Abstract
Background
Increased pathologic complete response (pCR) rates observed with neoadjuvant chemotherapy (NCT) for some subsets of patients with invasive breast cancer has prompted interest in whether patients with pCR can be identified preoperatively and potentially spared the morbidity of surgery. This multicenter retrospective study was performed to estimate the accuracy of preoperative MRI in predicting pCR in the breast.
Methods
MRI at baseline and after completion of NCT plus data regarding pathologic response was collected retrospectively from 746 women treated at 8 institutions between 2002–2011. Tumors were characterized by immunohistochemical (IHC) phenotype into 4 categories based on receptor expression: hormone (estrogen & progesterone) receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2) negative (n=327), HR-positive, HER2-positive, (n=148), HR-negative, HER2-positive, (n=101), and triple-negative (HR-negative, HER2-negative, n=155). 194/249 (78%) patients with HER2-positive tumors received trastuzumab. Univariate and multivariate analyses of factors associated with radiographic complete response (rCR) and pCR were performed.
Results
rCR and pCR for total group were 182/746 (24%) and 179/746 (24%), respectively, with the highest rate of pCR seen among triple-negative (57/155; 37%) and HER2 positive (38/101; 38%) subtypes. Overall accuracy of MRI for pCR prediction was 74%. Sensitivity, NPV, PPV, and accuracy differed significantly among tumor subtypes, with the greatest NPV in the TN (60%) and HER2 positive (62%) subtypes.
Conclusion
Overall accuracy of MRI for predicting pCR in invasive breast cancer patients receiving NCT was 74%. MR performance differed among subtypes possibly influenced by differences in pCR rates between groups. Future studies will determine whether MRI in combination with directed core biopsy improves predictive value for pathologic response.
Keywords: breast cancer, neoadjuvant chemotherapy, MRI, accuracy, pathologic complete response
Background
Neoadjuvant chemotherapy (NCT) is increasingly used for the treatment of invasive, high-risk breast cancer. The use of effective first-line chemotherapeutic agents as well as targeted therapies such as trastuzumab for HER2+ disease in the neoadjuvant setting have resulted in a high rate of pathologic complete response (pCR) ranging from 40–67% depending on the study population 1–4. It remains unknown whether excision of the tumor bed in the setting of pCR improves locoregional recurrence risk; thus there has been keen interest in determining whether negative imaging following systemic therapy could identify a patient subset that could be safely treated with radiation alone without surgery. Prior studies evaluating RT as the definitive modality in treating the breast in patients with a clinical complete response to NCT have resulted in unacceptably high locoregional failure rates5–7. This may in large part be attributed to poor patient selection due to the fact that clinical examination to detect residual disease or response is known to be limited8–10. A more sensitive tool to evaluate in-breast response could more effectively identify potential candidates for RT alone following a complete response to NCT.
One such tool is magnetic resonance imaging (MRI) which has been used with increasing frequency in recent years due to its high sensitivity in detection of breast cancers when compared to mammography or ultrasound11–14. The ability of a radiologic complete response (rCR) by MRI to predict pathologic complete response (pCR) has been the subject of active investigation. Several groups have evaluated the predictive accuracy of MRI for assessing response to NCT and found limited correlation between rCR after completion of neoadjuvant chemotherapy and pCR13, 15–17. Although the performance of MRI appeared better in some tumor phenotypes compared to others, these small retrospective studies lacked the power to detect a significant difference among clinically distinct subsets. In this study we sought to determine the performance of MRI following neoadjuvant chemotherapy in a larger multi-center dataset in order to better define the accuracy of post-treatment breast MRI in the prediction of pCR. Moreover, we wished to identify which tumor-related variables were associated with the highest correlation between radiologic and pathologic complete response in order to identify a patient population which may be most amenable to treatment with whole breast radiation without surgery based on demonstration of rCR.
Materials and Methods
Patient selection
Patients with pre- and post-neoadjuvant therapy MR breast imaging were retrospectively identified at 8 NCI-designated comprehensive cancer centers. Between January 2002 and Feb 2011, 770 women fulfilled study criteria. Identification of patients across institutions was stipulated by cross referencing billing codes of patients with a breast cancer diagnosis who were treated with chemotherapy and imaged with breast MRI. Among this group were 165 patients treated on the I-SPY trials which are prospective multicenter trials of women treated with neoadjuvant chemotherapy including radiographic and pathologic endpoints. All participating institutions were members of the Translational Breast Cancer Research Consortium (TBCRC) which sponsored the study, and included The University of Alabama at Birmingham, The University of Pittsburgh Medical Center, Dana-Farber Cancer Institute, The University of Texas MD Anderson Cancer Center, Duke University, The University of Chicago, The University of North Carolina Chapel Hill, and the University of California San Francisco. IRB approval for the study was obtained at each institution. In addition to pre- and post-NCT MR imaging, eligible patients were required to have undergone definitive surgery with pathology available for review. Patient, tumor, and treatment related variables were entered into a secure, password-protected on-line database. Documentation of baseline and post-treatment imaging with mammography, and ultrasound of the breast and lymph nodes were additionally recorded.
