Abstract
Background and Purpose
Our recent research revealed that adoptively-transferred regulatory T cells (Tregs) reduced acute ischemic brain injury by inhibiting neutrophil-derived MMP-9 and protecting against blood-brain barrier (BBB) damage. The mechanisms underlying Treg interactions with neutrophils remain elusive. This study evaluates the contribution of program death 1-ligand 1 (PD-L1) to Treg-mediated neutrophil inhibition and neuroprotection after cerebral ischemia.
Methods
In vitro experiments were carried out using a transwell system or a coculture system allowing cell-to-cell contact. Focal cerebral ischemia was induced in mice for 60min. Tregs (2×106) isolated from donor animals (wild-type or PD-L1−/−) were intravenously injected into ischemic recipients 2 h post-MCAO. MMP-9 production, BBB permeability and brain infarct were assessed at 1 or 3 days post-MCAO.
Results
In vitro experiments reveal that Treg-mediated inhibition of neutrophil MMP-9 required direct cell-to-cell contact. The suppression of MMP-9 was abolished when Tregs were pretreated with PD-L1 neutralizing Abs or when neutrophils were pre-treated with PD-1 Abs. In vivo studies confirmed that intravenous administration of Tregs pre-treated with PD-L1 Abs or Tregs isolated from PD-L1-deficient mice failed to inhibit MMP-9 production by blood neutrophils 1 day after 60 min middle cerebral artery occlusion (MCAO). Furthermore, the BBB damage after MCAO was greatly ameliorated in PD-L1-competent Treg-treated mice, but not in PD-L1-compromised Treg-treated mice. Consequently, PD-L1 dysfunction abolished Treg-mediated brain protection and neurological improvements 3 days after MCAO.
Conclusions
PD-L1 plays an essential role in the neuroprotection afforded by Tregs against cerebral ischemia by mediating the suppressive effect of Tregs on neutrophil-derived MMP-9.
Keywords: stroke, regulatory T cell, PD-L1, neutrophil, MMP-9
Introduction
Breakdown of the blood–brain barrier (BBB) is a significant contributor to brain injury after stroke. Disruption of the BBB after focal cerebral ischemia permits the leakage of plasma proteins and fluid into the brain parenchymal extracellular space, resulting in brain edema and further brain damage1. Matrix metalloproteinase-9 (MMP-9) levels are raised in the plasma and brain soon after ischemic stroke and help disrupt the BBB by degrading the extracellular matrix2-4. The early MMP-9 expression in the ischemic brain is derived mainly from peripheral leukocytes, especially neutrophils5. Infiltration of neutrophils enhances MMP-9 levels in the ischemic brain by causing the release of proform MMP-96-7. Recent data reveal that reperfusion after tissue plasminogen activator (tPA) treatment further promotes the degranulation and release of MMP-9 from human neutrophils, which may account for the hemorrhagic transformation that results from tPA treatment8. Therefore, neutrophil-derived MMP-9 might be a potential target for early stroke management.
Naturally-occurring CD4+CD25+ regulatory T cells (Tregs) are a specialized subpopulation of T cells that negatively regulate a number of physiological and pathological immune responses. The number of Tregs in the blood rises significantly several days after ischemic stroke, both in patients9 and in experimental animals10. Activation of peripheral Tregs is thought to reflect endogenous mechanisms to reduce cerebral inflammation following cerebral ischemia11. Our recent work demonstrates that adoptively-transferred Tregs protect against acute ischemic brain injury by inhibiting neutrophil-derived MMP-9 and thereby ameliorating BBB disruption12. However, the molecular mechanisms whereby Tregs, in particular, adoptively transferred Tregs, interact with neutrophils and inhibit their MMP-9 production after ischemic stroke remain elusive.
Programmed death–1 ligand 1 (PD-L1,or B7 homologue 1 [CD274]) and PD-L2 are members of the B7 family of costimulatory molecules. PD-L1 is broadly expressed on a variety of immune cells, including T cells, B cells, dendritic cells (DC), and monocytes, whereas the expression of PD-L2 is mainly restricted to macrophages, DC and specific B cell subpopulations13. The engagement of PD-L1/PD-L2 with their receptor, programmed death-1 (PD-1), relays inhibitory signals and leads to suppression of immune responses14. PD-L1 is expressed on Tregs and influences Treg function. For example, PD-L1 is important for Treg-mediated inhibition of the proliferation of effector T cells15. It has also been reported that PD-L1 is expressed on ex-vivo expanded human Tregs and directly induces PD-L1 expression on DC16. However, the importance of PD-L1 in the crosstalk between Tregs and neutrophils has not been established.
In the current study, we sought to investigate the molecular mechanism associated with the suppression of neutrophils by Tregs. Using a mouse model of focal transient ischemia and in vitro Treg-neutrophil co-cultures, we observed that PD-L1/PD-1 interactions are important for Treg-mediated inhibition of neutrophil-derived MMP-9. This inhibition, in turn, reduces post-ischemic BBB breakdown, leukocyte infiltration, and brain damage.
Methods
Murine model of transient focal ischemia
All animal experiments were approved by the University of Pittsburgh Institutional Animal Care and Use Committee and carried out in accordance with the NIH Guide for the Care and Use of Laboratory Animals. Male 8- to 10-week old C57BL/6J mice were purchased from The Jackson Laboratory. Breeding pairs of PD-L1 (B7-H1)−/− mice (C57BL/6 background) were generous gifts from Dr. Lieping Chen (Yale University). Mice were anesthetized with 1.5% isoflurane in a 30% O2/68.5% N2O mixture under spontaneous breathing conditions. Focal cerebral ischemia was produced by intraluminal occlusion of the left middle cerebral artery (MCAO) for 60 min as described previously17. Rectal temperature was maintained at ~37.0°C during MCAO surgery with a temperature-regulated heating pad. Regional cerebral blood flow was measured in all stroke animals using laser Doppler flowmetry. Animals that did not show a regional cerebral blood flow reduction to 30% of preischemia baseline levels during MCAO were excluded from further experimentation. Sham-operated animals underwent the same anesthesia and surgical procedures, with the exception of MCAO. Animals were assigned randomly to different treatment groups through the use of a lottery-drawing box. All assessments were performed by investigators who were ‘blinded’ to experimental group assignments.
