SUMMARY
The announcement of the results of the NLST, showing a 20% reduction in lung-cancer specific mortality with LDCT screening in a high risk population, marked a turning point in lung cancer screening. This was the first time that a randomized controlled trial had shown a mortality reduction with an imaging modality aimed at early detection of lung cancer. Current guidelines endorse LDCT screening for smokers and former smokers ages 55 to 74, with at least a 30 pack year smoking history. Adherence to published algorithms for nodule follow-up is strongly encouraged. Future directions for screening research include risk stratification for selection of the screening population, and improvements in the diagnostic follow-up for indeterminate pulmonary nodules. As with screening for other malignancies, screening for lung cancer with LDCT has revealed that there are indolent lung cancers which may not be fatal. More research is necessary if we are to maximize the risk-benefit ratio in lung cancer screening.
Keywords: Lung cancer screening, Lung cancer, Pulmonary nodule, Computed tomography, Thoracic imaging
INTRODUCTION
Lung cancer screening has been a hotly debated topic since early reports of lung cancer screening in the late 1990s, including screening programs in Japan and the Early Lung Cancer Action Program.[1–4] Since that report, much work has been done to determine the role CT should play in screening for lung cancer. Lung cancer screening remains controversial, as uncertainty remains about risks, cost-effectiveness, and application of screening in a clinical setting.
The fight to reduce the health care burden of lung cancer must be fought on several fronts. First and foremost, discouraging cigarette smoking and promoting smoking cessation is essential. Although lung cancer does occur in non-smokers, there is a much higher risk in cigarette smokers. The relative risk of death from lung cancer in men who are current smokers, compared with men who are never-smokers, was 24.97 for the decade from 2000 to 2010.[5] This rate was similar in women; women current smokers were 25.66 times more likely to die from lung cancer than women who had never smoked. In fact, mortality from all causes is increased in current smokers by a factor of 2.80 for men and 2.76 for women.
The second front in the battle against lung cancer must be early detection. When lung cancer is confined to the lung at the time of diagnosis, five-year survival is 53.5%. [6]This drops to 26.1% when there is regional nodal involvement and to 3.9% when there is distant metastatic disease. In the period from 2003 through 2009, only 15% of lung cancer cases were diagnosed at a localized stage. The goal of screening is to shift the timing of the diagnosis to an earlier point, so that the disease is localized to the lung, and appropriate therapy can reduce lung cancer mortality.
Results from a number of lung cancer screening trials around the world, including Japan, the United States, Italy, Denmark, and the Netherlands, have shown that screening for lung cancer with low-dose CT can result in an increase in the detection of lung cancer at an earlier stage, when it can be more effectively treated.[1–4, 7–9] Since these reports from single-arm (observational) trials, mortality data from a randomized controlled trial, the National Cancer Institute – funded National Lung Screening Trial (NLST), has shown a reduction in death from lung cancer in current and former smokers who were screened with LDCT in comparison with those screened with single view chest radiographs.[10] Although expectations were that the NLST would provide the definitive answer to the question of CT screening for lung cancer, questions remain about the costs of wide-spread screening, the radiation risks, the burden of working up incidental and false positive findings, and the potential for overdiagnosis.[TABLE 1].
TABLE 1.
Potential benefits and risks of LDCT screening for lung cancer in a population of older, heavy smokers.
| BENEFITS | RISKS |
|---|---|
| Reduced mortality from lung cancer | Radiation exposure |
|
Reduced morbidity from lung
cancer treatment |
Overdiagnosis |
|
Reduced morbidity and mortality
from other diseases discovered incidentally (e.g. COPD, coronary artery calcification, extrapulmonary malignancy) |
Risks associated with working up
positive findings – either false positive or true positive |
| Increased awareness of harms of smoking |
Potential for continued/renewed
smoking behavior |
| Reduced anxiety when screen is negative | Increased anxiety from positive test results |
|
Financial costs of screening and
subsequent evaluations |
|
| False negative test results |
The National Lung Screening Trial, which was conducted from 2002 through 2009, was a randomized, multi-institutional study designed to determine if screening with CT could reduce lung cancer mortality relative to screening with a single-view CXR in a high risk population.[10]Participants received either a low-dose spiral CT or a single-view CXR annually for three years. Although it would have been optimal to have the control arm receive usual care (no screening), the decision was made to provide chest radiography to the control arm to improve patient accrual and retention. The trial was funded through the National Cancer Institute and represented a collaboration of the NCI Division of Cancer Prevention and the NCI Division of Cancer Treatment and Diagnosis. The NCI Division of Cancer Prevention administered the Lung Screening Study (LSS) component of NLST and the NCI Division of Cancer Treatment and Diagnosis funded the American College of Radiology Imaging Network (ACRIN) component. The trial was conducted at 33 sites across the United States, and enrolled 53,456 participants between August 2002 and April 2004. Participants were between the ages of 55 and 74 years at the time of registration, had at least a 30 pack-year history of smoking, and were either current smokers or former smokers who had quit within the past 15 years. Additional eligibility criteria included no prior diagnosis of lung cancer, no treatment for any cancer within the past five years, with the exceptions of non-melanoma skin cancer and most in-situ carcinomas, no prior removal of any portion of the lung, and no requirement for home oxygen supplementation. Exclusion criteria included symptoms of lung cancer, such as unexplained weight loss of over 15 lbs within the past 12 months, or unexplained hemoptysis, any medical condition that would pose a significant risk of mortality during the trial period, or a prior chest CT within the preceding 18 months of study enrollment.
Of the total NLST population 26,733 participants were randomized to CXR, and 26,723 were randomized to CT. Randomization was stratified by sex and five year age group (55–59, 60–64, 65–69, 70–74). Of the entire study group, 59% were male, and 73% were younger than 65 years of age.[11] The mean age was 61.4, +/− 5.0 years, and the median age was 60. Ninety-one percent of the participants were white, 4.4% were black, and 1.7% were of Hispanic/Latino ethnicity.
There were 247 deaths from lung cancer per 100,000 person-years in the LDCT arm of the NLST, compared with 309 deaths per 100,000 person-years in the chest radiography arm.[10] This equates to a 20% relative reduction in mortality from lung cancer with LDCT relative to screening with a single view chest radiograph. The 6.7% relative reduction in all-cause mortality between the two arms was largely attributable to the reduction in lung cancer mortality. Comparison with the data from the Prostate, Lung, Colon and Ovarian (PLCO) Cancer Screening trial allows extrapolation to the usual care (no screening) arm of that trial. The lung-cancer specific mortality in a subset of PLCO participants who met NLST eligibility criteria was 361 per 100,000 person-years in the CXR arm, and 383 per 100,000 person-years. [12]Although there was no statistically significant difference in screening with chest radiographs versus usual care, comparison between the mortality in the usual care arm of the PLCO and the LDCT arm of the NLST raises the possibility that the mortality reduction demonstrated in the NLST could have been somewhat greater had the control arm received no screening.[TABLE 2]
Table 2.
Lung cancer-specific mortality was reduced in the low-dose CT arm of the NLST, relative to the CXR arm. Screening with chest radiography in a subset of PLCO participants who matched NLST eligibility criteria did not demonstrate a significant mortality reduction, compared with usual care (no screening). The potential reduction of lung-cancer specific mortality could be even greater when NLST data are compared with the usual care arm of this PLCO subset. [10, 12]
| NLST LDCT | NLST CXR | PLCO CXR | PLCO usual care |
|
|---|---|---|---|---|
|
Lung cancer deaths per |
246 | 308 | 361 | 383 |
|
100,000
person years |
GUIDELINES FOR LUNG CANCER SCREENING
Since the publication of the mortality reduction with LDCT screening in the NLST, a growing list of organizations have issued guidelines for lung cancer screening with LDCT, including the American Cancer Society (http://www.cancer.org), American Lung Association (http://www.lung.org), the National Comprehensive Cancer Network (http://www. nccn.org), and the American Association for Thoracic Surgery, largely following the eligibility criteria and structure of the National Lung Screening Trial. [13–15] The joint statement of the American College of Chest Physicians (ACCP) and the American Society of Clinical Oncology (ASCO) cautioned that screening should occur “only in settings that can deliver the comprehensive care provided to National Lung Screening Trial (NLST) participants.”[16]
In January 2013, the American Cancer Society issued the following guidelines for lung cancer screening; “Clinicians with access to high-volume, high-quality lung cancer screening and treatment centers should initiate a discussion about screening with apparently healthy patients aged 55 years to 74 years who have at least a 30–pack-year smoking history and who currently smoke or have quit within the past 15 years. A process of informed and shared decision-making with a clinician related to the potential benefits, limitations, and harms associated with screening for lung cancer with LDCT should occur before any decision is made to initiate lung cancer screening”.[14]
The United States Preventive Services Task Force (USPSTF) evaluated the body of literature regarding lung cancer screening in 2004 and determined that there was insufficient evidence to recommend for screening. Although the USPSTF has assembled a panel of experts to guide their next review, no statement has been issued as of date.
WHO SHOULD BE SCREENED?
Current recommendations for lung cancer screening use the eligibility criteria for the NLST to define high-risk individuals: current or former cigarette smokers between the ages of 55 and 74, with at least 30 pack-years smoking history. Former smokers should have quit smoking within the last 15 years. Ma et al estimated that approximately 8.6 million Americans (5.2 million men, 3.4 million women) met these criteria for lung cancer screening in 2010.[17] They calculated that, if screening were fully implemented, 12,250 deaths from lung cancer could be prevented or delayed. Pinsky and Berg calculated that the NLST criteria would include 6.2% of the US population over 40, but would detect only 26.7% of incident lung cancers. [18]
The NCCN guidelines also include LDCT screening, albeit based on lower level evidence, in individuals 50 or older, with at least a 20 pack-year smoking history, and one additional risk factor. [15]The additional risk factors include a personal history of cancer or lung disease, a family history of lung cancer, radon exposure, and occupational exposure to silica, cadmium, asbestos, arsenic, beryllium, chromium, diesel fumes, and nickel. These more inclusive guidelines were based on the results of the International Early Lung Cancer Action program which expanded eligibility guidelines from their earlier studies to include individuals 40 years of age and older, with either a history of cigarette smoking, occupational exposure (to asbestos, beryllium, uranium, or radon), or exposure to secondhand smoke. [19]
Screening with LDCT is not intended for individuals with symptoms suggestive of lung cancer, such as cough, weight loss, or chest pain. In these patients, standard-dose CT with intravenous contrast administration is the current standard of care.
