Abstract
Many diabetics are insensitive to aspirin's platelet anti-aggregation effects. The influence of co-administration of aspirin and fish oil (FO) on plasma lysophospholipids in subjects with diabetes is poorly characterized. Thirty adults with type 2 diabetes mellitus were treated with aspirin (81 mg/day) for seven days, then with FO (4 g/day) for 28 days, then in combination for another seven days. Lysophospholipids and platelet measures were determined after acute (4 hours) and chronic (7 days) ingestion of aspirin, FO, or both in combination. FO ingestion reduced all lysophosphatidic acid (LPA) concentrations, while EPA (20:5n-3) and DHA (22:6n-3) lysophosphatidylcholine (LPC) concentrations significantly increased after FO alone and in combination with aspirin. In vitro arachidonic acid-induced platelet aggregation was most strongly correlated with palmitoleic (16:1) and oleic (18:1) LPA and LPC concentrations at all time points. The ingestion of these agents may reduce cardiovascular disease risk in diabetic adults, with a disrupted lipid milieu, via lysolipid mediated mechanisms.
Keywords: Lysophosphatidylcholine, Lysophosphatidic acid, Omega-3 fatty acids, Eicosapentaenoic acid, Docosahexaenoic acid, Aspirin, Platelet function
INTRODUCTION
Phospholipids have a large range of cellular effects involving growth, survival, adhesion, and migration in a variety of tissues [1-3]. Phospholipids are the ultimate precursors for serum lysophosphatidic acids (LPAs) [4], lipid metabolites shown to play a primary role in the atherogenic process of platelet aggregation. The relatively few studies on LPAs focus upon the fatty acid side chain which may be responsible for specific biological activities [5]. The specific in vivo precursors of LPAs remain unclear. One likely precursor is lysophosphatidylcholine (LPC), a lysophospholipid contained in oxidized low density lipoprotein cholesterol (LDL-C) [4, 6, 7]. Circulating extra-cellular LPA is thought to be generated by the hydrolysis of LPC via the enzyme autotaxin (lysophospholipase D), which is produced and excreted into plasma by adipocytes [8-13]. As the role of LPA in atherosclerosis and acute coronary syndromes is rapidly emerging [14], there is intense interest in the development of new therapeutics to target LPA generation.
LPC and LPA species can play varying roles in disease processes based on their fatty acid chain [5, 15]. For example, polyunsaturated LPA species have been identified as more potent inducers of platelet aggregation and atherogenesis [7, 16] than saturated species [5, 17]. While the general consensus is that in humans total LPA exposure is related to acute arterial thrombosis, most of our understanding of LPC and LPA to date has been derived from animal studies, which in some instances have shown contradictory physiological effects [2]. Moreover, human studies have generally not focused on the different lysophospholipid species. Little is currently known about the effects of diet on circulating LPC or LPA levels in humans, and there are no data from those with diabetes mellitus. n-3 fatty acids derived from fish oils – containing primarily eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) – provide considerable benefit to heart health due to their anti-inflammatory and tissue-protective effects [18]. Aspirin has also been well-established in its ability to inhibit platelet aggregation [19], but little is known about its metabolomics when combined with fish oil. We recently investigated the impact of fish oil supplementation and aspirin, both alone and in combination, on plasma LPC and LPA in healthy adults without chronic disease and showed that levels of LPC, but not LPA, were regulated by n-3 dietary supplementation, suggesting a more complex pathway of LPA synthesis via LPC hydrolysis [20]. Because individuals at high risk for CVD likely have impaired metabolic function, particularly those with type 2 diabetes mellitus who do not benefit from the anti-platelet aggregation effects of aspirin alone [21], the effects of dietary supplementation and aspirin on lysophospholipids may differ in this vulnerable population compared to their healthy counterparts.
To our knowledge, there are no published studies that examine dietary and pharmacological influences on LPC and LPA concentrations in human diabetics. We hypothesized that the ingestion of fish oil, both alone and in combination with aspirin, reduces LPC and LPA plasma concentrations compared to baseline in a species-dependent manner, with greater effects on the n-3 LPCs and LPAs. We also hypothesized that changes in these lysophospholipid concentrations would correlate with measures of platelet aggregation.
PATIENTS AND METHODS
Patients
Thirty adults aged 40 to 80 years with type 2 diabetes mellitus were enrolled in this study. Details of recruitment, eligibility criteria, and data collection have been reported previously [21]. In short, a diagnosis of type 2 diabetes mellitus was based on the criteria from the Executive Committee of the American Diabetes Association Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus [22]. For the duration of the study, participants were instructed to avoid taking flax seed oil, fish oil other than the study capsules, vitamins, nutritional supplements, herbal preparations, and to limit fish intake to three servings/ week. The use of diabetic medications was permitted. The list of exclusion criteria was extensive and included: a diagnosis of CVD; recent history of malignancy, peptic ulcer, or bleeding disorder; use of antiplatelet or antithrombotic therapy, oral contraceptives, or NSAIDs; signs of any metabolic disease that would influence lipid metabolism; allergy to aspirin or fish oil; pregnancy; drug or alcohol abuse and tobacco use.
Protocol
This was an 8-week sequential-therapy clinical trial in which each subject served as his or her own control, as described previously by us [21]. The daily dosing regimens for each study agent were as follows: (1) one over-the-counter non-enteric 81 mg aspirin tablet and (2) four 1000 mg over-the-counter fish oil capsules (OmegaRx brand; Zone Labs, Marblehead, MA). Due to the inconsistent evidence regarding the effect of enteric coating on the bioavailability and biological activity of aspirin [23], non-enteric-coated aspirin was used. The timeline of study activities, all of which took place at the University of Rochester's Clinical Research Center, are summarized in Table 1. Briefly, following screening and recruitment (Day 1), participants underwent a 10-day aspirin-free ‘washout’ period. The night before each of the four study visits, subjects were required to eat a standardized low-fat meal, as directed by a registered dietician, and were instructed not to eat or drink (except water) for eight hours prior to each visit. On Day 11 (study visit 1), a baseline blood sample was obtained (blood draw 1, or BD1) before starting the seven-day aspirin regimen. Four hours after the ingestion of the first aspirin, BD2 was obtained. Participants returned on Day 18 (study visit 2), where BD3 was taken after seven days of aspirin ingestion. This was followed by the 28-day fish oil regimen, in which some participants (n=8) took aspirin as prescribed by their primary care physician but were instructed to remain aspirin-free for 10 days prior to study visit 3. On Day 46, an initial phlebotomy was done (BD4) reflecting the 28 days of fish oil consumption. Subjects then ingested a single dose (81 mg) of aspirin while continuing the fish oil and had the fifth phlebotomy (BD5) obtained four hours later. The combined fish oil plus aspirin regimen continued for seven days until Day 53 (study visit 4), in which the final phlebotomy (BD6) was taken and all study regimens were discontinued. Thus, BD2 and BD5 represent the acute (four hours) effects of aspirin ingestion, whereas BD3 and BD6 represent its chronic (seven days) effects.
