Abstract
Objectives
This study evaluated the Model for End-Stage Liver Disease (MELD) score and its modified versions, which are established measures of liver dysfunction, as a tool to assess heart transplantation (HTx) urgency in ambulatory patients with heart failure.
Background
Liver abnormalities have a prognostic impact on the outcome of patients with advanced heart failure.
Methods
We retrospectively evaluated 343 patients undergoing HTx evaluation between 2005 and 2009. The prognostic effectiveness of MELD and 2 modifications (MELDNa [includes serum sodium levels] and MELD-XI [does not include international normalized ratio]) for endpoint events, defined as death/HTx/ventricular assist device requirement, was evaluated in our cohort and in subgroups of patients on and off oral anticoagulation.
Results
The MELD and MELDNa scores were excellent predictors for 1-year endpoint events (areas under the curve: 0.71 and 0.73, respectively). High scores (>12) were strongly associated with poor survival at 1 year (MELD 69.3% vs. 90.4% [p < 0.0001]; MELDNa 70.4% vs. 96.9% [p < 0.0001]). Increased scores were associated with increased risk for HTx (hazard ratio: 1.10 [95% confidence interval: 1.06 to 1.14]; p < 0.0001 for both scores), which was independent of other known risk factors (MELD p = 0.0055; MELDNa p = 0.0083). Anticoagulant use was associated with poor survival at 1 year (73.7% vs. 86.4%; p = 0.0118), and the statistical significance of MELD/MELDNa was higher in patients not receiving oral anticoagulation therapy. MELD-XI was a fair but limited predictor of the endpoint events in patients receiving oral anticoagulation therapy.
Conclusions
Assessment of liver dysfunction according to the MELD scoring system provides additional risk information in ambulatory patients with heart failure.
Keywords: heart failure, liver dysfunction, MELD, prognosis
Various scoring models have been useful in assessing risk in patients with heart failure (HF). Peak oxygen consumption (VO2) collected during cardiopulmonary exercise testing, the Heart Failure Survival Score (HFSS), and the Seattle Heart Failure Model (SHFM) have all been shown to effectively identify patients at high risk for clinical events and death in cohorts of clinically stable, ambulatory HF patients (1–3). However, although the current models used during heart transplantation (HTx) evaluation incorporate a multitude of variables, they fail to fully address the impact of liver dysfunction. The cardio-hepatic syndrome, a condition characterized by the development of congestive hepatopathy and subsequent cirrhosis in patients with advanced HF, has long been recognized in clinical settings (4). Moreover, abnormal results on liver function tests in patients with HF have been linked to poor outcomes and higher risk of death (5–7).
However, data are limited regarding the usefulness of composite scoring systems of liver dysfunction in patients with HF. The established Model for End-Stage Liver Disease (MELD) scoring system, developed in patients with hepatic cirrhosis awaiting liver transplantation, may provide information in HF patients by measuring the progression of liver dysfunction based on a patient’s creatinine, total bilirubin, and international normalized ratio (INR) (8). These 3 laboratory parameters are noncardiac biomarkers representing hepatic and renal dysfunction and their impact on coagulation (9). This makes the MELD score suitable for the prognosis of advanced HF, a state of multiorgan dysfunction secondary to impaired cardiac function with known impairment of hepatic and renal function in advanced stages of the disease process. In addition to its established role in determining the urgency for liver transplantation, the MELD scoring system has also been shown to be a versatile tool for outcome prediction in cirrhotic patients undergoing cardiac surgery (10,11), patients with advanced HF undergoing left ventricular assist device (LVAD) implantation (12), and patients undergoing orthotopic HTx (13).
Furthermore, alternative MELD scoring systems may offer improved prognostic efficacy. This is particularly true for the MELD-XI score, which excludes INR as a variable and is thereby a more reliable marker of risk in patients with elevated INR secondary to anticoagulation (14). Furthermore, another modification of MELD, the MELDNa, may improve prognostic efficacy by incorporating low sodium levels indicating hyponatremia, a commonly cited marker for increased mortality in both liver cirrhosis and HF (15,16). In the current study, we analyzed the strength of these various MELD scoring systems in predicting the survival of clinically stable outpatients with advanced HF. We assessed MELD scores during the HTx evaluation process and followed up the cohort for a maximum of 3 years after the initial evaluation. In addition, we compared the prognostic power of MELD, MELDNa, and MELD-XI in patients on and off oral anticoagulation regimens.
Methods
Study design
We retrospectively evaluated 343 patients with HF referred for HTx evaluation at Columbia University Medical Center between 2005 and 2009. Patients with incomplete laboratory datasets (n = 83) were excluded from the study, leaving 260 patients in the cohort. The clinical characteristics, medical treatments, laboratory examination values, and hemodynamic data were collected by using an electronic chart review of data closest to the evaluation date. Survival data were collected by using the Social Security Death Index at the end of the 3-year observation period. If no death was indicated, the patient was recorded to be alive at the time of follow-up.
The patients in the cohort were categorized according to oral anticoagulation status, and 3 groups of subjects (all patients and patients on and off anticoagulation) were analyzed. The groups were further dichotomized according to MELD, MELDNa, and MELD-XI scores based on the optimal cutoff value derived from the receiver-operating characteristic (ROC) analysis. The laboratory and clinical characteristics at the time of HTx evaluation were compared between the groups.
The study was approved by the institutional review board of Columbia University Medical Center.
Assessment of liver dysfunction by using MELD scores
The standard MELD score was calculated by using the following formula (17): 11.2 · (ln INR) + 0.378 · (ln total bilirubin) + 0.957 · (ln creatinine) + 0.643. We applied the MELD modifications adopted by the United Network for Organ Sharing (18); the lower limit of all variables was set at 1.0 to prevent negative scores, and the upper limit for creatinine was set at 4.0 mg/dl. The MELDNa score was calculated by using the following formula (15): MELD – serum sodium − 0.025 · MELD · (140 – serum sodium) + 140. The limit for sodium was set between 125 and 140 mmol/l. The MELD-XI score formula developed by Heuman et al. (14) was used: 5.11 · (ln total bilirubin) + 11.76 · (ln creatinine) + 9.44.
