Abstract
Objective
To test the hypothesis that administration of indomethacin prophylaxis before 6 hours of life results in a lower incidence of intraventricular hemorrhage (IVH) compared with administration after 6 hours of life, and that the effects of early prophylaxis depend on gestational age (GA) and sex in very low birth weight infants (birth weight <1250 g).
Study design
Very low birth weight infants admitted to our neonatal intensive care unit between 2003 and 2010 who received indomethacin prophylaxis were analyzed retrospectively. Exclusion criteria included unknown time of indomethacin prophylaxis, death at <12 hours of life, congenital anomalies, and unavailable head ultrasound report. Infants were dichotomized based on the timing of indomethacin prophylaxis (<6 hours or >6 hours of life) to compare incidence of IVH all grades and severe (grade 3–4) IVH. Secondary analyses examined the effects of the time of indomethacin prophylaxis initiation by GA and sex on the incidence of IVH.
Results
A total of 868 infants (431 males and 437 females) met the criteria for analysis. Indomethacin prophylaxis was given at <6 hours of life in 730 infants and at >6 hours of life to 168 infants. The 2 groups differed with respect to antenatal steroid exposure, GA, outborn prevalence, and pneumothoraces. After multivariate analysis, there were no between-group differences in all-grade or severe IVH. However, females, but not males, treated at <6 hours of life had a lower incidence of severe IVH (P < .05), particularly at lower GAs.
Conclusion
Prophylactic indomethacin administered before 6 hours of life is not associated with lower incidence of IVH.
The incidence of intraventricular hemorrhage (IVH) in very low birth weight (VLBW) infants has decreased progressively since the early 1980s1,2 but no further decline has been reported over the last decade.3,4 Approximately 12 000 preterm infants develop IVH each year in the US.5 Preterm infants with grade 3–4 IVH are at high risk for death or impaired neurodevelopmental outcomes.6–9 Indomethacin prophylaxis reduces the incidence and severity of IVH in VLBW infants.10–12 Variations in clinical practice in the US make it difficult to estimate the use of indomethacin prophylaxis for IVH prevention. However, based on the Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network (NRN) database (2003–2010), approximately one-third of infants with a birth weight <1000 g receive indomethacin prophylaxis.
Indomethacin inhibits the cyclooxygenase pathways of prostaglandin synthesis, attenuates cerebral vascular hyperemic responses from hypoxia and hypercapnia, prevents ischemia-related impaired cerebral perfusion, and increases blood-brain barrier permeability.13–18 Indomethacin also promotes microvessel maturation in the germinal matrix.19 Hypoxia, hypercapnia, hypotension,20 and resulting changes in perfusion are more likely to occur shortly after birth in preterm infants. In addition, more than 50% of all cases of IVH in VLBW infants occur within the first 6–8 hours of life irrespective of gestational age (GA).21 One of the mechanisms underlying the increased risk of IVH may be high levels of vascular endothelial growth factor and angiopoietin 2 in the germinal matrix,5 which diminish within hours after birth.5 These changes could contribute to the lower risk of IVH with advancing postnatal age.
Based on 2 previous large randomized clinical trials, 2 time intervals were suggested for indomethacin prophylaxis: between 6 and 12 hours of life11 and <6 hours of life.12 However, in practice, the initial dose of indomethacin is administered over a wide range of time intervals. Because IVH develops most frequently during the first few hours of life and intravenous indomethacin has a rapid onset of action, it was plausible to consider that giving the drug within the first 6 hours of life would result in a lower incidence of IVH compared with administration after 6 hours of life. Therefore, the primary hypothesis of the present study was that administration of indomethacin prophylaxis before 6 hours of life results in a lower incidence of IVH than administration after 6 hours of life in infants weighing <1250 g at birth. A secondary objective was to examine the effects of the timing of the initiation of indomethacin prophylaxis by GA and sex on the incidence of IVH, because both of these variables are independent risk factors for IVH.22,23
Methods
In this retrospective cohort study, after receiving Internal Review Board approval and permission from the NRN, we retrieved the data for our center from the NRN’s Generic Database (2003–2010). This database is an ongoing survey of neonatal morbidity and mortality in which trained nurses abstract data from medical records guided by a manual of practice. We screen all VLBW infants for IVH by ultrasound on days 1, 3, 7, and 28 of life. Imaging is more frequent for infants with IVH, depending on severity. The cranial ultrasound image showing the most severe findings within the first 28 days after birth was used for determination of IVH. Ultrasound findings were used to establish the grade of IVH according to the classification scheme of Papile et al.24 For the purpose of this study, severe IVH includes grade 3 and grade 4 IVH.
We routinely prescribe indomethacin prophylaxis for preterm infants weighing <1250 g at birth to reduce the risk of IVH. However, the timing of administration varies depending on the infant’s clinical condition, birth site (inborn or outborn), and individual physician preference. We included all infants with birth weight <1250 g who received indomethacin prophylaxis at an identified time and had available cranial ultrasound results before 28 days of age. Exclusion criteria included death within 12 hours after birth, congenital anomalies, and an unavailable head ultrasound findings.
