Abstract
Reactive gliosis, a sign of neuroinflammation, has been observed in mice with adult-onset obesity as well as CNS injury. The hypothesis that obesity-derived metabolic factors exacerbate reactive gliosis in response to mechanical injury was tested here on cultured primary glial cells subjected to a well-established model of scratch wound injury. Cells treated with serum from mice with diet-induced obesity (DIO) showed higher immunoreactivity of CD11b (marker for microglia) and GFAP (marker for astrocytes), with morphological changes at both the injury border and areas away from the injury. The effect of DIO serum was greater than that of scratch injury alone. Leptin was almost as effective as DIO serum in inducing microgliosis and astrogliosis in a dose-response manner. By contrast, C-reactive protein (CRP) mainly induced microgliosis in noninjured cells; injury-induced factors appeared to attenuate this effect. The effect of CRP also differed from the effect of the antibiotic minocycline. Minocycline attenuated the microgliosis and to a lesser extent astrogliosis, particularly in CRP-treated cells, thus serving as a negative control. We conclude that blood-borne proinflammatory metabolic factors in obesity increase reactive gliosis and probably exacerbate CNS injury.
Keywords: Reactive astrogliosis, Microgliosis, Obesity, Trauma, Leptin, CRP
Introduction
Obesity is a worldwide endemic affecting millions of people and has serious metabolic and endocrine consequences. Obesity-related neuroinflammation has been recently recognized as a cause of cognitive dysfunction. The typical western diet, high in saturated fat and simple carbohydrate, is thought to be a culprit in obesity and correlates with neurobiological changes in the hippocampus and cognitive impairment (Kanoski et al. 2010; Kanoski and Davidson 2011). Obesity is not only associated with atrophy of selective brain regions, particularly gray matter (Walther et al. 2010), but also involves white matter such as the corona radiata (Raji et al. 2010). Obese or overweight elderly have decreased regional brain volume despite the lack of cognitive decline at the time of their magnetic resonance imaging (Raji et al. 2010).
Animal studies indicate that obesity is associated with neuroinflammation and oxidative stress (Farr et al. 2006, 2008; Pan et al. 2008; Lavin et al. 2011). In obese male mice vulnerable to diet-induced obesity (DIO), metabolic alterations are accompanied by deficits of learning and hippocampal synaptic plasticity (Hwang et al. 2010). Many adipokines are proinflammatory cytokines that cross the blood–brain barrier (BBB) (Kastin and Pan 2003, 2008; Pan and Kastin 2007). Several of them are known to induce reactive astrogliosis and microgliosis. In agouti viable yellow (Avy) mice with adult-onset obesity (Pan et al. 2008) and in DIO mice (Hsuchou et al. 2009a), we have observed reactive astrogliosis in selective regions of the hypothalamus and, to a lesser extent, the hippocampus and cerebral cortex (Pan et al. 2011). However, the resulting metabolic factors and their relative contributions to reactive gliosis in obese mice have not been characterized.
Obesity is associated with hyperleptinemia; increased leptin production plays a dual role in promoting neuroregeneration and memory functions and in acting as a proinflammatory factor (Pan et al. 2011, 2012). Leptin crosses the BBB by a saturable transport system (Banks et al. 1996), with about 0.5 % of the blood-borne leptin reaching brain parenchyma among various animal studies (Kastin et al. 2001; Kastin and Pan 2006; Pan and Kastin 2007). Obesity is also associated with increased concentrations of C-reactive protein (CRP). CRP is an acute phase protein involved in essentially all inflammatory and immune actions. With the higher global prevalence of obesity, a pressing question is whether metabolic disorders worsen the functional outcome in subjects with traumatic brain injury. In this study, we tested the hypothesis that leptin and CRP are among serum-derived factors contributing to reactive gliosis. We further examined the interactions between metabolic and traumatic causes of reactive gliosis.
Astrogliosis is characterized by rapid synthesis of glial fibrillary acidic protein (GFAP), which is an intermediate filament found in mature astrocytes that modulates astrocyte morphology and activity by providing stability to astrocytic processes (Eng et al. 1971, 2000). Besides induction by many cytokines during neuroinflammation, reactive astrogliosis is also a hallmark of CNS injury. A high fat diet is known to exacerbate brain injury (Hoane et al. 2011; Langdon et al. 2011), and this is an increasing problem in society as the prevalence of obesity increases. Although astrocytes are the most abundant cells in the brain, microglia show a rapid response to inflammatory and traumatic challenges (Streit 2002; Zhang et al. 2010), with changes in levels of leukocyte common antigen markers, particularly CD11b and CD45. To determine the interactions of traumatic and metabolic factors in reactive gliosis, we adapted a well-established scratch wound injury model (Yu et al. 1993; Malhotra et al. 1997; Katano et al. 1999; Kornyei et al. 2000; Tang et al. 2000; Lau and Yu 2001; Magdalena et al. 2003; Yang et al. 2009). This enabled us to determine the effects of individual factors, at least at pharmacological doses. Since the antibiotic minocycline is known to inhibit microglial activation (Henry et al. 2008; Chen et al. 2010; Tikka and Koistinaho 2001), we used it as a negative control and tested whether it abolishes reactive gliosis induced by DIO serum, leptin, or CRP. The results illustrate the mechanisms by which metabolic factors induce neuroinflammation and modulate glial reactivity after traumatic injury.
Materials and Methods
Primary Culture of Astrocytes and Microglia from Mouse Brain
C57BL/6 J (B6) mice were purchased from the Jackson Laboratory (Bar Harbor, ME). Mixed cultures of astrocytes and microglia were obtained as described previously (Tikka and Koistinaho 2001) with slight modification. In brief, eight hypothalami from 3- to 5-day old pups were collected in icy cold Hank’s balanced salt solution (Sigma-Aldrich, St. Louis, MO) containing 25 mM HEPES buffer (Sigma-Aldrich). After the meninges were carefully removed under a dissecting microscope, the hypothalami were transferred to icy cold Dulbecco’s Modified Eagle Medium (DMEM; Gibco, Grand Island, NY) containing 10 % fetal bovine serum (FBS; Atlanta Biologicals, Lawrenceville, GA) and antibiotic/antimycotic solution (Sigma-Aldrich), and minced to about 1-mm3 pieces. After trituration with a fire-polished Pasteur pipette, the homogenate was sieved through a 40-µm nylon cell strainer (BD Bioscience, Franklin Lakes, NJ). The cells passing through the cell strainer were seeded in a 75-mm2 Nunclon™Δ-treated flask (Nunc, Roskilde, Denmark) precoated with poly-D-lysine. After 2 to 3 weeks of culture, the fully confluent cells were shaken at 200 rpm at 37°C for 3 to 4 h to remove most of the microglia and then passed to poly-d-lysine precoated Nunclon™Δ-treated 3.5-mm culture dishes (Nunc) or 12-well plates and again grown to confluency for the following studies.