Isolation and adoptive transfer of Tregs
Single-cell suspensions were prepared from pooled inguinal and axillary lymph nodes and spleens of C57/BL6 mice (8-10 week old). CD4+CD25+ Treg populations were purified (>95% purity) by negative selection and positive selection with the regulatory T cell isolation kit (Miltenyi Biotec), according to the manufacturers' instructions. Recipient mice received a tail vein injection of 2×106 freshly-enriched Tregs or freshly-isolated splenocytes in 0.2 mL Dulbecco's Phosphate-Buffered Saline (DPBS) at 2 h after reperfusion. In selected experiments, isolated Tregs were pre-incubated with PD-L1 neutralization antibody (R&D System) or isotype control IgG (eBioscience) at 20 μg/mL for 1 h and then adoptively transferred into MCAO animals after 2h of reperfusion.
Primary neutrophil culture and treatments
Primary mouse neutrophils were isolated from bone marrow and blood of C57/BL6 mice (8-10 week old) using the EasySep Mouse Neutrophil Enrichment Kit (Stem Cell Technologies) according to the manufacturer's instructions. To determine the influence of T cell surface molecules on Treg-neutrophil interactions, isolated Tregs (1×105 per well) were plated in 96-well plates pre-coated with CD3/CD28 antibodies (Abs) (BD Bioscience) and IL-2 (200 IU/ml) for 3 days to reverse the hyporesponsiveness of Tregs while retaining their phenotype and suppressive activities18-20. Tregs were then incubated in the presence of PD-L1, or CTLA-4 or TGF-β neutralizing Abs (20 μg/ml, R&D Systems) or isotype control IgG (20 μg/ml, eBioscience) for 1 h prior to neutrophil addition. The enriched neutrophils (2×105 per well) were added to Treg cultures. Co-cultures were then treated with TNF-α (100 ng/ml) and maintained at 37 °C for 24 h. Alternatively, purified neutrophil cultures were pretreated with PD-1 Abs (20 μg/ml, R&D System) for 1 h and then cocultured with Tregs, followed by TNF-α stimulation for 24 h. For transwell experiments, neutrophils were added to the upper chambers of 96-well transwell trays and Tregs were added to the lower chambers. Transwell experiments helped to distinguish if cell-cell contact is required for Treg-afforded inhibition on neutrophil-derived MMP-9. In another set of experiments, purified neutrophil cultures were pretreated with different concentrations of recombinant mouse PD-L1 (R&D systems) for 1 h, followed by TNF-α stimulation. For all culture conditions, supernatants were collected 24 h after TNF-α exposure to quantify the extracellular release of MMP-9.
Cytokine enzyme-linked immunosorbent assay
Blood plasma and cell culture supernatants were collected. Concentrations of pro-MMP-9 were measured with commercial ELISA quantification kits (R&D Systems). Pro- and active MMP-9 activities were measured using commercial kits (GE Healthcare) according to the manufacturer's instructions.
Analysis of neutrophil-derived MMP-9 in vivo
Blood neutrophils from MCAO mice were isolated as described above. After hemacytometer cell counts, the neutrophils were lysed in lysis buffer (Cell Signaling Technology). Concentrations of pro-MMP-9 were measured with commercial ELISA kits (R&D Systems) according to the manufacturer's instructions.
Immunohistochemistry and cell counting
Immunohistochemistry was performed on 30μm free-floating sections. Rat anti-CD31 (1:200, BD PharMingen) and rabbit anti-MPO (1:100; Abcam) Abs were used to label endothelial cells and neutrophils, respectively. Other primary Abs includes mouse anti-ZO-1 (1:100, Invitrogen), rabbit anti-PD-1 (1:100, eBioscience), rabbit anti-PD-L1 (1:100, eBioscience), and goat anti-MMP-9 (1:200; R&D Systems). Biotin-conjugated anti-mouse IgG was used to detect extravascular IgG molecules. Biotin was detected with fluorescence-conjugated streptavidin (Jackson ImmunoResearch) or with an ABC kit (Vector) followed by the NovaRED peroxidase substrate kit (Vector) according to the manufacturer's instructions. All images were processed with Image J for cell-based counting of automatically recognized cells. The mean number of cells per mm2 was calculated from three fields in the cortex or striatum of each section.
Intravenous injection, quantification, and detection of tracers
Cadaverine conjugated to Alexa Fluor-555 (950 Da, Invitrogen, 200μg/mouse) was injected into the tail vein 22 h after reperfusion. For quantification of the tracer, animals were euthanized 24 h after reperfusion and perfused for 5 min with Hank's balanced salt solution (HBSS). Brains were removed and homogenized in 1% Triton X-100 in phosphate-buffered saline (PBS) and centrifuged at 16,000 rpm for 20 min. The relative fluorescence of the supernatant was measured at ex/em wavelengths of 544/590 nm.
Measurements of infarct volume
For 2,3,5-triphenyltetrazolium chloride (TTC) staining, brains were removed and sliced into 7 coronal sections, each 1 mm thick. Sections were immersed in pre-warmed 2% TTC (Sigma) in saline for 10 min, and then fixed in 4% paraformaldehyde in PBS, pH 7.4. For Cresyl Violet or MMP-2 staining, animals were sacrificed and perfused transcardially with 0.9% normal saline and 4% paraformaldehyde in PBS. Free-floating sections were prepared from the fixed and dehydrated brains and stained with Cresyl Violet (Sigma) or MMP-2 Abs (Santa Cruz Biotechnology). Infarct volume was determined with NIH Image J analysis by an observer ‘blinded’ to the experimental group assignment.