We divided the cohort into 2 groups based on the timing of the first dose of prophylactic indomethacin: indomethacin administration at <6 hours or >6 hours of life. Primary outcomes were all-grade IVH and severe IVH based on the worst head ultrasound image within the first 28 days of life. The incidences of all-grade and severe IVH were calculated in each group. Maternal variables, including labor and delivery, and neonatal variables were compared between the groups using t tests for continuous variables and χ2 tests for categorical variables. Multiple logistic regression analysis was used to adjust for significant differences between the 2 groups. We also performed a secondary analysis to examine the influence of the time of initiation of indomethacin prophylaxis on all-grade IVH and severe IVH separately for males and females at 3 GA ranges: <25 weeks, 25–27 weeks, and >27 weeks. Values are presented as mean ± SD or percent. Differences are considered statistically significant at P < .05. Results of logistic regressions are expressed as an OR and 95% CI.
Before initiating this retrospective study, power analysis was performed based on a 1-year sample from the NRN database at Brown University. In our neonatal intensive care unit, indomethacin prophylaxis was administered to 75% infants at <6 hours of life. At the same time, incidence of all grades of IVH was 32%. Therefore, for 80% power and α = 0.05, detection of a 10% reduction in IVH by administration of indomethacin prophylaxis at < 6 hours of life required 804 infants.
Results
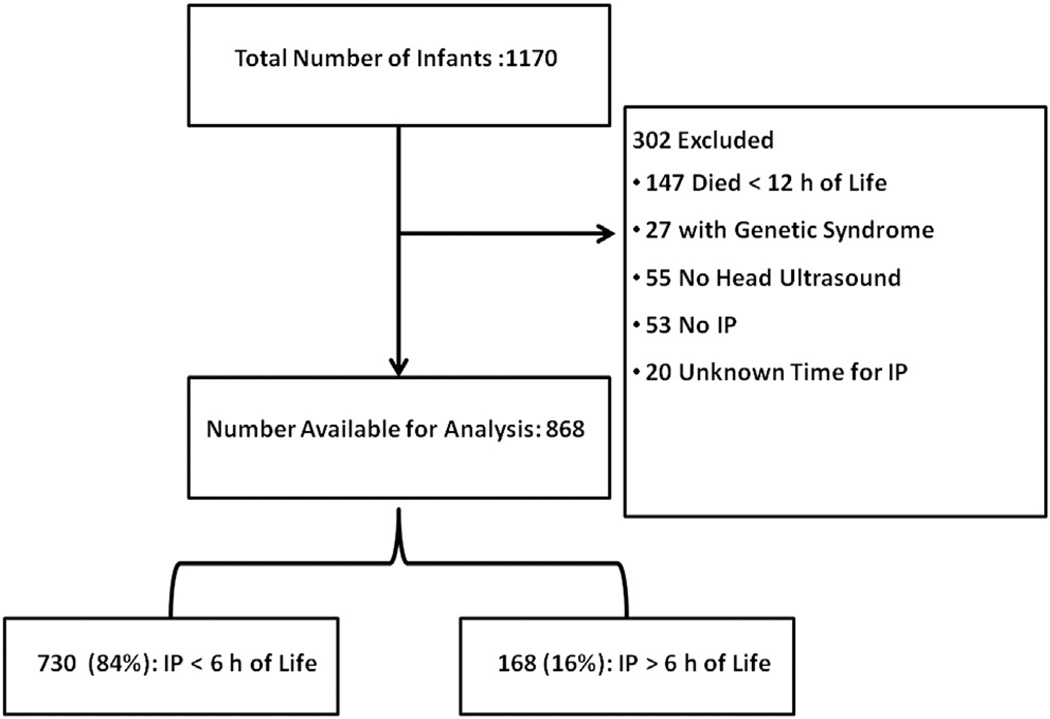
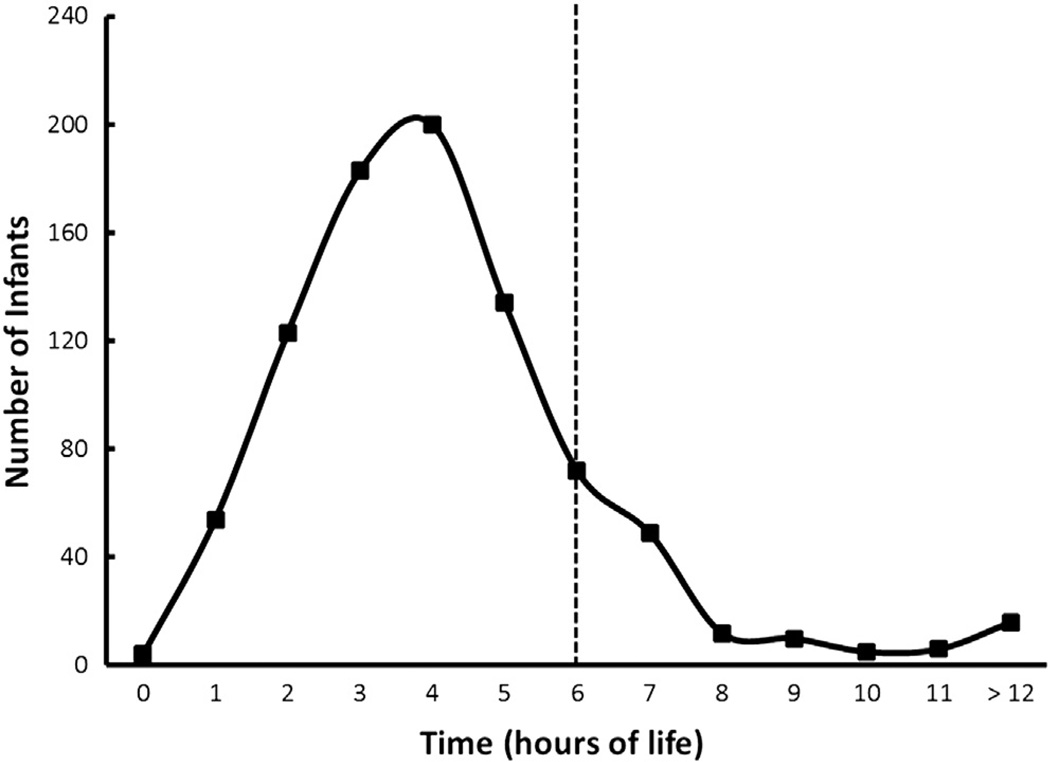