Peak VO2, HFSS, and SHFM
Peak VO2 was determined during the maximal treadmill exercise on a metabolic cart (Medical Graphics Corporation, Minneapolis, Minnesota). Three risk groups were defined according to the following values: peak VO2 >14 ml/min/kg (low risk), peak VO2 10 to 14 ml/min/kg (medium risk), and peak VO2 <10 ml/min/kg (high risk).
The HFSS score was calculated based on the following equation incorporating 7 variables: ([0.0216 · resting heart rate] + [−0.0255 · mean arterial blood pressure] + [−0.0464 · left ventricular ejection fraction] + [−0.0470 · serum sodium] + [−0.0546 · peak VO2] + [0.06083 · presence (1) or absence (0) of intraventricular conduction defect (QRS interval ≥120 ms due to left or right bundle branch block, nonspecific intraventricular conduction delay, or ventricular paced rhythm)] + [0.693 · presence (1) or absence (0) of ischemic cardiomyopathy]), as described previously (2). The absolute value of HFSS was used in the analysis. Patients were classified into 3 risk groups according to the following guidelines: low risk (≥8.10), medium risk (7.20 to 8.10), and high risk (≤7.19).
The SFHM score was calculated based on 24 variables, including clinical characteristics (age, gender, New York Heart Association functional class, weight, left ventricular ejection fraction, systolic blood pressure, ischemic etiology), medications (angiotensin-converting enzyme inhibitor, angiotensin receptor blocker, beta-blocker, statin, aldosterone blocker, loop diuretic–equivalent dose, allopurinol), device therapy (implantable cardioverter-defibrillator, cardiac resynchronization therapy), and laboratory data (lymphocytes percentage and serum sodium, hemoglobin, uric acid, total cholesterol) (3). Missing continuous variables were replaced with the mean value for all patients in the dataset. The SHFM scores were rounded to the nearest integer between 0 and 4 (scores <0 were considered as 0). Risk strata were defined as low risk (score 0), medium risk (score 1), or high risk (score ≥2).
Statistical analysis
Continuous variables were expressed as mean ± SD and categorical data as percentages. Continuous variables were compared between the groups by using the Student unpaired 2-tailed t test, whereas categorical variables were compared by using the Fisher exact test. A p value of <0.05 was considered statistically significant. The endpoint events were defined as death, HTx, and ventricular assist device (VAD) requirement. Pearson’s correlation analysis was performed between MELD models and right atrial pressure, pulmonary arterial mean pressure, and cardiac output.
The prognostic strength of the model and the individual variables composing the model were compared by calculating each area under the curve (AUC) from the ROC analysis for 1- and 3-year endpoint events. The ROC curves were quantitatively compared by using the DeLong test (19), and the optimal cutoff value for the model was determined by using the Youden criterion. For uniformity, the average of MELD, MELDNa, and MELD-XI optimal cutoff values derived from the 1-year ROC analysis in all patients (N = 260) was used to dichotomize the patients according to MELD score. The average optimal cutoff value (12) of MELD, MELDNa, and MELD-XI derived from the 1-year ROC analysis in all patients was set as the standard number to dichotomize the patients per their MELD score. The survival rates of 2 MELD score groups were compared by using Kaplan-Meier methods with a log-rank test. A Cox proportional hazards analysis was used to assess the association between the variables (MELD models, clinical and laboratory variables) and the occurrence of 3-year endpoint events in the group of subjects. Variables that achieved statistical significance in the univariate analysis by using all patients in the cohort were included in the multivariate analysis. We repeated the multivariate analysis including the MELD score but excluded any variables composing the model.
Statistical analyses were performed by using SAS software JMP version 7.0 (SAS Institute, Inc., Cary, North Carolina) and MedCalc for Windows version 9.5.0.0 (MedCacl Software, Mariakerke, Belgium).
Results
Baseline characteristics
Clinical characteristics for all patients evaluated for HTx eligibility (N = 343) are summarized in Table 1. The group consisted of ambulatory HF patients; 71% were male with a mean age of 56 years. Eighteen percent (n = 63) of the study subjects met the endpoint criteria in which 28% (n = 18) of the events were due to death. We selected only patients with complete laboratory and clinical data, and our study cohort (n = 260) was 72% male with a mean age of 54 years (Online Table 1). The baseline characteristics were further dichotomized according to MELD, MELDNa, and MELD-XI scores by using a cutoff value of 12. The comparison between groups revealed that patients with a higher MELD/MELDNa/MELD-XI score have poor prognoses. Higher MELD scores (>12) were associated with lower levels of heart rate, hemoglobin, albumin, and cholesterol, in addition to higher levels of blood urea nitrogen and B-type natriuretic peptide. A similar pattern was observed by using the MELDNa score, but elevated scores (>12) were also associated with lower levels of systolic blood pressure and platelets, as well as a higher level of aspartate aminotransferase. Patients with high MELD-XI scores were significantly older and likely male with higher INR levels in addition to the baseline characteristics seen in patients with high MELD scores.
Table 1.
Baseline Characteristics of All Patients Evaluated for HTx Eligibility (N = 343)
| Age (yrs) | 56 ± 14 |
| Male | 243 (71) |
| BMI (kg/m2) | 27.7 ± 5.6 |
| Diabetes | 75 (22) |
| Heart rate (beats/min) | 75.9 ± 14 |
| Systolic blood pressure (mm Hg) | 112 ± 19 |
| Diastolic blood pressure (mm Hg) | 72 ± 12 |
| Laboratory examination | |
| WBC (×103/μl) | 7.7 ± 2.1 |
| Hemoglobin (g/dl) | 13.6 ± 2.3 |
| Platelets (×103/μl) | 226 ± 71 |
| Serum sodium (mEq/l) | 138 ± 2.8 |
| BUN (mg/dl) | 25.3 ± 15 |
| Creatinine (mg/dl) | 1.3 ± 1.4 |
| Total bilirubin (mg/dl) | 0.9 ± 0.8 |
| AST (U/l) | 25 ± 12 |
| ALT (U/l) | 26 ± 15 |
| Albumin (g/dl) | 4.4 ± 0.5 |
| INR | 1.6 ± 0.8 |
| Total cholesterol (mg/dl) | 178 ± 51 |
| BNP (pg/ml) | 514 ± 814 |
| Medications | |
| ACE inhibitors | 215 (63) |
| ARBs | 55 (16) |
| Beta-blockers | 283 (83) |
| Statin | 140 (41) |
| Aldosterone antagonist | 136 (39) |
| Oral antidiabetic agents | 30 (8.7) |
| Insulin | 24 (7.0) |
| Prognostic factors | |
| Peak VO2 (ml/min/kg) | 12.9 ± 4.81 |
| HFSS | 7.45 ± 2.82 |
| SHFM | 0.43 ± 0.79 |
| Endpoint events count | 63 (26) |
| Endpoint events due to death | 18 (5.6) |
Values are mean ± SD or n (%).
