polarized release of cytokines either to the apical or basolateral chamber [45]. This indicates that exocytotic mechanisms may also differ among cytokines.
Polarized distribution of transporters is best known for efflux drug transporters [3,10]. For neuroinflammation, a good example is chemokine CXCL12. CXCL12 is localized to the basolateral surface of the normal BBB and functions to prevent leukocyte extravasation into the CNS. In active lesions of multiple sclerosis, however, CXCL12 is redistributed to the luminal side and is associated with activation (phosphorylation) of its receptor CXCR4 in infiltrating leukocytes [46]. This is reminiscent of the redistribution of tight junction proteins in inflammatory pain and other insults causing increased permeability of the BBB [47]. The field is at the beginning of understanding the cellular mechanisms mediating such changes [48]. In this review, we summarize recent work on cytokine signaling at the BBB and present new players and potential targets, including lipid rafts and nuclear hormone receptors.
2. CYTOKINE RECEPTORS AND ENDOTHELIAL MEMBRANE MICRODOMAINS
Cytokine receptors are localized in membrane microdomains and undergo ligand-dependent or independent endocytosis. The endocytic microdomains may include specific coat proteins, lipids, neither, or both. Depending on the coating, the membrane domains are either clathrin-coated pits or clathrin-independent lipid rafts. Lipid rafts are enriched in cholesterol and glycosphingolipids, and require sphingolipids for endocytosis [49,50]. Different types of lipid rafts are distinguished by the presence or absence of caveolin [51-53]. Dynamin and actin are major components of most endocytotic pathways. Dynamin is a GTPase mediating fission of vesicles from the plasma membrane [54,55], while actin is a highly conserved globular protein containing an ATP binding site. In addition, specific kinases play a role in activating clathrin- and caveolin-dependent endocytosis [56,57]. Nonetheless, internalization of glycosylphosphatidylinositol (GPI)-anchored proteins does not require dynamin, clathrin, or caveolin [58].
There is morphological evidence that caveolae may play a major role in transcytosis across the BBB [59]. Caveolin-1, the major component of caveolae, is highly expressed in brain microvessel endothelial cells [60]. Caveolin-dependent transcytosis is used by albumin [61], low density lipoprotein [62], and the Duffy antigen receptor along with chemokines to which it binds [63]. Proper functioning of caveolae involves phosphorylation of caveolin at the tyrosine residue Y [14] by src kinase to enable caveolae formation [64] and interaction of phosphorylated caveolin-1 with filamin A which in turn facilitates internalization and trafficking of caveolae [65]. Higher transcytosis activity through the BBB after brain injury correlates well with increased expression of phosphorylated caveolin-1 [66]. This emphasizes a role of caveolae in transport processes in brain microvessel endothelia.
Some cytokines and their receptors are endocytosed in a caveolin-dependent manner. In β cells, IL-1β and IL-1R1 induce phosphorylation of caveolin and subsequent caveolin-dependent endocytosis [67]. Accumulation of cytokines in lipid rafts may facilitate cell signaling. In astrocytes, for example, IL-1β induces translocation of IL-1R1 and recruitment of signaling molecules to caveolin-enriched lipid rafts. This is followed by caveolin-dependent endocytosis [68]. Intracellular signaling as a consequence of caveolin-dependent endocytosis is seen both in endothelia and other cells, such as human breast adenocarcinoma MCF-7 cells where inhibition of caveolin-dependent endocytosis abolishes IL-1β-dependent nuclear factor (NF)-κB activation [69]. Besides IL-1β, TNF also induces translocation of its receptors - TNFR1 and TNFR2 - to endothelial caveolae [70,71].
Endocytosis through clathrin-coated pits contributes to the termination of signaling by removal of cytokines and hormones and their receptors from the extracellular environment. Membrane receptors and their ligands that are endocytosed in a clathrin-dependent manner appear to be mostly degraded after passage through endocytic vesicles. This is seen for certain growth factors and their receptors which are tyrosine kinases. Nonetheless, rodent studies have shown saturable transport of nerve growth factor (NGF), neurotrophin-3, brain-derived growth factor (BDNF) [72-74], cilliary neurotrophic factor [34], and EGF [25]. Insulin-like growth factor (IGF)-1 also is transported across the BBB [75], and its transport system is not identical to that transporting insulin although there is partial sharing [76]. Even with passive diffusion, a significant amount of growth hormone can enter brain parenchyma in intact form [77,78].
There is a correlation between localization in and endocytosis from a specific microdomain and the intracellular fate of cytokines and their receptors. There are multiple examples: (1) When TNF is endocytosed via the clathrin-dependent pathway, it is degraded in lysosomes [79]; (2) TGFβ does not cross the BBB [33], but TGFβ receptors can be endocytosed by both caveolin- and clathrin-dependent mechanisms, which determine either signal transduction or receptor turnover, respectively [80,81]; (3) in eosinophils, IL-5 receptors are localized in and endocytosed from either clathrin-containing microdomains or lipid rafts. However, only those endocytosed in a clathrin-dependent manner are degraded [82]; (4) IL-7 induces down-regulation of its receptor, IL-7R, by rapid clathrin-dependent endocytosis and degradation [83]. Similarly, alternative mechanisms of receptor endocytosis may exist for growth factors and their receptors [84]. Nonetheless, the fate of a cytokine and its receptor may be distinctively different. Chemokines and their receptors are endocytosed in a clathrin-dependent manner, but chemokines are targeted to degradation whereas their receptors are recycled back to the membrane [85]. Leptin and its receptors are found in clathrin-coated pits. Both are endocytosed in a clathrin-dependent manner [86-88], but leptin may be either degraded intracellularly or exit the cell in intact form [89].
Lipid rafts lacking clathrin and caveolin are a third kind of microdomain important for cytokine receptors. Examples of clathrin- and caveolin- independent endocytosis are corticotropin-releasing hormone (CRH) receptors I and II [90] and the well studied cytokine receptors for IL-2Rβ and IL-2Rγ, receptors shared by IL-2 and IL-15. In T lymphoma cells, IL-15Rα, IL-2Rα, IL-2Rβ, and IL-2Rγ co-localize in lipid rafts [91]. Both IL-2Rβ and IL-2Rγ endocytosis requires dynamin, cortactin, and actin [92-94] as well as kinases that regulate actin polymerization [95,96]. Neither IL-15Rα nor IL-2Rα is effectively endocytosed [97], but rather rapidly recycle to the membrane via recycling endosomes. To date, the intracellular fate of cytokine receptors is poorly understood, although studies have addressed different aspects of endocytosis [93,98,99], recycling [99,100], and degradation by both lysosomes and proteasomes [101,102].
Taken together, cytokines and their receptors can accumulate in membrane microdomains, and cytokines can even induce translocation of their receptors into specific microdomains. The nature of the microdomain, then, may determine the fate of cytokines and their receptors after endocytosis, but little is known about the role of membrane microdomains for cytokine receptor function in endothelial cells of the BBB. Assuming that fundamental principles of cytokine receptor compartmentalization and its physiological significance are shared between tissues, much can be expected from further exploration of this subject in the BBB.
3. THE CONTEXT OF NEUROENDOCRINE REGULATION
The effects of cytokine signaling across the BBB on neuroendocrine regulation are best examplified by leptin. Leptin is the main cytokine exerting neuroendocrine regulation in the CNS on ingestion and presents close structural similarities with members of the long-chain helical cytokine family. Acting through the leptin receptor (ObR, or LR), a member of the class I cytokine receptor super-family, it controls food intake and energy expenditure by influencing several hypothalamic peptides.
To do so, leptin must cross the BBB from its primary origin in adipocytes. Although larger (16 kDa) than many smaller substances that do not cross, leptin not only enters the brain from blood in intact form, but does so by a saturable transport system [103,104]. This system is decreased by fasting [105], triglycerides [106], and the neonatal period of development [107]. It is increased by glucose [108], and is partially saturated in normal as well as obese animals [109,110]. The saturable transport system for leptin is also evident in vitro [111] and although primarily mediated by the ObRa isoform of the leptin receptor [112], it can also be mediated by ObRb, ObRc, and ObRd [89] and inhibited by the soluble receptor ObRe [113]. Even a tailless form of ObR can function as a transporting receptor [88]. In vivo, BBB transport of leptin shows regional [109,114] and diurnal variation [115] and is supplemented by a high capacity transport system at the choroid plexus [114]. A recent comprehensive study by Adam and Finlay clearly establishes the BBB as the primary site of leptin resistance [116], explaining why increased blood concentrations of leptin do not decrease food intake even though brain injections of leptin do so [117,118].
The neuroendocrine effects of leptin are exerted through several peptides. First affected by leptin in the arcuate nucleus of the hypothalamus are neuropeptide Y (NPY), agouti-related protein (AgRP), cocaine- and amphetamine-regulated transcript (CART), and proopiomelanocortin which is the precursor molecule for α-melanocyte stimulating hormone (MSH, melanocortin). In the lateral hypothalamus, orexin and melanocortin concentrating hormone (MCH) become involved, as can CRH and oxytocin in the paraventricular nucelus. Unlike leptin, which decreases food intake, NPY increases ingestion, and also unlike leptin, NPY enters the brain from blood by passive diffusion that is not saturable [119]. Most hypothalamic peptides enter the brain by passive diffusion based on physicochemical properties such as lipophilicity, hydrogen bonding, and conformation rather than by a saturable transport mechanism. These include AgRP [120], CART [121], α-MSH [122], orexin A [42], and urocortin II [123]. Some ingestive peptides do not seem to enter at all, including MCH [124], orexin B [42], and obestatin [125]. The neuroendocrine actions of leptin after it passes the BBB are mediated primarily by the ObRb isoform of the ObR leptin receptor through a Signal Transducer and Activation of Transcription (STAT)-3 mechanism [126]. This STAT3 signaling can be modified by Cdk5/p35 kinases [127], and, unexpectedly, by urocortin [128] and α-MSH [129], even though the main actions of these two peptides are through G-protein coupled receptors (GPCR) and cyclic AMP. Another overlooked observation is that adult onset obesity, either genetic [130] or diet-induced [131], induces astrocytic ObR [132] in dynamic relation with neuronal ObR.
But how does selective ablation of endothelial leptin signaling affect the CNS response to metabolic challenge? To determine this, we recently generated and characterized the metabolic phenotype of endothelial specific leptin receptor knockout (ELKO) mice by crossing Tie2-cre mice with ObR-floxed mice (Pan W et al, manuscript in review). The ELKO mice show a better response to diet-induced obesity with less weight and fat gain. They have higher oxygen consumption, carbon dioxide production, and heat dissipation than wildtype mice on a high-fat diet. The usual reduction of brain weight after prolonged exposure to the high-fat diet is also prevented. This partial resistance of ELKO mice to diet-induced obesity, therefore, suggests that endothelial leptin signaling may have a negative impact on obesity control. Overall, endothelial leptin signaling may counterbalance the neuronal effects of leptin.
The interaction of the cytokine leptin with the neuroendocrine peptide urocortin (urocortin I) is different from that of any of the peptides mentioned above and is, so far, unique. Leptin directly enhances the transport of urocortin across the BBB, and this is mediated by the CRH receptors for urocortin [133-136]. The transport system for urocortin is also activated by glucose [137] and TNF [134], the action of TNF requiring both TNF receptors. The release of CRH, structurally related to urocortin, also is affected by several cytokines [138]. Although there is a saturable transport system for CRH both in and out of the brain [123,139], it is not known whether the neuroendocrine actions of cytokines on CRH are exerted directly at the BBB. Thus, cytokines like leptin affect the neuroendocrine system both by direct and indirect actions.
4. RELAY OF NEUROINFLAMMATORY SIGNALS AND COOPERATION OF SIGNALING PATHWAYS ACTIVATED BY DIFFERENT LIGANDS
The BBB prevents the entry of some substances, allows passage of others, and generates secondary signals to affect CNS functions when interacting with a substance that may not cross the BBB. Perhaps the best characterized saturable transport system for cytokines is that for TNF in both mice [17,140] and rats [19]. The transport is receptor dependent [141], and abolished in knockout mice lacking both p55 (p60) and p75 (p80) TNF receptors [142]. There is species specificity in that the murine p55 receptor binds to murine TNF and human TNF with similar affinity, but the murine p75 receptor has strong species specificity for murine TNF [143]. Caveolae are involved in TNF endocytosis in BBB endothelia and eventual transport across the BBB from the luminal to abluminal side [71]. Different disorders, including experimental autoimmune encephalomyelitis [18], spinal cord injury [144-148], brain trauma [149], and stroke [150] induce region- and time-dependent upregulation of TNF transport. The details for TNF have been reviewed in earlier publications [20,151], and transport of cytokines in general across the BBB has been reviewed extensively elsewhere [7,21,152].