Assessment of neurological deficits
Neurological deficits were assessed in a blinded fashion using a 5-point-scale neurological score (0, no observable deficit; 1, torso flexion to right; 2, spontaneous circling to right; 3, leaning/falling to right; 4, no spontaneous movement; 5, death).
Statistical Analyses
Results are presented as means ± SEM. The difference in means between 2 groups was assessed by the two-tailed Student's ‘t’ test. Differences in means across multiple groups were analyzed using one- or two-way ANOVAs with time or treatment as independent factors. When the ANOVA revealed significant differences, pairwise comparisons between means were made by the post hoc Bonferroni/Dunn tests. In all analyses, p < 0.05 was considered statistically significant.
Results
PD-L1/PD-1 interactions are essential for Treg-mediated inhibition of neutrophil-derived MMP-9 in vitro
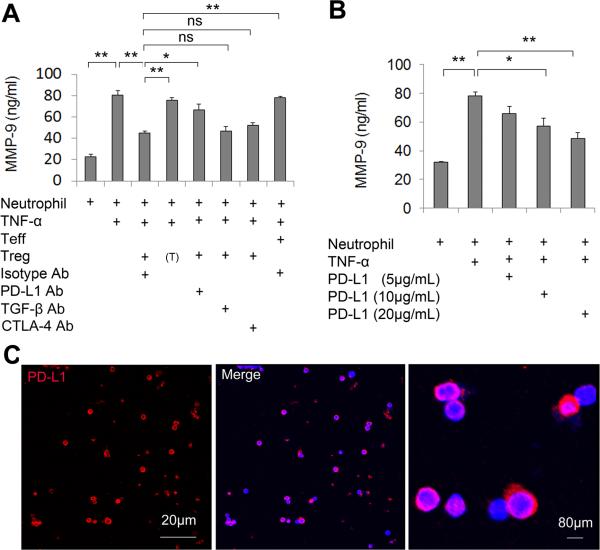
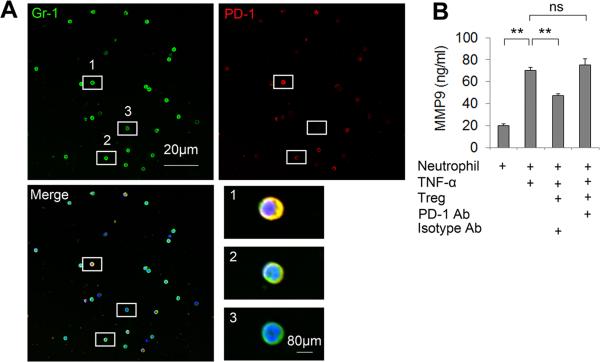
In vitro experiments revealed that cell-to-cell contacts were required for the inhibition of neutrophil MMP-9 by Tregs as Tregs lost their MMP-9 inhibitory effect when cocultured with neutrophils in transwells (Figure 1A). Therefore, one or more Treg surface molecule(s) might be critical for the regulation of neutrophils. Numerous cell surface molecules, including PD-L1, CTLA-4, and TGF-β, on Tregs have been shown to be important for the interaction of Tregs with other cells16, 21-22. We investigated the effect of these molecules on Treg-neutrophil interactions in Treg and neutrophil co-cultures. As shown in Figure 1A, PD-L1 blocking Abs were able to abolish the Treg-mediated suppression of MMP-9 production from neutrophils, whereas the blocking Abs for CTLA-4 and TGF-β showed no significant effects. In contrast, functional PD-L1 protein directly inhibited MMP-9 production from neutrophil cultures following TNF-α stimulation (Figure 1B). The expression of PD-L1 on stimulated Tregs was confirmed by immunocytochemical staining (Figure 1C). We also detected the expression of PD-1, the PD-L1 binding partner, on Gr1+ neutrophils (Figure 2A). Furthermore, pre-incubation of neutrophils with PD-1 blocking Abs abolished the Treg-mediated inhibition of neutrophil-derived MMP-9 (Figure 2B). These results indicate the importance of PD-L1/PD-1 in Treg-neutrophil interactions.
Figure 1. Tregs inhibit neutrophil-derived MMP-9 through PD-L1 in in vitro cultures.
(A) Isolated Tregs were stimulated with anti-CD3Ab and anti-CD28 Abs for 3 days and pretreated with various neutralizing Abs (anti-PD-L1, -TGF-β or -CTLA-4; 20 μg/mL) or isotype control IgG (20 μg/mL) for 1 h. Stimulated Tregs were co-cultured with neutrophils and then exposed to TNF-α (100 ng/mL) for 24 h. MMP-9 in the culture medium was measured by ELISA. (T) indicates that neutrophils were cultured in transwells. (B) Neutrophils were incubated with PD-L1 protein at indicated concentrations. Cells were then exposed to TNF-α (100 ng/mL) for 24 h. MMP-9 in the culture medium was measured by ELISA. n=6/group in 3 independent experiments. *P<0.05, **P<0.01. (C) Immunocytochemical staining of PD-L1 on Tregs. Red: PD-L1. Blue: DAPI.
Figure 2. PD-1 expressed on neutrophils mediates Treg-neutrophil interactions.
(A) Double staining of Gr-1 (a neutrophil marker) and PD-1 on neutrophils extracted from mice at 3 days after MCAO. High magnification images showing neutrophils with varying levels of PD-1 expression. 1, high expression; 2, low expression; 3, no expression. (B) Neutrophils isolated from mouse blood and bone marrow were pre-incubated with 20 μg/mL PD-1 Abs or 20 μg/mL isotype control IgG and then co-cultured with Tregs. Cells were then exposed to TNF-α (100 ng/mL) for 24 h. MMP-9 in the culture medium was measured by ELISA. n=6/group. *P<0.05, **P<0.01, ns: not significant.