Cell Treatment
The overall design tested the effects and interactions of three metabolic factors (serum from DIO mice, leptin, and CRP), scratch injury, and minocycline on reactive gliosis. To determine the effects of metabolic factors on the expression and distribution of CD11b or GFAP immunoreactivity by immunocytochemistry (ICC), four to five groups of cells were studied: cells incubated in control serum from lean B6 mice (that did not show any differences in microglial and astrocytic morphologies in comparison with cells grown in 10 % FBS), 10 % serum from DIO mice, recombinant mouse leptin (R&D, Minneapolis, MN), recombinant mouse CRP (10 µg/ml, R&D), or 10 % heat-inactivated DIO serum. In dose-response studies, two concentrations of leptin were used: 1 µg/ml (62.5 nM) or 8 µg/ml (0.5 µM). Otherwise, the more effective dose of 8 µg/ml was used. Following a protocol approved by the Institutional Animal Care and Use Committee, B6 mice were fed with 45 % high fat diet (Research Diets, New Brunswick, NJ) or regular rodent chow starting at 6 weeks of age and persisting until the mice were 4 months old. The mice were anesthetized by isofluorane inhalation, and arterial blood was collected after dissection of the carotid artery. The whole blood was refrigerated at 4°C overnight and centrifuged at 1,000×g for 15 min at 4°C to obtain serum. The sera from the DIO (DIO serum) and lean control (control serum) groups were filtered through a syringe filter of 0.22-µm pore size (Fisher Scientific, Pittsburgh, PA), aliquoted, and stored at −20°C until use for astrocyte culture. Inactivated DIO serum was used as a negative control in some studies. The inactivation procedure involved heating the serum at 56°C in a water bath for 30 min with gentle swirling every 10 min.
In studies involving scratch injury, the confluent astrocyte monolayer was scratched with a 200-µl pipette tip, following a grid design with each horizontal and vertical scratch spaced 4.5 mm apart across the entire coverslip or petri dish. The cells were rinsed with prewarmed phosphate buffered saline (PBS) twice and cultured in fresh DMEM containing 10 % B6 serum or DIO serum.
Minocycline (200 nM, Sigma-Aldrich) cotreatment was used to block the activity of microglia (Tikka and Koistinaho 2001). Treatment with minocycline started 1 h before other treatments and continued throughout the experiment.
ICC
The cultured cells were fixed with 4 % paraformaldehyde (Sigma-Aldrich, Inc.) 48 h after scratch injury and treatment by incubation at room temperature for 10 min. The cells were then incubated with 0.3 % glycine at room temperature for 10 min to reduce autofluorescence, permeablized with 0.3 % Triton-X 100 for 5 min, and incubated with 10 % normal donkey serum in PBS for 20 min. The cells were then incubated overnight with primary antibodies at 4°C and Alexa dye-labeled secondary antibodies (Invitrogen, Eugene, OR) for 1 h at room temperature, with thorough washes with PBS in between. The astrocyte intermediate filament, GFAP, was probed by use of either a rabbit polyclonal antibody (1:500 dilution; Chemicon, Temecula, CA) or a mouse monoclonal antibody (1:500 dilution, Sigma-Aldrich). The purity of the culture was also verified by use of a microglial marker CD11b (1:200 dilution; Abcam, Cambridge, MA). The specificity of the staining was confirmed by the lack of fluorescence in additional groups of cells preincubated with a blocking peptide for ObR or by omission of the primary antibody. The cells were mounted with DAPI-containing antifading fluorescent mounting medium (Vector Laboratories, Burlingame, CA) to show the nuclei of the cells. Cellular images were acquired on an Olympus FV1000 confocal microscope.
The number and percentage of CD11b (+) cells in randomly selected fields under a×20 objective lens 48 h after incubation in control serum, DIO serum, leptin (1 or 8 µg/ml), or CRP (10 µg/ml) were determined by manual counting (n=4/group). Images representing “away from the border” were captured equal distances away from the scratch borders near the center of the unscratched field.
qPCR
To determine the effect of leptin on GFAP and CD11b expression over time, primary astrocytes were treated with 8 µg/ml of leptin or medium only, without serum withdrawal. The time points were 0 (control), 6, 24, and 48 h (n=3/group). Total RNA extraction and qPCR for GFAP, CD11b, and the reference gene GAPDH were performed as described previously (Wu et al. 2010).
Statistical Analysis
Differences in CD11b cell numbers as a result of different metabolic factors and minocycline treatment were determined by two-way analysis of variance (ANOVA), followed by Tukey’s post hoc test. The mRNA expression of GFAP and CD11b over time was determined by one-way ANOVA.
Results
The Number and Fluorescent Intensity of CD11b–Positive Cells are Increased by Incubation with DIO Serum, Leptin, or CRP
To determine whether metabolic factors induce reactive microgliosis, eight groups of cells were studied. The cells were treated with 10 % serum from lean B6 mice or DIO mice, leptin (8 µg/ml), or CRP (10 µg/ml), and half of them received minocycline (200 nM) and the other half did not. Additional cells were also treated with heat-inactivated DIO serum as a negative control. The concentration of leptin used was based on published data of an effective dose to enhance microglial cytokine release (Lafrance et al. 2010). The dose of CRP was selected based on the literature for endothelial cells (Kibayashi et al. 2005), macrophages (Zhao et al. 2011), and a human leukemic monocytic cell line (Mahajan et al. 2010). Significant effects of metabolic factors and minocycline were seen by two-way ANOVA.
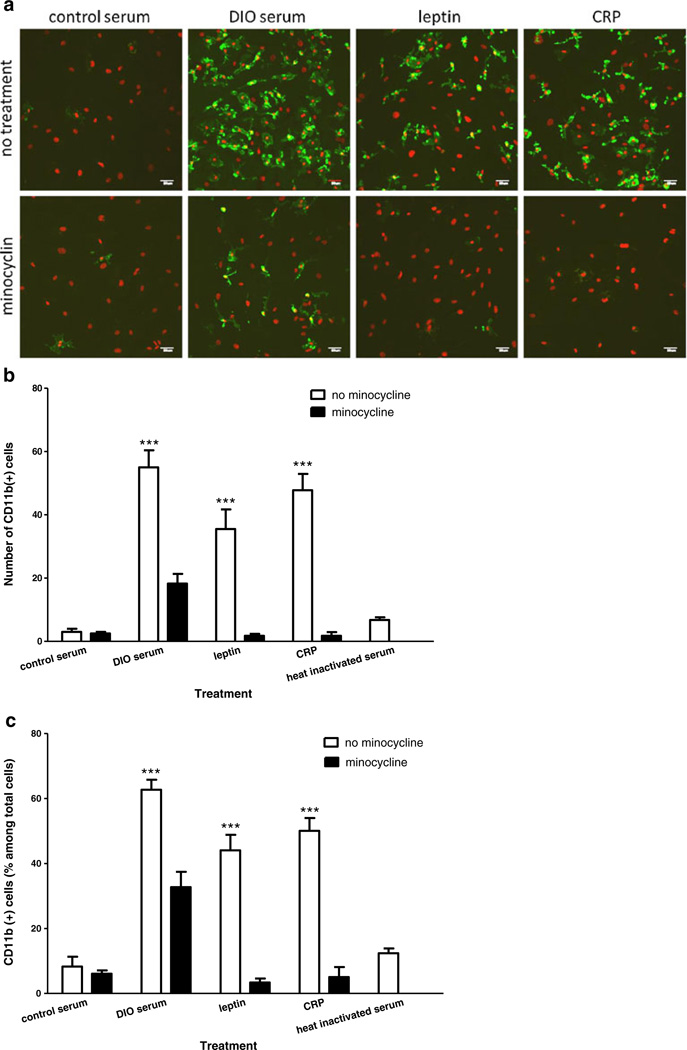
In mixed glial cells exposed to 10 % serum from DIO mice for 48 h, the total number and percentage of CD11b-positive cells were higher than for cells exposed to 10 % serum from lean mice. Incubation with leptin or CRP for 48 h produced a smaller increase of the percent of CD11b (+) cells. The total number of cells, shown by DAPI staining, was also increased, with CD11b (+) cells being the highest. The fluorescent intensity of CD11b ICC was increased in cells treated with DIO serum, leptin, or CRP. Cotreatment with minocycline attenuated CD11b expression in all groups. Representative ICC images are shown in Fig. 1a.
