Abstract
Rationale
Human ethanol withdrawal manifests as multiple behavioral deficits with distinct time courses. Most studies with mice index ethanol withdrawal severity with the handling-induced convulsion (HIC). Using the accelerating rotarod (ARR), we recently showed that ethanol withdrawal produced motor impairment.
Objectives
a) to characterize further the ARR withdrawal trait; b) to assess generalizability across additional behavioral assays; and c) to test the genetic correlation between ethanol withdrawal ARR impairment and HICs.
Results
Seventy of the ARR performance deficit depends on ethanol vapor dose and exposure duration, and lasts 1–4 days. Fatigue could not explain the deficits, which were also evident after intermittent exposure to ethanol vapor. Withdrawing mice were also impaired on a balance beam, but not on a static dowel or in foot slip errors per distance traveled in the parallel rod floor test, where they showed reduced locomotor activity. To assess genetic influences, we compared Withdrawal Seizure-Prone and -Resistant mice, genetically selected to express severe vs mild withdrawal HICs, respectively. The ARR scores were approximately equivalent in all groups treated with ethanol vapor, though WSP mice may have displayed a slightly more severe deficit as control-treated WSP mice performed better than control-treated WSR mice.
Conclusions
These studies show that ethanol withdrawal motor impairment is sensitive to a range of ethanol doses, and lasts for several days. Multiple assays of behavioral impairment are affected, but the effects depend on the assay employed. Genetic contributions to withdrawal-induced ARR impairment appear largely distinct from those leading to severe or mild HICs.
Keywords: Ethanol withdrawal, Mouse, Vapor inhalation, Rotarod, WSP and WSR selected lines, Motor impairment
Introduction
Alcoholism is a complex trait expressed through the interaction of multiple genes and environmental factors (Spanagel, 2009; Crabbe, 2002; Kranzler and Edenberg, 2010). Alcohol (ethanol) dependence is evidenced by the emergence of a withdrawal syndrome upon cessation of ethanol consumption or administration (Hasin et al., 2006). Ethanol withdrawal produces physiological and psychological symptoms based on neuroadaptations to exposure and subsequent abstinence (Fadda and Rossetti, 1998). Physiological withdrawal symptoms range from mild malaise to life threatening seizures (Dissanaike et al., 2006) and psychological withdrawal symptoms can manifest as stress, depression and anxiety (Heilig et al., 2010).
In mice, the handling-induced convulsion (HIC) is used as a very sensitive behavioral index of withdrawal seventy based on central nervous system hyperexcitability (Goldstein and Pal, 1971; Goldstein, 1973). Other ethanol withdrawal phenotypes in mice include decreased locomotor activity (Capaz et al., 1981; Hutchins et al., 1981; Kliethermes et al., 2004), increased startle response (Chester and Barrenha, 2007), increased susceptibility to drug-induced seizures (Crabbe et al., 1993; Kosobud and Crabbe, 1993), hypothermia (Ritzmann and Tabakoff, 1976), and anxiety-like (Kliethermes et al., 2004; Kliethermes, 2005) and depressive-like behaviors (Hirani et al., 2002). Mice have also shown increased voluntary drinking during ethanol withdrawal (Becker and Lopez, 2004; Griffin, III et al., 2009).
However, ethanol withdrawal indices other than the HIC have not been explored extensively. To add to the diversity of ethanol withdrawal phenotypes systematically characterized in mice, we recently used the accelerating rotarod (ARR) to assess motor performance and learning. We found a transient disruption in performance that was present several hours and days after withdrawal but not one week later. It did not appear to be the result of a learning deficit, as mice were impaired even when compared with controls whose practice on repeated trials was limited. Several inbred mouse strains differed in the seventy of the withdrawal ARR deficit, suggesting genetic influences underlying the phenotype (Philibin et al, 2008).
These results suggested that a deficit in motor performance was a promising phenotype for further investigation. Therefore, we explored its parameters in more detail. All our previous studies had employed continuous exposure to a single concentration of ethanol vapor for 72 h to establish physical dependence in the mice. Here, we varied the concentration or duration of ethanol vapor exposure, or the time course of the post-vapor withdrawal deficit. A fourth study assessed the potential contribution of fatigue to the ARR performance deficit. A fifth explored effects of intermittent ethanol vapor exposure. Systematic studies on inbred mice suggest that various behavioral assays of intoxication that have a motor component cannot be assessing the same set of physiological substrates (Crabbe et al 2005, 2008). Therefore, we explored three additional behavioral assays of motor performance, the dowel test, a balance beam, and the parallel rod floor, in an attempt to assess the generalizability of the withdrawal deficit in motor performance. Finally, we employed mice selectively bred for severe or mild ethanol withdrawal HIC to see whether the genetic contributions to motor deficits and HIC were independent.
Method
Subjects
Naïve outbred mice (WSC-2, or WSC-1 for Experiments 2 and 6) were used for most experiments. The WSC lines are genetically segregating controls for the two independent replicate lines of Withdrawal Seizure-Prone (WSP-1 and -2) and Withdrawal Seizure-Resistant (WSR-1 and -2) mice used for Experiment 8. The WSP and WSR lines were selectively bred to display severe (WSP) vs mild (WSR) HICs following 72 h continuous ethanol vapor inhalation (Crabbe et al., 1985). Naïve WSP and WSR mice from the 26th selected (114th–116th filial) generation were used in Experiment 8. Mice were housed 3–4 per polysulfone cage (28×17×11.5 cm) lined with sterile corncob bedding (Bedocob: Green Products Company, Conrad, Iowa, USA). Food (Purina 5001, PMI International, St. Louis, Missouri, USA) and water were available ad libitum. The colony was maintained on a 12h light/12 h dark cycle with lights on at 0600 h. Colony and procedure rooms were maintained at 22±1°C. All studies were carried out in male mice aged 50–140 days old. After ethanol vapor or air treatment (see next section), mice were group-housed with original cage mates in the colony room. Mice were moved into a procedure room 1 h prior to any behavioral testing, which occurred between 0800–2200 h. All mice used were bred and maintained at the Veterinary Medical Unit, Veterans Affairs Medical Center (Portland, Oregon, USA). Table 1 describes original and final sample sizes, together with subject loss, for each experiment.
Table 1.