PD-L1 mediates the suppressive effect of Tregs on neutrophil-derived MMP-9 after cerebral ischemia
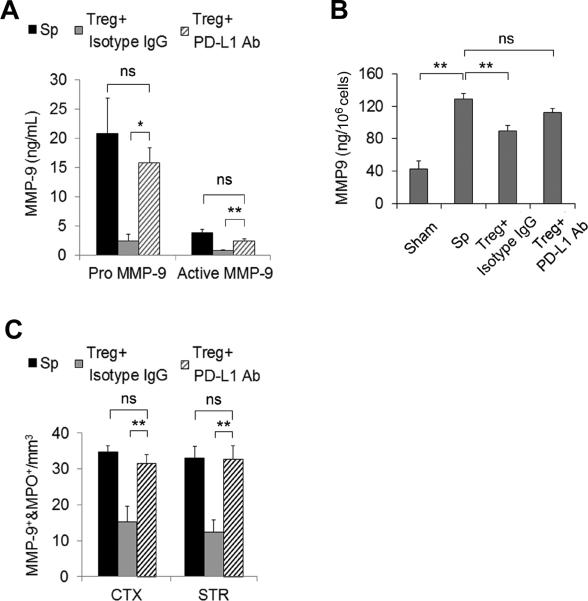
To further characterize the role of PD-L1 in Treg-conferred MMP-9 inhibition after cerebral ischemia, we pretreated Tregs with PD-L1 blocking Abs or isotype IgG controls prior to their adoptive transfer at 2 h after MCAO. ELISA assays demonstrated high levels of plasma pro-MMP-9 and activated MMP-9 24 h after ischemia. Both responses were inhibited by post-treatment with isotype-treated Tregs. PD-L1 Abs reduced the inhibitory effect of Tregs on plasma MMP-9 (Figure 3A). Furthermore, we isolated neutrophils from the blood of mice undergoing 1) sham operation, 2) MCAO followed by splenocyte treatment, 3) MCAO followed by isotype IgG-treated Treg treatment, or 4) MCAO followed by PD-L1 antibody-treated Treg treatment. As shown in Figure 3B, isotype IgG-treated Tregs significantly reduced the otherwise dramatic increase in MMP-9 in neutrophil lysates from MCAO mice. However, PD-L1 Ab-treated Tregs failed to inhibit neutrophil-derived MMP-9 production. Quantification of immunostaining confirmed that PD-L1 Abs abolished the Treg-mediated inhibition of the infiltration of MMP-9+ neutrophils into the brain parenchyma at 3 day after MCAO (Figure 3C).
Figure 3. PD-L1 blocking Abs abolish Treg-mediated inhibition of the rise in MMP-9 after MCAO.
Tregs (2×106/animal) were pre-incubated with PD-L1 neutralization Abs or isotype control IgG at 20 μg/mL and then adoptively transferred into MCAO animals after 2 h of reperfusion. (A) Quantification of plasma MMP-9 at 24 h after MCAO (n=7/group). (B) Quantification of blood neutrophil-derived MMP-9 at 24 h after MCAO (n=4/group). Neutrophils were isolated from the blood of MCAO and sham mice at 24 h after operation. MMP-9 was measured in the neutrophil lysate by ELISA. (C) The infiltration of MMP-9+MPO+ neutrophils into the cortex (CTX) and striatum (STR) was quantified at 3 days after MCAO (n=5/group). *P<0.05, **P<0.01, ns: not significant. Sp = splenocytes
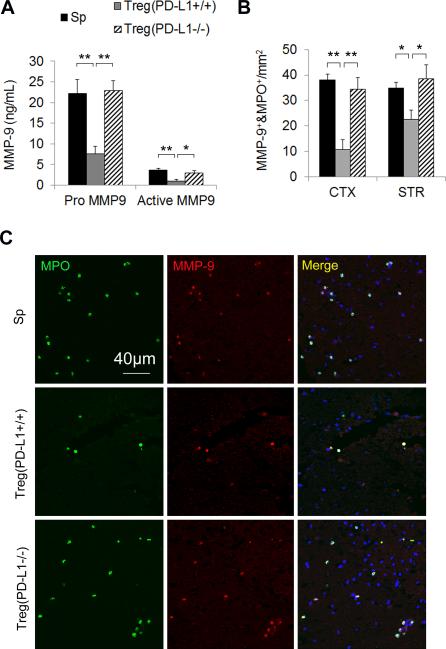
The role of PD-L1 in Treg-conferred inhibition of neutrophil MMP-9 was further confirmed in transfer experiments with Tregs from PD-L1−/− mice. Consistent with the results using PD-L1-Ab-pretreated Tregs, we observed that PD-L1 deficiency abrogated the inhibitory effect of Tregs on blood MMP-9 production (Figure 4A) and on the infiltration of MMP-9-loaded neutrophils after MCAO (Figure 4B and 4C).
Figure 4. Tregs prepared from PD-L1 deficient mice fail to inhibit the rise of blood MMP-9 after MCAO.
Tregs were prepared from PD-L1 knockout mice and then adoptively transferred into MCAO animals after 2 h of reperfusion. (A) Quantification of plasma MMP-9 1 day after MCAO (n=5/group). (B) The infiltration of MMP-9+MPO+ neutrophils into the cortex (CTX) and striatum (STR) was quantified at 3 days after MCAO (n=5/group). (C) Representative images of MMP-9 and MPO double staining on brain sections. Images are representative of brain sections from five mice in each group. Scale bar represents 40 μm *P<0.05, **P<0.01. Sp = splenocytes.
