Abstract
Pioneering work in the 1990’s first linked a novel microaerobic bacterium, Helicobacter hepaticus, with active chronic hepatitis and IBD in several murine models. Targeted H. hepaticus infection experiments subsequently demonstrated its ability to induce colitis, colorectal cancer, and extra-intestinal diseases in a number of mouse strains with defects in immune function and/or regulation. Helicobacter hepaticus is now widely utilized as a model system to dissect how intestinal microbiota interact with the host to produce both inflammatory and tolerogenic responses. This model has been used to make important advances in understanding factors that regulate both acquired and innate immune response within the intestine. Further, it has been an effective tool to help define the function of regulatory T cells, including their ability to directly inhibit the innate inflammatory response to gut microbiota. The complete genomic sequence of H. hepaticus has advanced the identification of several virulence factors and aided in the elucidation of H. hepaticus pathogenesis. Delineating targets of H. hepaticus virulence factors could facilitate novel approaches to treating microbially induced lower bowel inflammatory diseases.
Keywords: Helicobater hepaticus, mouse models, IBD
Initial isolation and characterization of H. hepaticus and H. bilis
Since the isolation and characterization of Helicobacter pylori, the etiologic agent of peptic ulcer disease in 1983, a number of other Helicobacter species have been isolated, many of which can produce serious non-gastric disease in their respective hosts. Helicobacter species that colonize the lower bowel and biliary tract of mice have been associated with the development of colitis resembling human inflammatory bowel disease (IBD) in susceptible hosts. Further, H. hepaticus infection has been demonstrated to exacerbate the development of cancer at both intestinal and extraintestinal sites. The most well studied member of this group of enterohepatic Helicobacter species (EHS) is Helicobacter hepaticus. In this review we will focus on the initial identification of H. hepaticus, the basis for H. hepaticus-induced lower bowel inflammation, and the characterization of bacterial virulence factors.
In the early 1990’s, an active chronic hepatitis was detected in several inbred strains of mice including A/JCr, originating from a barrier-maintained facility. A distinctive morphological pattern of liver damage was noted which had not been previously identified in mice. Using a special silver stain, a spiral-like organism was observed in the liver. Infected A/JCr mice were then shipped to our laboratory for characterization and diagnostic evaluation. A microaerobic bacterium was consistently isolated from the liver and colon of these mice between 3 and 18 months of age. By biochemical and molecular methodologies these bacteria were confirmed to represent a new Helicobacter sp. which we named H. hepaticus 1-3. Shortly after the isolation of H. hepaticus, we isolated a fusiform bacterium from the liver, bile, and lower intestine of aged, inbred mice. On the basis of 16S rRNA gene sequence analysis, the organism was classified as a novel Helicobacter sp., H. bilis.
Currently there are 9 formally named EHS isolated from mice as well as several other novel Helicobacter spp. awaiting formal naming. Most recently, H. pullorum, a human pathogen, has been isolated from commercial, barrier maintained mice 4. Surveys conducted in 1995 and 2007 indicated that H. hepaticus as well as other EHS have a worldwide distribution in academic and commercial mice 5, 6. Contrary to the earlier survey, no Helicobacter spp., however, were found in mice in 2007 from advertised Helicobacter-free production areas from two U.S. vendors. These results demonstrated that infection with EHS is wide spread in most if not all mouse colonies used for experimental investigations.
Spontaneous IBD in immunodeficient and genetically engineered mice: Association with Helicobacter spp
Prior to the isolation of EHS, it had been speculated that microflora might play an important role in the etiology of spontaneous colitis that developed in a number of immunodeficient laboratory mouse strains including IL-10-deficient, scid, and RAG. Berg et al. also demonstrated that inbred mice on different strain backgrounds with undefined intestinal flora influenced the degree of colitis and lower bowel adenocarcinoma noted in mice; C57BL IL10−/− were less prone to develop colitis and carcinoma, Balbc IL10−/− were intermediate in susceptibility and 129 IL10−/− mice developed the most severe disease 7. Once identified, we found that while H. hepaticus infection could be demonstrated in a number of immunologically normal mice without colitis, it was strongly associated with chronic proliferative typhlitis, colitis, and rectal prolapse in several immunodeficient mouse strains housed at NCI and MIT 8, 9. The specific pathogen-free health status of these colonies was verified to include lack of serum antibodies to murine viruses and negative cultures for respiratory pathogens, Salmonella sp., and Citrobacter rodentium. These findings suggested infection with H. hepaticus was strongly associated with the development of colitis in a number of laboratory mouse strains, and raised the question of whether H. hepaticus might be an etiologic agent of murine colitis.
Establishing the Link Between Helicobacter spp. and IBD
To establish the link between H. hepaticus and the development of colitis, we demonstrated that H. hepaticus inoculated into Helicobacter spp. free defined flora C.B. 17 scid mice reconstituted with CD45RBhigh T cells resulted in severe IBD, similar to that noted with human disease 10. The expression of typhlitis and colitis was statistically less severe in defined flora scid mice that had been reconstituted with T cells but not infected with H. hepaticus when compared to reconstituted scid mice infected with H. hepaticus 10, demonstrating that infection with this single species could significantly exacerbate colitis in a murine disease model 11, 12. Similar results were obtained following targeted infection of C57BL IL-10-deficient mice with H. hepaticus 13, 14. Targeted infection experiments have now demonstrated that H. hepaticus is able to induce colitis and in some cases lower bowel carcinoma in a number of mouse strains with defects in immune function and/or regulation (Table 1). Interestingly, the same strain susceptibility noted by Berg et al in IL10−/− mice was also documented in Rag−/− mice developing colitis associated lower bowel carcinoma when experimentally infected with H. hepaticus 7, 15.
Table 1.
H. hepaticus- and H. bilis-associated IBD in micea
| Genetic status of mice | Type of defect | Pathology | Reference |
|---|---|---|---|
| CD45RB (high)-reconstituted ICR defined flora scids |
Reconstitution with naïve CD4+ T cell | Typhlocolitis | 10 |
| TCRα, B mutants | Abnormal T cell receptors | Typhlocolitis | 88 |
| Scid ICR-defined florab | Lack T and B cell | Typhlocolitis | 89, 90 |
| C57BL/IL-10−/−c | Knockout | Typhlocolitis | 14, 18, 19, 36 |
| 129SvEv/Rag2−/− | Knockout | Typhlocolitis, colon cancer | 28, 56 |
| C57BLRag2−/− | Knockout | Typhlocolitis | 14 |
| IL-7−/−/RAG-2−/− | Double Knockout | None | 27 |
| A/JCr | Normal | Typhlitis | 3 |
| Swiss Webster gnotobiotic | Normal | Enterocolitis | 91 |
| 129SvEv/NF-κβ (p50−/−p65+/-) | Double Knockout | Typhlocolitis | 92 |
| mdrla−/−d | Lack P-glycoprotein | Typhlocolitis | 93 |
| SMAD3−/−d | Knockout | Typhlocolitis, colon cancer | 94 |
In mice of the same genetic status which had H. hepaticus (or other Helicobacter spp.)-negative microflora, no intestinaldisease was noted.
Mice infected with H. bilis also produced IBD89.