During the study period, 1170 infants were eligible for enrollment. Three hundred and two infants were excluded for a various reasons, leaving 868 infants available for analysis (Figure 1; available at www.jpeds.com). Of these, 730 (84%) received indomethacin prophylaxis at <6 hours and 168 (16%) received indomethacin prophylaxis at > 6 hours of age. The mean time of administration for the first prophylactic dose of indomethacin was 3.5 ± 1.3 hours (range 0.2–5.9 hours) in the <6-hour group, compared with 8.7 ± 4.6 hours (range 6.0–30.6 hours) in the >6-hour group. The numbers of infants treated at each hour of life are shown in Figure 2 (available at www.jpeds.com).
Figure 1.
Study subjects. IP, indomethacin prophylaxis.
Figure 2.
Number of infants (y-axis) by time of life, hours (x-axis) (n = 868).
Maternal characteristics are summarized in Table I (available at www.jpeds.com). Neonatal and perinatal characteristics are in Table II. The maternal characteristics were similar in the 2 groups, except for greater antenatal steroid exposure in the <6-hour group (P < .001). Neonatal characteristics also were similar in the 2 groups, except for lower GA (P < .03), fewer outborn infants (P < .001), and lower prevalence of pneumothoraces (P < .03) in the <6- hour group. All subsequent analyses of these 2 groups were adjusted for these 4 significant covariates (antenatal steroid exposure, GA, outborn status, and pneumothoraces). Multivariate analysis revealed no significant between-group differences in all-grade or severe IVH (all-grade IVH: <6 hours, 29% [n = 209] vs >6 hours, 29%, [n = 40], P = .92; severe IVH: <6 hours, 12% [n = 88] vs >6 hours, 14% [n = 20], P = .42).
Table I.
Maternal characteristics
| Time of indomethacin prophylaxis |
|||
|---|---|---|---|
| Characteristic | <6 h (n = 730) | >6 h (n = 138) | P value |
| Age, years, mean ± SEM | 28.3 ± 6.5 | 28.6 ± 7.2 | .64 |
| Diabetes, n (%) | 38 (5) | 7 (5) | .98 |
| Hypertension, preeclampsia/eclampsia, n (%) | 172 (24) | 37 (27) | .41 |
| Chorioamnionitis, n (%) | 47/455 (10) | 9/63 (14) | .34 |
| Antenatal steroids, n (%) | 672 (92) | 107 (78) | <.001 |
| Cesarean delivery, n (%) | 490 (67) | 101 (73) | .16 |
Table II.