Subjects, sample sizes, and sample losses across experiments
| Expt # | Subjects | Initial N | Final N | # Groups | Final N/Group | Subject Loss | |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Death/Illness | Other | ||||||
| 1 | WSC-2 | 71 | 64 | 4 | 14–19 | 5 | 2 |
| 2 | WSC-1 & WSC-2 | 111 | 87 | 4 | 18–22 | 3 | 21 |
| 3 | WSC-2 | 194 | 160 | 14 | 9–13 | 26 | 8 |
| 4 | WSC-2 | 60 | 45 | 4 | 8–15 | 6 | 9 |
| 5 | WSC-2 | 22 | 19 | 2 | 9–10 | 0 | 3 |
| 6 | WSC-1 | 44 | 34 | 2 | 5–17 | 2 | 8 |
| 7 | WSC-2 | 40 | 34 | 2 | 16–18 | 2 | 4 |
| 8 | WSP & WSR | 152 | 94 | 8 | 8–17 | 41 | 17 |
Ethanol vapor inhalation exposure
Ethanol vapor inhalation exposure was used to induce physical dependence as described previously (Metten et al., 2010; Metten and Crabbe, 2005). For all studies, mice in the ethanol groups were initially weighed and injected with an ethanol dose (g/kg, 20% [vol/vol] in saline, ip) to raise blood ethanol concentrations (BECs) to a target mg ethanol/mL blood. The alcohol dehydrogenase inhibitor pyrazole HCl (68.1 mg/kg, IP; Sigma, St. Louis, MO, USA) was administered in the ethanol solution to inhibit ethanol metabolism and stabilize BECs at the desired level (1.5 mg/mL unless otherwise specified). Mice were then placed in wire mesh cages within the Plexiglas chamber. Ethanol vapor concentrations were maintained as stably as possible by monitoring with gas chromatography. In continuous exposure experiments, EtOH-treated mice were removed from the chambers every 24 h, weighed, blood sampled (if applicable), injected with pyrazole in saline, and placed back in the ethanol vapor chambers. Control mice were injected with pyrazole in saline and placed in identical inhalation chambers exposed only to air. Ethanol vapor concentrations were adjusted daily based on BECs collected that day. The WSP and WSR selected lines used in Experiment 8 differ in rates of ethanol metabolism (Terdal and Crabbe, 1994). Therefore, to equate genotypes for inhaled ethanol dose, different ethanol vapor concentrations were used to avoid pharmacokinetic contributions to genotypic differences (for discussion, see Terdal and Crabbe, 1994). For Experiment 2, mice were continuously exposed for 24, 48 or 72 h. For Experiment 5, we exposed mice to chronic intermittent ethanol (CIE) vapor for 3 daily cycles of 16 h ethanol vapor (or air) exposure alternated with 8 hr exposure to air, as described previously (Metten et al., 2010). For this experiment, ethanol exposed mice were weighed and injected with pyrazole in ethanol before each daily 16 h exposure to ethanol vapor. They were removed, weighed, blood sampled, and then moved to an air chamber for the remaining 8 h of each day. Air exposed control mice were injected with pyrazole in saline and handled similarly. Due to behavioral testing time constraints, data were collected during multiple passes for some experiments and ethanol and air treatment groups were included for each pass.
Control mice are always exposed to pyrazole as this drug has behavioral effects including toxicity (Goldstein, 1980, and see Discussion). Mortality during or shortly after inhalation ranged from 0% to 13% (average = 8%) in Experiments 1–7 (see Results). In Experiment 8, we experienced a higher than expected rate of mortality (27%) for unknown reasons.
Blood ethanol concentrations
Blood samples (20 μl) were collected for BEC determination using a gas chromatographic assay detailed previously (Rustay and Crabbe, 2004). Mice were removed from the inhalation chambers daily at the assigned time (16 hr or 24 hr) and BEC was sampled from 10–20 ethanol-treated mice per chamber via tail vein (or peri-orbital sinus for Experiment 5 only) blood in order to make vapor concentration adjustments. In all experiments, all mice experienced a blood sampling procedure (with or without blood collection) regardless of treatment group after the last exposure period. We have found that BECs taken at the end of a 72 h period of continuous ethanol vapor exposure are well correlated with BECs experienced earlier during the prior 72 h, and that withdrawal BECs are significantly correlated with seventy of withdrawal assessed with the HIC (Crabbe et al., 1983). Thus, for all experiments, we report BECs at the time of withdrawal.
Apparatus and test procedures
Accelerating rotarod (ARR)
A modified Accurotor Rota Rod (Accuscan Instruments, Columbus, Ohio, USA) was used. Four mice were tested simultaneously per rotarod; two identical rotarods were used at a time. Once all mice were stationed on the rod, the motor was turned on and the rod was continuously accelerated from 0 revolutions per min (rpm) at a constant rate of 20 rpm/min. After all mice had fallen, mice were returned to their starting position on the static dowel for each consecutive trial after 30–120 s between trials. Latency to fall was automatically recorded by photo beam breaks. No mice were tested for handling induced convulsions (HICs) and were handled so as not to elicit a HIC inadvertently.
Dowel and balance beam
The 3/4 in diameter wooden dowel and the 19mm balance beam have been described previously (Crabbe et al., 2003a,b). One day before starting the inhalation procedure, mice were criterion tested on the dowel and pretrained on the balance beam. Each mouse was given 1–3 trials to establish whether it could remain on the dowel without falling for 1 min (Erwin and Deitrich, 1996). Mice were then immediately placed on the balance beam and required to traverse its length (104.1 cm) once. At 10 and 24 h of withdrawal, mice in Experiment 7 were given a single 1-min trial on the dowel and latency to fall was recorded. Mice were then given one trial on the balance beam, and foot slip errors were scored (Crabbe et al., 2003a).
Parallel rod floor
The parallel rod floor apparatus has been described in detail previously (Kamens and Crabbe, 2007; Kamens et al., 2005). Briefly, the floor consists of a series of parallel steel rods, a stainless steel base plate with an acrylic border raised 1 cm above the base plate, and a clear acrylic box with no bottom and a removable lid. TracerDAQ hardware and software were used to record locomotor activity and foot slips using a standard PC (Enu lnc.[Portland, OR] 3.01 GHz). A paw slip through the parallel rod floor is detected as the paw touches the base plate, completing a circuit. Two photocell beams cross the apparatus approximately 1.5 cm above the parallel rod floor. Locomotor activity is reported as beam interruptions.
At 12 h of withdrawal, each mouse was placed into the center of the apparatus; foot position and beam interruption status was recorded once each second during the 5 min test. The apparatus was cleaned between each test using 10% isopropyl alcohol.
Data analysis
Systat (Chicago, Illinois, USA) version 13 was used for statistical analyses. Data were analyzed using analysis of variance (ANOVA). Unless otherwise noted, significant main effects or interactions were further examined by Tukey’s honestly significant differences post hoc test. Statistical differences were considered significant at p < 0.05.
Accelerating rotarod
ARR testing involved multiple trials during each test session. In a few instances, data were lost during the ARR test because (i) the photocell beam was blocked and thus no latency to fall was recorded (a very rare event) or (ii) the mouse jumped from the rod during the trial. The latter problem was more pronounced in ethanol dependent mice experiencing withdrawal. Animals were retained despite jumping if there were no jumps on adjacent trials, if there were fewer than three trials missing, and if at least two of the first three and two of the last three trials were not missing. Mice whose data passed this criterion were included in analyses by averaging the two trials surrounding the missing trial and inserting the mean.
To simplify analysis, performance on the ARR was indexed by the average latency to fall across all trials during a particular test.
Other motor tests
For the dowel test, latency to fall was recorded. If the mouse did not fall, the maximum score of 60 s was assigned. For the balance beam, hind foot slips during the run across the beam were counted. For the parallel rod floor test, beam interruptions and number of foot slips was recorded: each was summed across the 5 min session for analysis. It has been previously noted that the number of foot slips is positively correlated with distance traveled (Kamens et al., 2005). Therefore, to index motor incoordination, a ratio was obtained by dividing the number of foot slips by the total number of beam breaks.
Experiment 1: Ethanol dose-response
To establish dose-dependence, four sets of WSC-2 mice were assigned to Air Control (Con) or Ethanol (Et) 1.0, Et 1.5, and Et 2.5 groups. Mice were continuously exposed for 72 h to air or different concentrations of ethanol vapor designed to yield BECs of 1.0, 1.5, and 2.5 mg ethanol/mL blood. Upon removal from the chambers after 72 h, mice were returned to their home cage until testing, and between tests. ARR testing was performed 10, 24 and 168 h later for all mice. For each test, mice were given 5 trials on the ARR.
Experiment 2: Ethanol exposure duration-response
To establish duration-dependence, four groups of WSC-1 and WSC-2 mice were treated. Ethanol vapor treatment start date was staggered so that all three groups receiving ethanol vapor completed their treatment of 24, 48, or 72 h on the same day. Group Et 24 was exposed to air for 48 h and then ethanol vapor during the last 24 h period. Group Et 48 was exposed to air for 24 h and then EtOH vapor during the final 48 h. Group Et 72 spent all 72 h in ethanol vapor. The air control (Con) group spent all 72 h exposed to air. Eight h after removal from the chambers, all mice were given 8 ARR trials as described.