Basis of the GI inflammatory response to H. hepaticus
The experiments described indicate that H. hepaticus infection can induce colitis in certain susceptible mouse strains, but the nature of the inflammatory response and why certain strains were susceptible required further investigation. Seminal experiments from the early 1990’s demonstrated that mice lacking IL-10 developed spontaneous colitis, which was significantly less severe when mice were housed in SPF conditions, suggesting enhanced susceptibility to microbiota induced inflammation 16. In contrast to H. hepaticus monoassociated C57BL IL10−/− mice which did not develop colitis, Kullberg and co-workers directly demonstrated that targeted infection of SPF housed C57BL IL-10 mice with H. hepaticus significantly exacerbated the development of colitis 13, 17. The inflammatory response in the H. hepaticus-infected C57BL IL-10-deficient mice was characterized by a Th1-like cytokine profile 13, 18. More recently these results have been replicated by demonstrating that wild-type (WT) C57BL mice treated with an antibody directed at the IL10 receptor (anti-IL-10R), but not those treated with a control antibody, develop colitis following infection with H. hepaticus 19. Colitis in anti-IL-10R treated mice is characterized by increased expression of mRNA coding for IFN-γ and IL-17 within the colon. Mesenteric lymph node cells (MLNs) derived from H. hepaticus-infected mice treated with anti-IL-10R secreted higher levels of IL-17 and IFN-γ compared to control mesenteric lymph node cells following exposure to soluble helicobacter antigen 19. Mice-deficient in IL-12p40 but not those deficient in IL-12p35 were resistant to H. hepaticus-induced colitis following anti-IL-10R treatment, which was associated with decreased expression of IFN-γ and IL-17, suggesting that IL-23 rather than IL-12p70 was necessary for the generation of both Th1 and Th17 responses observed following H. hepaticus infection. These experiments indicated that H. hepaticus can induce antigen-specific Th1 and Th17 responses in mice with impaired IL-10 signaling.
While H. hepaticus infection of mice with impaired IL-10 signaling induces CD4-mediated Th1 and Th17 responses, the exact role of both IFN-γ and IL-17 in H. hepaticus-mediated colitis has been more difficult to discern. Initial experiments with an IFN-γ depleting antibody suggested that IFN-γ was necessary for H. hepaticus to induce colitis in IL-10-deficient mice 13. However, the severity of H. hepaticus-induced colitis in mice doubly-deficient for IL-10 and IFN-γ was very similar to that in mice lacking IL-10 alone 18. Further, whereas H. hepaticus-induced inflammation within the colon of IFN-γ-deficient mice treated with anti-IL-10R was decreased compared to similarly treated WT mice, there was no difference in the level of inflammation in the cecum between genotypes 19. Although to our knowledge, the role of IL-17 in regulating H. hepaticus-induced inflammation in mice with impaired IL-10 signaling has not been directly tested, there are conflicting results from experiments testing the role of IL-17 in the development of colitis that develops follow the adoptive transfer of CD4+CD45RBhigh cells into lymphocyte-deficient hosts 20, 21. Further, as it has been suggested that other Th17 cell-derived cytokines such as Il-17F 20 and IL-22 22, 23 can modulate disease severity in T cell-dependent models of colitis, the role of these cytokines in H. hepaticus-dependent colitis requires additional study.
Although the exact role of T helper subsets in the development of H. hepaticus-induced colitis in susceptible hosts remains to be determined, increasing evidence has focused attention on the innate inflammatory response to H. hepaticus. Early studies demonstrated that H. hepaticus could induce colitis in certain strains of lymphocyte-deficient mice including scid and 129SvEv/Rag−/− 10, 24-27, and progressed to colonic carcinoma with neoplastic peritoneal invasion in some murine models 24, 28. H. hepaticus-induced innate colitis appeared to be dependent upon IL-23, as antibody depletion of both IL-12 p40 and IL-12 p19 (the components of IL-23) inhibited H. hepaticus-induced colitis in 129SvEv/Rag-/- mice 29. Antibody depletion assays have also demonstrated an important role for TNF in H. hepaticus-induced innate colitis 25.
Surprisingly, even in the innate colitis model, H. hepaticus infection induced robust levels of the T helper associated cytokines IL-17 and IFN-γ within the colon of 129SvEv/Rag−/− mice 29, and treatment of H. hepaticus-infected 129SvEv/Rag−/− mice with either IL-17 or IFN-γ depleting antibody ameliorated colitis 30. This suggested that cells of the innate immune system expresses IL-17 and IFN within the colon following H. hepaticus infection. Indeed, in 129SvEv/Rag−/− mice infected with H. hepaticus, there was a marked increase in the percentage of colonic lamina propria (LP) cells that stain positively for IL-17 alone or both IL-17 and IFN-γ following overnight stimulation with IL-23 30. These IL-17± cells lacked lineage markers (CD11b, GR1, B220), but expressed high levels of Thy1 30, and thus bear similarities to a previous described rare population of lamina propria cells termed Lymphoid Tissue inducer-like (LTi-like) cells 31, 32. Some LTi-like cells express CD4 on their cell surface 31, while these newly described IL-17+ innate lymphoid cells do not 30. However they share a number of other cell surface markers including IL-7Rα 30, 31. Interestingly, there was a marked increase in the frequency of Thy1high innate lymphoid cells within the colonic lamina propia of 129SvEv/Rag−/− following H. hepaticus infection, and when isolated by sorting, these cells produced IFN-γ, IL-17, and IL-22 after overnight culture, which was enhanced by treatment with IL-23 30.
It has previously been shown that the development of LTi and LTi-like cells, as well as classic Th17 cells require the transcription factor, RORγt 31-33. Interestingly, H. hepaticus-induced innate−Thy1high+ LP cells expressed higher levels of RORγt than remaining Thy1− LP cells 30, and both C57BL/6Rag−/− mice treated with anti-Thy1 antibody, or C57BL/6Rag−/− mice that lacked the gene for RORγt, were resistant to anti-CD40-induced innate colitis 30. These data strongly suggest that Thy1+ RORγt-expressing innate lymphoid cell plays a critical role in driving intestinal inflammation. Further, if these innate lymphoid cells require the IL-7 receptor for their survival and/or expansion in similar fashion to LTi cells 34, this could explain the earlier observation that H. hepaticus is unable to induce colitis in 129SvEv/Rag−/− mice that also lack IL-7 27.
These studies argue that innate immune mechanisms play a critical role in the ability of H. hepaticus to induce colitis in susceptible hosts. However, it is important to recognize that H. hepaticus infection does not induce colitis in most WT mouse strains despite persistent infection, suggesting that a lymphocyte-mediated mechanism inhibits H. hepaticus-induced innate inflammation. Consistent with this, several groups have shown that the adoptive transfer of CD4+CD25+ T regulatory-cells into 129SvEv/Rag−/− mice can inhibit the development of colitis following H. hepaticus infection 25, 28. Inhibition depends upon the ability of transferred cells to express IL-10, as IL-10-deficient CD4+CD25+ T cells are unable to inhibit H. hepaticus-induced innate inflammation, and in fact appear to exacerbate disease 24, 25. Remarkably, transfer of CD4+CD25+ T regulatory cells into H. hepaticus-infected 129SvEv/Rag−/− mice with colitis leads to rapid resolution of disease 24, 35. Several studies revealed that potency of CD4+CD25+ to protect against or treat IBD can be enhanced by H. hepaticus infection of donor mice 36, 37. Thus CD4+CD25+ T regulatory cells can both inhibit and treat H. hepaticus-induced innate inflammation in the lower bowel, based on an IL-10-dependent mechanism. Interestingly, CD25+ cells are not the only CD4+ subtype with regulatory activity, as it has been demonstrated that CD4+CD45RBlowCD25− cells harvested from H. hepaticus infected C57BL/10 mice are potent inhibitors of colitis induced by adoptive transfer of IL-10-deficient CD4+ T cells into H. hepaticus-infected C57BL/10Rag−/− mice 36.