It is well-known that BBB endothelia respond to inflammatory stimuli by generation of vasoactive substances, including endothelin-1 [153], prostaglandins, leukotrienes, and platelet-activating factor [154]. There is diverse intracellular signaling in response to different cytokines. For example, IL1β induces the activation of MyD88 [155], and IFNγ, IL-4, and IL-6 activate STAT1, STAT6, and STAT3, respectively, in hCMEC/D3 cells [156]. The stimuli may be generated by adjacent cells. Src-suppressed C-kinase substrate is a strong inducer of BBB function when overexpressed in astrocytes [157]. Perivascular macrophages mediate the effects of IL-1 on endothelial prostanoid production and thus provide conditioning to neuroinflammation [158]. The response of the BBB to inflammatory cells, neuroAIDS in particular, has been reviewed extensively elsewhere [159-161].
The BBB produces chemokines and cytokines in response to injury and inflammation [154,162,163]. In monkey brain endothelia, IL-1β, LPS, and hypoxia can stimulate the release of IL-6, and the effect is greater in endothelia from aged animals [164]. In the RBE4 cerebral endothelial cell line, TNF increases the expression of IL-15 and its receptors [165]. The best demonstration of polarized response to date is a Transwell study by Banks’ group, in which secretion of IL-1α, IL-10, granulocyte-macrophase colony-stimulating factor, IL-6, and TNF is shown under basal conditions and after LPS challenge. LPS stimulates more luminal than abluminal secretion of cytokines, but it is more effective when applied to the abluminal side [45]. Polarized function is determined by lateralized distribution of membrane microdomains, in particular lipid rafts, and other related structures [166].
For cytokines that cross the BBB by receptor-mediated transport, such as TNF [17,141,142,148,151], LIF [22,23], and leptin [88,89,103,107,112,115,167], the transporting receptor does not appear to differ from the signaling receptor. How does signaling affect the course of transcytosis? The data are still lacking, but it is clear that cellular signaling by one cytokine can modify the transport of another. In RBE4 cells treated with TNF, the expression of gp190, the specific receptor for LIF, is downregulated [168]. The gp130 co-receptor shared by the IL-6 family of cytokines, however, is upregulated [169]. The effect involves NFκB activation and increased proteasome activity. Nonetheless, the net effect is reduced LIF endocytosis [168]. By contrast, TNF and leptin can both facilitate the passage of urocortin into the brain without compromising the general permeability of the BBB. This increase is seen in both mice and cultured endothelial cells. Since urocortin uptake shows self-inhibition, it suggests activation of an innate transport system [28,90,133,170,171]. This is reminiscent of glucose-induced activation of urocortin transport [137].
Effects of cytokines on enhancing neuroinflammation by their interactions with the BBB also include impairment of tight junction activity, increase of paracellular permeation, facilitation of leukocyte migration, and induction of adsorptive endocytosis, as has been extensively reviewed recently [172,173].
Interactions of peptidergic signals at the BBB are illustrated by crosstalk between leptin and urocortin in the activation of STAT-1 and STAT-3. Although leptin activates urocortin transport across the BBB, urocortin potentiates leptin-induced STAT3 activation in RBE4 cerebral endothelial cells as well as HEK293 cells used as an initial model system. Urocortin can act through either receptor, CRHR-1 or CRHR-2, to increase STAT3-luciferase activity, but shows no effect on STAT1 activation. Leptin, on the other hand, can increase both STAT1 and STAT3 activation in cells overex-pressing the long transmembrane receptor isoform ObRb. When both ligands and receptor systems are present, both HEK293 cells and RBE4 cerebral endothelial cells show a greater increase of STAT1 and STAT3 activation than with either ligand alone. By contrast, leptin does not enhance cAMP production downstream to urocortin activation [128]. Though not yet tested in neurons expressing both receptors, one may anticipate that urocortin could provide partial compensation for the loss of signaling sensitivity in obese subjects with leptin resistance.
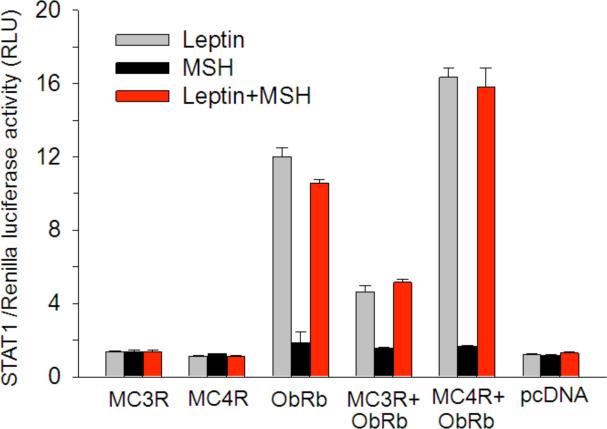
To further test this, crosstalk between leptin and α-MSH was determined by a similar approach. α-MSH binds to the melanocortin receptors (MC)-3R and MC-4R in the hypothalmus. Although ObRb, MC3R, and MC4R transcripts are all detectable in enriched cerebral microvessels, basal activation of STAT3 is low. In cells overexpressing ObRb and MC3R or MC4R, α-MSH potentiates leptin-induced STAT3 activation shown both by western blotting and luciferase reporter assays. The blocking effect of a specific MEK inhibitor suggests that the mitogen-activated kinase pathway is involved in the crosstalk. This contrasts with the lack of effect of a phosphoinositide-3 kinasee (PI3K) inhibitor. Similarly, there is a potentiating effect of α-MSH on leptin-induced STAT1 activation (Fig. 1). Like the urocortin-leptin interaction, leptin fails to further increase cAMP production induced by α-MSH in these cells. It is yet to be determined whether this represents a general principle that other G protein-coupled seven-transmembrane receptors also facilitate leptin-induced STAT3 signaling in the same cells [129].
Fig. (1).
Effects of leptin and αMSH on STAT1 activation in HEK293 cells transfected with MC3R, MC4R, ObRb or control pcDNA plasmids. Leptin induced STAT1 activation only in cells overexpressing ObRb. MSH alone had no effect on STAT1 activation. Co-transfection of MC3R or MC4R along with ObRb plasmid had differential effects on leptin-induced STAT1 activity in that MC3R overexpression decreased, whereas MC4R overexpression increased, leptin-induced STAT1. The same change was seen in cells treated with both leptin and αMSH (n = 3 /group).
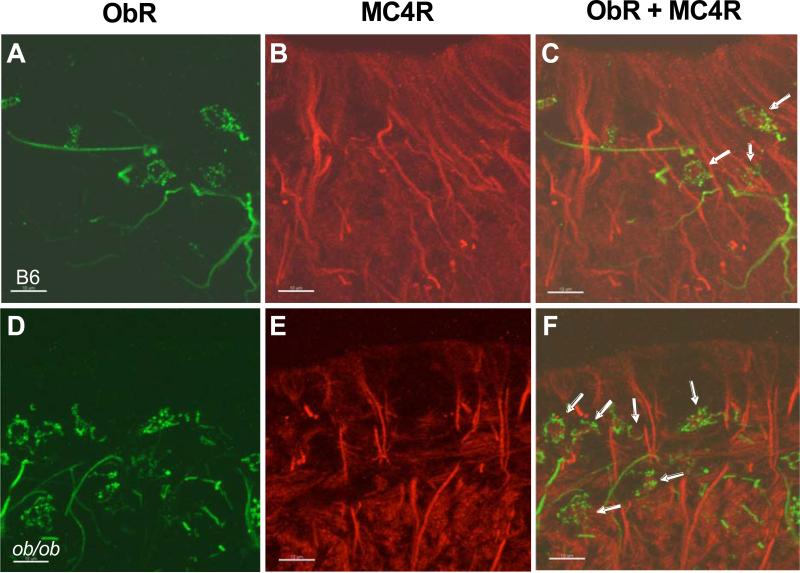
In the hypothalamus, we have not observed co-localization of ObR and melanocortin receptors except for the close interaction of MC4R fibers with ObR (+) neurons and astrocytes in the median eminence. Nevertheless, in ob/ob mice with their genetic loss of leptin production, there is an increase of ObR(+) neurons in the median eminence (Fig. 2). This suggests that obesity and nutrition-related disorders might change the interaction between leptin and α-MSH signaling crosstalk, at least in the median eminence. In terms of the regulatory changes of MC4R in the median eminence and adjacent hypothalamic regions, food restriction for 10 d and the fa/fa Zucker rat mutation both increase the density of MC4R in autoradiographs. These animal models have increased hunger, and appear to have receptor upregulation secondary to decreased release of α-MSH. By contrast, diet-induced obesity decreases the density of MC4R and thus shows an opposite regulatory mechanism [174]. Activation of MC4-R thus serves as a physiological regulator of feeding. Leptin also acts on the median eminence, shown by its dose-dependent effect in modulating the release of luteinizing hormone releasing hormone from median eminence-arcuate nucleus explants [175]. Although anatomical results show little spatial proximity of the two types of receptors, cellular assays in GT1-7 neurons of mouse hypothalamic origin indicate that α-MSH indeed potentiates leptin-induced STAT3 activation when both MC4R and ObRb are overexpressed (unpublished observations).
Fig. (2).
Confocal imaging showing the coexistence of MC4R and ObR in the median eminence of the mouse hypothalamus. MC4R (red) expression exhibited intense fiber-like staining and intermittent diffuse signaling in both wildtype (A) and ob/ob (D) mice. ObR (green) expression in wildtype mice (B) showed fiber and membrane-localized punctate staining, whereas ObR staining in ob/ob mice was more intense (E). The merged picture of MC4R and ObR staining shows the coexistence of both receptors in neuron-like cells (arrows) in the median eminence of both wildtype (C) and ob/ob mice (F). Scale bar: 10 μm.
5. INTERACTIONS OF CYTOKINES AND NUCLEAR HORMONE RECEPTORS AT THE BBB
Nuclear hormone receptors are ligand-activated transcription factors. Their ligands are hormones such as estrogen, progesterone, testosterone, and glucocorticoids, as well as retinoic acids and oxysterols. These hormones bind to receptors and regulate the expression of genes crucial for growth, metabolism, and many other vital functions. Many nuclear receptors are targets for drugs.
Few results describe nuclear receptor function in endothelial cells of the BBB. RT-PCR analysis shows that liver X receptors (LXR)α and LXRβ, retinoid X receptors (RXR), and peroxisome proliferator-activated receptors (PPAR)α and PPARβ are detectable in brain capillary fractions of 8 week old rats and in the brain endothelial cell line TR-BBB13 [176]. All three receptor types regulate the expression of genes for lipid metabolism and transport including ATP binding cassette (ABC) and scavenger receptor (SR-BI) transporters. In brain endothelial cells, activation of LXRs and PPARs induces ABC transporter A1 (ABCA1) and SR-BI mRNA levels [176-178]. In contrast with BBB endothelia, neurons in the hypothalamus, thalamus, amygdala, cholinergic basal forebrain, and hippocampus show high expression of ABCA1. Oxysterols (ligand for LXR) and 9-cis-retinoic acid (ligand for RXR) induce ABCA1 expression in cultured neurons and glial cells and increase the ef-flux of apoA-I- and apoE-specific cholesterol from the cells. Activation of LXR and RXR might also decrease amyloid β (Aβ) protein production and thereby decrease the amyloid burden in brain [179]. However, ABCA1 knockout mice do not show changes in A 1-40 efflux across the BBB [180]. Nonetheless, ABCA1 knockout mice are resistant to cerebral malaria, a brain disease resulting from sequestration of Plasmodium falciparum-parasitized red blood cells, leukocytes, and platelets within brain microvessels, and excessive cytokine release. This suggests that a deficit in ABCA1 prevents vesiculation upon infection by Plasmodium berghei ANKA [181] and supports a role of ABCA1 in the interactions of the intracellular pathogen with the BBB.
