Abstract
Obesity has profound negative consequences on female reproduction as well as on the metabolic health of offspring. Bariatric surgery is the most effective method for sustained weight loss. A critical question is whether bariatric surgery can reverse the deleterious effects of obesity on both female reproduction and subsequent offspring. Vertical sleeve gastrectomy (VSG) is a bariatric procedure rapidly growing in popularity because it provides weight loss and other metabolic benefits that are comparable to those offered by the more complicated Roux-en-Y gastric bypass (RYGB). Female rats rendered obese on a high-fat diet (HFD) underwent either VSG or sham surgery. Like their male counterparts, females had significant metabolic improvements including reduced adiposity and improved glucose tolerance. After VSG, female rats showed a more normal reproductive cycle. Despite these maternal benefits, the offspring of dams receiving VSG were born smaller and lighter than offspring of control dams that underwent sham surgery. When maintained on an HFD after puberty, these adult offspring had a greater propensity to develop glucose intolerance and increased adiposity than the offspring of lean mothers or obese mothers who underwent sham surgery. These data suggest that weight loss alone by obese mothers is not sufficient to reverse the deleterious effects of an HFD and obesity on their offspring.
INTRODUCTION
Obesity impairs health. In the greater than 25% of women of child-bearing age who are obese (1), obesity compromises reproductive health with abnormal menstrual cycles, inappropriate hormone levels, infertility, and high-risk pregnancies (1). The risks during pregnancy include increased morbidity and mortality to both mother and offspring because of gestational diabetes, hypertension, and preeclampsia in addition to difficulties in delivery of large-for-gestational age infants (2, 3) and increased rate of birth defects (4). Among many contributing factors, fetal exposure to maternal obesity leads to increased prevalence of obesity in childhood and adulthood and increased risk of obesity-related comorbidities including type 2 diabetes mellitus (5, 6), dyslipidemia, hypertension (7), nonalcoholic steatohepatitis (8), and psychosocial stress (9). Collectively, this represents a major health crisis and an economic burden.
Obesity and its comorbidities can be reversed and even averted for mother and offspring with sustained body weight reduction (10, 11). Diet and exercise, although effective, typically result in only modest improvement (12). In contrast, bariatric surgery causes sustained weight reduction and improved control of circulating glucose and lipids (13–15), and its therapeutic use has been increasing particularly among women (16).
We asked whether the weight loss and metabolic improvements after bariatric surgery can ameliorate the deleterious effects of obesity on female reproduction and the metabolic health of their offspring. Limited work has addressed these issues in female rodent models (13, 17). After either laparoscopic gastric banding or Roux-en-Y gastric bypass (RYGB), pregnant women showed reduced gestational diabetes and preeclampsia and decreased numbers of large-for-gestational age births, and there was an overall improvement in pregnancy-related outcomes (18). In a longitudinal study, the offspring that were born after their mothers had biliopancreatic diversion had less adiposity, increased insulin sensitivity, and an improved lipid profile compared to offspring that were born before their mother’s surgery (14, 15). Other retrospective studies of women receiving either open or laparoscopic gastric banding reported no adverse perinatal outcomes and decreased rates of gestational diabetes, hypertension, and macrosomia (18–20).
Here, we determined the impact of vertical sleeve gastrectomy (VSG) on female rats and their offspring. VSG is a bariatric procedure in which 80% of the stomach is excised along the greater curvature, leaving a “sleeve” of a stomach. VSG has gained popularity over the past decade because of the relative ease of the surgery and the weight loss and reductions in diabetes and hyperlipidemia that are comparable to those found with RYGB (21–25). Consequently, we asked whether VSG in female rats was able to reduce the impact of obesity on reproduction and the metabolic status of the offspring.
RESULTS
VSG induces fat loss and improves glucose tolerance in females
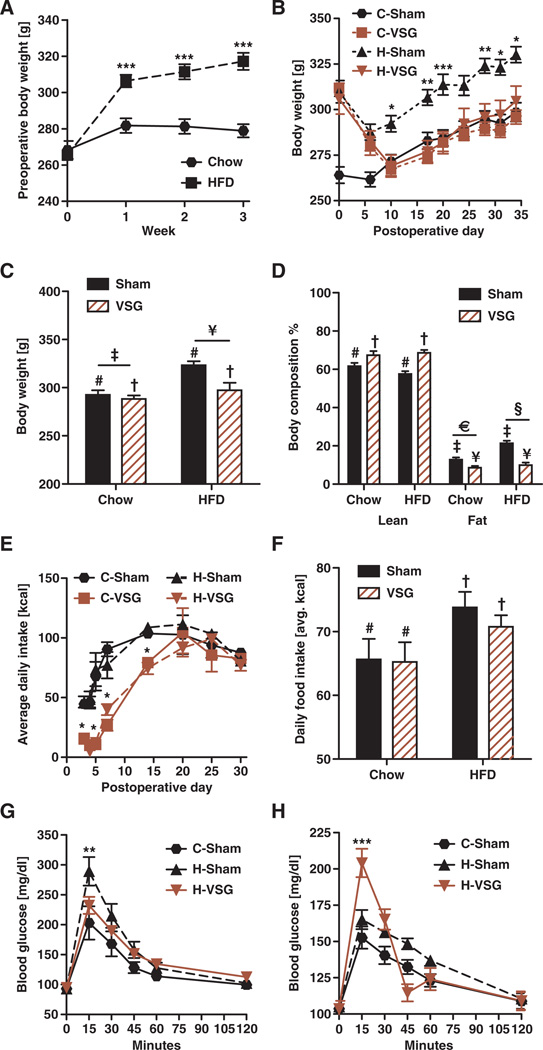
Consumption of HFD for 3 weeks before surgery resulted in a significant weight gain in young female rats (Fig. 1A) (effect of diet, P < 0.001; effect of time, P < 0.001). Animals receiving sham surgery were maintained on chow (C-Sham) or HFD (H-Sham) for the remainder of the study. Those receiving VSG were either maintained on HFD (H-VSG) or switched to chow after surgery (C-VSG). VSG resulted in a substantial 2-week weight loss relative to H-Sham rats despite maintenance on HFD (Fig. 1B) (effect of surgery, P < 0.001; effect of time, P < 0.001). By postoperative day 31, C-Sham, C-VSG, and H-VSG weighed significantly less than obese H-Sham animals (Fig. 1C) (effect of surgery, P < 0.01; effect of diet, P < 0.001). Sham animals had relatively less lean mass than lean C-VSG and H-VSG animals (Fig. 1D) (effect of surgery, P < 0.001). Whether maintained on chow or HFD after surgery, animals receiving VSG lost substantial fat mass, achieving a level of adiposity similar to that of C-Sham rats by 31 days after surgery (Fig. 1D) (effect of surgery, P < 0.001; effect of diet, P < 0.001). Weight loss was associated with decreased caloric intake over the first 3 weeks after VSG, and those animals never overate to compensate for the early decrease in calories (Fig. 1E) (group effect, P < 0.001; effect of time, P < 0.001). By postoperative day 31, food intakes were comparable for C-Sham and C-VSG (both consuming chow) and for H-Sham and H-VSG (both consuming HFD), and HFD-fed animals consumed more calories than did chow-fed animals (Fig. 1F) (effect of diet, P < 0.05).
Fig. 1. VSG in female rats resulted in metabolic improvements.
(A) Female rats placed on an HFD for 3 weeks before surgery showed body weight increases in comparison to chow-fed controls. Effect of diet (***P < 0.001) and time (***P < 0.001) by two-way repeated-measures ANOVA. ***P < 0.001, at weeks 1 to 3 with Bonferroni post hoc (n = 14 to 33 per group). (B) Female rats maintained on either chow or HFD after VSG had substantial body weight loss. Effect of group (***P < 0.001) and time (***P < 0.001) by two-way repeated-measures ANOVA. *P < 0.05, **P < 0.01, and ***P < 0.01, days 10 to 35 with Bonferroni post hoc (n = 13 to 15 per group). (C) By postoperative day 31, VSG animals weighed significantly less than obese H-Sham animals. Effect of surgery (P < 0.01, # versus †) and diet (P < 0.001, ‡ versus ¥) by two-way ANOVA (n = 13 to 15 per group). (D) Sham animals had relatively less lean mass than VSG-treated animals as measured by nuclear magnetic resonance (NMR). Effect of surgery (P < 0.001, # versus †) by two-way ANOVA (n = 13 to 15 per group). VSG-treated animals had reduced fat mass; overall, rats that continued to consume HFD had significantly greater levels of adiposity than chow-fed animals. Effect of surgery (P < 0.001, ‡ versus ¥) and diet (P < 0.001, € versus §) by two-way ANOVA (n = 13 to 15 per group). (E) Caloric intake was reduced in VSG-treated animals during the first 3 weeks. Effect of group (***P < 0.001) and time (***P < 0.001) by two-way repeated-measures ANOVA. *P < 0.05, days 0 to 15 with Bonferroni post hoc (n = 13 to 14 per group). (F) By postoperative day 31, HFD-fed animals were consuming more calories than chow-fed animals irrespective of surgery. Effect of diet (P < 0.05, # versus †) by two-way ANOVA (n = 12 to 15 per group). (G) We performed an intraperitoneal glucose tolerance test in 8-hour fasted rats at postoperative day 99. H-Sham animals were significantly glucose-intolerant relative to H-VSG and C-Sham rats. **P < 0.01 at the 15-min time point by two-way ANOVA with Bonferroni post hoc in H-Sham versus H-VSG and C-Sham (n = 5 to 6 per group). (H) We also performed an oral glucose tolerance test at postoperative day 30. H-VSG female rats had an early peak in blood glucose followed by a rapid decrease (***P < 0.01) at the 15-min time point by two-way ANOVA with Bonferroni post hoc in H-VSG versus C-Sham and H-Sham (n = 10 to 14 per group). Data are means ± SEM. Solid bars/black lines represent sham-operated females, and striped bars or red lines represent VSG-operated females. Unique symbol notations (for example, # versus †) denote statistical main effects by that variable (that is, diet or surgery). Same symbol notations denote no statistical main effect by that variable (that is, diet or surgery). Symbols do not denote significant differences in post hoc comparisons.