Experiments described above indicating that the recognition of H. hepaticus by the innate immune system plays a key role in regulating the ensuing inflammatory response in the lower bowel, suggest that understanding the innate inflammatory signaling pathways and receptors utilized by H. hepaticus could lead to important insights into the basis of microbiota-driven colonic inflammation. H. hepaticus challenge of ex vivo cultured bone marrow-derived macrophages induces activation of both the NF-κB and ERK pathways 26, 38. Evidence for both pro and anti-inflammatory roles for individual NF-κB subunits in the response to H. hepaticus come from experiments demonstrating that 129SvEv/Rag−/− mice that also lack the NF-κB subunit c-Rel are resistant to H. hepaticus-induced colonic inflammation 39, while 129SvEv mice that lack the p50/p105 subunit of NF-κB are sensitized to H. hepaticus-induced colitis, based on a defect within the innate immune system 26, 40.
Identifying the innate immune receptors responsible for inducing inflammatory signaling in response to Helicobacter spp. infection is an active area of reserach. Whole H. hepaticus bacteria are able to induce NF-κB activation in HEK293 cells transfected with TLR2 but not those transfected with TLR4 41. However, a recent study demonstrated that the absence of TLR2 in 129SvEv/Rag−/− mice does not influence the severity of the H. hepaticus-induced colitis 42. Nonetheless, there is strong evidence suggesting the involvement of TLRs in mediating the in vivo inflammatory response to H. hepaticus as irradiated 129SvEv/Rag−/− reconstituted with Myd88 deficient bone marrow cells are resistant to H. hepaticus-induced innate colitis 43.
Intracellular receptors of the Nod family plan an important role in recognizing bacterial products such as peptidoglycan that are injected by bacterial secretion systems44. It has recently been reported that the genome of H. hepaticus codes for a type VI secretion system that may function to inject substrates into host epithelial cells 45. Indeed, it has been suggested that the ability of H. muridarum, a murine EHS, to induce NF-kB reported activity in HEK293 cells depends upon Nod1 46. Although, of note, H. hepaticus is a very poor inducer of NF-kB activation in HEK293 cells 46, and a weak inducer of inflammatory gene expression in the murine intestinal crypt epithelial cells (m-ICcl2 cells) 47, suggesting that H. hepaticus is unlikely to engage Nod1 in these epithelial cell lines. In contrast, it has been found that H. hepaticus, as well as LPS derived from H. hepaticus, can inhibit TLR4 induced inflammatory gene expression in m-ICcl2 cells 47, raising the possibility that H. hepaticus may have prominent anti-inflammatory properties on epithelial cells. An anti-inflammatory role for an H. hepaticus-derived product is supported by studies showing that an H. hepaticus mutant lacking the type VI secretion system colonized the intestine of C57BL/6RAG-/- recipients of CD4+CD45RBhi T cells to higher levels than the WT H. hepaticus strain, and induced elevated levels of inflammatory gene expression 45. Finally, a role for Nod2 in recognition of H. hepaticus has been suggested by studies demonstrating higher levels of H. hepaticus in infected Nod2-deficient C57BL/6 mice than in infected WT mice, although intestinal pathology was not detected following infection of either WT or Nod2-deficient mice 48.
Helicobacter hepaticus infection modulates tumorigenesis at intestinal and extra-intestinal sites
At the time of initial characterization in A/JCr mice, it was recognized that in addition to active chronic hepatitis, infection with H. hepaticus was also associated with the development of hepatocellular carcinoma in A/JCr as well as live tumor development in AXB, B6C3 and a substrain of C57BL mice 1, 3, 49-52. Previous studies using germ-free mice link chronic bacterial infection with chronic inflammatory diseases in the lower bowel 53. Targeted bacterial infection with H. hepaticus in 129SvEv/Rag2−/− mice lead to colitis-associated colorectal cancer (CRC) with malignancy invading the peritoneal cavity 24, 28. Gut bacteria-induced lesions in 129SvEv/Rag2 -deficient mice resembled neoplasia in human patients 54 with IBD, in that carcinoma clearly arises from colitis-associated dysplastic epithelial foci that become locally invasive 55. The finding that 129SvEv/Rag2−/− mice lacking lymphocytes exhibited CRC demonstrated that innate immunity is sufficient for development of carcinoma 28. However, immunologically intact 129 wild type mice infected with H. hepaticus did not develop carcinoma, showing that IL-10-dependent activities of adaptive immune cells normally serve to reinforce homeostasis and prevent cancer in the lower bowel 28, 37. Adoptive transfer studies using CD4+ cell subsets demonstrated that an imbalance between IL-6, IL-10 and Tgf-β increased risk of neoplastic epithelial invasion 37. Interestingly, H. hepaticus-induced malignancy in the 129SvEv/Rag2−/− mice was readily reversible by blocking underlying bacteria-driven inflammation with antibodies directed at TNF and IL-6 37, 56.
Pathogenic gut microbial infections have also been shown to trigger development of intestinal adenomatous polyps in mice with a mutated Apc gene (ApcMin/+ or Min) 57. Mice heterozygous for Apc gene (ApcMin/+) develop a large number of intestinal polyps by 3 months of age 58. This pre-neoplastic process has been shown to be inhibited by adoptive transfer of interleukin 10 (IL-10)–dependent TReg cells, and depends on their ability to produce IL-10 59, 60. Targeted infection with H. hepaticus increases multiplicity and malignancy of intestinal polyps in the ApcMin/+ mouse model by an inflammation-associated TNF-α-dependent mechanism 57. Likewise, infection with other pathogenic enteric microbiota including Citrobacter rodentium 61 and an enterotoxigenic Bacteroides fragilis 62 have been shown to promote colon tumorigenesis in ApcMin/+ mice. Recent evidence points to key roles for IL-17 as well as IL-23 in colonic hyperplasia and tumor formation 62, 63. Taken together these models may provide novel mechanistic insights in colon carcinogenesis in humans.
More recently, experiments examining the ability of GI tract bacteria and subpopulations of immune cells to induce or inhibit IBD have revealed surprising insights into extra-intestinal carcinogenesis. For example, H. hepaticus infection was found to trigger mammary tumorigenesis in ApcMin/+ in the absence of IBD 57, 64. Mammary tumors also developed rapidly in susceptible mice after transfer of CD4+ CD45RBhigh T cells 57, 63. While WT Treg completely inhibited H. hepaticus-induced diseases in these models, IL-10-deficient Treg cells exacerbated rather than inhibited H. hepaticus-induced extra-intestinal pathology 24, 37. Interestingly, prior exposures of Treg donor mice to H. hepaticus enhanced their ability to inhibit extra-intestinal pathology 57, 63, 64, raising the possibility that therapies aimed towards intestinal homeostasis may offer sustained protection from systemic diseases.
Virulence Factors of Enterohepatic Helicobacters
Human IBD as well as IBD in mouse models is multifactorial, with genetic and environmental factors contributing to pathogenesis. The complexity of the system necessitates parallel investigations into bacterial virulence factors, gene expression changes in intestinal tissue, host innate and adaptive immune responses, changes in the gut microbial flora, and of course, lesion development in the intestine. Using the H. hepaticus genome, we have begun studying in detail, with the use of isogenic mutants, how different virulence genes play a role in chronic intestinal inflammation. The pathogenic potential of several virulence factors from H. hepaticus have been well characterized.