ACE = angiotensin-converting enzyme; ALT = alanine aminotransferase; ARB = angiotensin receptor blockers; AST = aspartate aminotransferase; BMI = body mass index; BNP = B-type natriuretic peptide; BUN = blood urea nitrogen; HFSS = Heart Failure Survival Score; HTx = heart transplantation; INR = international normalized ratio; VO2 = oxygen consumption; SHFM = Seattle Heart Failure Model; WBC = white blood cells.
The baseline characteristics for our cohort were also categorized according to oral anticoagulation therapy and further dichotomized according to MELD/MELDNa/MELD-XI scores using the same cutoff value specified earlier (Online Table 2A). Of 260 patients, 104 (40%) received anticoagulation therapy. Patients receiving anticoagulation had significantly lower serum sodium levels; otherwise, no significant differences in clinical and laboratory variables were observed. In patients off anticoagulation with high MELDNa scores, heart rate, and platelet counts were no longer significantly different, but the white blood cell count was elevated. The same baseline patterns were observed in patients not receiving anticoagulation, with high MELD-XI scores compared with those of all patients with high MELD-XI scores.
We observed fewer baseline differences between the dichotomized score groups in patients receiving anticoagulation (Online Table 2B). In this group of subjects, a MELD score >12 was associated with lower levels of hemoglobin and sodium along with higher levels of blood urea nitrogen; patients with higher MELDNa scores had significantly higher B-type natriuretic peptide levels. For MELD-XI, scores >12 were associated with lower body mass index, hemoglobin, and sodium, along with higher blood urea nitrogen and INR levels.
Hemodynamic profiles for right atrial pressure, pulmonary arterial mean pressure, and cardiac output were obtained from 52%, 53%, and 50% of the study subjects, respectively. The Pearson’s correlation analysis indicated that the MELD scores positively correlated with right atrial pressure (R = 0.26, p = 0.002 for MELD; R = 0.22, p = 0.006 for = MELDNa; and R = 0.20, p = 0.024 MELD-XI), pulmonary arterial pressure for (R = 0.26, p = 0.002 for MELD; R = 0.26, p = 0.002 for MELDNa; and R2 = 0.17, p = 0.035 for MELD-XI) but not with cardiac output. Comparison of AUC values of the MELD composite scoring system and the individual variables of the composite score. Table 2 summarizes results obtained from the ROC analysis of all MELD models and their individual variables for 1-year endpoint events. An improvement in AUC and p values for 1-year endpoint events was observed in the MELD scoring models relative to those of individual variables tested in all patients (N = 260). The AUCs for creatinine, total bilirubin, INR, and serum sodium were 0.62 (p = 0.5162), 0.66 (p = 0.0323), 0.70 (p = 0.0111), and 0.64 (p = 0.0158), respectively, whereas MELD, MELDNa, and MELD-XI had values of 0.71 (p < 0.0001), 0.73 (p < 0.0001), and 0.69 (p = 0.0002). When the ROC curves were compared by using DeLong’s method, MELDNa was a stronger predictor for the endpoint events than creatinine (p = 0.0496) and serum sodium (p = 0.0421) values alone (Online Table 3). This improvement with the composite scores was also seen at the 3-year endpoint analysis (Online Table 4).
Table 2.
Summary of ROC Analysis for 1-Year Death/HTx/LVAD Requirement Stratified According to Anticoagulation Treatment
| Total Patients (N = 260) |
Patients on Anticoagulation (n = 104) |
Patients off Anticoagulation (n = 156) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | AUC | Sensitivity (%) | Specificity (%) | p Value | AUC | Sensitivity (%) | Specificity (%) | p Value | AUC | Sensitivity (%) | Specificity (%) | p Value |
| MELD | 0.71 | 80.5 | 57.6 | <0.0001 | 0.55 | 39.1 | 76.5 | 0.1823 | 0.81 | 76.5 | 84.2 | 0.0001 |
| MELDNa | 0.73 | 95.1 | 46.0 | <0.0001 | 0.55 | 21.7 | 92.6 | 0.1752 | 0.84 | 94.1 | 64.8 | <0.0001 |
| MELD-XI | 0.69 | 62.5 | 65.9 | 0.0002 | 0.61 | 73.9 | 50.6 | 0.0740 | 0.77 | 64.7 | 84.2 | 0.0005 |
| Creatinine | 0.62 | 53.7 | 69.6 | 0.5162 | 0.60 | 60.9 | 61.7 | 0.1021 | 0.66 | 64.7 | 73.4 | 0.9068 |
| Total bilirubin | 0.66 | 48.8 | 74.1 | 0.0323 | 0.59 | 65.2 | 51.9 | 0.7316 | 0.71 | 47.1 | 92.8 | 0.0154 |
| INR | 0.70 | 92.5 | 55.5 | 0.0111 | 0.52 | 17.4 | 6.17 | 0.3442 | 0.84 | 82.4 | 82.7 | 0.1682 |
| Serum sodium | 0.64 | 61.0 | 62.5 | 0.0158 | 0.59 | 100 | 16.5 | 0.2893 | 0.68 | 70.6 | 65.4 | 0.0386 |
AUC = area under the curve; HTx = heart transplantation; LVAD = left ventricular assist device; MELD = Model for End-Stage Liver Dysfunction; MELDNa = MELD serum sodium score; MELD-XI = MELD without INR; ROC = receiver-operating characteristic; other abbreviations as in Table 1.
