Abstract
OBJECTIVE
To examine the dual-energy computed tomography (DECT) properties of 7 commonly used ureteral stents to optimize stent selection for calculi monitored using DECT. The use of DECT to evaluate renal and ureteral calculi has recently increased.
METHODS
Seven stents were individually placed in a fish bowl phantom and imaged using a Siemens Somatom Definition Flash CT scanner. DECT peak tube potentials of 80 and 140 kVp and 100 and 140 kVp were used, reflecting our current dual-energy protocols. These were compared to 31 in vivo stents of known composition. The data were reconstructed on a multimodality Work-Place (Siemens) using CT syngo Post-Processing Suite software.
RESULTS
The average patient age was 64 years (range 27–90). The average body mass index was 31.9 kg/m2 (range 24–51.6). Of the 27 patients, 4 had uric acid stones and 22 had calcium-based stones; 1 patient had undergone renal transplantation. No difference was seen in the dual-energy characterization of stents from the same manufacturer. All imaged Cook and Bard stents had a dual-energy characterization that approached that of calcium stones (blue). All Boston Scientific and Gyrus ACMI stents had a dual-energy characterization resembling that of uric acid stones (red).
CONCLUSION
The present study evaluated the stent appearance on DECT for various stent manufacturers. This information will aid in the optimal stent selection for patients undergoing treatment of renal calculi and followed up with DECT.
Previous investigators have reported the multiple benefits of using dual-energy computed tomography (DECT) to evaluate renal and ureteral calculi. Several groups have reported data suggesting that, compared with standard stone protocol CT scanning, DECT can be used to accurately distinguish uric acid (UA) stones from other stone types.1–6 The capability of pre-operatively identifying UA stones is important, because UA stones can be managed medically and other stone types often require stone removal or lithotripsy. Thus, the number of patients presenting with kidney stones who undergo DECT is likely to continue to increase steadily during the next decade.
The value of DECT notwithstanding, in cases of obstruction or a heavy stone burden, it is common practice for many urologists to place a ureteral stent, regardless of the suspected stone type. These ureteral stents are recognized by the DECT scanner and assigned a color value according to their density and chemical composition. More specifically, some stents are characterized as more closely resembling the dual energy characteristics (DECs) of non-UA stones (ie, they appear blue on the image), and others are characterized as more closely resembling the DEC of UA stones (ie, they appear red on the image). Thus, the ability to predict how the DECT scan will characterize a particular renal stent is important because such knowledge could help inform stent selection for individual patients. Placing a red stent in a patient with blue stones would allow for easy evaluation of stone migration and retention compared with using a blue stent, which could camouflage the stones and lead to decreased sensitivity and misdiagnosis.
Ureteral stents, just as for many pieces of medical equipment, are produced by multiple companies using many types of proprietary materials and coatings. The general materials include modified polymers, polyurethane, silicon, and metal, all with the option of embedding with surface property enhancers and antibacterial coating.7,8 Motivated by this variability in stent manufacturing and composition, we evaluate the DECT properties of the 7 most commonly used stents at our institution for the indication of obstructing renal calculi: Bard InLay Optima (Covington, GA), Boston Scientific Percuflex Plus (Natick, MA), Boston Scientific Polaris Loop, Cook Universa (Bloomington, IN), Cook Universa Amplatz-Ultrathane, Cook Endo-Sof, and Gyrus ACMI Telcoflex (Southborough, MA). The purpose of the present study was to help urologists optimize stent selection according to stone type when using DECT for monitoring.
MATERIAL AND METHODS
In Vitro
The 7 stents were individually placed in a fish bowl phantom. The phantom consisted of a Plexiglas fish bowl measuring 13.25 × 14.1875 × 10 in. (height × width × depth) and filled with warm water. The stents were placed in the center connected to a Plexiglas rod (Fig. 1). DECT was performed using the Somatom Definition Flash CT scanner (Siemens, Forchheim, Germany) and our DECT renal stone protocol. Each stent was evaluated twice, initially with tube potentials of 80 and 140 kVp and then at 100 and 140 kVp to reflect our current dual-energy protocols for patients according to their body mass index (BMI), higher potentials used for BMI >35 kg/m2. A slice thickness of 5 mm and interval of 5 mm (continuous) were chosen to be consistent with our clinical practice. The data were then reconstructed on a multimodality WorkPlace (Siemens) using CT syngo Post-Processing Suite software, version VE 36A (Siemens). Reconstructions used a 0.75-mm slice thickness and 0.5-mm interval, with a B30f convolution kernel for optimal data analysis. The color designations were determined by DECs of UA stones compared with other renal stones. Therefore, materials that more closely resemble the DEC of UA stones will be red and those that more closely resemble the DEC of non-UA stones will be blue.