Of the known EHS, H. hepaticus strain 3B1 (ATCC 51449), the protype of EHS, has been the most extensively investigated. This murine pathogen contains a circular genome of ~1.8 Mb with 1875 predicted open reading frames 65. The G+C content of the H. hepaticus genome is 35.9% which places it between the G+C content of H. pylori (39%) and C. jejuni (30.5%). In addition, ~ 50% of predicted H. hepaticus genes are orthologous to those in the genome of either H. pylori or C. jejuni, both of which cause significant gastrointestinal diseases in humans 65.
Cytolethal distending toxin (CDT)
Bacterial CDTs are present in multiple gram negative pathogens including strains of Salmonella spp, Shigella spp, C. jejuni, and E. coli, and belong to the AB2-type of toxins. In general these toxins are comprised of subunits CdtA and CdtC which together bind subunit CdtB and deliver CdtB, the active subunit of the toxin, intracellularly 66. Several biological functions of the CdtB subunit have been reported, including a DNase I-like function, cell cycle arrest, phosphatase activity and eventual cell death 67. The cdtB orthologue and CDT activity are found in several enterohepatic helicobacters, including H. hepaticus, H. bilis, H. canis, and H. marmotae as well as in H. cineadi and H. pullorum isolated from humans 68-70.
H. hepaticus 3B1 and an isogenic mutant of the cytolethal distending toxin gene (cdtB-deficient HhcdtBm7) were evaluated in outbred Swiss Webster mice. Whereas inoculation with WT 3B1 lead to persistent colonization, colonization by the mutant failed in female mice and was transient in males 71. Wildtype 3B1 infection caused significantly increased expression of ileal and cecal IFN-γ mRNA, higher Th1-induced IgG2a responses, and increased mucosal IgA secretion compared to the isogenic mutant 13, 71. CDT-deficient isogenic H. hepaticus mutants also lose their ability to persistently colonize the large intestine of B6.129-IL10tm1Cgn (IL10−/−) mice. Importantly, the mutant-infected mice developed significantly less severe typhlocolitis when compared to WT 3B1 infected mice 71-73. Mutation of the CDT gene of H. cinaedi, a human pathogen, also reduced its ability to induce lower bowel pathology in IL-10-deficient mice 74.
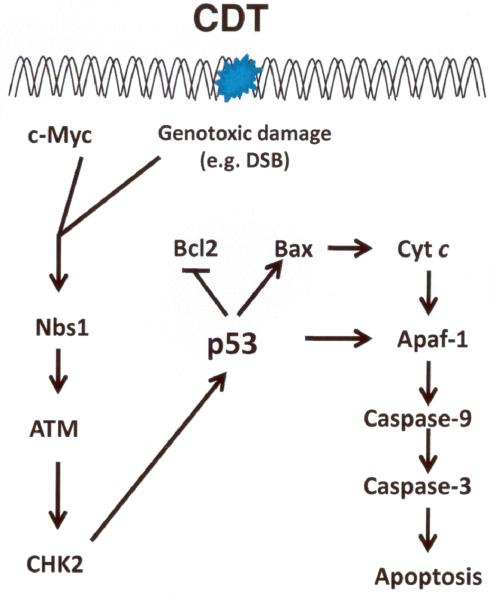
Molecular mechanisms operable in H. hepaticus CDT induced cell death are also being actively investigated. CDT activated the ATM-dependent DNA damage check point response in a myc-dependent fashion 75. In addition, exposure of INT407 cells to H. hepaticus CDT resulted in upregulation or pro-apoptotic Bax, release of Cyt c, and activation of caspases 3/7 and 9 76. Events operable in H. hepaticus CDT-induced cell arrest and apoptosis are summarized in Figure 1.
Figure 1.
ATM-dependent checkpoint responses and apoptosis triggered by H. hepaticus CDT-induced DNA damage 75, 76. DSB, double strand breakage; Nbs 1, Nijmegen Breakage Syndrome 1 protein; ATM, Ataxia Telangiectasia Mutated; CHK2, checkpoint kinase 2; Bcl2, B-cell leukemia/lymphoma 2; Bax, Bcl-2–associated X protein; Cyt c, Cytochrome c; Apaf-1, apoptotic peptidase activating factor 1.
H. hepaticus pathogenicity island (PAI)
PAIs are distinct genetic elements on bacterial chromosomes that encode one or more virulence-associated factors and often have a G+C content distinct from the rest of the core genome 65. Several PAIs, such as LEE in enteropathogenic Escherichia coli, cag in Helicobacter pylori and SaPl1 in Staphylococcus aureus, significantly contribute to pathogenicity of these infectious agents 77. In the genome of H. hepaticus 3B1, a 71-kb region, HHGI1, displays genetic features characteristic of PAIs 65. HHGI1 is highly variable among certain H. hepaticus isolates. Male A/JCr mice infected with H. hepaticus strain MIT 96-1809 lacking the entire HHGI1 or strain MIT 96-284 missing ~62 out of 71-kb of HHGI1 developed less-severe hepatitis than those infected with H. hepaticus 3B1 which has an intact HHGI1 78. An isogenic mutant (HhPAId1) of H. hepaticus 3B1 containing a deletion of 19 predicted genes (HH0250 to HH0268) within HHGI1 colonized the intestine of B6.129-IL10tm1Cgn (IL10−/−) mice at higher levels but provoked less severe typhlocolitis and hyperplasia when compared to H. hepaticus 3B1 79. The magnitude of the Th1-associated IgG2c response against this isogenic mutant (HhPAId1) was less than against wildtype H. hepaticus and was accompanied by suppressed cecal and colonic mRNA expression levels for proinflammatory IFN-γ, TNF-α and IL17a. These results demonstrate that HHGI1 contributes to the pathogenicity of H. hepaticus at least in part via up-regulation of proinflammatory mediators.
Recently, a study indicated that HHGI1 encodes for a set of 12 genes (HH0237, HH0242 to HH0245, HH0247 to HH0252, HH0291) which are homologous to known bacterial type VI secretion systems (T6SS) 45. T cell-reconstituted Rag1−/− mice infected with a T6SS-deficient ΔIcmF H. hepaticus mutant (encoded by HH0252) developed higher colonization levels of the mutant compared to wildtype H. hepaticus-infected mice, consistent with higher levels of mutant HhPAId1 cecal colonization 79. In addition, infection with ΔIcmF H. hepaticus induced higher expression in the colon of proinflammatory mediators including IL-17, IL-23, IL-1β, TNFα and IFNγ than infection with WT H. hepaticus 3B1 45. However, unlike the HhPAId1 mutant, the T6SS mutant ΔIcmF, and H. hepaticus 3B1 induced a similar degree of colitis by 2-4 weeks postinoculation 45, 79. Therefore, further investigations will be required to define the role(s) of T6SS and additional components in HHGI1 in the pathogenesis of H. hepaticus.