Table 1.
Study timeline of activities
| Regimen | Day | Study Visit | Activity |
|---|---|---|---|
| 1 | • Recruitment and screening • Collection of baseline demographic and clinical data |
||
| 1-10 | • Aspirin-free period of 10 days prior to Study Visit 1 | ||
| Aspirin only | 11 | 1 | • Blood draw 1 (baseline, before aspirin) • Ingestion single 81 mg dose aspirin • Blood draw 2 (4 hours post aspirin ingestion) |
| 12-17 | • Single 81 mg aspirin/day | ||
| Fish oil only | 18 | 2 | • Blood draw 3 (7 days aspirin ingestion) • Begin fish oil 4g/day and discontinue aspirin unless subject takes it as prescribed by their doctor |
| 19-45 | • 28 days of fish oil 4g/day | ||
| 36-45 | • Aspirin-free period of 10 days prior to Study Visit 3 | ||
| Aspirin + fish oil | 46 | 3 | • Blood draw 4 (4 weeks fish oil ingestion, before aspirin) • Continue fish oil and a single dose 81 mg aspirin • Blood draw 5 (4 hours post aspirin + fish oil ingestion) |
| 47-52 | • Continue aspirin and fish oil 4g/day | ||
| 53 | 4 | • Blood draw 6 (7 days aspirin + fish oil ingestion) • Discontinue fish oil and aspirin |
This study was approved by the Research Subject Review Board at the University of Rochester and registered with Clinicaltrials.gov (NCT01181882).
Laboratory Methods
Lysophospholipids
Eleven different acyl species of plasma LPC (in μM) and LPA (in nM) were measured simultaneously from each blood draw. The acyl chains analyzed were the saturated fatty acids palmitic acid (16:0) and stearic acid (18:0) and the unsaturated fatty acids 16:1, 18:1, linoleic acid (18:2n-6), 18:3, arachidonic acid (20:4n-6), EPA (20:5n-3), adrenic acid (22:4n-6), 22:5, and DHA (22:6n-3). Double bond position is not available directly from these or similar measurements, however because of the predominance of particular isomers in plasma and in LPC and LPA, we label them as shown. The 18:3 isomer is likely to be predominantly n-3 (α-linolenic acid) with some unknown contribution of gamma-linolenic acid (18:3n-6) while the mix of n-3 and n-6 22:5 cannot be estimated. The monoene isomers are typically dominated by products of 9-desaturation (16:1n-7 and 18:1n-9) with some 18:1n-7.
As previously specified in detail [20], a modified Bligh and Dyer extract of plasma was directly infused into and analyzed on an ABI (Life Technologies; Foster City, CA) QTrap 2000 MS/MS via an Advion Nanomate robotic device. Signals were calibrated against internal standards LPA-17:0 and LPC-17:0, obtained from Avanti Polar Lipids Inc. (Alabaster, AL). LPC and LPA were analyzed in positive and negative ion modes, respectively. Calibration on a molecular species-by-species basis was accomplished with an external standard mixture to obtain relative response factors. The response factor for LPC/LPA 18:0 was used for both saturates, 18:1 was used for LPC/LPA with one to three double bonds, and 20:4 for LPC/LPA with four to six double bonds. After relative response was calibrated, concentrations were calibrated against the internal standards. Under these conditions, negligible conversion (< 0.1%) of LPC to LPA was observed, and thus LPC and LPA accuracy was not compromised as a result of aberrant reactions in the ion source.
Platelet aggregation direct measures
Described in an earlier publication [21], whole blood electrical impedance platelet aggregation was conducted using a Chronolog Whole Blood Lumi-Aggregometer® (Model 560VS) with reagents from Chronolog Corporation (Havertown, PA). The following agonists were tested within two hours of the phlebotomy: ADP at 2.5 μM, 5 μM, and 10 μM, collagen at 1 and 2 mg/ml, and arachidonic acid at 0.5 mM. After incubation of each agonist with whole blood aliquots from a sodium citrate tube and saline, a baseline measurement of electrical impedance at 20Ω was recorded. The aggregation of platelets was measured from the change in impedance over a period of five minutes. The characteristics of the resultant platelet function curve were measured and calculated using Aggro/link software (version 5.1) from Chronolog Corporation.
Thromboxane B2 (TXB2)
A competitive enzyme linked immunosorbent assay (TXB2 EIA Kit, Cayman Chemical Co., Ann Arbor, MI) was used to analyze TXB2 from citrated plasma serially diluted, as necessary, in sample buffer to fall within the standard curve (typically 1:2). Using this method, there was no evidence suggesting interference in the assay and thus further purification of the plasma samples was not necessary (limit of detection (LOD) = 7.8 ng/ml).
Adiponectin and leptin
Commercially available ELISA kits (EMD Millipore Corporation, Billerica, MA) were used to measure serum levels of adiponectin after 1:500 dilution in assay buffer (LOD = 2.5 ng/ml) and leptin (LOD = 0.78 ng/ml).
Statistical Methods
Baseline characteristics of participants were described using relative frequencies for categorical measures, and means and standard deviations (SD) for continuous measures. Univariate analyses were used to examine the distribution of lysophospholipid and platelet aggregation/ function concentrations. Non-normally distributed variables, based on a Shapiro-Wilk statistic p-value > 0.05, were log transformed. All 11 species of LPC and LPA concentrations and total levels (sum of the 11 species) were analyzed using crude and adjusted mixed models to examine the treatment effects at each blood draw, controlling for statin use, blood draw, body mass index (BMI), presence of hypertension (HTN), gender, race, smoking status, alcohol frequency, fish consumption, and education in the adjusted models. In the mixed models, the unstructured correlation was assumed to account for within-participant variations of LPC and LPA concentrations measured at the different blood draws. P-values were reported to compare BD2-6 individually to baseline (BD1), with p ≤ 0.05 considered statistically significant.
