Abstract
Stroke is a leading cause of death and serious long-term disability. Ischemic stroke is the major subtype of stroke. Currently, its diagnosis is mainly dependent upon clinical symptoms and neuroimaging techniques. Despite these clinical and imaging modalities, often strokes are not recognized after initial onset. As early intervention of medical or surgical therapy is often associated with improved outcomes, there is an urgent need to improve the speed and accuracy of stroke diagnosis. Stroke is a complex pathophysiological process involving; energy failure, imbalance of ion homeostasis, acidosis, intracellular calcium overload, neuronal excitotoxicity, free radical-mediated lipid oxidation, inflammatory cell infiltration, and glial cell activation. These events ultimately lead to neuronal apoptotic cell death or necrosis. In this review, we have summarized the serum biomarkers according to the pathophysiological processes of stroke, which have been intensively studied in clinical trials of stroke over the past five years, and also used Medline’s ‘related article’ option to identify further articles. We focused on the potential biomarkers pertaining to vascular injury, metabolic changes, oxidative injury, and inflammation, and newly studied biomarkers, and discussed how these biomarkers could be used for the diagnosis or determining the prognosis of stroke.
Keywords: biomarkers, cerebral ischemia, clinical trial, pathophysiology, stroke
Stroke is a leading cause of death and long-term disability worldwide [1]. It is estimated that 750,000 new strokes occur annually in the USA, resulting in 150,000–200,000 deaths. There are two major types of stroke: ischemic and hemorrhagic stroke. Ischemic strokes, which include cryptogenic, lacunae and thromboembolic strokes, are caused by obstruction of blood flow to an area of the brain and account for 87% of all strokes [2]. Hemorrhagic strokes, which account for the remaining 13% of all strokes, are due to a lack of blood flow to an area of the brain that is triggered by a rupture or break in blood vessels [2]. Because ischemic strokes account for most of all strokes, the diagnosis and medical treatment of strokes have focused predominantly on ischemic stroke.
Ischemic stroke is a multifactorial disease. Risk factors for ischemic stroke include diabetes, atherosclerosis and hypertension. However, some patients with ischemic stroke have little or no risk factors. Thrombolytic therapy is currently the only approved acute treatment for ischemic stroke. As the treatment window is limited to 4.5 h or less after the onset of acute ischemic stroke, relatively few patients could actually benefit from recombinant tissue plasminogen activator (tPA) therapy. Thus, the rapid and accurate diagnosis of ischemic stroke is imperative if stroke outcome is to be improved.
Pathophysiology of ischemic stroke
Blockage of cerebral vessels trigger a cascade of ischemia-induced pathological events, including disturbance of ion homeostasis, neuronal excitotoxicity and intracellular calcium overload, peri-infarct depolarization, free radical generation, lipid peroxidation and altered protein synthesis that ultimately lead to irreversible neuronal injury. Some of these events occur within minutes of stroke onset, and in a time-dependent manner, are followed sequentially by other events. For example, neuronal excitotoxicity occurs within minutes, followed by a robust inflammatory response within hours, eventually leading to programmed cell death (apoptosis) that is maintained for several hours to days after stroke onset [3]. Because some of these pathological events in ischemic stroke occur in a sequential manner, the measurement of several serum biomarkers, which may reflect these pathophysiological events, offers promise for the early diagnosis of ischemic stroke.
Potential use of biomarkers in ischemic stroke
The monitoring trends and determinants of cardiovascular disease criteria for the diagnosis of stroke depends on typical presenting symptoms and signs in addition to at least one positive finding as determined by necropsy, CT scan or cerebrospinal fluid (CSF) analysis. Although brain CT scan is a very accurate method for diagnosing hemorrhagic stroke, it is only about 85% accurate in diagnosing ischemic stroke [4]. The main issue is that brain CT scan often cannot detect ischemic strokes within 6 h of stroke onset, and infarct lesions may not be evident until 12–24 h.
As mentioned, only a small number of stroke patients (1–2%) benefit from recombinant tPA treatment [5]. This is due, in part, to the delayed diagnosis of ischemic stroke and accompanying risks for hemorrhagic transformation. Thus, serum biomarkers, if validated, may help improve the early diagnostic certainty of stroke and/or be a good indicator of prognostic outcome. A good diagnostic biomarker should be sensitive and specific, and could also be used to help distinguish between different stroke subtypes, and potentially be used to exclude other neurological symptoms that mimic stroke such as conditions due to hypoglycemia, focal seizure, migraine, functional hemiparesis, psychogenic spells and other toxic metabolic conditions.
Potential serum biomarkers in stroke
Previous studies have examined the utility of several serum biomarkers in stroke. According to the pathological mechanism of stroke, these biomarkers are classified into markers related to vascular injury, metabolic changes, oxidative stress and inflammation. We also summarized some biomarkers recently intensively studied, such as miRNA and copeptin (Table 1).
Table 1.
Potential biomarkers in stroke.
| Biomarker | Changes in plasma level | Association | Ref. |
|---|---|---|---|
| Vascular markers | |||
| ADMA | Increased in IS and HS | Poor outcome | [8–10] |
| ET-1 | Increased in IS and HS | Lesion size | [15,16] |
| No change in IS | None | [17] | |
| vWF | Increased in IS | Risk of stroke | [21,23] |
| No change in IS | None | [22] | |
| tPA and PAI-1 | Increased in IS | Poor prognosis and re-occurrence | [25] |
| No change in mild or moderate | None | [26,27] | |
| D-dimer | Higher in CE stroke | Poor outcome | [32–34] |
| No effect | [36] | ||
| MMP-9 | Increased in IS and HS | Hemorrhage transformation and poor prognosis | [40–42] |
| c-Fn | Increased in IS | Hemorrhagic transformation | [40,44,45] |
| PENK-A | Increased in IS | Severity of cerebral injury | [46] |
| Metabolic markers | |||
| Cholesterol | <160 mg/dl in population | Risk factor for hemorrhagic stroke | [50] |
| TC >280 mg/dl | Risk of cerebral infarction | [50,51] | |
| Albumin | Reduced in IS | Poor outcome | [53] |
| Cortisol | Increased in IS | Poor outcome | [57,58] |
| Hcy | Increased in IS | Poor outcome | [65,67] |
| No effect | [66] | ||
| Oxidative markers | |||
| SOD | Reduced in IS | Poor outcome | [68] |
| NO | Increased in IS | Poor outcome | [69] |
| Increased in IS | None | [70] | |
| Bilirubin | High in population | Reduced risk of stroke | [71,72] |
| Increased in IS | Poor outcome | [73,74] | |
| Inflammatory markers | |||
| Lp-PLA2 | High in population | Risk of stroke occurrence | [76,77] |
| Increased in IS | Poor outcome | [78] | |
| High level after tPA treatment | Fail to re-canalize | [79] | |
| CRP | Increased in IS | Poor outcome | [80] |
| No change | None | [81,82] | |
| PTX3 | Higher in IS | Increased mortality | [85,86] |
| IL-6, TNF-α | Increased in IS and HS | Poor outcome | [87] |
| S100B | Increased in IS | Lesion size and poor outcome | [90] |
| sICAM-1 and sVCAM-1 | Increased or no change | Early death or not | [91–93] |
| Newly intensively studied markers | |||
| miRNA-210 | Higher in IS | Good outcome | [100] |
| Copeptin | Higher in IS | Poor outcome | [101–106] |
CE: Cardioembolic; c-Fn: Cellular-fibronectin; CRP: C-reactive protein; Hcy: Homocysteine; HS: Hemorrhagic stroke; IS: Ischemic stroke; Lp-PLA2: Lipoprotein-associated phospholipase A2; MMP: Matrix metalloproteinase; PAI-1: Plasminogen activator inhibitor type-1; PENK-A: Precursor neuropeptides proenkephalin A; PTX3: Pentraxin 3; SOD: Superoxide dismutase; TC: Total cholesterol; tPA: Tissue plasminogen activator.