Urease
Gastric helicobacters produce urease which is essential for establishing persistent colonization in the acidic milieu of the stomach 80. Approximately 40% of known EHS species, including H. hepaticus, are urease-positive 81. The H. hepaticus ure gene cluster is similar to that of H. pylori; however, regulatory mechanisms of urease activity in H. hepaticus are distinct from those in H. pylori. For example, H. hepaticus urease does not confer acid-resistance, transcription of ure genes is not induced by acid, and urease activity is suppressed by the transcriptional regulator Fur 82, 83.
By infecting male A/JCr mice with a urease-deficient, isogenic mutant (HhureNT9) of H. hepaticus 3B1, we demonstrated that the lack of urease activity did not affect the level of cecal colonization but its presence was essential for hepatic colonization 81. Consequently, the HhureNT9-infected mice developed less severe hepatitis and produced significantly lower hepatic mRNA levels of proinflammatory cytokines IFN-γ and TNF-α when compared to the H. hepaticus 3B1-infected mice 81.
Enzymes in oxidative stress resistance
To survive and establish colonization in the host, bacteria have evolved various mechanisms to combat oxidative stress that confers bactericidal effects via reactive oxygen species. The H. hepaticus catalase enzyme KatA, which converts hydrogen peroxide into molecular oxygen and water, plays a key role in this process. The amino acid sequence of H. hepaticus KatA contains three motifs (R-F-Y-D, RERIPER and VVHAKG) in the haem-ligand domain and three surface-predicted motifs that are shared among bacterial and mammalian catalases 84. KatA-deficient H. hepaticus are more sensitive to exposure of oxygen and H2O2 and also suffer severe DNA fragmentation by H2O2 treatment compared to H. hepaticus 3B1 85.
Bacterial hydrogenase, a hydrogen uptake hydrogenase, oxidizes molecular hydrogen to yield protons and electrons which have a key role in energy conservation in bacteria. H. hepaticus genes or HH0056 to HH0059 encode subunits of hydrogenase HyaA to HyaD. Mutation in the H. hepaticus hyaB (coding for the large subnit) does not affect cecal and hepatic colonization levels when compared to H. hepaticus 3B1 in male A/JCr mice; however, liver pathology is diminished in the hyaB mutant-infected mice 86. It is unknown how hydrogenase contributes to the pathogenesis of H. hepaticus, but it is speculated that energy released by hydrogen oxidation may be utilized to promote synthesis of virulence-related proteins or enzymes that result in hepatic or intestinal inflammation and necrosis 86.
Flagellar genes
All Helicobacter species possess flagella for motility; H. hepaticus is a spiral rod with bipolar sheathed flagella. In other related bacteria, flagellar genes are mainly regulated by the sigma factor FliA (σ28). H. hepaticus contains two identical copies of the gene encoding the major flagellin subunit FlaA (open reading frames HH1364 [flaA1] and HH1653 [flaA2]) 65, which is regulated by the sigma factor FliA (σ28). Mutations in fliA or both copies of the flaA gene resulted in no detectable synthesis of FlaA as well as severely truncated flagella, thus both mutants were nonmotile and unable to colonize mice 87. The flaA_1 H. hepaticus mutant, which has flagella but decreased motility, also does not colonize mice, indicating that full motility is a prerequisite for intestinal colonization by H. hepaticus, and that the presence of flagella alone is not sufficient.
Summary
As described in this review, Helicobacter hepaticus has played a central role in shaping our understanding of how intestinal microbiota interact with the host to produce both inflammatory and tolerant responses. The study of immunopathology that develops following H. hepaticus infection of susceptible mice has helped to define the importance of key inflammatory mediators including IL-23 and IL-17, and highlighted the importance of the innate immune response in the development of chronic intestinal inflammation. The use of H. hepaticus infection models has provided definitive evidence that regulatory T cells directly inhibit the innate inflammatory response to lower bowel microbiota and delineated the critical role of IL-10 in this process. Remarkably, H. hepaticus infection has powerful effects on carcinogenesis both within the intestine and at extraintestinal sites, and many of the same immunoregulatory pathways that influence the gastrointestinal inflammatory response to H. hepaticus also appear to influence the development of cancer. The complete genomic sequence of H. hepaticus has advanced the identification of potential virulence factors, and evaluating the function of these factors is ongoing. In addition, genome sequencing of five additional EHS isolated from humans with gastrointestinal disease (H. bilis, H. canadensis, H. cinaedi, H. pullorum and H. winghamensis) has been completed and these results can be found at http://www.broadinstitute.org/annotation/genome/Helicobacter_group/. Comparative genome analysis of these 5 genomes plus H. hepaticus will provide insight into the etiopathogenesis of EHS infection in humans. For example, preliminary data indicates that both H. cinaedi and H. pullorum have the existence of PAI’s with similar features to the HHGI found in H. hepaticus. Studies are needed to ascertain whether these PAI’s impart similar in vivo characteristics in humans. Additional areas for future investigation include defining the cellular and molecular pathways that contribute to host recognition of H. hepaticus, and determining how initial recognition events lead to activation of innate and acquired inflammatory effector mechanisms. Further, clarifying how responses to H. hepaticus infection influences carcinogenesis at local and distant sites could lead to important new insights into the relationship between specific microbiota and the development of cancer. Finally, delineating the targets of H. hepaticus associated virulence factors could lead to novel approaches to treating microbial induced inflammation in the lower bowel.
Acknowledgments
Grant Support
R01-DK052413 (JGF), P01-CA026731 (JGF), R01-CA067529 (JGF), P30-ES02109 (JGF), R01-A1052267 (BH, SE), R01-CA108854 (SE)
Footnotes
All authors verify that they have ‘no conflict’.
