Abstract
Pyrene, one of the most studied polycyclic aromatic hydrocarbons can damage biological macromolecules and cause toxicity when irradiated by light. The effect of substituents, 1-amino, 1-hydroxy, 1-nitro, and 1-bromo, on light-induced lipid peroxidation is studied. Degradation kinetics and photoproduct analyses were conducted to test how these substituents affect the photoreaction. All five compounds have different photodegradation rates, and these rates parallel their light absorptivity. Four out of the five compounds induce lipid peroxidation when irradiated with UVA light; whereas, 1-aminopyrene causes minimum or no lipid peroxidation. The relative amount of lipid peroxidation caused is: 1-Bromopyrene > Pyrene > 1-Nitropyrene ≈ 1-Hydroxypyrene > 1-Aminopyrene. This relative lipid peroxidation is dependent on structure due to the following factors: light absorptivity, relative rates of the competing processes in the excited states, nature of the photoreaction, and nature of the photoproducts.
Keywords: Mono-substituted pyrene, photoreaction rate, UVA radiation, lipid peroxidation
Introduction
It is estimated that more than 5 million pounds of polycyclic aromatic hydrocarbons (PAHs) were released into the environment [1]. PAHs are a class of persistent environmental mutagenic contaminants that are mainly produced from the incomplete combustion of matters such as fossil fuels, foods, tobacco smoke, and other sources [2–5]. They are thought to induce cancerous tumors, primarily in the lungs, bladder, and skin [3, 6, 7]. PAHs’ deleterious threats are not merely limited to metabolic activation, but photochemical as well [8–12]. Particularly, concomitant exposure to PAHs and light can lead to the generation of reactive intermediates [8] such as free radicals and reactive oxygen species [13], which may react with DNA, proteins, and lipids to cause various types of damages including lipid peroxidation [14–20].
Lipid peroxidation can become detrimental in human because they are associated with conditions including early liver problems, inflammation, athereosclerosis, and cancer [21–23]. Pyrene, one of the most widely studied and one of the most abundant PAHs, was found to generate light-induced lipid peroxidation in a previous study [18]. Studies on various structurally related PAHs, such as ethylchrysenes and methylbenz[a]anthracenes [16–19, 24], showed that light induced lipid peroxidation depends on PAH structure.
In this study, we report the light-induced lipid peroxidation by pyrene and its 1-amino, bromo, hydroxy, and nitro substituted derivatives (Figure 1). The level of light-induced peroxidation and photochemical reactions are compared, and a structure-activity relationship is discussed.
Figure 1.
Structures of pyrene and its mono-substituted derivatives.
1-Hydroxypyrene (1-HP) is the main metabolite of pyrene [25–29]. 1-HP is detected in urine, and is hence often used as a biomarker for PAH exposure [5, 25, 27, 30]. Additionally, 1-HP can be both acutely toxic and genotoxic [31, 32]. Nitro-PAHs, including 1-NP, can be directly formed by the incomplete combustion of petrochemicals; other nitro-PAHs, though a minor process, can also be indirectly generated in the atmosphere from the reaction of a PAH with NO2 or NO3− [33–35]. Nitro-PAHs have been found to be more mutagenic than their parent PAHs [33, 34, 36–40]. 1-NP can be reduced by nitro-reductases to 1-aminopyrene (1-AP) in living systems [41, 42]. Therefore, 1-AP is also included in this study. Most aromatic amines can be found within industrial chemicals, crude oil, and coal products and they can easily become widespread in the environment [43–45]. 1-AP can be oxidized into 1-hydroxyaminopyrene, a mutagenic intermediate, by cytochrome P450 enzymes [46, 47]. Unlike 1-NP, 1-AP was not found to induce cancerous tumors [36]. Although 1-bromopyrene (1-BP) occurs less abundantly compared to other compounds, it is chosen for comparison purposes.
Materials and Methods
Pyrene, 1-AP, 1-BP, 1-HP, 1-NP, methyl linoleate (ML), sodium azide (NaN3), and potassium iodide (KI) were purchased from Sigma-Aldrich (St. Louis, MO). The PAHs were with a purity of at least 96% and used without further purification. The methanol used was spectroscopic grade, and the water used (18 Ω) was deionized by a Barnstead Nanopure Infinity water deionization system (Dubuque, IA). UV spectra were attained from a CHEM USB4-UV-Vis (Ocean Optics, Winter Park, FL).
Photochemical Reaction
Pyrenes (50 µM) in methanol were prepared from the methanol stock solution stored in the refrigerator. Two milliliters of the 50 µM pyrenes in pure methanol was irradiated with UVA light for a total of 30 min in a quartz cuvette (10×35 mm). The light source was a model TL-365R 15 W Spectroline UVA transilluminator (Westbury, NY) that produces an emission band at 365 ± 25 nm. The UVA irradiance is 5.8 mW/cm2, measured with a Model PMA 2100 Radiometer (Solar Light, PA). An absorbance spectrum was obtained at 5 min intervals using UV-Vis spectrophotometer. The wavelength used for each compound to determine the first-order reaction kinetics was: pyrene: 330 nm; 1-AP: 360 nm; 1-BP: 346 nm; 1-HP: 350 nm; 1-NP: 400 nm. The photochemical reaction kinetics was analyzed based on a first-order reaction, ln (A0/At) = kt, where A0 and At refer to the absorption intensity of the pyrenes at irradiation times of 0 and t min, respectively; k is the first-order rate constant. The photodegradation rate was determined by plotting ln (A0/A) vs irradiation time, where the slope was the rate constant, k. The degradation half-life of the compounds was calculated by t1/2 = 0.693/k.