Taken together, the data presented here support a critical role for PD-L1 in Treg-afforded inhibition of neutrophil-derived MMP-9 following focal transient ischemia.
PD-L1 is critical for Tregs to preserve blood-brain barrier integrity after ischemia
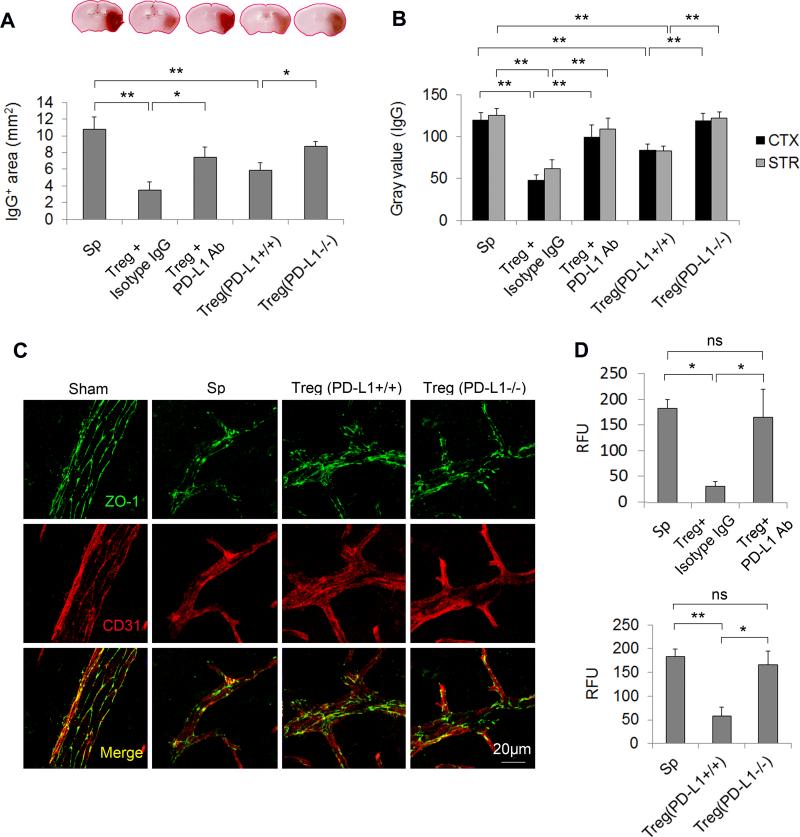
We showed previously that adoptively transferred Tregs ameliorated the BBB disruption through MMP-9 modulation in the early phase after stroke12. We therefore investigated the influence of PD-L1 in Treg-afforded BBB protection. As shown in Figure 5, PD-L1 blockade with PD-L1 neutralizing Abs or PD-L1 genetic deficiency abolished Treg-afforded protection of BBB integrity at 24 h after MCAO, as shown by enhanced leakage of plasma-derived IgG molecules (Figure 5A and 5B), increased extravasation of cadaverine Alexa-488 tracer (Figure 5D), and disrupted expression of the tight junction marker ZO-1 (Figure 5C) in animals treated with PD-L1-compromised Tregs. These results suggest that PD-L1 is critical for BBB protection by Tregs after cerebral ischemia.
Figure 5. PD-L1 is critical for Treg-afforded BBB preservation after MCAO.
To block PD-L1 signaling in Tregs, Tregs were pre-incubated with PD-L1 neutralizing Abs or prepared from PD-L1 knockout mice and then adoptively transferred into MCAO animals after 2 h of reperfusion. Isotype IgG-treated or wild-type Tregs were used as controls, respectively. (A-B) Quantification of IgG extravasation at 24 h after MCAO. (A) Quantification of endogenous IgG positive area determined by immunohistochemical staining of mouse IgG. n=5/group. (B) Quantification of gray values of IgG immunostaining in the cortex (CTX) and striatum (STR). n=5/group. (C) Representative Z-stack confocal images of the tight junction protein ZO-1 and endothelial cell marker CD31 in brain sections obtained 1 day after MCAO. Images are representative of brain sections from four mice in each group. (D) Cadaverine-Alexa-555 (950Da) was injected intravenously 22 h post-MCAO. Fluorescence intensities in brain lysates from the infarct area were measured after 2 h of circulation. n=5/group. *P<0.05, **P<0.01. Sp = splenocytes.
PD-L1 is essential for Treg-afforded neuroprotection
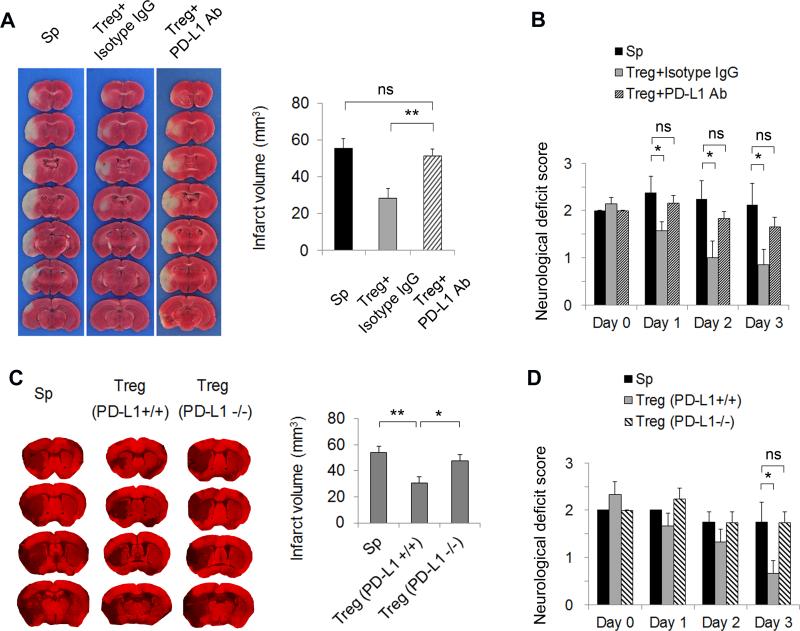
To assess if PD-L1 is essential for Treg-mediated protection of the brain against ischemic injury, we analyzed infarct size and neurological scores after MCAO. At 3 days after ischemic attack, the extent of brain damage, as measured by infarct area (Figure 6A), and neurological deficits, as measured by neurological severity scores (Figure 6B), were both reduced significantly in MCAO mice injected with isotype IgG-treated Tregs compared to mice injected with splenocytes. PD-L1 Ab-treated Tregs lost their protective effect in the ischemic brain. In another set of experiments using Tregs prepared from PD-L1 knockout mice, MAP2 staining was used to quantify brain tissue loss 3 days after MCAO (Figure 6C). Again, PD-L1 deficiency reduced the neuroprotective effects of Tregs significantly (Figure 6C) and led to a deterioration of the neurological severity scores (Figure 6D). Collectively, these results demonstrate the importance of PD-L1 in Treg-afforded neuroprotection against brain ischemia.
