Summary
Reasons for performing study
Infection with bovine papillomaviruses types 1 and 2 (BPV-1, BPV-2) can lead to the development of therapy-resistant skin tumours termed sarcoids and possibly other skin diseases in equids. Although sarcoids seriously compromise the welfare of affected animals and cause considerable economic losses, no prophylactic vaccine is available to prevent this common disease. In several animal species and man, immunisation with papillomavirus-like particles (VLP) has been shown to protect efficiently from papillomaviral infection.
Hypothesis
BPV-1 L1 VLPs may constitute a safe and highly immunogenic vaccine candidate for protection of horses against BPV-1/-2-induced disease.
Methods
Three groups of 4 horses each received 50, 100 or 150 μg of BPV-1 L1 VLPs, respectively, on Days 0, 28 and 168. Three control horses received adjuvant only. Horses were monitored on a daily basis for one week after each immunisation and then in 2 week intervals. Sera were collected immediately before, 2 weeks after each vaccination and one and 2 years after the final boost and analysed by pseudovirion neutralisation assay.
Results
None of the horses showed adverse reactions upon vaccination apart from mild and transient swelling in 2 individuals. Irrespective of the VLP dose, all VLP-immunised horses had developed a BPV-1-neutralising antibody titre of ≥1600 plaque forming units (pfu)/ml 2 weeks after the third vaccination. Eight of 10 trial horses still available for follow-up had neutralising antibody titres ≥1600 pfu/ml one year and ≥800 pfu/ml 2 years after the last immunisation.
Conclusion
Intramuscular BPV-1 L1 VLP vaccination in horses is safe and results in a long-lasting antibody response against BPV-1. Neutralisation titres were induced at levels that correlate with protection in experimental animals and man.
Potential relevance
BPV-1 L1 VLPs constitute a promising vaccine candidate for prevention of BPV-1/-2-induced disease in equids.
Keywords: horse, bovine papillomavirus, sarcoid, vaccine, virus-like particles
Introduction
Bovine papillomaviruses (BPV) are small nonenveloped viruses that consist of an icosahedral capsid harbouring a circular double-stranded DNA genome. The latter comprises an early (E) and a late (L) region coding for 6 functional (E1, E2, E4–E7) and 2 capsid proteins (L1, L2), respectively, and a long control region (LCR) required for replication and transcription of the viral genome (Campo 2006a). In cattle, BPV infection mainly results in the development of epithelial lesions. In immunocompetent animals, these lesions usually regress spontaneously. In some individuals, however, and particularly upon ingestion of immunosuppressants contained in bracken fern, lesions may progress to malignant carcinomas (Campo 2006b; Borzacchiello and Roperto 2008).
In addition to disease induced in cattle, BPVs of types 1 and 2 (BPV-1; BPV-2) contribute to the onset and progression of nonmetastasising yet locally aggressive skin tumours termed sarcoids (Nasir and Campo 2008). With a prevalence of 2–11.5% (Sullins et al. 1986; Studer et al. 2007), sarcoids represent the most common neoplasm in horses. Due to their location, extension and tendency to recur in a more progressive form upon therapeutical intervention or accidental trauma, sarcoids compromise the welfare of affected animals and lead to considerable economic losses (Scott and Miller 2003). This is even more so as no effective prophylaxis is available thus far.
Bovine papillomas are the result of a productive BPV infection, with high numbers of virions being assembled in the squamous epidermal layer and released (Campo 2006b). In equids, BPV infection is currently thought to be abortive, with virus solely residing in fibroblast in an episomal form (Amtmann et al. 1980; Lancaster 1981). However, there is increasing evidence towards sarcoids also harbouring and spreading infectious virions. Voss (1969) succeeded in transferring sarcoids from naturally affected to healthy horses by inoculating scarified skin with minced sarcoid suspension or cell free sarcoid extract supernatant. The possibility that infection of horses may be achieved by viral genome transduction has been refuted by Robl et al. (1972) who showed that tumour development can be experimentally induced by intracranial inoculation of hamsters with wild-type, but not with heat-denatured BPV-1 virion. In addition, we have recently shown that intradermal inoculation of foals with BPV-1 virion, but not with naked BPV-1 genome or sarcoid cells containing viral episomes, leads to pseudo-sarcoid formation (Hainisch et al. 2010). Using immunocapture PCR, we have detected BPV-1 genome-associated L1 capsomeres in a subset of sarcoids (Brandt et al. 2008). Moreover, we have recently demonstrated the presence of BPV-1 DNA and protein in sarcoid epidermis (Brandt et al. 2011a). Finally, co-stabling of sarcoid-affected with healthy donkeys has been shown to result in sarcoid development in the latter, thus providing strong evidence for a natural disease transmission from equid to equid (Nasir and Campo 2008) effected by de novo infection with virion.