Neonatal characteristics
| Characteristic | Time of indomethacin prophylaxis |
P value |
|
|---|---|---|---|
| <6 h (n = 730) | >6 h (n = 138) | ||
| GA, weeks, mean ± SEM | 26.3 ± 1.9 | 26.7 ± 2.3 | .03 |
| Birth weight, g, mean ± SEM | 861 ± 208 | 885 ± 225 | .21 |
| Male sex, n (%) | 363 (50) | 68 (49) | .92 |
| White race, n (%) | 623 (86) | 120 (88) | .53 |
| Outborn, n (%) | 25 (3) | 28 (20) | <.001 |
| 5-minute Apgar score <5, n (%) | 102 (14) | 21 (16) | .64 |
| Resuscitation in labor and delivery room, n (%) | |||
| Intubation | 527 (72) | 105 (77) | .28 |
| Chest compressions | 38 (5) | 12 (9) | .10 |
| Epinephrine | 11 (2) | 4 (3) | .24 |
| Respiratory support in the first 24 hours, n (%) | 716 (98) | 132 (96) | .08 |
| Surfactant use, n (%) | 590 (81) | 117 (85) | .27 |
| Pneumothorax, n (%) | 39 (5) | 14 (10) | .03 |
| Neonatal seizure, n (%) | 30 (4) | 8 (6) | .37 |
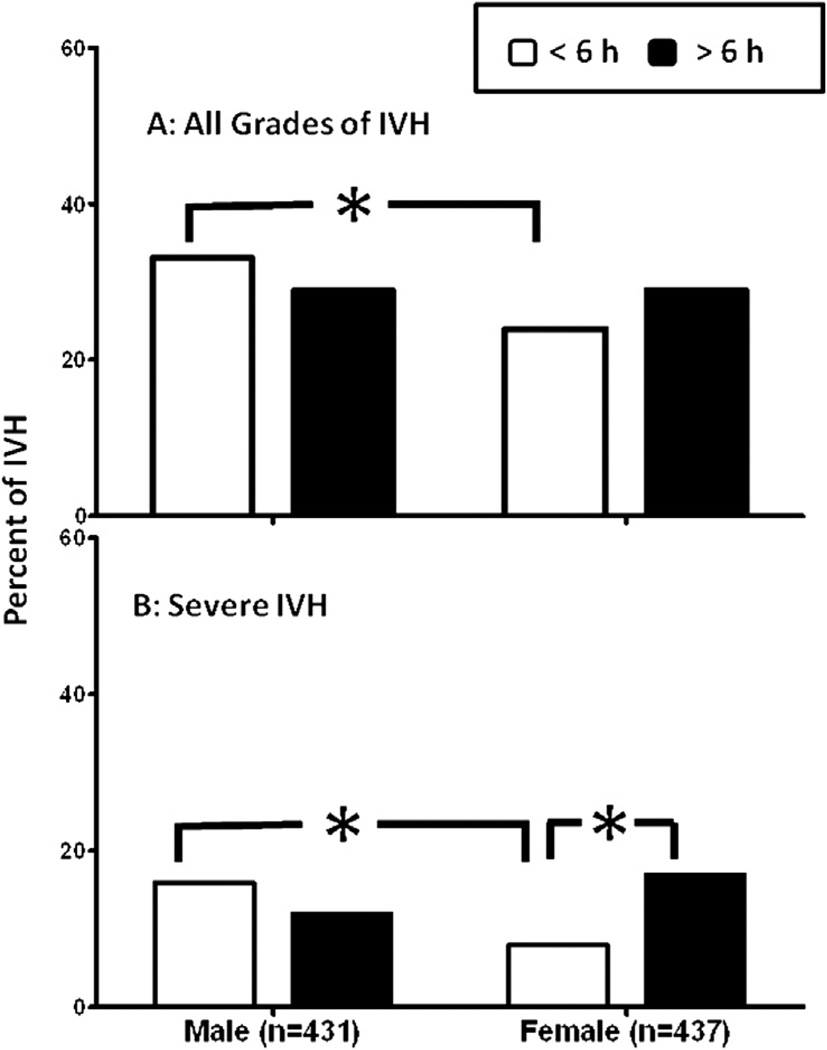
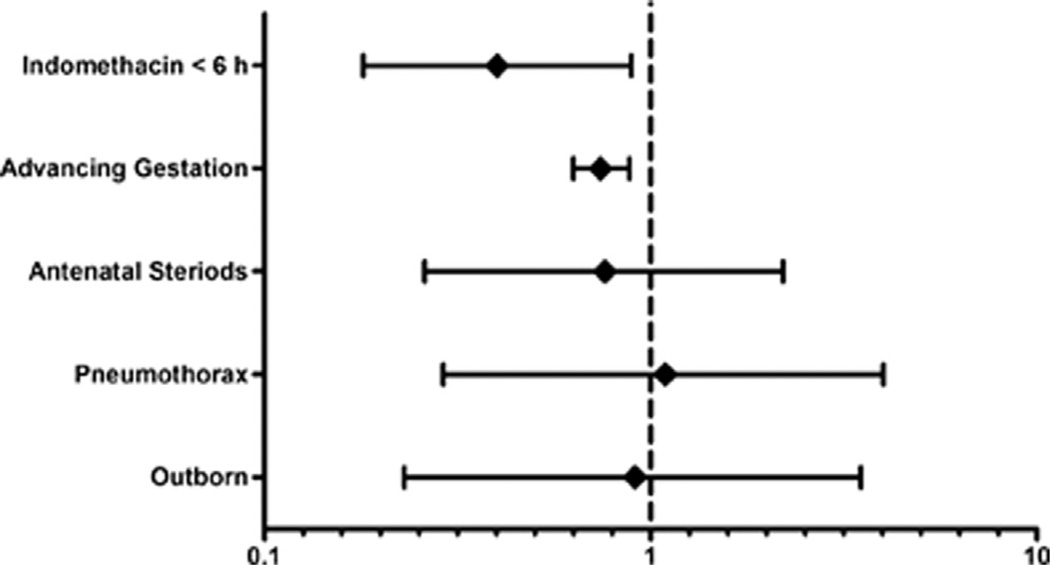
The incidences of all-grade and severe IVH in infants who received indomethacin prophylaxis at <6 hours of life were higher in males than in females (all-grade IVH, 33% in males vs 24% in females; severe IVH, 15% in males vs 8% in females; P < .01) (Figure 3). In contrast, there were no sex-based differences in all-grade IVH (P = .91) or severe IVH (P = .36) in infants who received indomethacin prophylaxis at >6 hours of life (Figure 3). The secondary analysis showed no difference in incidence of all-grade IVH between the male (P = .55) and female (P = .44) infants who were treated at <6 hours and >6 hours of life (Figure 3, A); however, the incidence of severe IVH was lower (P = .02) in female infants who received indomethacin prophylaxis at <6 hours compared with those who did so at >6 hours (8% vs 17%; Figure 3, B). The timing of administration of indomethacin prophylaxis was not associated with any differences in the incidence of severe IVH in the male infants (Figure 3, B). On multivariate analysis, indomethacin prophylaxis given at <6 hours of life and higher GA were independently associated with a reduced incidence of severe IVH in female infants (Figure 4).