Figure 6. PD-L1 is critical for Treg-afforded neuroprotection after MCAO.
(A-B) Tregs were pre-incubated with PD-L1 neutralizing Abs or isotype control IgG at 20 μg/mL and then adoptively transferred into MCAO animals after 2 h of reperfusion. (A) Representative TTC staining of brain infarct and quantification of infarct volume at 3 days after MCAO. n=7/group. (B) Neurological deficit scores over 3 days after MCAO. n=7/group. (C-D) Tregs were prepared from PD-L1 knockout mice and then adoptively transferred into MCAO animals after 2 h of reperfusion. (C) Representative MAP2 staining of brain infarct and quantification of infarct volume at 3 days after MCAO. n=5/group. (D) Neurological deficits scores over 3 days after MCAO. n=5/group. *P<0.05, **P<0.01, ns: not significant. Sp = splenocytes.
Discussion
Emerging evidence on the evolution of brain damage in ischemia supports the view that inflammatory responses are not restricted to resident immune cells but also involve the infiltration of peripheral immune cells into brain parenchyma. Peripheral cells represent viable targets for stroke treatment because they are such early responders to ischemic injury and are more accessible than cells in the CNS. We have shown recently that adoptively-transferred Tregs offer early protection against focal transient ischemia by inhibiting peripheral neutrophil-derived MMP-9 and thereby preserving BBB integrity12. In the current study, we discovered that PD-L1-mediated signaling is an integral part of this mechanism of protection in the ischemic brain.
PD-L1 signaling is known to be an important molecular mediator of Treg action23. PD-L1 signaling transmits inhibitory signals from Tregs to effector T cells15. Our in vitro data show for the first time that PD-L1 mediates the dialogue between Tregs and neutrophils and is critical for Treg-mediated inhibition of neutrophil-derived MMP-9. Most importantly, the transfer of Tregs purified from PD-L1 knockout mice or Tregs pretreated with PD-L1 neutralizing Abs confirmed a direct role for PD-L1 in blood neutrophil MMP-9 production and in Treg-mediated preservation of BBB integrity in an in vivo model of ischemic stroke.
A recent study reported that global PD-L1 knockout alleviated brain injury 4 days after MCAO, suggesting a detrimental effect of PD-L1 on the ischemic brain during early phases of stroke injury24. The pathogenic role of PD-L1 was attributed to inhibition of immunoregulatory CD8+CD122+ suppressor T cells. In contrast, the current study suggests that PD-L1 expression on Tregs is protective. One possible explanation for this discrepancy is that the functions of PD-L1 in the ischemic brain are cell-specific. Indeed, cell-specific protective effects of PD-L1 have already been reported in models of multiple sclerosis. For example, microglial PD-L1 ameliorates T cell activation in the CNS and reduces immunopathological damage in experimental autoimmune encephalomyelitis (EAE)25. PD-L1 expression on regulatory B cells was also shown to mediate estrogen-afforded neuroprotection in EAE mice26. In addition, PD-L1 and PD-L2 were upregulated on cultured brain endothelial cells under inflammatory stimulation, resulting in reduced migration of CD4+ T cells across the endothelium27.
Although the exact mechanism that permits Treg-derived PD-L1 to interact with neutrophils is not fully clear, our data strongly suggest that the activation of PD-1 signaling is important for neutrophil responses to Treg treatment. PD-1 is the most important receptor for PD-L1 and we detected its expression on neutrophils in the brain after MCAO. Moreover, Tregs failed to inhibit MMP-9 production from stimulated neutrophils that were pretreated with PD-1 neutralizing Abs. As such, Tregs may relay inhibitory signals to neutrophils through PD-L1/PD-1 interactions. Consistent with this interpretation, a recent publication demonstrated a protective role of PD-1 in the MCAO model of stroke28. It has been noted that PD-1 is broadly expressed in many types of immune cells that are actively involved in ischemic brain injury, including T cells, B cells, and macrophages29-30. Moreover, PD-1 expression on macrophages and microglia was strongly upregulated 4 days after MCAO28. It is therefore possible that Tregs exert more immunosuppressive effects than inhibition of neutrophil MMP-9 after ischemic brain injury. Further studies are warranted to confirm the involvement of PD-1 in Treg-afforded neuroprotection and to explore other targeted cells.
Although controversies still exist about the impact of Tregs in experimental stroke11-12, 31-32 due to different experimental setting and Treg kinetics, our recent studies strongly suggest that Treg post-treatment are protective against ischemic stroke12, 33. In this study, we further identify that PD-L1/PD-1 plays a critical role in the inhibitory effect of adoptively transferred Tregs on neutrophil-produced MMP-9 after ischemic brain injury. Thus, PD-L1 helps preserve BBB integrity and protects the brain from stroke through its essential role as a signaling molecule in adoptive Treg therapy.