Vascular injury-related markers
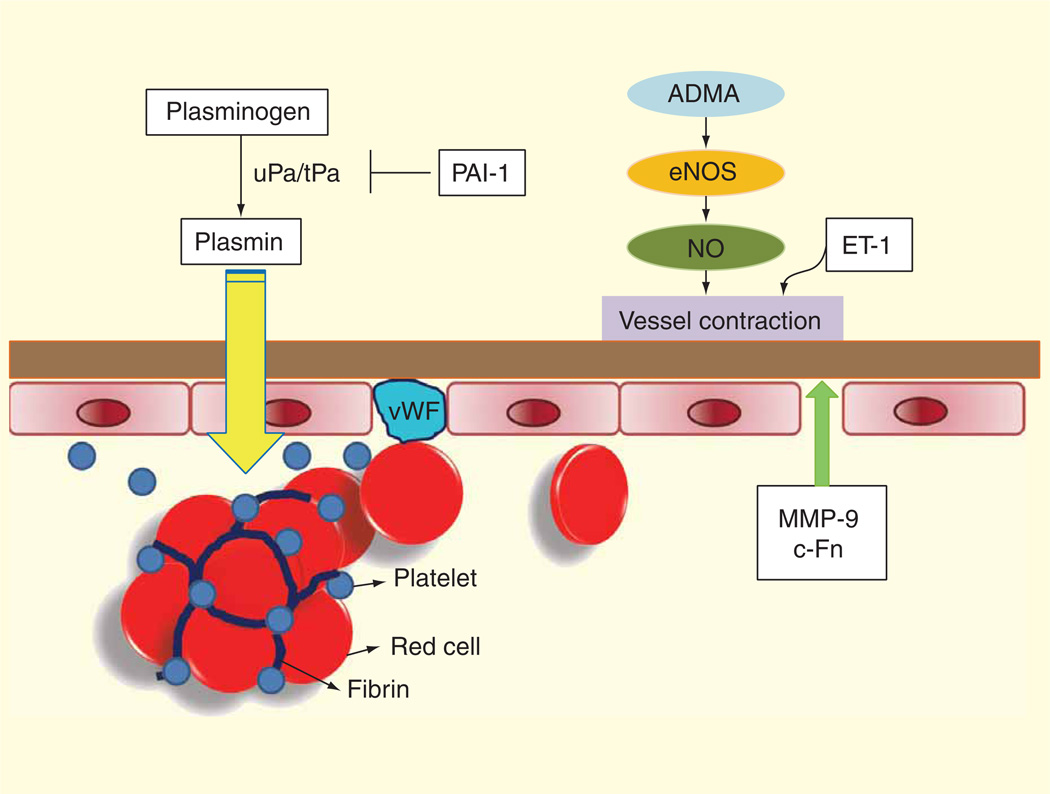
Ischemic stroke patients often have endothelial dysfunction due to underlying atherosclerosis in their carotid and cerebral arteries. Endothelial dysfunction could also be caused by elevated asymmetric dimethylarginine (ADMA), which inhibits the release of endothelium-derived nitric oxide (NO). In addition, dysfunctional endothelium releases endothelin-1 (ET-1), which along with decreased NO release, leads to vasoconstriction and further reduction of cerebral blood flow. Vascular injury could also lead to the degradation of the basal lamina by matrix metalloproteinases (MMPs) and the release of cellular-fibronectin (c-Fn). Subsequent clot formation from injured vessels could also activate the fibrinolytic and coagulation pathways. These potential serum biomarkers in response to vascular injury in ischemic stroke are shown in Figure 1.
Figure 1. Potential biomarkers in stroke.
At an early stage after stroke onset, the vascular endothelium becomes dysfunctional. The basal lamina is degraded by matrix metalloproteinases and cellular-fibronectin is released. The elevation of asymmetric dimethylarginine leads to endothelial NO synthase inhibition and decreased nitric oxide release. In addition, endothelin-1 is released by dysfunctional endothelium leading to vasoconstriction and reduction of blood flow. Thrombosis in vessels leads to the activation of both coagulation (PAI-1) and fibriolytic system (tPA). Increased von Willebrand factor (vWF) corresponds to injured endothelium. Thus, MMPs, c-Fn, ADMA, ET-1, NO, tPA, PAI-1 and vWF are potential biomarker of vascular injury in stroke.
Asymmetric dimethylarginine
ADMA, a circulating endogenous inhibitor of endothelial NO synthase, has been shown to cause endothelial dysfunction and atherosclerosis [6]. Increased concentration of ADMA has been found in several vascular-related diseases, such as diabetes, Alzheimer’s disease, heart failure and stroke. In clinical trials, serum ADMA concentration was found to be substantially higher in ischemic stroke patients than control subjects, and in particular, in stroke patients with cardioembolic or large-artery atherosclerotic etiologies [7]. An increase in plasma levels of both ADMA and its analog, symmetric dimethylarginine within the first 72 h after the onset of ischemic stroke predicts a poor outcome [8]. Similarly, another clinical trial found that ADMA was higher in patients with fatal strokes and was an independent predictor of all-cause mortality after stroke [9]. In some studies, a 0.15 µmol/l increase in baseline ADMA levels was associated with approximately 30% increase in cardiovascular risk in women [10]. In another study, increased concentrations of ADMA were observed in cardioembolic stroke and transient ischemic attacks (TIA), but not in non-cardioembolic stroke or hemorrhagic stroke. Interestingly, ADMA levels in the CSF were also increased in patients with ischemic stroke or TIA within 24 h after stroke onset. The ADMA levels, however, were found to be higher in patients with stroke than TIA [11]. Interestingly, in a small trial of about 13 patients with subarachnoid hemorrhage, CSF ADMA levels were also higher than those in the control group, suggesting that the increase in CSF ADMA levels may be due to endothelial dysfunction and vasospasm [12]. Despite these promising studies suggesting that ADMA may be a good biomarker for stroke, a recent multicenter study showed that there were no differences in plasma ADMA concentrations between stroke patients and control subjects [13]. Thus, it remains to be determined whether ADMA is a useful biomarker for ischemic stroke.
Endothelin-1
ET-1 is a potent and long-acting vasoconstrictor peptide, which is released from endothelial cell-specific storage granules in response to external physiological or pathophysiological stimuli [14]. Increased ET-1 expression reflects damages to endothelial cells within the ischemic tissue, causing vasoconstriction of collateral vessels, which further damages brain tissue. However, the use of ET-1 as a biomarker for ischemic stroke is somewhat controversial. Most clinical trials showed that ET-1 level is increased by 1.3- to 5.4-fold after ischemic stroke [15,16]. Furthermore, some studies suggest that plasma ET-1 level correlate with stroke size but not neurological deficit. However, one study of about 101 patients with ischemic stroke showed that ET-1 was not different from those of control subjects at any time point during stroke evolution [17]. Furthermore, a small study found that plasma ET-1 level was increased in hemorrhagic but not ischemic stroke [18]. In these studies, blood samples were collected between 18 and 72 h after onset of ischemic stroke, and different methods were used to measure ET-1 level. Because clinical trials in ischemic stroke have yielded conflicting results with ET-1 as a serum biomarker, future studies using ET-1 as a biomarker for stroke should adopt a more stringent and standardized protocol for enrolling patients, collecting samples, assaying for ET-1 and determining clinical follow-up.
von Willebrand factor
von Willebrand factor (vWF) is a plasma glycoprotein that plays critical roles in platelet adhesion and aggregation. Plasma vWF is mostly synthesized and secreted by endothelial cells under conditions of endothelial cell activation and damage. It also is associated with the occurrence of arterial thrombotic disease [19]. High vWF in young women is associated with an increased risk of ischemic stroke [20]. Some studies reported that vWF level is increased in ischemic stroke patients and higher plasma level of vWF is associated with a higher risk for ischemic stroke [21]. By contrast, some case–control and prospective studies did not find an association between vWF and ischemic stroke [22]. Recently, a large case–control study comprising 600 patients with 600 matched controls studied the relationship between vWF and etiologies and subtypes of ischemic stroke. The report showed that during the acute phase of stroke, vWF levels were significantly increased in all four subtypes of stroke: large vessel disease (LVD), cardioembolic (CE), cryptogenic stroke and small vessel disease (SVD). There were significant subtype-specific differences in vWF, with the highest levels in LVD and CE stroke after 3 months [23]. However, more studies are needed to determine whether vWF level is a useful predictor of stroke subtype.
tPA & plasminogen activator inhibitor type-1
The tPA is a serine protease found in endothelial cells. It catalyzes the conversion of plasminogen to plasmin, the major enzyme responsible for clot breakdown, and is also, an initiator of the fibrinolytic system. By contrast, plasminogen activator inhibitor type-1 (PAI-1) functions as the principal inhibitor of tPA by combining with tPA to inactivate tPA. Because of their roles in fibrinolysis and coagulation, tPA and PAI-1 are considered markers of clot formation and fibrinolysis [24]. Thrombolytic therapy in acute stroke is a first-line therapy since it accelerates clot lysis, which leads to the rapid restoration of blood flow. In several studies, high levels of tPA and PAI-1 antigen in acute ischemic stroke patients were observed. Furthermore, tPA concentrations were higher among men who later developed strokes than in control subjects. High tPA antigen in acute stroke patients was also associated with a worsening prognosis [25]. However, the levels of tPA or PAI-1 in mild-to-moderate ischemic stroke do not appear to be altered [26,27]. Thus, tPA and PAI-1 have not been shown to be definitive biomarkers of ischemic stroke.
D-dimer
D-dimer is a fibrin degradation product, which is not normally detected in healthy individuals, except when the coagulation system has been activated. Patients with various strokes and stroke-related diseases have increased plasma levels of D-dimer. In recent years, clinical trials showed that the sensitivities of D-dimer for predicting ischemic stroke tendency were very low of about 3.29%, but with a specificity of 100% [28]. D-dimer levels were significantly higher in CE stroke patients than in those with other etiologies [29–31]. D-dimer levels correlate with infarct volume in acute ischemic stroke patients [32], especially in CE stroke in non-valvular atrial fibrillation (AF) patients [33], and in-hospital death [34]. Low D-dimer levels are associated with an early improvement in AF-related stroke [35]. However, a total of 382 patients included in the Heparin in Acute Embolic Stroke trial found that D-dimer and other markers of hemostatic activation were not associated with stroke progression, recurrent stroke or death in patients with acute ischemic stroke and AF [36]. A systemic review published in 2009 about D-dimer in stroke patients suggests that plasma D-dimer levels are neither sensitive nor specific enough to be utilized in stroke diagnostics and cannot replace either clinical or radiological evaluation [37]. However, current available data suggest that D-dimer level in combination with AF may be useful for distinguishing cerebral infarction subtypes during the acute stage [38].