It would be helpful to further refine eligibility criteria for lung cancer screening, so that the number of diagnosed lung cancers per population screened would be higher, and fewer individuals would be unnecessarily exposed to the associated risks. In this regard, modeling can be used to estimate the impact of screening on a population, taking into account risk factors and the natural history of lung cancer. Bach et al used data on 18,172 subjects enrolled in the Carotene and Retinol Efficacy Trial (CARET), a large, randomized trial of lung cancer prevention, to derive a lung cancer risk prediction model. [20] Risk factors included the subject’s age, sex, asbestos exposure history, and smoking history. Examples of risk calculations included a 51-year-old female who smoked one pack per day for 28 years and quit smoking 9 years earlier, who was calculated to be in the 5th percentile of risk. Assuming that she remained nonsmoking, her 10-year risk of lung cancer would be 0.8%, or 1 in 120. In comparison, the 10-year risk of lung cancer for a 51 year old female never-smoker would be approximately 0.07% (1 in 1400). At the other extreme is a 68-year-old male current smoker who smoked two packs per day for 50 years, who would fall in the 95th percentile of lung cancer risk. His 10-year risk of lung cancer would be 11% (1 in 9) if he quit smoking immediately and 15% (1 in 7) if he continued to smoke at the current level. [TABLE 3]
TABLE 3.
Approximate 10-year risk of developing lung cancer. These tables assume that people who have quit smoking will remain nonsmoking for an additional 10 years and current smokers will keep smoking the same amount for the next 10 years. For individuals with occupational asbestos exposure, the risks should be multiplied by 1.24. Adapted from Bach PB et al.[20]
| Duration of smoking |
25 years former smoker |
25 years current smoker |
40 years former smoker |
40 years current smoker |
50 years former smoker |
50 years current smoker |
|---|---|---|---|---|---|---|
| Age (yrs) | 1 pack per day smoker | |||||
| 55 | < 1% | 1% | 3% | 5% | NA | NA |
| 65 | < 1% | 2% | 4% | 7% | 7% | 10% |
| 75 | 1% | 2% | 5% | 8% | 8% | 11% |
| 2 pack per day smoker | ||||||
| 55 | < 1% | 2% | 4% | 7% | NA | NA |
| 65 | 1% | 3% | 6% | 9% | 10% | 14% |
| 75 | 2% | 3% | 7% | 10% | 11% | 15% |
Although some have suggested that former smokers undergo a reduction in lung cancer risk relative to current smokers, Bach et al concluded that the difference in risk between continuing smokers and former smokers appears to be explained almost entirely by differences in duration of smoking between the two groups.[20]
Since then, additional models have been developed to calculate an individual’s lung cancer risk.[21] [22]These models have the potential of selecting patients not only for screening, but also for enrolment into lung cancer chemoprevention strategies. The Spitz model was published in 2007, and the Liverpool Lung Project (LLP) model in 2008 (Cassidy et al, 2008).[21, 22] All 3 models include risk factors such as age, smoking duration and occupational exposure. The Spitz model also included physician-diagnosed emphysema, and family history of cancer in first-degree relatives. The Liverpool Lung Project multivariate risk model included smoking duration (never, 1–20 years, 21–40 years, 41–60 years, > 60 years), prior diagnosis of pneumonia, occupational exposure to asbestos, prior diagnosis of malignant tumor, and family history of lung cancer (never, early onset [< 60 years], late onset [> 60 years]).
Risk prediction models can be evaluated by several qualities: discrimination, calibration, accuracy, and clinical utility.[23] Discrimination is the ability of the model to differentiate between those individuals who will develop disease versus those who will not develop disease. Calibration is the ability of the model to predict the probability of an event in the subject. Accuracy is the ability to correctly predict the total number of affected individuals. Clinical utility is the ability of the model to guide clinical decisions based on its output. D’Amelio et al compared the performance of these three lung cancer risk prediction models using independent data for 3,197 patients with lung cancer and 1,703 cancer-free controls.[24] The Spitz and Liverpool Lung Project had similar abilities to discriminate between former and current smoking cases and controls (discriminatory power = 0.69), whereas the Bach model had significantly lower power (0.66; P=0.02). In terms of accuracy, the Spitz model had higher positive predictive value (PPV) than both the LLP and Bach models among both types of ever smokers, but the LLP model had higher negative predictive value (NPV). In terms of clinical utility, the Spitz model had the lowest false-positive rate for risk estimates >2.5%, whereas the LLP model had the highest false-positive rate. The LLP model correctly identified a higher proportion of lung cancer patients at all levels of risk than did the other models, but also incorrectly identified a higher proportion of controls as lung cancer patients. Although the analysis by D’Amelio et al showed that lung cancer risk-prediction models performed reasonably well when compared with each other in an independent validation set, the relatively low discriminatory power shows that there is still progress to be made in terms of lung cancer risk prediction.
Tammemagi et al developed and validated a lung cancer risk-prediction model involving former and current smokers in the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial control and intervention groups, and further modified it in a combined population of PLCO and NLST participants.[25] Their model showed that the risk of lung cancer increased with age, black vs. white race, lower socioeconomic status (determined according to the level of education), lower BMI, self-reported history of COPD, personal history of cancer, family history of lung cancer, current smoking, increased smoking intensity (the average number of cigarettes smoked per day) and duration, and, in former smokers, shorter time since quitting. Application of the NLST eligibility criteria to the PLCO intervention group-smokers would include 482 of 678 lung cancers in a total population of 37, 332, for an overall positive predictive value of 3.4% Application of the PLCO-M2012 criteria to the PLCO intervention group-smokers, on the other hand, would identify 563 of 678 lung cancers in the same population, for an overall positive predictive value of 4.0%.
Risk estimates have been used to design large-scale randomized control lung cancer screening trials, and can potentially decrease the cost of screening by targeting those at highest risk. There is the potential to further refine risk modeling by including genetic profiles, as well as the results of the baseline screening examination. Risk modeling could define not only who should be screened, but the frequency of follow-up.
CT SCANNING TECHNIQUES
LDCT should allow identification of lung nodules using as low a radiation dose as can reasonably be achieved (ALARA). This is possible through the use of lower tube currents (mA) and lower tube voltages (kVp). The NLST decreased radiation dose through lowering tube current. [26]Typical techniques in the NLST included a kVp of 120 (140 kVp for larger patients), a tube current-time product of 40 mAs or less, and a pitch of 1.5. Depending on the scanner, the effective current-time product (tube current–time products/scan pitch) was typically 20 – 30 mAs. [26][TABLE 4] This resulted in a mean effective dose of 1.4 milliSievert (mSv).[27] Nominal reconstructed section width was 1.0 to 3.2 mm, with reconstruction scan intervals of 1.0 to 2.5 mm. Scans were performed in full inspiration without intravenous contrast material. In the ITALUNG trial, acquisition parameters were 120–140 kVP, 20–43 mAs, and a pitch of 1- 2. Calculated radiation doses were similar to those experienced in the NLST, ranging from 1.2 mSv in patients with less Z-axis coverage, to 1.4 mSv in patients with greater scan lengths. [28] In the NELSON trial, the kVp was adjusted according to patient body weight (80–90 kVp for <50 kg, 120 kVp for 50—80 kg, and 140 kVp for >80 kg). The mAs settings were 20 to 30, and were adjusted accordingly dependent on the machine used.[29] In a follow-up study of patients from the NELSON trial with GGO nodules, exposure settings were 30 mAs at 120 kVp for patients weighing less than 80 kg and 30 mAs at 140 kVp for those weighing more than 80 kg. Axial images of 1.0-mm thickness were reconstructed at a 0.7-mm increment.[30]
TABLE 4.
Technical parameters for LDCT screening: NLST & NELSON techniques, and a BMI-based protocol.[10, 29, 31]
| NLST std patient |
NLST large patient |
NELSON < 50 kg |
NELSON 50–80 kg |
NELSON >80 kg |
BMI ≤ 30 |
BMI > 30 – 34.9 |
BMI > 35 |
|
|---|---|---|---|---|---|---|---|---|
| kVp | 120 | 140 | 80–90 | 120 | 140 | 110 | 110 | 110 |
|
Effective mAs (mAs/pitch) |
20–30 | 20–30 | 20–30 | 20–30 | 20–30 | 30 | 30 – 40 | 40 –50 |
| Slice thickness | 1.0 – 3.2 mm |
1.0 – 3.2 mm |
1.0 mm | 1.0 mm | 1.0 mm | 1.2 mm | 1.2 mm | 1.2 mm |
|
Reconstruct ion scan interval |
1.0 – 2.5 mm |
1.0 – 2.5 mm |
0.7 mm | 0.7 mm | 0.7 mm | 2.0 mm | 2.0 mm | 2.0 mm |
|
Mean effective dose |
1.2 mSv |
1.4 mSv | <1.6 mSv | < 1.6 mSv | < 1.6 mSv | 1.3 mSv |
1.3 – 1.6 mSv |
1.6 – 2.0 mSv |
Manowitz et al implemented a BMI-driven protocol for lung cancer screening in a group of nuclear weapons workers. [31]Their guidelines included 110 kVp in all patients, and 30 mAs for all patients with a BMI < 35. If the patient’s BMI was greater than 35 kg/m2, technologists were allowed to raise the tube current above 30 mAs to as high as 70 mAs based on the BMI value and how the participant’s weight was distributed, such as above or below the waistline. The mean effective dose was 1.3 mSv for the standard settings of 110 kVp and 30 mAs. Mean effective doses increased with increased tube currents: 1.6 mSv (40 mAs), 2.0 mSv (50 mAs), 2.5 mSv (60 mAs), and 3.2 mSv (70 mAs).
Although every effort should be made to screen at the lowest possible radiation dose, this must be balanced with nodule conspicuity. Lower tube current and/or kVp produces increased image noise, which can degrade nodule detection, particularly for ground glass nodules. In a chest phantom study of detection of ground glass nodules at lower tube currents, Funama et al found that ground glass nodules were difficult to detect at tube current-time products of 21 and 45 mAs (120 kVP, 5 mm slice thickness reconstructed at 1.0 mm intervals) in comparison with standard imaging at 180 mAs.[32] Newer technology, such as iterative reconstruction, may allow modification of image noise so that radiation doses can be reduced even further.