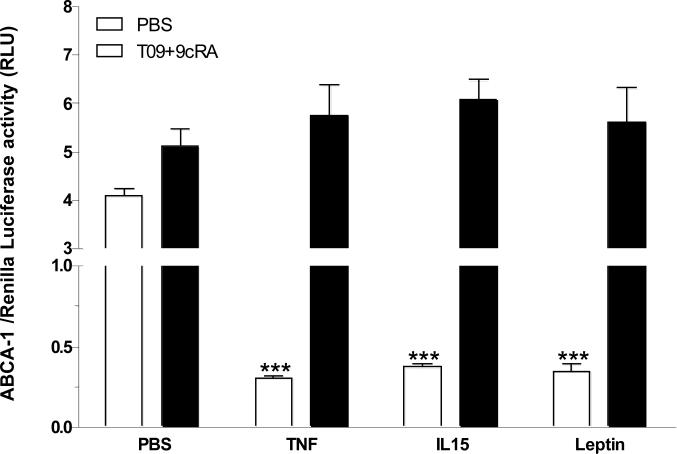
Interestingly, TNF, IL15, and leptin all depress ABCA-1 promoter luciferase activity [182] in RBE4 cerebral endothelial cells transfected with luciferase reporter plasmids (Fig. 3). In these cells, co-treatment with a synthetic ligand for LXR, T0903017 (T09), combined with the RXR ligand 9-cis-retinoic acid (9cRA) induces ABCA-1 promoter activity independently of cytokine treatment (Fig. 3, solid bars), indicating that LXR and RXR do not mediate the actions of cytokines to depress ABCA-1 activation. Cytokine effects may be mediated by either weak potential STAT binding elements or the inhibitory E-box that are both present in the reporter plasmid. Regardless of the mediating mechanisms, there is no doubt that cytokine signaling modulates cholesterol transporter expression and function. It remains possible that other nuclear hormone receptors are targets for cytokines, such as the glucocorticoid receptor (GR) that is present in brain microvessel endothelial cells (BMEC) and shows different modulation by glucocorticoids than in non-BMEC endothelial cells [183].
Fig. (3).
Effect of cytokines on ABCA-1 transporter promoter activity in RBE4 cells transfected with ABCA-1 luciferase promoter and control Renilla luciferase plasmids. Basal activity was significantly reduced when the cells were treated with TNF (5ng/ml), IL-15 (5ng/ml), or leptin 0.8μg/ml for 6 h (empty bars). Co-treatment of the cells with LXR ligand T0901317 (T09; 1 μM) and RXR ligand 9-cis retinoic acid (9cRA; 100 nM) induced a small but significant increase of ABCA-1 promoter activity in the phosphate-buffered saline (PBS) control group. Cytokine treatment did not affect this activation pattern (solid bars). ***: p < 0.005 in comparison with vehicle treatment (n = 3 /group).
Receptor phosphorylation changes the activity of many nuclear receptors, including PPAR [184], RXR [185], and the steroid hormone receptors for estrogen, progesterone, androgens, mineralocorticoids, and glucocorticoids [186]. For example, p38 mitogen-activated protein kinase (MAPK) and c-Jun N-terminal kinase modulate glucocorticoid receptor (GR) functions [187,188], and p38 MAPK phosphorylates PPARα to enhance its ligand-dependent activity [189]. JNK phosphorylates RXR [190]; this correlates with the inhibitory effects of IL1β on RXR function [191]. It is possible that cytokines activate different kinases to change the activities of target nuclear receptors, analogous to the effects of growth factor-activated kinases on nuclear receptor phosphorylation and signaling. In cancer cells, EGF-activated MAPK induces ligand-independent activity of phosphorylated estrogen receptor-α [192], and stimulates ligand-dependent progesterone receptor [193] and androgen receptor activities [194].
Nuclear receptors affect cytokine expression in various cell types. Both PPAR and LXR are anti-inflammatory. PPARs inhibit expression of inflammatory cytokines, chemokines, and cell-adhesion molecules through transcriptional repression of Toll-like receptor target genes [195]. LXR agonists modulate the expression of TNF, IL1, and NFκB in ileum tissue after ischemia [196]. Potential mechanisms include interference with NFκB pathways as shown in microglia and astrocytes [197], binding to a LXR response element in the TNF gene promoter as shown in monocytes [198], and modulation of posttranslational regulation of TNF as shown in Kupffer cells [199]. Similarly, estrogens can regulate the expression of pro-inflammatory cytokines [200,201].
Tight junctions are the main structural and functional elements regulating BBB integrity. Several nuclear receptors exert their anti-inflammatory actions by modulating the effects of inflammatory cytokines on the expression of tight junction proteins, including occludin and claudin. Whereas TNF reduces the expression of claudin-5, both the GR ligand dexamethasone and the estrogen receptor ligand estradiol increase its expression in cerebral but not myocardial endothelial cells [202,203]. The presence of a GR response element in the promoter region of occludin allows the GR ligand hydrocortisone to regulate occludin expression [202,204]. Progesterone acts through its receptor to inhibit the inflammatory response after stroke. This is achieved by reducing TNF expression and increasing claudin expression [205]. Nuclear receptors can also regulate tight junctions by inhibiting matrix metalloprotease (MMP)-9, a collagenase that digests components of basal lamina. The GR ligand dexamethasone induces the MMP inhibitor TIMP-1 in BMEC, thus reducing disruption of the BBB in neuroinflammatory conditions [206]. PPARα and PPARγ also protect the BBB by down-regulating MMP and proteasome activities [207]. In addition, activation of PPARγ inhibits monocyte adhesion to and migration across brain endothelia by inhibiting Rac1 and RhoA kinases [208]. Similarly, a PPARα agonist reduces the extent of barrier damage and paracellular permeability of the endothelial cell monolayer after hypoxic challenge [209].
Taken together, nuclear receptors are expressed in endothelial cells and play a role in tight junction protein, cytokine, and transporter expression. Signaling cascades initiated by cytokines may regulate nuclear receptor functions through phosphorylation. Therefore, the crosstalk between cytokine and nuclear receptor signaling is potentially important for regulating the functions of the BBB.
6. EFFECTS OF CYTOKINES ON EFFLUX DRUG TRANSPORTERS
The BBB endothelium is enriched with efflux transporters, which act as a transport barrier to both drugs and endogenous compounds entering the brain parenchyma. The primary active efflux pumps include P-glycoprotein (P-gp; ABCB1), breast cancer resistant protein (BCRP; ABCG2), and multidrug resistance associated proteins (MRP; ABCC1-6) belonging to the ABC family of transporters. The presence of these transporters has been implicated in limiting the entry of a broad range of drugs, including anticancer, antiviral, antibacterial, antiepileptic and analgesic substances [8,9]. In brain endothelial cells, P-gp is primarily located on the luminal side of the vasculature and on astrocytic end feet at the abluminal side of the BBB [210]. In addition, other brain structures also express P-gp, including the microglia of brain parenchyma and endothelia at the choroid plexus (blood-CSF barrier) [211-213]. P-gp is considered to be the primary transporter within this class given its capability to bind and efflux substrates with a wide variety of structural differences [214-216]. Subsequently, different classes of drugs ranging from anticancer to antiepileptic have been implicated as substrates of P-gp in various tissues, The regulation of P-gp expression and transport function is complex and varies depending upon the pathological condition [9]. Similar to P-gp, both BCRP and several isoforms of MRP are present on the luminal side of the BBB.
During inflammation, various cytokines, including TNF, IL-1 β, and IL-6 have been shown to modify the functional activity and expression of different efflux transporters [216]. The effects of TNF on multidrug resistance (MDR) proteins are probably the best studied. In RBE4 cells treated with TNF, microarray analysis showed that MDR1b is among the genes that have the highest level of upregulation. The increase of the MDR1b product P-gp was verified by qPCR and western blotting analyses. Consistent with this, cellular uptake of the P-gp substrate vinblastine is decreased [217]. In isolated rat brain capillaries, TNF shows biphasic effects on the expression and activity of P-gp, with low doses decreasing but longer exposure (6 h) increasing the expression and activity of P-gp. This is probably explained by differences in the signaling pathways activated [218]. At even longer exposure (72 h) to TNF, human endothelial cells show an increase of P-gp expression without a change in transport activity. By contrast, there was significant downregulation of BCRP after treatment with IL-1 β, IL-6, and TNF in BMEC, with concurrent reduction in drug uptake [219]. In another study, IL-1 β decreased the expression and activity of P-gp in vitro [220]. The findings indicate that cytokines could exert dose-and time-dependent modulation of efflux drug transporters.
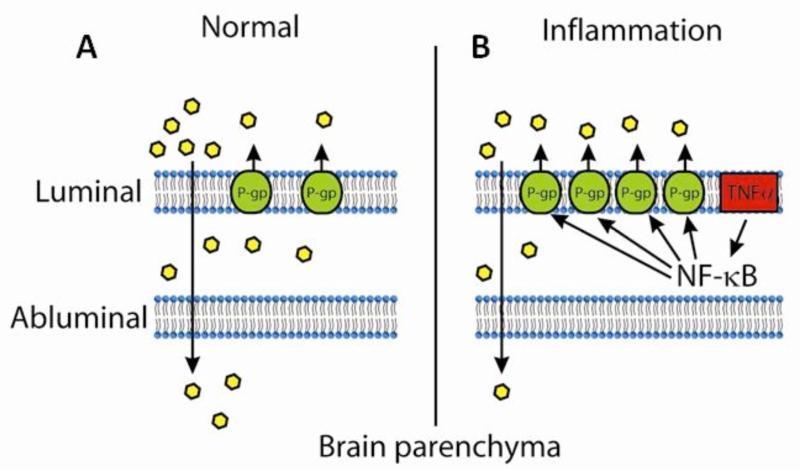
The activation of P-gp by TNF involves NFκB signaling (Fig. 4). In RBE4 cells transfected with luciferase constructs of various regions of rat MDR1b promoter, we showed that the maximal basal promoter activity is located within 476 bp upstream of the mdr1b transcriptional initiation site. In these cells, TNF induces NFκB translocation to the nucleus and increases promoter activity. Quinazoline, an inhibitor of NFκB activity, dampens the response. Deletion of the NFκB binding site completely abolishes the effect of TNF, whereas deletion of the p53 binding site has no effect. This suggests that the NFκB binding site of the mdr1b promoter is solely responsible for TNF-activation and most of the basal gene transcription. Both electrophoretic mobility shift and chromatin immunoprecipitation assays confirm the binding of the p65 subunit of NFκB to nuclear DNA from RBE4 cells [217]. An essential role of NFκB signaling is further shown in BMEC from wildtype and knockout mice with deletion of the p50 subunit of NFκB. In wild-type mice, LPS induces a mild increase of MDR1a mRNA and a robust increase of MDR1b mRNA, and it elevates the protein expression of P-gp which is a product of both genes. There is no effect of p50 knockout on the LPS-induced increase of P-gp. Rather, the uptake of vinblastine is increased by LPS in the knockout mice, indicating an inhibition of P-gp activity. Thus, a long duration and high dose of LPS treatment depresses, not increases, P-gp function with the help of NFκB [221]. In both situations, NFκB is a crucial modulator of P-gp function and a potential therapeutic target.
Fig. (4).
Schematic representation of regulation of P-gp during inflammation: Under normal physiological conditions, P-gp present on the luminal side of the BBB limits the brain uptake of drugs. In inflammatory diseases, TNF-α activates NFκB, which leads to transcriptional activation of P-gp. This leads to increased activity of P-gp and in turn causes further decrease in the brain penetration of drugs.
7. SUMMARY
We mainly reviewed cytokine signaling in BBB endothelial cells and summarized relevant aspects of cytokine transport across the BBB. We emphasized that endothelial membrane microdomains determine cytokine receptor localization, trafficking patterns, and the fate of intracellular degradation or transcytosis. We showed that cytokines alter endothelial function with several potential outcomes: increased permeability, generation of relaying signaling including another cytokine(s) and soluble mediators potentiating the effects of another cytokine(s), and modulation of efflux transporters. The effects of proinflammatory cytokines, particularly TNF and its inducing LPS, are well known to act through NFκB signaling to change P-gp activity at the BBB level. However, the inhibitory effect of TNF, IL-15, and leptin on ABCA-1 to potentially decrease cholesterol efflux is novel and may provide insight into how inflammatory signals cause metabolic disturbance. This is consistent with the neuroendocrine consequences of cytokine interactions with the BBB. Thus, this is an exciting field with new results yet to come.
Interactions of cytokines and the BBB are pertinent to many neurological disorders. Cytokines are intricately involved in autoimmune diseases, infection, inflammation associated with trauma, ischemia, hemorrhage, neurodegeneration, and certain genetic disorders. For instance, Tangier disease is associated with an ABCA1 mutation resulting in defective transport of high-density lipoprotein. Nutritionally related disorders, from obesity to cachexia, represent a special catogory in that many adipokines are cytokines produced by adipose tissue and other organs. Cytokines affect all aspects of brain physiology, including feeding behavior, sleep, thermoregulation, emotion, and memory, and their interactions with the BBB are an integral part of the cytokine network. With discussion of several unique aspects of BBB cytokine signaling and new trends in research, we hope that readers new to the field will be convinced that cytokine-induced BBB signaling is an important therapeutic target for human CNS diseases.
ACKNOWLEDGEMENT
Data included in this review were generated with sponsorship from NIH (DK54880 to AJK, and NS62291 to WP).