Experiment 3: Withdrawal time course
To establish the persistence of withdrawal deficits, WSC-2 mice were assigned to one of 7 withdrawal time point groups (8, 12, 24, 48, 72, 96, or 120 h) and were tested at that time on the ARR for 8 trials. Mice were continuously exposed for 72 h to air (Groups Con 8–Con 120) or ethanol vapor (groups Et 8–Et 120).
Experiment 4: Role of fatigue
Withdrawing mice could fail to show performance improvement across successive, closely-spaced rotarod trials because they become fatigued more easily than mice exposed to air. We exposed WSC-2 mice (Et groups) to ethanol vapor or air (Con groups) for 72 h continuously. Starting 8 h after removal from the chambers, mice were given 10 trials on the ARR. Half the mice (Massed groups) of each treatment group received their trials with a 30–120 s inter-trial interval (i.e., the normal test procedure). The other half of each treatment group (Spaced groups) was given 10 min between each rotarod trial.
Experiment 5: Withdrawal following chronic intermittent exposure (CIE) to ethanol vapor
To see whether ARR impairment was also seen following an intermittent ethanol vapor exposure pattern (Metten et al., 2010) we tested 22 WSC-2 mice. Half the mice were exposed only to air (Con group), and the other half to ethanol vapor (Et group). Each day, mice were removed from the chamber after only 16 h exposure to ethanol or air and returned to the air chamber. Eight h later, mice were placed again into ethanol vapor or air. All mice were given 8 ARR trials starting 8 h after removal from the 3rd 16 h vapor exposure (on day 3).
Experiment 6: Tests on dowel and balance beam
To see whether other motor behaviors were impaired, two groups of WSC-1 mice were tested. One group was exposed only to air (Con), and the other group to ethanol vapor (Et). Ten h after the end of the inhalation period, mice were given a one min test on the dowel followed by one traverse of the balance beam. These tests were repeated at 24 h.
Experiment 7: Tests on the parallel rod floor
To extend the survey to another motor response, WSC-2 mice were exposed to ethanol vapor (Et group) or air (Con group) for 72 h. Starting 12 h after removal from the inhalation chambers, mice were tested for 5 min in the parallel rod floor apparatus.
Experiment 8: Tests with WSP and WSR mice
To assess genetic contributions to motor impairment, mice from both replicates of the Withdrawal Seizure-Prone (WSP-1, WSP-2) and -Resistant (WSR-1, WSR-2) selected lines were exposed for 72 h to air (Con groups) or ethanol vapor (Et groups). Starting 12 h after withdrawal from the chambers, all mice were given 8 ARR trials. Mice were handled carefully so as not to elicit the HICs that both WSP-1 and WSP-2 mice were selectively bred to manifest during ethanol withdrawal: WSR-1 and WSR-2 mice show very minimal HIC scores.
Results
Experiment 1: Ethanol dose-response
BEC values at the time withdrawal commenced for the 1.0, 1.5 and 2.5 mg/mL dose groups were 0.89±0.07, 1.85±0.06, and 2.43±0.19, respectively. Groups differed significantly (F(2,42)=84.0; p <0.0001), and post hoc tests showed that each target dose group differed significantly from the other two. Although the group targeted for 1.5 mg/mL experienced slightly higher BECs than that, we refer to the three dose groups by their targeted values for simplicity.
Fig. 1a shows rotarod performance across trials when mice were initially tested 10 h into withdrawal. We analyzed the performance (average latency to fall across trials) across the three withdrawal time periods as a function of BEC (Fig. 1b). There were significant main effects of ethanol dose (F(3,60)=10.68; p <0.0001) and time (F(2,120)=131.25; p <0.0001), as well as a significant interaction (F(6,120)=8.02; p <0.0001). We therefore analyzed each time point with a separate ANOVA. There was a dose-dependent impairment 10 h after withdrawal (F(3,60)=28.80; p <0.0001). All groups differed significantly from all others (ps <0.01) with the exception of the Et 1.5 and Et 2.5 groups. Impairment remained significant at 24 h (F(3,60)=10.31, p <0.0001). Post hoc analysis now showed that all groups differed significantly from all others (ps<0.05) with the exception of the Et 1.0 and Et 1.5 groups, although the impairment of the Et 1.5 group versus controls only tended to reach significance (p =0.08). By 168 h (one week), there were no significant effects of ethanol treatment on performance (F(3,60)=1.68, NS).
Fig. 1.
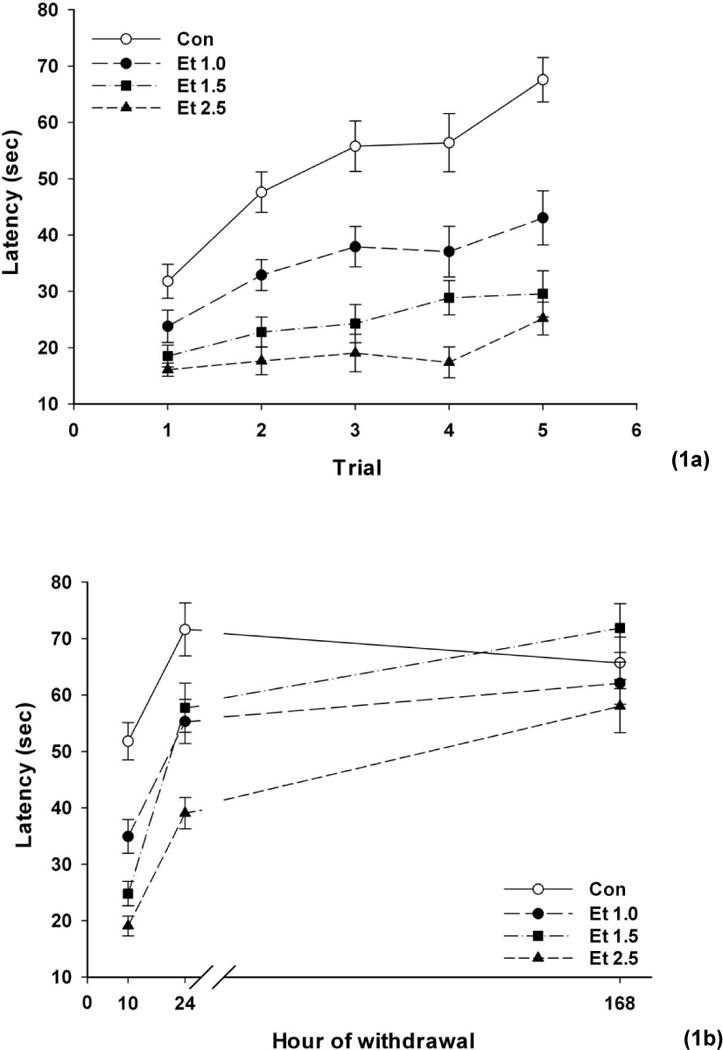
Rotarod performance (latency to fall) as a function of BEC after inhaled ethanol (Et) or air (Con group) and time of withdrawal test (10 h, 24 h, 168 h). 1a Performance across the 5 test trials at 10 h of withdrawal. 1b Performance across the three withdrawal tests. Means ± SEM are shown for 14–19 mice/group
We conclude that there was a regular ethanol dose-effect relationship to impair ARR performance during withdrawal.