References
- 1.Fox JG, et al. Helicobacter hepaticus sp. nov., a microaerophilic bacterium isolated from livers and intestinal mucosal scrapings from mice. J Clin Microbiol. 1994;1238:1238–1245. doi: 10.1128/jcm.32.5.1238-1245.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ward JM, et al. Chronic active hepatitis and associated liver tumors in mice caused by a persistent bacterial infection with a novel Helicobacter species. J Natl Cancer Inst. 1994;1222:1222–1227. doi: 10.1093/jnci/86.16.1222. [DOI] [PubMed] [Google Scholar]
- 3.Fox JG, et al. Chronic proliferative hepatitis in A/JCr mice associated with persistent Helicobacter hepaticus infection: a model of helicobacter-induced carcinogenesis. Infect Immun. 1996;1548:1548–1558. doi: 10.1128/iai.64.5.1548-1558.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boutin SR, et al. Helicobacter pullorum outbreak in C57BL/6NTac and C3H/HeNTac barrier-maintained mice. J Clin Microbiol. 48:1908–1910. doi: 10.1128/JCM.02531-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shames B, et al. Identification of widespread Helicobacter hepaticus infection in feces in commercial mouse colonies by culture and PCR assay. J Clin Microbiol. 1995;2968:2968–2972. doi: 10.1128/jcm.33.11.2968-2972.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taylor NS, Xu S, Nambiar P, Dewhirst FE, Fox JG. Enterohepatic Helicobacter species are prevalent in mice from commercial and academic institutions in Asia, Europe, and North America. J Clin Microbiol. 2007;2166:2166–2172. doi: 10.1128/JCM.00137-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berg DJ, et al. Enterocolitis and colon cancer in interleukin-10-deficient mice are associated with aberrant cytokine production and CD4(+) TH1-like responses. J Clin Invest. 1996;1010:1010–1020. doi: 10.1172/JCI118861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ward JM, et al. Inflammatory large bowel disease in immunodeficient mice naturally infected with Helicobacter hepaticus. Lab Anim Sci. 1996;15:15–20. [PubMed] [Google Scholar]
- 9.Foltz CJ, et al. Spontaneous inflammatory bowel disease in multiple mutant mouse lines: association with colonization by Helicobacter hepaticus. Helicobacter. 1998;69:69–78. doi: 10.1046/j.1523-5378.1998.08006.x. [DOI] [PubMed] [Google Scholar]
- 10.Cahill RJ, et al. Inflammatory bowel disease: an immunity-mediated condition triggered by bacterial infection with Helicobacter hepaticus. Infect Immun. 1997;3126:3126–3131. doi: 10.1128/iai.65.8.3126-3131.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Powrie F, Leach MW, Mauze S, Caddle LB, Coffman RL. Phenotypically distinct subsets of CD4+ T cells induce or protect from chronic intestinal inflammation in C. B-17 scid mice. Int Immunol. 1993;1461:1461–1471. doi: 10.1093/intimm/5.11.1461. [DOI] [PubMed] [Google Scholar]
- 12.Powrie F, et al. Inhibition of Th1 responses prevents inflammatory bowel disease in scid mice reconstituted with CD45RBhi CD4+ T cells. Immunity. 1994;553:553–562. doi: 10.1016/1074-7613(94)90045-0. [DOI] [PubMed] [Google Scholar]
- 13.Kullberg MC, et al. Helicobacter hepaticus triggers colitis in specific-pathogen-free interleukin-10 (IL-10)-deficient mice through an IL-12- and gamma interferon-dependent mechanism. Infect Immun. 1998;5157:5157–5166. doi: 10.1128/iai.66.11.5157-5166.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burich A, et al. Helicobacter-induced inflammatory bowel disease in IL-10- and T cell-deficient mice. Am J Physiol Gastrointest Liver Physiol. 2001;281:G764–778. doi: 10.1152/ajpgi.2001.281.3.G764. [DOI] [PubMed] [Google Scholar]
- 15.Erdman SE, Horwitz B, Poutahidis T, Rogers AB, Fox JG. Helicobacter induced adenocarcinoma in the colon of NFkB deficient mice. Gastroenterology. 2002;122:A429. [Google Scholar]
- 16.Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;263:263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 17.Dieleman LA, et al. Helicobacter hepaticus does not induce or potentiate colitis in interleukin-10-deficient mice. Infect Immun. 2000;5107:5107–5113. doi: 10.1128/iai.68.9.5107-5113.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kullberg MC, et al. Helicobacter hepaticus-induced colitis in interleukin-10-deficient mice: cytokine requirements for the induction and maintenance of intestinal inflammation. Infect Immun. 2001;4232:4232–4241. doi: 10.1128/IAI.69.7.4232-4241.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kullberg MC, et al. IL-23 plays a key role in Helicobacter hepaticus-induced T cell-dependent colitis. J Exp Med. 2006;2485:2485–2494. doi: 10.1084/jem.20061082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leppkes M, et al. ROR gamma-expressing Th17 cells induce murine chronic intestinal inflammation via redundant effects of IL-17A and IL-17F. Gastroenterology. 2009;257:257–267. doi: 10.1053/j.gastro.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 21.O'Connor W, Jr., et al. A protective function for interleukin 17A in T cell-mediated intestinal inflammation. Nat Immunol. 2009;603:603–609. doi: 10.1038/ni.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zenewicz LA, et al. Innate and adaptive interleukin-22 protects mice from inflammatory bowel disease. Immunity. 2008;947:947–957. doi: 10.1016/j.immuni.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sugimoto K, et al. IL-22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis. J Clin Invest. 2008;534:534–544. doi: 10.1172/JCI33194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Erdman SE, et al. CD4(+)CD25(+) regulatory lymphocytes require interleukin 10 to interrupt colon carcinogenesis in mice. Cancer Res. 2003;6042:6042–6050. [PubMed] [Google Scholar]
- 25.Maloy KJ, et al. CD4+CD25+ T(R) cells suppress innate immune pathology through cytokine-dependent mechanisms. J Exp Med. 2003;111:111–119. doi: 10.1084/jem.20021345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tomczak MF, et al. NF-kappa B is required within the innate immune system to inhibit microflora-induced colitis and expression of IL-12 p40. J Immunol. 2003;1484:1484–1492. doi: 10.4049/jimmunol.171.3.1484. [DOI] [PubMed] [Google Scholar]
- 27.von Freeden-Jeffry U, et al. IL-7 deficiency prevents development of a non-T cell non-B cell-mediated colitis. J Immunol. 1998;5673:5673–5680. [PubMed] [Google Scholar]
- 28.Erdman SE, et al. CD4+ CD25+ regulatory T lymphocytes inhibit microbially induced colon cancer in Rag2-deficient mice. Am J Pathol. 2003;691:691–702. doi: 10.1016/S0002-9440(10)63863-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hue S, et al. Interleukin-23 drives innate and T cell-mediated intestinal inflammation. J Exp Med. 2006;2473:2473–2483. doi: 10.1084/jem.20061099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buonocore S, et al. Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature. 2010;1371:1371–1375. doi: 10.1038/nature08949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eberl G, Littman DR. Thymic origin of intestinal alphabeta T cells revealed by fate mapping of RORgammat+ cells. Science. 2004;248:248–251. doi: 10.1126/science.1096472. [DOI] [PubMed] [Google Scholar]
- 32.Eberl G, et al. An essential function for the nuclear receptor RORgamma(t) in the generation of fetal lymphoid tissue inducer cells. Nat Immunol. 2004;64:64–73. doi: 10.1038/ni1022. [DOI] [PubMed] [Google Scholar]
- 33.Ivanov II, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;1121:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 34.Adachi S, et al. Essential role of IL-7 receptor alpha in the formation of Peyer's patch anlage. Int Immunol. 1998;1:1–6. doi: 10.1093/intimm/10.1.1. [DOI] [PubMed] [Google Scholar]