Fig 1.
Effects of metabolic factors, scratch injury, and minocycline on microgliosis. a Immunostaining of CD11b (green) 48 h after incubation in DIO serum, leptin (8 µg/ml), or CRP (10 µg/ml): all showed an increase of fluorescent intensity and number of cells in comparison with the group with control serum (upper panel). Minocycline (200 nM) cotreatment attenuated the increase of CD11b (lower panel). DAPI staining for all nucleated cells is shown in red Scale bar 50 µm. b The number of CD11b (+) cells was increased by DIO serum, leptin, or CRP treatment, whereas heat-inactivated DIO serum had minimal effect. The increase by DIO serum was greater than that by leptin (p<0.05). Minocycline (200 nM) cotreatment significantly attenuated the increase of CD11b (+) cells in all groups. ***p<0.005 compared with control serum. c The percentage of CD11b (+) cells (green) among total cells (shown by DAPI staining, red) was also increased by DIO serum, leptin, or CRP and inhibited by minocycline. ***p<0.005 in comparison with the control serum group
As seen in Fig. 1b, DIO serum, leptin, and CRP significantly increased the number of CD11b-positive cells compared with the group receiving control serum (p<0.0001). The increase after DIO serum was significantly higher than the increase after leptin (p<0.05). The increases after each treatment were blocked by minocycline cotreatment (p<0.0001). By contrast, heat-inactivated DIO serum did not result in a significant increase of CD11b staining, thus serving as a negative control. The number of nonCD11b cells was not affected by the treatments, indicating that the increase of DAPI (+) total cells was caused by the increase of microglia. When the number of CD11b (+) cells was evaluated as the percent of the total number of cells, the pattern of changes was the same as for the absolute number of microglia (Fig. 1c).
Overall, the results suggest that DIO serum, leptin, and CRP all induced reactive microgliosis, whereas the microglial activation inhibitor minocycline reversed the changes.
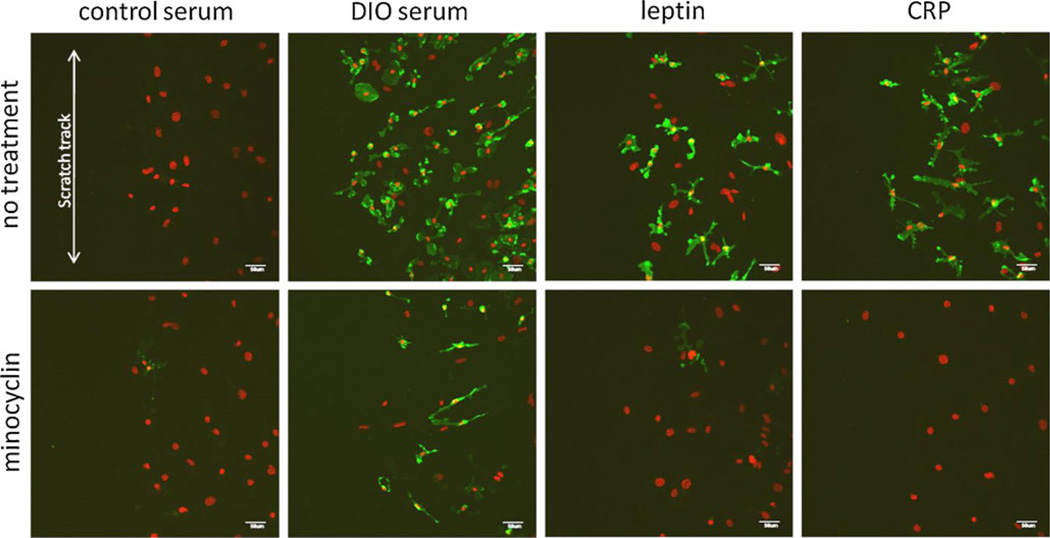
Since reactive gliosis is commonly seen after mechanical injury, we further tested whether in vitro scratch wound interacts with metabolic factors in reactive microgliosis. Scratch injury to a monolayer of cells is an established model widely used in the literature (Yu et al. 1993; Malhotra et al. 1997; Katano et al. 1999; Kornyei et al. 2000; Tang et al. 2000; Lau and Yu 2001; Magdalena et al. 2003;Yang et al. 2009). In this study, scratch was followed immediately by incubation of the cells with DIO serum, leptin, CRP, or control serum from lean mice. Scratch alone caused only a subtle increase in the level of CD11b expression 48 h later at the injury border. When DIO serum was added, there was a pronounced increase of CD11b fluorescent intensity at 48 h in comparison with the cells incubated with control serum after scratch. Leptin and CRP also increased CD11b fluorescent intensity, but not as much as the DIO serum-treated group. Minocycline attenuated the number and expression level of CD11b in cells treated with DIO serum after injury, almost completely abolished CD11b immunofluorescence in leptin-treated cells after injury, and completely blocked CD11b (+) cells in the CRP treatment group (Fig. 2). By contrast, heat-inactivated DIO serum did not have an apparent effect on CD11b expression. Altogether, the results show that metabolic factors had a greater effect than injury in inducing microgliosis and that DIO serum was a stronger inducer than leptin or CRP at the concentrations used for the study. This is also reflected by different degrees of inhibition of microgliosis by minocycline in the ascending order of DIO serum, leptin, and CRP.
Fig. 2.
