Abstract
Protein kinase C (PKC) is involved in the regulation of vascular smooth muscle contraction. However, the role of PKC in erectile function is poorly understood. This study investigated whether PKC mediates agonist-induced contractions in mouse penile tissue (corpora cavernosa). We also compared the effects of PKC activators and inhibitors on contractile responses in mouse corpus cavernosum with those in mouse aorta. Aortic rings and corpus cavernosal strips from C57BL/6J mice was isolated, mounted in the organ bath for isometric tension recording. Our data showed that a PKCα/β selective inhibitor, Gö6976 (10 µM), inhibited phenylephrine and 9,11-dideoxy-11α,9α-epoxymethanoprostaglandin F2α (U46619, a thromboxane mimetic)-induced contractions in mouse aorta, reducing the maximum contraction from 123 ± 2% of KCl-induced maximum contraction to 7 ± 2% and 13 ± 1%, respectively. A non-selective PKC inhibitor, chelerythrine (30 µM), also significantly reduced phenylephrine-and U46619-induced maximum contractions in mouse aorta. However, Gö6976 and chelerythrine had no significant effects on phenylephrine-and U46619-induced contractions in corpus cavernosum. Furthermore, a PKC activator, phorbol-12,13-dibutyrate (0.1 µM), significantly increased contractions in aorta (208 ± 14% of KCl-induced maximum contraction) but failed to cause contractions in corpus cavernosum at 1 and 10 µM. Western blot analysis data suggested that protein expression of PKC was similar in aorta and corpus cavernosum. Taken together, our data indicate that PKC does not have a significant role in agonist-induced contractions in mouse corpus cavernosum, whereas it mediates the contractile response to agonists in the aorta.
Keywords: aorta, penis, protein kinase C, smooth muscle, erectile dysfunction, corpus cavernosum
1. INTRODUCTION
Erectile dysfunction is recognized as a public health problem in recent years and increasing efforts have sought to treat this disease. Vascular dysfunction is one of the common causes of erectile dysfunction. Corpus cavernosum has a specialized vasculature (Andersson and Wagner, 1995). It is composed of numerous interconnected cavernosal spaces, which are lined by a layer of endothelial cells and separated by trabeculae, containing smooth muscle fibers, elastic fibers and fibroblasts. Penile smooth muscle stays contracted in the flaccid state, which is dominated by sympathetic neuronal activity. Erection occurs when the penile arteries and corpus cavernosal smooth muscle are relaxed by nitric oxide released from nerve endings and endothelial cells. This relaxation allows blood to flow into the penis under the driving force of systemic blood pressure. Vasculogenic erectile dysfunction is often due to an imbalance of contractile and relaxation mechanisms. Under pathophysiological conditions, increased release of adrenergic neurotransmitters such as norepinephrine or circulating constrictive factors lead to an increase in smooth muscle tone of penile tissue (Disanto, 2005; Jin and Burnett, 2006).
Protein kinase C (PKC) is an important molecule that transduces extracellular stimuli to intracellular signal cascades. It is involved in the signaling pathways that regulate cell growth, apoptosis, ion channel activities, actin cytoskeleton, and carcinogenesis. Functional studies have demonstrated that PKC mediates agonist-induced contractions in various arteries (Budzyn et al., 2006). Moreover, evidence suggests that increased PKC activity causes hyperactive contractility of vascular smooth muscle, contributing to the development of cardiovascular diseases (Salamanca and Khalil, 2005). Increased PKC activity in diabetic human penile tissue has also been reported (Angulo et al., 2006). PKC inhibitors may be a potential therapeutic treatment for vascular diseases.
Studies have demonstrated that rodents are valid animal models for the investigation of erectile physiology and pharmacology (Burnett, 2001; Giuliano, 2001). Although rats have been widely used for in vivo and in vitro assessments of erectile function, the mouse model has recently become attractive due to the accessibility of genetic manipulations. The knockout mouse models, such as nitric oxide synthase knockouts and cyclic GMP-dependent protein kinase knockouts, have provided valuable data at the molecular level on the specific signaling pathways involved in erection. However, there is a lack of information on physiological and pharmacological characterizations of mouse penile tissue. The functions of PKC in the regulation of smooth muscle tone in mouse penis are unknown. The goal of this study is to determine the involvement of PKC in mediating agonist-induced contractions in mouse corpus cavernosum and to compare its roles with those in mouse aorta, thereby providing basic information for future studies of erectile function in mice. We used the C57BL/6J mouse as our animal model since it is commonly used as the background strain for the genetic mutants.
2. MATERIALS AND METHODS
2.1. Animals and tissue preparation
C57BL/6J mice (8 to 10 weeks old) were purchased from Jackson Laboratory. The animals were cared for in accordance with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the U.S. National Institutes for Health.
The mice were anesthetized with sodium pentobarbital (50 mg/kg, i.p.). Aortae were rapidly excised and placed in cold physiological saline solution (PSS) of the following composition (mM): NaCl 118, KCl 4.7, KH2PO4 1.18, CaCl2·2H2O 1.6, MgSO4·7H2O 1.6, NaHCO3 25, dextrose 5.5, EDTA 0.03. After periadventitial adhering fat was removed, endothelial intact aortae were cut into 2 mm rings and mounted in myograph organ bath chambers (Danish Myo Technology A/S).
Mice penes were exposed and excised from skin and connective tissue. After removal of spongiosum and dorsal vein and arteries in cold PSS, mouse corpora cavernosa were cut longitudinally to obtain two identical strips, which were mounted on organ bath chambers.
2.2. Experimental protocols
The aortic rings or cavernosal smooth muscle strips were placed under an optimal passive tension of 5 mN or 2 mN, respectively, and allowed to equilibrate for 60 min in 5 ml PSS continuously bubbled with 95% O2 and 5% CO2 at 37 °C. After equilibration, aortic rings or cavernosal muscle strips were contracted to the maximum with 120 mM KCl to verify the viability of the preparations. After washing out KCl, the aortic rings or cavernosal strips were incubated with vehicle (1% DMSO), Gö6976 (a selective PKCα/βinhibitor), or chelerythrine (a non-selective PKC inhibitor) in the absence or presence of N(G)-nitro-L-arginine methyl ester (L-NAME) for 20 min. Cumulative concentration-response curves to phenylephrine (an α-adrenoceptor agonist) or 9,11-dideoxy-11α,9α-epoxymethanoprostaglandin F2α (U46619, a thromboxane mimetic) were constructed. In another set of experiments, phorbol-12,13-dibutyrate (PDBu) was added into organ bath chambers after verification of the viability of the aortic rings and cavernosal strips. The contractile responses to PDBu were monitored for 45 min after the administration of the chemical.