- 35.Maloy KJ, Antonelli LR, Lefevre M, Powrie F. Cure of innate intestinal immune pathology by CD4+CD25+ regulatory T cells. Immunol Lett. 2005;189:189–192. doi: 10.1016/j.imlet.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 36.Kullberg MC, et al. Bacteria-triggered CD4(+) T regulatory cells suppress Helicobacter hepaticus-induced colitis. J Exp Med. 2002;505:505–515. doi: 10.1084/jem.20020556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poutahidis T, et al. Rapid reversal of interleukin-6-dependent epithelial invasion in a mouse model of microbially induced colon carcinoma. Carcinogenesis. 2007;2614:2614–2623. doi: 10.1093/carcin/bgm180. [DOI] [PubMed] [Google Scholar]
- 38.Tomczak MF, et al. Defective activation of ERK in macrophages lacking the p50/p105 subunit of NF-kappaB is responsible for elevated expression of IL-12 p40 observed after challenge with Helicobacter hepaticus. J Immunol. 2006;1244:1244–1251. doi: 10.4049/jimmunol.176.2.1244. [DOI] [PubMed] [Google Scholar]
- 39.Wang Y, et al. c-Rel is essential for the development of innate and T cell-induced colitis. J Immunol. 2008;8118:8118–8125. doi: 10.4049/jimmunol.180.12.8118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tomczak MF, et al. Inhibition of Helicobacter hepaticus-induced colitis by IL-10 requires the p50/p105 subunit of NF-kappa B. J Immunol. 2006;7332:7332–7339. doi: 10.4049/jimmunol.177.10.7332. [DOI] [PubMed] [Google Scholar]
- 41.Mandell L, et al. Intact gram-negative Helicobacter pylori, Helicobacter felis, and Helicobacter hepaticus bacteria activate innate immunity via toll-like receptor 2 but not toll-like receptor 4. Infect Immun. 2004;6446:6446–6454. doi: 10.1128/IAI.72.11.6446-6454.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boulard O, Asquith MJ, Powrie F, Maloy KJ. TLR2-independent induction and regulation of chronic intestinal inflammation. Eur J Immunol. 2009;516:516–524. doi: 10.1002/eji.200939669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Asquith MJ, Boulard O, Powrie F, Maloy KJ. Pathogenic and Protective Roles of MyD88 in Leukocytes and Epithelial Cells in Mouse Models of Inflammatory Bowel Disease. Gastroenterology. 2010;519:519–529. doi: 10.1053/j.gastro.2010.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fritz JH, Ferrero RL, Philpott DJ, Girardin SE. Nod-like proteins in immunity, inflammation and disease. Nat Immunol. 2006;1250:1250–1257. doi: 10.1038/ni1412. [DOI] [PubMed] [Google Scholar]
- 45.Chow J, Mazmanian SK. A pathobiont of the microbiota balances host colonization and intestinal inflammation. Cell Host Microbe. 2010;265:265–276. doi: 10.1016/j.chom.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chaouche-Drider N, et al. A commensal Helicobacter sp. of the rodent intestinal flora activates TLR2 and NOD1 responses in epithelial cells. PLoS One. 2009;4:e5396. doi: 10.1371/journal.pone.0005396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sterzenbach T, et al. Inhibitory effect of enterohepatic Helicobacter hepaticus on innate immune responses of mouse intestinal epithelial cells. Infect Immun. 2007;2717:2717–2728. doi: 10.1128/IAI.01935-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Petnicki-Ocwieja T, et al. Nod2 is required for the regulation of commensal microbiota in the intestine. Proc Natl Acad Sci U S A. 2009;15813:15813–15818. doi: 10.1073/pnas.0907722106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Garcia A, et al. Genetic susceptibility to chronic hepatitis is inherited codominantly in Helicobacter hepaticus-infected AB6F1 and B6AF1 hybrid male mice, and progression to hepatocellular carcinoma is linked to hepatic expression of lipogenic genes and immune function-associated networks. Infect Immun. 2008;1866:1866–1876. doi: 10.1128/IAI.01044-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ihrig M, Schrenzel MD, Fox JG. Differential susceptibility to hepatic inflammation and proliferation in AXB recombinant inbred mice chronically infected with Helicobacter hepaticus. Am J Pathol. 1999;571:571–582. doi: 10.1016/S0002-9440(10)65152-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ward JM, Anver MR, Haines DC, Benveniste RE. Chronic active hepatitis in mice caused by Helicobacter hepaticus. Am J Pathol. 1994;959:959–968. [PMC free article] [PubMed] [Google Scholar]
- 52.Fox JG, et al. Gut microbes define liver cancer risk in mice exposed to chemical and viral transgenic hepatocarcinogens. Gut. 2010;88:88–97. doi: 10.1136/gut.2009.183749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Engle SJ, et al. Elimination of colon cancer in germ-free transforming growth factor beta 1-deficient mice. Cancer Res. 2002;6362:6362–6366. [PubMed] [Google Scholar]
- 54.Boivin GP, et al. Pathology of mouse models of intestinal cancer: consensus report and recommendations. Gastroenterology. 2003;762:762–777. doi: 10.1053/gast.2003.50094. [DOI] [PubMed] [Google Scholar]
- 55.Riddell RH, et al. Dysplasia in inflammatory bowel disease: standardized classification with provisional clinical applications. Hum Pathol. 1983;931:931–968. doi: 10.1016/s0046-8177(83)80175-0. [DOI] [PubMed] [Google Scholar]
- 56.Erdman SE, et al. Nitric oxide and TNF-alpha trigger colonic inflammation and carcinogenesis in Helicobacter hepaticus-infected, Rag2-deficient mice. Proc Natl Acad Sci U S A. 2009;1027:1027–1032. doi: 10.1073/pnas.0812347106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rao VP, et al. Innate immune inflammatory response against enteric bacteria Helicobacter hepaticus induces mammary adenocarcinoma in mice. Cancer Res. 2006;7395:7395–7400. doi: 10.1158/0008-5472.CAN-06-0558. [DOI] [PubMed] [Google Scholar]
- 58.Moser AR, Pitot HC, Dove WF. A dominant mutation that predisposes to multiple intestinal neoplasia in the mouse. Science. 1990;322:322–324. doi: 10.1126/science.2296722. [DOI] [PubMed] [Google Scholar]
- 59.Erdman SE, et al. CD4+CD25+ regulatory lymphocytes induce regression of intestinal tumors in ApcMin/+ mice. Cancer Res. 2005;3998:3998–4004. doi: 10.1158/0008-5472.CAN-04-3104. [DOI] [PubMed] [Google Scholar]
- 60.Rao VP, et al. Proinflammatory CD4+ CD45RB(hi) lymphocytes promote mammary and intestinal carcinogenesis in Apc(Min/+) mice. Cancer Res. 2006;57:57–61. doi: 10.1158/0008-5472.CAN-05-3445. [DOI] [PubMed] [Google Scholar]
- 61.Newman JV, Kosaka T, Sheppard BJ, Fox JG, Schauer DB. Bacterial infection promotes colon tumorigenesis in Apc(Min/+) mice. J Infect Dis. 2001;227:227–230. doi: 10.1086/321998. [DOI] [PubMed] [Google Scholar]
- 62.Wu S, et al. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat Med. 2009;1016:1016–1022. doi: 10.1038/nm.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Erdman SE, et al. Unifying roles for regulatory T cells and inflammation in cancer. Int J Cancer. 2010;1651:1651–1665. doi: 10.1002/ijc.24923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rao VP, Poutahidis T, Fox JG, Erdman SE. Breast cancer: should gastrointestinal bacteria be on our radar screen? Cancer Res. 2007;847:847–850. doi: 10.1158/0008-5472.CAN-06-3468. [DOI] [PubMed] [Google Scholar]
- 65.Suerbaum S, et al. The complete genome sequence of the carcinogenic bacterium Helicobacter hepaticus. Proc Natl Acad Sci U S A. 2003;7901:7901–7906. doi: 10.1073/pnas.1332093100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Smith JL, Bayles DO. The contribution of cytolethal distending toxin to bacterial pathogenesis. Crit Rev Microbiol. 2006;227:227–248. doi: 10.1080/10408410601023557. [DOI] [PubMed] [Google Scholar]