Experiment 2: Ethanol exposure duration-response
BECs did not differ significantly across the three ethanol groups on Day 3 (F(2,61)<1, NS). While BECs for the Et 48 group on Day 2 were also clearly similar to those of all groups on Day 3, group Et 72 appeared to have experienced higher blood alcohol levels during their first two days of vapor inhalation than on Day 3 and than did other groups (see Table 2). This impression was borne out by a repeated measure ANOVA across days for group Et 72 (F(2,42)=28.90, p <.0001). Post hoc analyses showed that the BECs for this group differed significantly on each day of exposure (all ps< 0.01).
Table 2.
Experiment 2 blood ethanol concentrations across days (mg/mL)
| Group | Day 1 | Day 2 | Day 3 |
|---|---|---|---|
| Con | |||
| Et 24 | 1.22 ± 0.05 | ||
| Et 48 | 1.30 ± 0.07 | 1.27 ± 0.08 | |
| Et 72 | 1.66 ± 0.08 | 1.53 ± 0.07 | 1.24 ± 0.06 |
Means ± SEM are shown. Group Con was exposed only to air. Groups Et 24–72 received 24, 48 or 72 h exposure to ethanol vapor, respectively
Withdrawal performance differed significantly in a duration-dependent fashion across treatment groups (see Fig. 2, F(3,83)=5.62; p <0.01). Performance was impaired in the Et 72 group as compared with the Et 24 group and the control group (ps<0.05), which did not differ from each other; the Et 48 group did not differ significantly from any other group.
Fig. 2.
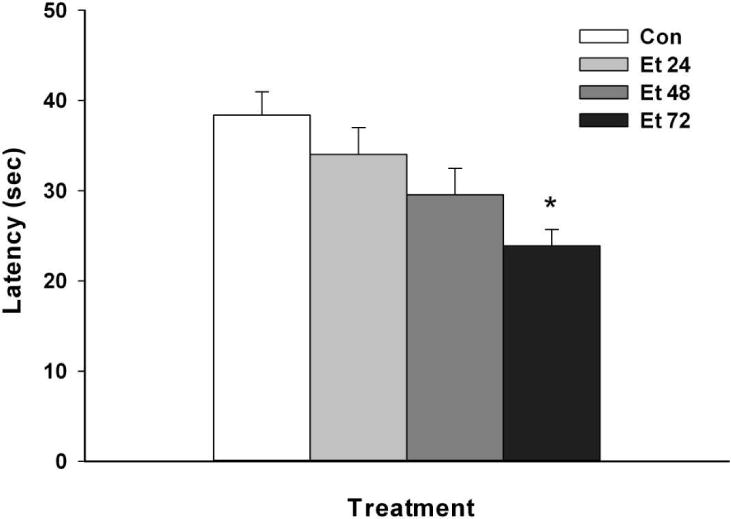
Rotarod performance (latency to fall) at 8 h of withdrawal as a function of duration of inhalation of ethanol vapor (Groups Et 24–72) or air (Group Con). Means ± SEM are shown for 18–22 mice/group. * p<0.05 vs Groups Et 24 and Con
Experiment 3: Withdrawal time course
Across all ethanol-exposed mice, there were no significant differences among the 7 time point groups in BEC at withdrawal (F(6,67)=0.10; NS).
Results are shown in Fig. 3. Ethanol withdrawing mice performed worse than controls (F(1,146)=33.4; p <0.0001). There were also significant main effects of withdrawal time and the interaction of treatment and withdrawal time (Fs(6,146) ≥3.92; ps≤0.001). We therefore compared each ethanol withdrawal time point group with its air-exposed control group. There were significant performance decrements at 8, 12, 24 and 72 h (Fs(1,19–22)≥7.71; ps≤0.01) but not at 48, 96 or 120 h (Fs(1,17–23)≤=0.55; NS).
Fig. 3.
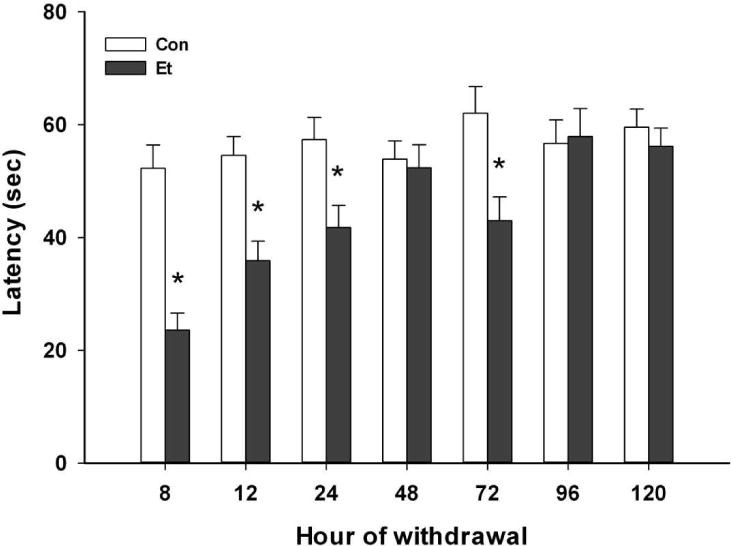
Rotarod performance (latency to fall) as a function of time (h) after inhalation. Mice were exposed for 72 h to ethanol (Groups Et) or air (Groups Con). Means ± SEM are shown for 9–13 mice per time X treatment condition. *p<0.01 vs respective Con group
We conclude that the withdrawal-related ARR impairment persisted for a least 24 h, possibly until 72 h, and had resolved by one week later.
Experiment 4: Role of fatigue
Results of the ARR tests are shown in Fig. 4. EtOH withdrawing mice performed worse than controls (F(1,41)=57.8; p <0.0001). There was a trend toward worse performance with massed versus spaced trials (F(1,41)=3.80; p =0.06), and no significant interaction of EtOH withdrawal and massed versus spaced trials (F(1,41)=0.02; NS). We conclude that fatigue is unlikely to explain the poor ARR performance of withdrawing mice.
Fig. 4.
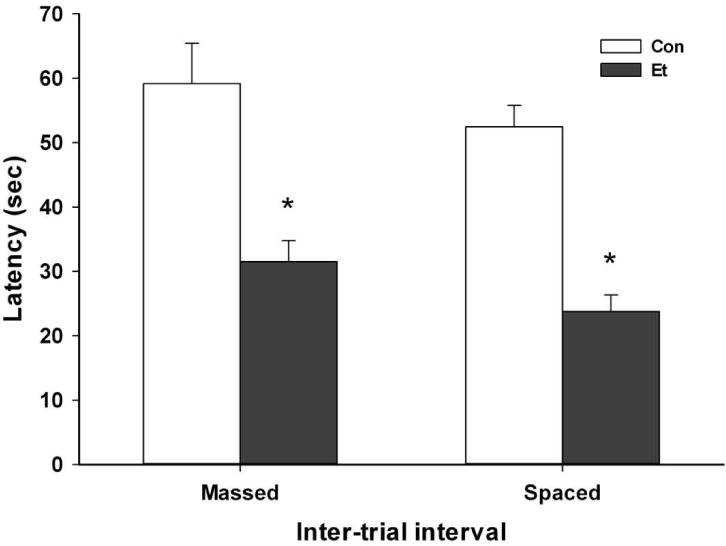
Effect of massed versus spaced trials during rotarod testing on performance. Mice were exposed for 72 h to ethanol (Groups Et; N = 12–15) or air (Groups Con; N = 8–10) and tested 8 h later. Means ± SEM are shown. *p<0.0001 for main effect, Et vs Con groups
Experiment 5: Withdrawal following chronic intermittent exposure to ethanol vapor
BECs from the ethanol-exposed mice were 2.29 ± 0.13, 1.82 ± 0.10, and 1.42 ± 0.15 mg/mL after the first, second, and third 16-h periods of vapor exposure, respectively. Data from the ARR tests with groups of 9–10 mice conducted 8 hr later showed that the ethanol withdrawing mice had worse performance than the mice exposed only to air ((Mean±SEM latency = 25.2±2.8 s vs 47.0±4.6 s, respectively; F(1,17)=16.7, p <0.001).