In Europe, sarcoids are mainly caused by BPV-1, with BPV-2 being detected in only ~10% of lesions (Otten et al. 1993; Chambers et al. 2003). However, in the western USA, BPV-2 is responsible for 62% of sarcoids (Carr et al. 2001). BPV-1 and BPV-2 share a genetic similarity of 87%. In vitro, we have recently demonstrated that immunisation of rabbits with BPV-1 L1 VLPs induces neutralising antibodies to BPV-1 that also cross-neutralise BPV-2. Similarly, anti BPV-2 VLP sera raised in rabbits were shown to neutralise BPV-2 virions and to cross-neutralise BPV-1. By this, we could demonstrate that BPV-1 and BPV-2 are related serotypes (Shafti-Keramat et al. 2009).
Neutralising antibodies are considered the main protection factor against experimental and natural infection in animals and man (Breitburd et al. 1995; Paavonen et al. 2007). Natural immunity against BPV-1 and -2 in equids appears to be poor. Sarcoid-affected horses examined in this regard revealed no measurable BPV-1 L1 antibodies (Ashrafi et al. 2008; Mattil-Fritz et al. 2008). This circumstance may help to explain why sarcoids usually present as persistent lesions (Chambers et al. 2003). BPV may escape from immune surveillance because of its paramount localisation in cutaneous cells (O’Brien and Campo 2002), yet also by its capacity to inhibit MHC class I-mediated antigen presentation via its major oncoprotein E5 (Marchetti et al. 2009).
In the early 1990s, the discovery was made that recombinant papillomavirus major capsid protein L1 spontaneously self-assembles into virus-like particles (VLPs) (Kirnbauer et al. 1992; Hagensee et al. 1993; Rose et al. 1993). Papillomavirus-like particles are morphologically and immunologically very similar to the ordered 72 pentamer array of L1 major capsid protein that (together with 12 L2 minor capsid protein monomers) forms the outer shell of the authentic virion. This discovery led the way to VLP-based prophylactic vaccines against PV infection and PV-associated tumours (Kirnbauer 1996). VLPs do not contain viral DNA and are, therefore, nonpathogenic. However, they resemble authentic virions in their ability to induce high titres of type-specific neutralising antibodies, the prerequisite for effective prophylactic vaccination (Zinkernagel 2003; Schiller 2007). VLP vaccination was successful in preventing infection with cottontail rabbit papillomavirus (CRPV) in domestic rabbits (Breitburd et al. 1995), canine oral papillomavirus (COPV) in dogs (Suzich et al. 1995) and BPV type 4 (BPV-4) in cattle (Kirnbauer et al. 1996). Bivalent and tetravalent HPV VLP (HPV-6, -11, -16, -18) vaccines (Villa et al. 2005) have been made commercially available worldwide.
However, VLPs were not successful in inducing regression of pre-existing tumours in cattle and human patients, thus lacking therapeutic efficacy (Schiller 2007). Chimeric BPV-1 L1 VLPs expressing E7 oncoprotein (CVLP) were assessed as a possible therapeutic agent in sarcoid-bearing horses (Mattil-Fritz et al. 2008) and donkeys (Ashrafi et al. 2008), but immunisation did not lead to a significant reduction in the number of sarcoids.
Based on the circumstance that equine sarcoids develop upon naturally acquired BPV-1/-2 infection, we hypothesised that BPV-1 VLPs are safe and immunogenic in horses and thus have the potential to protect equids from this common tumour disease. We addressed this assumption in a dose escalation trial in horses.
Materials and methods
The animal trial was approved by the institutional ethics committee, the Advisory Committee for Animal Experiments (§12 of Law for Animals experiments, Tierversuchsgesetz - TVG) and the Austrian Federal Ministry for Science and Research (animal experiment license No. BMBWK-68.205/ 0022-BrGT72007).
Animals
Twenty-four horses belonging to the Veterinary University of Vienna were clinically examined as to general health and the presence of BPV-associated malignancies. Full thickness punch biopsies were obtained from the skin of the neck and their DNA subjected to PCR for BPV-1/-2 DNA as described earlier (Brandt et al. 2008). Blood was taken and serum tested in a BPV-1 pseudovirion neutralisation assay as described below. From 23/24 animals that had scored negative by all tests, 8 were excluded on the grounds of various health problems. The remaining 15 horses were enrolled in the study and assigned to 4 different groups (Table 1).