- 67.Ge Z, Schauer DB, Fox JG. In vivo virulence properties of bacterial cytolethal-distending toxin. Cell Microbiol. 2008;1599:1599–1607. doi: 10.1111/j.1462-5822.2008.01173.x. [DOI] [PubMed] [Google Scholar]
- 68.Chien CC, et al. Identification of cdtB homologues and cytolethal distending toxin activity in enterohepatic Helicobacter spp. J Med Microbiol. 2000;525:525–534. doi: 10.1099/0022-1317-49-6-525. [DOI] [PubMed] [Google Scholar]
- 69.Taylor NS, Ge Z, Shen Z, Dewhirst FE, Fox JG. Cytolethal distending toxin: a potential virulence factor for Helicobacter cinaedi. J Infect Dis. 2003;1892:1892–1897. doi: 10.1086/379837. [DOI] [PubMed] [Google Scholar]
- 70.Young VB, et al. Cytolethal distending toxin in avian and human isolates of Helicobacter pullorum. J Infect Dis. 2000;620:620–623. doi: 10.1086/315705. [DOI] [PubMed] [Google Scholar]
- 71.Ge Z, et al. Cytolethal distending toxin is essential for Helicobacter hepaticus colonization in outbred Swiss Webster mice. Infect Immun. 2005;3559:3559–3567. doi: 10.1128/IAI.73.6.3559-3567.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pratt JS, Sachen KL, Wood HD, Eaton KA, Young VB. Modulation of host immune responses by the cytolethal distending toxin of Helicobacter hepaticus. Infect Immun. 2006;4496:4496–4504. doi: 10.1128/IAI.00503-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Young VB, et al. In vitro and in vivo characterization of Helicobacter hepaticus cytolethal distending toxin mutants. Infect Immun. 2004;2521:2521–2527. doi: 10.1128/IAI.72.5.2521-2527.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shen Z, et al. Cytolethal distending toxin promotes Helicobacter cinaedi-associated typhlocolitis in interleukin-10-deficient mice. Infect Immun. 2009;2508:2508–2516. doi: 10.1128/IAI.00166-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Guerra L, et al. Myc is required for activation of the ATM-dependent checkpoints in response to DNA damage. PLoS One. 2010;5:e8924. doi: 10.1371/journal.pone.0008924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liyanage NP, et al. Helicobacter hepaticus cytolethal distending toxin causes cell death in intestinal epithelial cells via mitochondrial apoptotic pathway. Helicobacter. 2010;98:98–107. doi: 10.1111/j.1523-5378.2010.00749.x. [DOI] [PubMed] [Google Scholar]
- 77.Gal-Mor O, Finlay BB. Pathogenicity islands: a molecular toolbox for bacterial virulence. Cell Microbiol. 2006;1707:1707–1719. doi: 10.1111/j.1462-5822.2006.00794.x. [DOI] [PubMed] [Google Scholar]
- 78.Boutin SR, et al. Different Helicobacter hepaticus strains with variable genomic content induce various degrees of hepatitis. Infect Immun. 2005;8449:8449–8452. doi: 10.1128/IAI.73.12.8449-8452.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ge Z, et al. Helicobacter hepaticus HHGI1 is a pathogenicity island associated with typhlocolitis in B6.129-IL10 tm1Cgn mice. Microbes Infect. 2008;726:726–733. doi: 10.1016/j.micinf.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Burne RA, Chen YY. Bacterial ureases in infectious diseases. Microbes Infect. 2000;533:533–542. doi: 10.1016/s1286-4579(00)00312-9. [DOI] [PubMed] [Google Scholar]
- 81.Ge Z, et al. Helicobacter hepaticus urease is not required for intestinal colonization but promotes hepatic inflammation in male A/JCr mice. Microb Pathog. 2008;18:18–24. doi: 10.1016/j.micpath.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Belzer C, et al. Differential regulation of urease activity in Helicobacter hepaticus and Helicobacter pylori. Microbiology. 2005;3989:3989–3995. doi: 10.1099/mic.0.28188-0. [DOI] [PubMed] [Google Scholar]
- 83.Belzer C, van Schendel BA, Kuipers EJ, Kusters JG, van Vliet AH. Iron-responsive repression of urease expression in Helicobacter hepaticus is mediated by the transcriptional regulator Fur. Infect Immun. 2007;745:745–752. doi: 10.1128/IAI.01163-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Alyamani EJ, et al. Helicobacter hepaticus catalase shares surface-predicted epitopes with mammalian catalases. Microbiology. 2007;1006:1006–1016. doi: 10.1099/mic.0.29184-0. [DOI] [PubMed] [Google Scholar]
- 85.Hong Y, Wang G, Maier RJ. A Helicobacter hepaticus catalase mutant is hypersensitive to oxidative stress and suffers increased DNA damage. J Med Microbiol. 2007;557:557–562. doi: 10.1099/jmm.0.46891-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mehta NS, Benoit S, Mysore JV, Sousa RS, Maier RJ. Helicobacter hepaticus hydrogenase mutants are deficient in hydrogen-supported amino acid uptake and in causing liver lesions in A/J mice. Infect Immun. 2005;5311:5311–5318. doi: 10.1128/IAI.73.9.5311-5318.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sterzenbach T, et al. Role of the Helicobacter hepaticus flagellar sigma factor FliA in gene regulation and murine colonization. J Bacteriol. 2008;6398:6398–6408. doi: 10.1128/JB.00626-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chin EY, Dangler CA, Fox JG, Schauer DB. Helicobacter hepaticus infection triggers inflammatory bowel disease in T cell receptor alpha\beta mutant mice. Comp Med. 2000;586:586–594. [PubMed] [Google Scholar]
- 89.Shomer NH, Dangler CA, Schrenzel MD, Fox JG. Helicobacter bilis-induced inflammatory bowel disease in scid mice with defined flora. Infect Immun. 1997;4858:4858–4864. doi: 10.1128/iai.65.11.4858-4864.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shomer NH, Dangler CA, Marini RP, Fox JG. Helicobacter bilis/Helicobacter rodentium co-infection associated with diarrhea in a colony of scid mice. Lab Anim Sci. 1998;455:455–459. [PubMed] [Google Scholar]
- 91.Fox JG, et al. Persistent hepatitis and enterocolitis in germfree mice infected with Helicobacter hepaticus. Infect Immun. 1996;3673:3673–3681. doi: 10.1128/iai.64.9.3673-3681.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Erdman S, Fox JG, Dangler CA, Feldman D, Horwitz BH. Typhlocolitis in NF-kappa B-deficient mice. J Immunol. 2001;1443:1443–1447. doi: 10.4049/jimmunol.166.3.1443. [DOI] [PubMed] [Google Scholar]
- 93.Maggio-Price L, et al. Helicobacter bilis infection accelerates and H. hsource>paticus infection delays the development of colitis in multiple drug resistance-deficient (mdr1a−/−) mice. Am J Pathol. 2002;739:739–751. doi: 10.1016/S0002-9440(10)64894-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Maggio-Price L, et al. Helicobacter infection is required for inflammation and colon cancer in SMAD3-deficient mice. Cancer Res. 2006;828:828–838. doi: 10.1158/0008-5472.CAN-05-2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fox JG, et al. A novel urease-negative Helicobacter species associated with colitis and typhlitis in IL-10-deficient mice. Infect Immun. 1999;1757:1757–1762. doi: 10.1128/iai.67.4.1757-1762.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Franklin CL, et al. Helicobacter typhlonius sp. nov., a Novel Murine Urease-Negative Helicobacter Species. J Clin Microbiol. 2001;3920:3920–3926. doi: 10.1128/JCM.39.11.3920-3926.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Whary MT, et al. Rapid onset of ulcerative typhlocolitis in B6.129P2-IL10tm1Cgn (IL-10-/-) mice infected with Helicobacter trogontum is associated with decreased colonization by altered Schaedler's flora. Infect Immun. 2006;6615:6615–6623. doi: 10.1128/IAI.01091-06. [DOI] [PMC free article] [PubMed] [Google Scholar]