Matrix metalloproteinase-9
MMPs, a family of zinc-dependent proteolytic enzymes, are capable of degrading certain extracellular matrix proteins. MMPs-mediated extracellular matrix degradation is one of pathophysiological mechanisms in atherosclerotic plaque progression and vulnerability. MMP-9 is responsible for degradation of type IV collagen, laminin and fibronectin, which are the major components of the basal lamina. The loss of integrity of the basal lamina is considered to be the primary cause of edema after focal cerebral ischemia and hemorrhagic transformation [39]. Several studies have examined MMP-9 level in ischemic stroke patients. MMP-9 level in blood of ischemic stroke patients was increased within 6 h after onset of stroke. Furthermore, MMP-9 levels of greater than 140 ng/ml before tPA administration were associated with significant hemorrhagic transformation [40]. Because tissue destruction in cerebral ischemia appears to be related to MMP expression, increased MMP-9 level has been shown to correlate with acute lesion volume [41] and neurological symptoms caused by unstable carotid plaques or atherosclerotic changes in other vessels [42]. Baseline MMP-9 was also a significant predictor of the hyperintense acute reperfusion injury marker at 24-h follow-up in acute ischemic cerebrovascular syndrome patients, supporting the hypothesis that MMP-9 is associated with blood–brain barrier (BBB) disruption [43]. These studies suggest that MMP-9 could be a good biomarker for hemorrhagic transformation after tPA treatment and a relatively good predictor of outcomes after ischemic strokes.
Cellular-fibronectin
c-Fn, another component of the basal lamina, is synthesized and secreted by endothelial cells. When the basal lamina is disrupted, c-Fn is released into the plasma, which leads to the recruitment of polymorphonuclear leukocytes to sites of vascular injury. Plasma c-Fn level is increased in acute ischemic stroke patients. Furthermore, high plasma c-Fn levels are independently associated with tPA-induced hemorrhagic transformation. In a retrospective study, plasma levels of c-Fn of equal or greater than 3.6 µg/ml have been associated with parenchymal hematoma after treatment with tPA in patients with acute ischemic stroke. c-Fn levels of greater than 6 µg/ml were independently associated with intracerebral hemorrhage enlargement [44]. Finally, plasma c-Fn levels of greater than 16.6 µg/ml were associated with the development of massive middle cerebral artery infarction [45]. As a marker of vascular integrity, c-Fn was shown to be an accurate predictor of hemorrhagic transformation. Thus, the combination of c-FN and MMP-9 levels may be a good indicator of hemorrhagic transformation in acute ischemic stroke patients receiving tPA.
Precursor neuropeptides proenkephalin A & protachykinin
Precursor neuropeptides proenkephalin A (PENK-A) and protachykinin are potential markers of BBB integrity. Both of these neuropeptides are active as neurotransmitters and are involved in nociception and immune stimulation. Previous study reported elevated plasma levels of methionine-enkephalin in patients after acute cerebral infarction, suggesting that these neuropeptides may play an important role in the evolution of stroke. Recently, in a prospective observational study with 189 acute stroke patients, plasma PENK-A, but not protachykinin, was significantly elevated in patients with ischemic stroke compared with patients with TIA and control subjects. Elevated PENK-A concentrations were associated with ischemic stroke and the severity of cerebral injury. Furthermore, PENK-A level was different between ischemic stroke patients and TIA patients, although there was substantial overlap between the maximum value and minimum levels of PENK-A between these two groups of patients [46]. Although PENK-A may be a biomarker for BBB disruption in stroke similar to c-Fn and MMP-9, it has not been shown to be useful in distinguishing stroke subtypes. More clinical trials are needed to determine whether PENK-A is a useful marker for hemorrhagic transformation.
Metabolism-related markers
Abnormal metabolism often occurs after the onset of stroke. Patients with ischemic stroke often exhibit increased levels of lactate, pyruvate, glycolate, formate and decreased levels of glutamine and methanol, which are suggestive of anaerobic glycolysis [47]. Metabolic products, therefore, may provide useful information regarding stroke prevention and/or prognosis.
Cholesterol
Several clinical trials have reported that serum total cholesterol (TC) levels are associated with increased risk of stroke [48]. However, TC level was not different in ischemic stroke subtypes in a study of Japanese general population [49]. In a recent large prospective Japanese population study, serum TC levels of less than 160 mg/dl were found to be a risk factor for hemorrhagic stroke, whereas TC levels of greater than 280 mg/dl were associated with an increased risk of ischemic stroke. Although high blood pressure was the strongest risk factor for any subtype of stroke, high blood pressure and low TC (<160 mg/dl) were highly predictive of hemorrhagic stroke [50]. Another large study reported that excess risk of ischemic stroke was observed in men, but not in women, with serum TC levels of greater than 240 mg/dl than those with TC levels of less than 180 mg/dl. Furthermore, a very high serum TC level is also a risk factor for ischemic stroke, especially in large-artery occlusive infarction in Japanese men [51]. However, average or moderately high serum TC level is a relatively weak marker for ischemic stroke in the Framingham Heart Study, suggesting that TC alone may not be a good biomarker in predicting stroke risks.
Albumin
Serum albumin functions as a shuttle for transporting drugs and endogenous compounds in the bloodstream. It also exerts anticoagulant and antioxidant effects, while maintaining microvascular integrity and supporting acid–base balance. Albumin is also involved in the scavenging of oxygen free radicals, which have been implicated in the pathogenesis of inflammatory diseases. In addition, serum albumin level is one of the biochemical markers of nutritional status, and malnutrition after acute stroke is a risk factor for poor outcome. Although hypoalbuminemia is commonly observed in critically ill patients, it is strongly associated with increased morbidity and mortality in patients with and without neurological conditions [52]. Many stroke studies indicate that patients with low serum levels of albumin have worse clinical outcomes. For example, patients with cardioembolic stroke showed lower albumin level and higher risk of mortality than non-cardioembolic stroke [53,54]. By contrast, high serum albumin level is associated with improved outcome and lower mortality in ischemic stroke patients. Some studies have suggested that high serum albumin may not only be a biomarker, but also, could be neuroprotective in ischemic stroke [55]. Indeed, albumin supplementation to near normal concentrations has been shown to improve outcomes in stroke patients [52]. An ongoing multicenter clinical trial investigating the effects of albumin in acute stroke will help address whether albumin is a biomarker or therapy for stroke patients [56].
Cortisol
Stroke could alter endocrine metabolism. The adrenal glucocorticoid stress reaction increases blood glucose and heart rate to protect the body from injury. However, these reactions could also aggravate ischemic damage to neurons. A recent study found that low serum albumin level in patients with ischemic stroke was associated with higher serum cortisol level [57]. Hypercortisolism was associated with cognitive dysfunction early after ischemic stroke. Furthermore, high circulating cortisol levels were associated with increased severity and mortality after stroke [58]. Cortisol, therefore, may be a good prognostic marker of functional outcome and mortality of patients with ischemic stroke.
Homocysteine
Homocysteine (Hcy) is a thiol-containing amino acid formed during methionine metabolism. Increased plasma concentrations of Hcy in patients seem to indicate dysfunction of brain metabolism. Hyperhomocysteinemia has been considered to be an independent risk factor for stroke. Women with total Hcy values in the highest tertile were almost three-times as likely to have lacunar infarcts compared with those in the lowest tertile [59]. Hyperhomocysteinemia (Hcy level ≥15.90 µmol/l) was more common in patients with large-vessel atherosclerotic stroke and CE stroke in the Turkish population [60]. By contrast, a study found that Hcy level was associated with SVDrelated stroke more strongly than LVD-related stroke [61]. Hyperhomocysteinemia was an independent risk factor for SVD [62]. Many studies reported that mean plasma Hcy levels were significantly higher in ischemic stroke patients [63,64]. High serum total Hcy levels were associated with increased mortality from ischemic stroke, which was reported by a large nested case–control study [65]. However, it has also been reported that Hcy levels do not have any correlations with functional disability at the third month after ischemic stroke [66]. A long-term observation found that acute phase elevated Hcy correlated with severity and prognosis in patients with atherothrombotic stroke [67]. Hcy is associated with risk of stroke, but whether Hcy affects stroke severity and prognosis remains controversial.
Oxidative stress-related markers
Superoxide dismutase
Superoxide dismutase (SOD) is an important antioxidant enzyme that limits oxidative stress. Oxidative stress is one of the mechanisms involved in neuronal damage induced by ischemia and reperfusion injury. However, clinical studies linking serum Cu/Zn SOD level with stroke are inconsistent. Some studies report that Cu/Zn SOD activity was reduced in red blood cells and serum of patients with acute stroke and that reduced Cu/Zn SOD levels were related to an enhancement and progression of cerebral infarctions. Other studies, however, reported that Cu/Zn SOD levels increased or was not changed in red blood cells after cerebrovascular events [68]. These studies suffer from small sample sizes, which preclude a definitive conclusion regarding the effects of Cu/Zn SOD in stroke. Other antioxidant enzymes such as glutathione peroxidase and products of lipid peroxidation such as malondialdehyde were increased after acute stroke. These studies suggest that oxidative stress is induced during stroke. Whether antioxidant enzyme level and/or activity could be a good serum biomarker for stroke remains to be determined.