The mean and SD of the actual baseline concentrations were reported. The effects of fish oil ingestion on these concentrations were calculated by subtracting baseline concentrations (BD1) from concentrations after 28 days of fish oil (BD4). Both unadjusted and adjusted p-values for the “change from baseline” were reported using the mixed model approach based on the log transformed values. A sensitivity analysis was conducted from a prior study of 15 non-diabetic otherwise healthy adults (similar dosing and timing of fish oil ingestion) [20] using this same methodology. Due to the limited amount of data collected in that study, the adjusted analysis only controlled for blood draw, age, BMI, gender, alcohol frequency, and fish consumption.
To determine which species of LPC and LPA were associated with platelet aggregation and platelet function measures, the change in concentrations relative to baseline were calculated for BD2-6, assuming there was no carry over effect for each treatment. Changes in each platelet aggregation and function measure were modeled against change in the lysophospholipids one by one using a mixed model to control for within subject clustering effects. The Akaike Information Criterion (AIC) was obtained and recorded from each model. For each platelet aggregation and function measure, the LPC and LPA metabolite with the smallest AIC represents the strongest association with the platelet aggregation or function measure (i.e. model with the best fit).
RESULTS
Thirty adults with type 2 diabetes mellitus completed the study protocol. The baseline characteristics of these study participants are shown in Table 2. The aspirin and fish oil study agents were well tolerated by all participants, and no adverse events were noted. The mean concentrations of DHA and EPA in the fish oil capsules were 406 ± 42 mg/ml and 330 ± 46 mg/ml, respectively, and we previously showed that ingestion of fish oil capsules resulted in increases from baseline of 106 ± 48 μg/ml to 190 ± 65 μg/ml after 28 days fish oil for plasma DHA and 13 ± 7 μg/ml to 61 ± 26 μg/ml EPA in this study [21].
Table 2.
Baseline participant characteristics (n = 30)
| Minimum | Maximum | ||
|---|---|---|---|
| Age, mean (SD) | 56.6 (8.9) | 40.0 | 74.5 |
| Male, n (%) | 15 (50.0) | ||
| Race, n (%) | |||
| African-American | 9 (30.0) | ||
| Asian | 2 (6.7) | ||
| Caucasian | 17 (56.7) | ||
| Other | 2 (6.7) | ||
| Smoking status, n (%) | |||
| Former | 11 (36.7) | ||
| Never | 19 (63.3) | ||
| Fish intake, n (%) | |||
| < Once/month | 11 (36.6) | ||
| 1-3 times/month | 10 (33.3) | ||
| Once/week | 7 (23.3) | ||
| Twice/week | 2 (6.7) | ||
| Physical activity (hrs/wk), mean (SD) | 5.12 (5.23) | ||
| Metformin use, n (%) | 25 (83.3) | ||
| Alcohol consumption, n (%) | |||
| None | 23 (76.7) | ||
| 1-3 days/month | 2 (6.7) | ||
| 1-4 days/week | 4 (13.3) | ||
| ≥5 days/week | 1 (3.3) | ||
| Education, n (%) | |||
| < High school | 2 (6.7) | ||
| High school or GED | 6 (20.0) | ||
| Bachelors or associates degree | 12 (40.0) | ||
| Graduate degree | 6 (20.0) | ||
| Systolic Blood Pressure, mean (SD) | 132.9 (14.0) | 98.0 | 171.0 |
| Diastolic Blood Pressure, mean (SD) | 76.0 (9.4) | 58.0 | 94.0 |
| BMI, mean (SD) | 34.6 (7.5) | 22.1 | 54.0 |
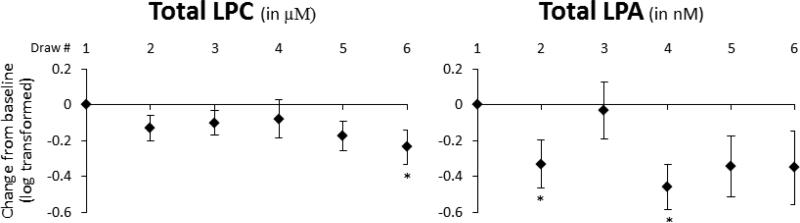
Figure 1 illustrates the effects of ingesting aspirin alone (BD2-3), fish oil alone (BD4), and aspirin + fish oil (BD5-6) on total levels of LPC and LPA, relative to baseline (BD1). Overall, concentrations of both LPC (in μM) and LPA (in nM) were reduced from baseline. While aspirin and fish oil alone did not cause a meaningful change in total LPC, we did observe a statistically significant reduction with chronic combined aspirin and fish oil ingestion (BD6, p = 0.04) and a trend for significance after acute ingestion (BD5, p = 0.06). Concentrations of total LPA were significantly reduced after acute aspirin ingestion (BD2, p = 0.04) and 28 days of fish oil ingestion (BD4, p = 0.01).
Figure 1.
The effects of aspirin alone (BD2-3), fish oil alone (BD4), and aspirin + fish oil (BD5-6) relative to baseline (as zero) on total LPC (in μM) and LPA (in nM) concentrations (log transformed). Data are expressed as mean concentration change from baseline and standard errors after log transformation. Asterisks (*) represent a significant difference from baseline blood draw (p < 0.05) using the t-test from a mixed model adjusting for statin use, blood draw, BMI, presence of hypertension, gender, race, smoking status, alcohol frequency, fish consumption, and education.