When the cohort was categorized according to anticoagulation therapy, we observed a notable difference between the treatment groups in the AUC value obtained from the 1-year analysis (Table 2). In patients not receiving anticoagulation, the MELD and MELDNa score became an impressive discriminator for 1-year death/HTx/VAD requirement (1-year AUC: 0.81 [p = 0.0001] and 0.84 [p < 0.0001], respectively). Furthermore, the AUC and the p value improved in the MELD/MELDNa score compared with creatinine, total bilirubin, INR, and serum sodium (1-year AUC: 0.66, p = 0.9068; 0.71, p = 0.0154; 0.84, p = 0.1682; and 0.68, p = 0.0386, respectively). This improvement was also significant between MELDNa and creatinine (p = 0.0184), as well as serum sodium (p = 0.0179) alone (Online Table 3). Similar results were observed in the 3-year outcome analysis (Online Table 4).
The MELD and MELDNa scores, however, were poor predictors for the 1-year events in patients on anticoagulation (1-year AUC: 0.55 [p = 0.1823] and 0.55 [p = 0.1752], respectively). As expected, the MELD-XI was the best predictor for 1-year endpoint events among all models and individual variables of MELD (1-year AUC: 0.61; p = 0.0740) (Table 2). However, the score was not as strong a predictor compared with the MELD/MELDNa in patients off anticoagulation, and no significant improvements in the AUC of MELD-XI were observed compared with those of any individual variables (Online Table 3). Furthermore, in addition to INR, other variables composing the model were no longer reliable predictors and therefore contributed to the limitation of MELD models in patients receiving anticoagulation (1-year AUC: 0.60 [p = 0.1021], 0.59 [p = 0.7316], and 0.59 [p = 0.2893] for creatinine, total bilirubin, and sodium, respectively).
It was recognized that factors including clinical decision processes and disease state may influence HTx and VAD events; therefore, we repeated the 1-year ROC analysis by using an endpoint event of death only to re-ensure the validity of the result obtained by using an endpoint of death/HTx/VAD requirement (Online Table 5). One-year ROC analysis using only death as an endpoint event confirmed the results obtained by using the death/HTx/VAD requirement as endpoint events.
Impact of dichotomized MELD, MELDNa, and MELD-XI scores
Results of the Kaplan-Meier analyses comparing survival of patients dichotomized according to MELD, MELDNa, and MELD-XI are illustrated in Figure 1. Patients with a high MELD score (>12) had significantly worse 1- and 3-year survival (1-year survival: 69.3% vs. 90.4%; 3-year survival: 54.6% vs. 77.1%; p < 0.0001). We found a similar survival difference between patients with high and low scores defined according to MELDNa (1-year survival: 70.4% vs. 96.6%; 3-year survival: 58.8% vs. 78.4%; p < 0.0001) and MELD-XI score (1-year survival: 70.4% vs. 88.1%; 3-year survival: 51.6% vs. 76.5%; p < 0.0001).
Figure 1. Kaplan-Meier Survival Curves of All Patients (N = 260) Dichotomized According to MELD Values.
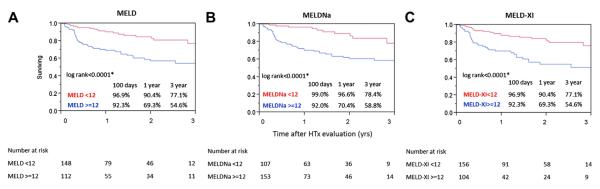
Data were dichotomized according to (A) Model for End-Stage Liver Dysfunction (MELD); (B) MELD serum sodium score (MELDNa); and (C) MELD without international normalized ratio score (MELD-XI) by using the average cutoff value (12) derived from the receiver-operating characteristic analysis for 1-year death/heart transplantation (HTx)/ventricular assist device requirement. The survivals are represented by the solid red line for patients with low scores (<12) and the solid blue line for patients with high scores (>12). The p values obtained by the log-rank test were (A) p < 0.0001, (B) p < 0.0001, and (C) p < 0.0001.
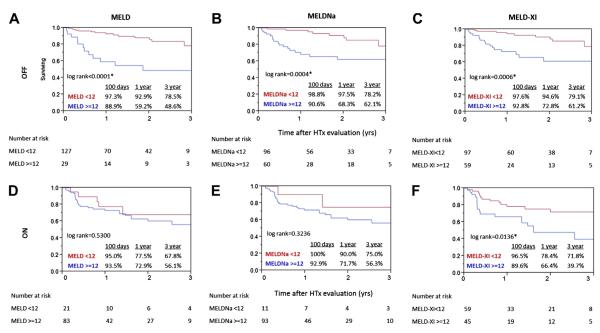
From the poor survival seen in patients on anticoagulation compared to those without anticoagulation (Fig. 1), we learned that anticoagulation itself may be associated with increased risks for the endpoint events. In fact, patients receiving anticoagulation had significantly worse survival than those not treated (1-year survival: 73.7% vs. 86.4%; 3-year survival: 58.2% vs. 72.4%; p = 0.0118) (Fig. 2). As a result, the risks associated with anticoagulation use may influence the effectiveness of MELD/MELDNa/MELDXI score as a predictor for HTx need. When categorized according to use of anticoagulation, patients off treatment were more effectively identified for events according to the MELD models (Figs. 3A to 3C). One-year and 3-year survival of patients off anticoagulation with high MELD scores (>12) were distinctly lower (1-year survival: 59.2% vs. 92.9%; 3-year survival: 48.6% vs. 78.5%; p < 0.0001). This pattern was also observed in patients with high MELDNa scores (>12) (1-year survival: 68.3% vs. 97.5%; 3-year survival: 62.1% vs. 78.2%; p = 0.0004) and high MELD-XI scores (>12) (1-year survival: 72.8% vs. 94.6%; 3-year survival: 61.2% vs. 79.1%; p = 0.0006). MELD-XI was the only model, however, that effectively stratified patients’ risk when they were treated with oral anticoagulation (1 year: 66.4% vs. 78.4%; 3 years: 39.7% vs. 71.8%; p = 0.0136) (Figs. 3D to 3F). No significant survival difference was observed in patients with higher MELD and MELDNa scores in the cohort on anticoagulation, indicating the impact of INR on these scores (p = 0.5300 and p = 0.3236, respectively).