Changes in isometric force were recorded by PowerLab data acquisition system (ADInstruments) and expressed as % of KCl-induced maximum contraction.
2.3. Western blot analysis for PKC protein expressions
Mouse aorta or corpus cavernosum was homogenized in RIPA buffer (Upstate) containing Tris-HCl, pH 7.4, 50 mM, NaCl 150 mM, deoxycholic acid 0.25%, NP-40 1%, EDTA 1 mM, phenylmethylsulfornyl fluoride 1 mM, and Na3VO4 1 mM. The tissue lysates were centrifuged at 10,000 g for 30 minutes at 4 °C and the supernatant was collected. Proteins (25 µg) were loaded on 7.5% SDS-polyacrylamide gel for Western blot analysis as described previously (Jin et al., 2006). Primary antibodies against PKCα (1:1,000 dilution) and PKCβ (1:500 dilution) isoforms (BD Biosciences) or β-actin (1:5,000, Sigma) were used to detect PKC and β-actin protein expression in mouse aorta or corpus cavernosum. Protein expression of PKCα or PKCβ was normalized by β-actin protein level.
2.4. Statistics
Data were expressed as the mean ± S.E.M. EC50 was obtained from the sigmoidal dose-response curve fit. Analysis of variance and Student’s t-test were used to evaluate the results. P < 0.05 was considered to be significant. GraphPAD Software was used for the statistical analysis of all data.
3. RESULTS
3.1. Effects of PKC inhibitors on agonist-induced contractions in mouse aorta
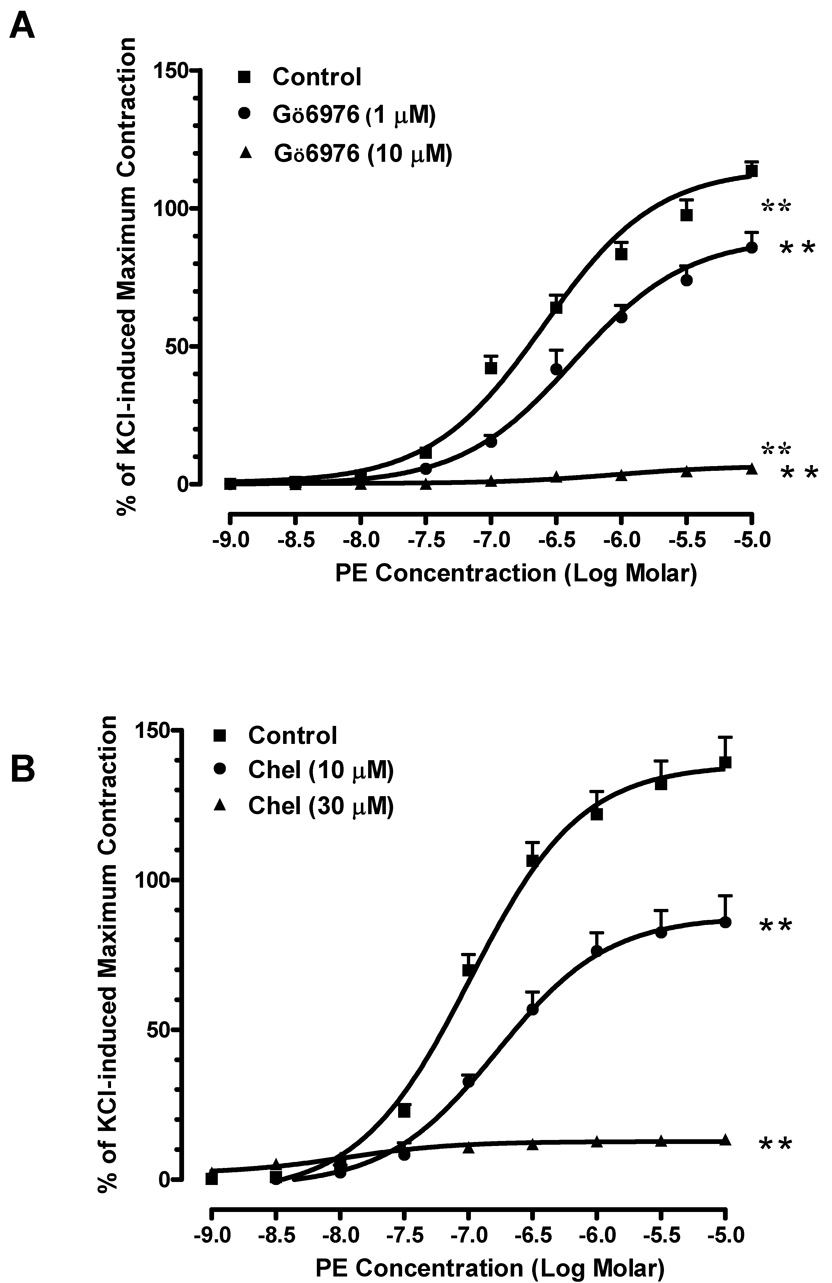
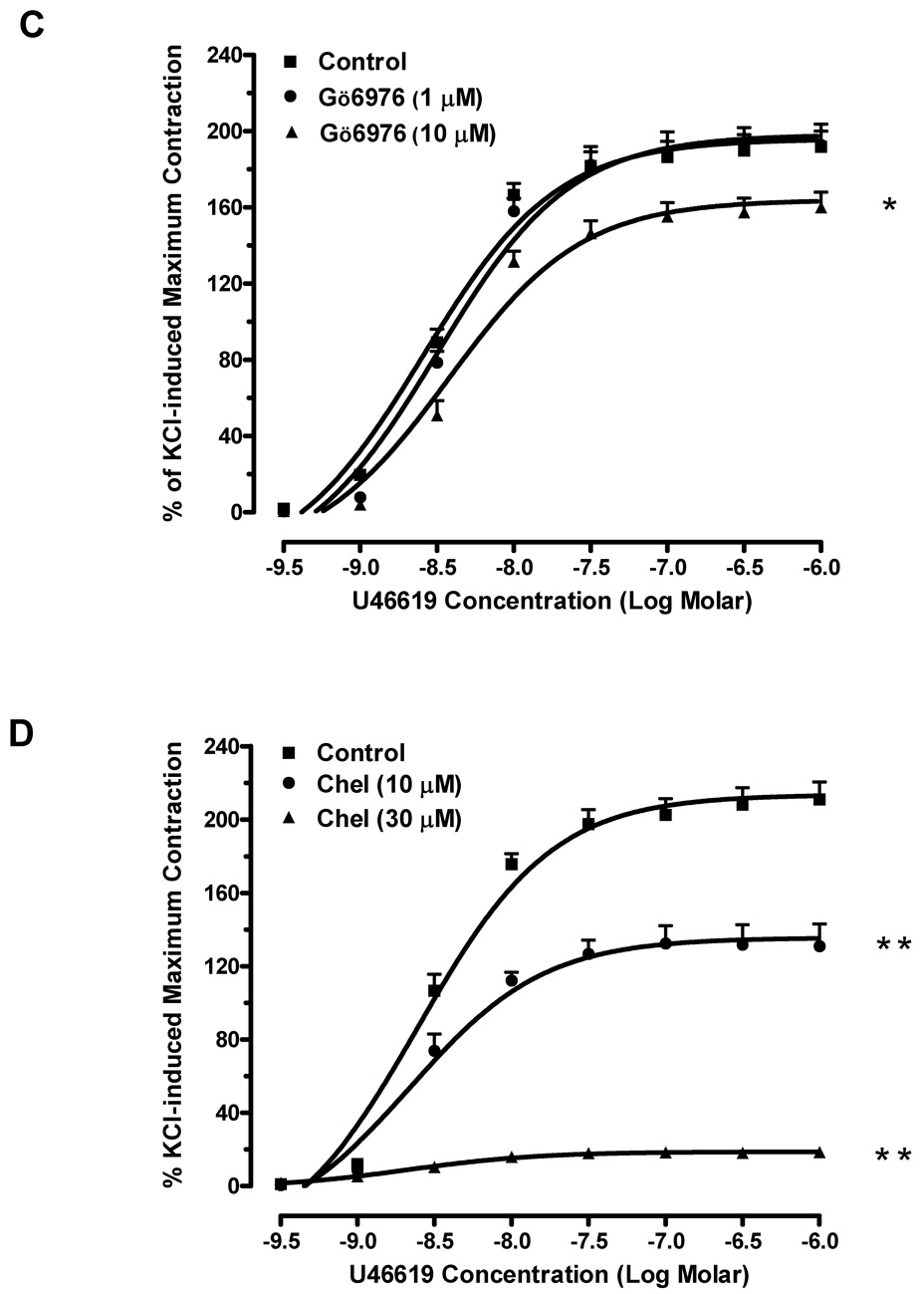
Phenylephrine produced a concentration-dependent contraction curve in mouse aorta with EC50 equal to 0.25 µM. The PKC inhibitor Gö6976 (1 µM) shifted the curve to the right and increased the EC50 of phenylephrine 1.7-fold (Fig. 1A). Additionally, the maximum contraction in response to phenylephrine was significantly reduced from 123 ± 2% of KCl-induced maximum contraction to 92 ± 5%. A higher concentration of Gö6976 (10 µM) almost blocked the contractile response to phenylephrine, decreasing the maximum contraction to 7 ± 2%. The inhibitory effect of another PKC