Photo-induced lipid peroxidation
Lipid peroxidation experiments were carried out using 0.2 mM solution of the pyrenes in the presence of 100 mM ML in methanol. Each reaction was irradiated with the UVA light for a total of 60 min in a quartz cuvette. Samples were collected at 20 min intervals and detected using high-performance liquid chromatography (HPLC), a Shimadzu SCL-10A VP HPLC system (Shimadzu Corporation, Houston, TX). The peak areas were converted to ML hydroperoxide concentrations based on the molar extinction coefficient at 235 nm as reported by Gibian and Vandenberg [48]. HPLC analyses were accomplished by injecting 10 µL of the reaction mixture after each irradiation time. The column was a reverse phase ODS column, 250×4.6 mm, 5 micron (Phenomenex, Torrance, CA). The mobile phase was isocratic 10% water in methanol (v/v). For 1-NP, it was 15% water in methanol for a better separation. The flow rate was set at 1.0 mL/min. The signals were monitored at 235 nm. The experiment was conducted in triplicate. All results were analyzed using an Analysis of Variance (ANOVA) and the Tukey t-test to distinguish the differences between the Pyrene derivatives available through the Statistical Analysis Software SAS version 9.1. These tests α-level = 0.05.
Lipid Peroxidation in the Presence of Quenchers
NaN3 or KI (50 mM) were incorporated into the solution mixture of 0.2 mM pyrenes and 100 mM ML in methanol. Each was irradiated with UVA light for 30 min in a quartz cuvette. Samples were collected and detected by HPLC analysis. The experiment was conducted in triplicate. All results were analyzed using an Analysis of Variance (ANOVA) and the Tukey t-test to distinguish the differences between the pyrene derivatives available through the Statistical Analysis Software SAS version 9.1. These tests α-value = 0.05.
Results and Discussion
Absorption Spectra
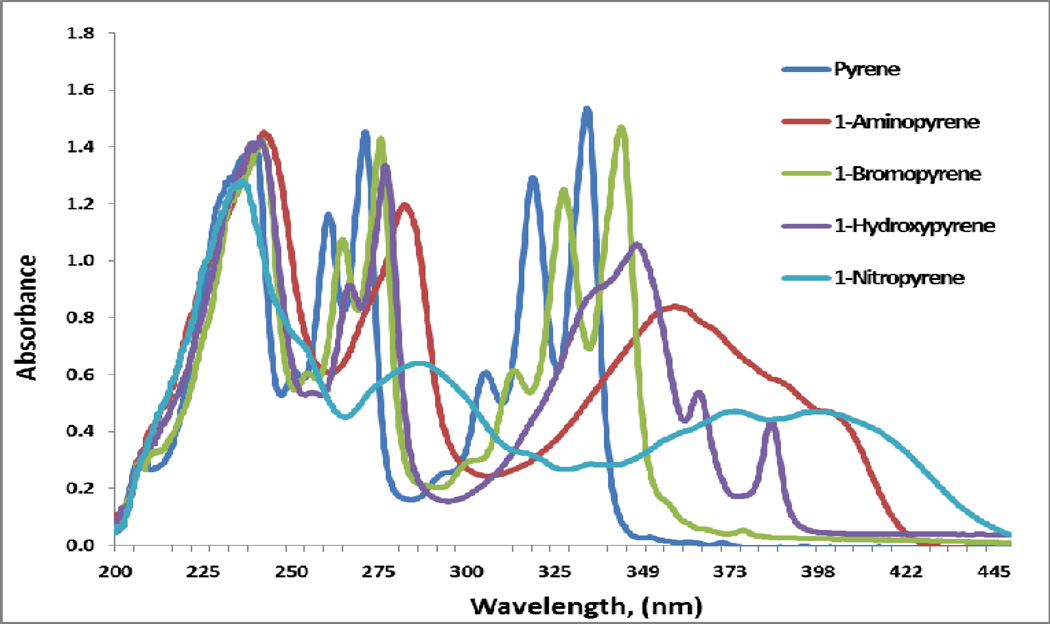
Pyrene and the four chosen 1-substituted Pyrenes all absorb light in the UV region with some extending into the visible region (Figure 2). The addition of a substituent, relative to Pyrene, results in a red-shifted spectrum, with the least and greatest shifts being due to the bromo and nitro groups, respectively. Pyrene has seven bands extending from 200–360 nm (238, 250, 260, 271, 304, 317, and 332 nm), while 1-BP has the same bands, but each red-shifted to 242, 255, 265, 273, 314, 329, and 343 nm, respectively. These seven bands can be further grouped into three main bands: 235–242, 250–271, and 304–343. 1-HP has six bands (241, 267, 277, 347, 365, and 385 nm). While the first two main bands at 241 and 267–277 are similar to that of 1-BP, the longer wavelength bands (347, 365, and 385) are not only red-shifted, but also have completely different shapes, indicating the effect of the extended conjugation of the hydroxyl group. The absorbance spectra of 1-NP and 1-AP have the same short wavelength bands at 235 nm, but the two other main bands are further red-shifted. In addition, the two longest wavelength bands lose the finger-print features. The relative absorbance of the UVA light sources (Irel) of these compounds were determined and listed in Table 1.
Figure 2.
The absorbance spectra of 50 µM pyrene and its derivatives in methanol.
Table 1.
Pyrene (50 µM in methanol) derivatives’ degradation rate constants and half-life values.
| PAH | K (min−1) | Hal-life (min) | Iabs (rel) |
|---|---|---|---|
| Pyrene (330 nm+) | 1.60×10−3 | 433 | 1.0 |
| 1-Bromopyrene (346 nm+) | 1.40×10−3 | 495 | 6.7 |
| 1-Nitropyrene (400 nm+) | 1.60×10−2 | 43.3 | 8.9 |
| 1-Hydroxypyrene (350 nm +) | 2.56×10−2 | 27.1 | 13.9 |
| 1-Aminopyrene (360 nm+) | 9.55×10−2* | 7.3* | 17.3 |
The wavelength used for each compound to determine the first-order reaction kinetics.
Determined by HPLC method.