Figure 3.
A, Incidence of IVH, all grades. Number of patients who received indomethacin prophylaxis <6 hours after birth: 120 males and 89 females; number who received indomethacin prophylaxis >6 hours after birth: 20 males and 20 females. B, Incidence of severe IVH. Number of patients who received indomethacin prophylaxis <6 hours after birth: 57 males and 31 females; number who received indomethacin prophylaxis >6 hours after birth: 8 males and 12 females. *P < .05. Values are percent of IVH.
Figure 4.
Forest plot for female infants, with severe IVH on the y-axis plotted against the OR (95% CI) on the x-axis.
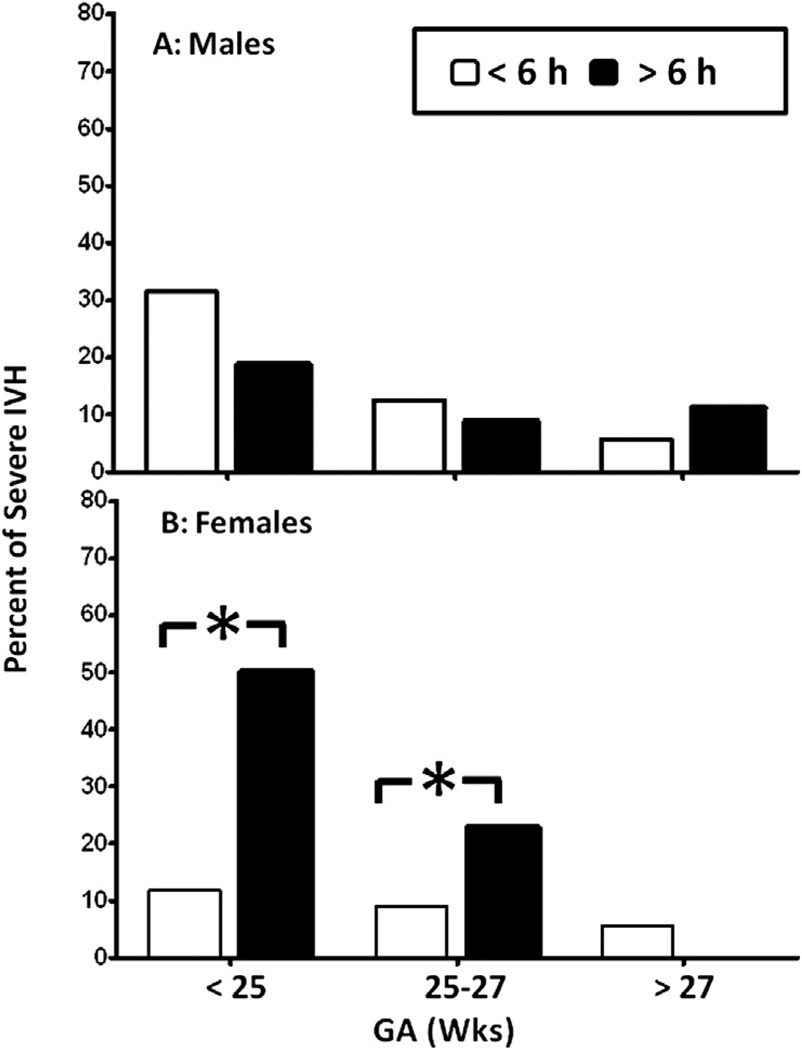
There were no differences in the incidence of all-grade IVH between male and female infants in any of the 3 GA groups given indomethacin prophylaxis at <6 hours or >6 hours of life (Table III; available at www.jpeds.com). The incidence of severe IVH also did not differ between male infants treated at <6 hours and those treated at >6 hours of life at any GA group (Figure 5, A); however, the incidence of severe IVH was lower in female infants treated at <6 hours, especially in the GA <27 weeks group (Figure 5, B).
Table III.