REFERENCES
- 1.Begley DJ. Peptides and the blood-brain barrier: the status of our understanding. Ann NY Acad Sci. 1994;739:89–100. doi: 10.1111/j.1749-6632.1994.tb19810.x. [DOI] [PubMed] [Google Scholar]
- 2.Abbott NJ. Dynamics of CNS barriers: evolution, differentiation, and modulation. Cell Mol Neurobiol. 2005;25(1):5–23. doi: 10.1007/s10571-004-1374-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37(1):13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 4.Neuwelt EA. Mechanisms of disease: the blood-brain barrier. Neurosurgery. 2004;54(1):131–40. doi: 10.1227/01.neu.0000097715.11966.8e. [DOI] [PubMed] [Google Scholar]
- 5.Minn A, Ghersi-Egea JF, Perrin R, Leininger B, Siest G. Drug metabolizing enzymes in the brain and cerebral microvessels. Brain Res Brain Res Rev. 1991;16(1):65–82. doi: 10.1016/0165-0173(91)90020-9. [DOI] [PubMed] [Google Scholar]
- 6.Kastin AJ, Pan W, Maness LM, Banks WA. Peptides crossing the blood-brain barrier: some unusual observations. Brain Res. 1999;848:96–100. doi: 10.1016/s0006-8993(99)01961-7. [DOI] [PubMed] [Google Scholar]
- 7.Pan W, Kastin AJ. Cytokine transport across the injured blood-spinal cord barrier. Curr Pharm Des. 2008;14(16):1620–4. doi: 10.2174/138161208784705450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith QR. Drug delivery to brain and the role of carrier-mediated transport. Adv Exp Med Biol. 1993;331:83–93. doi: 10.1007/978-1-4615-2920-0_14. [DOI] [PubMed] [Google Scholar]
- 9.Loscher W, Potschka H. Role of drug efflux transporters in the brain for drug disposition and treatment of brain diseases. Prog Neurobiol. 2005;76(1):22–76. doi: 10.1016/j.pneurobio.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 10.Begley DJ. ABC transporters and the blood-brain barrier. Curr Pharm Des. 2004;10(12):1295–312. doi: 10.2174/1381612043384844. [DOI] [PubMed] [Google Scholar]
- 11.Banks WA, Ortiz L, Plotkin SR, Kastin AJ. Human interleukin (IL)1a, murine IL-1a and murine IL-1b are transported from blood to brain in the mouse by a shared saturable mechanism. J Pharmacol Exp Ther. 1991;259:988–96. [PubMed] [Google Scholar]
- 12.Banks WA, Kastin AJ, Durham DA. Bidirectional transport of interleukin-1 alpha across the blood-brain barrier. Brain Res Bull. 1989;23:433–7. doi: 10.1016/0361-9230(89)90185-8. [DOI] [PubMed] [Google Scholar]
- 13.Banks WA, Kastin AJ, Gutierrez EG. Interleukin-1a in blood has direct access to cortical brain cells. Neurosci Lett. 1993;163:41–4. doi: 10.1016/0304-3940(93)90224-9. [DOI] [PubMed] [Google Scholar]
- 14.Banks WA, Kastin AJ, Ehrensing CA. Blood-borne interleukin-1a is transported across the endothelial blood-spinal cord barrier of mice. J Physiol (Lond) 1994;479:257–64. doi: 10.1113/jphysiol.1994.sp020293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gutierrez EG, Banks WA, Kastin AJ. Blood-borne interleukin-1 receptor antagonist crosses the blood-brain barrier. J Neuroimmunol. 1994;55:153–60. doi: 10.1016/0165-5728(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 16.Banks WA, Kastin AJ, Gutierrez EG. Penetration of interleukin-6 across the murine blood-brain barrier. Neurosci Lett. 1994;179:53–6. doi: 10.1016/0304-3940(94)90933-4. [DOI] [PubMed] [Google Scholar]
- 17.Gutierrez EG, Banks WA, Kastin AJ. Murine tumor necrosis factor alpha is transported from blood to brain in the mouse. J Neuroimmunol. 1993;47:169–76. doi: 10.1016/0165-5728(93)90027-v. [DOI] [PubMed] [Google Scholar]
- 18.Pan W, Banks WA, Kennedy MK, Gutierrez EG, Kastin AJ. Differential permeability of the BBB in acute EAE: enhanced transport of TNF-a. Am J Physiol. 1996;271:E636–42. doi: 10.1152/ajpendo.1996.271.4.E636. [DOI] [PubMed] [Google Scholar]
- 19.Osburg B, Peiser C, Dömling D, Schomburg L, Ko YT, Viogt K, Bickel U. Effect of endotoxin on expression of TNF receptors and transport of TNF-a at the blood-brain barrier of the rat. Am J Physiol. 2002;283:E899–908. doi: 10.1152/ajpendo.00436.2001. [DOI] [PubMed] [Google Scholar]
- 20.Pan W, Kastin AJ. In: In Blood-Spinal Cord and Brain Barriers in Health and Disease. Shanker H, Westman J, editors. Elsevier, Academic Press; San Diego, CA: 2003. pp. 395–407. [Google Scholar]
- 21.Pan W, Xiang S, Tu H, Kastin AJ. In: In Blood-Brain Barrier Interfaces: From Ontogeny to Artificial Barriers. Dermietzel R, Spray DC, Nedergaard M, editors. Wiley-VCH; Weinheim, Germany: 2006. pp. 247–64. [Google Scholar]
- 22.Pan W, Kastin AJ, Brennan JM. Saturable entry of leukemia inhibitory factor from blood to the central nervous system. J Neuroimmunol. 2000;106:172–80. doi: 10.1016/s0165-5728(00)00241-1. [DOI] [PubMed] [Google Scholar]
- 23.Pan W, Cain C, Yu Y, Kastin AJ. Receptor-mediated transport of LIF across blood-spinal cord barrier is upregulated after spinal cord injury. J Neuroimmunol. 2006;174:119–25. doi: 10.1016/j.jneuroim.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 24.Pan W, Yu C, Hsuchou H, Zhang Y, Kastin AJ. Neuroinflammation facilitates LIF entry into brain: role of TNF. Am J Physiol Cell Physiol. 2008;294(6):C1436–42. doi: 10.1152/ajpcell.00489.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pan W, Kastin AJ, Maness LM, Brennan JM. Saturable entry of ciliary neurotrophic factor into brain. Neurosci Lett. 1999;263:69–71. doi: 10.1016/s0304-3940(99)00083-x. [DOI] [PubMed] [Google Scholar]
- 26.Kastin AJ, Pan W. Feeding peptides interact in several ways with the blood-brain barrier. Curr Pharm Des. 2003;9:789–94. doi: 10.2174/1381612033455378. [DOI] [PubMed] [Google Scholar]
- 27.Pan W, Kastin AJ. Adipokines and the blood-brain barrier. Peptides. 2007;28(6):1317–30. doi: 10.1016/j.peptides.2007.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pan W, Kastin AJ. Urocortin and the brain. Prog Neurobiol. 2008;84(2):148–56. doi: 10.1016/j.pneurobio.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Banks WA, Kastin AJ. Interactions between the blood-brain barrier and endogenous peptides: emerging clinical implications. Am J Med Sci. 1988;295:459–65. doi: 10.1097/00000441-198805000-00008. [DOI] [PubMed] [Google Scholar]
- 30.Banks WA, Kastin AJ. Passage of peptides across the blood-brain barrier: pathophysiological perspectives. Life Sci. 1996;59:1923–43. doi: 10.1016/s0024-3205(96)00380-3. [DOI] [PubMed] [Google Scholar]
- 31.Pan W, Kastin AJ. Changing the chemokine gradient: CINC1 crosses the blood-brain barrier. J Neuroimmunol. 2001;115:64–70. doi: 10.1016/s0165-5728(01)00256-9. [DOI] [PubMed] [Google Scholar]
- 32.Pan W, Vallance K, Kastin AJ. TGFa and the blood-brain barrier: accumulation in cerebral vasculature. Exp Neurol. 1999;160:454–9. doi: 10.1006/exnr.1999.7215. [DOI] [PubMed] [Google Scholar]
- 33.Kastin AJ, Akerstrom V, Pan W. Circulating TGF-b1 does not cross the intact blood-brain barrier. J Mol Neurosci. 2003;21:43–8. doi: 10.1385/JMN:21:1:43. [DOI] [PubMed] [Google Scholar]
- 34.Pan W, Kastin AJ. Entry of EGF into brain is rapid and saturable. Peptides. 1999;20:1091–8. doi: 10.1016/s0196-9781(99)00094-7. [DOI] [PubMed] [Google Scholar]
- 35.Banks WA, Kastin AJ. Reversible association of the cytokines MIP-1a and MIP-1b with the endothelia of the blood-brain barrier. Neurosci Lett. 1996;205:202–6. doi: 10.1016/0304-3940(96)12410-1. [DOI] [PubMed] [Google Scholar]
- 36.Banks WA, Kastin AJ. In: In Circulating Regulatory Factors and Neuroendocrine Function. 1 ed Porter JC, Jezova D, editors. Plenum Press; New York: 1990. pp. 59–69. [Google Scholar]
- 37.Banks WA, Kastin AJ, Fasold MB, Barrera CM, Augereau G. Studies of the slow bidirectional transport of iron and transferrin across the blood-brain barrier. Brain Res Bull. 1988;21:881–5. doi: 10.1016/0361-9230(88)90021-4. [DOI] [PubMed] [Google Scholar]
- 38.Banks WA, Kastin AJ. Opposite direction of transport across the blood-brain barrier for Tyr-MIF-1 and MIF-1: comparison with morphine. Peptides. 1994;15:23–9. doi: 10.1016/0196-9781(94)90165-1. [DOI] [PubMed] [Google Scholar]
- 39.Banks WA, Robinson SM, Wolf KM, Bess JW, Jr, Arthur LO. Binding, internalization, and membrane incorporation of human immunodeficiency virus-1 at the blood-brain barrier is differentially regulated. Neuroscience. 2004;128(1):143–53. doi: 10.1016/j.neuroscience.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 40.Maness LM, Banks WA, Podlisny MB, Selkoe DJ, Kastin AJ. Passage of human amyloid b-protein 1-40 across the murine blood-brain barrier. Life Sci. 1994;55(21):1643–50. doi: 10.1016/0024-3205(94)00331-9. [DOI] [PubMed] [Google Scholar]
- 41.Banks WA, Robinson SM, Verma S, Morley JE. Efflux of human and mouse amyloid b proteins 1-40 and 1-42 from brain: impairment in a mouse model of Alzheimer's disease. Neurosci. 2003;121:487–92. doi: 10.1016/s0306-4522(03)00474-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kastin AJ, Akerstrom V. Orexin A but not Orexin B rapidly enters brain from blood by simple diffusion. J Pharmacol Exp Ther. 1999;289:219–23. [PubMed] [Google Scholar]
- 43.Kastin AJ, Akerstrom V, Hackler L, Pan W. Different mechanisms influencing permeation of PDGF-AA and PDGF-BB across the blood-brain barrier. J Neurochem. 2003;87:7–12. doi: 10.1046/j.1471-4159.2003.01933.x. [DOI] [PubMed] [Google Scholar]
- 44.Ueda F, Raja KB, Simpson RJ, Trowbridge IS, Bradbury MW. Rate of 59Fe uptake into brain and cerebrospinal fluid and the influence thereon of antibodies against the transferrin receptor. J Neurochem. 1993;60:106–13. doi: 10.1111/j.1471-4159.1993.tb05828.x. [DOI] [PubMed] [Google Scholar]
- 45.Verma S, Nakaoke R, Dohgu S, Banks WA. Release of cytokines by brain endothelial cells: A polarized response to lipopolysaccharide. Brain Behav. Immun. 2006;20:449–55. doi: 10.1016/j.bbi.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 46.McCandless EE, Piccio L, Woerner BM, Schmidt RE, Rubin JB, Cross AH, Klein RS. Pathological expression of CXCL12 at the blood-brain barrier correlates with severity of multiple sclerosis. Am J Pathol. 2008;172(3):799–808. doi: 10.2353/ajpath.2008.070918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brooks TA, Ocheltree SM, Seelbach MJ, et al. Biphasic cytoarchitecture and functional changes in the BBB induced by chronic inflammatory pain. Brain Res. 2006;1120(1):172–82. doi: 10.1016/j.brainres.2006.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ronaldson PT, Demarco KM, Sanchez-Covarrubias L, Solinsky CM, Davis TP. Transforming growth factor-beta signaling alters substrate permeability and tight junction protein expression at the blood-brain barrier during inflammatory pain. J Cereb. Blood Flow Metab. 2009;29(6):1084–98. doi: 10.1038/jcbfm.2009.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cheng ZJ, Singh RD, Sharma DK, et al. Distinct mechanisms of clathrin-independent endocytosis have unique sphingolipid requirements. Mol Biol Cell. 2006;17(7):3197–210. doi: 10.1091/mbc.E05-12-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Simons K, Gerl MJ. Revitalizing membrane rafts: new tools and insights. Nat Rev Mol Cell Biol. 2010;11(10):688–99. doi: 10.1038/nrm2977. [DOI] [PubMed] [Google Scholar]