Endothelium-dependent NO
NO is a multifunctional molecule, produced in neurons, glial, endothelial cells and macrophages. In the presence of oxygen, l-arginine is converted to NO and citrulline by endothelial NO synthase. NO plays an important role in homeostatic the regulation of blood flow. The half-life of NO is relatively short, making the detection of NO somewhat difficult. To circumvent this issue, stable NO metabolites (NO-m, nitrates and nitrites) have been measured instead of NO. In a recent clinical trial, NO-m was increased in the CSF of stroke patients. CSF NO-m concentrations were also significantly higher in stroke patients who subsequently suffered early neurologic worsening than in those with a stable course. Increased NO-m in CSF was also associated with greater cerebral injury and early neurological deterioration. In a small study, plasma NO in ischemic stroke patients was decreased compared with that of control subjects. But peroxynitrite (ONOO-), which is the product of the rapid reaction between NO and superoxide anion, was increased in ischemic stroke patients [69]. Although a large stroke trial recently showed that plasma NO-m (nitrate and nitrite) was increased after stroke, NO levels were not associated with either the outcome or severity in stroke patients. However, there was a significant association between elevated levels of the inflammatory biomarker, C-reactive protein (CRP) and NO with stroke [70]. Because NO can be produced by different NO synthase isoforms under different conditions, the measurement of serum NO levels may not correlate with neuroprotection or neurotoxicity. Furthermore, it is not know whether administration of NO could be beneficial in acute stroke. Thus, the role of NO as a biomarker for stroke requires further study.
Bilirubin
Bilirubin is a metabolic end product of heme degradation by heme-oxygenase. However, it could also serve as an important antioxidant, anti-inflammatory and neuroprotective molecule. Indeed, bilirubin has emerged as a possible endogenous defense mechanism against stroke. The function of bilirubin has been broadly investigated in cardiovascular disease, including stroke. For example, an increment in bilirubin level of 1.71 µmol/l (0.1 mg/dl) is associated with a 9% decrease in stroke incidence among, and is associated with a 10% decrease in adverse stroke outcome among patients with stroke. Higher serum total bilirubin level is associated with reduced stroke prevalence and improved stroke outcomes [71]. Another large prospective study showed that participants with a higher level of bilirubin showed lower hazard ratios for ischemic stroke in men, as well as all other stroke subtypes [72]. These findings suggest that serum bilirubin may exert some protective actions against stroke. However, when serum bilirubin was measured in patients after the onset of stroke, higher direct bilirubin levels were associated with greater stroke severity and poorer outcome at discharge, although there was no relationship between total bilirubin and stroke severity or outcome [73]. Similar results were observed in a study of acute ischemic stroke patients in China [74]. These findings suggest a relationship between serum bilirubin and ischemic stroke, but the relationship appears to differ whether the serum bilirubin levels were measured before or after stroke.
Inflammation-related marker
Inflammation is a key event in the ischemic cascade after cerebral ischemia and could play an important pathophysiological role in ischemic stroke. Some inflammatory-related molecules are produced and secreted in the neurovascular unit, which could activate immune system in response to cell damage. These inflammatory factors accumulate in the extracellular space and/or enter the bloodstream and CSF. These factors, therefore, could easily be detectible after brain ischemic stroke.
Lipoprotein-associated phospholipase A2
Lipoprotein-associated phospholipase A2 (Lp-PLA2) is produced by inflammatory cells and hydrolyzes oxidized phospholipids in low-density lipid. Lp-PLA(2) may participate in the development of atherosclerosis and plaque rupture. The Lp- PLA(2) blood test has been recently approved by the US FDA for assessing the risk of ischemic stroke and coronary artery disease. Indeed, the role of Lp-PLA2 as a biomarker of atherosclerosis and other cardiovascular disease has been extensively studied [75]. Some prospective studies support the role of Lp- PLA2 as a risk predictor, conferring about a twofold increase in stroke risk in patients with high Lp-PLA2 plasma level [76,77]. High mRNA expression level of Lp-PLA(2) in peripheral blood mononuclear cells is correlated with major adverse clinical outcome in patients with ischemic stroke [78]. Higher mass and activity of Lp-PLA2 were also found among patients who did not achieve complete recanalization with tPA treatment. Lp- PLA2 mass and the existence of a proximal occlusion at baseline were the most powerful predictors for persistent occlusions. Lp-PLA2 mass is associated with early arterial recanalization in intravenous tPA-treated stroke patients [79]. Overall, Lp-PLA2 appears to be a good risk predictor for both primary and secondary strokes, and is associated with worse outcome after tPA treatment.
Human C-reactive protein
Human CRP is a member of the pentraxin family, which plays a major role in the human innate immune response. Elevated CRP is associated with endothelial cell dysfunction and progression of atherosclerosis possibly by decreasing NO synthesis. Elevated plasma CRP concentration correlates with increased risk of cerebro- and cardiovascular events. For example, previous studies found high plasma levels of high-sensitivity CRP (hsCRP) in acute ischemic stroke patients and demonstrated that increased hsCRP level is a good predictor of first ever or recurrent cerebrovascular events. CRP also serves as a prognostic factor for functional outcome in the early phase of stroke. Indeed, a large clinical trial showed that elevated levels of hsCRP correlated with all stroke subtypes except for lacunar stroke, and was an independent prognostic factor of poor outcome at 3 months after stroke [80]. However, not all studies have found an association between elevated CRP levels and death or dependency after stroke [81]. In transient ischemic attack or patients with minor strokes, CRP and other biomarkers of inflammation such as IL-6, IL-1 and fibrinogen were not associated with recurrent vascular events [82]. Furthermore, genetic variants of CRP, which leads to changes in serum CRP levels, were not associated with ischemic stroke [83]. Thus, the role of CRP as a predictor of ischemic stroke is still uncertain.
Pentraxin 3
Pentraxin 3 (PTX3) is another member of the pentraxin superfamily. PTX3 is rapidly produced and released by several cell types in response to primary inflammatory signals [84]. PTX3 activates the complement pathway and facilitates pathogen recognition by macrophages and dendritic cells. Therefore, the release of PTX3 may reflect vascular damage. Recently, several studies suggested that PTX3 might be associated with increased mortality in patients with vascular diseases [85]. A recent study in 376 patients with ischemic stroke showed that higher levels of PTX3 were independently associated with increased mortality in ischemic stroke patients [86]. PTX3, therefore, appears to be a promising prognostic biomarker of ischemic stroke.
IL-6 & TNF-α
IL-6 and TNF-α are well-known inflammatory markers, which can be secreted by activated macrophages to stimulate immune response. Previous clinical studies showed that plasma concentrations of IL-6 and TNF-α were higher in patients with enlarging intracerebral hemorrhage. For ischemic strokes, IL-6 showed the strongest univariate association (>twofold increase) in a large sample study [87]. Higher plasma levels of TNF-α, IL-6 and IL-1β in acute ischemic stroke were associated predominantly with cardioembolic subtype, whereas lacunar subtype showed significantly lower plasma levels of these cytokines [21]. IL-6 and TNF-α are not sensitive and specific biomarkers for predicting the outcome of stroke.
S100B proteins
S100B belongs to a multigenic family of calcium-binding proteins (S100 proteins). In the CNS, S100B is expressed primarily in astrocytes, and to some extent, in Schwann cells, melanocytes, adipocytes and chondrocytes. The concentration of S100B is 40-fold higher in CSF than in serum. S100B is assumed to be a marker of generalized BBB dysfunction rather than specific glial damage [88]. S100B is stable in the bloodstream and is not affected by hemolysis. Therefore, S100 has been considered as a promising biomarker for acute ischemic stroke. Beer et al. reported that S100B is a good system inflammatory marker in acute ischemic stroke. Furthermore, S100B concentration is associated with the degree of systemic inflammation, independent of the size of the ischemic lesion [89]. Serum S100B levels were also significantly increased following ischemic stroke from 10 h to 2–3 days after stroke onset and correlated well with volume of infarction and outcome. Indeed, higher S100 values suggest significantly larger infarction volumes, more severe strokes and worsening functional outcome [90]. In a recent study, serum S100B level was higher in patients with large artery, cortical infarcts than that in patients with lacunar infarcts. The serum S100B level peaked earlier in traumatic brain injury (on day 1 or 2 after injury) than in stroke (on day 3 or 4 after stroke). These results also suggest that inflammation can aggravate tissue injury during cerebral ischemia. Altogether, these results suggest that S100B may have a role as a predictor of functional outcome after ischemic stroke. However, because S100B is also increased in brain trauma and other types of brain injury, its lack of specificity limits its utility as a diagnostic biomarker for stroke.
Cellular adhesion molecules
The expression of cellular adhesion molecules (CAMs) is induced under inflammatory conditions and mediates leukocyte recruitment, transendothelial migration and adhesion to the vascular endothelium. Thus, CAMs, which are shed into the bloodstream, may be a useful biomarker of stroke. Previous studies showed that serum levels of soluble intercellular adhesion molecule-1 (sICAM-1) and soluble vascular cell adhesion molecule-1 (sVCAM-1) in stroke patients on admission were significantly higher than those in control subjects. In recent reports, serum levels of sICAM-1 on admission of stroke patients were found to be significantly higher than those of healthy controls, and sICAM-1 and sVCAM-1 levels were significantly higher in stroke patients who died compared with those who survived. Only sICAM-1 levels, but not sVCAM-1 levels, were independently associated with early death [91,92]. A multicentered prospective observational study in patients with ischemic stroke not receiving anticoagulation therapy reported that baseline values of VCAM-1, but not sICAM-1, of greater than 1350 ng/ml increased the risk of new vascular event or death in patients with ischemic stroke by fourfold [93]. Although soluble CAMs appear to correlate with stroke and stroke severity, some studies do not find this association. Thus, the lack of consistency of plasma concentrations of CAMs in predicting stroke or stroke severity limits its clinical application.