NODULE MEASUREMENT AND CHARACTERIZATION
The Fleischner Society Glossary of Terms for use in CT defined a nodule as a “small, approximately spherical, circumscribed focus of abnormal tissue”. In this regard, linear and rectangular opacities encountered at screening do not meet the criteria of pulmonary nodules. Nodules may not be perfectly spherical, however, so that a long axis and short axis can be measured. Although some investigators use the longer axis to define nodule size, the Fleischner Society guidelines endorse using the average of the two diameters [(long axis + short axis)/2]. [33]
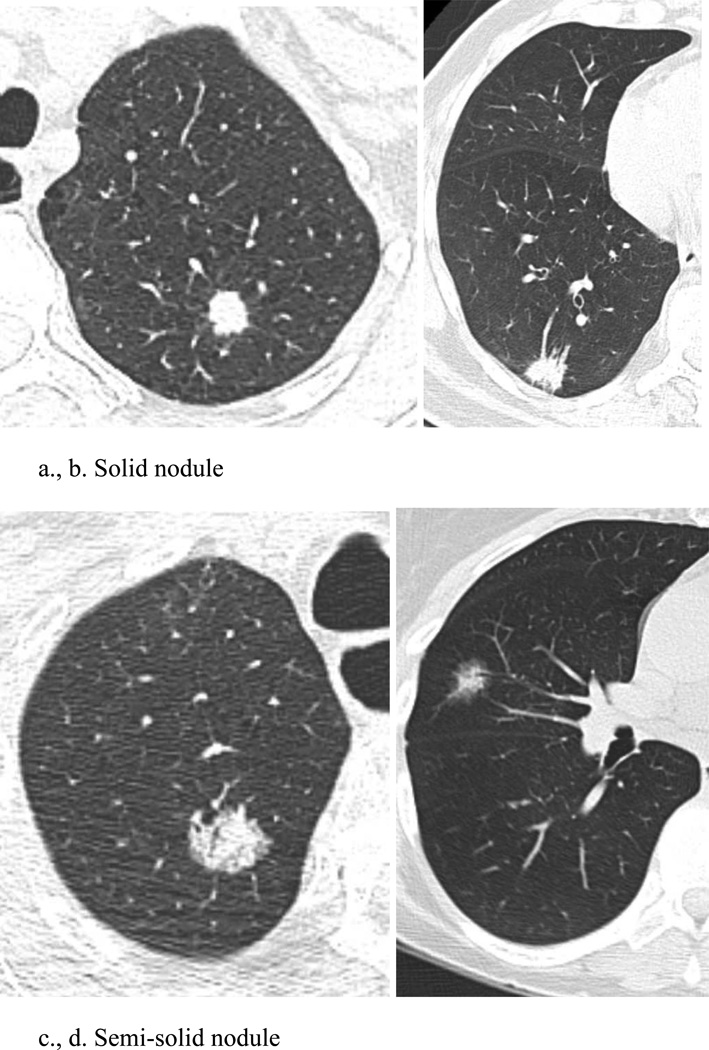
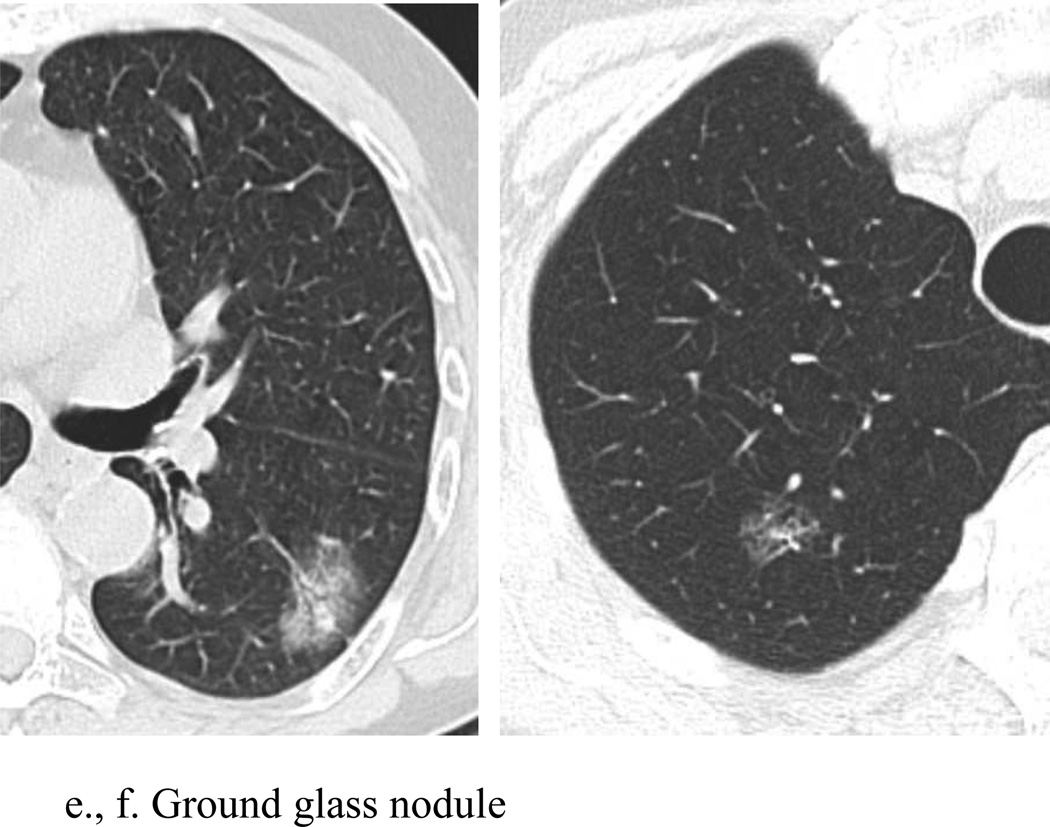
A pulmonary nodule can be characterized on CT as either solid (soft-tissue) attenuation, or subsolid.[TABLE 5] These subsolid nodules (SSNs) can be further categorized as either pure ground glass attenuation, through which the pulmonary vessels and bronchi may be seen, or semi-solid/part-solid, containing both ground glass and soft-tissue attenuation components. [FIGURE 1] Categorization of a pulmonary nodule into one of these three groups is important in that it predicts the likelihood of malignancy, and defines further management of the nodule. Optimal classification requires thin-section (< 2.5 mm) CT imaging, so that measurement of size and attenuation is accurate and reproducible.
TABLE 5.
Nodule characteristics
| SOLID | SEMI-SOLID | GROUND GLASS | |
|---|---|---|---|
| Appearance | Focal area of increased attenuation which completely obscures lung parenchyma |
Mixed solid and ground glass components |
Focal area of increased attenuation through which vessels and bronchi remain visible |
|
Likelihood
of malignancy |
7 – 11% | 48 – 63% | 18 – 59% |
|
VDT, days,
for malignant nodules |
149 | 457 | 813 |
Figure 1.
Examples of solid, semi-solid, and ground glass nodules
Even in high-risk patients, most lung nodules identified at screening will be benign. A number of features can be used to distinguish a nodule as benign or likely benign. [TABLE 6] The classic calcification patterns of benign nodules include uniform dense calcification, a laminated pattern, central calcification, and popcorn calcification.[34] Punctate calcification or eccentric calcification should prompt further work-up, however, as these patterns may be seen in malignant lesions. Diederich et al reported in 2002 that 14.3% of all nodules encountered in a lung cancer screening trial in Germany showed homogeneous calcification.[35] This prevalence can be expected to vary, due to geographic variations in histoplasma and tuberculous infections.
TABLE 6.
Characteristics of benign nodules
| Attenuation | Calcification: central, lamellar, solid or popcorn |
| Shape | Polygonal |
| Margin | Very smooth |
| Location | Perifissural |
| Vascular attachment | |
| Aspect ratio | Long axis: short axis diameter ratio > 1.78 |
| Growth rate | No growth over at least 2 years (solid nodules only) |
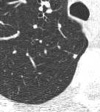
The location of a nodule with respect to the pleural surface is also helpful for nodule characterization. Ahn et al reported that up to one-third of all non-calcified nodules identified at screening are located adjacent to interlobar fissures, calling these nodules “perifissural”. [36] They described perifissural nodules (PFN) as well-circumscribed, smoothly marginated nodules in contact with or closely related to a fissure.[FIGURE 2] The PFNs were usually triangular or oval in shape, often showed a septal attachment, and were usually located below the level of the carina. After 7½ years of follow-up, no PFN had developed into a lung cancer. In the NELSON trial, de Hoop et al classified homogeneous solid nodules, attached to a fissure with a lentiform or triangular shape, as PFNs. At baseline screening, 19.7% of nodules were perifissural, with a mean size of 4.4 mm (range: 2.8–10.6 mm) and a mean volume of 43 mm3 (range: 13–405 mm3). None of the perifissural nodules were found to be malignant at follow-up, even though 15.5% demonstrated growth on subsequent CT.[37]
Figure 2.
Perifissural nodule. Perifissural nodules (PFN) are well-circumscribed, smoothly marginated nodules in contact with or closely related to a fissure. These are commonly encountered at LDCT for lung cancer screening, and are likely benign. This PFN was unchanged in 7 years of follow-up.
Other characteristics of benign nodules include a long axis to short axis ratio of > 1.78, and vascular attachment.[38]
The most compelling sign of benignity for solid nodules, however, is lack of growth on serial scanning over at least two years.[33] Slattery et al reported their experience with long-term follow-up of non-calcified pulmonary nodules (NCNs) less than 10 mm in diameter, discovered at the time of CT screening for lung cancer in Dublin, Ireland.[39] Eighty-three of 449 participants had NCNs smaller than 10 mm in diameter which were stable after two years of follow-up. The majority of the nodules in their series (132/141) were solid. Seven years after baseline screening, these patients were re-imaged using LDCT to assess for interval nodule growth. NCNs were unchanged in 78 subjects, had decreased in size in 4 subjects, and had shown interval growth (from 6 to 9 mm) in 1 subject. An additional two years of follow-up in that individual showed no further nodule growth. Slattery et al concluded that solid NCNs which are unchanged or decreasing in size during follow-up for a minimum of 24 months can be considered benign.
Nodules that do not exhibit these benign characteristics should be assumed to be potentially malignant, and further evaluation is warranted. Henschke et al reported a higher frequency of malignancy in part-solid and non-solid nodules in comparison with solid nodules.[40] The frequency of malignancy in solid nodules was 7%, compared with a 63% incidence (10/16) in part-solid nodules, and 18% (5/28) in non-solid, or ground glass, nodules.[TABLE 7] In their series, the majority (81%) of nodules were solid, with part-solid nodules representing 7% and non-solid nodules representing 12%. Li et al found a similar rate (11%) of malignancy in solid nodules in their analysis of 222 nodules from a lung cancer screening program in Nagano, Japan. [41] Mixed (part-solid) nodules occurred more commonly in their population, with 25% of the nodules determined to be part-solid. Almost half (48%) of these nodules were malignant. The rate of malignancy in ground-glass nodules was higher than in the Early Lung Cancer Action Program (ELCAP) population, with 59% (17/29) determined to be malignant.