Photochemical Reaction
With the exception of 1-AP, photodegradation kinetics of these Pyrenes were determined and calculated based on their absorbance obtained within the first 30 min of irradiation. Upon light irradiation, the 1-AP absorption decrease appeared to be small compared to that of 1-HP and 1-NP. HPLC analysis showed that new products were formed, and the absorbance spectra of 1-AP and its photoproducts were similar (Figure 4). Therefore, the degradation rate for 1-AP was determined by HPLC analysis. The 1-AP peak completely disappeared after 30 min of irradiation based on HPLC chromatogram. From this analysis, the degradation rate for 1-AP is 9.55×10−2 min−1 and the half-life is 7.3 min.
Figure 4.
Representative chromatograph of ML peroxide formation and absorption spectra for 1-AP and 1-BP.
As shown in Figure 3, the photodegradation rates are different for all five compounds. 1-AP has the fastest photodegradation while Pyrene and 1-BP degrade very slowly. Both 1-HP and 1-NP degrade moderately. All of these UV-Vis analyses were confirmed by HPLC analysis (Not shown). Table 1 lists the first-order rate constants and half-lives obtained from the photodegradation in methanol.
Figure 3.
Photodegradation rates of 50 µM 1-aminopyrene, 1-hydroxypyrene, 1-nitropyrene, pyrene, and 1-bromopyrene. The degradation rate for 1-AP was calculated from the peak area using HPLC analysis, while the others were based on their absorbance changes.
The absorption spectra and the degradation rates of all five compounds are substantially different. The light source used has an emission band at 365 ± 25 nm. These compounds’ relative absorbance (Irel) was determined by calculating the area under the absorption spectra within this region (340 – 390 nm). Thus, the calculated relative absorbance is: 1-AP > 1-HP > 1-NP > 1-BP > Pyrene (Pyrene’s relative absorbance was defined as 1.0 for comparison purposes). These relative absorbance values parallel the degradation rates. The absorbance spectrum of 1-BP is slightly more red-shifted than Pyrene, thus contributing to the higher relative absorbance value. Pyrene and 1-BP both have the lowest relative absorbance because they do not display an extensive red shift, and comparatively speaking, they have the smallest photodegradation rates. This clearly indicates that light absorption is crucial for their degradation.
Another reason for the difference in degradation rates is the nature of the substituents. For example, the amino and hydroxyl groups in the excited state Pyrene can be readily oxidized, while the nitro group can either rearrange or be replaced by a substituent [8, 49–53]. 1-AP, 1-HP, and to a lesser extent, 1-NP’s ability to absorb UVA light enables them to change the electronic distribution about the nucleus, employing bond breaking and creation [54]. This was demonstrated previously that 1-AP can be oxidized into 1-hydroxyaminopyrene, 1-nitrosopyrene, 1-nitropyrene, and 1-amino-x-hydroxypyrene [52]. The photoproducts of 1-HP are 1,6- and 1,8-pyrene quinones and a pyrene dimer [53], and the photo-induced reactions of 1-NP include pyrene, 1-HP, hydroxynitropyrenes, and pyrenediones [49, 50, 55]. Compared to the other 1-substituted Pyrenes, 1-NP was the least soluble in methanol [51]. In addition to the structural dependence, it has also been shown that the photoreaction of nitro-PAHs is dependent upon solvent polarity [49, 51]. For example, 1-NP photo-degrades faster in less polar and better soluble solvents such as chloroform.
Lipid Peroxidation
Upon UVA irradiation, ML can be transformed into cis and trans isomeric ML 9- and 13-hydroperoxides [13, 56]. Light-induced lipid peroxidation by Pyrene, 1-AP, 1-BP, 1-HP and 1-NP was monitored by HPLC (Figure 4). Each of the compounds was dissolved in methanol and irradiated by the UVA light, and a sample was taken at 0, 20, 40, and 60 min irradiation time for HPLC analysis. With the exception of 1-NP, the mobile phase used was 90% methanol and 10% water. At 90% methanol, 1-NP and ML hydroperoxides are not well-separated. Changing the mobile phase to 85% of methanol achieved good separation.
The amounts of ML hydroperoxides were calculated based on their HPLC peak area monitored at 235 nm [16, 17, 24]. Figure 5 plots the amount of ML hydroperoxides versus irradiation time during the 60 min of irradiation. 1-AP and 1-HP are nearly completely degraded within 60 min of irradiation based on their degradation half-lives of 7.3 min and 27.1 min, respectively (Table 1). It is also worth noting that neither Pyrene nor 1-BP degrades substantially during the 60 min of irradiation.
Figure 5.
Time-dependent ML Peroxide Formation by Pyrenes.
In comparison to the control, all compounds, except 1-AP, generate ML hydroperoxides (Figure 5). 1-BP generates the greatest amount of ML hydroperoxides. Pyrene is similar to 1-BP, but with less amounts of ML hydroperoxides, while the other compounds are less than Pyrene. It appears that the ability to generate lipid peroxidation is dependent on both the structure and these compounds’ photoreaction rate.
The rate at which 1-HP produces ML hydroperoxides increased steadily, similar to both Pyrene and 1-BP until 40 min, where it levels off. The half-life for 1-HP is 27 min, and this leveling off is due to the near completion of degradation of 1-HP. It is known that the photoproducts of 1-HP are 1,6- and 1,8-pyrene quinones and a Pyrene quinone dimer [53], and these quinones can also induced lipid peroxidation [16]. The leveling off at 40 min for the 1-HP induced lipid peroxidation is because the quinines, though formed as photoproducts, were not produced in significant amounts as it is indicated in the HPLC chromatograms in Figure 1S (supplementary materials). Similarly, 1-AP does not generate significant amount of ML hydroperoxides due to its relatively fast photochemical reaction, producing sequentially 1-hydroxyamino, 1-nitroso, and 1-NP [52]. It means that these photoproducts of 1-AP are not causing significant amount of lipid peroxidation.