Acknowledgement
P. L and L. M contribute equally to this manuscript. X.H, J.C and A.W.T. designed experiments. P.L, X.H, L.M, X.L, Y.Gan, J.Z and Y.Gao performed experiments. P.L, X.H , and Y.G analyzed data. X.H and P.L wrote the manuscript.
Funding Sources
This work was supported by the Veterans Health Administration (GRECC pilot grant to J.C), the National Institutes of Health Grants (NS36736, NS43802, and NS45048 to J.C), a grant from the American Heart Association (13SDG14570025 to X.H), and Shanghai Natural Science Foundation (13ZR1452200 to P. L).
Footnotes
Disclosures
None.
References
- 1.Fernandez-Lopez D, Faustino J, Daneman R, Zhou L, Lee SY, Derugin N, et al. Blood-brain barrier permeability is increased after acute adult stroke but not neonatal stroke in the rat. J Neurosci. 2012;32:9588–9600. doi: 10.1523/JNEUROSCI.5977-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asahi M, Wang X, Mori T, Sumii T, Jung JC, Moskowitz MA, et al. Effects of matrix metalloproteinase-9 gene knock-out on the proteolysis of blood-brain barrier and white matter components after cerebral ischemia. J Neurosci. 2001;21:7724–7732. doi: 10.1523/JNEUROSCI.21-19-07724.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosell A, Ortega-Aznar A, Alvarez-Sabin J, Fernandez-Cadenas I, Ribo M, Molina CA, et al. Increased brain expression of matrix metalloproteinase-9 after ischemic and hemorrhagic human stroke. Stroke; a journal of cerebral circulation. 2006;37:1399–1406. doi: 10.1161/01.STR.0000223001.06264.af. [DOI] [PubMed] [Google Scholar]
- 4.Park KP, Rosell A, Foerch C, Xing C, Kim WJ, Lee S, et al. Plasma and brain matrix metalloproteinase-9 after acute focal cerebral ischemia in rats. Stroke. 2009;40:2836–2842. doi: 10.1161/STROKEAHA.109.554824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosell A, Cuadrado E, Ortega-Aznar A, Hernandez-Guillamon M, Lo EH, Montaner J. Mmp-9-positive neutrophil infiltration is associated to blood-brain barrier breakdown and basal lamina type iv collagen degradation during hemorrhagic transformation after human ischemic stroke. Stroke. 2008;39:1121–1126. doi: 10.1161/STROKEAHA.107.500868. [DOI] [PubMed] [Google Scholar]
- 6.Gidday JM, Gasche YG, Copin JC, Shah AR, Perez RS, Shapiro SD, et al. Leukocyte-derived matrix metalloproteinase-9 mediates blood-brain barrier breakdown and is proinflammatory after transient focal cerebral ischemia. American journal of physiology. 2005;289:H558–568. doi: 10.1152/ajpheart.01275.2004. [DOI] [PubMed] [Google Scholar]
- 7.Justicia C, Panes J, Sole S, Cervera A, Deulofeu R, Chamorro A, et al. Neutrophil infiltration increases matrix metalloproteinase-9 in the ischemic brain after occlusion/reperfusion of the middle cerebral artery in rats. J Cereb Blood Flow Metab. 2003;23:1430–1440. doi: 10.1097/01.WCB.0000090680.07515.C8. [DOI] [PubMed] [Google Scholar]
- 8.Cuadrado E, Ortega L, Hernandez-Guillamon M, Penalba A, Fernandez-Cadenas I, Rosell A, et al. Tissue plasminogen activator (t-pa) promotes neutrophil degranulation and mmp-9 release. Journal of leukocyte biology. 2008;84:207–214. doi: 10.1189/jlb.0907606. [DOI] [PubMed] [Google Scholar]
- 9.Yan J, Greer JM, Etherington K, Cadigan GP, Cavanagh H, Henderson RD, et al. Immune activation in the peripheral blood of patients with acute ischemic stroke. J Neuroimmunol. 2009;206:112–117. doi: 10.1016/j.jneuroim.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 10.Offner H, Subramanian S, Parker SM, Wang C, Afentoulis ME, Lewis A, et al. Splenic atrophy in experimental stroke is accompanied by increased regulatory t cells and circulating macrophages. J Immunol. 2006;176:6523–6531. doi: 10.4049/jimmunol.176.11.6523. [DOI] [PubMed] [Google Scholar]
- 11.Liesz A, Suri-Payer E, Veltkamp C, Doerr H, Sommer C, Rivest S, et al. Regulatory t cells are key cerebroprotective immunomodulators in acute experimental stroke. Nature medicine. 2009;15:192–199. doi: 10.1038/nm.1927. [DOI] [PubMed] [Google Scholar]
- 12.Li P, Gan Y, Sun BL, Zhang F, Lu B, Gao Y, et al. Adoptive regulatory t-cell therapy protects against cerebral ischemia. Ann Neurol. 2013;74:458–471. doi: 10.1002/ana.23815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhong X, Tumang JR, Gao W, Bai C, Rothstein TL. Pd-l2 expression extends beyond dendritic cells/macrophages to b1 cells enriched for v(h)11/v(h)12 and phosphatidylcholine binding. European journal of immunology. 2007;37:2405–2410. doi: 10.1002/eji.200737461. [DOI] [PubMed] [Google Scholar]
- 14.Sharpe AH, Wherry EJ, Ahmed R, Freeman GJ. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nature immunology. 2007;8:239–245. doi: 10.1038/ni1443. [DOI] [PubMed] [Google Scholar]