TABLE 7.
| Solid nodules incidence |
Solid nodules malignant |
Semi-solid nodules incidence |
Semi-solid nodules malignant |
Ground glass nodules incidence |
Ground glass nodules malignant |
|
|---|---|---|---|---|---|---|
|
Henschke et al |
189/233 (81%) |
7% | 16/233 (7%) |
63% | 28/233 (12%) |
18% |
| Li et al | 137/222 (62%) |
11% | 56/222 (25%) |
48% | 29/222 (13%) |
59% |
CT features of GGNs can be used to help distinguish invasive adenocarcinomas from preinvasive lesions. To determine discriminating features of invasive adenocarcinomas, Lee et al retrospectively evaluated 64 pure GGNs and 208 part-solid GGNs in 253 patients. Pathologic confirmation identified 179 invasive pulmonary adenocarcinomas and 93 preinvasive lesions (21 atypical adenomatous hyperplasias and 72 adenocarcinomas in situ).[42] They found that, in pure GGNs, a lesion size of less than 10 mm was a very specific discriminator of preinvasive lesions from invasive pulmonary adenocarcinomas. Multivariate analysis of pure GGNs revealed that lesion size was the single significant differentiator of preinvasive lesions from invasive pulmonary adenocarcinomas. The optimal cut-off size for preinvasive lesions of less than 10 mm yielded a sensitivity of 53.33% and specificity of 100%. In part-solid GGNs, preinvasive lesions were accurately distinguished from invasive pulmonary adenocarcinomas by a number of factors, including smaller lesion size, smaller solid proportion, nonlobulated border, and nonspiculated margin.
In 2011, an international core panel of experts representing the International Association for the Study of Lung Cancer, American Thoracic Society, and European Respiratory Society published a revised classification of adenocarcinoma of the lung.[43] They introduced two new concepts, adenocarcinoma in situ (AIS) and minimally invasive adenocarcinoma (MIA), to describe small solitary adenocarcinomas with either pure lepidic growth (AIS) or predominant lepidic growth with <5 mm invasion (MIA). Patients with these lesions could be expected to have 100% or near 100% disease-specific survival, respectively, following complete resection. AIS, along with atypical adenomatous hyperplasia (AAH), are considered preinvasive lesions. The term AIS replaced the traditional terminology of Bronchioloalveolar cell carcinoma (BAC) for lesions < 3 cm in diameter.
From an imaging perspective, AAH is the earliest preinvasive lesion for lung adenocarcinoma that is detectable by thin-section CT. This term should be used for faintly seen, pure GGNs < 5 mm in size. The lesions of AIS are slightly more conspicuous than the faint GGNs of AAH. These nodules may be pure ground glass, part-solid, or bubble-like, and are generally smaller than 2 cm in diameter. AIS exhibits very slow growth on follow-up. Although imaging appearances are variable, MIA nodules may be part-solid, with a predominant ground-glass component and a small central solid component measuring 5 mm or less.[43] MIA lesions are also typically smaller than 2 cm in diameter.
GROWTH RATES OF NODULES
Measuring changes in the size of a nodule smaller than 10 mm in diameter is challenging. The size of a nodule can be indicated by (1) the longer diameter, (2) the average of the long axis and short axis diameters, (3) the area of the nodule [(π/4) (long axis multiplied by the short axis diameter)], which assumes a round nodule), or (4) the volume of the nodule. The Fleischner Society and the NCCN guidelines both endorse using the average of the long axis and short axis diameters, although there is increasing evidence that volume measurements are superior to 2D measurements in recognizing nodule growth. Ko et al have reported that growth rates suggestive of malignancy may be detected sooner using 3D volumetric analysis of both solid and subsolid nodules than by measurement of longer diameter with electronic calipers.[44] The apparent growth rate (GR) measured at any two points in time (T1, T2) was computed as
where V1 = the volume of the nodule at the first time point and V2 = the volume of the nodule at the second time point. For example, if we assume a spherical nodule 4 mm in diameter, the volume of the nodule is calculated from the formula , so that the volume of a 4 mm diameter nodule is 268 mm3. A 25% increase in diameter over the course of one year, from 4 to 5 mm, produces approximately 100% increase, or doubling, in volume, and the volume is now 523 mm3. The growth rate GR would be calculated as [100%(523-268)/268(1 year)], or roughly 100% per year.
Growth rate estimates may be less variable than measurements of volume doubling times, where
VDT is the time interval between the number of doublings, and is based on an exponential growth model. In our example of a 4 mm nodule increasing in size to 5 mm over the course of 365 days, the VDT would be calculated as [365 times log 2 divided by log (523/268)] or roughly 365 days. On-line calculators such as http://www.ldn4cancer.com/cancer-doubling-calculator.html are available to facilitate calculation of VDT. Calculations of volume doubling times are useful in the prediction of likelihood of malignancy for solid nodules. VDTs were calculated for 99 solid nodules and 12 subsolid nodules in the I-ELCAP database, and the median VDT was determined to be 98 days.[45] Of the 99 solid nodules, 85 had VDTs less than 200 days, 14 had VDTs between 200 and 399 days, and none were found to have VDTs longer than 400 days.
The nodule management strategy of the NELSON trial was based on initial nodule volume and also on volume-doubling time assessment. [29] Any participant with a nodule greater than 500 mm3 in volume on the baseline CT was referred for workup and diagnosis. If the nodule was intermediate in size, defined as between 50 and 500 mm3, a short-term follow-up CT was performed to evaluate growth. If the nodule exhibited a volume doubling time of less than <400 days on this short-term follow-up CT, the patient was also referred for workup and diagnosis. These investigators subsequently determined that the optimal cut-off for VDT was 232 days, rather than 400 days, and using this threshold would have resulted in fewer false positive referrals.[46]
Measuring a change in the size of a nodule between two time points raises the issue of reproducibility of nodule measurement, and interobserver variability. The interobserver variability in assessing maximal diameter of ground glass nodules is greater than that of solid nodules. Kakinuma et al evaluated the interobserver variability when 11 radiologists measured 10 ground glass nodules and determined that an increase in the length of the maximal diameter of the nodule of more than 1.72 mm would be necessary in order to state that the maximal diameter of a particular GGO had actually increased. [47]
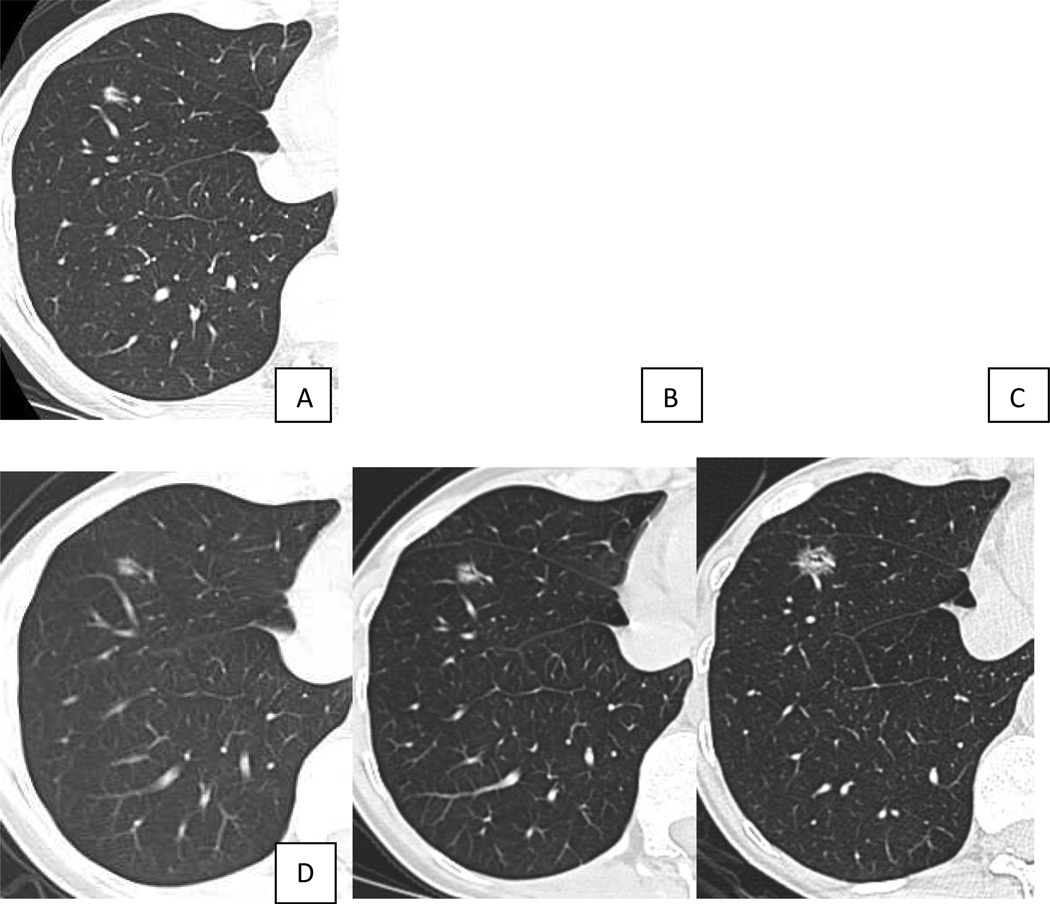
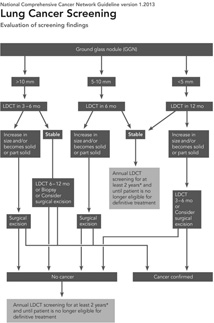
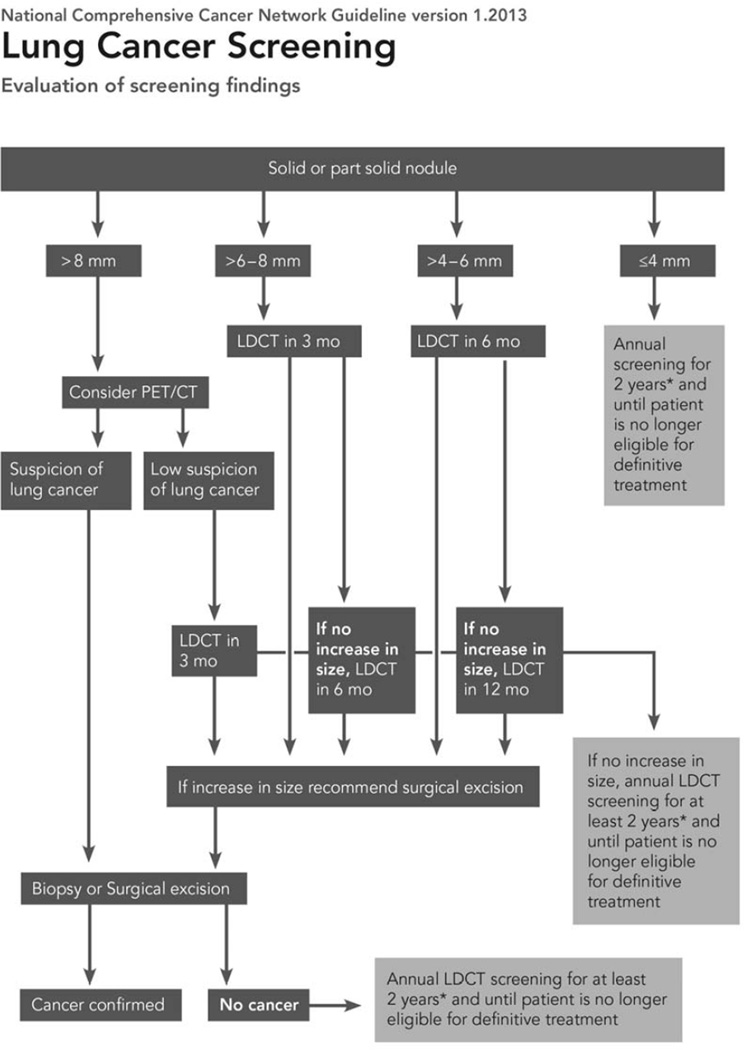
VDTs of malignant semi-solid and ground glass nodules can be significantly longer than those encountered in malignant solid nodules. Hasegawa et al reported VDTs of 813 days for pure ground glass malignancies, 457 days for semisolid malignant nodules, compared with 149 days for solid malignant nodules.[48] The slow growth rate of pure ground glass nodules mandates a different algorithm for management in comparison with solid and semi-solid nodules. [49–51][FIGURE 3, 4] Although 2 years of stability may be sufficient for solid nodules, longer follow-up is necessary for ground glass nodules. Godoy and Naidich recommend follow-up with LDCT for at least 3 to 5 years. [50] Either an increase in nodule size or density can indicate conversion from a premalignant lesion to an invasive adenocarcinoma.