In contrast, the initial rate at which 1-NP generates lipid peroxidation was slower compared to Pyrene, 1-BP, and 1-HP, but there is a significant increase in lipid peroxidation at 40–60 min. The half-life of 1-NP degradation is 43 min. The sharp rise in lipid peroxidation suggests that the photoproducts of 1-NP are formed after 40 min of irradiation and they are more photoactive than 1-NP itself. The nitro group in 1-NP is not co-planar to the Pyrene ring [57]. Nitro-PAHs with none co-planar nitro groups are known to undergo photochemical oxidation of the aromatic ring [51, 58]. Photoreaction products of 1-NP include Pyrene, 1-HP, hydroxynitropyrenes, and pyrenediones [50, 55]. These photoproducts, at least as it is known for 1-HP in this study, are more photoactive in causing lipid peroxidation.
Involvement of Oxygen/1O2
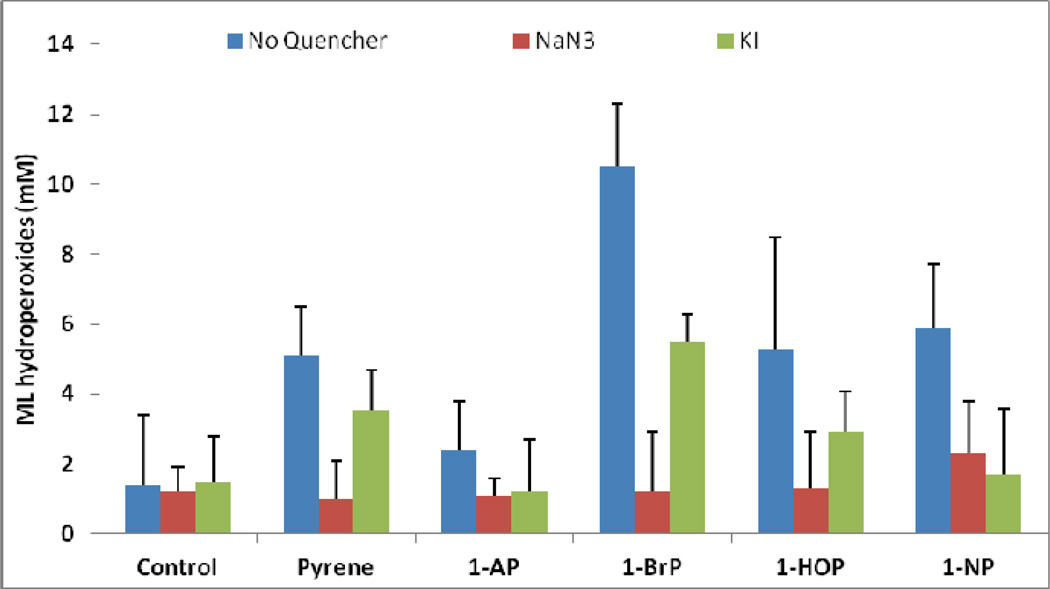
Quenchers, NaN3 and KI, were used to determine whether reactive oxygen species (ROS) are involved in the induction of lipid peroxidation. NaN3 is a singlet oxygen scavenger, while KI is a singlet state quencher. Samples were irradiated for a set time of 30 min. After taking into consideration the effects from the control, it is found that Pyrene, 1-BP, and 1-HP are quenched the most by NaN3 (Figure 6). 1-BP and 1-NP are the most responsive to KI quenching. Therefore, it seems that all of the studied Pyrenes can generate singlet oxygen. We believe that the heavy atom effect of the bromo group in 1-BP is the main reason for the more pronounced quenching effect. Specifically, the bromo atom promotes intersystem crossing and produces a higher triplet state population with a quantum efficiency of at least two orders of magnitudes higher than Pyrene [59], thus creating more lipid hydroperoxides.
Figure 6.
Effect of NaN3 and KI on light-induced peroxidation of ML by pyrenes after 30 min of irradiation (n=3).
ROS species, such as singlet oxygen, are highly reactive. Singlet oxygen can create damages by directly reacting with biological macromolecules or through the formation of longer-lived hydroxide radicals [21, 22, 60, 61]. It is postulated that disruption and damage to macromolecules by ROS can lead to deleterious effects, such as tumor formation [23, 62]. Adverse effects of lipid peroxidation can be just as detrimental in humans as it is within animal systems. For instance, lipid peroxidation caused by ROS can be associated with conditions such as early aging, liver problems, inflammation, athereosclerosis, ischemia, and cancer [22, 23].
Structure-Activity Relationship
Pyrene and 1-AP, 1-BP, 1-HP, and 1-NP can all absorb UVA light leading to lipid peroxidation. As determined previously on PAHs including Pyrene, both singlet oxygen and superoxide are involved in lipid peroxidation caused by light irradiation of PAHs [13, 16–19, 24]. Generally, singlet oxygen is the main reactive species leading to lipid peroxidation for most PAHs we tested. It is also the case with the mono-substituted Pyrenes in this study. In addition, 1-HP was also shown to induce DNA single strand cleavage via singlet oxygen [32]. The amounts of peroxidation of these compounds depend on the substituent, which governs the photochemical processes upon light excitation as shown in Scheme 1.