Table 1. Immunisation scheme.
| Group | VLP antigen dose | Horse | Age | Sex | Breed |
|---|---|---|---|---|---|
| 1 | Adjuvant only | 1 | 24 | G | WB |
| (Al(OH)3, 250 μg) | 2 | 8 | M | SB | |
| 3 | 18 | G | SB | ||
| 2 | 50 μg | 4 | 7 | M | SB |
| 5 | 7 | G | SB | ||
| 6 | 17 | G | WB | ||
| 7 | 19 | G | TB | ||
| 3 | 100 μg | 8 | 17 | G | WB |
| 9 | 18 | G | WB | ||
| 10 | 20 | M | WB | ||
| 11 | 20 | M | WB | ||
| 4 | 150 μg | 12 | 4 | G | SB |
| 13 | 4 | G | SB | ||
| 14 | 19 | G | WB | ||
| 15 | 30 | G | WB |
Incremental doses of antigen as indicated were injected deep intramuscularly into the left side of the neck (Groups 2, 3, 4). Three control horses (Group 1) received alum hydroxide adjuvant only. Age, gender and breed are indicated (G: gelding, M: mare; SB: Standardbred trotter, TB: Thoroughbred, WB: Warmblood).
Generation of BPV-1 L1 VLPs
To generate the VLPs, Sf9 cells were infected with BPV-1 L1 recombinant baculovirus, and high molecular weight complexes purified on density gradients. Aliquots were tested for VLP content by SDS-PAGE, Coomassie staining and transmission electron microscopy (TEM). Aliquots were then frozen and stored for further use at −24°C (Kirnbauer et al. 1992, 1993, 1996; Kirnbauer 1996).
Immunisation and monitoring
Vaccines were prepared freshly on the day of vaccination, with 250 μg Al(OH)3 per dose as adjuvant (Lindblad 2004). Phosphate buffered saline (PBS) was added to a final volume of 1.5 ml per dose. Three groups of 4 horses each (Groups 2–4), were immunised with doses of 50, 100 or 150 μg of BPV-1 VLPs, respectively, by deep intramuscular injection into the left side of the neck. Three horses formed the control group (Group 1) and received adjuvant in PBS. The horses were vaccinated 3 times, i.e. on Day 0, after 4 weeks and after 6 months. Horses were monitored on a daily basis for one week after each immunisation and then in 2 weeks intervals. Serum for neutralisation assay was collected immediately before and 14 days after each vaccination, as well as one and 2 years after the last boost.
Determining neutralising antibody titres
Neutralising antibody titres were determined by BPV-1 pseudovirion (PsV) neutralisation assay. PsVs are synthetic virus-like particles containing a plasmid vector encoding a reporter gene. Upon in vitro infection of permissive cell lines by PsVs, the plasmid is replicated to high copy numbers, which in turn leads to high-level expression of the reporter gene. Under the presence of PsV-neutralising serum, the expression of the reporter gene is correspondingly reduced, and thus inversely proportional to the amount of neutralising serum antibody (Pastrana et al. 2004). The BPV-1 PsVs used in this assay were produced as described by Buck et al. (2004, 2008) and contained a plasmid coding for secreted placental alkaline phosphatase (SEAP). BPV-1 PsVs were incubated in triplicates with 2-fold serial dilution of horse sera (1:50 to 1:25,600), plated on 293 TT cells for 48 h and alkaline phosphatase secreted into the cell culture medium was measured as mean ± s.d. optical density (OD) (Schiller 1997, 1998; Pastrana et al. 2004)
In order to be able to determine neutralising serum antibody titres and monitor inter-assay variability, the following controls have been included: 293TT cells in growth medium (background control), PsV-infected 293TT cells without antiserum (no serum control used as reference; maximal AP secretion of 100%), 293TT cells treated with PsV preincubated with neutralising monoclonal anti-BPV-1 L1 antibody 5B6 (positive control) and cells treated with PsV preincubated with unspecific monoclonal anti-HPV-16 antibody V5 (negative control). Serum dilutions causing at least a 50% reduction in SEAP in comparison to the no serum control were considered as being neutralising.