We conclude that CIE to ethanol vapor effectively yields withdrawal-induced ARR performance deficits.
Experiment 6: Tests on dowel and balance beam
Average BEC for the ethanol-exposed mice was 1.87±0.05 mg/mL. Anomalous behaviors of some mice in both tests at 10 h (see below) led us to analyze data from the 10 h and 24 h tests separately. Dowel performance is shown in Fig. 5A and balance beam data in Fig. 5B.
Fig. 5.
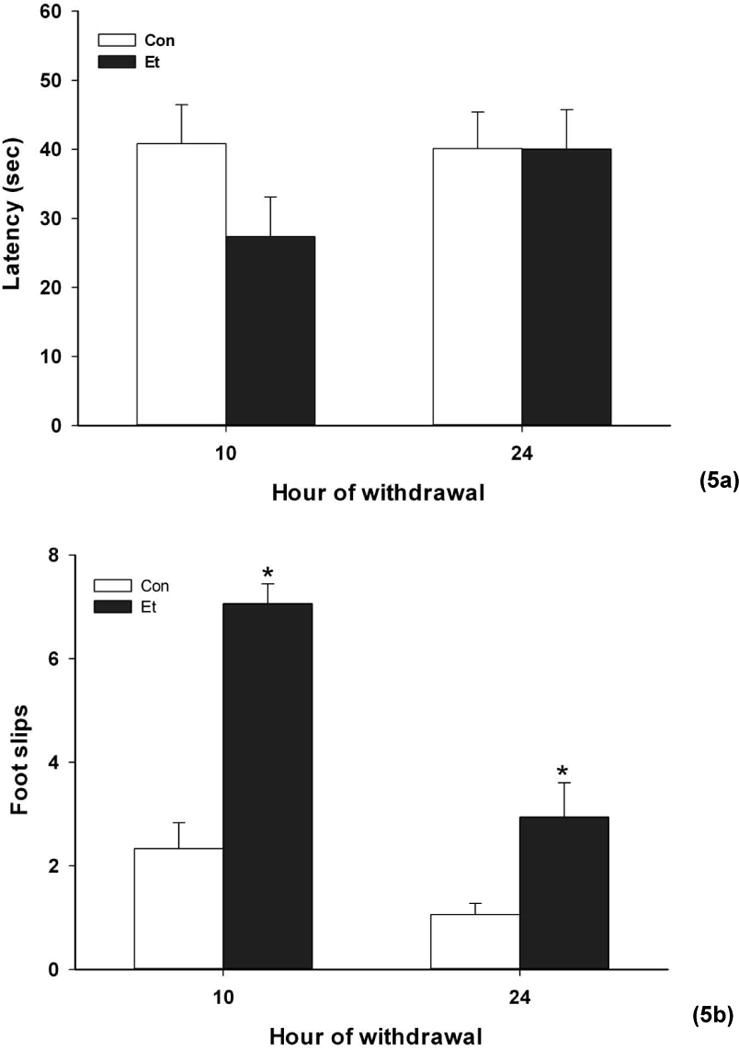
Performance on the a. static dowel and b. balance beam 10 and 24 h after 72 h exposure to ethanol vapor (Et groups) or air (Con groups). Means ± SEM are shown for 16–17 mice/group (5a). For the balance beam (5b), Et N = 5 at 10 h and 16 at 24 h vs 17 Con mice. *p<0.01 vs respective Con group
Dowel Test
Ten (5 ethanol, 5 air) of the remaining 34 mice had failed to pass the static dowel criterion testing, meaning that they did not stay on the dowel for 60 seconds during any of three trials given the day prior to vapor inhalation. Given some anomalies in performance at 10 h (see below), in order to maintain a reasonable N, we tested these mice notwithstanding.
At 10 h of withdrawal, there was no significant effect of treatment on latency to fall from the dowel (F(1,31)=2.81, NS). Eight mice jumped from the dowel during testing; this was recorded, and they were given another trial. Only one (air-treated) mouse refused to stay on the dowel during both trials, so its data were excluded. If the jumping mice from the ethanol-treated group were excluded from the analysis, there was no change in interpretation of the results (F(1,24)=0.59, NS). However, the proportion of animals displaying jumping (7/16 ethanol vs. 1/18 control) was significantly different (X2=6.87, df=1, p <0.01). At 24 h of withdrawal, only one (ethanol-treated) mouse jumped from the dowel, and there was no significant effect of treatment (F(1,32)=0.0001, NS).
While the dowel test may have detected withdrawal impairment at 10 h by showing a high proportion of mice that jumped, those mice that performed the task were not impaired at either 10 or 24 h.
Balance Beam Test
Many ethanol-treated mice dragged one or both hind feet alongside the sides of the beam rather than walking on the top surface. As this behavior competes with foot slips and makes it difficult for the observer to count them, we removed data from animals for which this was noted. The proportion of animals excluded for hind limb dragging during the hour 10 test (11/16 ethanol-treated vs. 0/18 controls) was significantly different (X2=18.3, df=1, p <0.0001). At 24 h of withdrawal, only one (ethanol) mouse displayed hind limb dragging. At 10 h, the remaining ethanol-treated mice made significantly more foot slips while crossing the balance beam (F(1,21)=17.60, p <0.001). There was also a significant impairment at 24 h (F(1,29)=7.21, p=0.01).
We conclude that the balance beam test showed significant motor impairment during ethanol withdrawal.
Experiment 7: Test on the parallel rod floor
BECs at withdrawal averaged 1.61 ± 0.09 mg/mL. Results are shown in Fig. 6. Ethanol-withdrawing mice tended to make fewer foot slips in the apparatus than Controls (F(1,32)=3.73, p =0.06) but they were significantly less active (F(1,32)=12.22, p <0.01). This resulted in no significant difference between groups in impairment expressed as foot slips corrected for activity (F(1,32)=0.02, NS).
Fig. 6.
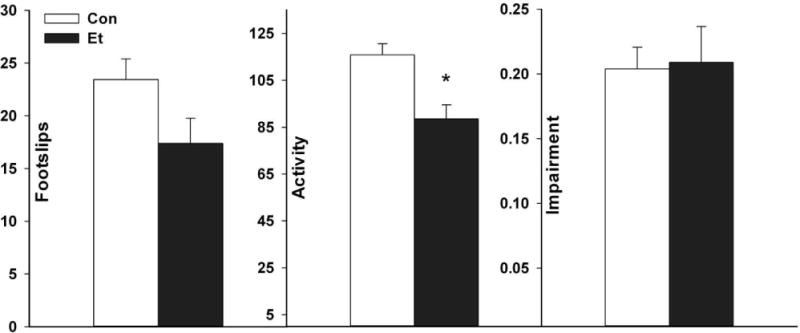
Performance in the parallel rod floor apparatus, a. Foot slips, b. Activity, and c. Impairment are shown in mice exposed to ethanol (Group Et) or air (Group Con). Data are from a 5 min test starting 12 h after removal from the chambers. Means ± SEM are shown for 16–18 mice/group. *p<0.01 vs Con
This task was insensitive to motor impairment during withdrawal, but did show a reduction in locomotor activity in withdrawing mice.