- 15.Yang ZZ, Novak AJ, Stenson MJ, Witzig TE, Ansell SM. Intratumoral cd4+cd25+ regulatory t-cell-mediated suppression of infiltrating cd4+ t cells in b-cell non-hodgkin lymphoma. Blood. 2006;107:3639–3646. doi: 10.1182/blood-2005-08-3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amarnath S, Costanzo CM, Mariotti J, Ullman JL, Telford WG, Kapoor V, et al. Regulatory t cells and human myeloid dendritic cells promote tolerance via programmed death ligand-1. PLoS Biol. 2010;8:e1000302. doi: 10.1371/journal.pbio.1000302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sawada M, Alkayed NJ, Goto S, Crain BJ, Traystman RJ, Shaivitz A, et al. Estrogen receptor antagonist ici182,780 exacerbates ischemic injury in female mouse. J Cereb Blood Flow Metab. 2000;20:112–118. doi: 10.1097/00004647-200001000-00015. [DOI] [PubMed] [Google Scholar]
- 18.Jordan MS, Boesteanu A, Reed AJ, Petrone AL, Holenbeck AE, Lerman MA, et al. Thymic selection of cd4+cd25+ regulatory t cells induced by an agonist self-peptide. Nat Immunol. 2001;2:301–306. doi: 10.1038/86302. [DOI] [PubMed] [Google Scholar]
- 19.Hombach AA, Kofler D, Hombach A, Rappl G, Abken H. Effective proliferation of human regulatory t cells requires a strong costimulatory cd28 signal that cannot be substituted by il-2. J Immunol. 2007;179:7924–7931. doi: 10.4049/jimmunol.179.11.7924. [DOI] [PubMed] [Google Scholar]
- 20.Tang Q, Henriksen KJ, Bi M, Finger EB, Szot G, Ye J, et al. In vitro-expanded antigen-specific regulatory t cells suppress autoimmune diabetes. J Exp Med. 2004;199:1455–1465. doi: 10.1084/jem.20040139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang Q, Boden EK, Henriksen KJ, Bour-Jordan H, Bi M, Bluestone JA. Distinct roles of ctla-4 and tgf-beta in cd4+cd25+ regulatory t cell function. Eur J Immunol. 2004;34:2996–3005. doi: 10.1002/eji.200425143. [DOI] [PubMed] [Google Scholar]
- 22.Tai X, Van Laethem F, Pobezinsky L, Guinter T, Sharrow SO, Adams A, et al. Basis of ctla-4 function in regulatory and conventional cd4(+) t cells. Blood. 2012;119:5155–5163. doi: 10.1182/blood-2011-11-388918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amarnath S, Costanzo CM, Mariotti J, Ullman JL, Telford WG, Kapoor V, et al. Regulatory t cells and human myeloid dendritic cells promote tolerance via programmed death ligand-1. PLoS biology. 8:e1000302. doi: 10.1371/journal.pbio.1000302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bodhankar S, Chen Y, Vandenbark AA, Murphy SJ, Offner H. Pd-l1 enhances cns inflammation and infarct volume following experimental stroke in mice in opposition to pd-1. Journal of neuroinflammation. 2013;10:111. doi: 10.1186/1742-2094-10-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Magnus T, Schreiner B, Korn T, Jack C, Guo H, Antel J, et al. Microglial expression of the b7 family member b7 homolog 1 confers strong immune inhibition: Implications for immune responses and autoimmunity in the cns. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:2537–2546. doi: 10.1523/JNEUROSCI.4794-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bodhankar S, Galipeau D, Vandenbark AA, Offner H. Pd-1 interaction with pd-l1 but not pd-l2 on b-cells mediates protective effects of estrogen against eae. Journal of clinical & cellular immunology. 2013;4:143. doi: 10.4172/2155-9899.1000143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pittet CL, Newcombe J, Prat A, Arbour N. Human brain endothelial cells endeavor to immunoregulate cd8 t cells via pd-1 ligand expression in multiple sclerosis. Journal of neuroinflammation. 2011;8:155. doi: 10.1186/1742-2094-8-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ren X, Akiyoshi K, Vandenbark AA, Hurn PD, Offner H. Programmed death-1 pathway limits central nervous system inflammation and neurologic deficits in murine experimental stroke. Stroke; a journal of cerebral circulation. 42:2578–2583. doi: 10.1161/STROKEAHA.111.613182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Agata Y, Kawasaki A, Nishimura H, Ishida Y, Tsubata T, Yagita H, et al. Expression of the pd-1 antigen on the surface of stimulated mouse t and b lymphocytes. Int Immunol. 1996;8:765–772. doi: 10.1093/intimm/8.5.765. [DOI] [PubMed] [Google Scholar]
- 30.Huang X, Venet F, Wang YL, Lepape A, Yuan Z, Chen Y, et al. Pd-1 expression by macrophages plays a pathologic role in altering microbial clearance and the innate inflammatory response to sepsis. Proc Natl Acad Sci U S A. 2009;106:6303–6308. doi: 10.1073/pnas.0809422106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ren X, Akiyoshi K, Vandenbark AA, Hurn PD, Offner H. Cd4+foxp3+ regulatory t-cells in cerebral ischemic stroke. Metabolic brain disease. 2011;26:87–90. doi: 10.1007/s11011-010-9226-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kleinschnitz C, Kraft P, Dreykluft A, Hagedorn I, Gobel K, Schuhmann MK, et al. Regulatory t cells are strong promoters of acute ischemic stroke in mice by inducing dysfunction of the cerebral microvasculature. Blood. 2013;121:679–691. doi: 10.1182/blood-2012-04-426734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li P, Mao L, Zhou G, Leak RK, Sun BL, Chen J, et al. Adoptive regulatory t-cell therapy preserves systemic immune homeostasis after cerebral ischemia. Stroke. 2013;44:3509–3515. doi: 10.1161/STROKEAHA.113.002637. [DOI] [PMC free article] [PubMed] [Google Scholar]