- 51.Nichols BJ, Lippincott-Schwartz J. Endocytosis without clathrin coats. Trends Cell Biol. 2001;11(10):406–12. doi: 10.1016/s0962-8924(01)02107-9. [DOI] [PubMed] [Google Scholar]
- 52.Pelkmans L, Burli T, Zerial M, Helenius A. Caveolin-stabilized membrane domains as multifunctional transport and sorting devices in endocytic membrane traffic. Cell. 2004;118(6):767–80. doi: 10.1016/j.cell.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 53.Kirkham M, Parton RG. Clathrin-independent endocytosis: new insights into caveolae and non-caveolar lipid raft carriers. Biochim Biophys Acta. 2005;1746(3):349–63. doi: 10.1016/j.bbamcr.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 54.Nabi IR, Le PU. Caveolae/raft-dependent endocytosis. J Cell Biol. 2003;161(4):673–7. doi: 10.1083/jcb.200302028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schafer DA. Regulating actin dynamics at membranes: a focus on dynamin. Traffic. 2004;5(7):463–9. doi: 10.1111/j.1600-0854.2004.00199.x. [DOI] [PubMed] [Google Scholar]
- 56.Pelkmans L, Fava E, Grabner H, Hannus M, Habermann B, Krausz E, Zerial M. Genome-wide analysis of human kinases in clathrinand caveolae/raft-mediated endocytosis. Nature. 2005;436(7047):78–86. doi: 10.1038/nature03571. [DOI] [PubMed] [Google Scholar]
- 57.Pelkmans L, Zerial M. Kinase-regulated quantal assemblies and kiss-and-run recycling of caveolae. Nature. 2005;436(7047):128–33. doi: 10.1038/nature03866. [DOI] [PubMed] [Google Scholar]
- 58.Sabharanjak S, Sharma P, Parton RG, Mayor S. GPI-anchored proteins are delivered to recycling endosomes via a distinct cdc42-regulated, clathrin-independent pinocytic pathway. Dev Cell. 2002;2(4):411–23. doi: 10.1016/s1534-5807(02)00145-4. [DOI] [PubMed] [Google Scholar]
- 59.Simionescu M, Popov D, Sima A. Endothelial transcytosis in health and disease. Cell Tissue Res. 2009;335(1):27–40. doi: 10.1007/s00441-008-0688-3. [DOI] [PubMed] [Google Scholar]
- 60.Frank PG, Pavlides S, Lisanti MP. Caveolae and transcytosis in endothelial cells: role in atherosclerosis. Cell Tissue Res. 2009;335(1):41–7. doi: 10.1007/s00441-008-0659-8. [DOI] [PubMed] [Google Scholar]
- 61.Predescu D, Vogel SM, Malik AB. Functional and morphological studies of protein transcytosis in continuous endothelia. Am J. Physiol Lung Cell Mol Physiol. 2004;287(5):L895–901. doi: 10.1152/ajplung.00075.2004. [DOI] [PubMed] [Google Scholar]
- 62.Candela P, Gosselet F, Miller F, et al. Physiological pathway for low-density lipoproteins across the blood-brain barrier: transcytosis through brain capillary endothelial cells in vitro. Endothelium. 2008;15(5-6):254–64. doi: 10.1080/10623320802487759. [DOI] [PubMed] [Google Scholar]
- 63.Pruenster M, Mudde L, Bombosi P, et al. The Duffy antigen receptor for chemokines transports chemokines and supports their promigratory activity. Nat Immunol. 2009;10(1):101–8. doi: 10.1038/ni.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hu G, Minshall RD. Regulation of transendothelial permeability by Src kinase. Microvasc. Res. 2009;77(1):21–5. doi: 10.1016/j.mvr.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 65.Sverdlov M, Shinin V, Place AT, Castellon M, Minshall RD. Filamin A regulates caveolae internalization and trafficking in endothelial cells. Mol Biol Cell. 2009;20(21):4531–40. doi: 10.1091/mbc.E08-10-0997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nag S, Manias JL, Stewart DJ. Expression of endothelial phosphorylated caveolin-1 is increased in brain injury. Neuropathol Appl Neurobiol. 2009;35(4):417–26. doi: 10.1111/j.1365-2990.2008.01009.x. [DOI] [PubMed] [Google Scholar]
- 67.Veluthakal R, Chvyrkova I, Tannous M, McDonald P, Amin R, Hadden T, Thurmond DC, Quon MJ, Kowluru A. Essential role for membrane lipid rafts in interleukin-1beta-induced nitric oxide release from insulin-secreting cells: potential regulation by caveolin-1+. Diabetes. 2005;54(9):2576–85. doi: 10.2337/diabetes.54.9.2576. [DOI] [PubMed] [Google Scholar]
- 68.Blanco AM, Perez-Arago A, Fernandez-Lizarbe S, Guerri C. Ethanol mimics ligand-mediated activation and endocytosis of IL-1RI/TLR4 receptors via lipid rafts caveolae in astroglial cells. J Neurochem. 2008;106(2):625–39. doi: 10.1111/j.1471-4159.2008.05425.x. [DOI] [PubMed] [Google Scholar]
- 69.Oakley FD, Smith RL, Engelhardt JF. Lipid rafts and caveolin-1 coordinate interleukin-1beta (IL-1beta)-dependent activation of NFkappaB by controlling endocytosis of Nox2 and IL-1beta receptor 1 from the plasma membrane. J Biol Chem. 2009;284(48):33255–64. doi: 10.1074/jbc.M109.042127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.D'Alessio A, Al Lamki RS, Bradley JR, Pober JS. Caveolae participate in tumor necrosis factor receptor 1 signaling and internalization in a human endothelial cell line. Am J Pathol. 2005;166(4):1273–82. doi: 10.1016/S0002-9440(10)62346-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pan W, Kastin AJ, Daniel J, Yu C, Baryshnikova LM, von Bartheld CS. TNF alpha trafficking in cerebral vascular endothelial cells. J Neuroimmunol. 2007;185:47–56. doi: 10.1016/j.jneuroim.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Poduslo JF, Curran GL. Permeability at the blood-brain and blood-nerve barriers of the neurotrophic factors: NGF, CNTF, NT-3, BDNF. Mol Brain Res. 1996;36:280–6. doi: 10.1016/0169-328x(95)00250-v. [DOI] [PubMed] [Google Scholar]
- 73.Pan W, Banks WA, Kastin AJ. Permeability of the blood-brain barrier to neurotrophins. Brain Res. 1998;788:87–94. doi: 10.1016/s0006-8993(97)01525-4. [DOI] [PubMed] [Google Scholar]
- 74.Pan W, Banks WA, Fasold MB, Bluth J, Kastin AJ. Transport of brain-derived neurotrophic factor across the blood-brain barrier. Neuropharmcol. 1998;37:1553–61. doi: 10.1016/s0028-3908(98)00141-5. [DOI] [PubMed] [Google Scholar]
- 75.Pan W, Kastin AJ. Interactions of IGF-1 with the blood-brain barrier in vivo and in situ. Neuroendocrinology. 2000;72:171–8. doi: 10.1159/000054584. [DOI] [PubMed] [Google Scholar]
- 76.Yu Y, Kastin AJ, Pan W. Reciprocal interactions of insulin and IGF-1 in receptor-mediated transport across the BBB. Endocrinol. 2006;147:2611–5. doi: 10.1210/en.2006-0020. [DOI] [PubMed] [Google Scholar]
- 77.Pan W, Yu Y, Cain CM, Nyberg F, Couraud P-O, Kastin AJ. Permeation of growth hormone across the blood-brain barrier. Endocrinol. 2005;146:4898–904. doi: 10.1210/en.2005-0587. [DOI] [PubMed] [Google Scholar]
- 78.Pan W, Yu Y, Nyberg F, Kastin AJ. In: In The Somatotrophic axis in brain function. Nyberg F, editor. Elsevier Academic Press; 2006. pp. 75–9. [Google Scholar]
- 79.Mosselmans R, Hepburn A, Dumont JE, Fiers W, Galand P. Endocytic pathway of recombinant murine tumor necrosis factor in L-929 cells. J Immunol. 1988;141:3096–100. [PubMed] [Google Scholar]
- 80.Di Guglielmo GM, Le RC, Goodfellow AF, Wrana JL. Distinct endocytic pathways regulate TGF-beta receptor signalling and turnover. Nat Cell Biol. 2003;5(5):410–421. doi: 10.1038/ncb975. [DOI] [PubMed] [Google Scholar]
- 81.Chen YG. Endocytic regulation of TGF-beta signaling. Cell Res. 2009;19(1):58–70. doi: 10.1038/cr.2008.315. [DOI] [PubMed] [Google Scholar]
- 82.Lei JT, Martinez-Moczygemba M. Separate endocytic pathways regulate IL-5 receptor internalization and signaling. J Leukoc. Biol. 2008;84(2):499–509. doi: 10.1189/jlb.1207828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Henriques CM, Rino J, Nibbs RJ, Graham GJ, Barata JT. IL-7 induces rapid clathrin-mediated internalization and JAK3-dependent degradation of IL-7Ralpha in T cells. Blood. 2010;115(16):3269–77. doi: 10.1182/blood-2009-10-246876. [DOI] [PubMed] [Google Scholar]
- 84.Orth JD, McNiven MA. Get off my back! Rapid receptor internalization through circular dorsal ruffles. Cancer Res. 2006;66(23):11094–6. doi: 10.1158/0008-5472.CAN-06-3397. [DOI] [PubMed] [Google Scholar]
- 85.Neel NF, Schutyser E, Sai J, Fan GH, Richmond A. Chemokine receptor internalization and intracellular trafficking. Cytokine Growth Factor Rev. 2005;16(6):637–58. doi: 10.1016/j.cytogfr.2005.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Barr VA, Lane K, Taylor SI. Subcellular localization and internalization of the four human leptin receptor isoforms. J Biol Chem. 1999;274:21416–24. doi: 10.1074/jbc.274.30.21416. [DOI] [PubMed] [Google Scholar]
- 87.Wilcke M, Walum E. Characterization of leptin intracellular trafficking. Eur J Histochem. 2000;44:325–34. [PubMed] [Google Scholar]
- 88.Tu H, Hsuchou H, Kastin AJ, Wu X, Pan W. Unique leptin trafficking by a tailless receptor. FASEB J. 2010;24:2281–91. doi: 10.1096/fj.09-143487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tu H, Pan W, Feucht L, Kastin AJ. Convergent trafficking pattern of leptin after endocytosis mediated by ObRa - ObRd. J Cell Physiol. 2007;212:215–22. doi: 10.1002/jcp.21020. [DOI] [PubMed] [Google Scholar]
- 90.Tu H, Kastin AJ, Pan W. CRH-R1 and CRH-R2 are both trafficking and signaling receptors for urocortin. Mol Endocrinol. 2007;21:700–11. doi: 10.1210/me.2005-0503. [DOI] [PubMed] [Google Scholar]
- 91.Bodnar A, Nizsaloczki E, Mocsar G, et al. A biophysical approach to IL-2 and IL-15 receptor function: localization: conformation and interactions. Immunol. Lett. 2008;116(2):117–25. doi: 10.1016/j.imlet.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 92.Subtil A, Hemar A, utry-Varsat A. Rapid endocytosis of interleukin 2 receptors when clathrin-coated pit endocytosis is inhibited. J Cell Sci. 1994;107(Pt 12):3461–8. doi: 10.1242/jcs.107.12.3461. [DOI] [PubMed] [Google Scholar]
- 93.Lamaze C, Dujeancourt A, Baba T, Lo CG, Benmerah A, Dautry-Varsat A. Interleukin 2 receptors and detergent-resistant membrane domains define a clathrin-independent endocytic pathway. Mol Cell. 2001;7(3):661–71. doi: 10.1016/s1097-2765(01)00212-x. [DOI] [PubMed] [Google Scholar]
- 94.Sauvonnet N, Dujeancourt A, Dautry-Varsat A. Cortactin and dynamin are required for the clathrin-independent endocytosis of gammac cytokine receptor. J Cell Biol. 2005;168(1):155–63. doi: 10.1083/jcb.200406174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Grassart A, Dujeancourt A, Lazarow PB, Dautry-Varsat A, Sauvonnet N. Clathrin-independent endocytosis used by the IL-2 receptor is regulated by Rac1, Pak1 and Pak2. EMBO Rep. 2008;9(4):356–62. doi: 10.1038/embor.2008.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Grassart A, Meas-Yedid V, Dufour A, Olivo-Marin JC, Dautry-Varsat A, Sauvonnet N. Pak1 phosphorylation enhances Cortactin-N-WASP interaction in clathrin-caveolin-independent endocytosis. Traffic. 2010;11(8):1079–91. doi: 10.1111/j.1600-0854.2010.01075.x. [DOI] [PubMed] [Google Scholar]