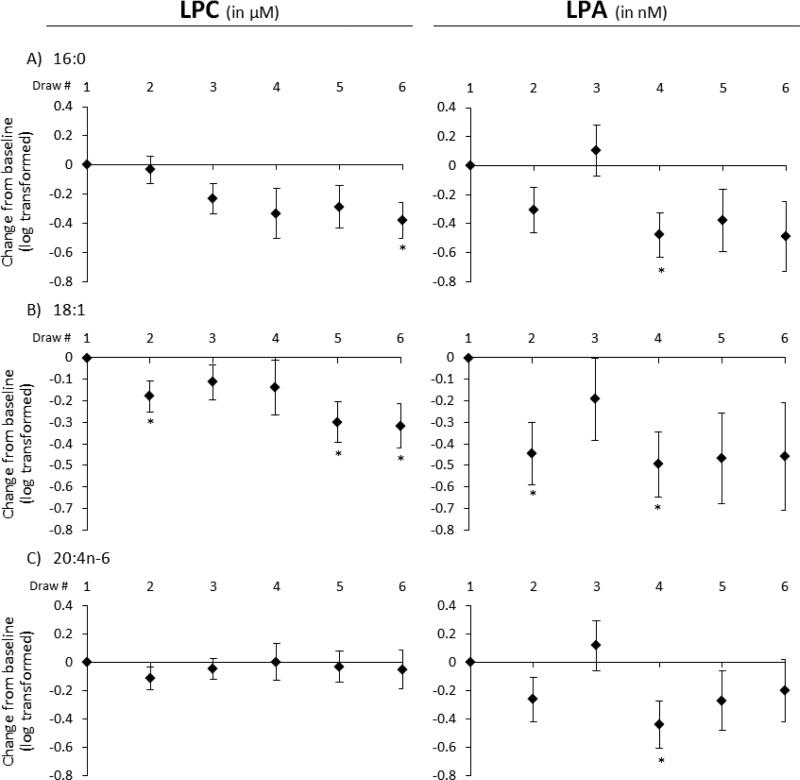
To detect variability among lysophospholipid species, individual metabolites were examined separately, with a particular focus on LPC and LPA metabolites that have previously been implicated in CVD risk [3-5, 17, 24, 25]. Among these (see Figure 2), palmitic acid (Fig. 2A), oleic acid (Fig. 2B), and arachidonic acid (Fig. 2C) side chain species demonstrated similar trends across the study period. While none of these LPC species were significantly changed after 28 days of fish oil ingestion (BD4), their LPA analogues showed significant reductions (p < 0.03). Of these species, acute aspirin ingestion (BD2) had the greatest trend in effect on LPC 18:1, which was reduced further with the addition of fish oil (BD5-6). Chronic aspirin ingestion (BD3) had discreet effects on concentrations of both LPC and LPA species relative to baseline. The combined aspirin and fish oil regimen demonstrated non-significant reductions in these LPA species both acutely and chronically. In all five of the other species measured (see Supplemental Figure 1), similar trends were noted, more so in the LPA configuration. Figure 3 separates the n-3 fatty acids EPA (Fig. 3A), DPA (Fig. 3B), and DHA (Fig. 3C) from the others. These LPC metabolites demonstrate quite different characteristics from those previously mentioned.
Figure 2.
The effects of aspirin alone (BD2-3), fish oil alone (BD4), and aspirin + fish oil (BD5-6) relative to baseline (as zero) on concentrations of three LPC (in μM) and LPA (in nM) species: (A) palmitic acid, (B) oleic acid, and (C) arachidonic acid. Data are expressed as mean concentration change from baseline and standard errors after log transformation. * indicates p < 0.05 using a mixed model as in Fig. 1.
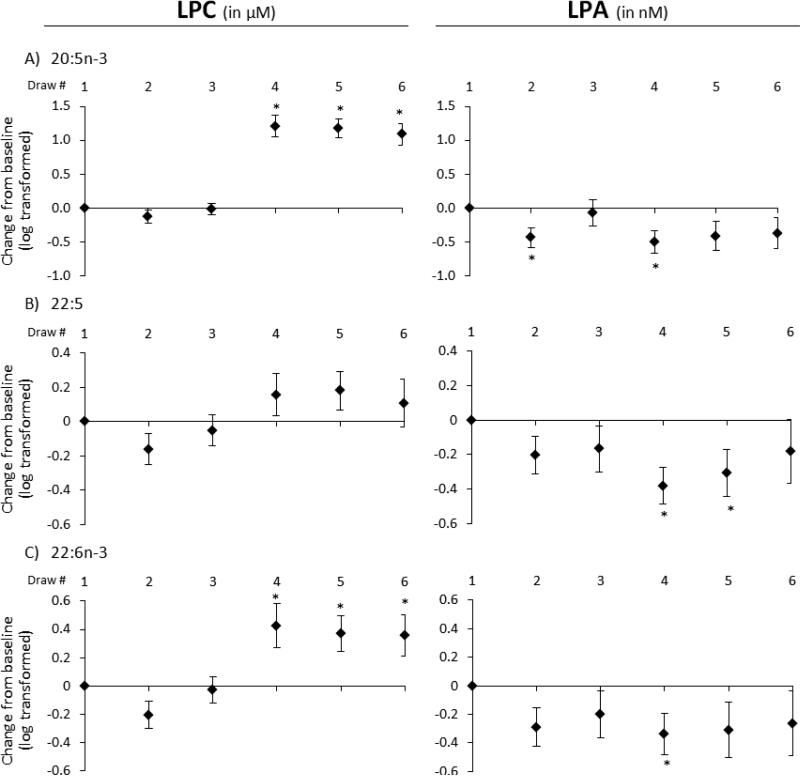
Figure 3.
The effects of aspirin alone (BD2-3), fish oil alone (BD4), and aspirin + fish oil (BD5-6) relative to baseline (as zero) on concentrations of n-3 fatty acid LPC (in μM) and LPA (in nM) species: (A) EPA, (B) DPA, and (C) DHA. Data are expressed as mean concentration change from baseline and standard errors after log transformation. * indicates p < 0.05 using a mixed model as in Fig. 1.
Although aspirin alone consistently caused a slight reduction in LPC concentrations relative to baseline, more so acutely than chronically, chronic fish oil ingestion without (BD4) and in combination with aspirin (BD5-6) led to increased levels, significantly so for LPC 20:5n-3 and LPC 22:6n-3. In contrast, concentrations of their LPA analogues behave similarly to total LPA, demonstrating significant decreases from baseline (p < 0.04) with 28 days of fish oil only.