inhibitor, chelerythrine, also occurred in a concentration-dependent fashion. Chelerythrine (10 µM) decreased phenylephrine-induced maximum contraction by 40% when compared to that of vehicle controls (Fig. 1B). At higher concentration (30 µM), chelerythrine almost abolished the phenylephrine-induced contraction.
Fig. 1. Effects of PKC inhibitors on agonist-induced contraction in mouse aorta.
(A) Pre-treatment of Gö6976 significantly decreased phenylephrine-induced contraction (■: control;●: pre-treated with 1 µM of Gö6976; ▲: pre-treated with 10 µM of Gö6976). n=4, ** P<0.01 vs. control. (B) Pre-treatment of chelerythrine reduced the contractile responses to phenylephrine (■: control; ●: pre-treated with 10 µM of chelerythrine; ▲: pre-treated with 30 µM of chelerythrine). n=4, ** P<0.01 vs. control. (C) Gö6976 reduced the contractile response to U46619 at 10 µM but had no effects at 1 µM (■: control; ●: pre-treated with 1 µM of Gö6976; ▲: pre-treated with 10 µM of Gö6976). n=4, * P<0.05 vs. control. (D) Chelerythrine concentration-dependently decreased the contractions induced by U46619 (■: control; ●: pre-treated with 10 µM of chelerythrine; ▲: pre-treated with 30 µM of chelerythrine). n=4, ** P<0.01 vs. control.
We also determined the effects of PKC inhibitors in the thromboxane receptor agonist-induced contractions. U46619 induced concentration-dependent contractions in mouse aorta with EC50 equal to 0.003 µM. At 10 µM, Gö6976 reduced the maximum smooth muscle contraction by 17% in response to U46619 and increased EC50 of U46619 to 0.004 µM in mouse aorta; however, it did not have a significant effect at 1 µM (Fig. 1C). Chelerythrine had profound effects on U46619-induced contractions, decreasing the maximum responses by 38% and 91% when compared to those effects in vehicle controls at 10 and 30 µM, respectively (Fig. 1D). The EC50 of U46619 was not significantly altered by chelerythrine. These data suggest that PKC mediates agonist-induced aortic smooth muscle contraction.