Tumor Classification
Histological tumor types were recorded as follows: invasive ductal carcinoma (IDC), pure invasive lobular carcinoma (ILC), invasive mammary carcinoma with ductal and lobular features, and invasive mammary carcinoma NOS. ER and PR status (positive or negative, with positive defined as ≥ 1%) and percentage of cells positive, and HER2 status (positive or negative) were also collected. HER2 status was determined by local testing according to the 2007 ASCO/CAP Guidelines18. All histopathology and biomarker assessments were performed at the individual sites. Pathologic response in the breast was categorized as: no residual invasive disease or DCIS; no residual invasive cancer with DCIS present; and residual invasive disease, including microscopic residual invasive disease19. To assess the primary endpoints of sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of MRI, pathologic complete response in the breast was defined as resolution of both invasive disease and DCIS.
Breast Imaging
All patients were required to have both baseline and post-treatment MRI to be eligible for the study. At presentation, all patients in the cohort had an enhancing lesion on MRI corresponding to the known biopsy-proven cancer. Baseline size of lesion defined as the maximal diameter in a single dimension by pretreatment MRI, mammogram, and ultrasound was recorded. A radiographic T classification was assigned based on largest imaging size by any modality. Patients with unknown primary lesions were excluded. Specific parameters for dynamic contrast-enhanced (DCE) breast imaging were not defined for eligibility; however, institutions included on this study have high levels of expertise in breast MR imaging. Central review of MR images was not performed. Complete MR response in the breast was defined as resolution of all areas of abnormal enhancement, mass, or distortion.
Systemic Treatment
All study patients were treated with preoperative systemic therapy. The number of cycles of chemotherapy prior to post-treatment MRI was recorded. Patients were additionally noted as having received trastuzumab, bevacizumab, or neoadjuvant hormonal therapy.
Statistical analysis
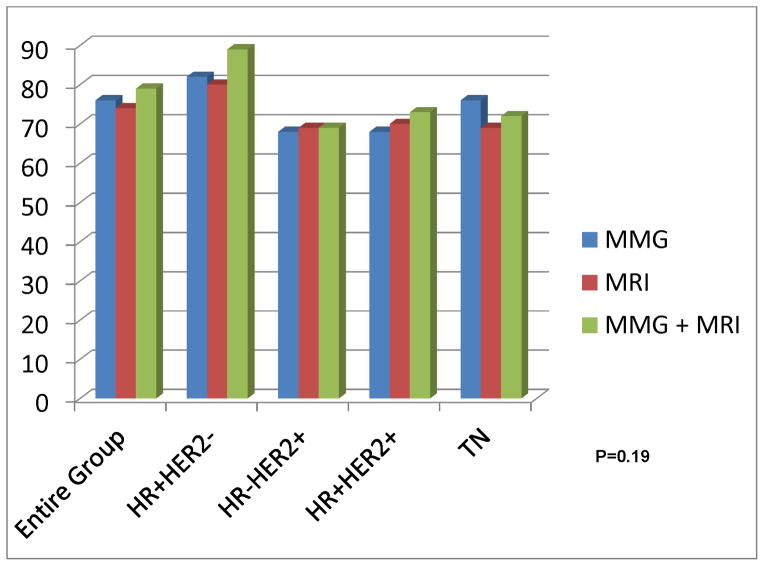
The sensitivity, specificity, PPV, NPV and accuracy of MRI for detecting residual disease in the breast were estimated from the data with pCR as defined above as complete resolution of both invasive cancer and DCIS. True negative (TN), false negative (FN), true positive (TP), and false positive (FP) were defined as follows: TN: negative on both MRI and pathology; FN: negative on MRI, but positive on pathology; TP: positive on both MRI and pathology; FP: positive on pathology, but negative on MRI. Accuracy was defined as the percentage of test results correctly identified by the test, i.e. (true positives + true negatives)/total test results =(TP+TN)/(TP+TN+FP+FN). Accuracy of MMG and MRI was defined separately for each individual modality (Figure 1). Combined accuracy of both modalities was scored as accurate if both modalities predicted pCR.
Figure 1.
Accuracy of Post-treatment Imaging for Prediction of pCR Between Tumor Subtypes for Individual and Combined Imaging Modalities
Comparisons of patient subsets are based on the Chi-Square test for contingency tables. Multivariable logistic regression was used to examine the simultaneous effects of multiple factors. SAS (version 9.2) was used for all analyses.