Because the effects of fish oil were more prominent than for aspirin, we present the changes in absolute concentrations caused by its ingestion in Table 3. The changes from baseline are mostly in the negative direction, with the exception of LPC 20:5n-3 and LPC 22:6n-3. For some species, namely 18:1 and 18:3, the p-values for change from baseline either lost significance (LPC configuration) or became significant (LPA configuration) after adjusting for potential confounders. In general, no significant changes were noted due to fish oil ingestion in eight of the 11 LPC species, while the concentrations of all 11 LPAs decreased significantly. In comparison, we re-analyzed data from our previous study of healthy individuals [20] using the same statistical approach of adjusting for potential confounders (Supplemental Table 1), and found that changes in absolute LPA levels from baseline after fish oil ingestion were all in the positive direction, though none were statistically significant. In the same prior study, among the four LPCs with significant changes from baseline, species 20:5n-3, 22:5, and 22:6n-3 (all n-3 fatty acids) were in the positive direction and 22:4n-6 was in the negative direction.
Table 3.
Effects of Fish Oil Ingestion by Diabetic Adults on Lysophospholipids
| Baseline Value |
Change from Baseline | Unadjusted p-value | Adjusted p-value* | ||
|---|---|---|---|---|---|
| Mean | SD | ||||
| LPCs (in μM) | |||||
| 16:0 | 142.86 | 100.96 | −48.81 | 0.052 | 0.083 |
| 16:1 | 9.61 | 7.08 | −58.42 | 0.003 | 0.024 |
| 18:0 | 58.11 | 28.52 | −11.87 | 0.093 | 0.146 |
| 18:1 | 70.47 | 69.58 | −82.34 | 0.001 | 0.302 |
| 18:2n-6 | 109.70 | 141.61 | −13.56 | 0.608 | 0.538 |
| 18:3 | 3.12 | 3.36 | −9.71 | 0.002 | 0.078 |
| 20:4n-6 | 70.01 | 121.18 | 0.07 | 0.851 | 0.972 |
| 20:5n-3 | 6.05 | 9.14 | 12.14 | <0.001 | <0.001 |
| 22:4n-6 | 4.45 | 6.71 | −1.40 | 0.047 | 0.069 |
| 22:5 | 10.73 | 17.94 | −1.52 | 0.163 | 0.227 |
| 22:6n-3 | 16.50 | 21.68 | 5.78 | 0.009 | 0.022 |
| Total LPC | 501.6 | 489.82 | −209.66 | 0.058 | 0.490 |
| LPAs (in nM) | |||||
| 16:0 | 42.73 | 33.98 | −19.94 | 0.003 | 0.012 |
| 16:1 | 10.74 | 8.89 | −30.68 | 0.549 | 0.010 |
| 18:0 | 43.85 | 33.10 | −17.24 | 0.011 | 0.038 |
| 18:1 | 10.40 | 7.91 | −27.64 | 0.506 | 0.009 |
| 18:2n-6 | 16.88 | 16.60 | −9.23 | 0.006 | 0.018 |
| 18:3 | 19.52 | 21.95 | −47.58 | 0.963 | 0.005 |
| 20:4n-6 | 19.12 | 14.85 | −7.80 | 0.012 | 0.027 |
| 20:5n-3 | 17.33 | 13.49 | −8.72 | 0.003 | 0.014 |
| 22:4n-6 | 17.38 | 15.26 | −9.09 | 0.002 | 0.011 |
| 22:5 | 30.40 | 17.82 | −10.35 | 0.001 | 0.005 |
| 22:6n-3 | 35.78 | 27.80 | −11.43 | 0.033 | 0.045 |
| Total LPA | 264.14 | 184.18 | −199.70 | 0.002 | 0.005 |
P-values were calculated using log transformed values
Adjusted for statin use, blood draw, BMI, presence of HTN, gender, race, smoking status, alcohol frequency, fish consumption, and education.
Finally, we conducted the model selection strategy to identify species of lysophospholipids that were most associated with platelet aggregation (using ADP, collagen, and arachidonic acid as agonists) and other platelet function measures (plasma TXB2, adiponectin, and leptin; see Methods). When examining change from baseline, the change in 18:1 and 16:1 LPC and LPA species consistently had the lowest AIC values with all six of these platelet measure changes individually at each time point, indicating the greatest association. 18:3 LPC and LPA species changes ranked among the lowest AIC values with all six platelet measures individually only at the BD4 time point (after ingestion of fish oil alone). Results were similar regardless of the concentration of ADP and collagen used. The models also showed that these species represent strong positive relationships with platelet aggregation and platelet function; hence, decreases in these LPC and LPA species are associated with a decrease in platelet aggregation activity. No major differences were noted between the six platelet measures at any time point.
DISCUSSION AND CONCLUSIONS
This study systematically investigated the effects of aspirin and fish oil supplementation, alone and in combination, on various LPC and LPA species, as well as their association with platelet aggregation and other function measures in adult subjects with type 2 diabetes mellitus. Our results suggest effects that appear different than in previous studies of healthy individuals without diabetes mellitus. Future research will be needed to determine their clinical importance for the prevention of CVD in subjects with diabetes mellitus given their high risk status and disrupted lipid milieu.
Aspirin alone had a greater acute (4 h) effect on both LPC and LPA levels than chronic (7 d) administration. Aspirin has long been considered a stalwart and inexpensive therapy for the prevention of CVD [26]. Low-dose aspirin (81-162 mg/d) has been shown to reduce vascular events in both primary and secondary prevention settings, with a 44% reduction in risk of myocardial infarction [27, 28], and is associated with a lower risk of gastrointestinal bleeding compared to higher doses [23]. Its benefits in preventing CVD have been most widely ascribed to its antiplatelet effects because it reduces the production of the very potent platelet function agonist thromboxane A2 through the acetylation of cyclooxygenase-1 (COX-1) [23]. By inhibiting COX-1, it reduces the production of other pro-inflammatory and pro-thrombotic prostaglandins from arachidonic acid [29-32]. However, excess thromboxane release has been shown to occur in type 2 diabetic patients with CVD [33]. It is therefore not surprising that meta-analyses of large-scale collaborative trials in men and women with diabetes support the view that low-dose aspirin therapy should be prescribed as a secondary prevention strategy, if no contra-indications exist. Our results suggest that the reduction of LPA concentrations by low-dose aspirin may play a role in its beneficial effects in individuals with acute coronary syndromes and should be further investigated given that approximately 50% of such individuals do not survive [34].