Other new intensively studied markers
miRNA
miRNA is a small non-coding RNA that participates in posttranscriptional regulation of gene expression via degradation or translational inhibition of their target mRNAs. One of the features of miRNA regulation is combinatorial regulation. As an miRNA can bind to multiple mRNAs and most mRNAs contain sequences for multiple miRNAs, a handful of miRNAs could effectively control a multitude of mRNAs. Consequently, alteration of miRNA expression could have profound effects on cellular transcription as well as translation. Recently, miRNA have been shown to be involved in multiple pathological processes including excitotoxicity, oxidative stress, inflammation and apoptosis [94]. For example, miRNA29 acts as a proapoptotic miRNA in the heart; miRNA145 acts as a proapoptotic miRNA in the brain; and miRNA494 is anti-apoptotic in the heart. In experimental ischemia/reperfusion injury, the expression of miR-290, miR-145 and miR-26 is upregulated, while the expression of miR-137, miR-27a and miR-18 is downregulated. Furthermore, miRNA shows different expression patterns in astrocytes and neurons after ischemic injury. For example, in neurons, the expression of miR-21 and miR-29b is upregulated, whereas the expression of miR-30b, miR-107 and miR-137 remains unaffected. However, in astrocytes, the expression of miR-21 and miR-29b is upregulated as in neurons, but the expression of miR-30b, miR-107 and miR-137 is also upregulated [94]. In addition, miRNA could play an important role in the pathogenesis of stroke such as atherosclerosis, hyperlipidemia, hypertension and plaque rupture [95]. Indeed, miRNA has served as useful biomarkers in cancer and diabetes.
Recent studies suggest that miRNAs may be promising biomarkers in stroke. By miRNA chip analyses of young ischemic stroke patients (18–49 years), there were 157 miRNAs associated with stroke with 138 miRNAs upregulated and 19 miRNAs downregulated. These expression patterns of miRNAs are reproducible and indicative of the disease state. In addition, the expression patterns of miRNAs were different in different types of stroke and were associated with stroke prognosis [96]. miRNA polymorphisms were also found in 678 patients with ischemic stroke and were associated with stroke prevalence and pathogenesis [97]. In particular, the level of miRNA-210 was significantly higher in patients with good outcome than in patients with poor outcome [98]. The sensitivity of miR-210 in predicting stroke outcome was 82.5% in patients with acute ischemic stroke. When other biomarkers such as FDP and IL-6 were used in conjunction with miR-210, the sensitivity of miR-210 in predicting ischemic stroke increased to 95.2% [99]. Thus, serum miRNA-210 appears to be a sensitive biomarker for the clinical diagnosis of acute cerebral ischemia. It remains to be determined whether other miRNA used alone or in combination could help increase the diagnostic accuracy for stroke. It should be noticed that the clinical trial about the role of miRNA remains very small, and lack validation.
Copeptin
The hypothalamic stress hormone copeptin is a stable byproduct of arginine-vasopressin synthesis and reflects its secretion. Copeptin is a novel, independent prognostic marker in acute ischemic stroke. A Swiss research group found that acute ischemic stroke patients with an unfavorable outcomes and non-survivors had significantly increased copeptin levels on admission [100]. Furthermore, Copeptin levels can predict functional outcome and mortality 1 year after stroke [101]. Additionally, copeptin levels were higher in ischemic stroke patients with a re-event compared with patients without re-event, suggesting copeptin levels predict the reoccurrence of stroke [102]. The observations from other groups also showed the consistent role of copeptin in predicting outcome and mortality of acute ischemic stroke [103–106]. Therefore, copeptin appears to be a promising new and accurate blood marker for prediction prognosis.
Expert commentary
In the past decade, considerable effort has been expended to find suitable serum biomarkers for stroke in order to enhance its diagnostic and prognostic accuracy. However, few, if any, of these biomarkers appear to be ready for clinical application. Stroke is a complex disease with different etiologies, which are affected by multiple risk factors. These differences, in turn, could affect the expression of biomarkers in plasma. Nevertheless, some of these biomarkers such as ADMA, vWF, MMP-9 and c-Fn appear to have some correlation with stroke subtype, stroke severity and hemorrhagic transformation. By contrast, some biomarkers such as ET-1 and S100B are inconsistent in their relationship to stroke. It is important to note that clinical trials using serum biomarkers have not been standardized to patient biases and blood sample collections. Thus, variation in the results using biomarkers in stroke could be due to these differences. Because the pathophysiology of stroke often involves temporal changes in neuronal excitotoxicity within minutes to inflammatory response within hours, and neuronal apoptosis in days, specific biomarkers, which reflect specific steps in this process may only be transiently useful. Furthermore, differences in results using biomarkers may be merely due to differences in assays used for their measurements. Thus, a standardized protocol use to measure biomarkers may help lessen the variability observed in clinical trials. Finally, it should be noted that stroke is a heterogeneous disease and that one should not expect one specific biomarker to reflect the complex process involved. It is likely that a combination of serum biomarkers in conjunction with physical examination and imaging studies will yield the greatest accuracy for predicting stroke and stroke outcome.
Five-year view
In recent years, epigenetic analysis and gene profiling are used as diagnostic tools in cardiovascular disease and cancer. Similar research tools are now beginning to be used for the diagnosis of stroke. In particular, miRNA has emerged as a potential biomarker for stroke as miRNAs could affect conditions that increase the risk of stroke such as atherosclerosis, hypertension, hyperlipidemia and inflammation. miRNAs could also play a role in BBB disruption and apoptosis which are pathological processes of ischemic stroke. Because miRNAs target multiple processes in the pathophysiological of stroke, it is likely that miRNA profiling will lead to the identification of specific miRNAs as useful biomarkers in stroke. Further studies are needed to determine whether miRNAs could predict stroke risks, subtypes and outcomes. It is also possible that miRNAs may not only be biomarkers for stroke, but also, could be used as therapeutic agents to decrease cerebral injury and improve stroke recovery. It is hoped that with standardized clinical trials using an accepted protocol for measuring biomarkers, the diagnosis and treatment of stroke will be considerably improved.
Key issues.
Cellular-fibronectin and matrix metalloproteinase-9 may be more indicative of hemorrhage transformation in acute ischemic stroke, especially in patients treated with tissue plasminogen activator.
Precursor neuropeptides proenkephalin A is a new inflammatory marker, which could also serve as a marker of blood–brain barrier disruption in stroke.
Markers of oxidative stress are not specific markers for stroke.
The lipoprotein-associated phospholipase A2 blood test has been approved by the US FDA for assessing the risk of ischemic stroke and coronary artery disease. There is considerable agreement among clinical trials that lipoprotein-associated phospholipase A2 is a good predictor of primary and secondary strokes, stroke outcome and recanalization after tissue plasminogen activator treatment.
A multiple biomarker strategy should increase the sensitivity and specificity for stroke diagnosis and prognosis.
miRNAs may potentially be a good biomarker for stroke, because miRNAs could regulate processes that affect cerebral injury after stroke.
Copeptin is an accurate blood marker for predicting stroke prognosis.
Biomarkers, to date, lack sensitivity or specificity to be of clinical use in stroke.
Acknowledgments
This work was supported by the National Institutes of Health (NS07001) and the China Scholarship Council.