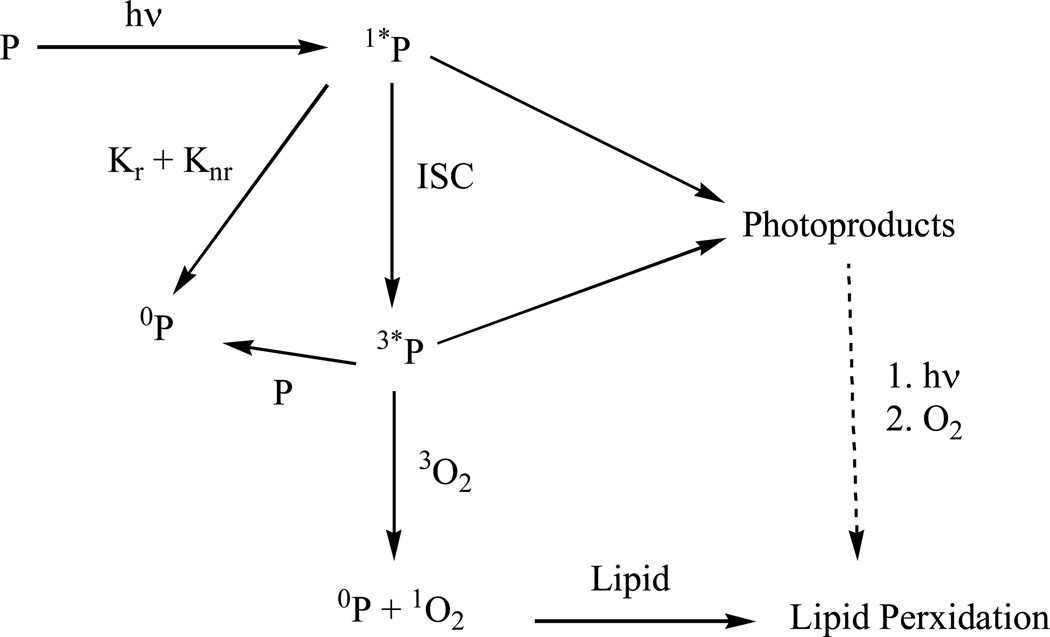
Scheme 1.
Light excitation of pyrene and its mono-substituted derivatives (represented by P) leading to lipid peroxidation.
Upon absorption of light, Pyrene or its mono-substituted derivatives (represented by P) is promoted to the excited singlet state, 1*P. The 1*P can undergo several competing processes including fluorescence (Kr), nonradiative decay (Knr), photochemical reaction to form photoproducts, and intersystem crossing (ISC) to reach the excited triplet state, 3*P. Processes such as photoreaction (K1 and K2) and phosphorescence (Kp) can occur from 3*P, but it can also react with ground state oxygen to produce singlet oxygen (1O2), which can react with ML to produce ML hydroperoxides. Some photoproducts can also go through the similar light activation processes, leading to lipid peroxidation.
Thus, there are several factors governing the light-induced lipid peroxidation by Pyrenes that are responsible for the structural dependence: light absorptivity, relative rates of the competing processes, and the photochemical reactions. 1.) Light absorptivity: As seen in Table 1, the photodegradation rate is parallel to light absorptivity. More efficient light absorption can produce more excited state molecules leading to lipid peroxidation. 2). Rates of competing processes: Competing processes leading to the triplet state include ISC versus Knr, Kr, and singlet state photoreaction. This is seen in 1-BP since the presence of the bromine atom promotes intersystem crossing, resulting in two orders of magnitude higher triplet quantum yield in 1-BP versus Pyrene [59]. This is also seen in 1-AP, whose photodegradation half-life is only 7.3 min. Therefore, 1-AP is not causing much lipid peroxidation during the 30 or 40 min of irradiation since most of it was degraded. 3) Photochemical reactions: Photoreactions lead to degradation (destruction of the parent compound) or generate products that can cause no, less, or more lipid peroxidation. For 1-HP, upon light irradiation, it degrades quickly and forms small amounts of quinones [32, 53]. This explains why the lipid peroxidation levels off after 40 min of irradiation. For 1-AP, upon UVA irradiation, it leads to the formation of 1-hydroxyamino, 1-nitroso, and 1-nitropyrene and some smaller fragmented molecules quickly [52], causing no lipid peroxidation. Conversely, 1-NP itself is a weak inducer of lipid peroxidation, but its photoproducts are stronger lipid peroxidation inducers, resulting in the ramping up of lipid peroxidation observed from 40–60 min of irradiation. Coincidently, one of the photoproducts of 1-NP is 1-HP [49], which is a good lipid peroxidation inducer. As a result of the combination of factors, the relative light-induced lipid peroxidation is: 1-BP > Pyrene > 1-NP ≈ 1-HP > 1-AP.
Finally, it is also worth noting that solvent polarity plays an important role on the photochemical reaction rate of PAHs, such as it was shown with nitro-PAHs [51]. Since the lipid peroxidation experiments were carried out in methanol, it can be inferred that the amount of lipid peroxidation induced by these Pyrene derivatives may be different in other solvents, and the relative ability of these compounds causing lipid peroxidation may be altered.
Table 2.
UVA light-induced ML hydroperoxides (mM) in the presence of Pyrenes (0.2 mM), expressed as means ± SD (n = 3). The ML hydroperoxide concentrations were obtained after zeroing the ML background.
| Samples | 0 min | 20 min | 40 min | 60 min |
|---|---|---|---|---|
| Control | 0.0 ± 0.0 | 1.1 ± 0.7 | 2.1 ± 0.9 | 3.0 ± 1.8 |
| Pyrene | 1.9 ± 0.8 | 7.1 ± 0.4 | 12.1 ± 1.0 | 18.1 ± 1.4 |
| 1-AP | 1.2 ± 0.0 | 2.0 ± 0.4 | 2.2 ± 0.5 | 3.3 ± 0.3 |
| 1-BrP | 1.2 ± 0.7 | 9.3 ± 0.6 | 18.6 ± 1.9 | 27.6 ± 2.8 |
| 1-HP | 0.8 ± 0.2 | 5.8 ± 0.6 | 10.4 ± 1.3 | 12.4 ± 1.0 |
| 1-NP | 1.3 ± 0.7 | 5.4 ± 1.0 | 7.9 ± 1.9 | 13.8 ± 2.3 |
Acknowledgements
Use of Research Core Facilities was made possible by NIH grant number G12 RR13459 from the NCRR/RCMI. Financial support for TEP was from the U.S. Department of Education Grant number P031B090210-11 through Title III-HBGI. The authors wish to thank Dr. Peter Fu of the National Center for Toxicological Research for useful discussions.
References
- 1.EPA US. Toxic Release Inventory. [Accessed April 2012];2009 http://www.epa.gov/triinter/tridata/index.htm.