Experiment 8: Tests with WSP and WSR mice
We removed data for seven mice to achieve matched BECs across gentotypes (Terdal and Crabbe, 1994). Final sample sizes per line/replication/treatment and their BECs at the time of withdrawal are shown in Table 3.
Table 3.
Group sizes, BEC at withdrawal, and rotarod performance for Experiment 8
| Line-Replicate | Con | Ethanol | BEC | Latency to fall (s) |
|---|---|---|---|---|
| WSP-1 | 12 | 8 | 1.01±0.07 | 49.1±4.1 |
| WSR-1 | 17 | 10 | 1.14±0.12 | 36.0±2.0 |
| WSP-2 | 17 | 8 | 1.38±0.18 | 31.9±2.5 |
| WSR-2 | 14 | 8 | 1.57±0.16 | 34.2±2.8 |
| WSP | 29 | 16 | 1.19±0.10 | 39.5±2.6 |
| WSR | 31 | 18 | 1.34±0.11 | 34.9±1.6 |
| Totals | 60 | 34 | 1.27±0.08 | 37.1±1.5 |
Con groups were exposed to air; Et groups were exposed to ethanol vapor. BEC = Mean ± SEM mg/mL blood ethanol concentration for the Et group only. For statistical results, see text.
BECs from the ethanol-treated mice differed significantly between replicates (F(1,29)=8.54, p <0.01), but not between lines (F(1,29)=1.40, NS). The interaction was also not significant (F(1,29)=0.03, NS). BECs averaged across the replicates and performance data are also shown in Table 2. Performance was significantly impaired in ethanol-treated groups and differed between replicates (Fs(1,86)≥16.5, p ≤0.0001). WSP mice tended to have better overall performance than WSR(F(1,86)=3.13, p =0.08). The interaction of line and replicate was also significant (F(1,86)=11.2, p =0.001). Significant differences involving replicate lines are not germane to the question of whether the treatment group differences depended on selected line. This question was addressed by the interaction of line and treatment, which approached significance (F(1,86)=3.86, p =0.05). Post hoc tests suggested that both WSP and WSR mice were significantly impaired. While WSP mice exposed to air performed significantly better than WSR (p =0.014), the selected lines did not differ in performance after withdrawal from ethanol vapor (p >0.10). Neither treatment X replicate nor the three-way interaction of treatment X line X replicate were significant (Fs(1,86)<0.54, NS).
These data suggest that impairment was greater in WSP mice than WSR, as the difference between air- and ethanol-treated WSP mice was greater, but may depend on the control group differences.
Discussion
The current studies further characterized the effects of ethanol withdrawal on motor performance using the ARR task in outbred mice. Together, the results of Experiments 1–5 clearly established performance on the ARR as an alternative test of ethanol withdrawal seventy. This test has some advantages over the HIC, as it may be less intrusive for the animal and therefore less disruptive than HIC scoring to other behavioral tests during withdrawal.
Experiment 1 offered evidence of dose dependency. We varied dose indirectly by manipulating ethanol vapor concentration and assessed the success of the manipulation with the BEC at removal from the inhalation chambers. This experiment also showed that the motor performance deficit lasts for 24 h but not as long as 1 week, consistent with our initial report (Philibin et al., 2008). In Experiment 2, we found a regular duration-dependent increase in seventy of behavioral impairment following 24, 48, and 72 h of inhalation. However, the BECs experienced by the 3 treatment groups were not entirely equivalent across all days of exposure. All 3 groups were well-matched for BECs during their final 24 h of exposure, and the 24 and 48 h groups experienced virtually the same BECs throughout their ethanol exposure (see Table 1). However, the latter groups did not differ significantly in withdrawal severity, nor was either significantly impaired as compared with the control group. The 72 h group experienced somewhat higher BECs during their first two days of inhalation, and displayed significant impairment vs either the 24 h group or the control group. While our experience and earlier data suggest that the BEC experienced during the final 24 h period is the most important value for predicting withdrawal HIC severity (Crabbe et al., 1983), we cannot rule out the role of an overall higher experienced dose by the 72 h group. Thus, this experiment is not definitive as to the role of exposure duration.
Experiment 3 sought to establish when between 1 day and 1 week the ARR withdrawal deficit resolved. Results here were also somewhat equivocal (Figure 3). The 7 different ethanol withdrawal groups experienced very similar BECs during inhalation (F=0.10), so the experiment successfully manipulated time of withdrawal independently of dose of inhaled ethanol. The deficit was clearly present at 8, 12, and 24 h, replicating Experiment 1, earlier studies (Philibin et al., 2008), and unpublished data. By 4 or 5 days, there was no significant deficit. However, the deficit seen at 24 and 72 h was absent in the 48 h test group. This puzzling departure from regularity in the results cannot be explained by repeated testing, as this was a between-groups analysis, and the pattern was apparent in all three passes of the experiment (data not shown). Some unexplained anomaly in performance at 48 h seems more likely to us than a transient recovery followed by a loss of function 24 h later.
We conclude that the ARR performance deficit lasts somewhere between 1 and 4 days. Given that the seventy of the withdrawal-induced motor impairment appears to be a function of both dose and duration of exposure to ethanol vapor, it is likely that the duration of impairment is a function of both as well. Withdrawal HIC seventy and duration are a joint function of ethanol dose and the duration of vapor exposure (Goldstein, 1972), so we suspect the ARR deficit is as well, although we did not formally test this. Other withdrawal signs have not been rigorously analyzed to establish their persistence, although many believe that withdrawal-associated anxiety-like behavior may persist for weeks in rodents [(Heilig et al., 2010); but see (Kliethermes, 2005)].
The nature of the performance deficit remains unclear. In our previous study, we ruled out one possibility, namely, that the ethanol-withdrawing mice could not acquire the motor learning that underlies improvement in latencies across the multiple trials on the ARR as efficiently as control mice (Philibin et al., 2008). In Experiment 4, we addressed another possible source of the performance deficit, fatigue. In fact, performance under massed trials tended to be worse than under spaced trials; however, the ethanol withdrawal deficit was equivalent in both test protocols. We conclude that fatigue is not likely to be the proximate cause of the motor impairment.
Interest has recently increased in studying the effect of multiple cycles of ethanol dependence and withdrawal to escalate voluntary ethanol drinking (Becker and Lopez 2004; Sommer et al. 2008). We have characterized withdrawal HIC seventy in multiple inbred mouse strains using the CIE exposure protocol, and a comparison of withdrawal seventy between CIE and standard 72 h continuous inhalation has shown that the two withdrawal phenotypes are partially correlated genetically (Metten et al., 2010). In Experiment 5, we demonstrated that the withdrawal ARR impairment is robust after CIE to ethanol, making this trait available to index relative dependence seventy in such experiments without needing to elicit HICs. It would be of interest to compare the severity of the ARR deficit in animals that had been exposed to equivalent doses of ethanol continuously vs intermittently.