H-Sham animals were glucose-intolerant relative to H-VSG and C-Sham rats at postoperative day 99 (Fig. 1G; **P < 0.01,15 min). When challenged with an oral bolus of dextrose at postoperative day 30, H-VSG female rats had an early peak in blood glucose followed by a rapid decrease, a pattern not observed in the other groups (Fig. 1H; ***P < 0.001, 15 min), with no differences in area under the curve. All groups responded similarly during an insulin tolerance test. They had comparable energy expenditure when adjusted for metabolic mass (fig. S1A), although H-VSG female rats had a lower respiratory quotient than did C-Sham animals (fig. S1B; *P < 0.05). H-VSG female rats had an increased number (fig. S1C; *P < 0.05) and reduced size (fig. S1D; **P < 0.01) of meals, as has been found in male rats after VSG (22).
VSG improves other metabolic parameters in nonpregnant rats
Fasting blood glucose was comparable among the different groups (Table 1). HFD female rats had increased fasting plasma insulin, but no statistical differences existed among the three groups. H-Sham rats had increased leptin concentrations relative to C-Sham and H-VSG (Table 1; ***P < 0.001). H-VSG animals had reduced cholesterol (***P < 0.001) and circulating triglycerides (***P < 0.001) (Table 1). Despite a more than twofold decrease in circulating triglycerides, both H-Sham and H-VSG rats had increased liver triglycerides, with those of H-VSG rats being the highest (Table 1; ***P < 0.001). No differences were detected in growth hormone and insulin-like growth factor 1 (IGF-1) concentrations or hematocrit in nonpregnant females (Table 1).
Table 1.
Blood profile and liver characteristics in nonpregnant female rats. Data are means ± SEM.
| C-Sham | H-Sham | H-VSG | |
|---|---|---|---|
| Blood glucose (mg/dl) | 105.2 ± 3.6 | 105.1 ± 2.7 | 98.3 ± 1.3 |
| Insulin (ng/ml) | 0.7 ± 0.1 | 0.9 ± 0.2 | 0.8 ± 0.2 |
| Leptin (ng/ml) | 4.9 ± 0.5 | 16.5 ± 2.4* | 4.7 ± 0.9 |
| Liver triglycerides (mg/dl) | 154.9 ± 7.3* | 288.1 ± 31.6 | 530.5 ± 71.0 |
| Triglycerides (mg/dl) | 152.1 ± 18.4 | 423.2 ± 66.7* | 92.5 ± 6.1 |
| Cholesterol (mg/dl) | 96.3 ± 2.8 | 108.0 ± 2.9* | 94.3 ± 4.2 |
| Growth hormone (ng/ml) | 12.2 ± 4.9 | 8.9 ± 5.3 | 8.8 ± 3.7 |
| IGF-1 (ng/ml) | 674.5 ± 74.1 | 780.0 ± 45.0 | 677.2 ± 87.2 |
| Hematocrit (%) | 44.9 ± 1.0 | 44.4 ± 0.71 | 45 ± 1.0 |
P < 0.001, one-way analysis of variance (ANOVA) (n = 7 to 14 per group).
Offspring of VSG female rats are small and growth-restricted during early postnatal life
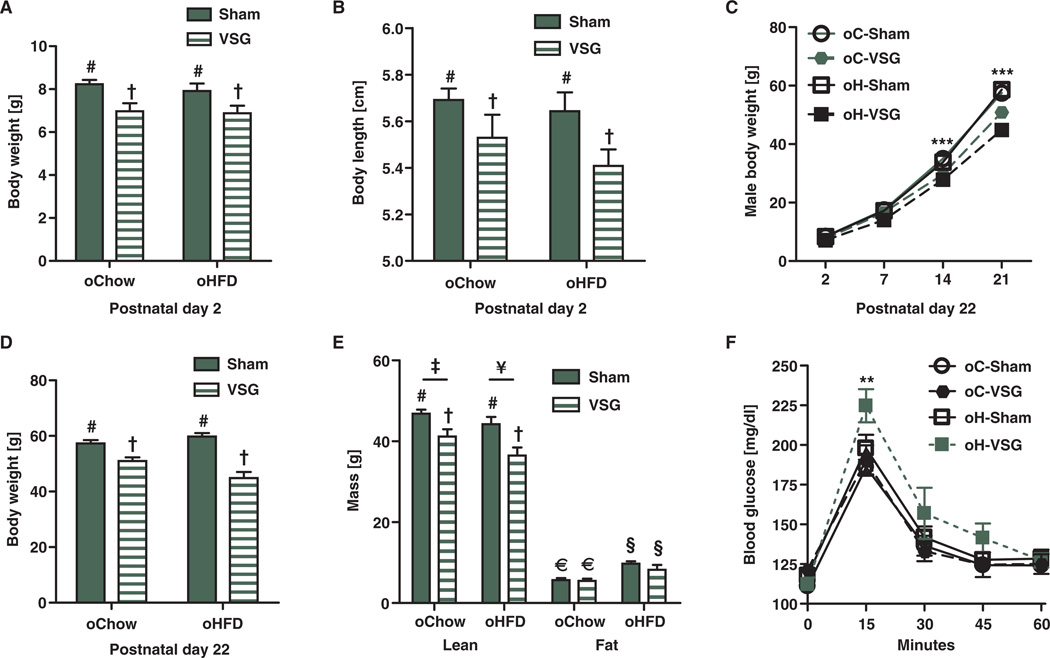
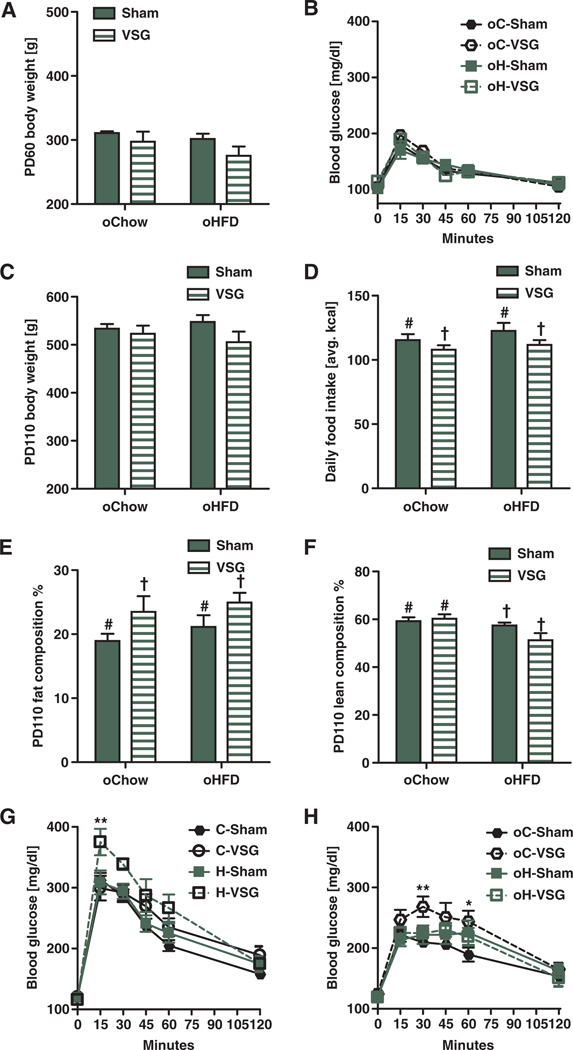
Five weeks after VSG or sham surgery, after stabilization of body weight and caloric intake, females were mated with young surgically naïve males and allowed to give birth naturally. On postnatal day 2, there were no statistical differences between the body weights of offspring of C-Sham (oC-Sham) or offspring of H-Sham (oH-Sham) female rats (males, Fig. 2A; females, fig. S3A). However, offspring of VSG females on either chow or HFD (oC-VSG and oH-VSG) were significantly lighter (males, Fig. 2A, effect of maternal surgery P < 0.001; females, fig. S3A, effect of maternal surgery P < 0.05, effect of maternal diet P < 0.05) and were also shorter (males, Fig. 2B, effect of maternal surgery P < 0.05; females, fig. S3B, effect of maternal surgery P < 0.05, effect of maternal diet P < 0.05) than offspring of the relevant controls. Litter size was comparable among all groups (Fig. 5B). oC-VSG and oH-VSG pups remained light throughout their postnatal life (males, Fig. 2C, ***P < 0.001; females, fig. S3C, **P < 0.01). At weaning, oC-VSG and oH-VSG weighed significantly less than offspring of sham-operated dams (males, Fig. 2D, effect of maternal surgery P < 0.001; females, fig. S3D, effect of maternal surgery P < 0.001). VSG pups had the lowest lean mass (males, Fig. 2E, effect of maternal surgery P < 0.001, effect of maternal diet P < 0.05; females, fig. S3E, effect of maternal surgery P < 0.001, effect of maternal diet P < 0.05). oH-Sham and oH-VSG had more body fat than their chow-fed controls (Fig. 2E, effect of maternal diet P < 0.001; fig. S3E, effect of maternal diet P< 0.001). During an intraperitoneal glucose tolerance test after a 4-hour fast at weaning, oH-VSG had higher blood glucose levels in comparison to other groups (Fig. 2F, **P < 0.01; fig. S3F, **P < 0.01). At weaning, the hypothalamus of oH-Sham and oH-VSG animals showed up-regulation of orexigenic peptide AgRP (***P < 0.001) and down-regulation of the anorexic gene POMC (**P < 0.01). The CART (**P < 0.01), MCR4 (*P < 0.05), and NPY (*P < 0.05) genes also were down-regulated in the hypothalamus of oH-Sham animals (fig. S2).
Fig. 2. Metabolic characteristics of male rodent offspring before weaning.