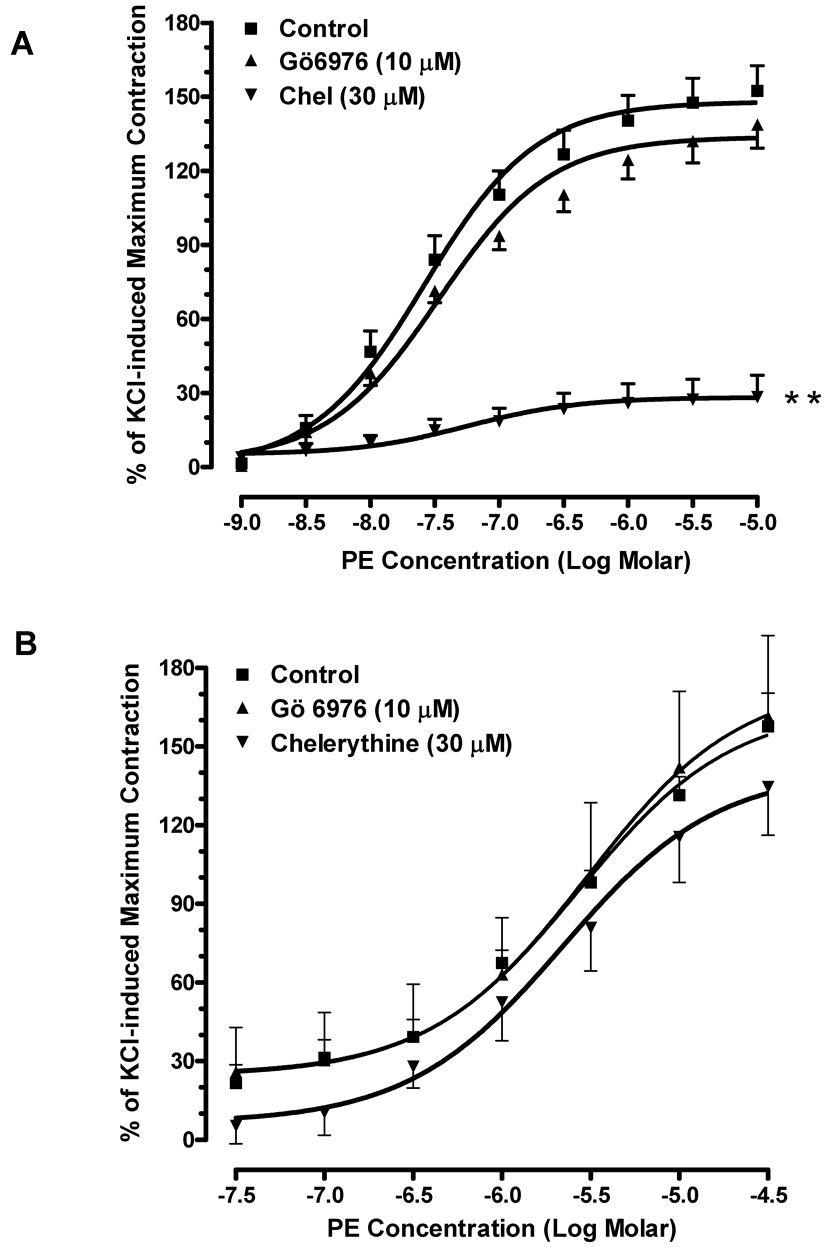
3.2. Effects of PKC inhibitors on agonist-induced contraction in mouse corpus cavernosum
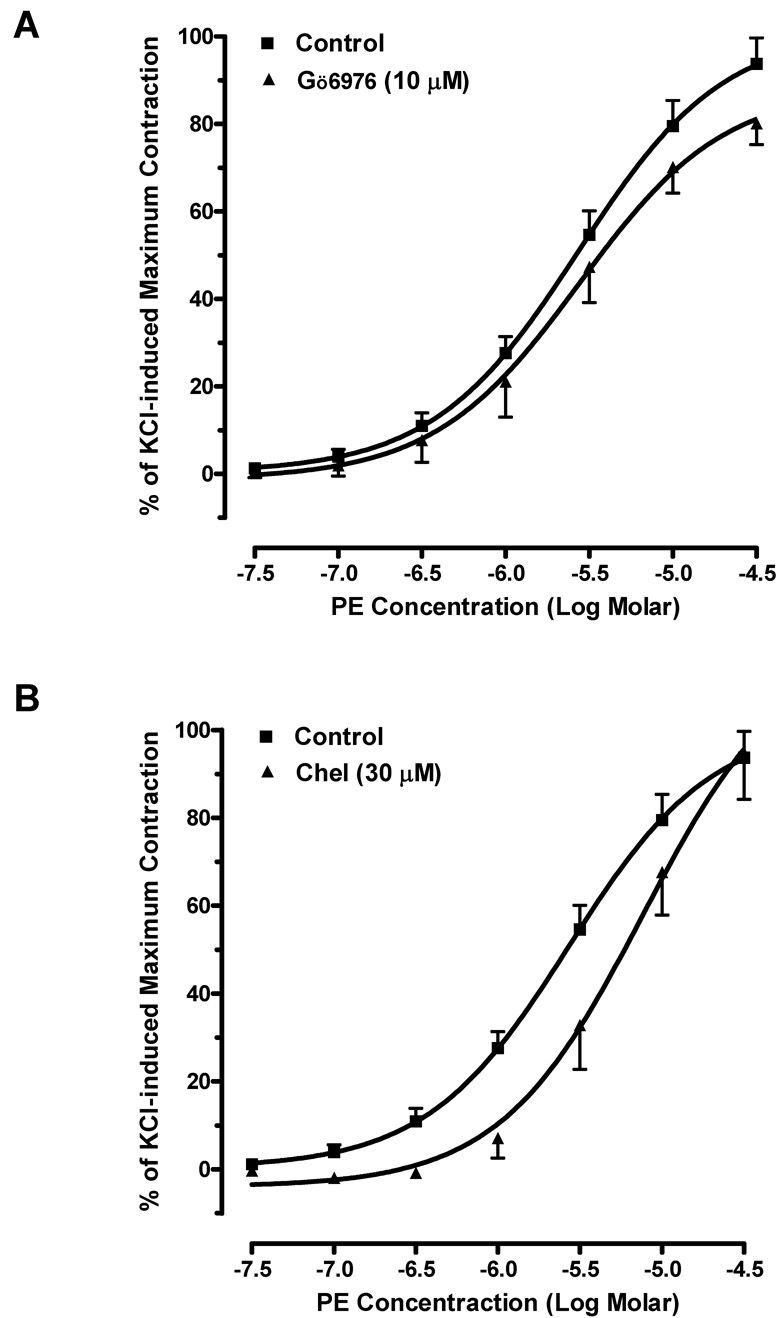
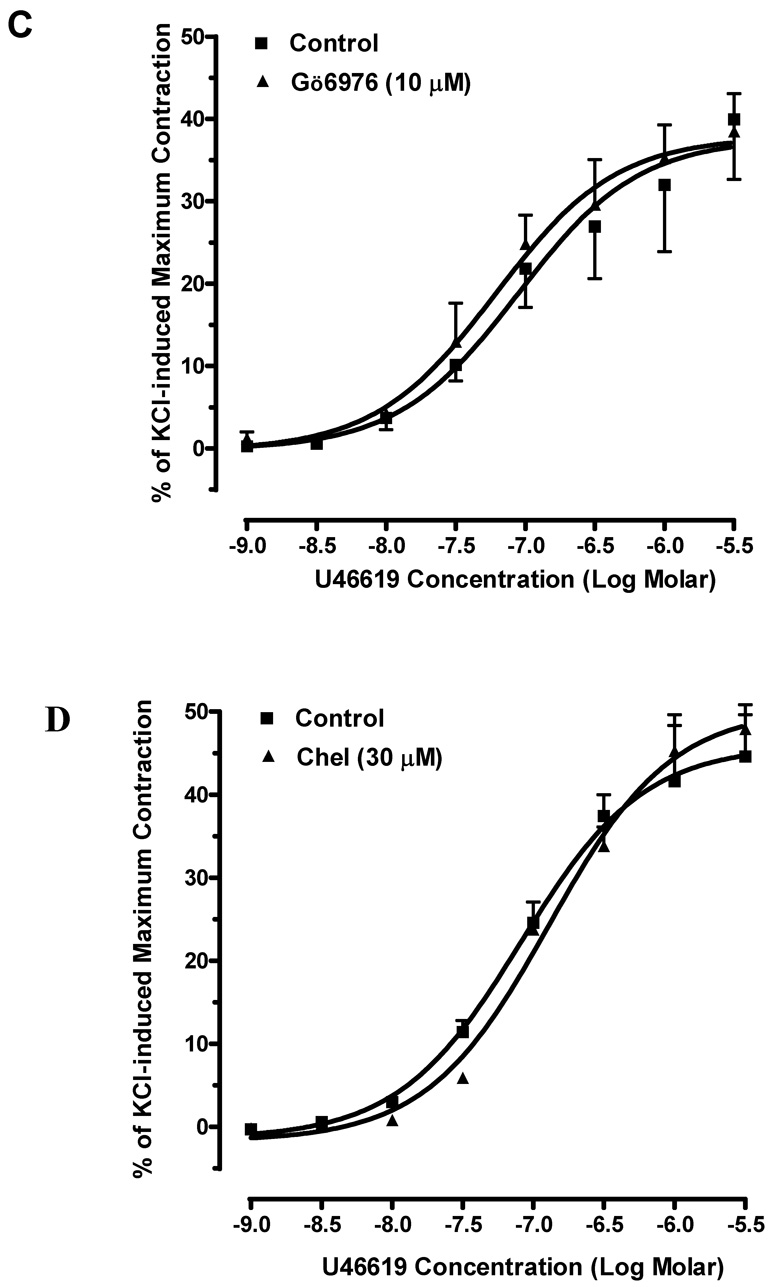
To investigate the role of PKC in mouse erectile function, we examined the effects of PKC inhibitors on phenylephrine or U46619-induced contractions in mouse corpus cavernosal smooth muscle. Phenylephrine elicited a concentration-dependent contraction curve in mouse cavernosal muscle strip with an EC50 of 0.27 µM. The maximum response to phenylephrine is 94 ± 6% of KCl-induced contraction. Gö6976 failed to alter the contractile actions of phenylephrine at the concentration (10 µM) that abolished the phenylephrine-induced contraction in mouse aorta (Fig. 2A). Pre-incubation with chelerythrine (30 µM) also had no significant effect on the contractions caused by phenylephrine (Fig. 2B). Moreover, the contractile responses to U46619 (EC50=0.081 µM) were unchanged in the corpus cavernosal smooth muscle strips in the presence of Gö6976 (10 µM) and chelerythrine (30 µM) (Fig. 2C and 2D).
Fig. 2. Effects of PKC inhibitors on agonist-induced contraction in mouse corpus cavernosum.
(A) Pre-treatment of Gö6976 did not alter phenylephrine-induced contraction (■: control; ▲: pre-treated with 10 µM of Gö6976). n=6. (B) Pre-treatment of chelerythrine had no significant effect on the contractile responses to phenylephrine. (■: control; ▲: pre-treated with 30 µM of chelerythrine). n=4. (C) Gö6976 had no effect on U4419-induced contraction (■: control; ▲: pre-treated with 10 µM of Gö6976). n=4. (D) Chelerythrine had no effect on the contractions-induced by U44619 (■: control; ▲: pre-treated with 30 µM of chelerythrine). n=4.