Results
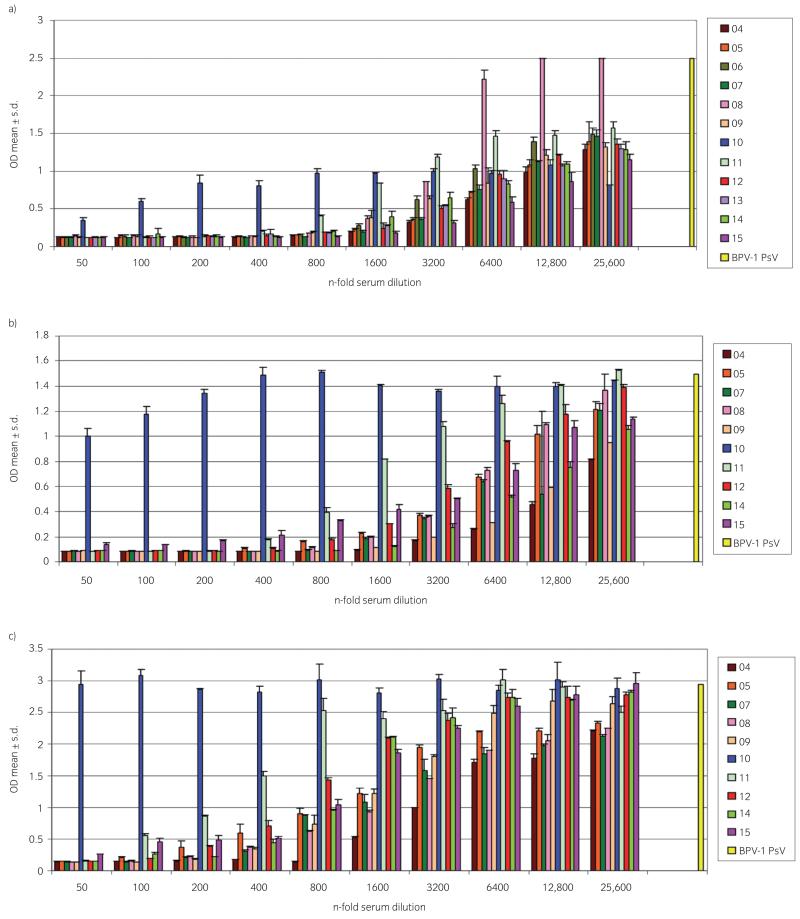
Apart from mild transient swelling in 2 horses on Day 10 after the second immunisation no adverse reactions, i.e. changes in body temperature or inflammation/swelling at the injection sites were recorded. All BPV-1 VLP-vaccinated horses seroconverted after the second shot, with detectable neutralising antibody titres ≥50 (data not shown). Two weeks after the third immunisation, all VLP-vaccinated horses had developed neutralising antibody titres ranging from 1600–12,800 plaque forming units (pfu)/ml (Fig 1a). As expected, none of the immune sera obtained from control horses (Horses 1, 2, 3) were neutralising at the lowest dilution 1:50 (data not shown).
Figure 1. VLP-induced neutralising serum antibody titres 2 weeks, one year and 2 years after the third immunisation.
BPV-1 pseudovirions (PsV) were incubated with serial dilutions of horse serum and then plated on 293 TT cells. Release of SEAP into culture medium was measured as mean ± s.d. OD and inversely correlates with PsV neutralisation. Sera were deemed neutralising when causing a SEAP reduction of at least 50% in comparison to the no serum control (BPV-1 PsV; 100%). a) Irrespective of the VLP dose, all sera revealed neutralising antibody titres ≥1600 pfu/ml. b) One year after the third immunisation, sera of all but one horse (Horse 10) showed neutralising antibody titres ≥800 pfu/ml. Unfortunately, Horses 6 and 13 were not available for long-term follow-up. c) 2 years after the third immunisation, all sera showed neutralising titres ≥800 pfu/ml apart from sera of Horses 10 and 11.
One year after the third vaccination, Horses 1, 6 and 13 were unavailable for follow-up due to reasons unrelated to this trial. Sera from 9 of 10 still available VLP-immunised horses revealed neutralising antibody titres ranging from 800–12,800 pfu/ml, while serum from Horse 10 had lost its neutralising capacity (Fig 1b). Two years after the third immunisation, sera from 8 of 10 horses still revealed neutralising antibody titres ranging from 800–3200 pfu/ml. As, expected serum of Horse 10 tested negative and serum of Horse 11 now revealed titres <400 pfu/ml (Fig 1c). The 2 remaining control horses scored negative in this assay, as anticipated (data not shown).
Discussion
Immunisation with PV VLPs has been shown to be well tolerated in equids (Ashrafi et al. 2008; Mattil-Fritz et al. 2008), rabbits (Breitburd et al. 1995), dogs (Suzich et al. 1995), cattle (Kirnbauer et al. 1996) and man (Villa et al. 2005). The fact that vaccination with BPV-1 VLPs produced no significant adverse reactions in our study is encouraging for its intended use as a prophylactic vaccine. Mattil-Fritz et al. (2008) have conducted a dose-escalation trial in 12 sarcoid-affected horses to determine the therapeutic potential of chimaeric virus-like particles (CVLPs of BPV-1 L1-E7). Using the described protocol herein, Mattil-Fritz et al. (2008) analysed 1:1000 diluted horse sera collected 3 weeks after the second immunisation (Day 21) for their ability to neutralise infectious BPV-1 L1/L2 pseudovirions. In agreement with the current data, seroconversion could be achieved in 11 out of 12 animals. Both reports also agree in that different CVLP doses (40–400 μg) induce comparable anti-L1 IgG levels (Mattil-Fritz et al. 2008; Fig 1). Whereas the work of Mattil-Fritz et al. (2008) otherwise focuses on the therapeutic effect of CVLPs, the current study was designed to specifically address the duration and stability of BPV-1 L1 VLPs as potential prophylactic vaccine candidate. Accordingly, presence of VLP-induced neutralising antibodies was monitored over a period of 2.5 years and antibody levels determined by titration at several points in time, i.e. before and 2 weeks after each of 3 immunisations and one and 2 years after the final boost.