Effects of metabolic factors on glial cells after scratch injury. CD11b immunostaining (green) was seen at the scratch border 48 h after incubation in control serum, DIO serum, leptin, or CRP. A similar increase of CD11b fluorescent intensity and number of cells as the nonscratched cells was seen in all treatment groups in comparison with the control serum group. Minocycline cotreatment inhibited the upregulation of CD11b. DAPI staining is shown in red. Scale bar 50 µm
DIO Serum, Leptin, and CRP Induce Reactive Astrogliosis Reflected by Greater GFAP Staining Intensity and Hypertrophic Cell Morphology
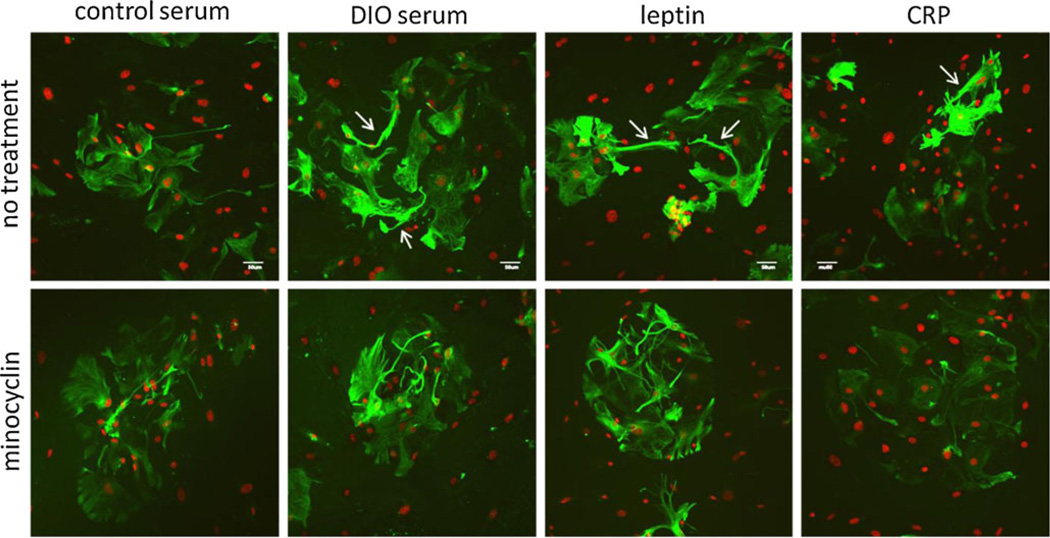
Primary astrocytes grown in control serum showed a homogeneous distribution of filamentous GFAP immunoreactivity in the cytoplasm. After incubation with DIO serum for 48 h, GFAP fluorescent intensity increased unevenly, and there were differences in adjacent cells. Most cells showed hypertrophic morphology. Like DIO serum, leptin also induced reactive changes in the percent of astrocytes involved, and the level of increased GFAP immunofluorescence was not as prominent as with DIO serum. In all three treatment groups, there were more processes extending outside the cytoplasm that were strongly GFAP (+) (arrows, upper panel of Fig. 3).
Fig. 3.
Effects of metabolic factors on astrogliosis shown by ICC of GFAP (green) 48 h after incubation in control serum, DIO serum, leptin, or CRP. All treatment groups showed an increase of GFAP fluorescent intensity and hypertrophic cell morphology in comparison with the control serum group. Arrows indicate the elongated processes of astrocytes. Minocycline cotreatment attenuated GFAP fluorescent intensity. DAPI staining is shown in red. Scale bar 50 µm
In the control group, minocycline did not decrease the uniform distribution of filamentous GFAP immunostaining in the cytoplasm of astrocytes. Rather, the subtle change appeared to be a minimal induction of astrogliosis. In astrocytes treated with DIO serum or leptin, minocycline cotreatment caused a mild decrease of hypertrophic GFAP (+) processes, but there was no significant quantifiable reduction. However, minocycline was able to almost completely abolish the reactive hypertrophic changes of increased GFAP expression and altered cell morphology in cells treated with CRP (Fig. 3, lower panel). This illustrates that inflammatory and metabolic factors showed differential effects on in vitro astrogliosis, and minocycline was more effective in reversing CRP-induced astrogliosis than after DIO serum and leptin induction.
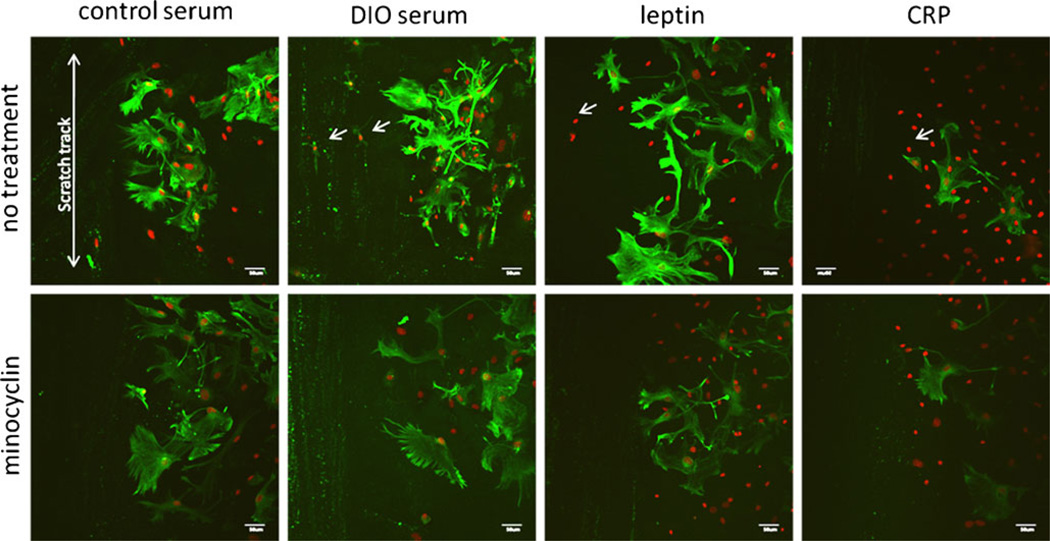
After scratch injury, a prominent upregulation of GFAP immunoreactivity was seen at the injury border in astrocytes grown in control serum (Fig. 4), but not in areas distant from injury, similar to that shown in Fig. 3. The apparent increase of GFAP was much greater than the minimal increase of CD11b at the injury border shown in Fig. 2. The combination of mechanical injury and DIO serum appeared to further induce an increase of GFAP fluorescent intensity, whereas leptin cotreatment of the injured cells enlarged the size of astrocytes. Surprisingly, CRP treatment attenuated the number and fluorescent intensity of GFAP (+) cells to a level even lower than that in the control serum group (Fig. 4, upper panel). In contrast to the findings from nonscratched cells treated with CRP shown in the upper right panel of Fig. 3, the results indicate that CRP inhibits reactive astrogliosis after scratch injury in mixed glial culture, an effect not seen with DIO serum or leptin.
Fig. 4.
Interactions of metabolic factors and scratch injury on GFAP (green) expression and distribution in astrocytes at the scratch border. Scratch alone increased GFAP immunofluorescence in cells incubated with control serum in comparison with nonscratched cells shown in the upper left corner of Fig. 3 that were processed simultaneously.DIO serum and leptin both induced further increases, whereas CRP decreased GFAP fluorescent intensity. Minocycline cotreatment attenuated GFAP expression and the extension of astrocytic processes in all groups. DAPI staining is shown in red. Scale bar 50 µm
In all four groups, minocycline cotreatment completely abolished reactive astrogliosis. The effect was most apparent in the cells treated with CRP immediately after injury, followed by leptin, the DIO serum, and control serum. The greater effect of CRP and minocycline in the injured glial cells is consistent with the speculation that CRP activates different cellular mechanisms for reactive astrogliosis than does DIO serum or leptin.