3.3. Effects of PKC inhibitors on mouse aorta and corpus cavernosum in the presence of L-NAME
Nitric oxide (NO), a major vasodilator, is constantly produced by endothelial cells in the aorta and the penis. To investigate whether the basal level of NO production influences the efficacy of PKC inhibitors, mouse aortic rings or cavernosal smooth muscle strips were pre-incubated with either L-NAME (500 µM) only or L-NAME plus a PKC inhibitor. In mouse aorta, pre-incubation of L-NAME only significantly increased phenylephrine-induced maximum contraction (126 ± 8% of control, P<0.01, data not shown). The inhibitory effects of Gö6976 (10 µM) and chelerythrine (30 µM) on phenylephrine-induced contraction were markedly decreased in the presence of L-NAME (Fig. 3A). However, L-NAME did not affect phenylephrine-induced maximum contraction in mouse cavernosal smooth muscle strips (99 ± 5% of control, data not shown). This suggests that unlike in aorta, the basal release of NO in cavernosal strips is insignificant. Gö6976 or chelerythrine did not alter the contractile response to phenylephrine in cavernosal smooth muscle in the presence of L-NAME (Fig. 3B), suggesting that NO does not cause the lack of inhibitory effects by the PKC inhibitors.
Fig. 3. Effects of PKC inhibitors on phenylephrine-induced contraction in the presence of the L-NAME.
(A) Aortic rings were pre-incubated with L-NAME (500 µM) only (■), L-NAME with Gö6976 (▲), or L-NAME with chelerythrine (▼) for 20 min. n=4, ** P<0.01 vs. control. (B) Corpus cavernosal smooth muscle strips were pre-incubated with L-NAME (500 µM) only (■), L-NAME with Gö6976 (▲),or L-NAME with chelerythrine (▼) for 20 min. n=4.