(A) Average body weight of postnatal day 2 offspring. Offspring of VSG weighed less in comparison to sham controls. Effect of maternal surgery (P < 0.001, # versus †) by two-way ANOVA (n = 9 to 19 per group). (B) Body length of postnatal day 2 VSG offspring was reduced in comparison to sham controls. Effect of surgery (P < 0.05, # versus †) by two-way ANOVA (n = 9 to 19 per group). (C) Body weight curves of offspring of chow-and HFD-fed, sham-operated, and VSG dams throughout postnatal life. Offspring of VSG-treated dams weighed less than those of sham-operated dams. Effect of maternal surgery (***P < 0.001) and time (***P < 0.001) by two-way repeated-measures ANOVA. ***P < 0.001, days 14 and 21 with Bonferroni post hoc (n = 7 to 18 per group). (D) Body weights at postnatal day 22 of male offspring of VSG were reduced in comparison to offspring of sham-operated dams. Effect of maternal group (P < 0.001) and an interaction between maternal surgery and maternal diet. P < 0.01, by two-way ANOVA (n = 7 to 18 per group). (E) Lean mass measured by NMR of postnatal day 22 male offspring. Effect of maternal surgery (P < 0.001, # versus †) and maternal diet (P < 0.05, ‡ versus ¥) by two-way ANOVA (n = 4 to 9). Fat mass was also measured by NMR. Effect of maternal diet (P < 0.001, € versus §) by two-way ANOVA (n = 4 to 9). (F) An intraperitoneal glucose tolerance test dosed at 1.25 g of dextrose per kilogram of body weight was performed at weaning. Animals were fasted for 4 hours before the test. **P < 0.01, at the 15-min time point by two-way repeated-measures ANOVA with Bonferroni post hoc in H-VSG versus H-Sham, C-VSG, and C-Sham (n = 7 to 15 per group). Data are means ± SEM; one male per litter. Solid bars represent sham offspring, and striped bars represent VSG offspring. Unique symbol notations (for example, # versus †) denote statistical main effects by that variable (that is, diet or surgery). Same symbol notations denote no statistical main effect by that variable (that is, diet or surgery). Symbols do not denote significant differences in post hoc comparisons.
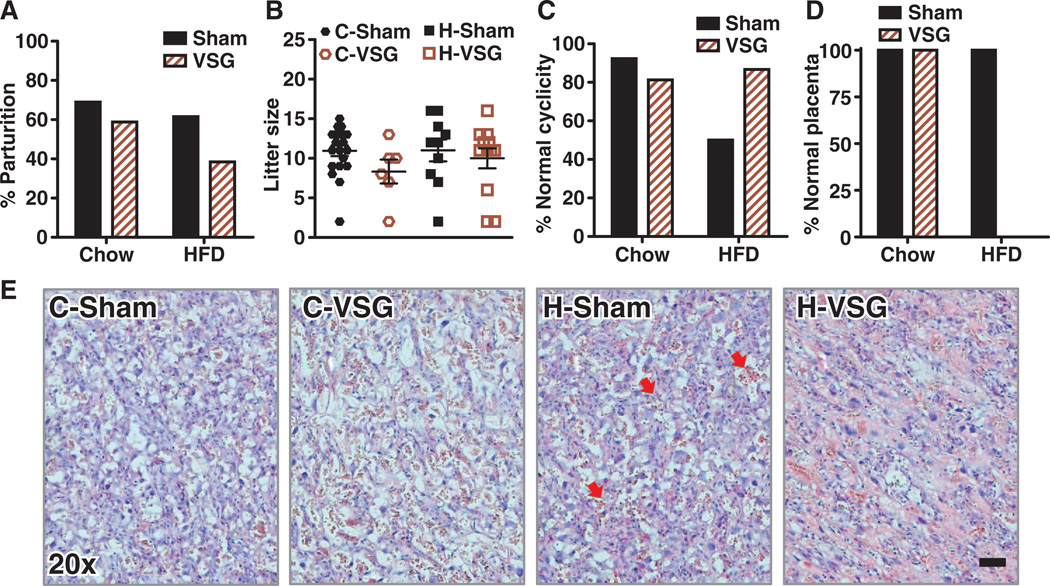
Fig. 5. Reproductive characteristics and placental histology of female rats after sham or VSG surgery.
(A) Percentage of dams maintained on either chow or HFD, which gave birth to live pups (n = 16 to 29). (B) Average litter size of each of the four groups (n = 6 to 20). (C) Percentage of animals with estrous cycles that appeared to be normal by vaginal cytology (n = 13 to 15). (D) Percentage of animals having normal placental histology; no normal H-VSG placentas were observed histologically (n = 3 to 5). (E) Representative photomicrographs of H&E-stained placentas at gestational days 16 to 18. Magnification, ×20; scale bar, 500 mm. Red arrows in H-Sham denote nucleated red blood cells.
Offspring of HFD-fed dams have hyperlipidemia and hepatic lipid accumulation at weaning
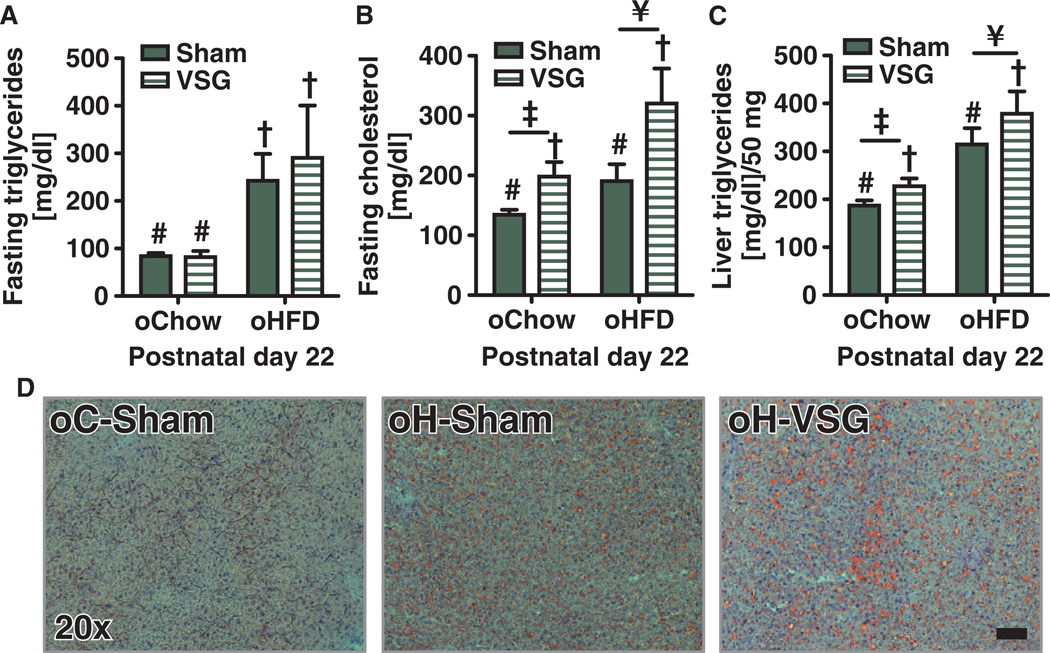
Throughout the postnatal period, oH-Sham and oH-VSG animals were suckled by dams maintained on an HFD. By postoperative day 16, all weanlings were supplementing their milk intake with the dam’s diet. oH-Sham and oH-VSG rat offspring had relatively high circulating triglycerides compared to controls fed a chow diet (males, Fig. 3A, effect of maternal diet P < 0.001; females, fig. S3G, *P < 0.05). oH-VSG offspring had the highest cholesterol among all groups, and there was a significant effect of both diet and surgery on cholesterol levels (Fig. 3B, effect of maternal surgery P < 0.01, effect of maternal diet P < 0.01). Female oH-VSG offspring trended toward elevated cholesterol levels, but there was no significant difference with respect to either chow- or HFD-fed control offspring (fig. S3H). Male oH-VSG offspring also had high liver triglycerides with a significant effect of both diet and surgery on these levels (males, Fig. 3C, effect of maternal surgery P < 0.05, effect of maternal diet P < 0.001; females, not determined). The increased liver triglycerides were also visualized with oil red O staining in pups from the first cohort (Fig. 3D).
Fig. 3. Circulating lipids and hepatic triglycerides in male rodent offspring before weaning.
(A) Circulating triglycerides after a 4-hour fast are increased in offspring of HFD-fed dams. Effect of maternal diet (P < 0.001, # versus †) by two-way ANOVA (n = 5 to 18). (B) Circulating cholesterol after a 4-hour fast. Effect of maternal surgery (P < 0.01, # versus †) and maternal diet (P < 0.01, ‡ versus ¥) by two-way ANOVA (n = 5 to 18). (C) Triglyceride content in the liver after a 4-hour fast. Effect of maternal surgery (P < 0.05, # versus †) and maternal diet (P < 0.001, ‡ versus ¥) by two-way ANOVA (n = 5 to 15). (D) Representative photomicrographs of lipids in the liver using oil red O staining. Magnification, ×20; scale bar, 500 µm. Data are means ± SEM; one male per litter. Solid bars represent sham offspring, and striped bars represent VSG offspring. Unique symbol notations (for example, # versus †) denote statistical main effects by that variable (that is, diet or surgery). Same symbol notations denote no statistical main effect by that variable (that is, diet or surgery). Symbols do not denote significant differences in post hoc comparisons.
Offspring of VSG dams have increased adiposity and impaired glucose tolerance in adulthood
At postnatal day 22, two animals from each litter were placed on a chow diet. At postnatal day 60, there was no residual effect of maternal diet or surgery on body weight of male offspring of VSG dams (Fig. 4A). Female offspring of VSG dams still tended to be smaller at postnatal day 60 than sham controls (fig. S3I, *P < 0.05). No differences were seen in glucose tolerance after maintenance on chow among the groups (males, Fig. 4B; females, fig. S3J). One offspring of each litter was then switched to an HFD. After 5 weeks on HFD (postnatal day 110), there remained no statistical differences between treatments with respect to body weight (males, Fig. 4C; females, fig. S3K). Offspring of VSG dams had reduced daily intake of HFD (males, *P < 0.05, Fig. 4D; females, not determined) and greater adiposity (males, Fig. 4E, effect of maternal surgery P < 0.05; females, fig. S3L, *P < 0.05) compared to offspring of sham-operated dams. Overall, oH-Sham and oH-VSG had the lowest lean mass (males, Fig. 4F, effect of maternal diet P < 0.01; females, fig. S3M, *P < 0.05). HFD-fed offspring of VSG dams had the greatest glucose intolerance during an intraperitoneal glucose tolerance test (males, Fig. 4G, **P < 0.01, 15 min; females, not determined); oC-VSG offspring had the greatest changes in blood glucose levels during an oral glucose tolerance test with respect to offspring of chow controls (males, Fig. 4H, **P < 0.01, 30 min, *P < 0.05, 60 min; females, fig. S3N, *P < 0.05, 15 min). Collectively, these data indicate an adverse effect of VSG on the adiposity and glucose tolerance of their offspring.
Fig. 4. Metabolic characteristics of adult male rat offspring of VSG and sham-operated dams.