Effect of indomethacin prophylaxis timing on IVH by GA and sex
| IVH, all grades | ||||
|---|---|---|---|---|
| GA, wks | Sex | <6 h | >6 h | P value |
| <25 | Male (n = 108) | 55/92 (59.8) | 8/16 (50) | .46 |
| Female (n = 81) | 31/67 (46.2) | 8/14 (57.1) | .45 | |
| 25–27 | Male (n = 218) | 55/184 (29.8) | 8/34 (23.5) | .45 |
| Female (n = 199) | 40/177 (22.6) | 7/22 (31.8) | .33 | |
| >27 | Male (n = 105) | 10/87 (11.4) | 4/18 (22.2) | .22 |
| Female (n = 157) | 18/123 (14.6) | 5/34 (14.7) | .99 | |
Values are n/total n (%).
Figure 5.
A, Males receiving indomethacin prophylaxis <6 hours after birth: GA <25 weeks, 29 of 92; GA 25–27 weeks, 23 of 184; GA >27 weeks, 5 of 87. Males receiving indomethacin prophylaxis >6 hours after birth: GA <25 weeks, 3 of 16; GA 25–27 weeks, 3 of 34; GA >27 weeks, 2 of 18. B, Females receiving indomethacin prophylaxis <6 hours after birth: GA <25 weeks, 8 of 67; GA 25–27 weeks, 16 of 177; GA >27 weeks, 7 of 123. Females receiving indomethacin prophylaxis >6 hours after birth: GA <25 weeks, 7 of 14; GA 25–27 weeks, 5 of 22; GA >27 weeks, 0 of 34. *P < .05. Values are percent of severe IVH.
Discussion
A meta-analysis of 19 clinical trials including 2872 infants found that indomethacin prophylaxis significantly reduces the incidence of grade 3–4 IVH in preterm infants.10 Current recommendations suggest administering indomethacin prophylaxis at 6–12 hours of life.25,26 This recommendation is based on findings from the first randomized clinical trial11 showing that indomethacin prophylaxis reduced the incidence of IVH in VLBW infants. In contrast, a subsequent larger randomized clinical trial initiated indomethacin prophylaxis before 6 hours of life.12 Although Ment et al27 also previously suggested giving indomethacin before 6 hours of life, indomethacin prophylaxis is currently initiated over a wide range of time intervals depending on local logistics and individual preferences. The most efficacious time to administer prophylactic indomethacin to prevent IVH has not been established. In this study, we aimed to delineate the potential beneficial effects of initiating indomethacin prophylaxis shortly after birth.
In our cohort, the incidence of all-grade IVH was 28%, and that of severe IVH was 12%. In contrast, in the first randomized controlled trial, Ment et al11 reported 12% all-grade IVH and <2% severe IVH in the treatment group, significantly lower than the rates found in our retrospective analysis. However, Ment et al excluded infants with preexisting IVH, which represented 52% of all-grade IVH in their cohort. Schmidt et al12 reported a 41% incidence of all-grade IVH and a 9% incidence of severe IVH in their cohort of infants treated with indomethacin. They did not exclude infants with preexisting IVH in their cohort, but did exclude those in whom indomethacin administration within 6 hours of birth was not possible.12
Our findings do not confirm our primary hypothesis that initiating indomethacin prophylaxis before 6 hours of age was associated with a lower incidence of IVH. The incidence of all-grade IVH was identical in the <6-hour (29%) and >6-hour (29%) indomethacin administration groups. The incidence of severe IVH also did not differ between the 2 groups (12% for <6 hours vs 14% for >6 hours).
In the present study, the incidences of all-grade IVH and severe IVH were significantly higher in males than females in the <6-hour prophylaxis group. Human and animal studies have identified male sex as an important risk factor for higher risk for and greater severity of IVH, as well as for more severe neurologic impairment in the perinatal period.23,28–31 A male predominance for IVH was not evident in our >6-hour prophylaxis group, however. The lack of difference in IVH incidence between male and female infants in the >6-hours group could be a reflection of the small group size.
Indomethacin prophylaxis had a greater effect in reducing the risk of IVH in male infants than in female infants.32,33 Consequently, we expected the beneficial effects of early indomethacin prophylaxis to be associated with a greater reduction in IVH rate in the male infants. Contrary to this expectation, however, the effect of early indomethacin prophylaxis appears to be associated with a lower incidence of severe IVH in the female infants. This association was persistent even after adjusting for the confounding variables. The mechanistic basis for the sex-specific beneficial effects in the <6-hour indomethacin prophylaxis group cannot be determined from our data. The lower incidence of severe IVH in the female infants in the <6-hour group was accentuated at lower GA (<27 weeks). In our cohort, a larger proportion of female infants received indomethacin at <6 hours (84%) than >6 hours (16%). Therefore, we cannot rule out the possibility that the larger number of female infants who received indomethacin before 6 hours of life compared with after 6 hours of life could have contributed to these findings.
Our study has some other limitations as well. Although our cohort spanned 8 years and included 868 infants, sample sizes were small in some of the subgroup analyses. Our power analysis was done conventionally to address the primary hypothesis, and thus whether sample size could be a significant factor in interpreting the interesting findings related to our secondary hypothesis is unclear. We used deidentified data from the NRN’s Generic Database, and thus a chart review was not feasible. Although the times of indomethacin administration were available, the time of onset of IVH could not be ascertained. Infants with preexisting IVH at the time of treatment were not identified, and thus we cannot exclude them to demonstrate the exclusive effects of the timing of indomethacin administration.