3.4. Effects of a PKC activator on mouse aorta and corpus cavernosum
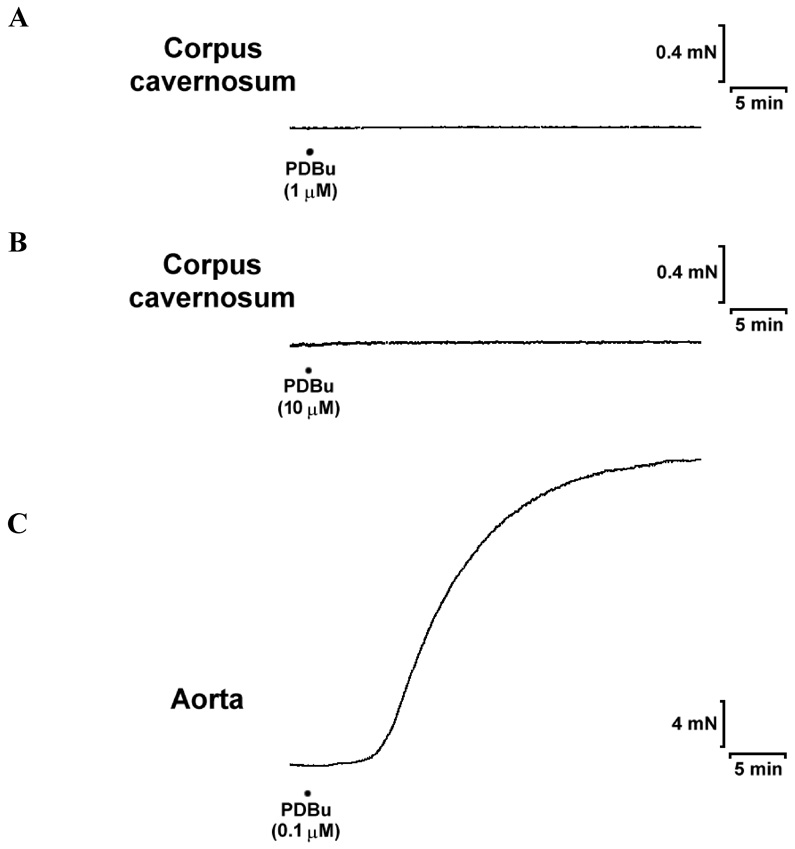
To further compare the role of PKC in mediating contractions in mouse aorta and corpus cavernosum, the effects of a specific activator of PKC, PDBu, were examined. The addition of PDBu (0.1 µM) caused a strong and sustained contraction in the aorta, and the maximum contraction reached to 208 ±14% of KCl-induced maximum contraction. However, PDBu failed to induce contraction in the cavernosal smooth muscle strips even when the concentrations were increased to 1 or 10 µM (Fig. 4).
Fig. 4. Effects of PDBu on the contractility of mouse aorta and corpus cavernosum.
Panels (A) and (B) show the representative tracings obtained in corpus cavernosal strips after stimulation with PDBu at 1 and 10 µM,respectively. PDBu failed to cause contractions at both concentrations. n=4. Panel (C) shows the representative tracing obtained in aortic rings after stimulation with 0.1 µM of PDBu. The maximum contraction is 208 ±14% KCl-induced contraction. n=4.
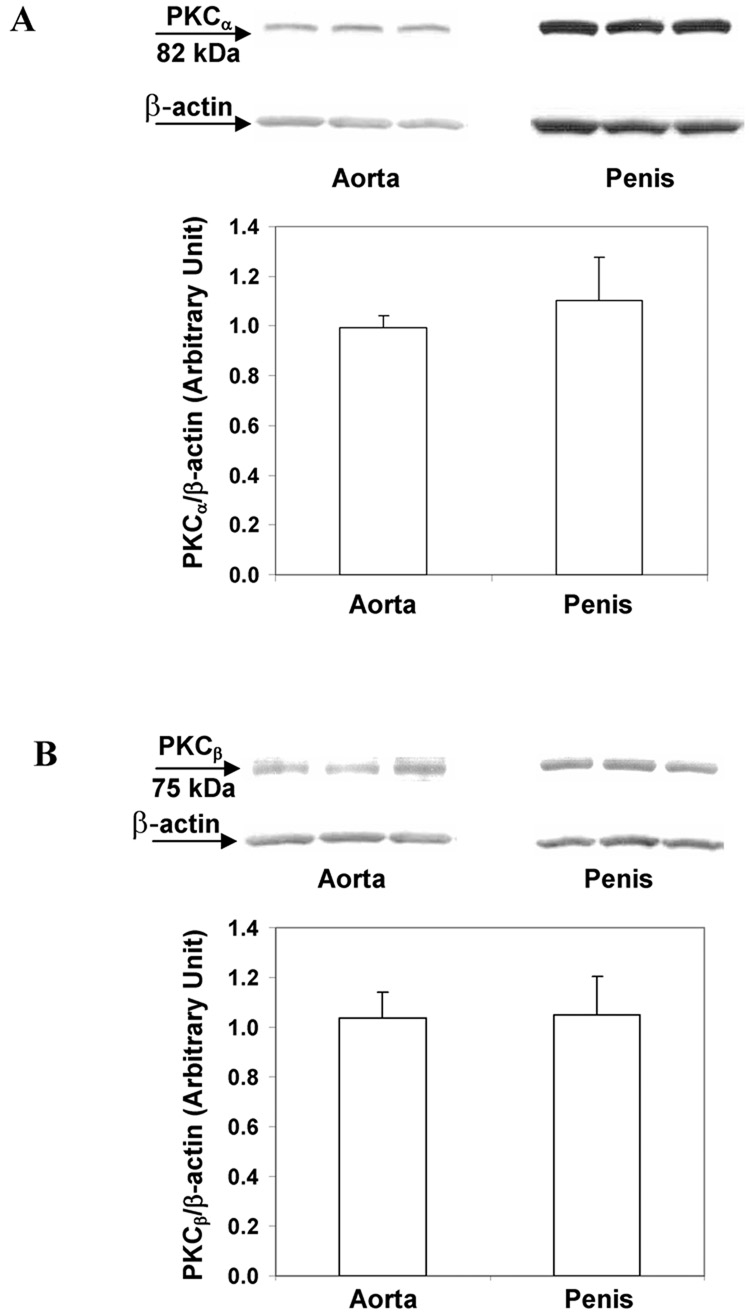
3.5. PKC protein expression in mouse aorta and corpus cavernosum
To determine whether the PKC’s differential mediation of agonist-induced contractions in mouse aorta and corpus cavernosum is due to different levels of protein content, we determined and compared PKC protein expression in those tissues. Data from Western blot analysis suggested that both PKCαand PKCβ were expressed in the aorta and corpus cavernosum. The levels of PKC protein expression were similar in mouse aorta and corpus cavernosum after normalization by β-actin protein level (Fig. 5).