Whereas we have previously shown concentrations of only EPA and DHA species of LPC, but not LPA, to be modified after fish oil ingestion in healthy individuals [20], here we demonstrate increases in the concentration of n-3 LPC species and decreases in their LPA analogues. Other LPC species, namely 16:1 LPC concentrations were reduced by 28 days of fish oil ingestion. An interesting finding was that all LPA species, including total LPA, were significantly reduced by fish oil ingestion alone, however this finding was not evident in healthy subjects, suggesting differences in effects on phospholipids as an important element in the pathophysiology of atherosclerosis in those with diabetes. Since 28 days of fish oil ingestion demonstrated a profound decrease in LPA concentrations, and lower plasma levels of LPA are associated with antithrombotic properties [2, 15], the effects of fish oil supplementation alone in this study are consistent with its already ascribed benefits in a population of diabetics. The unremarkable findings in healthy subjects are not well-understood and require further examination.
Adding aspirin to the fish oil regimen revealed unexpected lysolipid species-specific changes. Overall, the addition of aspirin enhanced the beneficial effects of fish oil in total LPC while slightly weakening the effects in total LPA. Aspirin acetylates COX and blocks the metabolism of arachidonic acid into a variety of pro-inflammatory and thrombosis-enhancing mediators including thromboxane [29-32]. The n-3 fatty acids EPA and DHA compete with arachidonic acid for the same COX pathways and can also inhibit the production of inflammatory mediators. Therefore, in combination, aspirin and fish oil work in parallel to shift the fatty acid metabolic balance toward a less inflammatory environment. Our data, in contrast, suggest that the combination of these agents may affect the conversion of LPC to LPA via an unknown mechanism that may apply for only some LPC species (16:0, 16:1, 18:0, 18:1, 18:2n-6, and 18:3), although this should be validated in vivo using a tracer study. Though the direction of effects was variable between LPC and LPA species in this population, no significant changes were previously noted between fish oil and aspirin + fish oil in healthy individuals [20]. It remains unknown whether these characteristics are specific to the diabetic population where metabolic abnormalities are most common and susceptible to change.
The use of one or more phospholipids as biomarkers for early detection of CVD or as independent causal but modifiable risk factors in humans has not been an area of substantial study among CVD investigators. Previous preclinical studies have demonstrated unsaturated LPA species (16:1, 18:1, and 18:2) to potentially dedifferentiate vascular smooth muscle cells and stimulate neointima formation in vivo through activation of ERK and p38MAPK, whereas saturated LPA species (16:0 and 18:0) did not manifest these atherogenic properties [5, 17]. Although our results suggest some consistent evidence for specific metabolites (i.e. 16:1, 18:1, and 18:3) to be associated with platelet function and aggregation after controlled changes in dietary intake, it is clear that lysolipid class behaves independently. For example, although LPC species both increase and decrease after fish oil ingestion, all LPA concentrations are reduced, suggesting that fish oil consumption may modulate autotaxin expression or activity, which has been shown to be a primary mediator for converting LPC to LPA [2, 35]. Autotaxin, a part of the ectonucleotide pyrophosphatase/phosphodiesterase (Enpp) family, has the ability to hydrolyze LPC produced from cholesterol esterification or phospholipase type-A [1]. In human liver cells, it has been shown that transgenic overexpression of Enpp using the α1-anti-trypsin inhibitor promoter increases circulating autotaxin and LPA levels simultaneously [36], suggesting autotaxin to regulate blood LPA concentrations. This evidence, however, is based on total LPC and LPA concentrations, which as we show do not necessarily reflect functions of their individual metabolites. Thus, regulation of lysophospholipid metabolism in human subjects may be more complex than previously suggested from in vitro and animal studies.
Our study has some strengths and limitations. The lack of a placebo control group limits our ability to determine whether our findings are due to the actual treatment regimens. However, we used a sequential clinical trial design, where individuals’ baseline values represent their own control, and mixed modeling to account for the small sample size and enhance statistical power because we analyzed multiple outcomes at each time point. At the same time, as our previous study conducted with only 15 healthy subjects likely had reduced statistical power, it is possible that this may explain the lack of association in healthy individuals compared to the diabetics in the current study. However, no major trends, which parallel what was seen in the group of diabetics, were present. Although all subjects were carefully characterized diabetics who were seen in a controlled research setting where study protocol adherence was strictly documented, medication adherence between study visits was based on self-report. Although validated measures of lysophospholipids, platelet aggregation and platelet function were used, the local production of lysophospholipids in inflamed or injured tissues may not be faithfully reflected in circulation levels, which has a very short half-life [37, 38]. Nonetheless, levels of LPC and LPA are increased in certain disease states including patients with acute coronary syndromes [39]. The possibility of measurement error also exists, but efforts were made to minimize this by using reagents from the same batch and the same analytical machine to process specimens, via the methods referenced in our team's prior publication.
In conclusion, our study is the first to describe the effects of low-dose aspirin and fish oil ingestion, alone and combined, on plasma concentrations of lysophospholipid species in individuals with a chronic and epidemic disease characterized by a metabolically disrupted and adverse lipid milieu. We have shown that diabetics, but not healthy individuals, ingesting EPA and DHA via fish oil supplementation, experienced a substantial lowering of all LPA species, and that the effects of LPCs were species-dependent. Our exploratory analyses with platelet function variables indicated three species of lysophospholipids (palmitoleic acid, oleic acid, and -linolenic acid) to potentially be directly related with platelet function and aggregation. Knowledge of the effects of aspirin and other medications on lysophospholipid species, particularly the three mentioned here, will further our understanding of the metabolic factors that influence the concentrations of potent lipid mediators in humans at high risk of CVD, including those with diabetes mellitus. Since EPA, DHA, and aspirin have potent cardioprotective effects and these may be, at least partially, related to their effects on lysophospholipid metabolism, it is important to know if particular species of lysophospholipids are cardioprotective or detrimental and if manipulation of their levels is a promising therapeutic option. Answering these questions via larger clinical trials will be important in the future in individuals with diabetes and potentially other high risk groups.