- 2.Harvey R. Polycyclic Aromatic Hydrocarbons. New York: Wiley-VCH; 1996. [Google Scholar]
- 3.Dipple A. Polycyclic Aromatic Hydrocarbons and Carcinogenesis. Washington, DC: 1985. [Google Scholar]
- 4.Angerer J, Mannschreck C, Gundel J. Biological monitoring and biochemical effect monitoring of exposure to polycyclic aromatic hydrocarbons. Int. Arch. Occup. Environ. Health. 1997;70:365–377. doi: 10.1007/s004200050231. [DOI] [PubMed] [Google Scholar]
- 5.Talaska G, Underwood P, Maier A, Lewtas J, Rothman N, Jaeger M. Polycyclic aromatic hydrocarbons (PAHs), nitro-PAHs and related environmental compounds: Biological markers of exposure and effects. Environ. Health Perspect. 1996;104:701–908. doi: 10.1289/ehp.96104s5901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Warshawsky D. polycyclic Aromatic Hydrocarbons in Carcinogenesis. Environ. Health Perspect. 1999;107:317–320. doi: 10.1289/ehp.99107317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.US Department of Health and Human Services P. Toxicological profile for polycyclic aromatic hydrocarbons (PAHs) Atlanta, GA: 1995. ATSDR. [Google Scholar]
- 8.Yu H. Environmental carcinogenic polycyclic aromatic hydrocarbons: photochemistry and phototoxicity. J. Environ. Sci. Health, Part C: Environ. Carcinog. Ecotoxic. Revs. 2002;C20:149–183. doi: 10.1081/GNC-120016203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arfsten DP, Schaeffer DJ, Mulveny DC. The effects of near ultraviolet radiation on the toxic effects of polycyclic aromatic hydrocarbons in animals and plants. A review. Ecotoxicol. Environ. Safety. 1996;33:1–24. doi: 10.1006/eesa.1996.0001. [DOI] [PubMed] [Google Scholar]
- 10.Ankley GT, Collyard SA, Monson PD, Kosian PA. Influence of ultraviolet light on the toxicity of sediments contaminated with polycyclic aromatic hydrocarbons. Envin. Toxicol. Chem. 1994;13:1791–1796. [Google Scholar]
- 11.Betowski LD, Enlow M, Riddick L. The phototoxicity of polycyclic aromatic hydrocarbons: a theoretical study of excited states and correlation to experiment. Comp. Chem. 2002;26:371–377. doi: 10.1016/s0097-8485(02)00002-5. [DOI] [PubMed] [Google Scholar]
- 12.Krylov S, Huang X, Zeiler L, Dixon D, Greenberg B. Mechanistic quantitative structure-activity relationship model for the photoinduced toxicity of polycyclic aromatic hydrocarbons: I. physical model based on chemical kinetics in a two-compartment system. Environ. Toxicol. Chem. 1997;16:2283–2295. [Google Scholar]
- 13.Yu H, Xia Q, Yan J, Herreno-Saenz D, Wu YS, et al. Photoirradiation of polycyclic aromatic hydrocarbons with UVA- A Pathway leading to the generation of reactive oxygen species, lipid peroxidation, and DNA damage. Int. J. Environ. Res. Public Health. 2006;3:348–354. doi: 10.3390/ijerph2006030045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Toyooka T, Ibuki Y, Goto R. Coexposure to benzo[a]pyrene and UVA induces DNA damage; first proof of double-strand breaks in a cell-free system. Environ. Mol. Mutagen. 2006;47:38–47. doi: 10.1002/em.20166. [DOI] [PubMed] [Google Scholar]
- 15.Newsted JL, Giesy JP. Predictive models for photoinduced acute toxicity of polycyclic aromatic hydrocarbons to Daphnia magna, Strauss (Cladocera, Crustacea) Environ. Toxicol. Chem. 1987;13:1791–1796. [Google Scholar]
- 16.Zhao Y, Xia Q, Yin JJ, Yu H, Fu PP. Photoirradiation of polycyclic aromatic hydrocarbon diones by UVA light leading to lipid peroxidation. Chemosphere. 2011;85:83–91. doi: 10.1016/j.chemosphere.2011.05.040. [DOI] [PubMed] [Google Scholar]
- 17.Xia Q, Chou MW, Yin JJ, Howard PC, Yu H, Fu PP. Photoirradiation of representative polycyclic aromatic hydrocarbons and twelve isomeric methylbenz[a]anthracene with UVA light: formation of lipid peroxidation. Toxicol. Ind. Health. 2006;22:147–156. doi: 10.1191/0748233706th259oa. [DOI] [PubMed] [Google Scholar]
- 18.Yan J, Xia Q, Cherng S, Warner WG, Howard PC, et al. Photoirradiation of representative polycyclic aromatic hydrocarbons and twelve isomeric methylbenz[a]anthracenes with UVA light; formation of lipid peroxidation. Toxicol. Ind. Health. 2006;22:147–156. doi: 10.1191/0748233706th259oa. [DOI] [PubMed] [Google Scholar]
- 19.Yin JJ, Xia Q, Cherng SH, Tang IW, Fu PP, et al. UVA photoirradiation of oxygenated benz[a]anthracene and 3-methylcholanthene- Generation of singlet oxygen and induction of lipid peroxidation. Int.J. Environ. Res. Public Health. 2008;5:26–31. doi: 10.3390/ijerph5010026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yan J, Wang L, Fu PP, Yu H. Photomutagenicity of sixteen polycyclic aromatic hydrocarbons from the US EPA's priority pollutant list. Mutat. Res. 2004;557:99–108. doi: 10.1016/j.mrgentox.2003.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rokitskaya TBM, Antonenko YN, Kotova EA, Pohl P. Photosensitizer binding to lipid bilayers as a precondition for the photoinactivation of membrane channels. Biophys. J. 2000;78:2572–2580. doi: 10.1016/S0006-3495(00)76801-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Girotti AW. Photosensitized oxidation of membrane lipids: reaction pathways, cytotoxic effects, and cytoprotective mechanisms. J. Photochem. Photobiol. B: Biology. 2001;63:103–113. doi: 10.1016/s1011-1344(01)00207-x. [DOI] [PubMed] [Google Scholar]