- 97.Subtil A, Dautry-Varsat A. Several weak signals in the cytosolic and transmembrane domains of the interleukin-2-receptor beta chain allow for its efficient endocytosis. Eur J Biochem. 1998;253(2):525–30. doi: 10.1046/j.1432-1327.1998.2530525.x. [DOI] [PubMed] [Google Scholar]
- 98.Duprez V, Smoljanovic M, Lieb M, Dautry-Varsat A. Trafficking of interleukin 2 and transferrin in endosomal fractions of T lymphocytes. J Cell Sci. 1994;107(Pt 5):1289–95. doi: 10.1242/jcs.107.5.1289. [DOI] [PubMed] [Google Scholar]
- 99.Dubois S, Mariner J, Waldmann TA, Tagaya Y. IL-15Ralpha recycles and presents IL-15 In trans to neighboring cells. Immunity. 2002;17(5):537–47. doi: 10.1016/s1074-7613(02)00429-6. [DOI] [PubMed] [Google Scholar]
- 100.Hemar A, Subtil A, Lieb M, Morelon E, Hellio R, Dautry-Varsat A. Endocytosis of interleukin 2 receptors in human T lymphocytes: Distinct intracellular localization and fate of the receptor alpha, beta and gamma chains. J Cell Biol. 1995;129:55–64. doi: 10.1083/jcb.129.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Subtil A, Rocca A, Dautry-Varsat A. Molecular characterization of the signal responsible for the targeting of the interleukin 2 receptor beta chain toward intracellular degradation. J Biol Chem. 1998;273(45):29424–9. doi: 10.1074/jbc.273.45.29424. [DOI] [PubMed] [Google Scholar]
- 102.Rocca A, Lamaze C, Subtil A, Dautry-Varsat A. Involvement of the ubiquitin/proteasome system in sorting of the interleukin 2 receptor beta chain to late endocytic compartments. Mol Biol Cell. 2001;12(5):1293–301. doi: 10.1091/mbc.12.5.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Banks WA, Kastin AJ, Huang W, Jaspan JB, Maness LM. Leptin enters the brain by a saturable system independent of insulin. Peptides. 1996;17:305–11. doi: 10.1016/0196-9781(96)00025-3. [DOI] [PubMed] [Google Scholar]
- 104.Kastin AJ, Akerstrom V, Pan W. Validity of multiple-time regression analysis in measurement of tritiated and iodinated leptin crossing the blood-brain barrier: meaningful controls. Peptides. 2001;22:2127–36. doi: 10.1016/s0196-9781(01)00569-1. [DOI] [PubMed] [Google Scholar]
- 105.Kastin AJ, Akerstrom V. Fasting, but not adrenalectomy, reduces transport of leptin into the brain. Peptides. 2000;21:679–82. doi: 10.1016/s0196-9781(00)00195-9. [DOI] [PubMed] [Google Scholar]
- 106.Banks WA, Coon AB, Robinson SM, et al. Triglycerides induce leptin resistance at the blood-brain barrier. Diabetes. 2004;53:1253–60. doi: 10.2337/diabetes.53.5.1253. [DOI] [PubMed] [Google Scholar]
- 107.Pan W, Hsuchou H, Tu H, Kastin AJ. Developmental changes of leptin receptors in cerebral microvessels: unexpected relation to leptin transport. Endocrinology. 2008;149(3):877–85. doi: 10.1210/en.2007-0893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kastin AJ, Akerstrom V. Glucose and insulin increase the transport of leptin through the blood-brain barrier in normal mice but not in streptozotocin-diabetic mice. Neuroendocrinology. 2001;73:237–42. doi: 10.1159/000054640. [DOI] [PubMed] [Google Scholar]
- 109.Banks WA, Clever CM, Farrell CL. Partial saturation and regional variation in the blood-to-brain transport of leptin in normal weight mice. Am J. Physiol. 2000;278:E1158–65. doi: 10.1152/ajpendo.2000.278.6.E1158. [DOI] [PubMed] [Google Scholar]
- 110.Banks WA, DiPalma CR, Farrell CL. Impaired transport of leptin across the blood-brain barrier in obesity. Peptides. 1999;20:1341–5. doi: 10.1016/s0196-9781(99)00139-4. [DOI] [PubMed] [Google Scholar]
- 111.Maresh GA, Maness LM, Zadina JE, Kastin AJ. In vitro demonstration of a saturable transport system for leptin across the blood-brain barrier. Life Sci. 2001;69:67–73. doi: 10.1016/s0024-3205(01)01093-1. [DOI] [PubMed] [Google Scholar]
- 112.Kastin AJ, Pan W, Maness LM, Koletsky RJ, Ernsberger P. Decreased transport of leptin across the blood-brain barrier in rats lacking the short form of the leptin receptor. Peptides. 1999;20:1449–53. doi: 10.1016/s0196-9781(99)00156-4. [DOI] [PubMed] [Google Scholar]
- 113.Tu H, Kastin AJ, Hsuchou H, Pan W. Soluble receptor inhibits leptin transport. J Cell Physiol. 2008;214(2):301–5. doi: 10.1002/jcp.21195. [DOI] [PubMed] [Google Scholar]
- 114.Zlokovic BV, Jovanovic S, Miao W, Samara S, Verma S, Farrell CL. Differential regulation of leptin transport by the choroid plexus and blood-brain barrier and high affinity transport systems for entry into hypothalamus and across the blood-cerebrospinal fluid barrier. Endocrinol. 2000;141:1434–41. doi: 10.1210/endo.141.4.7435. [DOI] [PubMed] [Google Scholar]
- 115.Pan W, Kastin AJ. Diurnal variation of leptin entry from blood to brain involving partial saturation of the transport system. Life Sci. 2001;68:2705–14. doi: 10.1016/s0024-3205(01)01085-2. [DOI] [PubMed] [Google Scholar]
- 116.Adam CL, Findlay PA. Decreased blood-brain leptin transfer in an ovine model of obesity and weight loss: resolving the cause of leptin resistance. Int. J Obes. (Lond) 2010;34:980–8. doi: 10.1038/ijo.2010.28. [DOI] [PubMed] [Google Scholar]
- 117.Van Heek M, Compton DS, France CF, Tedesco RP, Fawzi AB, Graziano MP, Sybertz EJ, Strader CD, Davis HR., Jr. Diet-induced obese mice develop peripheral, but not central, resistance to leptin. J Clin. Invest. 1997;99:385–90. doi: 10.1172/JCI119171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Halaas J, Boozer C, Blair-West J, Fidahusein N, Denton DA, Friedman JM. Physiological response to long-term peripheral and central leptin infusion in lean and obese mice. Proc Natl Acad Sci USA. 1997;94:8878–83. doi: 10.1073/pnas.94.16.8878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kastin AJ, Akerstrom V. Nonsaturable entry of neuropeptide Y into the brain. Am J. Physiol. 1999;276:E479–82. doi: 10.1152/ajpendo.1999.276.3.E479. [DOI] [PubMed] [Google Scholar]
- 120.Kastin AJ, Akerstrom V, Hackler L. Agouti-related protein (83-132) aggregates and crosses the blood-brain barrier slowly. Metabolism. 2000;49:1444–8. doi: 10.1053/meta.2000.16556. [DOI] [PubMed] [Google Scholar]
- 121.Kastin AJ, Akerstrom V. Entry of CART into brain is rapid but not inhibited by excess CART or leptin. Am J. Physiol. 1999;277:E901–4. doi: 10.1152/ajpendo.1999.277.5.E901. [DOI] [PubMed] [Google Scholar]
- 122.Kastin AJ, Nissen C, Nikolics K, et al. Distribution of [3H]a-MSH in rat brain. Brain Res Bull. 1976;1:19–26. doi: 10.1016/0361-9230(76)90045-9. [DOI] [PubMed] [Google Scholar]
- 123.Kastin AJ, Akerstrom V. Differential interactions of urocortin/corticotropin-releasing hormone peptides with the blood-brain barrier. Neuroendocrinology. 2002;75:367–74. doi: 10.1159/000059433. [DOI] [PubMed] [Google Scholar]
- 124.Kastin AJ, Akerstrom V, Hackler L, Zadina JE. Phe13,Tyr19- Melanin-concentrating hormone and the blood-brain barrier: role of protein binding. J Neurochem. 2000;74:385–91. doi: 10.1046/j.1471-4159.2000.0740385.x. [DOI] [PubMed] [Google Scholar]
- 125.Pan W, Tu H, Kastin AJ. Differential BBB interactions of three ingestive peptides: obestatin, ghrelin, and adiponectin. Peptides. 2006;27:911–6. doi: 10.1016/j.peptides.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 126.Vaisse C, Halaas JL, Horvath CM, Darnell JE, Jr, Stoffel M, Friedman JM. Leptin activation of Stat3 in the hypothalamus of wild-type and ob/ob mice but not db/db mice. Nat Genet. 1996;14(1):95–7. doi: 10.1038/ng0996-95. [DOI] [PubMed] [Google Scholar]
- 127.He Y, Kastin AJ, Hsuchou H, Pan W. The Cdk5/p35 kinases modulate leptin-induced STAT3 signaling. J Mol Neurosci. 2009;39:49–58. doi: 10.1007/s12031-008-9174-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Pan W, Tu H, Hsuchou H, Daniel J, Kastin AJ. Unexpected amplification of leptin-induced Stat3 signaling by urocortin: implications for obesity. J Mol Neurosci. 2007;33(3):232–8. doi: 10.1007/s12031-007-0071-y. [DOI] [PubMed] [Google Scholar]
- 129.Zhang Y, Wu X, He Y, et al. Melanocortin potentiates leptin-induced STAT3 signaling via MAPK pathway. J Neurochem. 2009;110(1):390–9. doi: 10.1111/j.1471-4159.2009.06144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Pan W, Hsuchou H, He Y, Sakharkar A, Cain C, Yu C, Kastin AJ. Astrocyte Leptin Receptor (ObR) and Leptin Transport in Adult-Onset Obese Mice. Endocrinology. 2008;149(6):2798–806. doi: 10.1210/en.2007-1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hsuchou H, He Y, Kastin AJ, et al. Obesity induces functional astrocytic leptin receptors in hypothalamus. Brain. 2009;132:889–902. doi: 10.1093/brain/awp029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hsuchou H, Pan W, Barnes MJ, Kastin AJ. Leptin receptor mRNA in rat brain astrocytes. Peptides. 2009;30:2275–80. doi: 10.1016/j.peptides.2009.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kastin AJ, Akerstrom V, Pan W. Activation of urocortin transport into brain by leptin. Peptides. 2000;21(12):1811–7. doi: 10.1016/s0196-9781(00)00349-1. [DOI] [PubMed] [Google Scholar]
- 134.Pan W, Akerstrom V, Zhang J, Pejovic V, Kastin AJ. Modulation of feeding-related peptide /protein signals by the blood-brain barrier. J Neurochem. 2004;90:455–61. doi: 10.1111/j.1471-4159.2004.02502.x. [DOI] [PubMed] [Google Scholar]
- 135.Tu H, Kastin AJ, Pan W. CRH-R1 and CRH-R2 are both trafficking and signaling receptors for urocortin. Mol Endocrinol. 2007;21:700–11. doi: 10.1210/me.2005-0503. [DOI] [PubMed] [Google Scholar]
- 136.Tu H, Kastin AJ, Bjorbaek C, Pan W. Urocortin trafficking in cerebral microvessel endothelial cells. J Mol Neurosci. 2007;31:171–82. doi: 10.1385/jmn/31:02:171. [DOI] [PubMed] [Google Scholar]
- 137.Kastin AJ, Akerstrom V. Pretreatment with glucose increases entry of urocortin into mouse brain. Peptides. 2001;22(5):829–34. doi: 10.1016/s0196-9781(01)00397-7. [DOI] [PubMed] [Google Scholar]
- 138.Spinedi E, Hadid R, Daneva T, Gaillard RC. Cytokines stimulate the CRH but not the vasopressin neuronal system: evidence for a median eminence site of interleukin-6 action. Neuroendocrinology. 1992;56(1):46–53. doi: 10.1159/000126207. [DOI] [PubMed] [Google Scholar]
- 139.Martins JM, Kastin AJ, Banks WA. Unidirectional specific and modulated brain to blood transport of corticotropin-releasing hormone. Neuroendocrinology. 1996;63:338–48. doi: 10.1159/000126974. [DOI] [PubMed] [Google Scholar]