(A) Body weights of postnatal day 60 male rat offspring after maintenance on chow from weaning to postnatal day 60 (n = 4 to 10). (B) Intraperitoneal glucose tolerance test was performed with dextrose (1.25 g/kg) after an 8-hour fast. No differences between groups existed. (C) Body weights of male rat offspring after maintenance on an HFD from postnatal days 60 to 110. No differences between groups existed. (D) Average daily food intake by male rat offspring after switching from chow to HFD. Offspring of VSG-operated dams consumed significantly less food than sham-operated dam offspring: effect of maternal surgery (P < 0.05, # versus †) by two-way ANOVA (n = 7 to 18). (E) Body fat composition of male offspring after 4 weeks on HFD. Offspring of VSG-operated rats had greater levels of adiposity. Effect of maternal surgery (P < 0.05, # versus †) (n = 7 to 18). (F) Lean body composition percentage. Offspring of HFD-fed dams had less lean body mass composition in comparison to offspring of chow-fed dams. Effect of diet (P < 0.01, # versus †) by two-way ANOVA (n = 7 to 18). (G) Intraperitoneal glucose tolerance test was performed after consumption of HFD for 4 weeks, with dosing at 1.25 g of dextrose per kilogram of individual body weight with an 8-hour fast. **P < 0.01, at the 15-min time point using a two-way repeated-measures ANOVA with Bonferroni post hoc in H-VSG versus C-Sham and H-Sham (n = 7 to 15). (H) Oral glucose tolerance test after 5 weeks on HFD, with dosing at 1.25 g of dextrose per kilogram of average body weight after an 8-hour fast. **P < 0.01 at the 30-min time point and *P < 0.05 at the 60-min time point by two-way repeated-measures ANOVA with Bonferroni post hoc in C-VSG versus C-Sham. Solid bars represent sham offspring, and striped bars represent VSG offspring (n = 7 to 15). Unique symbol notations (for example, # versus †) denote statistical main effects by that variable (that is, diet or surgery). Same symbol notations denote no statistical main effect by that variable (that is, diet or surgery). Symbols do not denote significant differences in post hoc comparisons.
Consumption of an HFD after VSG negatively affects the rate of live births
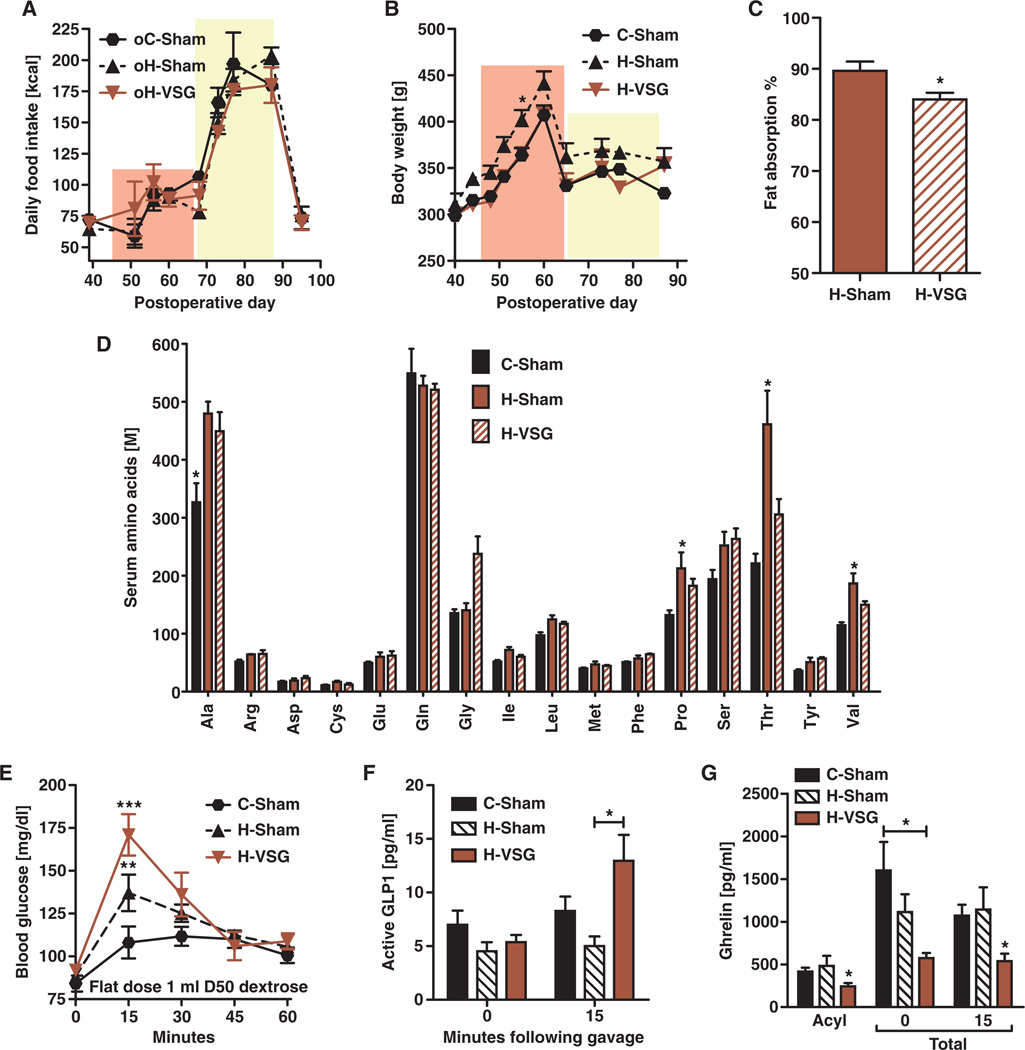
H-VSG females had the lowest rate of live births (Fig. 5A). Unlike the intrauterine growth restriction phenotype that prevailed in both C-VSG and H-VSG offspring, the low birth number was specific to H-VSG rat dams. Of the 26 healthy H-VSG females mated, only 10 gave birth to live litters. Despite this difficulty, litter sizes were similar among groups, averaging around 10 pups (Fig. 5B). H-Sham females had higher rate of irregular reproductive cycles determined by vaginal cytology (Fig. 5C). C-VSG and H-VSG animals had normal estrous cycles compared to C-Sham females. When mated, the H-VSG female dams gained early postpartum weight following time spent in the male cage. However, after about 16 days, in comparison to the other groups, fewer animals continued their gestational weight trajectory. We sacrificed one cohort of animals during pregnancy and harvested and sectioned placentas and stained them with hematoxylin and eosin (H&E stain). Placentas from C-Sham, C-VSG, and H-Sham female rats had little to no pathology, although nucleated red blood cells were present in H-Sham animal placentas (Fig. 5E). H-VSG animals had hemorrhagic placentas with gross pathology; none were normal (Fig. 5D). Indeed, the placentas from H-VSG dams appeared to be in a state of pup resorption that would reduce the chance of viable births.
Intrauterine growth restriction may be multifactorial
Dams who underwent VSG gave birth to small pups irrespective of their maintenance diet. There are many causes of intrauterine growth restriction in humans and rodents, including malnutrition (26). During gestation, however, VSG rat dams consumed similar calories as control sham-operated dams (Fig. 6A, pink area). In addition, all animals were able to mount an hyperphagic response during lactation, highlighting the fact that the “gastric restriction” due to VSG does not prevent the animal from ingesting additional calories when the situation warrants (Fig. 6A, yellow area). During gestation and lactation, H-Sham weighed slightly more than either C-Sham or H-VSG dams, but this was only statistically significant on day 55 (Fig. 6B, pink area, *P < 0.05, day 55). There was no difference between C-Sham and H-VSG dams at any time (Fig. 6B). We next assessed nutritional possibilities. To assess calorie malabsorption as has been reported after some bariatric surgeries, we first considered lipid absorption. However, only 5% more fat calories were lost in the feces of H-VSG females compared to H-Sham females (Fig. 6C; *P < 0.05). With regard to possible protein deficiency, another cause of intrauterine growth restriction, fasting plasma amino acids were similar among groups. H-Sham dams had higher levels of proline, threonine, and valine (*P < 0.05) than C-Sham or H-VSG dams (Fig. 6D). Both H-Sham and H-VSG dams had significantly elevated levels of alanine in comparison to C-Sham animals (Fig. 6D). However, it was never the case that H-VSG dams had levels lower than C-Sham dams, eliminating major protein malnutrition as a primary cause of the intrauterine growth restriction (Fig. 6D). In addition, when gavaged with a protein load, all three groups achieved similar concentrations of circulating amino acids.
Fig. 6. Metabolic characteristics of pregnant female rats.
(A) Average daily kilocalories consumed by female rats during gestation and lactation (n = 6 per group). (B) Average body weight of female rats during gestation and lactation. (C) Percentage of fat absorbed by pregnant adult female rats at gestational days 16 to 17. *P < 0.05, Student's t test (n = 3 to 5 per group). (D) Circulating amino acids were measured after 6 hours of fasting (n = 4 to 6 per group). Significant differences are denoted by asterisks and measured by one-way ANOVA. (E) Oral glucose tolerance test was performed at gestational days 15 to 16 using a 1-ml volume of 50% dextrose after a 6-hour fast. ***P < 0.001 C-Sham versus H-VSG and **P < 0.01 C-Sham versus H-Sham, at the 15-min time point by two-way ANOVA (n = 4 to 5 per group). (F) Active GLP1 after a 6-hour fast (t = 0) and after a 1-ml gavage of mixed nutrient meal, Ensure Plus. *P < 0.05, at the 15-min time point by two-way ANOVA in C-Sham versus H-VSG (n = 4 to 7 per group). (G) Fasting acyl ghrelin levels were measured after a 6-hour fast. *P < 0.05, by one-way ANOVA (n = 5 to 11 per group). Total ghrelin levels were also measured after a 6-hour fast (t = 0 and 15 min) and after a 1-ml gavage of a mixed nutrient meal, Ensure Plus. *P < 0.05, by one-way ANOVA (n = 5 to 7). Unique symbol notations (for example, # versus †) denote statistical main effects by that variable (that is, diet or surgery). Same symbol notations denote no statistical main effect by that variable (that is, diet or surgery). Symbols do not denote significant differences in post hoc comparisons.