We also extended the findings to other behavioral assays of motor performance, and corroborated our earlier finding that withdrawal HIC severity and motor disruption are influenced by largely distinct sets of genes (Philibin et al. 2008). Few behaviors other than HICs have been rigorously characterized during ethanol withdrawal. Experiments 6 and 7 extended the range of assays tested for the withdrawal deficit to the dowel test, balance beam test, and a new behavioral assay, the parallel rod floor test (Kamens et al., 2005). The dowel test yielded equivocal results. Significantly more (nearly half) of the ethanol-withdrawing mice than control mice (only one) elected to jump from the dowel during the 10 h withdrawal test. Although Fig. 5a gave the appearance of a deficit at 10 h of withdrawal, this difference was not significant. By 24 h, there was no difference in performance or behavior. On the balance beam, many withdrawing mice also displayed aberrant behavior at 10 h, and those withdrawing mice that performed the task made more foot slip errors than controls. Competing behavior had normalized by 24 h, and the withdrawing mice displayed a clear deficit by displaying more foot slip errors at this time point (Fig. 5b). In the parallel rod floor test, withdrawing mice showed fewer foot slips, but also had significantly reduced activity as compared with controls (Fig. 6). Motor performance when corrected for activity did not reveal a significant deficit in withdrawing mice. Thus, Experiments 6 and 7 showed that the withdrawal deficit was evident in one additional task, the balance beam, and potentially in a second, the dowel test. Experiment 7 also replicated the earlier findings of reduced activity in a novel apparatus during withdrawal (Kliethermes et al, 2004; 2005). It should be noted that task-specific deficits for these and other tasks may only be seen at times other than those we tested.
It is possible that the neurobiological substrates for acute ethanol intoxication are similar to those underlying the compromised motor performance seen during withdrawal from chronic ethanol exposure. If this hypothesis is true, we would predict that those tasks where sensitivity to ethanol intoxication had been found to be correlated genetically would show a similar pattern of sensitivity to ethanol withdrawal. The current data offer some support for this hypothesis. We have compared sensitivity to ethanol in 18 intoxication measures derived from 11 behavioral assays and assessed in 8 inbred mouse strains (Crabbe et al., 2005; Crabbe et al., 2008). Here, we found that performance on the ARR and on the balance beam were both compromised during withdrawal. The earlier intoxication survey reported a genetic correlation between sensitivities on these tasks (r = 0.67). Overall, the hypothesized commonality of biological substrates between acute ethanol intoxication and ethanol withdrawal is consistent with some, but not all of the current data. If the hypothesis is true, based on the acute sensitivity correlations, we would predict that withdrawing mice would show abnormal ambulation in an observer-rated ataxia assay (Metten et al. 2004), but would not show a withdrawal deficit on the screen test (Crabbe et al. 2003a).
We previously reported that male Withdrawal Seizure-Prone and -Resistant selected lines were affected similarly by ethanol withdrawal on the ARR after 72 h chronic continuous ethanol vapor inhalation exposure (Philibin et al., 2008). However, there were very few WSP-2 (N=4) mice in that study, dictating an analysis that collapsed data across replicates of the selection. Testing both pairs of independently-selected replicate lines allows the experimenter to perform a much stronger test of the hypothesis of divergent responses between lines, which would suggest genetically correlated traits (Crabbe et al., 1990). Therefore, we repeated the experiment with WSP and WSR lines. The current experiment included more mice from each replicate line. It differed from the previous study in that BECs were generally lower (WSP=1.19 mg/mL; WSR=1.34 mg/mL) as compared with earlier values of 1.68 and 1.71 mg/mL, respectively. We also tested later during withdrawal (12 h vs 8 h). We obtained essentially the same result. All 4 selected lines showed marked impairment during withdrawal. There was a tendency toward greater impairment in WSP than WSR mice. This could have been due to the significantly better performance of WSP-1 mice in the control group (see Table 2), as the 4 ethanol-treated groups did not differ significantly, but the difference between air- and ethanol-treated groups tended to be greater in WSP than WSR mice. This result suggests that while there may be some genes that influence both withdrawal HIC severity and the ARR performance deficit, the two traits are predominantly influenced by different genes, consistent with the previously-noted lack of a genetic correlation between inbred strain mean withdrawal deficits in the ARR task (Philibin et al., 2008) and in HIC seventy (Metten and Crabbe, 2005).
We note that these findings may be specific to withdrawal from ethanol dependence induced by vapor inhalation and that different results might be seen if a different method were used (e.g., exposure to a liquid diet containing ethanol). All the animals tested here had received daily pyrazole injections to inhibit alcohol dehydrogenase activity. The potential contributing role of ethanol metabolites to the neurobiological sequelae of chronic ethanol exposure remains an area of active interest (Deitrich, 2011). Acute injection of pyrazole to rats, at a higher dosage (120 mg/kg) than we use in mice (68.1 mg/kg), has been reported to cause motor incoordination in the tilting plane test (Goldberg et al. 1972). It both potentiated and prolonged the intoxication induced by 1.5 g/kg ethanol. Thus, specifics of the results reported here should be generalized with care to other situations.
This report adds to the arsenal of behavioral tests used to measure motor responses known to be sensitive to disruption during withdrawal from chronic ethanol vapor exposure and therefore should facilitate characterization of the complex phenotype. As knowledge of the mechanisms underlying these various behavioral tasks advances, this should enable us to gain parallel increments in our understanding of withdrawal from chronic ethanol. One of our goals in the immediate future is to develop a battery of tests assessing a range of behaviors in mice that could be used to create a general profile of ethanol withdrawal severity. The tests will cover the domains of central nervous system excitability (e.g., the HIC), motor performance (the ARR and balance beam), locomotor activity, anxiety- and depression-like behavior, motivation, and cognitive performance. Early studies suggest that such a battery may be possible to achieve (Hutchins et al. 1981) and would improve the parallelism between preclinical and clinical studies of alcohol dependence.
Acknowledgments
Support for this work was from the NIH (AA010760, AA013519, T32AA05828, and F32AA17021) and a grant from the US Department of Veterans Affairs. Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC) principles of laboratory animal care were followed, as well as the Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research (National Research Council 2003). The experiments comply with the current laws of the United States. We thank Lauren Brown and Stephanie Spence for assistance with the experiments, and Mark Rutledge-Gorman for assistance with the manuscript.
Footnotes
The authors have no conflicts of interest to declare.