Lack of Transcriptional Upregulation of CD11b and GFAP
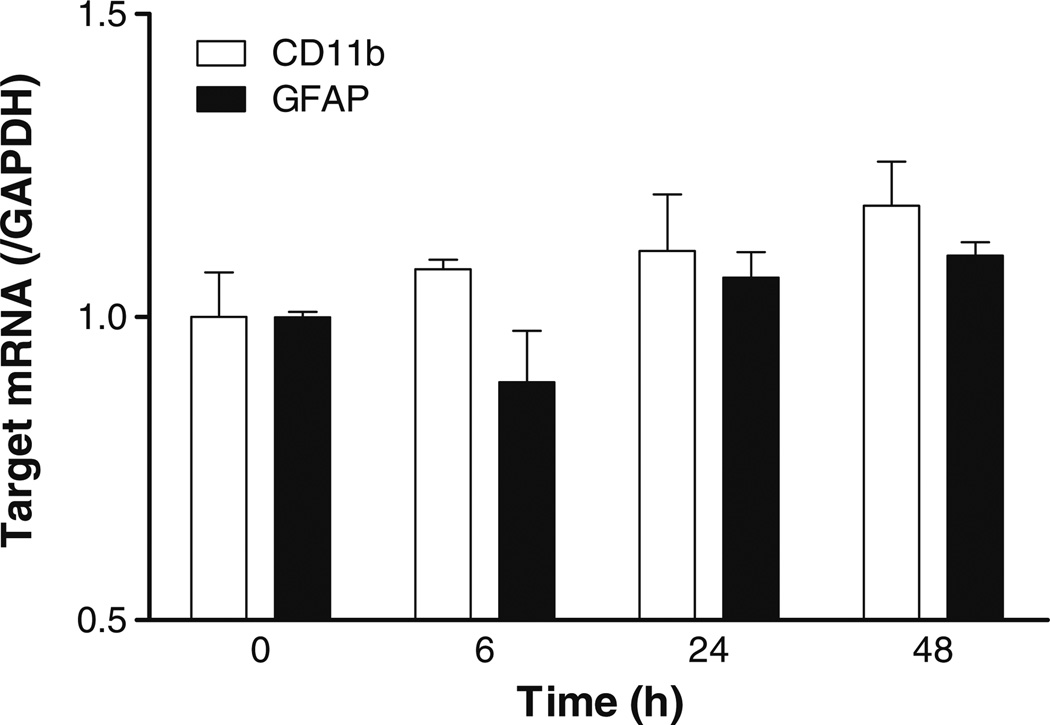
To determine whether the reactive changes with higher immunoreactivity of CD11b and GFAP were mediated by upregulation of glial mRNA expression, qPCR was performed on P1 cells receiving 8 µg/ml of leptin for 0, 6, 24, or 48 h (n=3/group). There was no significant increase of either CD11b or GFAP mRNA in the mixed primary glial cell culture without scratch injury (Fig. 5).
Fig. 5.
mRNA of CD11b and GFAP was determined in mixed glial culture at 0, 6, 24, and 48 h after leptin treatment (8 µg/ml). There was no significant increase of either glial marker
Dose Response of Leptin and Spatial Relationship Between Microglia and Astrocytes
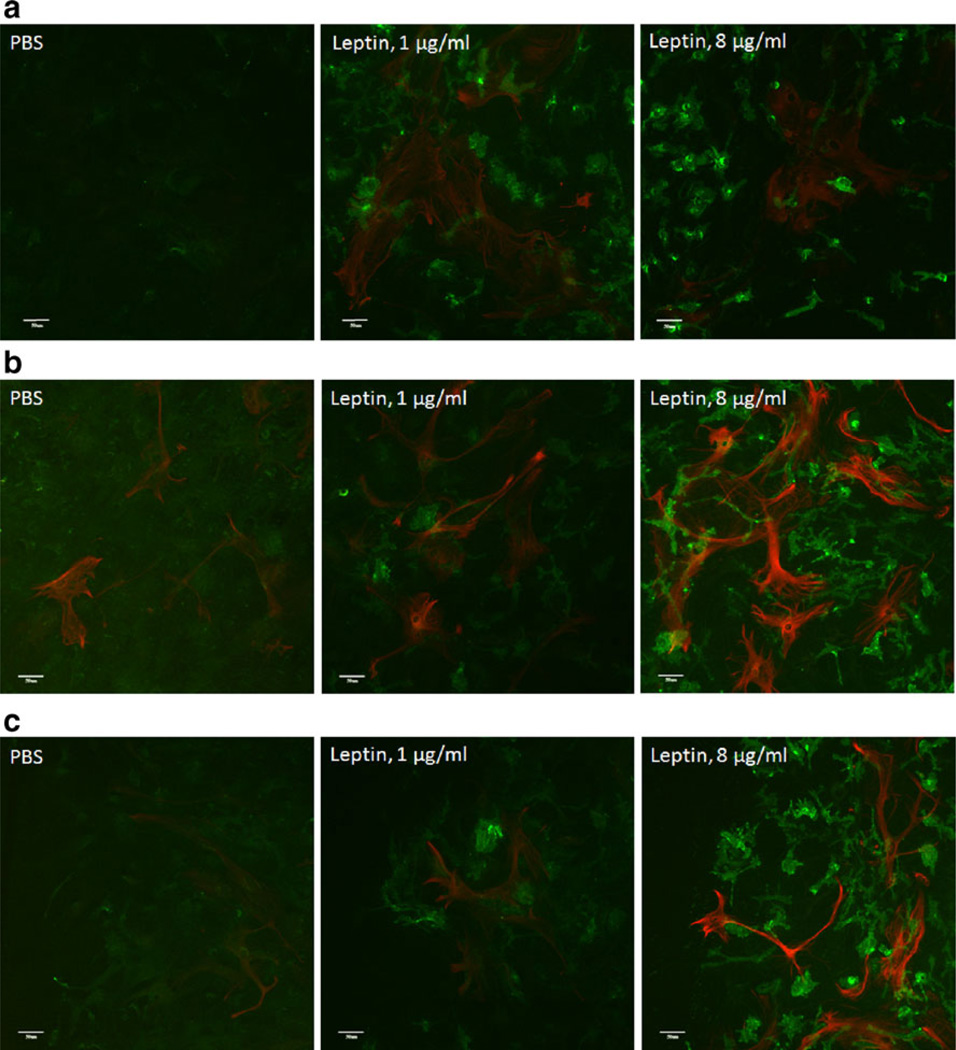
Coimmunostaining of CD11b and GFAP was performed in groups of cells without scratch injury and in those 48 h after scratch injury and concomitant leptin treatment (0, 1, or 8 µg/ml; n=3/group). In the absence of scratch injury, leptin caused a greater increase of CD11b (+) cells (green pseudocoloring) than it did to GFAP (+) cells (red pseudocoloring). There was more of an increase of CD11b immunofluorescent intensity in cells treated with 8 µg/ml of leptin than in those treated with 1 µg/ml of leptin (Fig. 6a). In cells sustaining scratch injury, in areas away from the scratch border, both CD11b and GFAP immunoreactivities showed remarkable increases that were greater in cells treated with the higher dose than the lower dose of leptin. Microglia and astrocytes interspersed with each other in nonoverlapping domains (Fig. 6b). At the scratch border, the increase of both the amount and immunofluorescent intensity of CD11b was higher than that of GFAP, and the higher dose of leptin was more effective than the lower dose. GFAP (+) astrocytes appeared to extend their processes toward CD11b (+) microglia (Fig. 6c).