Figure 3.
Slowly growing ground glass nodule. Ground glass nodule in the anterior basilar segment of the right lower lobe in (a) October 2003, (b) April 2004, and (c) January 2006 had shown no growth in over 2 years. Repeat chest CT in October 2011 showed interval growth of the ground glass nodule, suggesting malignancy. Wedge resection of the nodule revealed adenocarcinoma in situ, non-mucinous.
Figure 4.
National Comprehensive Cancer Network guidelines for nodule management.
Reprinted with permission from Wood, D.E., et al., Lung cancer screening. J Natl Compr Canc Netw, 2012. 10(2): p. 240-65.[15]
De Hoop et al propose using a new measurement, nodule mass, to evaluate growth of ground glass nodules.[30] They define nodule mass as nodule volume multiplied by the physical density of the nodule (CT attenuation of the nodule in HU plus 1,000). In their analysis, there was less variability among readers in measurement of nodule mass as compared with manual measurements of nodule volume or diameter.
PATIENT MANAGEMENT
Although CT screening for lung cancer may be effectively performed within the framework of a diagnostic radiology department, the patient with positive findings, either pulmonary nodules or incidental findings, is best cared for within a multidisciplinary clinic. The clinic should include representatives from a number of specialties, including pulmonary medicine, cardiology, thoracic surgery, interventional radiology, and smoking cessation. This will allow appropriate nodule follow-up, either with serial CT imaging, PET-CT or biopsy, as well as management of incidental findings and other smoking-related diseases, such as COPD or cardiovascular disease. Nodule follow-up should be performed following established guidelines, such as those developed by NCCN, for solid, semi-solid, and ground glass nodules. [FIGURE 4]Information about smoking cessation should be provided to all current smokers at the time of screening, but the multidisciplinary clinic affords a second opportunity to counsel patients about the benefits of quitting smoking. If a nodule is found to be malignant at biopsy, then further evaluation by a multidisciplinary thoracic oncology clinic, which includes medical oncology, radiation oncology, thoracic surgery, pathology and diagnostic radiology, can allow the patient to make an informed decision about his/her care.
INCIDENTAL FINDINGS
CHRONIC OBSTRUCTIVE PULMONARY DISEASE
Respiratory illness, and particularly chronic obstructive pulmonary disease (COPD) is a major cause of chronic morbidity and mortality in the lung cancer screening population. COPD is currently the fourth leading cause of death in the world, and this ranking can be expected to rise even higher over the next decade with the aging of the population, and increased exposure to risk factors such as cigarette smoking. Thun et al reported a relative risk of death from COPD of 25.61 in male current smokers, compared with male never-smokers, and a similar relative risk (22.35) in women current smokers compared with women never-smokers. [5] COPD is underdiagnosed in the general population, and participants in screening trials uncommonly self-report a history of COPD. [52, 53] Earlier recognition of COPD may play a role in both smoking cessation and in earlier treatment. Although the diagnosis of COPD is based on spirometry, screening spirometry is not currently recommended. In this regard, recognition of CT features of COPD can contribute to an earlier diagnosis of this disease.
COPD is increasingly recognized as a heterogeneous disease, and multiple phenotypes have been described, which can be used to predict likelihood of acute exacerbations and mortality. In addition to clinical phenotypes, imaging-based phenotypes of COPD have been described. These include emphysema-predominant COPD, airway-predominant COPD, and a mixed phenotype. This can be assessed either qualitatively or quantitatively. Quantitative CT metrics of COPD include measurements of emphysema severity, airway wall thickness, and air-trapping.
Emphysema and airway-predominant COPD can both be detected on LDCT. An ancillary study of the Dutch and Belgian Lung Cancer Screening Trial included low-dose inspiratory and expiratory CT scans and spirometry.[54] In 1,140 men, COPD risk scores were calculated from quantitative CT data and patient characteristics, and correlated with same-day pulmonary function testing. The CT emphysema score was defined as the percentage of voxels on inspiratory CT with attenuation values below −950 HU. The CT airtrapping score was defined as the expiratory:inspiratory ratio of mean lung density. COPD was characterized as mild obstruction if the FEV1 was ≥80% than predicted (GOLD stage 1), moderate obstruction if the FEV1was ≥50% and <80% of predicted (GOLD stage 2), and severe obstruction if the FEV1 was <50% predicted (GOLD stages 3 and 4). Of the 437 patients (38%) diagnosed with COPD on the basis of pulmonary function testing, 63% were classified as mild obstruction, 31% as moderate obstruction, and 6% as severe obstruction. Only 41 of the 1,140 participants (3.6%) self-reported physician-diagnosed emphysema and 93 (8.2%) self-reported bronchitis. The final model, which included 5 factors independently associated with obstructive pulmonary disease: CTemphysema, CT air trapping, BMI, pack-years, and smoking status (current vs. former), predicted COPD with 78% accuracy (sensitivity 63%, specificity 88%, PPV 76%, NPV 79%). Diagnosis in this population did require additional CT scanning in exhalation, with associated increase in radiation dose. The combined inhalation and exhalation scans yielded an estimated effective dose of 1.2 to 2.0 mSv, of which 0.3 to 0.65 mSv was accounted for by the exhalation scan.
The relationship between emphysema and lung cancer is uncertain. A recent meta-analysis of 7 studies, including 7368 subjects (2809 with emphysema on CT and 870 with a diagnosis of lung cancer) reported an adjusted odds ratio for lung cancer in the presence of emphysema on CT of 2.11 (95% CI 1.10–4.04). Smith et al reported that emphysema detected visually on CT was independently associated with significantly increased odds of lung cancer but this was not the case with quantitative assessment of emphysema.[55] Visual detection of emphysema yielded an OR of 3.50 (95% CI 2.71–4.51), whereas CT densitometry yielded an OR of 1.16 (95% CI 0.48–2.81). Detection of COPD at the time of lung cancer screening may play a role in the future in developing risk-based guidelines for patient follow-up.
CORONARY ARTERY CALCIFICATION
Cigarette smoking is a risk factor for atherosclerotic coronary artery disease. Coronary artery calcification (CAC) is predictive of coronary artery disease and can be detected on LDCT performed for lung cancer screening. Jacobs et al performed a case-control study of 958 participants in the NELSON trial, and showed that CAC is a strong and independent predictor of all-cause mortality and cardiovascular events in current and former smokers 50 years and older.[56] Using the Agatston scoring method for CAC, subjects were considered very low risk (CAC = 0), low risk (CAC = 1 –100), moderate to high risk (101 – 1,000), and very high risk (CAC >1,000). Multivariate adjusted hazard ratios (HR), adjusted for age, sex, smoking, hypertension, hypercholesterolemia, and diabetes, for subjects with CAC scores of 1–100, 101–1,000, and more than 1,000 were 3.00 (95% CI, 0.61–14.93), 6.13 (95% CI, 1.35–27.77), and 10.93 (95% CI, 2.36–50.60), respectively. Multivariate-adjusted HRs for coronary events were 1.38 (95% CI, 0.39–4.90), 3.04 (95% CI, 0.95–9.73), and 7.77 (95% CI, 2.44–24.75), respectively. Adding CAC scoring in the interpretation of LDCT for lung cancer screening can be used to identify patients at risk for cardiovascular events, who might benefit from preventive therapy such as antihypertensive or lipid-lowering medication.
McEvoy et al evaluated the relationship of smoking, CAC and all-cause mortality in a study cohort of 44,042 asymptomatic individuals referred for noncontrast cardiac CT at 3 different centers in the US. [57] The average age was 54 (+/−) 10 years. Approximately 14% of subjects were smokers (6,020 smokers, and 38,022 nonsmokers). Subjects were followed for a mean of 5.6 years. The primary endpoint was all-cause mortality. In concordance with other studies, they found that smokers with any CAC had significantly higher mortality than smokers without CAC. Hazard ratios for all-cause mortality in smokers with CAC (as compared with the reference group of smokers with CAC = 0) were 2.04 in the group with CAC = 1 – 100, 2.57 in the group with CAC = 101 – 400, and 4.25 in the group with CAC > 400. However, they also suggested that the absence of CAC might not be useful as a “negative risk factor” in current smokers. Smokers with CAC = 0 had an all-cause mortality rate of 3.31 deaths/1,000 person-years, in contrast to 0.67 deaths/1,000 person-years for nonsmokers with CAC = 0.
Agatston scoring may not be required for cardiovascular event risk prediction on LDCT screening. Shemesh et al reported their results with a visual scoring system for CAC. [58] Calcification in each of 4 coronary arteries (left main, left anterior descending, circumflex, and right) was categorized as absent (0), mild (1), moderate (2), or severe (3), based on the extent of vessel involvement. If less than one-third of the length of the entire artery showed calcification, the disease was categorized as mild, moderate when one-third to two-thirds of the artery showed calcification, and severe when calcification was visible in more than two-thirds of the artery. Each subject received a CAC score ranging from 0 to 12. With a CAC score of 0 as the reference group, a CAC score of at least 4 was a significant predictor of cardiovascular death (odds ratio [OR] 4.7; 95% confidence interval: 3.3, 6.8; P < .0001).