Neutralising antibodies raised by PV VLP vaccination are protective and usually type-restricted. For example, rabbits vaccinated with CRPV VLP produced a significantly lower number of skin lesions after experimental infection with CRPV virions and none developed cancer. In contrast, immunisation of rabbits with BPV-1 VLP could not prevent CRPV infection and subsequent papilloma formation (Breitburd et al. 1995). Vaccination of dogs with COPV VLPs resulted in complete protection against COPV-induced oral mucosal papillomas (Suzich et al. 1995). Similarly, 13 of 15 BPV-4 VLP-immunised calves did not develop oral papillomas upon experimental infection, whereas disease developed in 9 of 10 control animals (Kirnbauer et al. 1996). In young women immunised with a quadrivalent VLP vaccine, the combined incidence of persistent infection with or disease induced by HPV-6, -11, -16 or -18 decreased by 90% in comparison to the placebo group (Villa et al. 2005).
High titres of neutralising antibody were obtained in all of the vaccinated horses after the third vaccination. No correlation between antibody titres and administered antigen dose has been observed, indicating that the lowest dose of 50 μg/BPV1 VLP may suffice to achieve maximum immune responses. This agrees with the observation that PV VLPs are highly immunogenic even at small doses and without the use of adjuvant (Schiller 2007).
One year after the third immunisation, high titres of neutralising antibodies were still detectable in VLP-immunised horses, except in one 20-year-old mare. It has been shown that older horses have an impaired immune response to vaccinations (Fermaglich and Horohov 2002), although Horse 15 (age 30 years; neutralising antibody titre of 800 pfu/ml 2 years after the last immunisation) does not respect this rule. As with in young human subjects, longer lasting antibody titres may be expected in younger horses (Villa et al. 2005).
The minimal antibody titres required for VLP vaccination-mediated protection against PV infection have not yet been determined for man or animals. This is mainly due to the high prophylactic efficacy of VLP-based vaccines. Although a substantial number of vaccinates have become sero-negative to HPV-18 in one vaccine trial at end-of-study, protection against HPV-18 induced disease remained high, indicating protection through an anamnestic response following exposure (or re-challenge by vaccination) (Joura et al. 2008).
Antibody responses to VLP vaccination are mainly type-restricted, yet cross-neutralising immune responses have been observed that confer partial cross-protection to closely related HPV types in human vaccine trials (Roden et al. 1996; Pastrana et al. 2005; Villa et al. 2005). BPV-1 and BPV-2 are closely related genotypes. Their respective L1 capsid protein sequences have homology of 92%, which is comparable to that between L1s of closely related HPV types that share several cross-neutralisation epitopes. We have recently re-evaluated the serological relationship of BPV-1 and BPV-2. Using BPV-1 and BPV-2 neutralisation assays, we have demonstrated that VLP vaccination with either type was able to induce neutralising antibodies to both the homologous and the heterologous type (Shafti-Keramat et al. 2009). This established that BPV-1 and BPV-2 are closely related serotypes and indicates that vaccination with BPV-1 L1 VLP can protect against infections with homologous BPV-1 and heterologous BPV-2.
Bovine papillomavirus type 1 DNA has also been detected in some cases of equine inflammatory skin disease (Yuan et al. 2007) and was consistently found in lesions, intact skin and blood of horses affected by hoof canker (Brandt et al. 2011b). These findings indicate that BPV-1 may also be involved in the pathogenesis of other equine skin diseases. In this case, a prophylactic BPV-1 vaccine may have an even broader protective significance.
In the present study, we were able to demonstrate that BPV-1 VLPs in horses are safe and highly immunogenic in the vast majority of horses. In addition, we provide further evidence that low VLP doses (≤50 μg) may be sufficient to induce protection. In man, a 3 dose regimen of quadrivalent HPV vaccine has been shown to induce stable neutralising anti-HPV antibody levels for at least 5 years and a robust immune memory (Olsson et al. 2007). This finding and high neutralising antibody titres still present in most horse sera 2 years after the third immunisation are indicative for a long-lasting effect of vaccination. A virus challenge study aiming at determining the prophylactic potential of BPV-1 VLPs in equids is currently in preparation.
Acknowledgments
Source of funding: This study was funded by AVIR Green Hills Biotechnology, Vienna, and the Centre for Innovation and Technology of the City of Vienna (ZIT), Austria.
Footnotes
Authors’ declaration of interests: No conflicts of interest have been declared.