Fig. 6.
Dose response of reactive gliosis after leptin treatment initiated immediately after scratch injury. Scale bar 50 µm. a In the absence of scratch injury, leptin treatment caused a major increase of CD11b immunoreactivity (green) and a mild increase of GFAP immunoreactivity (red). A higher dose (8 µg/ml) was more effective than a lower dose (1 µg/ml). b In areas away from the scratch border, leptin increased both CD11b (green) and GFAP (red), and it was more pronounced at the higher dose. c At the scratch border, leptin caused a greater increase of CD11b (+) microglia and their fluorescent intensity (green) than it did to GFAP (+) astrocytes. The GFAP (+) processes appeared to extend toward the microglia
Discussion
In this study, we showed that microgliosis and astrogliosis were induced by 48-h exposure to DIO serum, an excess concentration of leptin, or pathological levels of CRP seen in obesity and inflammation, although these metabolic factors differ in the extent of reactive gliosis. DIO serum was more effective in inducing gliosis, not unexpected since leptin and CRP are only individual components of the many factors present in DIO serum. Heat inactivation essentially abolished the increase of CD11b or GFAP (+) cells, indicating that most inducers were heat labile.
We chose to study leptin and CRP as two metabolic factors elevated in obesity, though they have no structural or signaling similarity. Although the concentrations of these proteins at the interstitial space in the CNS are not yet known, there is ample evidence from the literature suggesting the choice of the doses, as discussed below. In addition, dose-response studies shown in Fig. 6 also suggest that 8 µg/ml of leptin had more robust effects in inducing reactive gliosis than the 1-µg/ml dose. Leptin is a 16-kDa polypeptide mainly produced by adipocytes. Obesity increases the production of leptin, and a concentration of 100 ng/ml is seen in morbidly obese subjects that is nearly 30 times higher than the normal range (Banks 2001). In infants newly born to mothers with type I diabetes, serum leptin concentrations reach 24.9 µg/l or 24.9 ng/ml (Tapanainen et al. 2001). Nonetheless, 0.1 to 3 µg/ml of leptin has been used to induce the release of interleukin (IL)-1β from microglia (Lafrance et al. 2010). Leptin binds to a single transmembrane cytokine type I receptor (ObR or LR) that activates Janus kinase and Signal transducers and activator of transcription, as well as many other pathways (Ahima and Osei 2004; Pan et al. 2007; He et al. 2009; Zhang et al. 2009). ObR mRNA is present in mouse brain astrocytes (Hsuchou et al. 2009b), and upregulated astrocytic ObR protein expression is seen in obesity (Pan et al. 2008; Hsuchou et al. 2009a). Although the mechanisms by which leptin induces reactive astrogliosis have not been studied, there have been multiple reports that leptin activates microglia with secretion of cytokines and other proinflammatory molecules. High doses of leptin (1 to 10 µg/ml) induce microglial release of IL-1β (Pinteaux et al. 2007; Lafrance et al. 2010), IL-6 (Tang et al. 2007), and IL-1β, as well as IL-1RA mRNA expression in mixed glial culture (Hosoi et al. 2003). Leptin treatment (10 µg/ml for 22 h) increases nitric oxide production and expression of inducible nitric oxide synthase, and the presence of TNF-α or IL-1 β potentiates such increase (Mattace et al. 2006). All these results support the finding that high doses of leptin can induce reactive microgliosis and astrogliosis.
The monomer of CRP is a 21 kDa acute phase protein in the pentraxin family with levels correlating with cardiovascular risk and endothelial dysfunction (Ohnishi et al. 1988). CRP exists as a cyclic pentomer with five identical non-glycosylated protein subunits that is mainly produced in liver in response to IL-6 with synergy by IL-1β. Though the mean value of CRP in healthy adults is about 800 ng/ml with the 90 percentile less than 3 µg/ml, inflammation or injury can lead to a surge to 300 µg/ml that peaks at 24–48 h and can serve both as a ligand and a receptor (Pepys 1981). Structurally similar to serum amyloid P component and tissue amyloid P component, CRP binds to phosphocholine and Fc receptors, activates complements, and facilitates phagocytosis and release of proinflammatory cytokines (Marnell et al. 2005). The multitude of actions and signal amplification by cytokines and other secondary mediators make CRP an interface between innate and adaptive immunity (Peisajovich et al. 2008).
Although leptin and CRP showed similar effects in inducing microgliosis that was inhibited by minocycline, leptin appeared to be a stronger inducer of astrogliosis than CRP, and CRP-induced astrogliosis was more susceptible to inhibition by minocycline. This indicates different cellular mechanisms of action of these two metabolic factors. More interestingly, CRP showed an unexpected inhibitory effect on astrogliosis at the border of the scratch injury, suggesting a negative regulation of injury-induced local factors.
Differences in microglial and astrocytic responses may reflect the time course, mobility, and secretion of different secondary mediators in response to metabolic, inflammatory, and traumatic factors. An unusual finding, however, was a significant increase of CD11b (+) cells without an elevation of GFAP (+) cells in response to metabolic factors or scratch injury. This suggests that microglia show a more robust response with increased cell proliferation as well as reactive changes, whereas astrocytic proliferation is less affected. Overall, the results extend our understanding of differential cellular responses to CNS insults, and the interactions of metabolic factors with scratch injury may be applicable to determination of the prognosis of obese human subjects after CNS trauma.
In summary, we showed that leptin, CRP, and serum from DIO mice are strong inducers of microgliosis and astrogliosis. Leptin and DIO serum show additive effects with scratch injury, whereas CRP attenuates reactive astrogliosis along the injury border. The microglial response involves reactive changes with upregulation of cellular proteins, including CD11b, and cellular proliferation. The astroglial response reflects mainly reactive changes that are also susceptible to inhibition by minocycline. Neuroinflammation induced by metabolic factors may be a crucial first step of decreased CNS function in obesity.
Acknowledgments
Grant support was provided by NIH (DK54880 and DK92245 to AJK and NS62291 to WP.