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
- 1.Donnan GA, Fisher M, Macleod M, Davis SM. Stroke. Lancet. 2008;371:1612–1623. doi: 10.1016/S0140-6736(08)60694-7. [DOI] [PubMed] [Google Scholar]
- 2.Lloyd-Jones D, Adams RJ, Brown TM, et al. Heart disease and stroke statistics–2010 update: a report from the American Heart Association. Circulation. 2010;121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 3.Brouns R, De Deyn PP. The complexity of neurobiological processes in acute ischemic stroke. Clin Neurol Neurosurg. 2009;111(6):483–495. doi: 10.1016/j.clineuro.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 4.Latchaw RE, Alberts MJ, Lev MH, et al. Recommendations for imaging of acute ischemic stroke: a scientific statement from the American Heart Association. Stroke. 2009;40(11):3646–3678. doi: 10.1161/STROKEAHA.108.192616. [DOI] [PubMed] [Google Scholar]
- 5.Jaffer H, Morris VB, Stewart D, Labhasetwar V. Advances in stroke therapy. Drug Deliv Transl Res. 2011;1(6):409–419. doi: 10.1007/s13346-011-0046-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aldámiz-Echevarría L, Andrade F. Asymmetric dimethylarginine, endothelial dysfunction and renal disease. Int J Mol Sci. 2012;13(9):11288–11311. doi: 10.3390/ijms130911288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scherbakov N, Sandek A, Martens-Lobenhoffer J, et al. Endothelial dysfunction of the peripheral vascular bed in the acute phase after ischemic stroke. Cerebrovasc Dis. 2012;33:37–46. doi: 10.1159/000332809. [DOI] [PubMed] [Google Scholar]
- 8.Worthmann H, Chen S, Martens-Lobenhoffer J, et al. High plasma dimethylarginine levels are associated with adverse clinical outcome after stroke. J Atheroscler Thromb. 2011;18(9):753–761. doi: 10.5551/jat.8144. [DOI] [PubMed] [Google Scholar]
- 9.Schulze F, Carter AM, Schwedhelm E, et al. Symmetric dimethylarginine predicts all-cause mortality following ischemic stroke. Atherosclerosis. 2010;208(2):518–523. doi: 10.1016/j.atherosclerosis.2009.06.039. [DOI] [PubMed] [Google Scholar]
- 10.Leong T, Zylberstein D, Graham I, et al. Asymmetric dimethylarginine independently predicts fatal and nonfatal myocardial infarction and stroke in women:24-year follow-up of the population study of women in Gothenburg. Arterioscler Thromb Vasc Biol. 2008;28(5):961–967. doi: 10.1161/ATVBAHA.107.156596. [DOI] [PubMed] [Google Scholar]
- 11.Brouns R, Marescau B, Possemiers I, et al. Dimethylarginine levels in cerebrospinal fluid of hyperacute ischemic stroke patients are associated with stroke severity. Neurochem Res. 2009;34:1642–1649. doi: 10.1007/s11064-009-9954-3. [DOI] [PubMed] [Google Scholar]
- 12.Jung CS, Oldfield EH, Harvey-White J, et al. Association of an endogenous inhibitor of nitric oxide synthase with cerebral vasospasm in patients with aneurysmal subarachnoid hemorrhage. J Neurosurg. 2007;107(5):945–950. doi: 10.3171/JNS-07/11/0945. [DOI] [PubMed] [Google Scholar]
- 13.Rueda-Clausen CF, Cordoba-Porras A, Bedoya G, et al. Increased plasma levels of total homocysteine but not asymmetric dimethylarginine in Hispanic subjects with ischemic stroke FREC-VI sub-study. Eur J Neurol. 2012;19:417–425. doi: 10.1111/j.1468-1331.2011.03534.x. [DOI] [PubMed] [Google Scholar]
- 14.Pernow J, Shemyakin A, Böhm F. New perspectives on endothelin-1 in atherosclerosis and diabetes mellitus. Life Sci. 2012;91(13–14):507–516. doi: 10.1016/j.lfs.2012.03.029. [DOI] [PubMed] [Google Scholar]
- 15.Moldes O, Sobrino T, Millán M, et al. High serum levels of endothelin-1 predict severe cerebral edema in patients with acute ischemic stroke treated with t-PA. Stroke. 2008;39(7):2006–2010. doi: 10.1161/STROKEAHA.107.495044. [DOI] [PubMed] [Google Scholar]
- 16.Sapira V, Cojocaru IM, Lilios G, et al. Study of endothelin-1 in acute ischemic stroke. Rom J Intern Med. 2010;48(4):329–332. [PubMed] [Google Scholar]
- 17.Haapaniemi E, Tatlisumak T, Hamel K, et al. Plasma endothelin-1 levels neither increase nor correlate with neurological scores, stroke risk factors, or outcome in patients with ischemic stroke. Stroke. 2000;31(3):720–725. doi: 10.1161/01.str.31.3.720. [DOI] [PubMed] [Google Scholar]
- 18.Paczkowska E, Gołąb-Janowska M, Bajer-Czajkowska A, et al. Increased circulating endothelial progenitor cells in patients with haemorrhagic and ischaemic stroke: the role of Endothelin-1. J Neurol Sci. 2013;325(1–2):90–99. doi: 10.1016/j.jns.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 19.Valentijn KM, Sadler JE, Valentijn JA, et al. Functional architecture of Weibel-Palade bodies. Blood. 2011;117(19):5033–5043. doi: 10.1182/blood-2010-09-267492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andersson HM, Siegerink B, Luken BM, et al. High VWF, low ADAMTS13, and oral contraceptives increase the risk of ischemic stroke and myocardial infarction in young women. Blood. 2012;119(6):1555–1560. doi: 10.1182/blood-2011-09-380618. [DOI] [PubMed] [Google Scholar]
- 21.Licata G, Tuttolomondo A, Di Raimondo D, et al. Immuno-inflammatory activation in acute cardio-embolic strokes in comparison with other subtypes of ischaemic stroke. Thromb Haemost. 2009;101:929–937. [PubMed] [Google Scholar]
- 22.Yip HK, Lai SL, Lan MY, et al. Time course of platelet activation and von Willebrand factor in patients with non-valvular atrial fibrillation after ischemic stroke. Circ J. 2007;71:321–326. doi: 10.1253/circj.71.321. [DOI] [PubMed] [Google Scholar]
- 23.Hanson E, Jood K, Karlsson S, et al. Plasma levels of von Willebrand factor in the etiologic subtypes of ischemic stroke. J Thromb Haemost. 2011;9(2):275–281. doi: 10.1111/j.1538-7836.2010.04134.x. [DOI] [PubMed] [Google Scholar]
- 24.Van De Craen B, Declerck PJ, Gils A. The Biochemistry, Physiology and Pathological roles of PAI-1 and the requirements for PAI-1 inhibition in vivo. Thromb Res. 2012;130(4):576–585. doi: 10.1016/j.thromres.2012.06.023. [DOI] [PubMed] [Google Scholar]
- 25.Abdullah WZ, Idris SZ, Bashkar S, Hassan R. Role of fibrinolytic markers in acute stroke. Singapore Med J. 2009;50(6):604–609. [PubMed] [Google Scholar]
- 26.Haapaniemi E, Soinne L, Syrjälä M, et al. Serial changes in fibrinolysis and coagulation activation markers in acute and convalescent phase of ischemic stroke. Acta Neurol Scand. 2004;110(4):242–247. doi: 10.1111/j.1600-0404.2004.00304.x. [DOI] [PubMed] [Google Scholar]
- 27.Ilhan D, Ozbabalik D, Gulcan E, et al. Evaluation of platelet activation, coagulation, and fibrinolytic activation in patients with symptomatic lacunar stroke. Neurologist. 2010;16(3):188–191. doi: 10.1097/NRL.0b013e318198d8bc. [DOI] [PubMed] [Google Scholar]
- 28.Meng R, Li ZY, Ji X, et al. Antithrombin III associated with fibrinogen predicts the risk of cerebral ischemic stroke. Clin Neurol Neurosurg. 2011;113(5):380–386. doi: 10.1016/j.clineuro.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 29.Alvarez-Perez FJ, Castelo-Branco M, Alvarez-Sabin J. Usefulness of measurement of fibrinogen, D-dimer, D-dimer/fibrinogen ratio, C reactive protein and erythrocyte sedimentation rate to assess the pathophysiology and mechanism of ischaemic stroke. J Neurol Neurosurg Psychiatry. 2011;82(9):986–992. doi: 10.1136/jnnp.2010.230870. [DOI] [PubMed] [Google Scholar]
- 30.Hirano K, Takashima S, Dougu N. Study of hemostatic biomarkers in acute ischemic stroke by clinical subtype. J Stroke Cerebrovasc Dis. 2012;21(5):404–410. doi: 10.1016/j.jstrokecerebrovasdis.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 31.Isenegger J, Meier N, Lämmle B, et al. D-dimers predict stroke subtype when assessed early. Cerebrovasc Dis. 2010;29(1):82–86. doi: 10.1159/000256652. [DOI] [PubMed] [Google Scholar]
- 32.Park YW, Koh EJ, Choi HY. Correlation between serum D-dimer level and volume in acute ischemic stroke. J Korean Neurosurg Soc. 2011;50(2):89–94. doi: 10.3340/jkns.2011.50.2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sakaguchi M, Okazaki S. Relationship between plasma (D)-dimer level and cerebral infarction volume in patients with nonvalvular atrial fibrillation. Cerebrovasc Dis. 2013;35(1):64–72. doi: 10.1159/000345336. [DOI] [PubMed] [Google Scholar]
- 34.Hasan N, McColgan P, Bentley P, et al. Towards the identification of blood biomarkers for acute stroke in humans: a comprehensive systematic review. Br J Clin Pharmacol. 2012;74(2):230–240. doi: 10.1111/j.1365-2125.2012.04212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoon CW, Kim SJ, Bang OY, et al. Premorbid warfarin use and lower D-dimer levels are associated with spontaneous early improvement in atrial fibrillation-related stroke. J Thromb Haemost. 2012 doi: 10.1111/j.1538-7836.2012.04909.x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 36.Krarup LH, Sandset EC, Sandset PM, Berge E. D-dimer levels and stroke progression in patients with acute ischemic stroke and atrial fibrillation. Acta Neurol Scand. 2011;124(1):40–44. doi: 10.1111/j.1600-0404.2010.01409.x. [DOI] [PubMed] [Google Scholar]
- 37.Haapaniemi E, Tatlisumak T. Is D-dimer helpful in evaluating stroke patients? A systematic review. Acta Neurol Scand. 2009;119(3):141–150. doi: 10.1111/j.1600-0404.2008.01081.x. [DOI] [PubMed] [Google Scholar]
- 38.Dougu N, Takashima S, Sasahara E, et al. Differential diagnosis of cerebral infarction using an algorithm combining atrial fibrillation and D-dimer level. Eur J Neurol. 2008;15(3):295–300. doi: 10.1111/j.1468-1331.2008.02063.x. [DOI] [PubMed] [Google Scholar]
- 39.Romi F, Helgeland G, Gilhus NE. Serum levels of matrix metalloproteinases: implications in clinical neurology. Eur Neurol. 2012;67(2):121–128. doi: 10.1159/000334862. [DOI] [PubMed] [Google Scholar]