To determine whether VSG impairs the expansive capacity of the gut and hence limits the absorptive capacity requisite for healthy pregnancy and gestation, we measured gut weight during pregnancy. H-Sham and H-VSG females had comparable gut weights, and both were less than those of C-Sham females (mean gut weight ± SEM: C-Sham, 8.3 ± 0.22; H-Sham, 7.7 ± 0.12; and H-VSG, 7.8 ± 0.33). Poor glycemic control during pregnancy also has implications for intrauterine growth failure. In humans, prolonged glucose intolerance with hyperinsulinemia leads to gestational diabetes and increased prevalence of macrosomic offspring, and intermittent periods of hypoglycemia and hypoinsulinemia can stunt in utero growth. Fasting glucose was comparable among groups. Following a 1-ml gastric gavage of 50% dextrose, H-VSG dams had the highest glucose excursions (Fig. 6E; min 15, H-VSG versus C-Sham ***P < 0.001, H-VSG versus H-Sham **P < 0.01) and increased concentrations of insulin (Table 2) and significantly increased levels of glucagon-like peptide 1 (GLP1) (Fig. 6F; *P < 0.05, 15 min). Although potentially problematic, these data do not support poor glucose control as the cause of intrauterine growth restriction.
Table 2.
Blood profile characteristics of pregnant female rats. Data are means ± SEM. Effect of maternal diet (P < 0.05, # versus †) by two-way ANOVA (n = 4 to 6 per group). Some groups were unavailable at the time of analysis. ND, not determined.
| C-Sham | C-VSG | H-Sham | H-VSG | |
|---|---|---|---|---|
| Fasting insulin (mg/dl) |
3.7 ± 1.4 | ND | 2.4 ± 0.6 | 3.9 ± 1.1 |
| Insulin (t = 15) (mg/dl) |
6.5 ± 1.4 | ND | 8.3 ± 2.3 | 14.2 ± 2.6 |
| Vitamin B12 (ng/ml) |
1,941 ± 42.4 | 2,114 ± 25.8 | 1,612 ± 9.5 | 1,730 ± 190 |
| Folate (mg/dl) |
26,301 ± 734# | 25,287 ± 1,354# | 23,312 ± 1,139† | 22,195 ± 661† |
| Ferritin (ng/ml) |
827.9 ± 293 | ND | 1,426 ± 323 | 329 ± 50.4 |
| Hematocrit (%) | 45.2 ± 1.2 | ND | 42.8 ± 0.8 | 40.9 ± 0.5* |
P < 0.05.
Because low vitamin levels are a factor in intrauterine growth restriction and some bariatric surgeries such as gastric bypass and biliopancreatic diversion, we assessed vitamin B12 and folate levels. Vitamin B12 levels were comparable among groups, whereas folate levels were significantly reduced in HFD-fed animals (Table 2, *P < 0.05). However, this did not vary by surgery. Ferritin levels both during pregnancy and in the nonpregnant state suggested that H-VSG animals had low circulating iron (Table 2). However, depending on the cohort, the animals had hematocrits that were normal or only slightly below the normal range (Tables 1 and 2). These findings are complex because low ferritin without appreciable impact on red blood cell volume may not translate to intrauterine growth restriction in the rodent. Finally, we hypothesized that gut hormones might directly affect the growth axis of the dam and developing fetus. Similar to what occurs in males (27), total ghrelin (*P < 0.05) and acyl ghrelin [*P < 0.05, 0 (C-Sham versus H-VSG) and 15 min (H-VSG versus H-Sham and C-Sham] are decreased during pregnancy (Fig. 6G).
DISCUSSION
Our study indicates that VSG is effective for improving many of the comorbidities of the metabolic syndrome in female rats as has been previously reported in males (21–23). Excess weight is lost during the early postoperative phase and then remains reduced despite VSG animals being maintained on an HFD. Body weight lost is almost exclusively fat and is reflected by reduced circulating leptin. The reduction in body fat can be attributed to reduced food intake in the initial weeks after surgery. As with males (22), females respond to VSG by reducing the size of individual meals while increasing meal frequency. VSG females have improved glucose homeostasis and reduced hyperlipidemia and hypercholesterolemia despite being maintained on an HFD. This supports a growing literature documenting the positive benefits of VSG as being comparable to those of RYGB despite being a less invasive surgery that does not alter the route of nutrients through the gastrointestinal tract.
Given this spectrum of benefits, we reasoned that VSG would reverse the deleterious effects of obesity and metabolic dysfunction on offspring. Obesity during pregnancy has implications for morbidity and mortality for both mother and offspring, with a doubling of the risk of stillbirth and neonatal death (28, 29). Fetal exposure to maternal obesity increases the risk of childhood metabolic syndrome by twofold (5, 30). High maternal body mass index, even in the absence of maternal gestational diabetes, is a risk factor for large-for-gestational age infants who are highly susceptible to lifelong disease (5, 31). Finally, women with poor glycemic control and/or gestational diabetes have a high risk for having large-for-gestational age babies that are at risk for developing obesity and insulin resistance later in life (32–34). Work from rodents and nonhuman primates recapitulates these human findings (35–38). The clear implication is that reversing obesity and its associated metabolic problems with surgery would be predicted to have a beneficial impact on the offspring.
Contrary to these predictions, our current data suggest that VSG increases the rate of small-for-gestational age offspring in rats. Pups born to females giving birth 8 weeks after VSG were small-for-gestational age because of intrauterine growth restriction in comparison to pups from either lean or obese control dams. Because this occurred on both diets, it points to an important impact of surgery on the maternal environment that appears to be independent of the level of dietary fat. During the postnatal period, whether VSG dams were maintained on chow or HFD, offspring of VSG dams failed to catch up in weight, and they had less lean mass at weaning. Maternal diet adversely affected the adiposity of the weanlings because pups whose dams consumed HFD had the greatest adiposity and circulating triglycerides irrespective of surgery.
The offspring of VSG dams fed an HFD (oH-VSG) had the worst outcomes. Despite being smaller, the offspring of H-VSG dams were glucose-intolerant at weaning with respect to control offspring and had hypercholesterolemia and hepatic steatosis similar to obese control offspring. This suggests that there is an interaction between maternal surgery and maternal/early postnatal diet.
When offspring were maintained on chow after weaning, there were few effects of either maternal diet or maternal surgery; that is, at postnatal day 60, there were no significant differences between the four groups. The smaller male oC-VSG and oH-VSG animals experienced “catchup” growth after being weaned, as they had made up for their lower body weight by postnatal day 49 and there were no longer differences by postnatal day 60. The females were still smaller at postnatal day 60 but “caught up” by postnatal day 70. There also appeared to be no lingering effect on glucose homeostasis, at least at postnatal day 60. We then challenged cohorts of each group with an HFD beginning at postnatal day 60. Offspring of VSG dams developed the greatest adiposity and had the poorest glucose control, implying that VSG increases the risk of subsequent offspring being more susceptible to the deleterious effects of an HFD.
Numerous factors in the maternal environment can result in intra-uterine growth restriction including calorie or macronutrient malnutrition (26, 39). However, neither of these can explain what happens with VSG dams; that is, by the time they were mated, VSG females had calorie intakes that were identical to those of sham controls. For this reason, we did not include a pair-fed group because pair-feeding is an additional metabolic stressor that results in durable effects in offspring. Furthermore, VSG dams were able to mount sufficient pregnancy and lactation-induced hyperphagia, and the weights of H-VSG animals were similar to those of C-Sham controls during pregnancy. Previous studies showed that pair-fed dams had normal-sized pups with reduced adiposity and improved metabolic parameters despite maternal and postnatal maintenance on an HFD (40). Collectively, these lines of evidence do not support the hypothesis that reduced maternal food consumption caused the reduced birth weights and sizes of these VSG offspring.
We carefully evaluated other possible causes of intrauterine growth restriction. Although there was a small (5%) fat malabsorption during gestation, only 40% of the calories in the HFD are from lipids, and this would produce only a 2% reduction in realizable calories. Protein malnutrition can also cause intrauterine growth restriction (26), but circulating amino acids were normal at baseline and 1 hour after protein gavage. Finally, although there was a modest suggestion of anemia after VSG during pregnancy, there were no differences in hematocrit before pregnancy (Table 1), and vitamin B12 was normal in VSG dams (Table 2).
Ghrelin administered to pregnant dams increases neonatal birth weight, and this was also true in ghrelin-injected pair-fed animals (41). When available circulating ghrelin was reduced with ghrelin antibodies, the neonates were small-for-gestational age, implicating ghrelin as a regulator of intrauterine growth and development (41). VSG results in the loss of most ghrelin-producing cells, and we have observed that VSG females have lower circulating total ghrelin in both nonpregnant and pregnant females (27). In addition, ghrelin levels remain low after refeeding or nutrient gavage. Thus, the dynamic range of ghrelin levels is greatly diminished after VSG. Our current data are consistent with a role for maternal ghrelin to stimulate the growth axis in the offspring, this being reduced after VSG and perhaps contributing to the observed intrauterine growth restriction.
An experiment to replace ghrelin levels in VSG females and observe the effect on the offspring has a number of technical complications. Ghrelin circulates in multiple forms, and each is reduced after VSG (27). Thus, it is not entirely obvious which form should be infused. Further, ghrelin secretion is highly pulsatile (42, 43), and this is an important part of many of its biological actions. It is quite difficult to provide exogenous ghrelin to dams that would adequately mimic the composition and secretion pattern of endogenous ghrelin. Thus, future experiments will require both rat and mouse models that would allow for loss- or gain-of-function studies of ghrelin, GOAT (the enzyme that posttranslationally modifies ghrelin), and the ghrelin receptor.
Few studies have addressed indices of reproductive health after bariatric surgery. In general, bariatric surgery reduces the rate of irregular cyclicity and anovulation, the degree of improvement being linked to successful weight loss after surgery (44). Although there is evidence that polycystic ovarian syndrome may be resolved with bariatric surgery (45, 46), a closer analysis of these purported improvements has revealed that not all obesity-related dysfunction is reversed despite significant weight loss (47). Overall, the human literature suggests that although significant ovulatory improvements can be realized after bariatric surgery, some aspects of dysfunctional reproductive health are not reversed.