Reference List
- Becker HC, Lopez MF. Increased ethanol drinking after repeated chronic ethanol exposure and withdrawal experience in C57BL/6 mice. Alcohol Clin Exp Res. 2004;28:1829–1838. doi: 10.1097/01.alc.0000149977.95306.3a. [DOI] [PubMed] [Google Scholar]
- Capaz FR, Vanconcellos LE, De MS, Neto JP. The open field: a simple method to show ethanol withdrawal symptoms. Arch Int Pharmacodyn Ther. 1981;251:228–236. [PubMed] [Google Scholar]
- Chester JA, Barrenha GD. Acoustic startle at baseline and during acute alcohol withdrawal in replicate mouse lines selectively bred for high or low alcohol preference. Alcohol Clin Exp Res. 2007;31:1633–1644. doi: 10.1111/j.1530-0277.2007.00462.x. [DOI] [PubMed] [Google Scholar]
- Crabbe JC. Genetic contributions to addiction. Annual Review of Psychology. 2002;53:435–462. doi: 10.1146/annurev.psych.53.100901.135142. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Cameron AJ, Munn E, Bunning M, Wahlsten D. Overview of mouse assays of ethanol intoxication. Curr Protoc Neurosci. 2008:9.26.1–9.26.18. doi: 10.1002/0471142301.ns0926s42. Unit. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Cotnam CJ, Cameron AJ, Schlumbohm JP, Rhodes JS, Metten P, Wahlsten D. Strain differences in three measures of ethanol intoxication in mice, the screen, dowel and grip strength tests. Genes, Brain Behav. 2003a;2:201–213. doi: 10.1034/j.1601-183x.2003.00023.x. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Kosobud A, Young ER, Tam BR, McSwigan JD. Bidirectional selection for susceptibility to ethanol withdrawal seizures in Mus musculus. Behav Genet. 1985;15:521–536. doi: 10.1007/BF01065448. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Merrill CM, Belknap JK. Effect of acute alcohol withdrawal on sensitivity to pro- and anticonvulsant treatments in WSP mice. Alcohol Clin Exp Res. 1993;17:1233–1239. doi: 10.1111/j.1530-0277.1993.tb05235.x. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Metten P, Cameron AJ, Wahlsten D. An analysis of the genetics of alcohol intoxication in inbred mice. Neurosci Biobehav Rev. 2005;28:785–802. doi: 10.1016/j.neubiorev.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Metten P, Yu C-H, Schlumbohm JP, Cameron AJ, Wahlsten D. Genotypic differences in ethanol sensitivity in two tests of motor incoordination. J Appl Physiol. 2003b;95:1338–1351. doi: 10.1152/japplphysiol.00132.2003. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Phillips TJ, Kosobud A, Belknap JK. Estimation of genetic correlation: interpretation of experiments using selectively bred and inbred animals. Alcohol Clin Exp Res. 1990;14(2):141–151. doi: 10.1111/j.1530-0277.1990.tb00461.x. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Young ER, Kosobud A. Genetic correlations with ethanol withdrawal severity. Pharmacol Biochem Behav. 1983;18(Suppl. 1):541–547. doi: 10.1016/0091-3057(83)90233-2. [DOI] [PubMed] [Google Scholar]
- Dissanaike S, Halldorsson A, Frezza EE, Griswold J. An ethanol protocol to prevent alcohol withdrawal syndrome. J Am Coll Surg. 2006;203:186–191. doi: 10.1016/j.jamcollsurg.2006.04.025. [DOI] [PubMed] [Google Scholar]
- Erwin VG, Deithch RA. Genetic selection and characterization of mouse lines for acute functional tolerance to ethanol. J Pharmacol Exp Ther. 1996:1310–1317. [PubMed] [Google Scholar]
- Fadda F, Rossetti ZL. Chronic ethanol consumption: from neuroadaptation to neurodegeneration. Prog Neurobiol. 1998;56:385–431. doi: 10.1016/s0301-0082(98)00032-x. [DOI] [PubMed] [Google Scholar]
- Goldstein DB. Relationship of alcohol dose to intensity of withdrawal signs in mice. J Pharmacol Exp Ther. 1972;180:203–215. [PubMed] [Google Scholar]
- Goldstein DB. Quantitative study of alcohol withdrawal signs in mice. Ann N Y Acad Sci. 1973;215:218–223. doi: 10.1111/j.1749-6632.1973.tb28276.x. [DOI] [PubMed] [Google Scholar]
- Goldstein DB, Pal N. Alcohol dependence produced in mice by inhalation of ethanol: grading the withdrawal reaction. Science. 1971;172:288–290. doi: 10.1126/science.172.3980.288. [DOI] [PubMed] [Google Scholar]
- Griffin WC, III, Lopez MF, Becker HC. Intensity and duration of chronic ethanol exposure is critical for subsequent escalation of voluntary ethanol drinking in mice. Alcohol Clin Exp Res. 2009;33:1893–1900. doi: 10.1111/j.1530-0277.2009.01027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin D, Hatzenbuehler ML, Keyes K, Ogburn E. Substance use disorders: Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV) and International Classification of Diseases, tenth edition (ICD-10) Addiction. 2006;101(Suppl 1):59–75. doi: 10.1111/j.1360-0443.2006.01584.x. [DOI] [PubMed] [Google Scholar]
- Heilig M, Egli M, Crabbe JC, Becker HC. Acute withdrawal, protracted abstinence and negative affect in alcoholism: are they linked? Addict Biol. 2010;15:169–184. doi: 10.1111/j.1369-1600.2009.00194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirani K, Khisti RT, Chopde CT. Behavioral action of ethanol in Porsolt’s forced swim test: modulation by 3 alpha-hydroxy-5 alpha-pregnan-20-one. Neuropharmacology. 2002;43:1339–1350. doi: 10.1016/s0028-3908(02)00330-1. [DOI] [PubMed] [Google Scholar]
- Hutchins JB, Allen DL, Cole-Harding LS, Wilson JR. Behavioral and physiological measures for studying ethanol dependence in mice. Pharmacol Biochem Behav. 1981;15:55–59. doi: 10.1016/0091-3057(81)90338-5. [DOI] [PubMed] [Google Scholar]
- Kamens HM, Crabbe JC. The parallel rod floor test: a measure of ataxia in mice. Nature Protocols. 2007;2:277–281. doi: 10.1038/nprot.2007.19. [DOI] [PubMed] [Google Scholar]
- Kamens HM, Phillips TJ, Holstein SE, Crabbe JC. Characterization of the parallel rod floor apparatus to test motor incoordination in mice. Genes Brain Behav. 2005;4:253–266. doi: 10.1111/j.1601-183X.2004.00100.x. [DOI] [PubMed] [Google Scholar]
- Kliethermes CL. Anxiety-like behaviors following chronic ethanol exposure. Neurosci Biobehav Rev. 2005;28:837–850. doi: 10.1016/j.neubiorev.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Kliethermes CL, Cronise K, Crabbe JC. Anxiety-like behavior in mice in two apparatuses during withdrawal from chronic ethanol vapor inhalation. Alcohol Clin Exp Res. 2004;28:1012–1019. doi: 10.1097/01.alc.0000131976.40428.8f. [DOI] [PubMed] [Google Scholar]
- Kosobud AE, Crabbe JC. Sensitivity to N-methyl-D-aspartic acid-induced convulsions is genetically associated with resistance to ethanol withdrawal seizures. Brain Res. 1993;610:176–179. doi: 10.1016/0006-8993(93)91235-k. [DOI] [PubMed] [Google Scholar]
- Kranzler HR, Edenberg HJ. Pharmacogenetics of alcohol and alcohol dependence treatment. Curr Pharmaceut Des. 2010;16:2141–2148. doi: 10.2174/138161210791516387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metten P, Crabbe JC. Alcohol withdrawal severity in inbred mouse (Mus musculus) strains. Behav Neurosci. 2005;119:911–925. doi: 10.1037/0735-7044.119.4.911. [DOI] [PubMed] [Google Scholar]
- Metten P, Sorensen ML, Cameron AJ, Yu C-H, Crabbe JC. Withdrawal severity after chronic intermittent ethanol in inbred mouse strains. Alcohol Clin Exp Res. 2010;34:1552–1564. doi: 10.1111/j.1530-0277.2010.01240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philibin SD, Cameron AJ, Metten P, Crabbe JC. Motor impairment: a new ethanol withdrawal phenotype in mice. Behav Pharmacol. 2008;19:604–614. doi: 10.1097/FBP.0b013e32830ded27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritzmann RF, Tabakoff B. Ethanol, serotonin metabolism, and body temperature. Annals NY Acad Sci. 1976;273:247–255. doi: 10.1111/j.1749-6632.1976.tb52888.x. [DOI] [PubMed] [Google Scholar]
- Rustay NR, Crabbe JC. Genetic analysis of rapid tolerance to ethanol’s incoordinating effects in mice: Inbred strains and artificial selection. Behav Genet. 2004;34:441–451. doi: 10.1023/B:BEGE.0000023649.60539.dd. [DOI] [PubMed] [Google Scholar]
- Spanagel R. Alcoholism: a systems approach from molecular physiology to addictive behavior. Physiol Rev. 2009;89:649–705. doi: 10.1152/physrev.00013.2008. [DOI] [PubMed] [Google Scholar]
- Terdal ES, Crabbe JC. Indexing withdrawal in mice: matching genotypes for exposure in studies using ethanol vapor inhalation. Alcohol Clin Exp Res. 1994;18:542–547. doi: 10.1111/j.1530-0277.1994.tb00907.x. [DOI] [PubMed] [Google Scholar]