Results
Patients
Data from 770 women treated at 8 tertiary NCI comprehensive cancer centers were collected. After excluding patients with missing data (MRI response after NCT or final pathology), 746 patients remained evaluable for the primary endpoint. The median age of the cohort was 49 years (range 20–86). Histologic subtype rendered on initial core biopsy was generally representative of newly diagnosed cancers. 61% were estrogen receptor (ER) positive, 54% were progesterone receptor positive, 34% were HER2 positive. A summary of patient and tumor characteristics is shown in Table 1.
Table 1.
Patient Characteristics and in-Breast Radiographic and Pathologic Response
| Patient Characteristics | Entire Cohort | Complete MR Response (%) | p* | Complete Pathologic Response (%) | p* |
|---|---|---|---|---|---|
| Age | 0.29 | 0.56 | |||
| 18–40 | 168 | 47 (28) | 43 (26) | ||
| 40–60 | 463 | 104 (22) | 105 (23) | ||
| >60 | 112 | 30 (27) | 30 (27) | ||
| Race | 0.16 | < 0.0001 | |||
| White | 591 | 155 (26) | 120 (20) | ||
| Black | 87 | 15 (17) | 34 (39) | ||
| Hispanic | 22 | 4 (18) | 11 (50) | ||
| Other | 46 | 8 (17) | 14 (30) | ||
| T Stage | 0.0002 | 0.04 | |||
| I | 65 | 26 (40) | 19 (27) | ||
| II | 418 | 113 (27) | 112 (27) | ||
| III | 226 | 39 (17) | 39 (17) | ||
| IV | 35 | 4 (11) | 9 (26) | ||
| Histology | 0.41 | 0.13 | |||
| Invasive ductal | 637 | 151 (24) | 159 (25) | ||
| Invasive lobular | 61 | 20 (33) | 7 (11) | ||
| Mixed ducto-lobular | 40 | 10 (25) | 10 (25) | ||
| Invasive mammary carcinoma NOS | 7 | 1 (14) | 2 (29) | ||
| Tumor Grade | 0.004 | <0.0001 | |||
| Grade I | 49 | 9 (18) | 4 (8) | ||
| Grade II | 298 | 56 (19) | 37 (12) | ||
| Grade III | 386 | 113 (29) | 134 (35) | ||
| Receptor Status | |||||
| ER | 0.005 | <0.0001 | |||
| Positive | 455 | 95 (21) | 70 (15) | ||
| Negative | 290 | 87 (30) | 109 (38) | ||
| PR | 0.01 | <0.0001 | |||
| Positive | 398 | 82 (21) | 57 (14) | ||
| Negative | 145 | 99 (29) | 121 (35) | ||
| HER2 | 0.05 | 0.004 | |||
| Positive | 250 | 72 (29) | 76 (30) | ||
| Negative | 485 | 108 (22) | 101 (21) | ||
| Breast Cancer Subtype | 0.007 | <0.0001 | |||
| HR(+)/ HER2(−) | 327 | 60 (18) | 44 (13) | ||
| HR(−)/HER2(+)** | 101 | 29 (29) | 38 (38) | ||
| HR(+)/ HER2(+) | 148 | 43 (29) | 37 (25) | ||
| Triple Negative | 155 | 47 (30) | 57 (37) |
p-value based on chi-square test
HR status unknown in one HER2+ patient
Systemic Therapy
The most common chemotherapy regimen delivered included doxorubicin, cyclophosphamide, and a taxane (51%), other regimens (35%), taxane and carboplatin (9%), and doxorubicin and cyclophosphamide (5%). Five percent of patients received bevacizumab, and 18 patients received neoadjuvant hormonal therapy only. Of 248 HER2 positive breast tumors, 194 (78%) received trastuzumab. The remaining 54 HER2 positive breast tumors were treated prior to 2005 and did not receive trastuzumab.
Surgery
The median interval between posttreatment scans and surgery was 20 days. Definitive surgery was mastectomy in 54% of women with or without reconstruction and breast conservation in 46%. Nineteen percent underwent sentinel lymph node biopsy prior to starting systemic treatment, 42% underwent sentinel node biopsy as part of definitive surgery and 72% had level I–II axillary node dissections.
Performance of Breast MRI
Overall, post-treatment MRI detected residual disease in the breast with a sensitivity of 83%, specificity of 47%, PPV of 47%, NPV of 83%, and accuracy of 74%. Table 2 additionally illustrates the performance of MRI of the breast when stratified by IHC phenotypes. There were significant differences in sensitivity, NPV, PPV and accuracy among subtypes. NPV was highest for patients with HR(−) HER2(+) and TN breast cancers. HER2(+) breast tumors differed according to receipt of trastuzumab (Table 3). The addition of trastuzumab in HER2+ breast tumors significantly increased the rate of rCR as compared with no trastuzumab (32.5% vs 15%, respectively, p<0.0001) and was reflected in the increased rate of pCR (33.5% vs 18.5%, respectively, p=0.01). However, this did not translate into a difference in accuracy of MRI for predicting pCR among patients.