Figure 1.
Plexiglas fish bowl phantom filled with warm water, with stents placed in center connected to Plexiglas rod. Image shows Boston Scientific stent.
In Vivo
The institutional review board approved the present study. A total of 27 patients with 31 ureteral stents underwent imaging with our standard DECT stone protocol. The stents used included 4 Boston Scientific Percuflex Plus, 18 Cook Universa Firm, 2 Cook Universa Amplatz-Ultrathane, 1 Cook Endo-Sof, and 7 Bard InLay Optima stents. The patients underwent imaging with either 80/140 kVp or 100/140 kVp, according to their BMI. All other parameters were identical to the DECT protocol described in the previous section. Noncontrast images from the diaphragm to the pubic symphysis were acquired. The images were reconstructed on a multimodality WorkPlace (Siemens) using CT syngo Post-Processing Suite software, version VE 36A (Siemens). All images were evaluated by 1 radiologist (J.C.C.).
Post Processing
Dual-source DECT acquires data simultaneously from 2 x-ray tubes and detector arrays at perpendicular angles. Data can be generated as pure low kVp (80 or 100 kVp), pure high kVp(140 kVp), or as a weighted average (usually 70% high kVp and 30% low kVp). CT numbers from the high and low kVp are then used to determine the material characterization.
A simple method of understanding the DECT characterization of materials is the calculated dual-energy index (dual-energy index = [X80 − X140]/[X80 + X140 + 2000], where X80 is the Hounsfield units of a region of interest at 80 kVp, and X140 is the Hounsfield units of that same region of interest at 140 kVp).2,4 This formula allows the calculation of the effective atomic mass number and, in turn, the chemical composition. However, this is a rather rigid formula and does not allow for the mixed composition of many stones or the presence of pores, which allows mixing with urine.
The syngo Post-Processing Suite software program (Siemens) uses a proprietary 3-material (UA, calcium, and urine) decomposition algorithm for material characterization.4,6,9 This algorithm provides more accurate in vivo results, allowing a stone that is porous to be considered as a mixture of urine, UA, and calcium. A range of values for each material is determined from stones of known composition. The voxel values are plotted on a graph, with the x axis, the CT numbers at 140 kVp and the y axis, the CT numbers at 80 kVp. The image voxels are color coded according to where they lie on the graph; those within the range of UA are characterized as red, and those within the range of calcium are characterized as blue. Voxel values outside of either range are shown on the gray scale. Voxel attenuation values are not produced as a part of the final diagnostic image; rather, color-coding allows for image interpretation.
RESULTS
The average patient age was 64 years (range 27–90). The average BMI was 31.9 kg/m2 (range 24–51.6). Of the 27 patients, 4 had UA stones and 22 had calcium-based stones; 1 patient had undergone renal transplantation. The characteristics of each stent are listed in Table 1. The Boston Scientific Percuflex Plus ureteral stents were characterized as resembling the DECs of UA stones (red) using the dual-energy protocol both in vivo and in vitro (Fig. 2A). The Boston Scientific Polaris Loop and Gyrus ACMI Telcoflex stents were only evaluated in vitro but also appeared red with DECT. All Bard InLay Optima, Cook Universa Amplatz-Ultrathane, Cook Universa Firm (Fig. 2B), and Cook Endo-Sof ureteral stents were characterized as resembling the DECs of calcium (blue) in vivo and in vitro using the dual-energy protocol. Patient age, BMI, sex, stone disease type, duration since stent placement, and stent location had no effect (Table 2). No discrepancy was between the in vivo and in vitro findings. Varying the kVp value had no effect on stent characterization. We did not note any difference in the dual-energy CT characterization of stents from the same manufacturer. All Cook and Bard stents that we imaged appeared blue, and all Boston Scientific and Gyrus ACMI stents appeared red.
Table 1.
DECT findings of stents
| Stent | Color
|
|
|---|---|---|
| 80/140 kVp | 100/140 kVp | |
| Bard InLay Optima | Blue | Blue |
| Boston Scientific Percuflex | Red | Red |
| Boston Scientific Polaris Loop | Red | Red |
| Cook Universa Firm | Blue | Blue |
| Cook Universa Amplatz-Ultrathane | Blue | Blue |
| Cook Endo-Sof | Blue | Blue |
| Gyrus ACMI Telcoflex (infrared) | Red | Red |
Figure 2.