References
- Amtmann E, Müller H, Sauer G. Equine connective tissue tumours contain unintegrated bovine papilloma virus DNA. J. Virol. 1980;35:962–964. doi: 10.1128/jvi.35.3.962-964.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashrafi GH, Piuko K, Burden F, Yuan Z, Gault EA, Muller M, Trawford A, Reid SW, Nasir L, Campo MS. Vaccination of sarcoid-bearing donkeys with chimeric virus-like particles of bovine papillomavirus type 1. J. Gen. Virol. 2008;89:148–157. doi: 10.1099/vir.0.83267-0. [DOI] [PubMed] [Google Scholar]
- Borzacchiello G, Roperto F. Bovine papillomaviruses, papillomas and cancer in cattle. Vet. Res. 2008;39:45. doi: 10.1051/vetres:2008022. doi: 10.1051/vetres:2008022. [DOI] [PubMed] [Google Scholar]
- Brandt S, Haralambus R, Shafti-Keramat S, Steinborn R, Stanek C, Kirnbauer R. A subset of equine sarcoids harbours BPV-1 DNA in a complex with L1 major capsid protein. Virology. 2008;375:433–441. doi: 10.1016/j.virol.2008.02.014. [DOI] [PubMed] [Google Scholar]
- Brandt S, Tober R, Corteggio A, Burger S, Sabitzer S, Walter I, Kainzbauer C, Steinborn R, Nasir L, Borzacchiello G. BPV-1 infection is not confined to the dermis but also involves the epidermis of equine sarcoids. Vet. Microbiol. 2011a;150:35–40. doi: 10.1016/j.vetmic.2010.12.021. doi:10.1016/j.vetmic.2010.12.021. [DOI] [PubMed] [Google Scholar]
- Brandt S, Schoster A, Tober R, Kainzbauer C, Burgstaller JP, Haralambus R, Steinborn R, Hinterhofer C, Stanek C. Consistent detection of bovine papillomavirus in lesions, intact skin and peripheral blood mononuclear cells of horses affected by hoof canker. Equine vet. J. 2011b;43:202–209. doi: 10.1111/j.2042-3306.2010.00147.x. doi: 10.1111/j.2042-3306.2010.00147.x. [DOI] [PubMed] [Google Scholar]
- Breitburd F, Kirnbauer R, Hubbert NL, Nonnenmacher B, Trin-Dinh-Desmarquet C, Orth G, Schiller JT, Lowy DR. Immunization with virus-like particles from cottontail rabbit papillomavirus (CRPV) can protect against experimental CRPV infection. J. Virol. 1995;69:3959–3963. doi: 10.1128/jvi.69.6.3959-3963.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck CB, Pastrana DV, Lowy DR, Schiller JT. Efficient intracellular assembly of papillomaviral vectors. J. Virol. 2004;78:751–757. doi: 10.1128/JVI.78.2.751-757.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck CB, Pastrana DV, Lowy DR, Schiller JT. [Last update 2008, 01.02.2009. Accessed 2009, 12/02];Production of papillomaviral vectors (pseudoviruses) 2008 http://home.ccr.cancer.gov/lco/pseudovirusproduction.htm
- Campo MS. Introduction. In: Campo MS, editor. Papillomavirus Research: From Natural History to Vaccines and Beyond. Caister Academic Press; Norfolk: 2006a. pp. 1–2. [Google Scholar]
- Campo MS. Bovine papillomavirus: Old system, new lessons? In: Campo MS, editor. Papillomavirus Research: From Natural History to Vaccines and beyond. Caister Academic Press; Norfolk: 2006b. pp. 373–387. [Google Scholar]
- Carr EA, Theon AP, Madewell BR, Griffey SM, Hitchcock ME. Bovine papillomavirus DNA in neoplastic and nonneoplastic tissues obtained from horses with and without sarcoids in the western United States. Am. J. vet. Res. 2001;62:741–744. doi: 10.2460/ajvr.2001.62.741. [DOI] [PubMed] [Google Scholar]
- Chambers G, Ellsmore VA, O’Brien PM, Reid SW, Love S, Campo MS, Nasir L. Association of bovine papillomavirus with the equine sarcoid. J. Gen. Virol. 2003;84:1055–1062. doi: 10.1099/vir.0.18947-0. [DOI] [PubMed] [Google Scholar]
- Fermaglich DH, Horohov DW. The effect of aging on immune responses. Vet. Clin. N. Am.: Equine Pract. 2002;18:621–630. doi: 10.1016/s0749-0739(02)00027-5. [DOI] [PubMed] [Google Scholar]
- Hagensee ME, Yaegashi N, Galloway DA. Self-assembly of human papillomavirus type 1 capsids by expression of the L1 protein alone or by coexpression of the L1 and L2 capsid proteins. J. Virol. 1993;67:315–322. doi: 10.1128/jvi.67.1.315-322.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hainisch EK, Hartl B, Kainzbauer C, Tober R, Shafti-Keramat S, Kirnbauer R, Brandt S. Intact BPV-1 virion is required for experimental tumour induction in foals and causes viraemia. Proceedings of the 26th International Papillomavirus Conference; Montreal, Canada. July 3-8 2010; 2010. [Google Scholar]