Table 2.
Comparison of Post-Treatment Breast MRI Performance between Tumor Subtypes
| Tumor Subtype | Sensitivity (%) | Specificity (%) | NPV (%) | PPV (%) | Accuracy (%) |
|---|---|---|---|---|---|
| Total Group | 470/567 (83) | 85/179 (47) | 85/182 (47) | 470/564 (83) | 555/746 (74) |
| HR+/HER2− | 243/283 (86) | 20/44 (45) | 20/160 (33) | 243/267 (91) | 263/327 (80) |
| HR−/HER2+ | 52/63 (83) | 18/38 (47) | 18/29 (62) | 52/72 (72) | 70/101 (69) |
| HR+/HER2+ | 86/111 (77) | 18/37 (49) | 18/43 (42) | 86/105 (82) | 104/148 (70) |
| TN | 79/98 (81) | 28/57 (49) | 28/47 (60) | 79/108 (73) | 107/155 (69) |
| p value* | 0.02 | NS | 0.014 | <0.0001 | 0.01 |
p values compare the 4 molecular subtypes for each performance measure (sensitivity, specificity, NPV, PPV, and accuracy) with MR breast imaging using the Chi-square test.
Table 3.
Comparison of Post-treatment Breast MRI Performance for pCR* in Patients with HER2+ Tumors Treated With and Without Trastuzumab
| Trastuzumab Use** HR+/−Her2+ | Sensitivity | Specificity | NPV | PPV | Accuracy |
|---|---|---|---|---|---|
| Yes | 98/129 (76) | 32/65 (49) | 32/63 (51) | 98/131 (75) | 130/194 (67) |
| No | 39/44 (89) | 3/10 (30) | 3/8 (38) | 39/46 (85) | 42/54 (78) |
| p value | 0.07 | NS | NS | 0.16 | 0.12 |
pCR: resolution of invasive disease and DCIS
Use of trastuzumab unknown in one HR+HER2+ patient
As a secondary aim we also assessed whether complete response on post-NCT mammogram or ultrasound (when available) in combination with MRI increased accuracy for predicting pCR. The accuracy of mammogram and MRI, separately and in combination for determining pCR is listed in Figure 1. A complete response on both mammogram and MRI, did not significantly increase the ability to detect patients with pCR over MRI alone although as expected, rCR by mammogram (p< 0.0001) or ultrasound (p< 0.0001) were also significantly associated with rCR on MRI (data not shown). The small number of patients imaged post-treatment with all three imaging modalities (MRI, MMG, and ultrasound) precluded formal evaluation of trimodality performance.
Covariates Associated with rCR and pCR
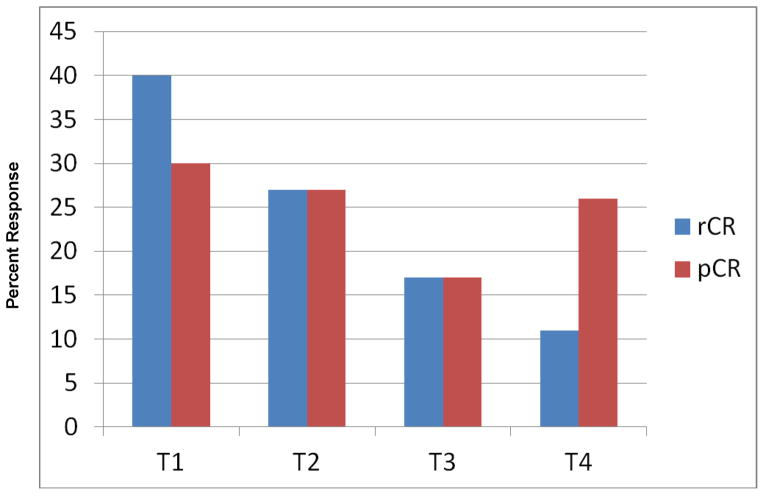
rCR and pCR for the total group were 182/746 (24%) and 179/746 (24%), respectively. Tumor characteristics significantly associated with both complete radiographic and pathologic response on MRI included lower radiographic baseline T classification, high grade, ER and/or PR negative status, and HR(−)HER2(+) or TN IHC phenotype (Table 1). On univariate analysis, race was associated with pCR but not rCR. HER2(+) breast tumors had a trend towards increased rate of rCR (p=0.05). Treatment regimens associated with highest rates of rCR included TC chemotherapy (p<0.0001) and use of trastuzumab containing regimens among HER2+ breast tumors (p=0.0001). Size of tumor by pretreatment MRI was also evaluated as a continuous variable to determine whether a significant cutoff for rCR could be determined. Patients whose tumors were ≤ 5 cm in size by pretreatment MR imaging had a higher chance of obtaining an rCR (p=0.09) and for achieving a pCR in those who achieved an rCR (p = 0.06), although these findings did not reach statistical significance (Figure 2).