Figure 2. Kaplan-Meier Survival Curve of All Patients (N = 260) Stratified According to Anticoagulation Use.
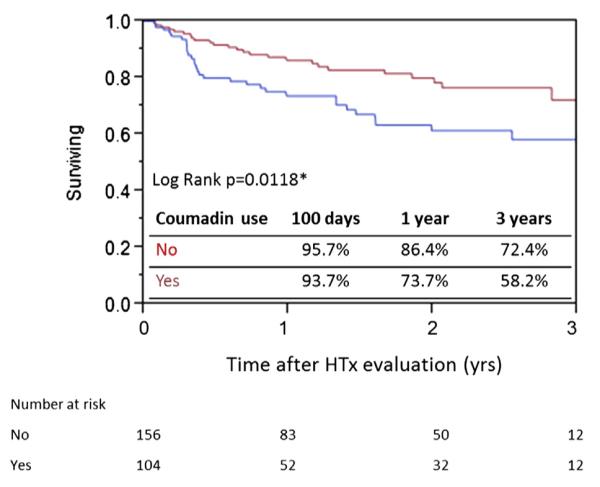
The survivals are represented by the solid red line for patients not undergoing anticoagulation treatment (n = 156) and the solid blue line for patients receiving anticoagulation (n = 104). *Statistically significant (log rank test). HTx = heart transplantation.
Figure 3. Kaplan-Meier Survival Curves of Patients Off and On Anticoagulation.
Values given for patients off anticoagulation (n = 156; top) and on anticoagulation (n = 104; bottom) further dichotomized by MELD (left), MELDNa (middle), and MELD-XI (right) scores by using the cutoff value of 12. The survivals are represented by the solid red line for patients with low scores (<12) and the solid blue line for patients with high scores (>12). The p value obtained by using the log-rank test in patients off anticoagulation was (A) p < 0.0001, (B) p = 0.0004, and (C) p = 0.0006. The p value obtained by using the log-rank test in patients on anticoagulation was (D) p = 0.5300, (E) p = 0.3236, and (F) p = 0.0136. *Statistically significant (log rank test). Abbreviations as in Figure 1.
Impact of MELD/MELDNa/MELD-XI on survival prediction
Cox proportional hazards analysis revealed that an elevation in MELD/MELDNa/MELD-XI score was associated with an increased risk for clinical events (hazard ratio [HR]: 1.10 [95% confidence interval (CI): 1.06 to 1.14], p < 0.0001 for MELD; HR: 1.10 [95% CI: 1.06 to 1.14], p < 0.0001 for MELDNa; HR: 1.13 [95% CI: 1.07 to 1.19], p = 0.0001 for MELD-XI). Body mass index, mean arterial blood pressure, hemoglobin, blood urea nitrogen, serum sodium, INR, B-type natriuretic peptide, cholesterol, and peak VO2 consumption were individually associated with increased risks for endpoint events (Table 3), but only mean arterial blood pressure, INR, and peak VO2 were independent predictors in the multivariate analysis (Online Table 5). In the repeated multivariate analysis including the composite models, the MELD and MELDNa scores were independent predictors for clinical events (Table 4).
Table 3.
Univariate Analysis of Factors Associated With 3-Year Death/HTx/VAD Requirement
| All Patients (N = 260) |
Patients off Anticoagulation (n = 156) |
Patients on Anticoagulation (n = 104) |
||||
|---|---|---|---|---|---|---|
| Variable | HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value |
| MELD | 1.10 (1.06–1.14) | <0.0001 | 1.19 (1.10–1.29) | <0.0001 | 1.06 (0.99–1.13) | 0.0816 |
| MELDNa | 1.10 (1.06–1.14) | <0.0001 | 1.20 (1.11–1.30) | <0.0001 | 1.06 (1.00–1.13) | 0.0620 |
| MELD-XI | 1.13 (1.07–1.19) | <0.0001 | 1.14 (1.05–1.22) | 0.0020 | 1.13 (1.03–1.23) | 0.0098 |
| Age | 1.01 (0.99–1.03) | 0.4751 | 1.00 (0.98–1.03) | 0.7488 | 1.01 (0.98–1.04) | 0.6499 |
| Male | 0.89 (0.51–1.63) | 0.6936 | 0.98 (0.43–2.53) | 0.9717 | 0.76 (0.36–1.74) | 0.4926 |
| BMI | 0.93 (0.88–0.97) | 0.0030 | 0.94 (0.87–1.00) | 0.0688 | 0.89 (0.81–0.96) | 0.0046 |
| Diabetes | 1.44 (0.78–2.52) | 0.2304 | 2.26 (0.95–5.02) | 0.0634 | 0.95 (0.38–2.11) | 0.9142 |
| Heart rate | 0.99 (0.97–1.01) | 0.4903 | 1.00 (0.97–1.02) | 0.9042 | 0.99 (0.97–1.02) | 0.5401 |
| Mean arterial BP | 0.95 (0.93–0.97) | <0.0001 | 0.95 (0.91–0.98) | 0.0006 | 0.95 (0.92–0.98) | 0.0006 |
| Atrial fibrillation | 1.49 (0.81–2.68) | 0.1942 | 1.39 (0.40–3.67) | 0.5630 | 0.91 (0.40–2.14) | 0.8240 |
| Laboratory examination | ||||||
| WBC | 0.94 (0.82–1.08) | 0.4203 | 0.98 (0.80–1.19) | 0.8597 | 0.88 (0.71–1.08) | 0.2306 |
| Hemoglobin | 0.69 (0.60–0.81) | <0.0001 | 0.59 (0.48–0.74) | <0.0001 | 0.80 (0.63–1.00) | 0.0482 |
| Platelets | 1.00 (1.00–1.00) | 0.8401 | 1.00 (1.00–1.01) | 0.4890 | 1.00 (0.99–1.01) | 0.8764 |
| Serum sodium | 0.87 (0.80–0.96) | 0.0062 | 0.85 (0.72–1.02) | 0.0758 | 0.91 (0.82–1.02) | 0.1081 |
| BUN | 1.03 (1.01–1.04) | <0.0001 | 1.02 (1.00–1.04) | 0.0189 | 1.05 (1.03–1.07) | <0.0001 |
| Creatinine | 1.09 (0.89–1.22) | 0.3506 | 1.04 (0.73–1.22) | 0.7521 | 1.83 (1.15–2.62) | 0.0155 |
| Total bilirubin | 1.24 (1.03–1.43) | 0.0237 | 1.25 (0.94–1.53) | 0.1113 | 1.21 (0.92–1.46) | 0.1487 |
| AST | 1.01 (0.99–1.03) | 0.2662 | 1.02 (0.99–1.06) | 0.1202 | 1.00 (0.97–1.02) | 0.9332 |
| ALT | 1.00 (0.98–1.02) | 0.9180 | 0.99 (0.96–1.01) | 0.3889 | 1.01 (0.98–1.02) | 0.4987 |