- 40.Castellanos M, Sobrino T, Millán M, et al. Serum cellular fibronectin and matrix metalloproteinase-9 as screening biomarkers for the prediction of parenchymal hematoma after thrombolytic therapy in acute ischemic stroke: a multicenter confirmatory study. Stroke. 2007;38(6):1855–1859. doi: 10.1161/STROKEAHA.106.481556. [DOI] [PubMed] [Google Scholar]
- 41.Bogoslovsky T, Spatz M, Chaudhry A, et al. Circulating CD133+CD34+ progenitor cells inversely correlate with soluble ICAM-1 in early ischemic stroke patients. J Transl Med. 2011;9:145. doi: 10.1186/1479-5876-9-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jaroslav P, Christian R, Stefan O, et al. Evaluation of serum biomarkers for patients at increased risk of stroke. Int J Vasc Med. 2012;906954 doi: 10.1155/2012/906954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barr TL, Latour LL, Lee KY, et al. Blood-brain barrier disruption in humans is independently associated with increased matrix metalloproteinase-9. Stroke. 2010;41(3):e123–e128. doi: 10.1161/STROKEAHA.109.570515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Silva Y, Leira R, Tejada J, et al. Stroke Project, Cerebrovascular Diseases Group of the Spanish Neurological Society. Molecular signatures of vascular injury are associated with early growth of intracerebral hemorrhage. Stroke. 2005;36(1):86–91. doi: 10.1161/01.STR.0000149615.51204.0b. [DOI] [PubMed] [Google Scholar]
- 45.Serena J, Blanco M, Castellanos M, et al. The prediction of malignant cerebral infarction by molecular brain barrier disruption markers. Stroke. 2005;36(9):1921–1926. doi: 10.1161/01.STR.0000177870.14967.94. [DOI] [PubMed] [Google Scholar]
- 46.Doehner W, von Haehling S, Suhr J, et al. Elevated plasma levels of neuropeptide proenkephalin a predict mortality and functional outcome in ischemic stroke. J Am Coll Cardiol. 2012;60(4):346–534. doi: 10.1016/j.jacc.2012.04.024. [DOI] [PubMed] [Google Scholar]
- 47.Jung JY, Lee HS, Kang DG, et al. 1H-NMR-based metabolomics study of cerebral infarction. Stroke. 2011;42(5):1282–1288. doi: 10.1161/STROKEAHA.110.598789. [DOI] [PubMed] [Google Scholar]
- 48.Cui R, Iso H, Toyoshima H, et al. Serum total cholesterol levels and risk of mortality from stroke and coronary heart disease in Japanese: the JACC study. Atherosclerosis. 2007;194:415–420. doi: 10.1016/j.atherosclerosis.2006.08.022. [DOI] [PubMed] [Google Scholar]
- 49.Tanabe N, Iso H, Okada K, et al. Serum total and non-high-density lipoprotein cholesterol and the risk prediction of cardiovascular events – the JALS-ECC. Circ J. 2010;74:1346–1356. doi: 10.1253/circj.cj-09-0861. [DOI] [PubMed] [Google Scholar]
- 50.Suzuki K, Izumi M, Sakamoto T, Hayashi M. Blood pressure and total cholesterol level are critical risks especially for hemorrhagic stroke in Akita. Japan. Cerebrovasc Dis. 2011;31(1):100–106. doi: 10.1159/000321506. [DOI] [PubMed] [Google Scholar]
- 51.Cui R, Iso H, Yamagishi K, et al. High serum total cholesterol levels is a risk factor of ischemic stroke for general Japanese population: the JPHC study. Atherosclerosis. 2012;221(2):565–569. doi: 10.1016/j.atherosclerosis.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 52.Powner DJ. In my opinion: serum albumin should be maintained during neurocritical care. Neurocrit Care. 2011;14(3):482–488. doi: 10.1007/s12028-011-9513-z. [DOI] [PubMed] [Google Scholar]
- 53.Babu MS, Kaul S, Dadheech S, et al. Serum albumin levels in ischemic stroke and its subtypes: correlation with clinical outcome. Nutrition. 2013;29(6):872–875. doi: 10.1016/j.nut.2012.12.015. [DOI] [PubMed] [Google Scholar]
- 54.Alvarez-Perez FJ, Castelo-Branco M, Alvarez-Sabin J. Albumin level and stroke. Potential association between lower albumin level and cardioembolic aetiology. Int J Neurosci. 2011;121(1):25–32. doi: 10.3109/00207454.2010.523134. [DOI] [PubMed] [Google Scholar]
- 55.Idicula TT, Waje-Andreassen U, Brogger J, et al. Serum albumin in ischemic stroke patients: the higher the better. The Bergen Stroke Study. Cerebrovasc Dis. 2009;28(1):13–17. doi: 10.1159/000215938. [DOI] [PubMed] [Google Scholar]
- 56.Ginsberg MD, Palesch YY, Martin RH, et al. The albumin in acute stroke (ALIAS) multicenter clinical trial: safety analysis of part 1 and rationale and design of part 2. Stroke. 2011;42(1):119–127. doi: 10.1161/STROKEAHA.110.596072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dziedzic T, Pera J, Wnuk M, et al. Serum albumin as a determinant of cortisol release in patients with acute ischemic stroke. Atherosclerosis. 2012;221(1):212–214. doi: 10.1016/j.atherosclerosis.2011.12.017. [DOI] [PubMed] [Google Scholar]
- 58.Neidert S, Katan M, Fluri F, et al. Cortisol as a prognostic marker of outcome in acute ischemic cerebrovascular events. Endocrine Abstracts. 2009;20:OC3.6. [Google Scholar]
- 59.Zylberstein DE, Skoog I, Björkelund C, et al. Homocysteine levels and lacunar brain infarcts in elderly women: the prospective population study of women in Gothenburg. J Am Geriatr Soc. 2008;56(6):1087–1091. doi: 10.1111/j.1532-5415.2008.01724.x. [DOI] [PubMed] [Google Scholar]
- 60.Tascilar N, Ekem S, Aciman E, et al. Hyperhomocysteinemia as an independent risk factor for cardioembolic stroke in the Turkish population. Tohoku J Exp Med. 2009;218(4):293–300. doi: 10.1620/tjem.218.293. [DOI] [PubMed] [Google Scholar]
- 61.Feng C, Bai X, Xu Y, et al. Hyperhomocysteinemia associates with small vessel disease more closely than large vessel disease. Int J Med Sci. 2013;10(4):408–412. doi: 10.7150/ijms.5272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ma Y, Zhao X, Zhang W, et al. Homocysteine and ischemic stroke subtype: a relationship study in Chinese patients. Neurol Res. 2010;32(6):636–641. doi: 10.1179/016164109X12445616596445. [DOI] [PubMed] [Google Scholar]
- 63.Fekih-Mrissa N, Mrad M, Klai S, et al. Methylenetetrahydrofolate reductase (C677T and A1298C) polymorphisms, hyperhomocysteinemia, and ischemic stroke in Tunisian patients. J Stroke Cerebrovasc Dis. 2013;22(4):465–469. doi: 10.1016/j.jstrokecerebrovasdis.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 64.Omrani HQ, Shandiz EE, Qabai M, et al. Hyperhomocysteinemia, folateo and B12 vitamin in Iranian patients with acute ischemic stroke. ARYA Atheroscler. 2011;7(3):97–101. [PMC free article] [PubMed] [Google Scholar]
- 65.Cui R, Moriyama Y, Koike KA, et al. Serum total homocysteine concentrations and risk of mortality from stroke and coronary heart disease in Japanese: the JACC study. Atherosclerosis. 2008;198(2):412–418. doi: 10.1016/j.atherosclerosis.2007.09.029. [DOI] [PubMed] [Google Scholar]
- 66.Unal E, Mungan S, Bilen S, et al. The effects of lipoprotein(a) and homocysteine on prognosis and risk factors in acute ischemic stroke. Int J Neurosci. 2013;123(8):532–536. doi: 10.3109/00207454.2013.772609. [DOI] [PubMed] [Google Scholar]
- 67.Wu XQ, Ding J, Ge AY, et al. Acute phase homocysteine related to severity and outcome of atherothrombotic stroke. Eur J Intern Med. 2013;24(4):362–367. doi: 10.1016/j.ejim.2013.01.015. [DOI] [PubMed] [Google Scholar]
- 68.Ciancarelli I, Di Massimo C, De Amicis D, et al. Evidence of redox unbalance in post-acute ischemic stroke patients. Curr Neurovasc Res. 2012;9(2):85–90. doi: 10.2174/156720212800410885. [DOI] [PubMed] [Google Scholar]
- 69.Taffi R, Nanetti L, Mazzanti L, et al. Plasma levels of nitric oxide and stroke outcome. J Neurol. 2008;255(1):94–98. doi: 10.1007/s00415-007-0700-y. [DOI] [PubMed] [Google Scholar]
- 70.Rajeshwar K, Kaul S, Al-Hazzani A, et al. C-reactive protein and nitric oxide levels in ischemic stroke and its subtypes: correlation with clinical outcome. Inflammation. 2012;35(3):978–984. doi: 10.1007/s10753-011-9401-x. [DOI] [PubMed] [Google Scholar]
- 71.Perlstein TS, Pande RL, Creager MA, et al. Serum total bilirubin level, prevalent stroke, and stroke outcomes: NHANES 1999–2004. Am J Med. 2008;121(9):781–788. doi: 10.1016/j.amjmed.2008.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kimm H, Yun JE, Jo J, Jee SH. Low serum bilirubin level as an independent predictor of stroke incidence: a prospective study in Korean men and women. Stroke. 2009;40(11):3422–3427. doi: 10.1161/STROKEAHA.109.560649. [DOI] [PubMed] [Google Scholar]
- 73.Pineda S, Bang OY, Saver JL, et al. Association of serum bilirubin with ischemic stroke outcomes. J Stroke Cerebrovasc Dis. 2008;17(3):147–152. doi: 10.1016/j.jstrokecerebrovasdis.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Luo Y, Li JW, Lu ZJ, et al. Serum bilirubin after acute ischemic stroke is associated with stroke severity. Curr Neurovasc Res. 2012;9(2):128–132. doi: 10.2174/156720212800410876. [DOI] [PubMed] [Google Scholar]
- 75.Vittos O, Toana B, Vittos A, Moldoveanu E. Lipoprotein-associated phospholipase A2 (Lp-PLA2): a review of its role and significance as a cardiovascular biomarker. Biomarkers. 2012;17(4):289–302. doi: 10.3109/1354750X.2012.664170. [DOI] [PubMed] [Google Scholar]
- 76.Elkind MS, Tai W, Coates K, et al. Lipoprotein-associated phospholipase A2 activity and risk of recurrent stroke. Cerebrovasc Dis. 2009;27(1):42–50. doi: 10.1159/000172633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Thompson A, Gao P, Orfei L, et al. Lp-PLA2 Studies Collaboration. Lipoprotein-associated phospholipase A(2) and risk of coronary disease, stroke, and mortality: collaborative analysis of 32 prospective studies. Lancet. 2010;375:1536–1544. doi: 10.1016/S0140-6736(10)60319-4. • Well-designed meta-analysis that reports the association of lipoprotein-associated phospholipase A2 activity with pro-atherogenic lipids and vascular disease, including stroke.