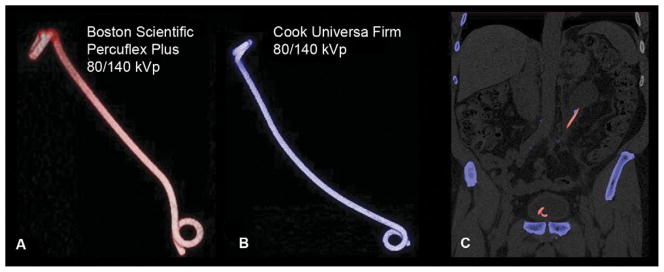
Appearance of stents using DECT. (A) Boston Scientific Percuflex Plus stent at 80/140 kVp on DECT. Note, in vitro appearance of stent with DECs similar to UA calculi (appears red). (B) Cook Universa Firm stent at 80/140 kVp on DECT. Note, in vitro appearance of stent with DECs similar to non-UA calculi (appears blue). (C) DECT of stent with calculi in ureter showing clinical applicability of DECT for stent/stone contrasting. Note, red stent contrasting with blue calculus in ureter (Color figure available online).
Table 2.
In vivo stent findings
| Stent | Patients (n) | Stents (n) | Mean BMI (kg/m2) | BMI Range (kg/m2) | Age (y) | Age Range (y) | Gender (M/F) | Red (%) | Blue (%) |
|---|---|---|---|---|---|---|---|---|---|
| Bard InLay Optima | 7 | 7 | 32.5 | 27.2–40.6 | 58 | 47–73 | 5/2 | 0 | 100 |
| Boston Scientific Percuflex | 4 | 4 | 28.2 | 24.7–30.5 | 68 | 54–77 | 4/1 | 100 | 0 |
| Boston Scientific Polaris Loop | 0 | — | — | — | — | — | — | — | — |
| Cook Universa Firm | 17 | 18 | 32.1 | 24–44.6 | 66.2 | 50–90 | 11/6 | 0 | 100 |
| Cook Universa Amplatz-Ultrathane (infrared) | 2 | 2 | 38.1 | 24.7–51.6 | 59.5 | 42–77 | 1/1 | 0 | 100 |
| Cook Endo-Sof | 1 | 1 | 27.6 | — | 27 | — | 1/0 | 0 | 100 |
| Gyrus ACMI Telcoflex (infrared) | 0 | — | — | — | — | — | — | — | — |
BMI, body mass index.
COMMENT
The introduction of DECT scanners has changed the ability to characterize genitourinary stones. Moreover, as the usage of DECT increases, current radiopaque ureteral stents might need to be modified to fit this usage pattern. One value of DECT is the ability to more readily select stone therapy according to the stone type. For instance, one might forgo extracorporeal shock wave lithotripsy in favor of percutaneous nephrostolithotomy for large calculi with DECT properties consistent with a heavy calcium composition or choose dissolution therapy for UA stones. Currently, knowledge regarding the ureteral stent properties using DECT protocols is lacking. As illustrated by our results, if a stent has a different DEC than the stone it is near, treatment of that stone can be more easily monitored. Figure 2C demonstrates an example of a red stent contrasted with blue stone material that could possibly make the stone materials easier to differentiate and clinically monitor with imaging.
As DECT becomes more widely used in the diagnosis of renal calculi, ureteral stents must not only meet the requirement of being radiopaque, but also be of a material that allows for optimal dual-energy characterization. Dual-energy characterization is based on the change in attenuation of a material at 2 separate kVp. With our renal stone protocol, we use syngo Post-Processing software (Siemens) with a 3-material (UA, calcium, and urine) decomposition algorithm as our guideline for assigning color to the images. For an imaged material to be characterized with a color, it must closely resemble the change in attenuation experienced by calcium or UA.
Most ureteral stents are composed of modified polymers, polyurethane, silicon, and metal embedded, all with the option of surface property enhancers and antibacterial coating.7,8 During our investigation, we found only the Boston Scientific Polaris Loop, Boston Scientific Percuflex, and Gyrus ACMI Telcoflex were identified by the dual-energy postprocessing algorithm as within the range of UA. The tested Cook and Bard products were within the range of non-UA stones (blue). The clinical importance of this is that that it will be easier to monitor the progress of calcium stone fragments (blue) in the proximity of a red stent on the CT scan.
Although our data are informative to the contemporary urologist using DECT to identify and manage kidney stones, it is worth noting that in the DECT imaging domain for nephrolithiasis, the ideal stent material would not display on the image as red or blue but would appear as a separate color all together. Moreover, the characteristics of the ideal stent would be to maintain its color characterization over time, regardless of encrustation, infection, or patient population. Changing the current renal stone protocols to narrow the range of UA and calcium identification would decrease the ability of the imaging study to diagnose renal calculi. Thus, identifying new protocols to recharacterize current ureteral stent materials would seem a poor option; however, it is 1 that might be worth examining in the future. Thus, additional investigation of other stent materials is needed to construct a stent reference library for clinical use.