- 23.Chung FC, Chen HJC, Guttenplan JB, Nishikawa A, Hard GC. 2,3-epoxy-4-hydroxynonanal as a potential tumor-initiating agent of lipid-peroxidation. Carcinogenesis. 1993;14:2073–2077. doi: 10.1093/carcin/14.10.2073. [DOI] [PubMed] [Google Scholar]
- 24.Chen HC, Xia Q, Cherng SH, Chen S, Lai CC, et al. Synthesis and photoirradiation of isomeric ethylchrysenes by UVA light leading to lipid peroxidation. Int. J. Environ. Res. Public Health. 2007;4:145–152. doi: 10.3390/ijerph2007040009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jongeneelen F. Biological monitoring of environmental exposure to polycyclic aromatic hydrocarbons: 1-hydroxypyrene in urine of people. Toxicol. Lett. 1994;72:205–211. doi: 10.1016/0378-4274(94)90030-2. [DOI] [PubMed] [Google Scholar]
- 26.Angerer J, Mannschreck C, Gundel J. Occupational exposure to polycyclic aromatic hydrocarbons in a graphite-electrode producing plant: biological monitoring of 1-hydroxypyrene and monohydroxylated metabolites of phenanthrene. Int. Arch. Occup. Environ. Health. 1997;69:323–331. doi: 10.1007/s004200050155. [DOI] [PubMed] [Google Scholar]
- 27.Merlo F, Anderson A, Weston A, Pan CF, Haugen A, et al. Urinary excretion of 1-hydroxypyrene as a marker for exposure to urban air levels of polycyclic aromatic hydrocarbons. Cancer Epidemiol. Biomark. Prev. 1998;7:147–155. [PubMed] [Google Scholar]
- 28.Nakajima D, Kojima E, Suzuki S. Presence of 1-hydroxypyrene conjugates in woody plant leaves and seasonal changes in their concentrations. J. Envirn. Sci. Tech. 1996;30:1675. [Google Scholar]
- 29.Roggi C, Minoia C, Sciarra G, Apostoli P, Maccarini L, et al. Urinary 1-hydroxypyrene as a marker of exposure to pyrene: an epidemiological survey on a general population group. Sci. Total Environ. 1997;199:247–254. doi: 10.1016/s0048-9697(97)05458-2. [DOI] [PubMed] [Google Scholar]
- 30.Dor F, Dab W, Empereur-Bissonnet P, Zmirou D. Validity of biomarkers in environmental health studies: the case of PAHs and benzenes. Crit. Rev. Toxicol. 1999;29:129–168. doi: 10.1080/10408449991349195. [DOI] [PubMed] [Google Scholar]
- 31.Lambert M, Kremer S, Anke H. Antimicrobial, phytotoxic, nematicidal, cytotoxic, and mutagenic activities of 1-hydroxypyrene, the initial metabolite in pyrene metabolism by the basidiomycete Crinipellis stipitaria. Bull. Environ. Contam. Toxicol. 1995;55:251–257. doi: 10.1007/BF00203017. [DOI] [PubMed] [Google Scholar]
- 32.Dong S, Hwang HM, Shi X, Holloway L, Yu H. UVA-induced DNA single strand cleavage by 1- hydroxypyrene and formation of covalent adducts between DNA and 1-hydroxypyrene. Chem. Res. Toxicol. 2000;13:585–593. doi: 10.1021/tx990199x. [DOI] [PubMed] [Google Scholar]
- 33.Fan Z, Chen D, Birla P, Kamens RM. Modeling of nitro-polycyclic aromatic hydrocarbons formation and decay in the atmosphere. Atmos. Environ. 1995;29:1171–1181. [Google Scholar]
- 34.Fu PP, Herreno-Saenz D. Nitro-polycyclic aromatic hydrocarbons: A class of genotoxic environmental pollutants. J. Env. Sci. Health, Part C: Environ. Carcinog. Ecotox. Rev. 1999;C17:1–43. [Google Scholar]
- 35.Okinaka RT, Nickols JW, Whaley TW, Strinste GF. 1-Nitropyrene: a mutagenic product induced by the action of near ultraviolet light on 1-aminopyrene. Mutat. Res. 1986;173:93–98. doi: 10.1016/0165-7992(86)90083-7. [DOI] [PubMed] [Google Scholar]
- 36.El-Bayoumy K, Revenson A, Johnson B, DiBello J, Little P, Hecht SS. Comparative Tumorigenicity of 1-nitropyrene-1-nitrosopyrene, and 1-aminopyrene administered by gavage to spraguedawley rats. Cancer Res. 1988;48:4256–4260. [PubMed] [Google Scholar]
- 37.Fu PP. Metabolism of nitropolycyclic aromatic hydrocarbons. Drug Metab. Rev. 1990;22:209–268. doi: 10.3109/03602539009041085. [DOI] [PubMed] [Google Scholar]
- 38.Fu PP, Chou MW, Beland FA. In: Polycyclic Aromatic Hydrocarbon Carcinogensis: Structure - Activity Relationships. Yang SK, Silverman BD, editors. Boca Raton, Florida: CRC Press; 1988. pp. 37–65. [Google Scholar]
- 39.Fu PP, Herrero-Saenz D, von Tungeln LS, Hart RW, Lin S-D. DNA adducts and carcinogenicity of nitro-polycyclic aromatic hydrocarbons. Polycycl. Arom. Comp. 1994;6:71–78. doi: 10.1289/ehp.94102s6177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fu PP, Qui F-Y, Jung H, von Tungeln LS, Zhan D-J, et al. Metabolism of isomeric nitrobenzo[a]pyrenes leading to DNA adducts and mutagenesis. Mutat. Res. 1997;376:43–51. doi: 10.1016/s0027-5107(97)00024-9. [DOI] [PubMed] [Google Scholar]