- Joura EA, Kjaer SK, Wheeler CM, Sigurdsson K, Iversen O, Hernandez-Avila M, Perez G, Brown DR, Koutsky LA, Tay EH, García P, Ault KA, Garland SM, Leodolter S, Olsson SE, Tang GW, Ferris DG, Paavonen J, Lehtinen M, Steben M, Bosch X, Dillner J, Kurman RJ, Majewski S, Muñoz N, Myers ER, Villa LL, Taddeo FJ, Roberts C, Tadesse A, Bryan J, Lupinacci LC, Giacoletti KE, Lu S, Vuocolo S, Hesley TM, Haupt RM, Barr E. HPV antibody levels and clinical efficacy following administration of a prophylactic quadrivalent HPV vaccine. Vaccine. 2008;26:6844–6851. doi: 10.1016/j.vaccine.2008.09.073. [DOI] [PubMed] [Google Scholar]
- Kirnbauer R. Papillomavirus-like particles for serology and vaccine development. Intervirology. 1996;39:54–61. doi: 10.1159/000150475. [DOI] [PubMed] [Google Scholar]
- Kirnbauer R, Booy F, Cheng N, Lowy DR, Schiller JT. Papillomavirus L1 major capsid protein self-assembles into virus-like particles that are highly immunogenic. Proc. Natl. Acad. Sci. USA. 1992;89:12180–12184. doi: 10.1073/pnas.89.24.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirnbauer R, Chandrachud LM, O’Neil BW, Wagner ER, Grindlay GJ, Armstrong A, McGarvie GM, Schiller JT, Lowy DR, Campo MS. Virus-like particles of bovine papillomavirus type 4 in prophylactic and therapeutic immunization. Virology. 1996;219:37–44. doi: 10.1006/viro.1996.0220. [DOI] [PubMed] [Google Scholar]
- Kirnbauer R, Taub J, Greenstone H, Roden R, Durst M, Gissmann L, Lowy DR, Schiller JT. Efficient self-assembly of human papillomavirus type 16 L1 and L1-L2 into virus-like particles. J. Virol. 1993;67:6929–6936. doi: 10.1128/jvi.67.12.6929-6936.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster WD. Apparent lack of integration of bovine papillomavirus DNA in virus induced equine and bovine tumour cells and virus transformed mouse cells. Virology. 1981;108:251–255. doi: 10.1016/0042-6822(81)90433-5. [DOI] [PubMed] [Google Scholar]
- Lindblad EB. Aluminium compounds for use in vaccines. Immunol. Cell Biol. 2004;82:497–505. doi: 10.1111/j.0818-9641.2004.01286.x. [DOI] [PubMed] [Google Scholar]
- Marchetti B, Gault EA, Cortese MS, Yuan Z, Ellis SA, Nasir L, Campo MS. Bovine papillomavirus type 1 oncoprotein E5 inhibits equine MHC class I and interacts with equine MHC I heavy chain. J. Gen. Virol. 2009;90:2865–2870. doi: 10.1099/vir.0.014746-0. [DOI] [PubMed] [Google Scholar]
- Mattil-Fritz S, Scharner D, Piuko K, Thones N, Gissmann L, Müller H, Müller M. Immunotherapy of equine sarcoid: Dose-escalation trial for the use of chimeric papillomavirus-like particles. J. Gen. Virol. 2008;89:138–147. doi: 10.1099/vir.0.83266-0. [DOI] [PubMed] [Google Scholar]
- Nasir L, Campo MS. Bovine papillomaviruses: Their role in the aetiology of cutaneous tumours of bovids and equids. Vet. Dermatol. 2008;19:243–254. doi: 10.1111/j.1365-3164.2008.00683.x. [DOI] [PubMed] [Google Scholar]
- O’Brien PM, Campo MS. Evasion of host immunity directed by papillomavirus-encoded proteins. Virus Res. 2002;88:103–117. doi: 10.1016/s0168-1702(02)00123-5. [DOI] [PubMed] [Google Scholar]
- Olsson SE, Villa LL, Costa RL, Petta CA, Andrade RP, Malm C, Iversen OE, Høye J, Steinwall M, Riis-Johannessen G, Andersson-Ellstrom A, Elfgren K, von Krogh G, Lehtinen M, Paavonen J, Tamms GM, Giacoletti K, Lupinacci L, Esser MT, Vuocolo SC, Saah AJ, Barr E. Induction of immune memory following administration of a prophylactic quadrivalent human papillomavirus (HPV) types 6/11/16/18 L1 virus-like particle (VLP) vaccine. Vaccine. 2007;25:4931–4939. doi: 10.1016/j.vaccine.2007.03.049. [DOI] [PubMed] [Google Scholar]
- Otten N, von Tscharner C, Lazary S, Antczak DF, Gerber H. DNA of bovine papillomavirus type 1 and 2 in equine sarcoids: PCR detection and direct sequencing. Arch. Virol. 1993;132:121–131. doi: 10.1007/BF01309847. [DOI] [PubMed] [Google Scholar]