- 140.Pan W, Banks WA, Kastin AJ. Permeability of the blood-brain and blood-spinal cord barriers to interferons. J Neuroimmunol. 1997;76:105–11. doi: 10.1016/s0165-5728(97)00034-9. [DOI] [PubMed] [Google Scholar]
- 141.Pan W, Tu H, Yu C, Hsuchou H, Yang Y, Kastin AJ. Differential role of TNF receptors in cellular trafficking of intact TNF. Cell. Phyiosiol. Biochem. 2007;20:559–68. doi: 10.1159/000107539. [DOI] [PubMed] [Google Scholar]
- 142.Pan W, Kastin AJ. TNFa transport across the blood-brain barrier is abolished in receptor knockout mice. Exp Neurol. 2002;174:193–200. doi: 10.1006/exnr.2002.7871. [DOI] [PubMed] [Google Scholar]
- 143.Lewis M, Tartaglia LA, Lee A, et al. Cloning and expression of cDNAs for two distinct murine tumor necrosis factor receptors demonstrate one receptor is species specific. Proc Natl Acad Sci USA. 1991;88(7):2830–4. doi: 10.1073/pnas.88.7.2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Pan W, Banks WA, Kastin AJ. Blood-brain barrier permeability to ebiratide and TNF in acute spinal cord injury. Exp Neurol. 1997;146:367–73. doi: 10.1006/exnr.1997.6533. [DOI] [PubMed] [Google Scholar]
- 145.Pan W, Kastin AJ, Bell RL, Olson RD. Upregulation of tumor necrosis factor a transport across the blood-brain barrier after acute compressive spinal cord injury. J Neurosci. 1999;19(9):3649–55. doi: 10.1523/JNEUROSCI.19-09-03649.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Pan W, Kastin AJ. Increase in TNFa transport after SCI is specific for time, region, and type of lesion. Exp Neurol. 2001;170(2):357–63. doi: 10.1006/exnr.2001.7702. [DOI] [PubMed] [Google Scholar]
- 147.Pan W, Zhang L, Liao J, Csernus B, Kastin AJ. Selective increase in TNFa permeation across the blood-spinal cord barrier after SCI. J Neuroimmunol. 2003;134:111–7. doi: 10.1016/s0165-5728(02)00426-5. [DOI] [PubMed] [Google Scholar]
- 148.Pan W, Csernus B, Kastin AJ. Upregulation of p55 and p75 receptors mediating TNF alpha transport across the injured blood-spinal cord barrier. J Mol Neurosci. 2003;21(2):173–84. doi: 10.1385/JMN:21:2:173. [DOI] [PubMed] [Google Scholar]
- 149.Pan W, Kastin AJ, McLay RN, Rigai T, Pick CG. Increased hippocampal uptake of TNFa and behavioral changes in mice. Exp Brain Res. 2003;149:195–9. doi: 10.1007/s00221-002-1355-7. [DOI] [PubMed] [Google Scholar]
- 150.Pan W, Ding Y, Yu Y, Ohtaki H, Nakamachi T, Kastin AJ. Stroke upregulates TNF alpha transport across the blood-brain barrier. Exp Neurol. 2006;198:222–33. doi: 10.1016/j.expneurol.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 151.Pan W, Kastin AJ. Upregulation of the transport system for TNFa at the blood-brain barrier. Arch Physiol Biochem. 2001;109(4):350–3. doi: 10.1076/apab.109.4.350.4238. [DOI] [PubMed] [Google Scholar]
- 152.Banks WA. Blood-brain barrier transport of cytokines: a mechanism for neuropathology. Curr Pharm Des. 2005;11(8):973–84. doi: 10.2174/1381612053381684. [DOI] [PubMed] [Google Scholar]
- 153.Didier N, Banks WA, Créminon C, Dereudder-Bosquet N, Mabondzo A. HIV-1-induced production of endothelin-1 in an in vitro model of the human blood-brain barrier. NeuroReport. 2002;13:1179–83. doi: 10.1097/00001756-200207020-00022. [DOI] [PubMed] [Google Scholar]
- 154.Stanimirovic D, Satoh K. Inflammtory mediators of cerebral endothelium: a role in ischemic brain inflammation. Brain Pathol. 2000;10:113–26. doi: 10.1111/j.1750-3639.2000.tb00248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Gosselin D, Rivest S. MyD88 signaling in brain endothelial cells is essential for the neuronal activity and glucocorticoid release during systemic inflammation. Mol Psychiatry. 2008;13(5):480–97. doi: 10.1038/sj.mp.4002122. [DOI] [PubMed] [Google Scholar]
- 156.Fasler-Kan E, Suenderhauf C, Barteneva N, Poller B, Gygax D, Huwyler J. Cytokine signaling in the human brain capillary endothelial cell line hCMEC/D3. Brain Res. 2010;1354:15–22. doi: 10.1016/j.brainres.2010.07.077. [DOI] [PubMed] [Google Scholar]
- 157.Lee SW, Kim WJ, Choi YK, et al. SSeCKS regulates angiogenesis and tight junction formation in blood-brain barrier. Nat Med. 2003;9(7):900–6. doi: 10.1038/nm889. [DOI] [PubMed] [Google Scholar]
- 158.Serrats J, Schiltz JC, Garcia-Bueno B, van RN, Reyes TM, Sawchenko PE. Dual roles for perivascular macrophages in immune-to-brain signaling. Neuron. 2010;65(1):94–106. doi: 10.1016/j.neuron.2009.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Banks WA, Ercal N, Price TO. The blood-brain barrier in neuroAIDS. Curr HIV. Res. 2006;4(3):259–66. doi: 10.2174/157016206777709447. [DOI] [PubMed] [Google Scholar]
- 160.Ricardo-Dukelow M, Kadiu I, Rozek W, et al. HIV-1 infected monocyte-derived macrophages affect the human brain microvascular endothelial cell proteome: new insights into blood-brain barrier dysfunction for HIV-1-associated dementia. J Neuroimmunol. 2007;185(1-2):37–46. doi: 10.1016/j.jneuroim.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Persidsky Y, Ramirez SH, Haorah J, Kanmogne GD. Blood-brain barrier: structural components and function under physiologic and pathologic conditions. J Neuroimmune. Pharmacol. 2006;1(3):223–36. doi: 10.1007/s11481-006-9025-3. [DOI] [PubMed] [Google Scholar]
- 162.Fabry Z, Fitzsimmons KM, Herlein JA, Moninger TO, Dobbs MB, Hart MN. Production of the cytokines interleukin 1 and 6 by murine brain microvessel endothelium and smooth muscle pericytes. J Neuroimmunol. 1993;47(1):23–34. doi: 10.1016/0165-5728(93)90281-3. [DOI] [PubMed] [Google Scholar]
- 163.van Dam AM, De Vries HE, Kuiper J, et al. Interleukin-1 receptors on rat brain endothelial cells: a role in neuroimmune interaction? FASEB J. 1996;10(2):351–6. doi: 10.1096/fasebj.10.2.8641570. [DOI] [PubMed] [Google Scholar]
- 164.Reyes TM, Fabry Z, Coe CL. Brain endothelial cell production of a neuroprotective cytokine, interleukin-6, in response to noxious stimuli. Brain Res. 1999;851(1-2):215–20. doi: 10.1016/s0006-8993(99)02189-7. [DOI] [PubMed] [Google Scholar]
- 165.Pan W, Yu C, Hsuhou H, Khan RS, Kastin AJ. Cerebral microvascular IL15 is a novel mediator of TNF action. J Neurochem. 2009;111:819–27. doi: 10.1111/j.1471-4159.2009.06371.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Dodelet-Devillers A, Cayrol R, van HJ, et al. Functions of lipid raft membrane microdomains at the blood-brain barrier. J Mol Med. 2009;87(8):765–74. doi: 10.1007/s00109-009-0488-6. [DOI] [PubMed] [Google Scholar]
- 167.Banks WA, Niehoff ML, Martin D, Farrell CL. Leptin transport across the blood-brain barrier of the Koletsky rat is not mediated by a product of the leptin receptor gene. Brain Res. 2002;950:130–6. doi: 10.1016/s0006-8993(02)03013-5. [DOI] [PubMed] [Google Scholar]
- 168.Yu C, Kastin AJ, Tu H, Pan W. Opposing effects of proteasomes and lysosomes on LIFR: modulation by TNF. J Mol Neurosci. 2007;32:80–9. doi: 10.1007/s12031-007-0017-4. [DOI] [PubMed] [Google Scholar]
- 169.Yu C, Kastin AJ, Pan W. TNF reduces LIF endocytosis despite increasing NFkappaB-mediated gp130 expression. J Cell Physiol. 2007;213:161–6. doi: 10.1002/jcp.21105. [DOI] [PubMed] [Google Scholar]
- 170.Kastin AJ, Pan W, Akerstrom V, Hackler L, Wang CF, Kotz CM. Novel peptide-peptide cooperation may transform feeding behavior. Peptides. 2002;23(12):2189–96. doi: 10.1016/s0196-9781(02)00247-4. [DOI] [PubMed] [Google Scholar]
- 171.Tu H, Kastin AJ, Bjorbaek C, Pan W. Urocortin trafficking in cerebral microvessel endothelial cells. J Mol Neurosci. 2007;31:171–82. doi: 10.1385/jmn/31:02:171. [DOI] [PubMed] [Google Scholar]
- 172.Willis CL, Davis TP. Chronic inflammatory pain and the neurovascular unit: a central role for glia in maintaining BBB integrity? Curr Pharm Des. 2008;14(16):1625–43. doi: 10.2174/138161208784705414. [DOI] [PubMed] [Google Scholar]
- 173.Banks WA, Erickson MA. The blood-brain barrier and immune function and dysfunction. Neurobiol Dis. 2010;37(1):26–32. doi: 10.1016/j.nbd.2009.07.031. [DOI] [PubMed] [Google Scholar]
- 174.Harrold JA, Widdowson PS, Williams G. Altered energy balance causes selective changes in melanocortin-4(MC4-R), but not melanocortin-3 (MC3-R), receptors in specific hypothalamic regions: further evidence that activation of MC4-R is a physiological inhibitor of feeding. Diabetes. 1999;48(2):267–71. doi: 10.2337/diabetes.48.2.267. [DOI] [PubMed] [Google Scholar]
- 175.Yu WH, Kimura M, Walczewska A, Karanth S, McCann SM. Role of leptin in hypothalamic-pituitary function. Proc Natl Acad Sci USA. 1997;94(3):1023–8. doi: 10.1073/pnas.94.3.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Akanuma S, Hori S, Ohtsuki S, Fujiyoshi M, Terasaki T. Expression of nuclear receptor mRNA and liver X receptor-mediated regulation of ABC transporter A1 at rat blood-brain barrier. Neurochem. Int. 2008;52(4-5):669–74. doi: 10.1016/j.neuint.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 177.Panzenboeck U, Balazs Z, Sovic A, et al. ABCA1 and scavenger receptor class B, type I, are modulators of reverse sterol transport at an in vitro blood-brain barrier constituted of porcine brain capillary endothelial cells. J Biol Chem. 2002;277:42781–9. doi: 10.1074/jbc.M207601200. [DOI] [PubMed] [Google Scholar]
- 178.Panzenboeck U, Kratzer I, Sovic A, et al. Regulatory effects of synthetic liver X receptor- and peroxisome-proliferator activated receptor agonists on sterol transport pathways in polarized cerebrovascular endothelial cells. Int. J Biochem. Cell Biol. 2006;38(8):1314–29. doi: 10.1016/j.biocel.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 179.Koldamova RP, Lefterov IM, Ikonomovic MD, et al. 22R-hydroxycholesterol and 9-cis-retinoic acid induce ATP-binding cassette transporter A1 expression and cholesterol efflux in brain cells and decrease amyloid beta secretion. J Biol Chem. 2003;278(15):13244–56. doi: 10.1074/jbc.M300044200. [DOI] [PubMed] [Google Scholar]