Fig. 5. PKC protein expressions in mouse aorta and corpus cavernosum.
(A) A representative immunoblot of PKCα protein expression from 3 mice and the summary of data. n=5. (B) A representative immunoblot of PKCβ protein expression from 3 mice and the summary of data. n=7. Protein expressions of PKC were normalized by β-actin level.
4. DISCUSSION
In response to receptor activation, contraction in most types of smooth muscle involves an increase in intracellular Ca2+ levels and enhancement of Ca2+ sensitivity. Activation of G protein coupled receptor by agonists increases phospholipase activity and sequentially inositol phospoholipid hydrolysis, leading to the formation of inositol trisphosphate (IP3) and diacylglycerol (DAG). IP3 increases intracellular Ca2+ release from sarcoplasmic reticulum, which thereby increases Ca2+-dependent PKC activity; meanwhile, DAG is able to activate PKC through direct physical binding. Evidence suggests that PKC mediates Ca2+ sensitization pathways and increases smooth muscle contraction either by inhibition of myosin light chain phosphatase or by inhibition of actomyosin ATPase (Somlyo and Somlyo, 2003; Wier and Morgan, 2003).
The study by Budzyn et al. (Budzyn et al., 2006) suggests that the degree to which PKC is involved in contractile response depends upon the sizes of arteries. The contribution of PKC to agonist-induced contractions is increased when the size of arteries is decreased. In our study, we compared the roles of PKC in mediating contractile response in different tissues. We found that PKC inhibitors significantly decreased phenylephrine-and U46619-induced contractions in mouse aorta, suggesting an important role for PKC in mediating aortic smooth muscle contraction. However, PKCα/βmay play a less significant role in U46619-induced contraction, since Gö6976, a PKCα/βselective inhibitor, was less effective in reducing the contractions. Consistent with our observations, previous studies have shown that PKC mediates the contractile responses upon agonist stimulations in various arteries, including aorta, mesenteric, femoral, and tail arteries isolated from rat, canine, or guinea pig (Jiang et al., 1994; Merkel et al., 1991; Sato et al., 2001; Shima and Blaustein, 1992). In addition, data from us and other groups show that PKC activators have potent contractile effects on aortic smooth muscle. It has been suggested that abnormally enhanced PKC activity contributes to increased vascular reactivity in disease states. For example, the response to phorbol esters is greater in the arteries from hypertensive animals as well as diabetic animals when compared to those in their normal counterparts (Bilder et al., 1990; Suzuki et al., 1994; Turla and Webb, 1987; 1991; White and Carrier, 1990). In diabetic mouse aorta, increased phenylephrine-induced contractions were abolished by chronic treatment with a PKCβselective inhibitor, suggesting that PKC activity is responsible for the increased agonist-induced contraction in diabetic aorta (Nangle et al., 2003).
On the other hand, little is known about the role of PKC in penile tissue, such as the effects of PKC on agonist-induced corpus cavernosal smooth muscle contraction. Studies done by Holmquist et al. (1990) showed that application of a PKC inhibitor, H7, abolished the endothelin-1-induced contraction in rabbit corpus cavernosum in the Ca2+ free medium. These observations indicate that PKC may be involved in Ca2+ sensitization mechanisms of endothelin-1-induced contraction. However, the authors concluded that the non-specificity of H7, which also inhibits cyclic AMP or cyclic GMP dependent protein kinases, limited the value of these observations. Nangle et al. (Nangle et al., 2003) reported that the contractile responses to phenylephrine were unchanged in cavernosal muscle strips isolated from diabetic mice or diabetic mice chronically treated with a PKCβ inhibitor when compared to those from the control mice. Because there was no comparison made to the cavernosal muscle strips from control mice treated with the PKCβ inhibitor, it is not clear whether the lack of changes in phenylephrine-induced contractions in diabetic cavernosum or PKCβinhibitor-treated diabetic cavernosum is because PKC does not mediate agonist-induced contractions in erectile tissue.
In the present study, we found that PKC inhibitors had no effects on phenylephrine-or U46619-evoked contractions and PDBu failed to induce contractions in mouse corpus cavernosal smooth muscle strip. Furthermore, inhibition of NO synthase by L-NAME did not change the contractile responses in the presence of PKC inhibitors. Cavernosal smooth muscle strips pre-incubated with L-NAME did not response to PDBu either (data not shown). These data suggest that PKC plays a minimal role in contractile responses to these agonists in mouse corpus cavernosum. Consistent with our findings, recent studies done by Angulo et al. (Angulo et al., 2006) showed that contractile response to U46619 in non-diabetic human cavernosal muscle was not altered by PKC inhibition. Interestingly, their results suggest that PKC is responsible for the increased contraction induced by U46619 in diabetic human cavernosal muscle because the PKC inhibitor completely reversed this hypersensitive response. Therefore, it is likely that PKC is not involved in the regulation of agonist-induced contraction in normal mouse or human cavernosal tissue, while activation of PKC may be an underlying mechanism for increased contractility of caverosal tissue in disease state.
In rat corpus cavernosum, PKC was suggested to mediate phenylephrine-induced contraction, yet the potency of the PKC inhibitor, chelerythrine, was not compared between rat aorta and corpus cavernosum (Husain et al., 2004). The different effect of PKC on corpus cavernosal contraction probably is due to species difference.
In conclusion, our study provides the pharmacological characterizations of PKC in agonist-induced contractions in both mouse corpus cavernosum and mouse aorta. Our data suggest that activation of PKC is required for agonist-induced contractions in mouse aorta; however, PKC is not involved in corpus cavernosal smooth muscle contractile responses to the agonists. Further investigations are needed to examine the functions of PKC in penile tissue and its role in erectile dysfunction.