In addition to direct improvements in metabolic health in our rat model, VSG resulted in improved estrous cyclicity. This was not surprising in C-VSG dams that were switched to chow after the surgery, but VSG also improved cyclicity in H-VSG dams, restoring it to levels similar to those of C-Sham animals. These diet-independent effects suggest that VSG-induced weight loss per se can improve reproductive cyclicity. One of the marked results of our data is that despite improved estrous cyclicity, H-VSG dams had difficulty bringing viable litters to term relative to other animals with normal cycles. When harvested before parturition, the placentas from H-VSG dams were in the process of being resorbed, whereas there were no observed problems in the other groups. Further, there were no pathologic findings in the C-Sham, H-Sham, or C-VSG placentas, although H-Sham placentas had a small number of nucleated red blood cells, potentially indicating some level of hypoxia in the fetus. However, C-VSG placentas were indistinguishable from those of C-Sham. In contrast, all H-VSG placentas were hemorrhagic and resorbing, indicating an interaction between surgery and the HFD in predisposing to deleterious consequences for pregnancy.
Few studies have reported relevant outcomes for offspring of mothers after bariatric surgery. In most studies, mothers had adjustable gastric banding, which results in less weight loss and only a modest change in key metabolic hormones. Although the rates of macrosomia, gestational diabetes, and preeclampsia are reportedly improved after adjustable gastric band, there is nonetheless a trend toward increased intrauterine growth restriction and low birth weight (18–20). Following more invasive surgeries like RYGB and biliary-pancreatic diversion, there are also reduced rates of gestational diabetes and preeclampsia in mothers who received the surgery before pregnancy (14, 15, 48). Those studies also reported less pregnancy-related weight gain and a decreased rate of macrosomia. Although these maternal improvements do show comparable beneficial outcomes for the offspring (14, 15) with significant genome-wide methylation changes that may herald metabolic improvements (49), as we have observed in rats, the human studies also indicate a trend toward increased rates of small-for-gestational age offspring born after surgery (17.5%) versus before surgery (11.1%) (14). In a recent chart review, a significant increase was similarly found: 5% of births in morbidly obese patients were small-for-gestational age versus 17.4% of births from bariatric surgery patients (48). These data raise concerns given the rising numbers of women of childbearing age receiving bariatric procedures. In humans born either small-for-gestational age or large-for-gestational age, there is an increased risk for diabetes, obesity, and other components of the metabolic syndrome later in life. Indeed, the risk of being born large-for-gestational age appears to be reduced by bariatric surgery, but the current data indicate that this does not readily translate to improved metabolic outcomes because they are more at risk of small-for-gestational age with its concomitant metabolic risks.
Our study does have some limitations for consideration. First, there are a variety of weight loss surgeries. Here, we evaluated only VSG. In the laboratory setting, VSG is a high-throughput surgery with reliable, metabolic improvements that appear similar to the more commonly used RYGB. Whether RYGB and other metabolic surgeries have similar results in offspring will need to be the focus of future research. Furthermore, in the laboratory setting, we evaluated the effects of VSG using two controlled dietary conditions with no food choice for the animals. In contrast, humans consume calories in a wide spectrum of macronutrient components and can alter food choice during pregnancy. Finally, because of forced consumption of the high-fat diet (HFD) to evaluate the interaction between diet and surgery, we observed reduced fecundity. This meant that it was necessary to make animals pregnant 5 weeks after the surgery. Although this was enough for the animals’ food intake and body weights to stabilize, physicians typically counsel patients to wait 18 to 24 months after bariatric surgery before attempting to become pregnant.
Collectively, the present data highlight that although VSG has many beneficial effects in females, it is not uniformly beneficial for their offspring. The important conclusion is that weight loss alone may not be sufficient to reduce the impact of maternal consumption of an HFD on the increased risk of metabolic disease in their offspring. This has implications for both the clinical use of these procedures and the nature of how maternal environments can affect susceptibility to metabolic disease in their offspring.
MATERIALS AND METHODS
Study design
The research objective of this controlled laboratory study was to determine whether similar metabolic improvements were realized by female rats as previously reported in males after VSG in the laboratory rat. Additionally, we sought to evaluate the impact of surgical weight loss on reproductive health, in utero growth, lactation capability, and metabolic parameters of the resulting offspring. Although power analyses based on previous work with male rodents dictated the original cohort size, the unanticipated reproductive difficulties in females after VSG resulted in the use of three separate cohorts of rats and their offspring. The primary endpoints for both the dam and offspring were body weight, body composition, food intake, glucose tolerance, and lipid profiling.
Animals
All procedures for animal use were approved by the University of Cincinnati Institutional Animal Care and Use Committee. Three cohorts (C1, C2, and C3) of adult female Long-Evan rats (Harlan Laboratories; 225 to 250 g) were individually housed and maintained in a room on a 12:12-hour light/dark cycle at 25°C and 50 to 60% humidity. Following acclimatization to the facilities, animals were given ad libitum access to water and either low-fat chow (#7012, Harlan Teklad, 3.41 kcal/g; 5.67% fat) or palatable HFD (#D03082706, Research Diets, 4.54 kcal/g; 41% fat) for 3 weeks before surgery. Animals in C1 were assorted to three groups: (i) maintained on chow and receiving sham-VSG (C-Sham), (ii) maintained on HFD and receiving sham-VSG (H-Sham), and (iii) maintained on HFD and receiving VSG surgery (H-VSG). C2 and C3 had an additional group of animals maintained on HFD and receiving VSG and then switched to chow (C-VSG). C1 and C3 generated pups, whereas C2 was sacrificed during gestational days 16 to 17.
Surgical procedures
Preoperative care
One week before surgery, animals were exposed to Osmolite OneCal liquid diet for two 24-hour periods. Four days before surgery, body composition was assessed with an EchoMRI analyzer. Animals were solid food–restricted for 24 hours and given Osmolite.
Vertical sleeve gastrectomy
VSG was performed as previously described (22). Briefly, it consisted of a midline abdominal laparotomy with exteriorization of the stomach. The lateral 80% of the stomach was excised with an ETS 35-mm staple gun, leaving a tubular gastric remnant in continuity with the esophagus. This gastric sleeve was then reintegrated into the abdominal cavity, and the abdominal wall was closed in layers.
Sham-VSG
An abdominal laparotomy was performed with exteriorization of the stomach. Light pressure with forceps was applied to the exteriorized stomach. It was then reintegrated, and the abdomen was closed in layers.
Postoperative care
Following surgery, rats received care for 3 days, consisting of twice-daily subcutaneous injections of 5 ml of saline, 0.25 ml of Buprenex (0.05 mg/kg), and 0.25 ml of Metacam (once daily, 0.5 mg/kg). Animals were maintained on Osmolite until food was returned 3 days after surgery.
Body weight, body composition, and food intake
Food intake and body weights of females having received either sham or VSG were measured daily for the first week after surgery and then measured weekly for the remainder of the study. Offspring were weighed on postnatal days 2, 7, 14, and 22 of postnatal life. After weaning, they were weighed weekly for the remainder of the study. Body composition (fat and lean mass) was determined with NMR technology (Echo NMR). NMR was performed in adult females (postoperative 26), postnatal day 22 pups, and postnatal day 59 and 110 offspring.
Husbandry and breeding
Animals were allowed to recover for 4 weeks before glucose tolerance testing. Five weeks after surgery, breeding commenced. Singly housed males were caged with one female for 4 to 8 days. Females were returned to their home cage for the remainder of gestation. C1 and C3 were bred to produce offspring. During gestation, these dams were not tested in any way. C3 was generated and tested during gestation and sacrificed before parturition. The day of birth was denoted as postnatal day 0. Animals were culled to eight pups on postnatal day 2, four males and four females when possible. Animals were weaned on postnatal day 22.
Measures of glucose and insulin tolerance
Adult females were fasted for 8 hours after the onset of the light. Baseline blood glucose was measured with an ACCU-CHEK glucometer. Females were administered either an intraperitoneal dose of dextrose (1.5 g/kg) (postoperative day 99) or an oral gavage of 1.5 g/kg of the mean body weight of the animals on the day of the experiment (postoperative day 30). Postnatal day 22 pups were fasted for 4 hours before intra-peritoneal administration of dextrose (1.25 g/kg). Adult offspring were fasted for 8 hours and given intraperitoneal dextrose (1.25 g/kg) (postnatal days 60 and 88) or an oral gavage of dextrose equaling 1.25 g/kg of the mean weight on the day of the experiment (postnatal day 95). Blood glucose was measured at 15, 30, 45, 60, and 120 min after dextrose administration. Plasma for insulin measurement was taken at 0 and 15 min. For the insulin tolerance test, females were fasted for 8 hours after light onset. Following a baseline sample, 0.75 U of insulin (Sigma) was administered, and blood glucose was measured at 15, 30, 45, and 60 min.
Metabolic analysis
Female rats (postoperative days 100 to 104) were housed in LabMaster chambers (TSE Systems), which allow simultaneous measurement of metabolic performance, home cage activity, and feeding behavior as described (50).
Hormone assays
Commercially available assays were used. Plasma insulin (#90060, Crystal Chem), total ghrelin (#EZRGRT-91K, Millipore), active ghrelin (#EZRGRA-90K, Millipore), IGF-1 (#MG100, R&D Systems), active GLP1 (#K150FAC-1, Mesoscale), vitamin B12 (#30-KIF012, ALPCO Diagnostics), and ferritin (#41-FERRT-E01, ALPCO Diagnostics) were performed according to the manufacturers’ specifications. For active GLP1, 180 ml blood into 20 ml antiproteolytic cocktail (4.65 g of EDTA + 92 mg of aprotinin + 40,000 U of heparin in 50 ml of saline). GLP1(7–36) was measured by an electrochemiluminescence assay (Meso Scale Discovery). For acyl ghrelin, blood was collected in 10 ml of 200 mM 4-(2-aminoethyl)-benzenesulfonyl fluoride (AEBSF) in EDTA-treated tubes, and then 1 N HCl was added to 100 ml of plasma.
Lipid analysis
Plasma triglycerides and cholesterol were analyzed with Infinity Liquid Stable Reagents (Thermo Scientific) according to the manufacturer’s protocol with Infinity internal standards.
Hepatic triglyceride determination
Liver triglycerides were measured with Pointe Scientific Triglyceride reagent set (#T7532-120) according to the manufacturer’s specifications.
Hematocrit determination
Blood was collected from the tail vein into heparinized microcapillary tubes. After centrifugation, hematocrit values were determined with a Micro-Hematocrit Reader.