References
- Ahima RS, Osei SY. Leptin signaling. Physiol Behav. 2004;81:223–241. doi: 10.1016/j.physbeh.2004.02.014. [DOI] [PubMed] [Google Scholar]
- Banks WA. Leptin transport across the blood-brain barrier: implications for the cause and treatment of obesity. Curr Pharm Des. 2001;7:125–133. doi: 10.2174/1381612013398310. [DOI] [PubMed] [Google Scholar]
- Banks WA, Kastin AJ, Huang W, Jaspan JB, Maness LM. Leptin enters the brain by a saturable system independent of insulin. Peptides. 1996;17:305–311. doi: 10.1016/0196-9781(96)00025-3. [DOI] [PubMed] [Google Scholar]
- Chen X, Pi R, Liu M, Ma X, Jiang Y, Liu Y, Mao X, Hu X. Combination of methylprednisolone and minocycline synergistically improves experimental autoimmune encephalomyelitis in C57 BL/6 mice. J Neuroimmunol. 2010;226:104–109. doi: 10.1016/j.jneuroim.2010.05.039. [DOI] [PubMed] [Google Scholar]
- Eng LF, Vanderhaeghen JJ, Bignami A, Gerstl B. An acidic protein isolated from fibrous astrocytes. Brain Res. 1971;28:351–354. doi: 10.1016/0006-8993(71)90668-8. [DOI] [PubMed] [Google Scholar]
- Eng LF, Ghirnikar RS, Lee YL. Glial fibrillary acidic protein: GFAP-thirty-one years (1969–2000) Neurochem Res. 2000;25:1439–1451. doi: 10.1023/a:1007677003387. [DOI] [PubMed] [Google Scholar]
- Farr SA, Banks WA, Morley JE. Effects of leptin on memory processing. Peptides. 2006;27:1420–1425. doi: 10.1016/j.peptides.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Farr SA, Yamada KA, Butterfield DA, Abdul HM, Xu L, Miller NE, Banks WA, Morley JE. Obesity and hypertriglyceridemia produce cognitive impairment. Endocrinology. 2008;149:2628–2636. doi: 10.1210/en.2007-1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Kastin AJ, Hsuchou H, Pan W. The Cdk5/p35 kinases modulate leptin-induced STAT3 signaling. J Mol Neurosci. 2009;39:49–58. doi: 10.1007/s12031-008-9174-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry CJ, Huang Y, Wynne A, Hanke M, Himler J, Bailey MT, Sheridan JF, Godbout JP. Minocycline attenuates lipopoly-saccharide (LPS)-induced neuroinflammation, sickness behavior, and anhedonia. J Neuroinflammation. 2008;5:15. doi: 10.1186/1742-2094-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoane MR, Swan AA, Heck SE. The effects of a high-fat sucrose diet on functional outcome following cortical contusion injury in the rat. Behav Brain Res. 2011;223:119–124. doi: 10.1016/j.bbr.2011.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoi T, Okuma Y, Wada S, Nomura Y. Inhibition of leptin-induced IL-1beta expression by glucocorticoids in the brain. Brain Res. 2003;969:95–101. doi: 10.1016/s0006-8993(03)02282-0. [DOI] [PubMed] [Google Scholar]
- Hsuchou H, He Y, Kastin AJ, Tu H, Markadakis EN, Rogers RC, Fossier PB, Pan W. Obesity induces functional astrocytic leptin receptors in hypothalamus. Brain. 2009a;132:889–902. doi: 10.1093/brain/awp029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsuchou H, Pan W, Barnes MJ, Kastin AJ. Leptin receptor mRNA in rat brain astrocytes. Peptides. 2009b;30:2275–2280. doi: 10.1016/j.peptides.2009.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang LL, Wang CH, Li TL, Chang SD, Lin LC, Chen CP, Chen CT, Liang KC, Ho IK, Yang WS, Chiou LC. Sex differences in high-fat diet-induced obesity, metabolic alterations and learning, and synaptic plasticity deficits in mice. Obes Silver Spring. 2010;18:463–469. doi: 10.1038/oby.2009.273. [DOI] [PubMed] [Google Scholar]
- Kanoski SE, Davidson TL. Western diet consumption and cognitive impairment: links to hippocampal dysfunction and obesity. Physiol Behav. 2011;103:59–68. doi: 10.1016/j.physbeh.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanoski SE, Zhang Y, Zheng W, Davidson TL. The effects of a high-energy diet on hippocampal function and blood-brain barrier integrity in the rat. J Alzheimers Dis. 2010;21:207–219. doi: 10.3233/JAD-2010-091414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastin AJ, Pan W. Feeding peptides interact in several ways with the blood-brain barrier. Curr Pharm Des. 2003;9:789–794. doi: 10.2174/1381612033455378. [DOI] [PubMed] [Google Scholar]
- Kastin AJ, Pan W. Intranasal leptin: blood-brain barrier bypass (BBBB) for obesity? Endocrinol. 2006;147:2086–2087. doi: 10.1210/en.2006-0208. [DOI] [PubMed] [Google Scholar]
- Kastin AJ, Pan W. Blood-brain barrier and feeding: regulatory roles of saturable transport systems for ingestive peptides. Curr Pharm Des. 2008;14:1615–1619. doi: 10.2174/138161208784705423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastin AJ, Akerstrom V, Pan W. Validity of multiple-time regression analysis in measurement of tritiated and iodinated leptin crossing the blood-brain barrier: meaningful controls. Peptides. 2001;22:2127–2136. doi: 10.1016/s0196-9781(01)00569-1. [DOI] [PubMed] [Google Scholar]
- Katano H, Fujita K, Kato T, Asai K, Kawamura Y, Masago A, Yamada K. Traumatic injury in vitro induces IEG mRNAs in cultured glial cells, suppressed by co-culture with neurons. NeuroReport. 1999;10:2439–2448. doi: 10.1097/00001756-199908200-00002. [DOI] [PubMed] [Google Scholar]
- Kibayashi E, Urakaze M, Kobashi C, Kishida M, Takata M, Sato A, Yamazaki K, Kobayashi M. Inhibitory effect of pitavastatin (NK-104) on the C-reactive-protein-induced interleukin-8 production in human aortic endothelial cells. Clin Sci Lond. 2005;108:515–521. doi: 10.1042/CS20040315. [DOI] [PubMed] [Google Scholar]
- Kornyei Z, Czirok A, Vicsek T, Madarasz E. Proliferative and migratory responses of astrocytes to in vitro injury. J Neurosci Res. 2000;61:421–429. doi: 10.1002/1097-4547(20000815)61:4<421::AID-JNR8>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Lafrance V, Inoue W, Kan B, Luheshi GN. Leptin modulates cell morphology and cytokine release in microglia. Brain Behav Immun. 2010;24:358–365. doi: 10.1016/j.bbi.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Langdon KD, Clarke J, Corbett D. Long-term exposure to high fat diet is bad for your brain: exacerbation of focal ischemic brain injury. Neuroscience. 2011;182:82–87. doi: 10.1016/j.neuroscience.2011.03.028. [DOI] [PubMed] [Google Scholar]
- Lau LT, Yu AC. Astrocytes produce and release interleukin-1, interleukin-6, tumor necrosis factor alpha and interferon-gamma following traumatic and metabolic injury. J Neurotrauma. 2001;18:351–359. doi: 10.1089/08977150151071035. [DOI] [PubMed] [Google Scholar]