- 41.Howard PC, Beland FA, Cerniglia CE. Reduction of the carcinogen 1-nitropyrene to 1- aminopyrene by rat intestinal bacteria. Carcinogenesis. 1983;4:985–990. doi: 10.1093/carcin/4.8.985. [DOI] [PubMed] [Google Scholar]
- 42.Manning BW, Cerniglia CE, Federle TW. Biotransformation of 1-nitropyrene to 1-aminopyrene and N-formyl-1-aminopyrene by the human intestinal microbiota. J. Toxicol. Environ. Health. 1986;18:339–346. doi: 10.1080/15287398609530875. [DOI] [PubMed] [Google Scholar]
- 43.Weisburger JH. Comments on the history and importance of aromatic and heterocyclic amines in public health. Mutat. Res. 2002;506-507:9–20. doi: 10.1016/s0027-5107(02)00147-1. [DOI] [PubMed] [Google Scholar]
- 44.Later DW, Lee ML. Selective detection of amino polycyclic aromatic compounds in solvent refined coal. Anal. Chem. 1982;54:117–123. [Google Scholar]
- 45.Haugen DA, Peak MJ, Suhrbler KM. Isolation of mutagenic aromatic amines from a coal conversion oil by cation exchange chromatography. Anal. Chem. 1982;54:32–37. [Google Scholar]
- 46.Benigni R, Passerini L. Carcinogenicity of the aromatic amines: from structure-activity relationship to mechanisms of action and risk assessment. Mutat. Res. 2002;511:191–206. doi: 10.1016/s1383-5742(02)00008-x. [DOI] [PubMed] [Google Scholar]
- 47.Kadlubar FF, Fu PP, Jung H, Shaikh AU, Beland FA. The metabolic N-oxidation of carcinogenic arylamines in relation to nitrogen charge density and oxidation potential. Environ. Health Perspect. 1990;87:233–236. doi: 10.1289/ehp.9087233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gibian MJ, Vandenberg P. Product yield in oxygenation of linoleate by soybean lipoxygenase: The value of the molar extinction coefficient in the spectrophotometric assay. Anal. Biochem. 1987;163:343–349. doi: 10.1016/0003-2697(87)90234-x. [DOI] [PubMed] [Google Scholar]
- 49.Arce R, Pino EF, Valle C, Ágreda J. Photophysics and photochemistry of 1-nitropyrene. J. Phys. Chem. 2008;112:10294–10304. doi: 10.1021/jp803051x. [DOI] [PubMed] [Google Scholar]
- 50.Kameda T, Akiyama A, Toriba A, Tang N, Hayakawa K. Atmospheric formation of hydroxynitropyrenes from a photochemical reaction of particle-associated 1-nitropyrene. Environ. Sci. Technol. 2011;45:3325–3332. doi: 10.1021/es1042172. [DOI] [PubMed] [Google Scholar]
- 51.Stewart G, Smith K, Chornes A, Harris T, Honeysucker T, et al. Photochemical reaction of nitropolycyclic aromatic hydrocarbons: effect by solvent and structure. Environ. Chem. Lett. 2010;8:301–306. doi: 10.1007/s10311-009-0221-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zeng K, Hwang HM, Dong S, Shi X, Wilson K, et al. Photochemical transformation and phototoxicity of 1-aminopyrene. Envin. Toxicol. Chem. 2004;23:1400–1407. doi: 10.1897/03-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zeng K, Hwang HM, Fu F, Yu H. Identification of 1-hydroxypyrene photoproducts and study of the effect of humic substances on its photolysis. Polycycl. Arom. Compd. 2002;22:459–467. [Google Scholar]
- 54.Turro N, Ramamurthy V, Scaiano JC. Principles of molecular photochemistry: an introduction. Sausalito, CA: University Science Books; 2009. pp. 15–32. [Google Scholar]
- 55.van den Braken-van Leersum AM, Tintel C, van't Zelfde M, Cornelisse J, Lugtenburg J. Spectroscopic and photochemical properties of mononitropyrenes. Recl. Trav. Chim. Pays-Bas. 1987;106:120–128. [Google Scholar]
- 56.Tokita M MM. Identification of new geometric isomers of methyl linoleate hydroperoxides and their chromatographic behavior. Biosci. Biotechnol. Biochem. 2000;64:1044–1046. doi: 10.1271/bbb.64.1044. [DOI] [PubMed] [Google Scholar]
- 57.Yu H. Environmental carcinogenic polycyclic aromatic hydrocarbons: photochemistry and phototoxicity. J. Environ. Sci. & Health, Part C-Environ. Carcinog. & Ecotoxic. Revs. 2002;C20:149–183. doi: 10.1081/GNC-120016203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chapman OL, Heckert DC, Reasoner JW, Thackaberry SP. Photochemical studies on 9- anthracene. J. Am. Chem. Soc. 1966;88:5550–5554. [Google Scholar]
- 59.Thony A, Rossi MJ. UV photon-assisted incineration of polycyclic aromatic hydrocarbons at elevated temperatures between 150 and 800 °C. J. Photochem. Photobiol. A.: Chemistry. 1997;109:267–280. [Google Scholar]
- 60.Rahmanto AS, Morgan PE, Hawkins CL, Davies MJ. Cellular effects of photogenerated oxidants and long-lived, reactive, hydroperoxide photoproducts. Free Rad. Biol. Med. 2010;49:1505–1515. doi: 10.1016/j.freeradbiomed.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 61.Davies M. Reactive species formed on proteins exposed to singlet oxygen. Photochem. Photobiol. Sci. 2004;3:17–25. doi: 10.1039/b307576c. [DOI] [PubMed] [Google Scholar]
- 62.Shore R, Gardner MJ, Pannett B. Ethylene oxide: an assessment of the epidemiological evidence on carcinogenicity. Br. J. Ind. Med. 1993;50:971–997. doi: 10.1136/oem.50.11.971. [DOI] [PMC free article] [PubMed] [Google Scholar]