OTHER CANCERS
It is intuitive that lesions within the thyroid gland, breasts, mediastinum, liver, kidneys and adrenal glands may be visible on unenhanced LDCT of the chest. The cost-effectiveness of evaluating these abnormalities remains a subject of study. The risks and costs associated with evaluating additional findings on LDCT must be weighed against the benefit of detecting clinically significant abnormalities. The frequency of extrapulmonary tumors on LDCT performed in a high risk population is typically less than 1%.[9, 59, 60] Priola et al reported finding six malignancies (thymoma, renal cell carcinoma, adrenal metastasis) in their study population of 519 current and former smokers aged 55 and older.[59] Incidental findings were reported in 307 patients (59.2%) at baseline screening, with a mean of 1.49 findings per participant. Of these 307 participants, 63 (20.5%) had 64 clinically relevant incidental findings. Based on national reimbursement rates in Italy, the authors calculated that the work-up of incidental findings was U.S. $15.85 per patient for all screening rounds (U.S. $12.67 at baseline examination, and U.S. $3.18 at annual follow-up for 5 years). They concluded that a substantial portion of the total cost was directed toward diagnosing lesions that were truly important, and that the workup of abnormal incidental findings seemed economically feasible.
Within the COSMOS (the Continuous Observation of Smoking Subjects) study in Milan, Italy, 5,201 asymptomatic heavy smokers aged 50 years or older underwent annual LDCT for 5 consecutive years.[61] After 5 years of CT screening, 27 unsuspected extrapulmonary malignancies were diagnosed, representing 0.5% (27 of 5201 subjects; 95% CI: 0.34%, 0.75%) of volunteers enrolled. These included renal cell carcinoma (n = 7), lymphoma (n = 5), thyroid cancer (n = 3), thymoma (n = 2), pancreatic tumor (n = 2), schwannoma (n = 1), hepatocellular carcinoma (n = 1), gastrointestinal stromal tumor (n = 1), prostate cancer (n = 1), urinary tract tumor (n = 1), breast cancer (n = 1), adrenal gland tumor (n = 1), and ovarian cancer (n = 1). The economic aspects of the diagnostic work-ups were not addressed.
REPORTING RESULTS
McKee et al created a standardized CT lung screening reporting system (LungRADS) modeled on BI-RADS®.[62] This incorporated the NCCN guidelines into categories for nodule description and management, and also included a category (S) for clinically significant incidental findings. [TABLE 8] A structured reporting system such as this has many potential advantages, including improved adherence to recommended guidelines for nodule management, and improved communication of examination results to health care providers. Additionally, it allows automatic generation of results-specific patient notification letters, and facilitates structured database storage and tracking of findings.
TABLE 8.
Structured reporting of findings on lung cancer screening CT (Reprinted from J Am Coll Radiol, McKee, B.J., et al., Initial Experience With a Free, High-Volume, Low-Dose CT Lung Cancer Screening Program, epub ahead of print, 2013, with permission from Elsevier).[62].
| CATEGORY | FINDINGS | RECOMMENDATION |
|---|---|---|
|
LungRADS1: Negative |
|
Next LDCT in 12 months |
| LungRADS2:Benign |
|
Next LDCT in 12 months |
|
LungRADS
3: Positive, likely benign (< 4% chance of malignancy) |
|
Next LDCT in 3 – 6 months |
|
Next LDCT in 6 – 12 months |
|
|
next LDCT in 1- 2 months, consider antibiotics |
|
|
LungRADS
4: Positive, suspicious for malignancy (> 4% chance of malignancy) |
|
Pulmonary consultation advised |
|
LungRADS 5:
Known cancer |
||
|
Significant
incidental findings "Category S": Positive(P) or Negative(N) |
|
BARRIERS TO SCREENING
FINANCIAL COSTS
The mortality benefit of screening can only be realized if individuals at risk actually participate in screening programs. Jonnalagadda et al surveyed 108 individuals at risk for lung cancer, of which 40% were Black and 34% were Hispanic regarding their attitudes about lung cancer screening.[63] The majority (82%) would undergo CT screening for lung cancer if recommended by their physician, a number that was similar across racial and ethnic groups (76% non-minority, 90% black, 77% Hispanic; p = 0.19). However, only 32% would undergo screening if it were at their own expense. This percentage was even lower for Hispanics (15%). Financial costs are a huge barrier to screening, especially when downstream costs are considered. Reimbursement by third party payers, and especially by the Centers for Medicare and Medicaid Services (CMMS) will alleviate this to some extent. Concerns about radiation effects and the discomfort of the screening process were also correlated with reluctance to undergo screening. Individuals who held fatalistic beliefs towards developing lung cancer (e.g., “If I develop lung cancer, I am not supposed to know why, I am just supposed to accept it,”) were also less likely to undergo screening.
RISKS ASSOCIATED WITH CT SCREENING
RADIATION EXPOSURE
The primary concern with the radiation dose from CT screening is the possibility of radiation-induced carcinogenesis. [64, 65] Although the initial screening CT is performed at a low dose, subsequent examinations to work up positive findings contribute to the patient’s lifetime exposure. Although the NLST results were based on scanning annually for three years, it can be expected that annual screening might continue for decades in a current smoker, so that the cumulative dose of even these low-dose scans contributes risk.
Larke et al calculated the effective dose associated with a single screening LDCT examination of average-size participants in the NLST, as well as individual organ doses for both males and females. [27] The CT effective dose was calculated using the following formulas:
where dose length product = CT dose indexvolume x scan length, and k = 0.014 mSv/mGy-cm. A typical chest CT scan length of 35 cm was used. These calculations yielded an average effective dose from one screening CT of 1.4 mSv (standard deviation = 0.5 mSv). This can be compared with the average effective dose for a standard chest CT examination, which is estimated to be 7 mSv (range, 4–18 mSv). [66] The investigators used CT-Expo software to calculate organ dose. This software takes into account scanner model, body part scanned, length of scan, and technique factors (i.e., collimation, mAs, pitch, kVp) to calculate CTDIvol and organ dose. Individual organ doses ranged from zero to nearly 5 mGy, with the greatest dose to the female breast. The significant dose to the breasts of the adult female (4.9 mGy vs near zero for male) was the primary factor distinguishing the organ doses between the two genders. The dose to the female breast from screening CT is comparable to two-view digital mammography and screen-film mammography, which deliver average mean glandular radiation doses of 3.7 and 4.7 mGy, respectively.[67] Annual screening mammography (either digital or screen-film) performed in women aged 50–80 years has been associated with a lifetime attributable risk of fatal breast cancer of 10–12 cases in 100,000.
It has long been thought that the risk of radiation-induced malignancy diminishes with increasing age of exposure, not only because children have a greater number of dividing cells, and are therefore more radiosensitive, but also because they have a longer life expectancy and therefore more years to develop a cancer. More recently, it has been determined that radiation risks after exposure in younger individuals are dominated by radiation-induced premalignant cells (initiation processes), whereas radiation risks after exposure at later ages are more influenced by promotion of preexisting premalignant cells. Analyses of Japanese atomic bomb survivors suggest that the radiation-related excess relative risk (ERR) for cancer induction decreases with increasing age at exposure only until exposure ages of 30–40 years. At older ages at exposure, the ERR may actually increase for many individual cancer sites. For radiation-related breast cancers, initiation processes are thought to be the predominant factor, whereas for radiation-related lung cancer, promotion of preexisting premalignant cells may dominate. Radiation-induced breast cancer risks decrease therefore, with age at exposure at all ages, but radiation-induced lung cancer risks do not. [68] Shuryak et al suggest that the excess lifetime risks of lung cancer may peak at around age 50 years, the most likely age for individuals to undergo CT screening.
The increased risk of fatal lung cancer from annual LDCT must be weighed against the inherent likelihood of fatal lung cancer related to the individual’s smoking history and the benefit gained from early detection of disease.
OVERDIAGNOSIS
Overdiagnosis is the detection of indolent/occult disease that would not otherwise have become clinically significant/impacted patient outcome. This is an inherent part of any screening program. The downside of overdiagnosis is that it may cause unnecessary morbidity (and mortality in rare cases), cost, anxiety, and labels a patient with a disease that otherwise would never have been detected.[69, 70] Overdiagnosis is difficult to measure, even in a controlled trial. Within the NLST, there were an excess number of lung cancers in the CT arm relative to the CXR arm. There were 110 bronchioloalveolar cell carcinomas in the CT arm, 95 of which were screen-detected.[10] In the CXR arm, there were only 35 bronchioloalveolar cell carcinomas, 13 of which were screen-detected. Were these evidence of overdiagnosis, or would they have progressed to invasive adenocarcinomas within the lifetimes of these patients? Although there was a median follow-up period of 6.5 years in the NLST, perhaps this is insufficient when the cancers grow so slowly. Veronesi et al determined that 10.8% of lung cancers detected at screening were indolent, with VDTs greater than 600 days, and 15% were slow-growing with VDTs in the 400 – 599 day range.[70] This is similar to the results in the Mayo lung cancer screening trial, in which 13 of 48 (27%) had VDTs greater than 400 days. [71] Henschke et al reported that only 3% of lung cancers in the international –ELCAP had VDTs greater than 400 days.[72] Hazelton et al assume indolent cancers initially grow like other cancers, but their growth slows down and perhaps stops, so that the tumors reach a maximum diameter of about 1.5 cm, rather than continuing exponential growth. [73] They estimate that approximately 33% of the CT detected cancers diagnosed among females during baseline and first annual CT screens are indolent, compared with approximately 7% among males. As we move forward with lung cancer screening on a clinical basis, we need to be able to recognize which tumors are indolent, and develop different treatment guidelines for these tumors.