Figure 2.
Association of Radiographic and Pathologic Complete Response with Baseline Tumor Size
Multivariate logistic regression analysis for variables significantly influencing rCR and pCR was performed with the final model including baseline T classification, age, race, and tumor subtype. Variables independently associated with rCR included TN or HER2+ tumor subtype (p=0.03) and lower T stage at presentation (p=0.002). Variables independently associated with likelihood of pCR, were TN or HER2+ tumor subtype (p<0.0001), lower T stage (p=0.01), and African American race (p=0.0003).
Discussion
The increasingly high rate of pCR in some subsets of patients receiving NCT for invasive breast cancer has raised the question of whether patients who achieve pCR require surgical resection of the tumor bed, or whether the breast can be appropriately managed with radiation therapy alone 17. However safe omission of surgery in patients treated with neoadjuvant therapy who achieve a rCR depends on the ability to accurately estimate pCR preoperatively. MRI has an increased sensitivity in detecting residual disease in the breast when compared to either mammogram or ultrasound making it a potentially useful tool in this setting.20–25. The performance of MRI in predicting pathologic complete response in breast cancer patients treated with NCT has been the topic of several publications over the past several years12, 14, 15, 17, 26–36. These mostly included small single institution studies which reported that post-treatment MRI correlated more closely with final pathology than physical exam, mammogram, or ultrasound. However, a broad range of predictive accuracy (58–93%) was identified. One of the best characterized studies is the I-SPY 1 trial29–31, 37 which found in 216 evaluable patients that MRI findings were a better indicator of pathologic response to NCT than clinical assessment30. However, I-SPY 1 was underpowered to detect significant subset differences.
The present analysis was performed to inform the feasibility of using MRI in-breast response in a prospective trial omitting breast surgery by using radiotherapy alone in patients who achieved a radiographic complete response after NCT. Because omission of surgery in patients with a rCR would not be considered feasible if residual DCIS remained in the breast, we defined pCR as resolution of both invasive disease and DCIS. This definition of pCR has also been associated with an improvement in disease-free survival compared to patients with resolution of invasive disease but with residual DCIS in the breast following NCT19. We also wished to determine whether there were differences in MRI accuracy between tumor subtypes.
We found a statistically significant difference in MRI sensitivity, NPV, PPV and accuracy for detecting pCR among different IHC phenotypes when pCR was defined according to either the standard definition of resolution of invasive disease only (data not shown) or more rigorously as resolution of both invasive disease and DCIS. Previous trials have suggested differences in the performance of MRI among different IHC phenotypes15, 31, 33, 38. We confirmed that among patients with rCR, positive HR status and low tumor grade were most commonly associated with residual disease at surgery, suggesting that rCR on preoperative MRI in these patient populations should be interpreted with caution. However, it is important to note that differences in NPV, PPV and accuracy among phenotype subsets are impacted by varying prevalence of pCR among subtypes. Similarly, comparisons showing significant differences among variables for complete radiographic response are reflective of disease sensitivity to NCT rather than differing MR assessment quality across these subtypes. Although retrospective, the present study represents the single largest analysis addressing this question.
There were several important limitations to note. The study did not include either central radiology review or central pathology review. However, data collection was performed across 8 NCI comprehensive cancer centers, each with substantial expertise in MR imaging techniques and interpretation, as well as significant expertise in surgical breast pathology. Our goal was to create a dataset that was generalizable across different situations and institutions, and endpoints chosen were objective and unambiguous, minimizing potential for subjective bias. In addition, we did not collect data on markers of proliferation, such as Ki-67, that would allow further characterization of ER+ tumors into luminal A and B subtypes and facilitate analyses of differences between these subsets in imaging accuracy. Finally, the specificities of the MR coil strength, contrast type, and infusion protocol for every institution were not collected on this retrospective study and indeed, they changed over time at each institution. While these limitations should be acknowledged, they nevertheless allow for greater generalizability of our findings across many different clinical settings.
In this study, the addition of mammographic rCR to MRI in a subset of patients did not improve the ability to detect residual microscopic disease. The role of ultrasound could not be evaluated, as few patients in the cohort had post-treatment ultrasound in addition to MRI. While our findings emphasize that rCR on MRI alone is not sufficient to reliably rule out residual disease, it nevertheless remains a useful and sensitive imaging modality in this setting, particularly among certain breast cancer subtypes.
Conclusions
The accuracy of breast MRI was 74% in prediction of pCR in patients undergoing preoperative chemotherapy for invasive breast cancer. Sensitivity, NPV, PPV, and accuracy of MRI for predicting pCR differed significantly among molecular breast cancer subtypes, with the highest NPV among the HR(−)HER2(+) (62%) and TNBC phenotypes (60%). The observed NPV of MRI overall following neoadjuvant systemic therapy does not support using MRI alone to accurately identify patients who may be candidates for the study of radiation alone in the context of a clinical trial. However, the high sensitivity of MRI, particularly in HR(−) and HER2(+) phenotypes, supports its potential role in a prospective trial to evaluate the omission of surgery in women who achieve rCR following neoadjuvant chemotherapy, possibly in combination with other tests such as tumor bed biopsy.