Liver histology
Fresh-frozen samples were cryostat-sectioned. Oil red O staining was performed by the Cincinnati Children’s Hospital Medical Center (CCHMC) Pathology Core.
Placental histology
Tissue samples were fixed in 4% paraformaldehyde and stored in phosphate-buffered saline. Sections were paraffin-blocked and counterstained with H&E by CCHMC Pathology Core. Photomicrographs were acquired with a digital camera coupled to a Zeiss microscope.
Fecal analysis
Rats were temporarily placed on a similar diet to which they were accustomed but also containing 5% sucrose polybehenate (behenic acid). After 24 hours of acclimation to the diet, cages were changed and fecal pellets were collected after another 24 hours. Fecal samples of about 10 mg were collected, and fecal lipid content was assayed by gas chromatography of fatty acid methyl esters by the University of Cincinnati Mouse Metabolic Phenotyping Center. Fat absorption was calculated from the ratio of behenic acid to other fatty acids in the diet and feces.
Amino acid analysis
After a 6-hour fast, tail vein samples were collected. Animals were gavaged with 4 ml (1 g/kg) of a protein mixture (Muscle Milk, CytoSport) and samples were collected after 60 min. Samples were processed by the CCHMC Clinical Assay Core. Briefly, this methodology used ion exchange chromatography. The samples were deproteinized with 33% sulfosalicylic acid, internal standard was added and spun, and supernatant was poured off and then filtered with a Whatman Anotop 10IC syringe filter. The samples were run through a Hitachi L8800 Amino Acid Analyzer.
Statistical analyses
All statistical analyses were performed with GraphPad Prism version 4.0 (GraphPad Software). Differences between two treatments were assessed with unpaired Student’s t test and two-tailed distribution. Differences between three treatments were analyzed with one-way ANOVA followed by a Tukey post hoc test. To observe time-wise differences, two-way repeated-measures ANOVA (variables: surgical/diet group and time) with Bonferroni post hoc test was used. In the case of maternal and pup analysis where a 2 × 2 design was possible (Chow/HFD × Sham/VSG), two-way ANOVA (variables: maternal surgery and maternal diet) with Bonferroni post hoc test was used. Unique symbol notations (for example, # versus †) denote statistical differences by that variable (diet or surgery). Same symbol notations denote no statistical significance by that variable. All results are given as means ± SEM. Results were considered statistically significant when P < 0.05.
Supplementary Material
Acknowledgments
We thank J. Berger, A. Lewis, K. Parks, K. Smith, and M. Toure for their surgical expertise; N. Bedel, A. Chambers, R. Gutierrez-Aguilar, A. Hakala-Finch, I. Ressler, and K. Ryan for assistance with glucose tolerance tests; and R. Krishna for execution of assays. Also, we extend sincere gratitude to P. Pfluger for metabolic cage analysis and T. Morgan for rating placental pathology. We also thank S. Benoit, D. D'Alessio, and A. Menon for helpful discussion concerning these studies. Finally, we thank G. Doerman for assistance with graphics conversions.
Funding: The work of the laboratory is supported in part by NIH Awards DK56863, DK57900, U01CA141464, DK082480, MH069860, DK08248, and DK017844 and also by Ethicon Endo-Surgery Inc., F. Hoffman-La Roche Ltd., Pfizer Inc., and Novo Nordisk A/S. B.E.G. is supported by NIH Award 1F32HD68103.
Footnotes
Author contributions: B.E.G. and K.M.S. performed all experiments. B.E.G., S.C.W., and R.J.S. planned all experiments. B.E.G., S.C.W., and R.J.S. are responsible for interpreting and analyzing the data. B.E.G., S.C.W., and R.J.S. drafted and revised the manuscript. B.E.G., K.M.S., S.C.W., and R.J.S. approved the final draft. Competing
interests: R.J.S. has received research support from Ethicon Endo-Surgery, Mannkind, Novo Nordisk, Ablaris, Pfizer, and Roche. R.J.S. has served on scientific advisory boards for Ethicon Endo-Surgery, Angiochem, Novartis, and Novo Nordisk. R.J.S. is a paid speaker for Merck, Ethicon Endo-Surgery, Pfizer, and Novo Nordisk.
SUPPLEMENTARY MATERIALS
www.sciencetranslationalmedicine.org/cgi/content/full/5/199/199ra112/DC1
Materials and Methods
Fig. S1. Metabolic cage analysis of females.
Fig. S2. Reverse transcription polymerase chain reaction analysis of hypothalamic brain blocks of postnatal day 22 pups.
Fig. S3. Metabolic characteristics of female rodent offspring.
REFERENCES AND NOTES
- 1.Metwally M, Li TC, Ledger WL. The impact of obesity on female reproductive function. Obes. Rev. 2007;8:515–523. doi: 10.1111/j.1467-789X.2007.00406.x. [DOI] [PubMed] [Google Scholar]
- 2.Plagemann A, Harder T, Kohlhoff R, Rohde W, Dörner G. Glucose tolerance and insulin secretion in children of mothers with pregestational IDDM or gestational diabetes. Diabetologia. 1997;40:1094–1100. doi: 10.1007/s001250050792. [DOI] [PubMed] [Google Scholar]
- 3.Cedergren MI. Maternal morbid obesity and the risk of adverse pregnancy outcome. Obstet. Gynecol. 2004;103:219–224. doi: 10.1097/01.AOG.0000107291.46159.00. [DOI] [PubMed] [Google Scholar]
- 4.Watkins ML, Rasmussen SA, Honein MA, Botto LD, Moore CA. Maternal obesity and risk for birth defects. Pediatrics. 2003;111:1152–1158. [PubMed] [Google Scholar]
- 5.Boney CM, Verma A, Tucker R, Vohr BR. Metabolic syndrome in childhood: Association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics. 2005;115:e290–e296. doi: 10.1542/peds.2004-1808. [DOI] [PubMed] [Google Scholar]
- 6.Sinha R, Fisch G, Teague B, Tamborlane WV, Banyas B, Allen K, Savoye M, Rieger V, Taksali S, Barbetta G, Sherwin RS, Caprio S. Prevalence of impaired glucose tolerance among children and adolescents with marked obesity. N. Engl. J. Med. 2002;346:802–810. doi: 10.1056/NEJMoa012578. [DOI] [PubMed] [Google Scholar]
- 7.Juonala M, Magnussen CG, Berenson GS, Venn A, Burns TL, Sabin MA, Srinivasan SR, Daniels SR, Davis PH, Chen W, Sun C, Cheung M, Viikari JS, Dwyer T, Raitakari OT. Childhood adiposity adult adiposity and cardiovascular risk factors. N. Engl. J. Med. 2011;365:1876–1885. doi: 10.1056/NEJMoa1010112. [DOI] [PubMed] [Google Scholar]
- 8.Gupta R, Bhangoo A, Matthews NA, Anhalt H, Matta Y, Lamichhane B, Malik S, Narwal S, Wetzler G, Ten S. The prevalence of non-alcoholic fatty liver disease and metabolic syndrome in obese children. J. Pediatr. Endocrinol. Metab. 2011;24:907–911. doi: 10.1515/jpem.2011.282. [DOI] [PubMed] [Google Scholar]
- 9.Puder JJ, Munsch S. Psychological correlates of childhood obesity. Int. J. Obes. 2010;34(Suppl. 2):S37–S43. doi: 10.1038/ijo.2010.238. [DOI] [PubMed] [Google Scholar]
- 10.Clark AM, Thornley B, Tomlinson L, Galletley C, Norman RJ. Weight loss in obese infertile women results in improvement in reproductive outcome for all forms of fertility treatment. Hum. Reprod. 1998;13:1502–1505. doi: 10.1093/humrep/13.6.1502. [DOI] [PubMed] [Google Scholar]
- 11.Clark AM, Ledger W, Galletly C, Tomlinson L, Blaney F, Wang X, Norman RJ. Weight loss results in significant improvement in pregnancy and ovulation rates in anovulatory obese women. Hum. Reprod. 1995;10:2705–2712. doi: 10.1093/oxfordjournals.humrep.a135772. [DOI] [PubMed] [Google Scholar]
- 12.Jeffery RW, Drewnowski A, Epstein LH, Stunkard AJ, Wilson GT, Wing RR, Hill DR. Long-term maintenance of weight loss: Current status. Health Psychol. 2000;19:5–16. doi: 10.1037/0278-6133.19.suppl1.5. [DOI] [PubMed] [Google Scholar]
- 13.Brinckerhoff TZ, Bondada S, Lewis CE, French SW, DeUgarte DA. Metabolic effects of sleeve gastrectomy in female rat model of diet-induced obesity. Surg. Obes. Relat. Dis. 2013;9:108–112. doi: 10.1016/j.soard.2011.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith J, Cianflone K, Biron S, Hould FS, Lebel S, Marceau S, Lescelleur O, Biertho L, Simard S, Kral JG, Marceau P. Effects of maternal surgical weight loss in mothers on intergenerational transmission of obesity. J. Clin. Endocrinol. Metab. 2009;94:4275–4283. doi: 10.1210/jc.2009-0709. [DOI] [PubMed] [Google Scholar]
- 15.Kral JG, Biron S, Simard S, Hould FS, Lebel S, Marceau S, Marceau P. Large maternal weight loss from obesity surgery prevents transmission of obesity to children who were followed for 2 to 18 years. Pediatrics. 2006;118:e1644–e1649. doi: 10.1542/peds.2006-1379. [DOI] [PubMed] [Google Scholar]
- 16.Smoot TM, Xu P, Hilsenrath P, Kuppersmith NC, Singh KP. Gastric bypass surgery in the United States 1998–s2002. Am. J. Public Health. 2006;96:1187–1189. doi: 10.2105/AJPH.2004.060129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Asarian L, Abegg K, Geary N, Schiesser M, Lutz TA, Bueter M. Estradiol increases body weight loss and gut-peptide satiation after Roux-en-Y gastric bypass in ovariectomized rats. Gastroenterology. 2012;143:325–327. doi: 10.1053/j.gastro.2012.05.008. e2. [DOI] [PubMed] [Google Scholar]