- 78.Tsai TH, Chen YL, Lin HS, et al. Link between lipoprotein-associated phospholipase A2 gene expression of peripheral-blood mononuclear cells and prognostic outcome after acute ischemic stroke. J Atheroscler Thromb. 2012;19(6):523–531. doi: 10.5551/jat.10751. [DOI] [PubMed] [Google Scholar]
- 79.Delgado P, Chacón P, Penalba A, et al. Temporal profile and prognostic value of Lp-PLA2 mass and activity in the acute stroke setting. Atherosclerosis. 2012;220(2):532–536. doi: 10.1016/j.atherosclerosis.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 80.Rajeshwar K, Kaul S, Al-Hazzani A, et al. C-reactive protein and nitric oxide levels in ischemic stroke and its subtypes: correlation with clinical outcome. Inflammation. 2012;35(3):978–984. doi: 10.1007/s10753-011-9401-x. [DOI] [PubMed] [Google Scholar]
- 81.Topakian R, Strasak AM, Nussbaumer K, et al. Prognostic value of admission C-reactive protein in stroke patients undergoing iv thrombolysis. J Neurol. 2008;255(8):1190–1196. doi: 10.1007/s00415-008-0866-y. [DOI] [PubMed] [Google Scholar]
- 82. Selvarajah JR, Smith CJ, Hulme S, et al. Does inflammation predispose to recurrent vascular events after recent transient ischaemic attack and minor stroke? The North West of England transient ischaemic attack and minor stroke (NORTHSTAR) study. Int J Stroke. 2011;6(3):187–194. doi: 10.1111/j.1747-4949.2010.00561.x. • Well-designed study suggesting that circulating inflammatory markers are not associated with recurrent vascular events in transient ischemic attack or minor stroke patients.
- 83.Ladenvall C, Jood K, Blomstrand C, et al. Serum C-reactive protein concentration and genotype in relation to ischemic stroke subtype. Stroke. 2006;37(8):2018–2023. doi: 10.1161/01.STR.0000231872.86071.68. [DOI] [PubMed] [Google Scholar]
- 84.Kunes P, Holubcova Z, Kolackova M, Krejsek J. Pentraxin 3 (PTX 3): an endogenous modulator of the inflammatory response. Mediators Inflamm. 2012;920517 doi: 10.1155/2012/920517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kanbay M, Ikizek M, Solak Y, et al. Uric acid and pentraxin-3 levels are independently associated with coronary artery disease risk in patients with stage 2 and 3 kidney disease. Am J Nephrol. 2011;33(4):325–331. doi: 10.1159/000324916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ryu WS, Kim CK, Kim BJ, et al. Pentraxin 3: a novel and independent prognostic marker in ischemic stroke. Atherosclerosis. 2012;220(2):581–586. doi: 10.1016/j.atherosclerosis.2011.11.036. [DOI] [PubMed] [Google Scholar]
- 87.Welsh P, Barber M, Langhorne P, et al. Associations of inflammatory and haemostatic biomarkers with poor outcome in acute ischaemic stroke. Cerebrovasc Dis. 2009;27(3):247–253. doi: 10.1159/000196823. [DOI] [PubMed] [Google Scholar]
- 88.Ehrenreich H, Kästner A, Weissenborn K, et al. Circulating damage marker profiles support a neuroprotective effect of erythropoietin in ischemic stroke patients. Mol Med. 2011;17(11–12):1306–1310. doi: 10.2119/molmed.2011.00259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Beer C, Blacker D, Bynevelt M, et al. Systemic markers of inflammation are independently associated with S100B concentration: results of an observational study in subjects with acute ischaemic stroke. J Neuroinflammation. 2010;7:71. doi: 10.1186/1742-2094-7-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nash DL, Bellolio MF, Stead LG. S100 as a marker of acute brain ischemia: a systematic review. Neurocrit Care. 2008;8(2):301–307. doi: 10.1007/s12028-007-9019-x. [DOI] [PubMed] [Google Scholar]
- 91.Rallidis LS, Zolindaki MG, Vikelis M, et al. Elevated soluble intercellular adhesion molecule-1 levels are associated with poor short-term prognosis in middle-aged patients with acute ischaemic stroke. Int J Cardiol. 2009;132(2):216–220. doi: 10.1016/j.ijcard.2007.11.031. [DOI] [PubMed] [Google Scholar]
- 92.Wang JY, Zhou DH, Li J, et al. Association of soluble intercellular adhesion molecule 1 with neurological deterioration of ischemic stroke: the Chongqing Stroke Study. Cerebrovasc Dis. 2006;21(1–2):67–73. doi: 10.1159/000090005. [DOI] [PubMed] [Google Scholar]
- 93.Castillo J, Alvarez-Sabín J, Martínez-Vila E, et al. Inflammation markers and prediction of post-stroke vascular disease recurrence: the MITICO study. J Neurol. 2009;256(2):217–224. doi: 10.1007/s00415-009-0058-4. [DOI] [PubMed] [Google Scholar]
- 94. Weiss JB, Eisenhardt SU, Stark GB, et al. MicroRNAs in ischemia-reperfusion injury. Am J Cardiovasc Dis. 2012;2(3):237–247. • A good review of miRNA and its target genes in different tissues after ischemia-reperfusion injury.
- 95.Ziu M, Fletcher L, Rana S, et al. Temporal differences in microRNA expression patterns in astrocytes and neurons after ischemic injury. PLoS ONE. 2011;6:e14724. doi: 10.1371/journal.pone.0014724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Cameron Rink and Savita Khanna. MicroRNA in ischemic stroke etiology and pathology. Physiol Genomics. 2011;43(10):521–528. doi: 10.1152/physiolgenomics.00158.2010. • Overview of the function of miRNAs in pathophysiology of ischemic stroke.
- 97.Tan KS, Armugam A, Sepramaniam S, et al. Expression profile of microRNAs in young stroke patients. PLoS ONE. 2009;4:e7689. doi: 10.1371/journal.pone.0007689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jeon YJ, Kim OJ, Kim SY, et al. Association of the miR-146a, miR-149, miR-196a2, and miR-499 Polymorphisms With Ischemic Stroke and Silent Brain Infarction Risk. Arterioscler Thromb Vasc Biol. 2013;33(2):420–430. doi: 10.1161/ATVBAHA.112.300251. [DOI] [PubMed] [Google Scholar]
- 99.Zeng L, Liu J, Wang Y, et al. MicroRNA-210 as a novel blood biomarker in acute cerebral ischemia. Front Biosci (Elite Ed) 2011;3:1265–1272. doi: 10.2741/e330. [DOI] [PubMed] [Google Scholar]
- 100.Katan M, Fluri F, Morgenthaler NG, et al. Copeptin: a novel, independent prognostic marker in patients with ischemic stroke. Ann Neurol. 2009;66(6):799–808. doi: 10.1002/ana.21783. [DOI] [PubMed] [Google Scholar]
- 101.Urwyler SA, Schuetz P, Fluri F, et al. Prognostic value of copeptin: one-year outcome in patients with acute stroke. Stroke. 2010;41(7):1564–1567. doi: 10.1161/STROKEAHA.110.584649. [DOI] [PubMed] [Google Scholar]
- 102.Katan M, Nigro N, Fluri F, et al. Stress hormones predict cerebrovascular re-events after transient ischemic attacks. Neurology. 2011;76(6):563–566. doi: 10.1212/WNL.0b013e31820b75e6. [DOI] [PubMed] [Google Scholar]
- 103.Dong X, Tao DB, Wang YX, et al. Plasma copeptin levels in Chinese patients with acute ischemic stroke: a preliminary study. Neurol Sci. 2013;34(9):1591–1595. doi: 10.1007/s10072-013-1291-2. [DOI] [PubMed] [Google Scholar]
- 104.De Marchis GM, Katan M, Weck A, et al. Copeptin adds prognostic information after ischemic stroke: results from the CoRisk study. Neurology. 2013;80(14):1278–1286. doi: 10.1212/WNL.0b013e3182887944. [DOI] [PubMed] [Google Scholar]
- 105.Zhang JL, Yin CH, Zhang Y, et al. Plasma copeptin and long-term outcomes in acute ischemic stroke. Acta Neurol Scand. 2013;128(6):372–380. doi: 10.1111/ane.12132. [DOI] [PubMed] [Google Scholar]
- 106.Tu WJ, Dong X, Zhao SJ, et al. Prognostic value of plasma neuroendocrine biomarkers in patients with acute ischemic stroke. J Neuroendocrinol. 2013;25(9):771–778. doi: 10.1111/jne.12052. [DOI] [PubMed] [Google Scholar]