| Albumin | 0.91 (0.45–1.91) | 0.8082 | 0.53 (0.19–1.55) | 0.2443 | 1.74 (0.62–5.23) | 0.3004 |
| INR | 1.48 (1.13–1.88) | 0.0060 | 2.13 (0.91–3.97) | 0.0766 | 1.21 (0.80–1.74) | 0.3452 |
| Total cholesterol | 0.99 (0.98–0.99) | <0.0001 | 0.98 (0.98–0.99) | 0.0002 | 0.99 (0.98–1.00) | 0.0150 |
| BNP | 1.00 (1.00–1.00) | <0.0001 | 1.00 (1.00–1.00) | <0.0001 | 1.00 (1.00–1.00) | 0.0576 |
| Peak VO2 | 0.84 (0.78–0.90) | <0.0001 | 0.79 (0.70–0.88) | <0.0001 | 0.90 (0.81–0.99) | 0.0327 |
Table 4.
Summary of the Multivariate Analysis Including MELD Models With the Factors Associated With 3-Year Death/HTx/VAD Requirement
| All Patients (N = 260) |
Patients off Anticoagulation (n = 156) |
Patients on Anticoagulation (n = 104) |
||||
|---|---|---|---|---|---|---|
| Variable | HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value |
| MELD | 1.07 (1.02–1.13) | 0.0055 | 1.25 (1.09–1.42) | 0.0025 | 0.99 (0.92–1.07) | 0.8739 |
| MELDNa | 1.06 (1.02–1.11) | 0.0083 | 1.24 (1.07–1.42) | 0.0055 | 0.98 (0.92–1.05) | 0.5975 |
| MELD-XI | 1.05 (0.95–1.16) | 0.3154 | 1.20 (1.02–1.41) | 0.0326 | 0.98 (0.86–1.11) | 0.7895 |
The elevated MELD/MELDNa/MELD-XI scores were also strongly correlated with clinical events in patients off anticoagulation (HR: 1.19 [95% CI: 1.10 to 1.29], p < 0.0001 for MELD; HR: 1.20 [95% CI: 1.11 to 1.30], p < 0.0001 for MELDNa; and HR: 1.14 [95% CI: 1.05 to 1.22], p = 0.0020 for MELD-XI). Mean arterial blood pressure, hemoglobin, serum sodium, cholesterol, blood urea nitrogen, B-type natriuretic peptide, and peak VO2 were individually associated with increased risk for endpoint events (Table 3), but only mean arterial blood pressure, B-type natriuretic peptide, and peak VO2 were independent predictors (Online Table 6). When the multivariate analysis included the risk models, all MELD models were independent predictors for HTx need in this group of patients (Table 4).
On the contrary, MELD-XI was the only model that was individually associated with the endpoint events in patients receiving anticoagulation, along with body mass index, mean arterial blood pressure, blood urea nitrogen, and peak VO2. However, MELD-XI score was no longer a significant factor for events in the multivariate analysis (Table 4), and only body mass index, blood urea nitrogen, and total cholesterol remained independent predictors for the endpoint events (Online Table 6).
Discussion
Liver abnormalities in patients with HF have a strong impact on prognosis and risk assessment. In this retrospective study, we found that MELD, an established scoring system for liver dysfunction, was used to successfully risk-stratify ambulatory patients with HF. MELD scores correlate with hemodynamic variables indicative of right ventricular dysfunction. The composite models were improved discriminators for the death/HTx/VAD requirement compared with individual variables comprising the score. Elevated MELD scores were associated with poor survival and greater risk of clinical events. Notably, these relationships were more prominent in patients not treated with oral anticoagulation. For patients treated with anticoagulation, MELD-XI provided moderate but limited risk information on HTx need.
Previous studies have demonstrated that the combination of risk models performs better as a predictor for clinical events in HF patients. Additional evaluation using the HFSS in patients with high-risk peak VO2 (<10 ml/min/kg) further stratified subjects at higher risk for HTx need (20). Furthermore, the combination of HFSS and SHFM has been shown to be a better predictor of events, particularly in the medium-risk group (21). In the current study, we found that the MELD model provides risk information that is independent of the variables such as peak VO2 used in the current HTx evaluation models; therefore, MELD is an excellent tool to complement the existing evaluation methods. When MELD scores and peak VO2/HFSS/SHFM were combined, low MELD score (<12) within the low-risk group confirmed patient clinical stability (<1 year), whereas high scores (>12) within the medium-risk group identified patients with increased risk (Online Figs. 1 and 2). This additional risk information would be particularly helpful in determining patients at higher risk within the current medium-risk group based on assessment of peak VO2/HFSS/SHFM, which is often considered to be the “gray area.”