Acknowledgments
We are grateful to all the patients who were included in this study and to the TBCRC investigators and data managers for their efforts. We are very appreciative of funding support to the TBCRC from The AVON Foundation, The Breast Cancer Research Foundation, and Susan G. Komen for the Cure.
We gratefully acknowledge ACRIN for granting permission to include patients treated on ACRIN 6657/ I-SPY TRIAL (National Cancer Institute grants U01 CA079778 and U01 CA080098).
Footnotes
Conflict of Interest: None
Contributor Information
Jennifer F. De Los Santos, Email: jdelossantos@uabmc.edu, Department of Radiation Oncology, University of Alabama at Birmingham Kirklin Clinic at Acton Rd, Comprehensive Cancer Center, 2145 Bonner Way, Birmingham, AL 35243, Phone: 205-978-0250, Fax: 205-978-3928.
Alan Cantor, University of Alabama at Birmingham, Division of Biostatistics/Preventive Medicine
Keith D. Amos, University of North Carolina Chapel Hill, Department of Surgical Oncology
Andres Forero, University of Alabama at Birmingham, Division of Hematology/Clinical Oncology
Mehra Golshan, Harvard Medical School, Department of Surgery
Janet K. Horton, Duke University Medical School, Department of Radiation Oncology
Clifford A. Hudis, Memorial Sloan-Kettering Cancer Center, Department of Medicine
Nola M. Hylton, University of California at San Francisco, Department of Radiology and Biomedical Imaging
Kandace McGuire, University of Pittsburgh Cancer Institute and UPMC Cancer Center, Department of Surgical Oncology
Funda Meric-Bernstam, MD Anderson Cancer Center, Department of Surgical Oncology
Ingrid M. Meszoely, Vanderbilt University Medical Center, Department of Surgical Oncology
Rita Nanda, University of Chicago, Section of Hematology/Oncology
E. Shelley Hwang, Duke University, Division of Surgical Oncology
References
- 1.Baselga J, Bradbury I, Eidtmann H, et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised, open-label, multicentre, phase 3 trial. Lancet. 2012;379:633–640. doi: 10.1016/S0140-6736(11)61847-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buzdar AU, Ibrahim NK, Francis D, et al. Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: results of a randomized trial in human epidermal growth factor receptor 2-positive operable breast cancer. J Clin Oncol. 2005;23:3676–3685. doi: 10.1200/JCO.2005.07.032. [DOI] [PubMed] [Google Scholar]
- 3.Gianni L, Eiermann W, Semiglazov V, et al. Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet. 2010;375:377–384. doi: 10.1016/S0140-6736(09)61964-4. [DOI] [PubMed] [Google Scholar]
- 4.Semiglazov V, Eiermann W, Zambetti M, et al. Surgery following neoadjuvant therapy in patients with HER2-positive locally advanced or inflammatory breast cancer participating in the NeOAdjuvant Herceptin (NOAH) study. Eur J Surg Oncol. 2011;37:856–863. doi: 10.1016/j.ejso.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 5.Baillet F, Rozec C, Ucla L, Chauveinc L, Housset M, Weil M. Treatment of locally advanced breast cancer without mastectomy. 5- and 10-year results of 135 tumors larger than 5 centimeters treated by external beam therapy, brachytherapy, and neoadjuvant chemotherapy. Ann N Y Acad Sci. 1993;698:264–270. doi: 10.1111/j.1749-6632.1993.tb17217.x. [DOI] [PubMed] [Google Scholar]
- 6.Mauriac L, MacGrogan G, Avril A, et al. Neoadjuvant chemotherapy for operable breast carcinoma larger than 3 cm: a unicentre randomized trial with a 124-month median follow-up. Institut Bergonie Bordeaux Groupe Sein (IBBGS) Ann Oncol. 1999;10:47–52. doi: 10.1023/a:1008337009350. [DOI] [PubMed] [Google Scholar]
- 7.Ring A, Webb A, Ashley S, et al. Is surgery necessary after complete clinical remission following neoadjuvant chemotherapy for early breast cancer? J Clin Oncol. 2003;21:4540–4545. doi: 10.1200/JCO.2003.05.208. [DOI] [PubMed] [Google Scholar]