SMOKING BEHAVIOURS
There is concern that screening could change patients’ attitudes towards smoking, giving them an opportunity to continue or resume smoking. An opposing, but optimistic, view is that CT screening would improve quit rates by increasing patient awareness of the harms of smoking, and providing a “teachable moment” for altering smoking behaviors. In reality, there is little evidence to support either side. The Dutch–Belgian Randomised Controlled Lung Cancer Screening Trial (NELSON trial) evaluated smoking behaviors two years after randomization in 550 male current smokers who had received negative screening results, and in 440 male current smokers who had received indeterminate test results. [74] Although smokers with an indeterminate test result reported more quit attempts, the prolonged abstinence rate in smokers receiving a negative test (8.9%) was comparable with the abstinence rate in smokers with one or more indeterminate results (11.5%). The quit rates of those in the experimental (CT) arm of the Danish Lung Cancer Screening Trial were compared with the quit rates of the control (no imaging) arm at one year follow-up, and were found to be similar (11.9% vs 11.8%). [75]
Smoking behaviors are complex, and screening is just one variable in the puzzle. Participants in the Prostate, Lung, Colon and Ovarian (PLCO) Cancer Screening trial completed a baseline questionnaire at trial enrollment and a supplemental questionnaire 4–14 years after enrollment. Of the 31,694 former smokers on the baseline questionnaire, 1,042 (3.3%) had relapsed, and resumed smoking. [76] Of the 6,807 current smokers on the baseline questionnaire, 4,439 (65.2%) reported continued smoking. Relapse was more likely among those who were younger at completion of the baseline questionnaire, black or Hispanic, less educated, unmarried, and those with a lower income, a lower BMI, or no family history of lung cancer. Tobacco-related variables were also associated with relapse, including more secondhand smoke exposure, fewer cigarettes smoked per day, more pack-years, and smoking light or ultralight cigarettes or pipes or cigars. The same variables were associated with higher likelihood of continued smoking by those who were current smokers at baseline.
Nevertheless, any lung cancer screening program should be closely affiliated with a smoking cessation program to utilize this “teachable moment” and hopefully alter smoking behavior in its participants.
FALSE POSITIVES
Within the NLST, 96% of the positive results in the LDCT arm were false positives.[10] In the majority of cases, positive screens were managed with at least one follow-up CT to determine stability of the pulmonary nodules. False positive results can be reduced when prior imaging is available. After two rounds of screening, there are fewer false positives as a result of comparison with the baseline screening CT, which may demonstrate two years of nodule stability. Reducing the number of false positive screens is an area for future research. An ongoing trial, Detection of Early Lung Cancer Among Military Personnel Study 1 (DECAMP-1), is designed to improve the efficiency of the diagnostic follow-up of patients with indeterminate pulmonary nodules. In this trial, 500 smokers with indeterminate pulmonary nodules (0.7cm-2cm) on chest CT will undergo fiberoptic bronchoscopy and be followed for two years, to determine whether biomarkers for lung cancer diagnosis that are measured in minimally invasive biospecimens are able to distinguish malignant from benign pulmonary nodules. This trial will continue through 2016.
CONCLUSION
The announcement of the results of the NLST, showing a 20% reduction in lung-cancer specific mortality with LDCT screening in a high risk population, marked a turning point in lung cancer screening. This was the first time that a randomized controlled trial had shown a mortality reduction with an imaging modality aimed at early detection of lung cancer. This is not the end of the story, however. There are improvements to be made, not only in the selection of the screening population, but also in management of imaging findings. As with screening for other malignancies, screening for lung cancer with LDCT has revealed that there are indolent lung cancers which may not be fatal. More research is necessary if we are to maximize the risk-benefit ratio in lung cancer screening.
KEY POINTS.
The National Lung Screening Trial reported a 20% reduction in lung-cancer specific mortality with LDCT in a high risk population of current and former smokers, ages 55 to 74, with at least 30 pack-years of smoking history.
Screening centers should adhere to published guidelines for determining the population to be screened, CT screening techniques and management of screening findings.
Future directions for lung cancer screening include (1) improved patient selection with modeling and risk stratification, (2) identification and management of indolent tumors, and (3) reduction in the number of false positive screening examinations.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Henschke CI, et al. Early Lung Cancer Action Project: overall design and findings from baseline screening. Lancet. 1999;354(9173):99–105. doi: 10.1016/S0140-6736(99)06093-6. [DOI] [PubMed] [Google Scholar]
- 2.Kaneko M, et al. Peripheral lung cancer: screening and detection with low-dose spiral CT versus radiography. Radiology. 1996;201(3):798–802. doi: 10.1148/radiology.201.3.8939234. [DOI] [PubMed] [Google Scholar]
- 3.Sone S, et al. Mass screening for lung cancer with mobile spiral computed tomography scanner. The Lancet. 1998;351(9111):1242–1245. doi: 10.1016/S0140-6736(97)08229-9. [DOI] [PubMed] [Google Scholar]
- 4.Nawa T, et al. Lung cancer screening using low-dose spiral CT: results of baseline and 1-year follow-up studies. Chest. 2002;122(1):15–20. doi: 10.1378/chest.122.1.15. [DOI] [PubMed] [Google Scholar]
- 5.Thun MJ, et al. 50-year trends in smoking-related mortality in the United States. N Engl J Med. 2013;368(4):351–364. doi: 10.1056/NEJMsa1211127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.2013 Available from: http://seer.cancer.gov/csr/1975_2010/results_merged/sect_15_lung_bronchus.pdf.
- 7.Henschke CI, et al. Early lung cancer action project: initial findings on repeat screenings. Cancer. 2001;92(1):153–159. doi: 10.1002/1097-0142(20010701)92:1<153::aid-cncr1303>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 8.Sone S, et al. Results of three-year mass screening programme for lung cancer using mobile low-dose spiral computed tomography scanner. Br J Cancer. 2001;84(1):25–32. doi: 10.1054/bjoc.2000.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Swensen SJ, et al. Lung cancer screening with CT: Mayo Clinic experience. Radiology. 2003;226(3):756–761. doi: 10.1148/radiol.2263020036. [DOI] [PubMed] [Google Scholar]
- 10.National Lung Screening Trial Research. T, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Lung Screening Trial Research. T, et al. Baseline characteristics of participants in the randomized national lung screening trial. J Natl Cancer Inst. 2010;102(23):1771–1779. doi: 10.1093/jnci/djq434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oken MM, Screening MM, et al. Screening by chest radiograph and lung cancer mortality: the Prostate, Lung, Colorectal, and Ovarian (PLCO) randomized trial. JAMA. 2011;306(17):1865–1873. doi: 10.1001/jama.2011.1591. [DOI] [PubMed] [Google Scholar]
- 13.Jaklitsch MT, et al. The American Association for Thoracic Surgery guidelines for lung cancer screening using low-dose computed tomography scans for lung cancer survivors and other high-risk groups. J Thorac Cardiovasc Surg. 2012;144(1):33–38. doi: 10.1016/j.jtcvs.2012.05.060. [DOI] [PubMed] [Google Scholar]
- 14.Wender R, et al. American Cancer Society lung cancer screening guidelines. CA Cancer J Clin. 2013;63(2):106–117. doi: 10.3322/caac.21172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wood DE, et al. Lung cancer screening. J Natl Compr Canc Netw. 2012;10(2):240–265. doi: 10.6004/jnccn.2012.0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bach PB, et al. Benefits and harms of CT screening for lung cancer: a systematic review. JAMA. 2012;307(22):2418–2429. doi: 10.1001/jama.2012.5521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma J, et al. Annual number of lung cancer deaths potentially avertable by screening in the United States. Cancer. 2013;119(7):1381–1385. doi: 10.1002/cncr.27813. [DOI] [PubMed] [Google Scholar]
- 18.Pinsky PF, Berg CD. Applying the National Lung Screening Trial eligibility criteria to the US population: what percent of the population and of incident lung cancers would be covered? J Med Screen. 2012;19(3):154–156. doi: 10.1258/jms.2012.012010. [DOI] [PubMed] [Google Scholar]
- 19.International Early Lung Cancer Action Program. I, et al. Survival of patients with stage I lung cancer detected on CT screening. N Engl J Med. 2006;355(17):1763–1771. doi: 10.1056/NEJMoa060476. [DOI] [PubMed] [Google Scholar]
- 20.Bach PB, et al. Variations in lung cancer risk among smokers. J Natl Cancer Inst. 2003;95(6):470–478. doi: 10.1093/jnci/95.6.470. [DOI] [PubMed] [Google Scholar]
- 21.Spitz MR, et al. A risk model for prediction of lung cancer. J Natl Cancer Inst. 2007;99(9):715–726. doi: 10.1093/jnci/djk153. [DOI] [PubMed] [Google Scholar]
- 22.Cassidy A, et al. The LLP risk model: an individual risk prediction model for lung cancer. Br J Cancer. 2008;98(2):270–276. doi: 10.1038/sj.bjc.6604158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Etzel CJ, Bach PB. Estimating individual risk for lung cancer. Semin Respir Crit Care Med. 2011;32(1):3–9. doi: 10.1055/s-0031-1272864. [DOI] [PubMed] [Google Scholar]
- 24.D’Amelio AM, Jr., et al. Comparison of discriminatory power and accuracy of three lung cancer risk models. Br J Cancer. 2010;103(3):423–429. doi: 10.1038/sj.bjc.6605759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tammemagi MC, et al. Selection criteria for lung-cancer screening. N Engl J Med. 2013;368(8):728–736. doi: 10.1056/NEJMoa1211776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.National Lung Screening Trial Research. T, et al. The National Lung Screening Trial: overview and study design. Radiology. 2011;258(1):243–253. doi: 10.1148/radiol.10091808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Larke FJ, et al. Estimated radiation dose associated with low-dose chest CT of average-size participants in the National Lung Screening Trial. AJR Am J Roentgenol. 2011;197(5):1165–1169. doi: 10.2214/AJR.11.6533. [DOI] [PubMed] [Google Scholar]