- 180.Akanuma S, Ohtsuki S, Doi Y, et al. ATP-binding cassette transporter A1 (ABCA1) deficiency does not attenuate the brain-to-blood efflux transport of human amyloid-beta peptide (1-40) at the blood-brain barrier. Neurochem. Int. 2008;52(6):956–61. doi: 10.1016/j.neuint.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 181.Combes V, Coltel N, Faille D, Wassmer SC, Grau GE. Cerebral malaria: role of microparticles and platelets in alterations of the blood-brain barrier. Int J Parasitol. 2006;36(5):541–6. doi: 10.1016/j.ijpara.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 182.Miller A, Crumbley C, Prufer K. The N-terminal nuclear localization sequences of liver X receptors alpha and beta bind to importin alpha and are essential for both nuclear import and transactivating functions. Int J Biochem. Cell Biol. 2009;41(4):834–43. doi: 10.1016/j.biocel.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 183.Forster C, Waschke J, Burek M, Leers J, Drenckhahn D. Glucocorticoid effects on mouse microvascular endothelial barrier permeability are brain specific. J Physiol. 2006;573(Pt 2):413–25. doi: 10.1113/jphysiol.2006.106385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Burns KA, Vanden Heuvel JP. Modulation of PPAR activity via phosphorylation. Biochim Biophys Acta. 2007;1771(8):952–60. doi: 10.1016/j.bbalip.2007.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Shimizu M, Takai K, Moriwaki H. Strategy and mechanism for the prevention of hepatocellular carcinoma: phosphorylated retinoid X receptor alpha is a critical target for hepatocellular carcinoma chemoprevention. Cancer Sci. 2009;100(3):369–74. doi: 10.1111/j.1349-7006.2008.01045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ward RD, Weigel NL. Steroid receptor phosphorylation: Assigning function to site-specific phosphorylation. Biofactors. 2009;35(6):528–36. doi: 10.1002/biof.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Itoh M, Adachi M, Yasui H, Takekawa M, Tanaka H, Imai K. Nuclear export of glucocorticoid receptor is enhanced by c-Jun N-terminal kinase-mediated phosphorylation. Mol Endocrinol. 2002;16(10):2382–92. doi: 10.1210/me.2002-0144. [DOI] [PubMed] [Google Scholar]
- 188.Miller AL, Webb MS, Copik AJ, Wang Y, Johnson BH, Kumar R, Thompson EB. p38 Mitogen-activated protein kinase (MAPK) is a key mediator in glucocorticoid-induced apoptosis of lymphoid cells: correlation between p38 MAPK activation and site-specific phosphorylation of the human glucocorticoid receptor at serine 211. Mol Endocrinol. 2005;19(6):1569–83. doi: 10.1210/me.2004-0528. [DOI] [PubMed] [Google Scholar]
- 189.Barger PM, Browning AC, Garner AN, Kelly DP. p38 mitogen-activated protein kinase activates peroxisome proliferator-activated receptor alpha: a potential role in the cardiac metabolic stress response. J Biol Chem. 2001;276(48):44495–501. doi: 10.1074/jbc.M105945200. [DOI] [PubMed] [Google Scholar]
- 190.Bruck N, Bastien J, Bour G, et al. Phosphorylation of the retinoid x receptor at the omega loop: modulates the expression of retinoic-acid-target genes with a promoter context specificity. Cell Signal. 2005;17(10):1229–39. doi: 10.1016/j.cellsig.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 191.Li D, Zimmerman TL, Thevananther S, Lee HY, Kurie JM, Karpen SJ. Interleukin-1 beta-mediated suppression of RXR:RAR transactivation of the Ntcp promoter is JNK-dependent. J Biol Chem. 2002;277(35):31416–22. doi: 10.1074/jbc.M204818200. [DOI] [PubMed] [Google Scholar]
- 192.Bunone G, Briand PA, Miksicek RJ, Picard D. Activation of the unliganded estrogen receptor by EGF involves the MAP kinase pathway and direct phosphorylation. EMBO J. 1996;15(9):2174–83. [PMC free article] [PubMed] [Google Scholar]
- 193.Daniel AR, Qiu M, Faivre EJ, Ostrander JH, Skildum A, Lange CA. Linkage of progestin and epidermal growth factor signaling: phosphorylation of progesterone receptors mediates transcriptional hypersensitivity and increased ligand-independent breast cancer cell growth. Steroids. 2007;72(2):188–201. doi: 10.1016/j.steroids.2006.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Ponguta LA, Gregory CW, French FS, Wilson EM. Site-specific androgen receptor serine phosphorylation linked to epidermal growth factor-dependent growth of castration-recurrent prostate cancer. J Biol Chem. 2008;283(30):20989–1001. doi: 10.1074/jbc.M802392200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Straus DS, Glass CK. Anti-inflammatory actions of PPAR ligands: new insights on cellular and molecular mechanisms. Trends Immunol. 2007;28(12):551–8. doi: 10.1016/j.it.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 196.Crisafulli C, Di PR, Mazzon E, Paterniti I, Galuppo M, Genovese T, Bramanti P, Cappellani A, Cuzzocrea S. Liver X receptor agonist treatment reduced splanchnic ischemia and reperfusion injury. J Leukoc. Biol. 2010;87(2):309–21. doi: 10.1189/jlb.0609438. [DOI] [PubMed] [Google Scholar]
- 197.Zhang-Gandhi CX, Drew PD. Liver X receptor and retinoid X receptor agonists inhibit inflammatory responses of microglia and astrocytes. J Neuroimmunol. 2007;183(1-2):50–9. doi: 10.1016/j.jneuroim.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Landis MS, Patel HV, Capone JP. Oxysterol activators of liver X receptor and 9-cis-retinoic acid promote sequential steps in the synthesis and secretion of tumor necrosis factor-alpha from human monocytes. J Biol Chem. 2002;277(7):4713–21. doi: 10.1074/jbc.M108807200. [DOI] [PubMed] [Google Scholar]
- 199.Wang YY, Dahle MK, Steffensen KR, et al. Liver X receptor agonist GW3965 dose-dependently regulates lps-mediated liver injury and modulates posttranscriptional TNF-alpha production and p38 mitogen-activated protein kinase activation in liver macrophages. Shock. 2009;32(5):548–53. doi: 10.1097/SHK.0b013e3181a47f85. [DOI] [PubMed] [Google Scholar]
- 200.Johnson AB, Bake S, Lewis DK, Sohrabji F. Temporal expression of IL-1beta protein and mRNA in the brain after systemic LPS injection is affected by age and estrogen. J Neuroimmunol. 2006;174(1-2):82–91. doi: 10.1016/j.jneuroim.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 201.Arnal JF, Fontaine C, Billon-Gales A, et al. Estrogen receptors and endothelium. Arterioscler. Thromb. Vasc. Biol. 2010;30(8):1506–12. doi: 10.1161/ATVBAHA.109.191221. [DOI] [PubMed] [Google Scholar]
- 202.Burek M, Forster CY. Cloning and characterization of the murine claudin-5 promoter. Mol Cell Endocrinol. 2009;298(1-2):19–24. doi: 10.1016/j.mce.2008.09.041. [DOI] [PubMed] [Google Scholar]
- 203.Burek M, Arias-Loza PA, Roewer N, Forster CY. Claudin-5 as a novel estrogen target in vascular endothelium. Arterioscler. Thromb. Vasc. Biol. 2010;30(2):298–304. doi: 10.1161/ATVBAHA.109.197582. [DOI] [PubMed] [Google Scholar]
- 204.Forster C, Silwedel C, Golenhofen N, et al. Occludin as direct target for glucocorticoid-induced improvement of blood-brain barrier properties in a murine in vitro system. J Physiol. 2005;565(Pt 2):475–86. doi: 10.1113/jphysiol.2005.084038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Jiang C, Wang J, Li X, Liu C, Chen N, Hao Y. Progesterone exerts neuroprotective effects by inhibiting inflammatory response after stroke. Inflamm. Res. 2009;58(9):619–24. doi: 10.1007/s00011-009-0032-8. [DOI] [PubMed] [Google Scholar]
- 206.Forster C, Kahles T, Kietz S, Drenckhahn D. Dexamethasone induces the expression of metalloproteinase inhibitor TIMP-1 in the murine cerebral vascular endothelial cell line cEND. J Physiol. 2007;580(Pt.3):937–49. doi: 10.1113/jphysiol.2007.129007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Huang W, Eum SY, Andras IE, Hennig B, Toborek M. PPARalpha and PPARgamma attenuate HIV-induced dysregulation of tight junction proteins by modulations of matrix metalloproteinase and proteasome activities. FASEB J. 2009;23(5):1596–606. doi: 10.1096/fj.08-121624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Ramirez SH, Heilman D, Morsey B, Potula R, Haorah J, Persidsky Y. Activation of peroxisome proliferator-activated receptor gamma (PPARgamma) suppresses Rho GTPases in human brain microvascular endothelial cells and inhibits adhesion and transendothelial migration of HIV-1 infected monocytes. J Immunol. 2008;180(3):1854–65. doi: 10.4049/jimmunol.180.3.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Mysiorek C, Culot M, Dehouck L, et al. Peroxisome-proliferator-activated receptor-alpha activation protects brain capillary endothelial cells from oxygen-glucose deprivation-induced hyperpermeability in the blood-brain barrier. Curr Neurovasc. Res. 2009;6(3):181–93. doi: 10.2174/156720209788970081. [DOI] [PubMed] [Google Scholar]
- 210.Golden PL, Pardridge WM. P-Glycoprotein on astrocyte foot processes of unfixed isolated human brain capillaries. Brain Res. 1999;819(1-2):143–6. doi: 10.1016/s0006-8993(98)01305-5. [DOI] [PubMed] [Google Scholar]
- 211.Beaulieu E, Demeule M, Ghitescu L. Beliveau RP-glycoprotein is strongly expressed in the luminal membranes of the endothelium of blood vessels in the brain. Biochem. J. 1997;326(Pt 2):539–44. doi: 10.1042/bj3260539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Rao VV, Dahlheimer JL, Bardgett ME, et al. Choroid plexus epithelial expression of MDR1 P glycoprotein and multidrug resistance-associated protein contribute to the blood-cerebrospinal-fluid drug-permeability barrier. Proc Natl Acad Sci USA. 1999;96(7):3900–5. doi: 10.1073/pnas.96.7.3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Lee G, Schlichter L, Bendayan M, Bendayan R. Functional expression of P-glycoprotein in rat brain microglia. J Pharmacol Exp Ther. 2001;299(1):204–12. [PubMed] [Google Scholar]
- 214.Gottesman MM, Pastan I. The multidrug transporter, a double-edged sword. J Biol Chem. 1988;263(25):12163–6. [PubMed] [Google Scholar]
- 215.Endicott JA, Ling V. The biochemistry of P-glycoprotein-mediated multidrug resistance. Annu. Rev Biochem. 1989;58:137–71. doi: 10.1146/annurev.bi.58.070189.001033. [DOI] [PubMed] [Google Scholar]
- 216.Miller DS, Bauer B, Hartz AM. Modulation of P-glycoprotein at the blood-brain barrier: opportunities to improve central nervous system pharmacotherapy. Pharmacol Rev. 2008;60(2):196–209. doi: 10.1124/pr.107.07109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Yu C, Pan W, Tu H, Waters S, Kastin AJ. TNF activates MDR1 (P-glycoprotein) in cerebral microvascular endothelial cells. Cell Physiol Biochem. 2007;20(6):853–8. doi: 10.1159/000110445. [DOI] [PubMed] [Google Scholar]
- 218.Bauer B, Hartz AM, Miller DS. Tumor necrosis factor alpha and endothelin-1 increase P-glycoprotein expression and transport activity at the blood-brain barrier. Mol Pharmacol. 2007;71(3):667–75. doi: 10.1124/mol.106.029512. [DOI] [PubMed] [Google Scholar]
- 219.Poller B, Drewe J, Krahenbuhl S, Huwyler J, Gutmann H. Regulation of BCRP (ABCG2) and P-glycoprotein (ABCB1) by cytokines in a model of the human blood-brain barrier. Cell Mol Neurobiol. 2010;30(1):63–70. doi: 10.1007/s10571-009-9431-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.von Wedel-Parlow M, Wolte P, Galla HJ. Regulation of major efflux transporters under inflammatory conditions at the blood-brain barrier in vitro. J Neurochem. 2009;111(1):111–8. doi: 10.1111/j.1471-4159.2009.06305.x. [DOI] [PubMed] [Google Scholar]
- 221.Yu C, Argyropoulos G, Zhang Y, Kastin AJ, Hsuchou H, Pan W. Neuroinflammation activates Mdr1b efflux transport through NFkappaB: promoter analysis in BBB endothelia. Cell Physiol Biochem. 2008;22(5-6):745–56. doi: 10.1159/000185558. [DOI] [PMC free article] [PubMed] [Google Scholar]