- 8.Chagpar AB, Middleton LP, Sahin AA, et al. Accuracy of physical examination, ultrasonography, and mammography in predicting residual pathologic tumor size in patients treated with neoadjuvant chemotherapy. Ann Surg. 2006;243:257–264. doi: 10.1097/01.sla.0000197714.14318.6f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuhl C, Weigel S, Schrading S, et al. Prospective Multicenter Cohort Study to Refine Management Recommendations for Women at Elevated Familial Risk of Breast Cancer: The EVA Trial. J Clin Oncol. 2010;28:1450–1457. doi: 10.1200/JCO.2009.23.0839. [DOI] [PubMed] [Google Scholar]
- 10.Segara D, Krop IE, Garber JE, et al. Does MRI predict pathologic tumor response in women with breast cancer undergoing preoperative chemotherapy? J Surg Oncol. 2007;96:474–480. doi: 10.1002/jso.20856. [DOI] [PubMed] [Google Scholar]
- 11.Londero V, Bazzocchi M, Del Frate C, et al. Locally advanced breast cancer: comparison of mammography, sonography and MR imaging in evaluation of residual disease in women receiving neoadjuvant chemotherapy. Eur Radiol. 2004;14:1371–1379. doi: 10.1007/s00330-004-2246-z. [DOI] [PubMed] [Google Scholar]
- 12.Nakahara H, Yasuda Y, Machida E, et al. MR and US imaging for breast cancer patients who underwent conservation surgery after neoadjuvant chemotherapy: comparison of triple negative breast cancer and other intrinsic subtypes. Breast Cancer. 2011;18:152–160. doi: 10.1007/s12282-010-0235-4. [DOI] [PubMed] [Google Scholar]
- 13.Partridge SC, Gibbs JE, Lu Y, Esserman LJ, Sudilovsky D, Hylton NM. Accuracy of MR imaging for revealing residual breast cancer in patients who have undergone neoadjuvant chemotherapy. AJR Am J Roentgenol. 2002;179:1193–1199. doi: 10.2214/ajr.179.5.1791193. [DOI] [PubMed] [Google Scholar]
- 14.Yeh E, Slanetz P, Kopans DB, et al. Prospective comparison of mammography, sonography, and MRI in patients undergoing neoadjuvant chemotherapy for palpable breast cancer. AJR Am J Roentgenol. 2005;184:868–877. doi: 10.2214/ajr.184.3.01840868. [DOI] [PubMed] [Google Scholar]
- 15.Chen JH, Feig B, Agrawal G, et al. MRI evaluation of pathologically complete response and residual tumors in breast cancer after neoadjuvant chemotherapy. Cancer. 2008;112:17–26. doi: 10.1002/cncr.23130. [DOI] [PubMed] [Google Scholar]
- 16.Cheung YC, Chen SC, Su MY, et al. Monitoring the size and response of locally advanced breast cancers to neoadjuvant chemotherapy (weekly paclitaxel and epirubicin) with serial enhanced MRI. Breast Cancer Res Treat. 2003;78:51–58. doi: 10.1023/a:1022153327339. [DOI] [PubMed] [Google Scholar]
- 17.De Los Santos J, Bernreuter W, Keene K, et al. Accuracy of breast magnetic resonance imaging in predicting pathologic response in patients treated with neoadjuvant chemotherapy. Clinical Breast Cancer. 2011;11:312–319. doi: 10.1016/j.clbc.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 18.Wolff AC, Hammond ME, Schwartz JN, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25:118–145. doi: 10.1200/JCO.2006.09.2775. [DOI] [PubMed] [Google Scholar]
- 19.von Minckwitz G, Untch M, Blohmer JU, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012;30:1796–1804. doi: 10.1200/JCO.2011.38.8595. [DOI] [PubMed] [Google Scholar]
- 20.Berg WA, Gutierrez L, NessAiver MS, et al. Diagnostic accuracy of mammography, clinical examination, US, and MR imaging in preoperative assessment of breast cancer. Radiology. 2004;233:830–849. doi: 10.1148/radiol.2333031484. [DOI] [PubMed] [Google Scholar]
- 21.Brennan ME, Houssami N, Lord S, et al. Magnetic resonance imaging screening of the contralateral breast in women with newly diagnosed breast cancer: systematic review and meta-analysis of incremental cancer detection and impact on surgical management. J Clin Oncol. 2009;27:5640–5649. doi: 10.1200/JCO.2008.21.5756. [DOI] [PubMed] [Google Scholar]
- 22.Kriege M, Brekelmans CT, Boetes C, et al. Efficacy of MRI and mammography for breast-cancer screening in women with a familial or genetic predisposition. N Engl J Med. 2004;351:427–437. doi: 10.1056/NEJMoa031759. [DOI] [PubMed] [Google Scholar]
- 23.Lehman CD, Blume JD, Weatherall P, et al. Screening women at high risk for breast cancer with mammography and magnetic resonance imaging. Cancer. 2005;103:1898–1905. doi: 10.1002/cncr.20971. [DOI] [PubMed] [Google Scholar]