- 18.Weintraub AY, Levy A, Levi I, Mazor M, Wiznitzer A, Sheiner E. Effect of bariatric surgery on pregnancy outcome. Int. J. Gynaecol. Obstet. 2008;103:246–251. doi: 10.1016/j.ijgo.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 19.Sheiner E, Menes TS, Silverberg D, Abramowicz JS, Levy I, Katz M, Mazor M, Levy A. Pregnancy outcome of patients with gestational diabetes mellitus following bariatric surgery. Am. J. Obstet. Gynecol. 2006;194:431–435. doi: 10.1016/j.ajog.2005.08.056. [DOI] [PubMed] [Google Scholar]
- 20.Sheiner E, Levy A, Silverberg D, Menes TS, Levy I, Katz M, Mazor M. Pregnancy after bariatric surgery is not associated with adverse perinatal outcome. Am. J. Obstet. Gynecol. 2004;190:1335–1340. doi: 10.1016/j.ajog.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 21.Stefater MA, Sandoval DA, Chambers AP, Wilson-Pérez HE, Hofmann SM, Jandacek R, Tso P, Woods SC, Seeley RJ. Sleeve gastrectomy in rats improves postprandial lipid clearance by reducing intestinal triglyceride secretion. Gastroenterology. 2011;41:939–949. doi: 10.1053/j.gastro.2011.05.008. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stefater MA, Pérez-Tilve D, Chambers AP, Wilson-Pérez HE, Sandoval DA, Berger J, Toure M, Tschöp M, Woods SC, Seeley RJ. Sleeve gastrectomy induces loss of weight and fat mass in obese rats but does not affect leptin sensitivity. Gastroenterology. 2010;138:2426–2436. doi: 10.1053/j.gastro.2010.02.059. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chambers AP, Jessen L, Ryan KK, Sisley S, Wilson-Pérez HE, Stefater MA, Gaitonde SG, Sorrell JE, Toure M, Berger J, D'Alessio DA, Woods SC, Seeley RJ, Sandoval DA. Weight-independent changes in blood glucose homeostasis after gastric bypass or vertical sleeve gastrectomy in rats. Gastroenterology. 2011;141:950–958. doi: 10.1053/j.gastro.2011.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bayham BE, Greenway FL, Bellanger DE, O'Neil CE. Early resolution of type 2 diabetes seen after Roux-en-Y Gastric bypass and vertical sleeve gastrectomy. Diabetes Technol. Ther. 2012;14:30–34. doi: 10.1089/dia.2011.0151. [DOI] [PubMed] [Google Scholar]
- 25.Schauer PR, Kashyap SR, Wolski K, Brethauer SA, Kirwan JP, Pothier CE, Thomas S, Abood B, Nissen SE, Bhatt DL. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N. Engl. J. Med. 2012;366:1567–1576. doi: 10.1056/NEJMoa1200225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kramer MS. Determinants of low birth weight: Methodological assessment and meta-analysis. Bull. World Health Organ. 1987;65:663–737. [PMC free article] [PubMed] [Google Scholar]
- 27.Chambers AP, Kirchner H, Wilson-Perez HE, Willency JA, Hale JE, Gaylinn BD, Thorner MO, Pfluger PT, Gutierrez JA, Tschöp MH, Sandoval DA, Seeley RJ. The effects of vertical sleeve gastrectomy in rodents are ghrelin independent. Gastroenterology. 2013;144:50–52. doi: 10.1053/j.gastro.2012.09.009. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cnattingius S, Bergström R, Lipworth L, Kramer MS. Prepregnancy weight and the risk of adverse pregnancy outcomes. N. Engl. J. Med. 1998;338:147–152. doi: 10.1056/NEJM199801153380302. [DOI] [PubMed] [Google Scholar]
- 29.Kristensen J, Vestergaard M, Wisborg K, Kesmodel U, Secher NJ. Pre-pregnancy weight and the risk of stillbirth and neonatal death. BJOG. 2005;112:403–408. doi: 10.1111/j.1471-0528.2005.00437.x. [DOI] [PubMed] [Google Scholar]
- 30.Wei JN, Sung FC, Li CY, Chang CH, Lin RS, Lin CC, Chiang CC, Chuang LM. Low birth weight and high birth weight infants are both at an increased risk to have type 2 diabetes among schoolchildren in Taiwan. Diabetes Care. 2003;26:343–348. doi: 10.2337/diacare.26.2.343. [DOI] [PubMed] [Google Scholar]
- 31.Ehrenberg HM, Mercer BM, Catalano PM. The influence of obesity and diabetes on the prevalence of macrosomia. Am. J. Obstet. Gynecol. 2004;191:964–968. doi: 10.1016/j.ajog.2004.05.052. [DOI] [PubMed] [Google Scholar]
- 32.Plagemann A, Harder T, Kohlhoff R, Rohde W, Dörner G. Overweight and obesity in infants of mothers with long-term insulin-dependent diabetes or gestational diabetes. Int. J. Obes. Relat. Metab. Disord. 1997;21:451–456. doi: 10.1038/sj.ijo.0800429. [DOI] [PubMed] [Google Scholar]
- 33.Gillman MW, Rifas-Shiman S, Berkey CS, Field AE, Colditz GA. Maternal gestational diabetes birth weight and adolescent obesity. Pediatrics. 2003;111:e221–e226. doi: 10.1542/peds.111.3.e221. [DOI] [PubMed] [Google Scholar]
- 34.Lindsay RS, Hanson RL, Bennett PH, Knowler WC. Secular trends in birth weight BMI and diabetes in the offspring of diabetic mothers. Diabetes Care. 2000;23:1249–1254. doi: 10.2337/diacare.23.9.1249. [DOI] [PubMed] [Google Scholar]
- 35.Sun B, Purcell RH, Terrillion CE, Yan J, Moran TH, Tamashiro KL. Maternal high-fat diet during gestation or suckling differentially affects offspring leptin sensitivity and obesity. Diabetes. 2012;61:2833–2841. doi: 10.2337/db11-0957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McCurdy CE, Bishop JM, Williams SM, Grayson BE, Smith MS, Friedman JE, Grove KL. Maternal high-fat diet triggers lipotoxicity in the fetal livers of nonhuman primates. J. Clin. Invest. 2009;119:323–335. doi: 10.1172/JCI32661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gorski JN, Dunn-Meynell AA, Levin BE. Maternal obesity increases hypothalamic leptin receptor expression and sensitivity in juvenile obesity-prone rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007;292:R1782–R1791. doi: 10.1152/ajpregu.00749.2006. [DOI] [PubMed] [Google Scholar]
- 38.Chen H, Simar D, Morris MJ. Hypothalamic neuroendocrine circuitry is programmed by maternal obesity: Interaction with postnatal nutritional environment. PLoS One. 2009;4:e6259. doi: 10.1371/journal.pone.0006259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fernandez SF, Menendez MF, Fernandez BM, Patterson AM. Malnutrition in utero and during lactation in the rat: Relationship of dams weight gain and development of suckling. Nutr. Res. 1985;5:413–421. [Google Scholar]
- 40.White CL, Purpera MN, Morrison CD. Maternal obesity is necessary for programming effect of high-fat diet on offspring. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009;296:R1464–R1472. doi: 10.1152/ajpregu.91015.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakahara K, Nakagawa M, Baba Y, Sato M, Toshinai K, Date Y, Nakazato M, Kojima M, Miyazato M, Kaiya H, Hosoda H, Kangawa K, Murakami N. Maternal ghrelin plays an important role in rat fetal development during pregnancy. Endocrinology. 2006;147:1333–1342. doi: 10.1210/en.2005-0708. [DOI] [PubMed] [Google Scholar]
- 42.Bagnasco M, Kalra PS, Kalra SP. Ghrelin and leptin pulse discharge in fed and fasted rats. Endocrinology. 2002;143:726–729. doi: 10.1210/endo.143.2.8743. [DOI] [PubMed] [Google Scholar]
- 43.Tolle V, Bassant MH, Zizzari P, Poindessous-Jazat F, Tomasetto C, Epelbaum J, Bluet-Pajot MT. Ultradian rhythmicity of ghrelin secretion in relation with GH feeding behavior and sleep-wake patterns in rats. Endocrinology. 2002;143:1353–1361. doi: 10.1210/endo.143.4.8712. [DOI] [PubMed] [Google Scholar]
- 44.Teitelman M, Grotegut CA, Williams NN, Lewis JD. The impact of bariatric surgery on menstrual patterns. Obes. Surg. 2006;16:1457–1463. doi: 10.1381/096089206778870148. [DOI] [PubMed] [Google Scholar]
- 45.Escobar-Morreale HF, Botella-Carretero JI, Alvarez-Blasco F, Sancho J, San Millán JL. The polycystic ovary syndrome associated with morbid obesity may resolve after weight loss induced by bariatric surgery. J. Clin. Endocrinol. Metab. 2005;90:6364–6369. doi: 10.1210/jc.2005-1490. [DOI] [PubMed] [Google Scholar]
- 46.Eid GM, Cottam DR, Velcu LM, Mattar SG, Korytkowski MT, Gosman G, Hindi P, Schauer PR. Effective treatment of polycystic ovarian syndrome with Roux-en-Y gastric bypass. Surg. Obes. Relat. Dis. 2005;1:77–80. doi: 10.1016/j.soard.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 47.Rochester D, Jain A, Polotsky AJ, Polotsky H, Gibbs K, Isaac B, Zeitlian G, Hickmon C, Feng S, Santoro N. Partial recovery of luteal function after bariatric surgery in obese women. Fertil. Steril. 2009;92:1410–1415. doi: 10.1016/j.fertnstert.2008.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lesko J, Peaceman A. Pregnancy outcomes in women after bariatric surgery compared with obese and morbidly obese controls. Obstet. Gynecol. 2012;119:547–554. doi: 10.1097/AOG.0b013e318239060e. [DOI] [PubMed] [Google Scholar]
- 49.Guénard F, Deshaies Y, Cianflone K, Kral JG, Marceau P, Vohl MC. Differential methylation in glucoregulatory genes of offspring born before vs. after maternal gastrointestinal bypass surgery. Proc. Natl. Acad. Sci. U.S.A. 2013;110:11439–11444. doi: 10.1073/pnas.1216959110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tang-Christensen M, Vrang N, Ortmann S, Bidlingmaier M, Horvath TL, Tschöp M. Central administration of ghrelin and agouti-related protein (83–132) increases food intake and decreases spontaneous locomotor activity in rats. Endocrinology. 2004;145:4645–4652. doi: 10.1210/en.2004-0529. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.