The best applications to date for DECT are to differentiate UA from non-UA calculi. However, more recent studies have expanded stone characterization to more groups of stones, including cystine stones, calcium oxylate monohydrate stones, and hydroxylapatite stones.10,11 Although the sensitivity and specificity of detecting stone composition using DECT is not 100%, the results have been favorable for most stone types, with the largest variability occurring with mixed composition calculi.11 The usefulness of stent/calculi contrasting might not have a clinical benefit in the management of very small calculi after treatment; however, nonvisualization of small calculi after lithotripsy could lead to premature ureteral stent removal and future morbidity from obstructive uropathy. It remains to be seen whether DECT and the associated stent/stone color contrast will aid in calculi treatment success and decreased the morbidity associated with premature stent removal in the presence of potential obstructing stone fragments.
One possible shortfall of the present study is the small number of in vitro stents of each type that were examined. Although the present study noted a 100% correlation of the blue and red color assignment of the stents between the in vitro and in vivo data, it is certainly possible that the clinical application might not be 100% accurate once more patients undergo imaging in the clinical setting. Our sample sizes were very small, and increased numbers in the in vitro cohort are needed to validate our findings.
CONCLUSION
Stents from different manufacturers can be characterized by the dual-energy algorithm using DECT imaging and, therefore, have color that mimic UA or non-UA calculi on imaging. We have provided the first data to aid urologists in optimal stent selection according to stone type present on DECT.
Acknowledgments
To John C. Lieske, M.D., and Cynthia H. McCollough, Ph.D., Department of Medicine, Division of Nephrology and Hypertension, and Department of Radiology, Mayo Clinic College of Medicine, for helpful discussions, with thanks.
Funding Support: This project was supported by a grant from the National Institutes of Health (Mayo Clinic Urology Research Center grant DK 83007) and the Mayo Clinic.
Footnotes
Financial Disclosure: The authors declare that they have no relevant financial interests.
References
- 1.Boll DT, Patil NA, Paulson EK, et al. Renal stone assessment with dual-energy multidetector CT and advanced postprocessing techniques: improved characterization of renal stone composition—pilot study. Radiology. 2009;250:813–820. doi: 10.1148/radiol.2503080545. [DOI] [PubMed] [Google Scholar]
- 2.Eiber M, Holzapfel K, Frimberger M, et al. Targeted dual-energy single-source CT for characterisation of urinary calculi: experimental and clinical experience. Eur Radiol. 2012;22:251–258. doi: 10.1007/s00330-011-2231-2. [DOI] [PubMed] [Google Scholar]
- 3.Eliahou R, Hidas G, Duvdevani M, et al. Determination of renal stone composition with dual-energy computer tomography: an emerging application. Semin Ultrasound CT MR. 2010;31:315–320. doi: 10.1053/j.sult.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 4.Graser A, Johnson TRC, Bader M, et al. Dual energy CT characterization of urinary calculi: initial in vitro and clinical experience. Invest Radiol. 2008;43:112–119. doi: 10.1097/RLI.0b013e318157a144. [DOI] [PubMed] [Google Scholar]
- 5.Matlaga BR, Kawamoto S, Fishman E. Dual source computer tomography: a novel technique to determine stone composition. J Urol. 2008;5:1164–1168. doi: 10.1016/j.urology.2008.03.051. [DOI] [PubMed] [Google Scholar]
- 6.Primak AN, Fletcher JG, Vriska TJ, et al. Noninvasive differentiation of uric acid versus non-uric acid kidney stones using dual-energy CT. Acad Radiol. 2007;14:1441–1447. doi: 10.1016/j.acra.2007.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Al-Aown A, Kyriazis I, Kallidonis P, et al. Ureteral stents: new ideas, new designs. Ther Adv Urol. 2010;2:85–92. doi: 10.1177/1756287210370699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Venkatesan N, Shroff S, Jayachandran K, et al. Polymers as ureteral stents. J Endourol. 2010;24:191–198. doi: 10.1089/end.2009.0516. [DOI] [PubMed] [Google Scholar]
- 9.Liu XY, Yu L, Primak AN, McCollough CH, et al. Quantitative imaging of element composition and mass fraction using dual-energy CT: three-material decomposition. Med Phys. 2009;36:1602–1609. doi: 10.1118/1.3097632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vrtiska TJ, Takahashi NT, Fletcher JG, et al. Genitourinary applications of dual-energy CT. AJR Am J Roentgenol. 2010;194:1434–1442. doi: 10.2214/AJR.10.4404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manglaviti G, Tresoldi S, Guerrer CS, et al. In vivo evaluation of the chemical composition of urinary stones using dual-energy CT. AJR Am J Roentgenol. 2011;197:W76–W83. doi: 10.2214/AJR.10.5217. [DOI] [PubMed] [Google Scholar]