We conclude that administration of prophylactic indomethacin before the first 6 hours of life was not associated with a lower incidence of IVH in infants with birth weight ≤1250 g compared with later administration (>6 hours).
Acknowledgments
Supported by the National Institutes of Health (NIH; U10 HD027904 and 1R01 HD057100). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
We thank the Eunice Kennedy Shriver National Institutes of Child Health and Human Development NRN for granting permission to access their data.
Glossary
- GA
Gestational age
- IVH
Intraventricular hemorrhage
- NRN
Neonatal Research Network
- VLBW
Very low birth weight
Footnotes
The authors declare no conflicts of interest.
References
- 1.Philip AG, Allan WC, Tito AM, Wheeler LR. Intraventricular hemorrhage in preterm infants: declining incidence in the 1980s. Pediatrics. 1989;84:797–801. [PubMed] [Google Scholar]
- 2.Marba ST, Caldas JP, Vinagre LE, Pessoto MA. Incidence of periventricular/intraventricular hemorrhage in very low birth weight infants: a 15-year cohort study. J Pediatr (Rio J) 2011;87:505–511. doi: 10.2223/JPED.2137. [DOI] [PubMed] [Google Scholar]
- 3.Jain NJ, Kruse LK, Demissie K, Khandelwal M. Impact of mode of delivery on neonatal complications: trends between 1997 and 2005. J Matern Fetal Neonatal Med. 2009;22:491–500. doi: 10.1080/14767050902769982. [DOI] [PubMed] [Google Scholar]
- 4.McCrea HJ, Ment LR. The diagnosis, management, and postnatal prevention of intraventricular hemorrhage. Clin Perinatol. 2008;35:777–792. doi: 10.1016/j.clp.2008.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ballabh P. Intraventricular hemorrhage in premature infants: mechanism of disease. Pediatr Res. 2010;67:1–8. doi: 10.1203/PDR.0b013e3181c1b176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shaver DC, Bada HS, Korones SB, Anderson GD, Wong SP, Arheart KL. Early and late intraventricular hemorrhage: the role of obstetric factors. Obstet Gynecol. 1992;80:831–837. [PubMed] [Google Scholar]
- 7.Ment LR, Duncan CC, Ehrenkranz RA, Lange RC, Taylor KJ, Kleinman CS, et al. Intraventricular hemorrhage in the preterm neonate: timing and cerebral blood flow changes. J Pediatr. 1984;104:419–425. doi: 10.1016/s0022-3476(84)81109-9. [DOI] [PubMed] [Google Scholar]
- 8.Dolfin T, Skidmore MB, Fong KW, Hoskins EM, Shennan AT. Incidence, severity, and timing of subependymal and intraventricular hemorrhages in preterm infants born in a perinatal unit as detected by serial real-time ultrasound. Pediatrics. 1983;71:541–546. [PubMed] [Google Scholar]
- 9.Vohr B, Allan WC, Scott DT, Katz KH, Schneider KC, Makuch RW, et al. Early-onset intraventricular hemorrhage in preterm neonates: incidence of neurodevelopmental handicap. Semin Perinatol. 1999;23:212–217. doi: 10.1016/s0146-0005(99)80065-2. [DOI] [PubMed] [Google Scholar]
- 10.Fowlie PW, Davis PG. Prophylactic indomethacin for preterm infants: a systematic review and meta-analysis. Arch Dis Child Fetal Neonatal Ed. 2003;88:F464–F466. doi: 10.1136/fn.88.6.F464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ment LR, Oh W, Ehrenkranz RA, Philip AG, Vohr B, Allan W, et al. Low-dose indomethacin and prevention of intraventricular hemorrhage: a multicenter randomized trial. Pediatrics. 1994;93:543–550. [PubMed] [Google Scholar]
- 12.Schmidt B, Davis P, Moddemann D, Ohlsson A, Roberts RS, Saigal S, et al. Long-term effects of indomethacin prophylaxis in extremely-low-birth-weight infants. N Engl J Med. 2001;344:1966–1972. doi: 10.1056/NEJM200106283442602. [DOI] [PubMed] [Google Scholar]
- 13.Coyle MG, Oh W, Petersson KH, Stonestreet BS. Effects of indomethacin on brain blood flow, cerebral metabolism, and sagittal sinus prostanoids after hypoxia. Am J Physiol. 1995;269:H1450–H1459. doi: 10.1152/ajpheart.1995.269.4.H1450. [DOI] [PubMed] [Google Scholar]
- 14.Coyle MG, Oh W, Stonestreet BS. Effects of indomethacin on brain blood flow and cerebral metabolism in hypoxic newborn piglets. Am J Physiol. 1993;264:H141–H149. doi: 10.1152/ajpheart.1993.264.1.H141. [DOI] [PubMed] [Google Scholar]