- 24.Lehman CD, Gatsonis C, Kuhl CK, et al. MRI evaluation of the contralateral breast in women with recently diagnosed breast cancer. N Engl J Med. 2007;356:1295–1303. doi: 10.1056/NEJMoa065447. [DOI] [PubMed] [Google Scholar]
- 25.Pediconi F, Catalano C, Roselli A, et al. Contrast-enhanced MR mammography for evaluation of the contralateral breast in patients with diagnosed unilateral breast cancer or high-risk lesions. Radiology. 2007;243:670–680. doi: 10.1148/radiol.2433060838. [DOI] [PubMed] [Google Scholar]
- 26.Akazawa K, Tamaki Y, Taguchi T, et al. Preoperative evaluation of residual tumor extent by three-dimensional magnetic resonance imaging in breast cancer patients treated with neoadjuvant chemotherapy. Breast J. 2006;12:130–137. doi: 10.1111/j.1075-122X.2006.00220.x. [DOI] [PubMed] [Google Scholar]
- 27.Belli P, Costantini M, Malaspina C, Magistrelli A, Latorre G, Bonomo L. MRI accuracy in residual disease evaluation in breast cancer patients treated with neoadjuvant chemotherapy. Clin Radiol. 2006;61:946–953. doi: 10.1016/j.crad.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 28.Bhattacharyya M, Ryan D, Carpenter R, Vinnicombe S, Gallagher CJ. Using MRI to plan breast-conserving surgery following neoadjuvant chemotherapy for early breast cancer. Br J Cancer. 2008;98:289–293. doi: 10.1038/sj.bjc.6604171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hylton NF, Blume J, Gatsonis C, et al. MRI tumor volume for predicting response to neoadjuvant chemotherapy in locally advanced breast cancer: Findings from ACRIN 6657/CALGB 150007. J Clin Oncol. 2009;27(15S):13s. [Google Scholar]
- 30.Hylton NM, Blume JD, Bernreuter WK, et al. Locally advanced breast cancer: MR imaging for prediction of response to neoadjuvant chemotherapy--results from ACRIN 6657/I-SPY TRIAL. Radiology. 2012;263:663–672. doi: 10.1148/radiol.12110748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lehman D, Marques H, Bernreuter W, Pisano E, Rosen M, Hylton N. Assessing residual disease in breast cancer patients post neoadjuvant chemotherapy prior to surgery: findings of the American College of Radiology Imaging Network (ACRIN) Trial 6657. Radiology. 2009 [Google Scholar]
- 32.Loo CE, Teertstra HJ, Rodenhuis S, et al. Dynamic contrast-enhanced MRI for prediction of breast cancer response to neoadjuvant chemotherapy: initial results. AJR Am J Roentgenol. 2008;191:1331–1338. doi: 10.2214/AJR.07.3567. [DOI] [PubMed] [Google Scholar]
- 33.Moon HG, Han W, Lee JW, et al. Age and HER2 expression status affect MRI accuracy in predicting residual tumor extent after neo-adjuvant systemic treatment. Ann Oncol. 2009;20:636–641. doi: 10.1093/annonc/mdn683. [DOI] [PubMed] [Google Scholar]
- 34.Schott AF, Roubidoux MA, Helvie MA, et al. Clinical and radiologic assessments to predict breast cancer pathologic complete response to neoadjuvant chemotherapy. Breast Cancer Res Treat. 2005;92:231–238. doi: 10.1007/s10549-005-2510-1. [DOI] [PubMed] [Google Scholar]
- 35.Straver ME, Loo CE, Rutgers EJ, et al. MRI-Model to Guide the Surgical Treatment in Breast Cancer Patients After Neoadjuvant Chemotherapy. Ann Surg. 2010;251:701–707. doi: 10.1097/SLA.0b013e3181c5dda3. [DOI] [PubMed] [Google Scholar]
- 36.Moon HG, Han W, Ahn SK, et al. Breast Cancer Molecular Phenotype and the Use of HER2-Targeted Agents Influence the Accuracy of Breast MRI After Neoadjuvant Chemotherapy. Ann Surg. 2012 Sep 10; doi: 10.1097/SLA.0b013e3182686bd9. In Press. [DOI] [PubMed] [Google Scholar]
- 37.Esserman LJ, Berry DA, Cheang MC, et al. Chemotherapy response and recurrence-free survival in neoadjuvant breast cancer depends on biomarker profiles: results from the I-SPY 1 TRIAL (CALGB 150007/150012; ACRIN 6657) Breast Cancer Res Treat. 2012;132:1049–1062. doi: 10.1007/s10549-011-1895-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peters NH, Borel Rinkes IH, Zuithoff NP, Mali WP, Moons KG, Peeters PH. Meta-analysis of MR imaging in the diagnosis of breast lesions. Radiology. 2008;246:116–124. doi: 10.1148/radiol.2461061298. [DOI] [PubMed] [Google Scholar]