ACKNOWLEDGMENT
This study is supported by grants from the American Heart Association (0530007N to LJ) and the National Institutes of Health (DK-73531 to LJ and HL-74167 to RCW). CET was funded by a Postdoctoral Fellowship from the American Heart Association Southeast Affiliate. In the memory of Cleber E. Teixeira, an inspiring young scientist and a wonderful friend and colleague.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Andersson KE, Wagner G. Physiology of penile erection. Physiol Rev. 1995;75:191–236. doi: 10.1152/physrev.1995.75.1.191. [DOI] [PubMed] [Google Scholar]
- Angulo J, Cuevas P, Fernandez A, Allona A, Moncada I, Martin-Morales A, La Fuente JM, de Tejada IS. Enhanced Thromboxane Receptor-Mediated Responses and Impaired Endothelium-Dependent Relaxation in Human Corpus Cavernosum from Diabetic Impotent Men: Role of Protein Kinase C Activity. J Pharmacol Exp Ther. 2006;319:783–789. doi: 10.1124/jpet.106.108597. [DOI] [PubMed] [Google Scholar]
- Bilder GE, Kasiewski CJ, Perrone MH. Phorbol-12,13-dibutyrate-induced vasoconstriction in vivo: characterization of response in genetic hypertension. J Pharmacol Exp Ther. 1990;252:526–530. [PubMed] [Google Scholar]
- Budzyn K, Paull M, Marley PD, Sobey CG. Segmental differences in the roles of Rho-kinase and protein kinase C in mediating vasoconstriction. J Pharmacol Exp Ther. 2006;317:791–796. doi: 10.1124/jpet.105.100040. [DOI] [PubMed] [Google Scholar]
- Burnett AL. General use of animal models for investigation of the physiology of erection. Int J Impot Res. 2001;13:135–139. doi: 10.1038/sj.ijir.3900678. [DOI] [PubMed] [Google Scholar]
- Disanto ME. Contractile mechanisms in diabetes-related erectile dysfunction. Curr Pharm Des. 2005;11:3995–4010. doi: 10.2174/138161205774913417. [DOI] [PubMed] [Google Scholar]
- Giuliano F. Rodents in impotence research: functional and genetic aspects. Int J Impot Res. 2001;13:143–145. doi: 10.1038/sj.ijir.3900680. [DOI] [PubMed] [Google Scholar]
- Husain S, Young D, Wingard CJ. Role of PKCalpha and PKCiota in phenylephrine-induced contraction of rat corpora cavernosa. Int J Impot Res. 2004;16:325–333. doi: 10.1038/sj.ijir.3901164. [DOI] [PubMed] [Google Scholar]
- Jiang MJ, Chan CF, Chang YL. Intracellular calcium and myosin light chain phosphorylation during U46619-activated vascular contraction. Life Sci. 1994;54:2005–2013. doi: 10.1016/0024-3205(94)90135-x. [DOI] [PubMed] [Google Scholar]
- Jin L, Burnett AL. RhoA/Rho-kinase in erectile tissue: mechanisms of disease and therapeutic insights. Clin Sci (Lond) 2006;110:153–165. doi: 10.1042/CS20050255. [DOI] [PubMed] [Google Scholar]
- Jin L, Liu T, Lagoda GA, Champion HC, Bivalacqua TJ, Burnett AL. Elevated RhoA/Rho-kinase activity in the aged rat penis: mechanism for age-associated erectile dysfunction. Faseb J. 2006;20:536–538. doi: 10.1096/fj.05-4232fje. [DOI] [PubMed] [Google Scholar]
- Merkel LA, Rivera LM, Colussi DJ, Perrone MH. Protein kinase C and vascular smooth muscle contractility: effects of inhibitors and down-regulation. J Pharmacol Exp Ther. 1991;257:134–140. [PubMed] [Google Scholar]
- Nangle MR, Cotter MA, Cameron NE. Protein kinase C beta inhibition and aorta and corpus cavernosum function in streptozotocin-diabetic mice. Eur J Pharmacol. 2003;475:99–106. doi: 10.1016/s0014-2999(03)02113-7. [DOI] [PubMed] [Google Scholar]
- Salamanca DA, Khalil RA. Protein kinase C isoforms as specific targets for modulation of vascular smooth muscle function in hypertension. Biochem Pharmacol. 2005;70:1537–1547. doi: 10.1016/j.bcp.2005.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K, Dohi Y, Suzuki S, Miyagawa K, Takase H, Kojima M, van Breemen C. Role of Ca2+-sensitive protein kinase C in phenylephrine enhancement of Ca2+ sensitivity in rat tail artery. J Cardiovasc Pharmacol. 2001;38:347–355. doi: 10.1097/00005344-200109000-00003. [DOI] [PubMed] [Google Scholar]
- Shima H, Blaustein MP. Contrasting effects of phorbol esters on serotonin- and vasopressin-evoked contractions in rat aorta and small mesenteric artery. Circ Res. 1992;70:978–990. doi: 10.1161/01.res.70.5.978. [DOI] [PubMed] [Google Scholar]
- Somlyo AP, Somlyo AV. Ca2+ Sensitivity of Smooth Muscle and Nonmuscle Myosin II: Modulated by G Proteins, Kinases, and Myosin Phosphatase. Physiol Rev. 2003;83:1325–1358. doi: 10.1152/physrev.00023.2003. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Takata Y, Kubota S, Ozaki S, Kato H. Characterization of the alpha-1 adrenoceptors in the mesenteric vasculature from deoxycorticosterone-salt hypertensive rats: studies on vasoconstriction, radioligand binding and postreceptor events. J Pharmacol Exp Ther. 1994;268:576–583. [PubMed] [Google Scholar]
- Turla MB, Webb RC. Enhanced vascular reactivity to protein kinase C activators in genetically hypertensive rats. Hypertension. 1987;9:150–154. doi: 10.1161/01.hyp.9.6_pt_2.iii150. [DOI] [PubMed] [Google Scholar]
- Turla MB, Webb RC. Vascular responsiveness to protein kinase C activators in mineralocorticoid-hypertensive rats. J Hypertens. 1991;9:209–215. doi: 10.1097/00004872-199103000-00003. [DOI] [PubMed] [Google Scholar]
- White RE, Carrier GO. Vascular contraction induced by activation of membrane calcium ion channels is enhanced in streptozotocin-diabetes. J Pharmacol Exp Ther. 1990;253:1057–1062. [PubMed] [Google Scholar]
- Wier WG, Morgan KG. Alpha1-adrenergic signaling mechanisms in contraction of resistance arteries. Rev Physiol Biochem Pharmacol. 2003;150:91–139. doi: 10.1007/s10254-003-0019-8. [DOI] [PubMed] [Google Scholar]