- Lavin DN, Joesting JJ, Chiu GS, Moon ML, Meng J, Dilger RN, Freund GG. Fasting induces an anti-inflammatory effect on the neuroimmune system which a high-fat diet prevents. Obes Silver Spring. 2011;19:1586–1594. doi: 10.1038/oby.2011.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magdalena J, Millard TH, Etienne-Manneville S, Launay S, Warwick HK, Machesky LM. Involvement of the Arp2/3 complex and Scar2 in Golgi polarity in scratch wound models. Mol Biol Cell. 2003;14:670–684. doi: 10.1091/mbc.E02-06-0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahajan N, Bahl A, Dhawan V. C-reactive protein (CRP) up-regulates expression of receptor for advanced glycation end products (RAGE) and its inflammatory ligand EN-RAGE in THP-1 cells: inhibitory effects of atorvastatin. Int J Cardiol. 2010;142:273–278. doi: 10.1016/j.ijcard.2009.01.008. [DOI] [PubMed] [Google Scholar]
- Malhotra SK, Luong LT, Bhatnagar R, Shnitka TK. Up-regulation of reactive astrogliosis in the rat glioma 9L cell line by combined mechanical and chemical injuries. Cytobios. 1997;89:115–134. [PubMed] [Google Scholar]
- Marnell L, Mold C, Du Clos TW. C-reactive protein: ligands, receptors and role in inflammation. Clin Immunol. 2005;117:104–111. doi: 10.1016/j.clim.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Mattace RG, Esposito E, Iacono A, Pacilio M, Coppola A, Bianco G, Diano S, Di Carlo R, Meli R. Leptin induces nitric oxide synthase type II in C6 glioma cells. Role for nuclear factor-kappaB in hormone effect. Neurosci Lett. 2006;396:121–126. doi: 10.1016/j.neulet.2005.11.022. [DOI] [PubMed] [Google Scholar]
- Ohnishi S, Maeda S, Nishiguchi S, Arao T, Shimada K. Structure of the mouse C-reactive protein gene. Biochem Biophys Res Commun. 1988;156:814–822. doi: 10.1016/s0006-291x(88)80917-3. [DOI] [PubMed] [Google Scholar]
- Pan W, Kastin AJ. Adipokines and the blood-brain barrier. Peptides. 2007;28:1317–1330. doi: 10.1016/j.peptides.2007.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan W, Tu H, Hsuchou H, Daniel J, Kastin AJ. Unexpected amplification of leptin-induced Stat3 signaling by urocortin: implications for obesity. J Mol Neurosci. 2007;33:232–238. doi: 10.1007/s12031-007-0071-y. [DOI] [PubMed] [Google Scholar]
- Pan W, Hsuchou H, He Y, Sakharkar A, Cain C, Yu C, Kastin AJ. Astrocyte leptin receptor (ObR) and leptin transport in adult-onset obese mice. Endocrinology. 2008;149:2798–2806. doi: 10.1210/en.2007-1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan W, Hsuchou H, He Y, Kastin AJ. Glial leptin receptors and obesity. In: Preedy VR, editor. Modern insights into disease from molecules to man: Adipokines. Enfield: Science Publishers; 2011. pp. 185–196. [Google Scholar]
- Pan W, Hsuchou H, Jayaram B, Khan RS, Huang EYK, Wu X, Chen C, Kastin AJ. Leptin action on non-neuronal cells in the CNS: potential clinical implications. Ann N Y Acad Sci. 2012 doi: 10.1111/j.1749-6632.2012.06472.x. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peisajovich A, Marnell L, Mold C, Du Clos TW. C-reactive protein at the interface between innate immunity and inflammation. Expert Rev Clin Immunol. 2008;4:379–390. doi: 10.1586/1744666X.4.3.379. [DOI] [PubMed] [Google Scholar]
- Pepys MB. C-reactive protein fifty years on. Lancet. 1981;1:653–657. doi: 10.1016/s0140-6736(81)91565-8. [DOI] [PubMed] [Google Scholar]
- Pinteaux E, Inoue W, Schmidt L, Molina-Holgado F, Rothwell NJ, Luheshi GN. Leptin induces interleukin-1beta release from rat microglial cells through a caspase 1 independent mechanism. J Neurochem. 2007;102:826–833. doi: 10.1111/j.1471-4159.2007.04559.x. [DOI] [PubMed] [Google Scholar]
- Raji CA, Ho AJ, Parikshak NN, Becker JT, Lopez OL, Kuller LH, Hua X, Leow AD, Toga AW, Thompson PM. Brain structure and obesity. Hum Brain Mapp. 2010;31:353–364. doi: 10.1002/hbm.20870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit WJ. Microglia as neuroprotective, immunocompetent cells of the CNS. Glia. 2002;40:133–139. doi: 10.1002/glia.10154. [DOI] [PubMed] [Google Scholar]
- Tang H, Fu WY, Ip NY. Altered expression of tissue-type plasminogen activator and type 1 inhibitor in astrocytes of mouse cortex following scratch injury in culture. Neurosci Lett. 2000;285:143–146. doi: 10.1016/s0304-3940(00)00998-8. [DOI] [PubMed] [Google Scholar]
- Tang CH, Lu DY, Yang RS, Tsai HY, Kao MC, Fu WM, Chen YF. Leptin-induced IL-6 production is mediated by leptin receptor, insulin receptor substrate-1, phosphatidylinositol 3-kinase, Akt, NF-kappaB, and p300 pathway in microglia. J Immunol. 2007;179:1292–1302. doi: 10.4049/jimmunol.179.2.1292. [DOI] [PubMed] [Google Scholar]
- Tapanainen P, Leinonen E, Ruokonen A, Knip M. Leptin concentrations are elevated in newborn infants of diabetic mothers. Horm Res. 2001;55:185–190. doi: 10.1159/000049993. [DOI] [PubMed] [Google Scholar]
- Tikka TM, Koistinaho JE. Minocycline provides neuroprotection against N-methyl-D-aspartate neurotoxicity by inhibiting microglia. J Immunol. 2001;166:7527–7533. doi: 10.4049/jimmunol.166.12.7527. [DOI] [PubMed] [Google Scholar]
- Walther K, Birdsill AC, Glisky EL, Ryan L. Structural brain differences and cognitive functioning related to body mass index in older females. Hum Brain Mapp. 2010;31:1052–1064. doi: 10.1002/hbm.20916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Kastin AJ, He Y, Hsuchou H, Rood JC, Pan W. Essential role of interleukin-15 receptor in normal anxiety behavior. Brain Behav Immun. 2010;24:1340–1346. doi: 10.1016/j.bbi.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Cheng XP, Li JW, Yao Q, Ju G. De-differentiation response of cultured astrocytes to injury induced by scratch or conditioned culture medium of scratch-insulted astrocytes. Cell Mol Neurobiol. 2009;29:455–473. doi: 10.1007/s10571-008-9337-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu AC, Lee YL, Eng LF. Astrogliosis in culture: I. The model and the effect of antisense oligonucleotides on glial fibrillary acidic protein synthesis. J Neurosci Res. 1993;34:295–303. doi: 10.1002/jnr.490340306. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Wu X, He Y, Kastin AJ, Hsuchou H, Rosenblum CI, Pan W. Melanocortin potentiates leptin-induced STAT3 signaling via MAPK pathway. J Neurochem. 2009;110:390–399. doi: 10.1111/j.1471-4159.2009.06144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Hu X, Qian L, O'Callaghan JP, Hong JS. Astrogliosis in CNS pathologies: is there a role for microglia? Mol Neurobiol. 2010;41:232–241. doi: 10.1007/s12035-010-8098-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao XQ, Zhang MW, Wang F, Zhao YX, Li JJ, Wang XP, Bu PL, Yang JM, Liu XL, Zhang MX, Gao F, Zhang C, Zhang Y. CRP enhances soluble LOX-1 release from macrophages by activating TNF-alpha converting enzyme. J Lipid Res. 2011;52:923–933. doi: 10.1194/jlr.M015156. [DOI] [PMC free article] [PubMed] [Google Scholar]