Supplementary Material
Supplemental Figure 1
The effects of aspirin alone (BD2-3), fish oil alone (BD4), and aspirin + fish oil (BD5-6) relative to baseline (as zero) on concentrations of other LPC (in μM) and LPA (in nM) species: (A) palmitoleic acid, (B) stearic acid, (C) linoleic acid, (D) -linolenic acid, and (E) adrenic acid. Data are expressed as mean concentration change from baseline and standard errors after log transformation. * indicates p < 0.05 using a mixed model as in Fig. 1.
ACKNOWLEDGEMENTS
The authors gratefully thank the Inflammation Research Foundation for supplying the OmegaRx Zone capsules. A special thanks to Carlos M. Swanger, MD, clinical co-director of the Greater Rochester Practice-Based Research Network, for his patient referrals.
Sources of Support
This publication was made possible by Grant Number 5R21HL102582-02 and, in part, by Grant Number T32HL007937 from the National Heart, Lung, and Blood Institute. The project described in this publication was supported by the University of Rochester CTSA award number KL2 RR024136 from the National Center for Research Resources and the National Center for Advancing Translational Sciences of the National Institutes of Health. Other support from the NIH was provided by R01 HL071933, R21 ES023032 and P30 ES001247. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NHLBI or NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
None
Trial Registration: Clinicaltrials.gov: NCT01181882
REFERENCES
- 1.Aoki J. Mechanisms of lysophosphatidic acid production. Semin. Cell. Dev. Biol. 2004;15:477–489. doi: 10.1016/j.semcdb.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 2.Morris AJ, Panchatcharam M, Cheng HY, et al. Regulation of blood and vascular cell function by bioactive lysophospholipids. J. Thromb. Haemost. 2009;7(Suppl 1):38–43. doi: 10.1111/j.1538-7836.2009.03405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smyth SS, Cheng HY, Miriyala S, Panchatcharam M, Morris AJ. Roles of lysophosphatidic acid in cardiovascular physiology and disease. Biochim. Biophys. Acta. 2008;1781:563–570. doi: 10.1016/j.bbalip.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siess W, Zangl KJ, Essler M, et al. Lysophosphatidic acid mediates the rapid activation of platelets and endothelial cells by mildly oxidized low density lipoprotein and accumulates in human atherosclerotic lesions. Proc. Natl. Acad. Sci. U. S. A. 1999;96:6931–6936. doi: 10.1073/pnas.96.12.6931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hayashi K, Takahashi M, Nishida W, et al. Phenotypic modulation of vascular smooth muscle cells induced by unsaturated lysophosphatidic acids. Circ. Res. 2001;89:251–258. doi: 10.1161/hh1501.094265. [DOI] [PubMed] [Google Scholar]
- 6.Zhang C, Baker DL, Yasuda S, et al. Lysophosphatidic acid induces neointima formation through PPARgamma activation. J. Exp. Med. 2004;199:763–774. doi: 10.1084/jem.20031619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou Z, Subramanian P, Sevilmis G, et al. Lipoprotein-derived lysophosphatidic acid promotes atherosclerosis by releasing CXCL1 from the endothelium. Cell Metab. 2011;13:592–600. doi: 10.1016/j.cmet.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 8.Umezu-Goto M, Kishi Y, Taira A, et al. Autotaxin has lysophospholipase D activity leading to tumor cell growth and motility by lysophosphatidic acid production. J. Cell Biol. 2002;158:227–233. doi: 10.1083/jcb.200204026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tokumura A, Majima E, Kariya Y, et al. Identification of human plasma lysophospholipase D, a lysophosphatidic acid-producing enzyme, as autotaxin, a multifunctional phosphodiesterase. J. Biol. Chem. 2002;277:39436–39442. doi: 10.1074/jbc.M205623200. [DOI] [PubMed] [Google Scholar]
- 10.van Meeteren LA, Ruurs P, Stortelers C, et al. Autotaxin, a secreted lysophospholipase D, is essential for blood vessel formation during development. Mol. Cell Biol. 2006;26:5015–5022. doi: 10.1128/MCB.02419-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hausmann J, Kamtekar S, Christodoulou E, et al. Structural basis of substrate discrimination and integrin binding by autotaxin. Nat. Struct. Mol. Biol. 2011;18:198–204. doi: 10.1038/nsmb.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nishimasu H, Okudaira S, Hama K, et al. Crystal structure of autotaxin and insight into GPCR activation by lipid mediators. Nat. Struct. Mol. Biol. 2011;18:205–212. doi: 10.1038/nsmb.1998. [DOI] [PubMed] [Google Scholar]
- 13.Ferry G, Tellier E, Try A, et al. Autotaxin is released from adipocytes, catalyzes lysophosphatidic acid synthesis, and activates preadipocyte proliferation. Up-regulated expression with adipocyte differentiation and obesity. J. Biol. Chem. 2003;278:18162–18169. doi: 10.1074/jbc.M301158200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schober A, Siess W. Lysophosphatidic acid in atherosclerotic diseases. Br. J. Pharmacol. 2012;167:465–482. doi: 10.1111/j.1476-5381.2012.02021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin ME, Herr DR, Chun J. Lysophosphatidic acid (LPA) receptors: signaling properties and disease relevance. Prostaglandins Other Lipid Mediat. 2010;91:130–138. doi: 10.1016/j.prostaglandins.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khandoga AL, Pandey D, Welsch U, Brandl R, Siess W. GPR92/LPA(5) lysophosphatidate receptor mediates megakaryocytic cell shape change induced by human atherosclerotic plaques. Cardiovasc. Res. 2011;90:157–164. doi: 10.1093/cvr/cvq369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoshida K, Nishida W, Hayashi K, et al. Vascular remodeling induced by naturally occurring unsaturated lysophosphatidic acid in vivo. Circulation. 2003;108:1746–1752. doi: 10.1161/01.CIR.0000089374.35455.F3. [DOI] [PubMed] [Google Scholar]