- 28.Mascalchi M, et al. Dose exposure in the ITALUNG trial of lung cancer screening with low-dose CT. Br J Radiol. 2012;85(1016):1134–1139. doi: 10.1259/bjr/20711289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu DM, et al. Nodule management protocol of the NELSON randomised lung cancer screening trial. Lung Cancer. 2006;54(2):177–184. doi: 10.1016/j.lungcan.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 30.de Hoop B, et al. Pulmonary Ground-Glass Nodules: Increase in Mass as an Early Indicator of Growth1. Radiology. 2010;255(1):199–206. doi: 10.1148/radiol.09090571. [DOI] [PubMed] [Google Scholar]
- 31.Manowitz A, et al. Use of BMI guidelines and individual dose tracking to minimize radiation exposure from low-dose helical chest CT scanning in a lung cancer screening program. Acad Radiol. 2012;19(1):84–88. doi: 10.1016/j.acra.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 32.Funama Y, et al. Detection of nodules showing ground-glass opacity in the lungs at low-dose multidetector computed tomography: phantom and clinical study. J Comput Assist Tomogr. 2009;33(1):49–53. doi: 10.1097/RCT.0b013e31815e6291. [DOI] [PubMed] [Google Scholar]
- 33.MacMahon H, et al. Guidelines for management of small pulmonary nodules detected on CT scans: a statement from the Fleischner Society. Radiology. 2005;237(2):395–400. doi: 10.1148/radiol.2372041887. [DOI] [PubMed] [Google Scholar]
- 34.Good CA, Wilson TW. The solitary circumscribed pulmonary nodule; study of seven hundred five cases encountered roentgenologically in a period of three and one-half years. J Am Med Assoc. 1958;166(3):210–215. doi: 10.1001/jama.1958.02990030008003. [DOI] [PubMed] [Google Scholar]
- 35.Diederich S, et al. Screening for early lung cancer with low-dose spiral CT: prevalence in 817 asymptomatic smokers. Radiology. 2002;222(3):773–781. doi: 10.1148/radiol.2223010490. [DOI] [PubMed] [Google Scholar]
- 36.Ahn MI, et al. Perifissural nodules seen at CT screening for lung cancer. Radiology. 2010;254(3):949–956. doi: 10.1148/radiol.09090031. [DOI] [PubMed] [Google Scholar]
- 37.de Hoop B, et al. Pulmonary perifissural nodules on CT scans: rapid growth is not a predictor of malignancy. Radiology. 2012;265(2):611–616. doi: 10.1148/radiol.12112351. [DOI] [PubMed] [Google Scholar]
- 38.Takashima S, et al. Small solitary pulmonary nodules (< or =1 cm) detected at population-based CT screening for lung cancer: Reliable high-resolution CT features of benign lesions. AJR Am J Roentgenol. 2003;180(4):955–964. doi: 10.2214/ajr.180.4.1800955. [DOI] [PubMed] [Google Scholar]
- 39.Slattery MM, et al. Long-term follow-up of non-calcified pulmonary nodules (<10 mm) identified during low-dose CT screening for lung cancer. Eur Radiol. 2012;22(9):1923–1928. doi: 10.1007/s00330-012-2443-0. [DOI] [PubMed] [Google Scholar]
- 40.Henschke CI, et al. CT screening for lung cancer: frequency and significance of part-solid and nonsolid nodules. AJR Am J Roentgenol. 2002;178(5):1053–1057. doi: 10.2214/ajr.178.5.1781053. [DOI] [PubMed] [Google Scholar]
- 41.Li F, et al. Malignant versus benign nodules at CT screening for lung cancer: comparison of thin-section CT findings. Radiology. 2004;233(3):793–798. doi: 10.1148/radiol.2333031018. [DOI] [PubMed] [Google Scholar]
- 42.Lee SM, et al. Invasive Pulmonary Adenocarcinomas versus Preinvasive Lesions Appearing as Ground-Glass Nodules: Differentiation by Using CT Features. Radiology. 2013 doi: 10.1148/radiol.13120949. [DOI] [PubMed] [Google Scholar]
- 43.Travis WD, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol. 2011;6(2):244–285. doi: 10.1097/JTO.0b013e318206a221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ko JP, et al. Pulmonary Nodules: growth rate assessment in patients by using serial CT and three-dimensional volumetry. Radiology. 2012;262(2):662–671. doi: 10.1148/radiol.11100878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Henschke CI. <VDTs in ELCAP.pdf> [Google Scholar]
- 46.Heuvelmans MA, et al. Optimisation of volume-doubling time cutoff for fast-growing lung nodules in CT lung cancer screening reduces false-positive referrals. Eur Radiol. 2013 doi: 10.1007/s00330-013-2799-9. [DOI] [PubMed] [Google Scholar]
- 47.Kakinuma R, et al. Measurement of focal ground-glass opacity diameters on CT images: interobserver agreement in regard to identifying increases in the size of ground-glass opacities. Acad Radiol. 2012;19(4):389–394. doi: 10.1016/j.acra.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 48.Hasegawa M, et al. Growth rate of small lung cancers detected on mass CT screening. Br J Radiol. 2000;73(876):1252–1259. doi: 10.1259/bjr.73.876.11205667. [DOI] [PubMed] [Google Scholar]
- 49.Takahashi S, et al. Long term follow-up for small pure ground-glass nodules: implications of determining an optimum follow-up period and high-resolution CT findings to predict the growth of nodules. Jpn J Radiol. 2012;30(3):206–217. doi: 10.1007/s11604-011-0033-8. [DOI] [PubMed] [Google Scholar]
- 50.Godoy MC, Naidich DP. Overview and strategic management of subsolid pulmonary nodules. J Thorac Imaging. 2012;27(4):240–248. doi: 10.1097/RTI.0b013e31825d515b. [DOI] [PubMed] [Google Scholar]
- 51.Godoy MC, Sabloff B, Naidich DP. Subsolid pulmonary nodules: imaging evaluation and strategic management. Curr Opin Pulm Med. 2012;18(4):304–312. doi: 10.1097/MCP.0b013e328354a5f2. [DOI] [PubMed] [Google Scholar]
- 52.Soriano JB, Zielinski J, Price D. Screening for and early detection of chronic obstructive pulmonary disease. Lancet. 2009;374(9691):721–732. doi: 10.1016/S0140-6736(09)61290-3. [DOI] [PubMed] [Google Scholar]
- 53.Tammemagi CM, et al. Lung cancer risk prediction: Prostate, Lung, Colorectal And Ovarian Cancer Screening Trial models and validation. J Natl Cancer Inst. 2011;103(13):1058–1068. doi: 10.1093/jnci/djr173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mets OM, et al. Identification of chronic obstructive pulmonary disease in lung cancer screening computed tomographic scans. JAMA. 2011;306(16):1775–17781. doi: 10.1001/jama.2011.1531. [DOI] [PubMed] [Google Scholar]
- 55.Smith BM, et al. Emphysema detected on computed tomography and risk of lung cancer: a systematic review and meta-analysis. Lung Cancer. 2012;77(1):58–63. doi: 10.1016/j.lungcan.2012.02.019. [DOI] [PubMed] [Google Scholar]
- 56.Jacobs PC, et al. Coronary artery calcium can predict all-cause mortality and cardiovascular events on low-dose CT screening for lung cancer. AJR Am J Roentgenol. 2012;198(3):505–511. doi: 10.2214/AJR.10.5577. [DOI] [PubMed] [Google Scholar]
- 57.McEvoy JW, et al. Mortality rates in smokers and nonsmokers in the presence or absence of coronary artery calcification. JACC Cardiovasc Imaging. 2012;5(10):1037–1045. doi: 10.1016/j.jcmg.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shemesh J, et al. Ordinal scoring of coronary artery calcifications on low-dose CT scans of the chest is predictive of death from cardiovascular disease. Radiology. 2010;257(2):541–548. doi: 10.1148/radiol.10100383. [DOI] [PubMed] [Google Scholar]
- 59.Priola AM, et al. Clinical Implications and Added Costs of Incidental Findings in an Early Detection Study of Lung Cancer by Using Low-Dose Spiral Computed Tomography. Clin Lung Cancer. 2012 doi: 10.1016/j.cllc.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 60.van de Wiel JC, et al. Neglectable benefit of searching for incidental findings in the Dutch-Belgian lung cancer screening trial (NELSON) using low-dose multidetector CT. Eur Radiol. 2007;17(6):1474–1482. doi: 10.1007/s00330-006-0532-7. [DOI] [PubMed] [Google Scholar]
- 61.Rampinelli C, et al. Extrapulmonary malignancies detected at lung cancer screening. Radiology. 2011;261(1):293–299. doi: 10.1148/radiol.11102231. [DOI] [PubMed] [Google Scholar]
- 62.McKee BJ, et al. Initial Experience With a Free, High-Volume, Low-Dose CT Lung Cancer Screening Program. J Am Coll Radiol. 2013 doi: 10.1016/j.jacr.2013.02.015. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 63.Jonnalagadda S, et al. Beliefs and attitudes about lung cancer screening among smokers. Lung Cancer. 2012;77(3):526–531. doi: 10.1016/j.lungcan.2012.05.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brenner DJ. Radiation risks potentially associated with low-dose CT screening of adult smokers for lung cancer. Radiology. 2004;231(2):440–445. doi: 10.1148/radiol.2312030880. [DOI] [PubMed] [Google Scholar]
- 65.Brenner DJ, Hall EJ. Computed tomography--an increasing source of radiation exposure. N Engl J Med. 2007;357(22):2277–2284. doi: 10.1056/NEJMra072149. [DOI] [PubMed] [Google Scholar]
- 66.Mettler FA, Jr., et al. Effective doses in radiology and diagnostic nuclear medicine: a catalog. Radiology. 2008;248(1):254–263. doi: 10.1148/radiol.2481071451. [DOI] [PubMed] [Google Scholar]
- 67.Hendrick RE. Radiation doses and cancer risks from breast imaging studies. Radiology. 2010;257(1):246–253. doi: 10.1148/radiol.10100570. [DOI] [PubMed] [Google Scholar]
- 68.Shuryak I, Sachs RK, Brenner DJ. Cancer risks after radiation exposure in middle age. J Natl Cancer Inst. 2010;102(21):1628–1636. doi: 10.1093/jnci/djq346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bleyer A, Welch HG. Effect of three decades of screening mammography on breast-cancer incidence. N Engl J Med. 2012;367(21):1998–2005. doi: 10.1056/NEJMoa1206809. [DOI] [PubMed] [Google Scholar]
- 70.Veronesi G, et al. Estimating overdiagnosis in low-dose computed tomography screening for lung cancer: a cohort study. Ann Intern Med. 2012;157(11):776–784. doi: 10.7326/0003-4819-157-11-201212040-00005. [DOI] [PubMed] [Google Scholar]
- 71.Lindell RM, et al. Five-year lung cancer screening experience: CT appearance, growth rate, location, and histologic features of 61 lung cancers. Radiology. 2007;242(2):555–562. doi: 10.1148/radiol.2422052090. [DOI] [PubMed] [Google Scholar]
- 72.Henschke CI, et al. Lung cancers diagnosed at annual CT screening: volume doubling times. Radiology. 2012;263(2):578–583. doi: 10.1148/radiol.12102489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hazelton WD, et al. Longitudinal multistage model for lung cancer incidence, mortality, and CT detected indolent and aggressive cancers. Math Biosci. 2012;240(1):20–34. doi: 10.1016/j.mbs.2012.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.van der Aalst CM, et al. The impact of a lung cancer computed tomography screening result on smoking abstinence. Eur Respir J. 2011;37(6):1466–1473. doi: 10.1183/09031936.00035410. [DOI] [PubMed] [Google Scholar]
- 75.Ashraf H, et al. Effect of CT screening on smoking habits at 1-year follow-up in the Danish Lung Cancer Screening Trial (DLCST) Thorax. 2009;64(5):388–392. doi: 10.1136/thx.2008.102475. [DOI] [PubMed] [Google Scholar]
- 76.Barry SA, et al. Predictors of adverse smoking outcomes in the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial. J Natl Cancer Inst. 2012;104(21):1647–1659. doi: 10.1093/jnci/djs398. [DOI] [PMC free article] [PubMed] [Google Scholar]