- 15.Leffler CW, Mirro R, Shibata M, Parfenova H, Armstead WM, Zuckerman S. Effects of indomethacin on cerebral vasodilator responses to arachidonic acid and hypercapnia in newborn pigs. Pediatr Res. 1993;33:609–614. doi: 10.1203/00006450-199306000-00016. [DOI] [PubMed] [Google Scholar]
- 16.McCalden TA, Nath RG, Thiele K. The role of prostacyclin in the hypercapnic and hypoxic cerebrovascular dilations. Life Sci. 1984;34:1801–1807. doi: 10.1016/0024-3205(84)90672-6. [DOI] [PubMed] [Google Scholar]
- 17.Furlow TW, Jr, Hallenbeck JM. Indomethacin prevents impaired perfusion of the dogs’s brain after global ischemia. Stroke. 1978;9:591–594. doi: 10.1161/01.str.9.6.591. [DOI] [PubMed] [Google Scholar]
- 18.Zuckerman SL, Mirro R, Armstead WM, Shibata M, Leffler CW. Indomethacin reduces ischemia-induced alteration of blood-brain barrier transport in piglets. Am J Physiol. 1994;266:H2198–H2203. doi: 10.1152/ajpheart.1994.266.6.H2198. [DOI] [PubMed] [Google Scholar]
- 19.Ment LR, Stewart WB, Ardito TA, Huang E, Madri JA. Indomethacin promotes germinal matrix microvessel maturation in the newborn beagle pup. Stroke. 1992;23:1132–1137. doi: 10.1161/01.str.23.8.1132. [DOI] [PubMed] [Google Scholar]
- 20.Ando Y, Takashima S, Takeshita K. Cerebral blood flow velocity in preterm neonates. Brain Dev. 1985;7:385–391. doi: 10.1016/s0387-7604(85)80135-2. [DOI] [PubMed] [Google Scholar]
- 21.Ment LR, Oh W, Ehrenkranz RA, Philip AG, Schneider K, Katz KH, et al. Risk period for intraventricular hemorrhage of the preterm neonate is independent of gestational age. Semin Perinatol. 1993;17:338–341. [PubMed] [Google Scholar]
- 22.Kadri H, Mawla AA, Kazah J. The incidence, timing, and predisposing factors of germinal matrix and intraventricular hemorrhage (GMH/IVH) in preterm neonates. Childs Nerv Syst. 2006;22:1086–1090. doi: 10.1007/s00381-006-0050-6. [DOI] [PubMed] [Google Scholar]
- 23.Mohamed MA, Aly H. Male gender is associated with intraventricular hemorrhage. Pediatrics. 2010;125:e333–e339. doi: 10.1542/peds.2008-3369. [DOI] [PubMed] [Google Scholar]
- 24.Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978;92:529–534. doi: 10.1016/s0022-3476(78)80282-0. [DOI] [PubMed] [Google Scholar]
- 25.Tschudy MM, Arcara KM. The Harriet Lane handbook: A manual for pediatric house officers. 19th ed. Philadelphia: Mosby Elsevier; 2012. [Google Scholar]
- 26.Young TE, Mangum B. Neofax. 24th ed. Raleigh (NC): Thompson Reuters; 2011. p. 192. [Google Scholar]
- 27.Ment LR, Oh W, Ehrenkranz RA, Phillip AG, Vohr B, Allan W, et al. Low-dose indomethacin therapy and extension of intraventricular hemorrhage: a multicenter randomized trial. J Pediatr. 1994;124:951–955. doi: 10.1016/s0022-3476(05)83191-9. [DOI] [PubMed] [Google Scholar]
- 28.Tioseco JA, Aly H, Essers J, Patel K, El-Mohandes AA. Male sex and intraventricular hemorrhage. Pediatr Crit Care Med. 2006;7:40–44. doi: 10.1097/01.pcc.0000192341.67078.61. [DOI] [PubMed] [Google Scholar]
- 29.Zhu C, Xu F, Wang X, Shibata M, Uchiyama Y, Blomgren K, et al. Different apoptotic mechanisms are activated in male and female brains after neonatal hypoxia-ischaemia. J Neurochem. 2006;96:1016–1027. doi: 10.1111/j.1471-4159.2005.03639.x. [DOI] [PubMed] [Google Scholar]
- 30.Li H, Pin S, Zeng Z, Wang MM, Andreasson KA, McCullough LD. Sex differences in cell death. Ann Neurol. 2005;58:317–321. doi: 10.1002/ana.20538. [DOI] [PubMed] [Google Scholar]
- 31.Cuestas E, Bas J, Pautasso J. Sex differences in intraventricular hemorrhage rates among very low birth weight newborns. Gender Med. 2009;6:376–382. doi: 10.1016/j.genm.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 32.Ment LR, Vohr BR, Makuch RW, Westerveld M, Katz KH, Schneider KC, et al. Prevention of intraventricular hemorrhage by indomethacin in male preterm infants. J Pediatr. 2004;145:832–834. doi: 10.1016/j.jpeds.2004.07.035. [DOI] [PubMed] [Google Scholar]
- 33.Ohlsson A, Roberts RS, Schmidt B, Davis P, Moddeman D, Saigal S, et al. Male/female differences in indomethacin effects in preterm infants. J Pediatr. 2005;147:860–862. doi: 10.1016/j.jpeds.2005.07.032. [DOI] [PubMed] [Google Scholar]