- Paavonen J, Jenkins D, Bosch FX, Naud P, Salmerón J, Wheeler CM, Chow S, Apter DL, Kitchener HC, et al. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: An interim analysis of a phase III double-blind, randomised controlled trial. Lancet. 2007;369:2161–2170. doi: 10.1016/S0140-6736(07)60946-5. [DOI] [PubMed] [Google Scholar]
- Pastrana DV, Buck CB, Pang YY, Thompson CD, Castle PE, Fitzgerald PC, Kruger Kjaer S, Lowy DR, Schiller JT. Reactivity of human sera in a sensitive, high-throughput pseudovirus-based papillomavirus neutralization assay for HPV16 and HPV18. Virology. 2004;321:205–216. doi: 10.1016/j.virol.2003.12.027. [DOI] [PubMed] [Google Scholar]
- Pastrana DV, Gambhira R, Buck CB, Pang Y-S, Thompson CD, Culp TD, Christensen ND, Lowy DR, Schiller JT, Roden RBS. Cross-neutralization of cutaneous and mucosal papillomavirus types with anti-sera to the amino terminus of L2. Virology. 2005;337:365–372. doi: 10.1016/j.virol.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Robl MG, Gordon DE, Lee KP, Olson C. Intracranial fibroblastic neoplasms in the hamster from bovine papilloma virus. Cancer Res. 1972;32:2221–2225. [PubMed] [Google Scholar]
- Roden RBS, Hubbert NL, Kirnbauer R, Christensen ND, Lowy DR, Schiller JT. Assessment of the serological relatedness of genital human papillomaviruses by hemagglutination inhibition. J. Virol. 1996;70:3298–3301. doi: 10.1128/jvi.70.5.3298-3301.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose RC, Bonnez W, Reichman RC, Garcea RL. Expression of human papillomavirus type 11 L1 protein in insect cells: In vivo and in vitro assembly of virus-like particles. J. Virol. 1993;67:1936–1944. doi: 10.1128/jvi.67.4.1936-1944.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller JT. [Last update 1997, 30.6.1997. Accessed 2009, 12/02];pSHELL. 1997 http://home.ccr.cancer.gov/lco/pSheLL.htm
- Schiller JT. [Last update 1998, 10.3.1998. Accessed 2009, 12/02];pYSEAP. 1998 http://home.ccr.cancer.gov/lco/pYSEAP.htm
- Schiller JT. Papillomavirus vaccines. In: Garcia R, DiMaio D, editors. The Papillomaviruses. Springer; New York: 2007. pp. 337–369. [Google Scholar]
- Scott DW, Miller WH., Jr . Equine Dermatology. Saunders, Elsevier Science; St Louis: 2003. Sarcoid; pp. 719–731. [Google Scholar]
- Shafti-Keramat S, Schellenbacher C, Handisurya A, Christensen N, Reininger B, Brandt S, Kirnbauer R. Bovine papillomavirus type 1 (BPV1) and BPV2 are closely related serotypes. Virology. 2009;393:1–6. doi: 10.1016/j.virol.2009.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studer S, Gerber V, Straub R, Brehm W, Gaillard C, Luth A, Burger D. Prevalence of hereditary diseases in three-year-old Swiss Warmblood horses. Schweiz. Arch. Tierheilkd. 2007;149:161–171. doi: 10.1024/0036-7281.149.4.161. [DOI] [PubMed] [Google Scholar]
- Sullins KE, Roberts SM, Lavach JD, Severin GA. Equine sarcoid. Equine Pract. 1986;8:21–27. [Google Scholar]
- Suzich JA, Ghim SJ, Palmer-Hill FJ, White WI, Tamura JK, Bell JA, Newsome JA, Jenson AB, Schlegel R. Systemic immunization with papillomavirus L1 protein completely prevents the development of viral mucosal papillomas. Proc. Natl. Acad. Sci. USA. 1995;92:11553–11557. doi: 10.1073/pnas.92.25.11553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa LL, Costa RL, Petta CA, Andrade RP, Ault KA, Giuliano AR, Wheeler CM, Koutsky LA, Malm C. Prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in young women: A randomised double-blind placebo-controlled multicentre phase II efficacy trial. Lancet Oncol. 2005;6:271–278. doi: 10.1016/S1470-2045(05)70101-7. [DOI] [PubMed] [Google Scholar]
- Voss JL. Transmission of equine sarcoid. Am. J. vet. Res. 1969;30:183–191. [PubMed] [Google Scholar]
- Yuan Z, Philbey AW, Gault EA, Campo MS, Nasir L. Detection of bovine papillomavirus type 1 genomes and viral gene expression in equine inflammatory skin conditions. Virus Res. 2007;124:245–249. doi: 10.1016/j.virusres.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Zinkernagel RM. On natural and artificial vaccinations. Annu. Rev. Immunol. 2003;21:515–546. doi: 10.1146/annurev.immunol.21.120601.141045. [DOI] [PubMed] [Google Scholar]