Most notably, MELD scores can be used to continuously evaluate HF patients’ risk over the course of their disease progression. Contrary to the SHFM and HFSS models, which can only evaluate relatively stable ambulatory HF patients, the MELD score has been shown to identify risk in end-stage HF patients undergoing LVAD implantation (12) and orthotopic HTx (13) as well. Moreover, comparison of presurgical and postsurgical MELD scores was shown to reflect the reversibility of HF progression of patients with improved HF after intervention (12,13). Along with the relative ease of obtaining the score, this feature would help clinicians to monitor improvement or advancement in the patient’s risk between early, late, and post-LVAD/HTx stage of HF. Hence, we strongly support the use of the MELD score as a routine tool to complement the peak VO2 value during the evaluation of HF patients for HTx. To our knowledge, this is the first study supporting the use of MELD scores as a tool for HTx evaluation in a cohort of ambulatory patients with advanced HF.
Of note, MELD-XI was the only MELD score with significant power to predict lower survival in patients receiving anticoagulation therapy. However, it has limited prognostic power in patients undergoing anticoagulation treatment compared with patients not receiving anticoagulation therapy. In fact, anticoagulation itself was a risk factor for poorer outcome in patients with HF (Fig. 2). Anticoagulation was likely prescribed to patients who were sicker due to multiple other comorbidities requiring anticoagulation; in particular, atrial fibrillation was more frequently observed in patients taking warfarin (p < 0.0001), which indicates greater risk for either ischemic stroke or hemorrhage secondary to anticoagulation (Online Table 2A). The WASH (Warfarin/Aspirin Study in Heart Failure) and WARCEF (Warfarin Versus Aspirin in Reduced Cardiac Ejection Fraction) studies showed no significant benefit of warfarin use in HF patients with sinus rhythm compared with aspirin use and because of a reduced risk of ischemic stroke with warfarin, use may have been offset by an increased risk of hemorrhage secondary to anticoagulation (22,23). The MELD score, which quantifies the degree of hepatic and renal dysfunction, is likely limited in addressing risk contributed by sudden cardiovascular events such as stroke or hemorrhage. Further analysis revealed that patients’ risk identified by using the HFSS score, which includes etiology of HF, was not significantly influenced by anticoagulation (Online Fig. 2). Therefore, to address this limitation, MELD-XI should be used in combination with several other risk models such as HFSS to evaluate risk in HF patients treated with anticoagulation.
Our study also supports the incorporation of serum sodium in the MELD score to provide additional risk information for ambulatory patients with advanced HF. Hyponatremia is a common condition in HF patients caused by activation of the renin-angiotensin system associated with the disease (24). Decreased cardiac output of HF leads to a continued release of vasopressin and activation of multiple neuronal hormones that cause fluid retention despite the reduction in serum osmolarity; this imbalance is further exaggerated by the reduction in glomerular filtration rate. Not only is hyponatremia common, but it is also a marker of increased risk for poor outcome in HF patients (25,26). In OPTIME-CHF (Outcomes of a Prospective Trial of Intravenous Milrinone for Exacerbations of Chronic Heart Failure), patients with the lowest admission serum sodium levels had the poorest outcome (27). In the current study, we found that the incorporation of serum sodium with the MELDNa resulted in improved prognostic power (Table 2), and we support the use of MELDNa for patients not treated with anticoagulation to evaluate their risk.
Study limitations
Due to its retrospective observational analysis, the study could not establish causal relationships and is subject to inherent biases. Our database does not include echocardiographic or invasive data or information on the degree of HF status. In addition, the components of the MELD score are subject to laboratory variations. For example, serum sodium levels are highly variable by time, and the use of fluid restriction and diuretic agents may bypass the purpose of the sodium component in the model. Lastly, due to the ambulatory nature of our cohort, there is limited information on the medications prescribed by the primary physicians.
Conclusions
This single-center, retrospective study demonstrated the prognostic effectiveness of various MELD scoring systems in a cohort of ambulatory patients with HF. An elevated MELD/MELDNa score was strongly associated with an increased risk that is independent of the current HTx evaluation models; this relationship was strengthened when patients were not undergoing oral anticoagulant therapy. For patients receiving anticoagulation therapy, MELD-XI provided moderate but limited risk information. The use of the MELD scoring system might complement and enhance the current HTx evaluation models for ambulatory patients with advanced HF.
Supplementary Material
Abbreviations and Acronyms
- AUC
area under the curve
- CI
confidence interval
- HF
heart failure
- HFSS
Heart Failure Survival Score
- HR
hazard ratio
- HTx
heart transplantation
- INR
international normalized ratio
- LVAD
left ventricular assist device
- MELD
Model for End-Stage Liver Dysfunction
- ROC
receiver-operating characteristic
- SHFM
Seattle Heart Failure Model
- VAD
ventricular assist device
- VO2
oxygen consumption
Footnotes
APPENDIX For supplementary tables and figures, please see the online version of this article.