- 18.Kris-Etherton PM, Harris WS, Appel LJ. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation. 2002;106:2747–2757. doi: 10.1161/01.cir.0000038493.65177.94. [DOI] [PubMed] [Google Scholar]
- 19.Li ZG, Yu ZC, Wang DZ, et al. Influence of acetylsalicylate on plasma lysophosphatidic acid level in patients with ischemic cerebral vascular diseases. Neurol. Res. 2008;30:366–369. doi: 10.1179/174313208X300369. [DOI] [PubMed] [Google Scholar]
- 20.Block RC, Duff R, Lawrence P, et al. The effects of EPA, DHA, and aspirin ingestion on plasma lysophospholipids and autotaxin. Prostaglandins Leukot. Essent. Fatty Acids. 2010;82:87–95. doi: 10.1016/j.plefa.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Block RC, Abdolahi A, Smith B, et al. Effects of low-dose aspirin and fish oil on platelet function and NF-kappaB in adults with diabetes mellitus. Prostaglandins Leukot. Essent. Fatty Acids. 2013;89:9–18. doi: 10.1016/j.plefa.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.American Diabetes Association, Executive summary: Standards of medical care in diabetes – 2012. Diabetes Care. 2012;32:S4–S10. doi: 10.2337/dc12-s004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Campbell CL, Smyth S, Montalescot G, Steinhubl SR. Aspirin dose for the prevention of cardiovascular disease: a systematic review. JAMA. 2007;297:2018–2024. doi: 10.1001/jama.297.18.2018. [DOI] [PubMed] [Google Scholar]
- 24.Pamuklar Z, Lee JS, Cheng HY, et al. Individual heterogeneity in platelet response to lysophosphatidic acid: evidence for a novel inhibitory pathway. Arterioscler. Thromb. Vasc. Biol. 2008;28:555–561. doi: 10.1161/ATVBAHA.107.151837. [DOI] [PubMed] [Google Scholar]
- 25.Barber MN, Risis S, Yang C, et al. Plasma lysophosphatidylcholine levels are reduced in obesity and type 2 diabetes. PLoS One. 2012;7:e41456. doi: 10.1371/journal.pone.0041456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.[Anonymous] Collaborative overview of randomised trials of antiplatelet therapy--I: Prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. Antiplatelet Trialists’ Collaboration. BMJ. 1994;308:81–106. [PMC free article] [PubMed] [Google Scholar]
- 27.Grundy SM, Brewer HB, Jr., Cleeman JI, Smith SC, Jr., Lenfant C. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109:433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 28.Steering Committee of the Physicians’ Health Study Research Group, Final report on the aspirin component of the ongoing Physicians’ Health Study. Steering Committee of the Physicians’ Health Study Research Group. N. Engl. J. Med. 1989;321:129–135. doi: 10.1056/NEJM198907203210301. [DOI] [PubMed] [Google Scholar]
- 29.Gurbel PA, Bliden KP, DiChiara J, et al. Evaluation of dose-related effects of aspirin on platelet function: results from the Aspirin-Induced Platelet Effect (ASPECT) study. Circulation. 2007;115:3156–3164. doi: 10.1161/CIRCULATIONAHA.106.675587. [DOI] [PubMed] [Google Scholar]
- 30.Ohmori T, Yatomi Y, Nonaka T, et al. Aspirin resistance detected with aggregometry cannot be explained by cyclooxygenase activity: involvement of other signaling pathway(s) in cardiovascular events of aspirin-treated patients. J. Thromb. Haemost. 2006;4:1271–1278. doi: 10.1111/j.1538-7836.2006.01958.x. [DOI] [PubMed] [Google Scholar]
- 31.Dyerberg J, Bang HO, Stoffersen E, Moncada S, Vane JR. Eicosapentaenoic acid and prevention of thrombosis and atherosclerosis? Lancet. 1978;2:117–119. doi: 10.1016/s0140-6736(78)91505-2. [DOI] [PubMed] [Google Scholar]
- 32.Terano T, Hirai A, Hamazaki T, et al. Effect of oral administration of highly purified eicosapentaenoic acid on platelet function, blood viscosity and red cell deformability in healthy human subjects. Atherosclerosis. 1983;46:321–331. doi: 10.1016/0021-9150(83)90181-8. [DOI] [PubMed] [Google Scholar]
- 33.Colwell JA. Aspirin therapy in diabetes. Diabetes Care. 2004;27(Suppl 1):S72–73. doi: 10.2337/diacare.27.2007.s72. [DOI] [PubMed] [Google Scholar]
- 34.Antman EM, Anbe DT, Armstrong PW, et al. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1999 Guidelines for the Management of Patients with Acute Myocardial Infarction) Circulation. 2004;110:e82–292. [PubMed] [Google Scholar]
- 35.Tokumura A, Harada K, Fukuzawa K, Tsukatani H. Involvement of lysophospholipase D in the production of lysophosphatidic acid in rat plasma. Biochim. Biophys. Acta. 1986;875:31–38. [PubMed] [Google Scholar]
- 36.Pamuklar Z, Federico L, Liu S, et al. Autotaxin/lysopholipase D and lysophosphatidic acid regulate murine hemostasis and thrombosis. J. Biol. Chem. 2009;284:7385–7394. doi: 10.1074/jbc.M807820200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tomsig JL, Snyder AH, Berdyshev EV, et al. Lipid phosphate phosphohydrolase type 1 (LPP1) degrades extracellular lysophosphatidic acid in vivo. Biochem. J. 2009;419:611–618. doi: 10.1042/BJ20081888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Albers HM, Dong A, van Meeteren LA, et al. Boronic acid-based inhibitor of autotaxin reveals rapid turnover of LPA in the circulation. Proc. Natl. Acad. Sci. U. S. A. 2010;107:7257–7262. doi: 10.1073/pnas.1001529107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dohi T, Miyauchi K, Ohkawa R, et al. Increased circulating plasma lysophosphatidic acid in patients with acute coronary syndrome. Clin. Chim. Acta. 2012;413:207–212. doi: 10.1016/j.cca.2011.09.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1
The effects of aspirin alone (BD2-3), fish oil alone (BD4), and aspirin + fish oil (BD5-6) relative to baseline (as zero) on concentrations of other LPC (in μM) and LPA (in nM) species: (A) palmitoleic acid, (B) stearic acid, (C) linoleic acid, (D) -linolenic acid, and (E) adrenic acid. Data are expressed as mean concentration change from baseline and standard errors after log transformation. * indicates p < 0.05 using a mixed model as in Fig. 1.