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
REFERENCES
- 1.Mancini DM, Eisen H, Kussmanul W, Mull R, Edmunds LH, Wilson JR. Value of peak exercise oxygen consumption for optimal timing of cardiac transplantation in ambulatory patients with heart failure. Circulation. 1991;83:778–86. doi: 10.1161/01.cir.83.3.778. [DOI] [PubMed] [Google Scholar]
- 2.Aaronson KD, Schwartz JS, Chen TM, Wong KL, Goin JE, Mancini DM. Development and prospective validation of a clinical index to predict survival in ambulatory patients referred for cardiac transplant evaluation. Circulation. 1997;95:2660–7. doi: 10.1161/01.cir.95.12.2660. [DOI] [PubMed] [Google Scholar]
- 3.Levy WC, Mozaffarian D, Linker DT, et al. The Seattle Heart Failure Model: prediction of survival in heart failure. Circulation. 2006;113:1424–33. doi: 10.1161/CIRCULATIONAHA.105.584102. [DOI] [PubMed] [Google Scholar]
- 4.van Deursen VM, Damman K, Hillege HL, et al. Abnormal liver function in relation to hemodynamic profile in heart failure patients. J Card Fail. 2010;16:84–90. doi: 10.1016/j.cardfail.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 5.Poelzl G, Ess M, Mussner-Seeber C, Pachinger O, Frick M, Ulmer H. Liver dysfunction in chronic heart failure: prevalence, characteristic and prognostic significance. Eur J Clin Invest. 2012;42:153–63. doi: 10.1111/j.1365-2362.2011.02573.x. [DOI] [PubMed] [Google Scholar]
- 6.Shinagawa H, Inomata T, Koitabashi T, et al. Prognostic significance of increased serum bilirubin levels coincident with cardiac decompensation in chronic heart failure. Circ J. 2008;72:364–9. doi: 10.1253/circj.72.364. [DOI] [PubMed] [Google Scholar]
- 7.Allen LA, Felker GM, Pocock S, et al. Liver function abnormalities and outcome in patients with chronic heart failure: data from the Candesartan in Heart Failure: assessment of Reduction in Mortality and Morbidity (CHARM) program. Eur J Heart Fail. 2009;11:170–7. doi: 10.1093/eurjhf/hfn031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malinchoc M, Kamath PS, Gordon FD, Peine CJ, Rank J, ter Borg PC. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. 2000;31:864–71. doi: 10.1053/he.2000.5852. [DOI] [PubMed] [Google Scholar]
- 9.Nagarajan V, Tang WH. Biomarkers in advanced heart failure: diagnostic and therapeutic insights. Congest Heart Fail. 2011;17:169–74. doi: 10.1111/j.1751-7133.2011.00244.x. [DOI] [PubMed] [Google Scholar]
- 10.Suman A, Barnes DS, Zein NN, Levinthal GN, Connor JT, Carey WD. Predicting outcome after cardiac surgery in patients with cirrhosis: a comparison of Child-Pugh and MELD scores. Clin Gastroenterol Hepatol. 2004;2:719–23. doi: 10.1016/s1542-3565(04)00296-4. [DOI] [PubMed] [Google Scholar]
- 11.Teh SH, Nagorney DM, Stevens SR, et al. Risk factors for mortality after surgery in patients with cirrhosis. Gastroenterology. 2007;132:1261–9. doi: 10.1053/j.gastro.2007.01.040. [DOI] [PubMed] [Google Scholar]
- 12.Matthews JC, Pagani FD, Haft JW, Koelling TM, Naftel DC, Aaronson KD. Model for End-Stage Liver Disease score predicts left ventricular assist device operative transfusion requirements, morbidity, and mortality. Circulation. 2010;121:214–20. doi: 10.1161/CIRCULATIONAHA.108.838656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chokshi A, Cheema FH, Schaefle KJ, et al. Hepatic dysfunction and survival after orthotopic heart transplantation: application of the MELD scoring system for outcome prediction. J Heart Lung Transplant. 2012;31:591–600. doi: 10.1016/j.healun.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heuman DM, Mihas AA, Habib A, et al. MELD-XI: a rational approach to “sickest first” liver transplantation in cirrhotic patients requiring anticoagulant therapy. Liver Transpl. 2006;13:30–7. doi: 10.1002/lt.20906. [DOI] [PubMed] [Google Scholar]
- 15.Kim WR, Biggins SW, Kremers WK, et al. Hyponatremia and mortality among patients on the liver-transplant waiting list. N Engl J Med. 2008;359:1018–26. doi: 10.1056/NEJMoa0801209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Romanovsky A, Bagshaw S, Rosner MH. Hyponatremia and congestive heart failure: a marker of increased mortality and a target for therapy. Int J Nephrol. 2011;2011:732746. doi: 10.4061/2011/732746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kammath PS, Wiesner RH, Malinhoc M, et al. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464–70. doi: 10.1053/jhep.2001.22172. [DOI] [PubMed] [Google Scholar]
- 18.United Network for Organ Sharing (UNOS) [Accessed April 21, 2013];MELD/PELD Calculator Documentation. Available at: http://www.unos.org/docs/MELD_PELD_Calculator_Documentation.pdf. Updated January 28, 2009.
- 19.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–45. [PubMed] [Google Scholar]
- 20.Lund LH, Aaronson KD, Mancini DM. Validation of peak oxygen consumption and the Heart Failure Survival Score for serial risk stratification in advanced heart failure. Am J Cardiol. 2005;95:734–41. doi: 10.1016/j.amjcard.2004.11.024. [DOI] [PubMed] [Google Scholar]
- 21.Goda A, Williams P, Mancini D, Lund LH. Selecting patients for heart transplantation: comparison of the Heart Failure Survival Score (HFSS) and the Seattle Heart Failure Model (SHFM) J Heart Lung Transplant. 2011;30:1236–43. doi: 10.1016/j.healun.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 22.Cleland JG, Findlay I, Jafri S, et al. The Warfarin/Aspirin Study in Heart Failure (WASH): a randomized trial comparing antithrombotic strategies for patients with heart failure. Am Heart J. 2004;148:157–64. doi: 10.1016/j.ahj.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 23.Homma S, Thompson JL, Pullicino PM, Levin B, et al. WARCEF Investigators Warfarin and aspirin in patients with heart failure and sinus rhythm. N Engl J Med. 2012;366:1859–69. doi: 10.1056/NEJMoa1202299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sica DA. Sodium and water retention in heart failure and diuretic therapy: basic mechanisms. Clev Clin J Med. 2006;73:S30–3. doi: 10.3949/ccjm.73.suppl_2.s2. [DOI] [PubMed] [Google Scholar]
- 25.Lee WH, Packer M. Prognostic importance of serum sodium concentration and its modification by converting-enzyme inhibition in patients with severe chronic heart failure. Circulation. 1986;73:257–67. doi: 10.1161/01.cir.73.2.257. [DOI] [PubMed] [Google Scholar]
- 26.Gheorghiade M, Rossi JS, Cotts W, et al. Characterization and prognostic value of persistent hyponatremia in patients with severe heart failure in the ESCAPE trial. Arch Intern Med. 2007;167:1998–2005. doi: 10.1001/archinte.167.18.1998. [DOI] [PubMed] [Google Scholar]
- 27.Klein L, O’Connor CM, Leimberger JD, et al. Lower serum sodium is associated with increased short-term mortality in hospitalized patients with worsening heart failure: results from the Outcomes of a Prospective Trial of Intravenous Milrinone for Exacerbations of Chronic Heart Failure (OPTIME-CHF) Study. Circulation. 2005;111:2454–60. doi: 10.1161/01.CIR.0000165065.82609.3D. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.