Abstract
Aging is the common denominator and the highest risk factor for macular degeneration and Alzheimers Disease (AD). Important pathological hallmarks common to both diseases are the presence of amyloid β (Aβ) in the senile plaques of the AD brain and in the drusen of age-related macular degeneration (AMD) patients, oxidative stress, and apoptotic cell death. Data suggest that a common pathogenic mechanism might exist between AMD and AD. Brain and eye depend on redox electrons from pyridinic and flavinic nucleotides to produce ATP, and reactive oxygen intermediates (ROI). Disorganization of mitochondrial structure and decline in mitochondrial oxidative phosphorylation (OXPHOS) functioning, as well as hypometabolism and alterations in mitochondrial DNA are aging features. Because ROI damage and mitochondrial dysregulation are prominent in AMD and AD and their relationship to the redox state is unclear we addressed a new hypothesis according to which the interaction of melatonin vs Aβ are intertwined to balance of the intra- and extra-mitochondrial energy production. This balance would be impaired by the ageing process and environmental/genetic factors, ultimately leading to AD and /or AMD.
Key Words: Malatonin, Age related macular degeneration, Alzheimers
INTRODUCTION
Aging is a common risk factor in both age-related macular degeneration (AMD) and Alzheimer's disease (AD). The incidence of AMD is increasing in the aging population. The World Health Organization has stated that AMD is the most common form of blindness, with 1.75 million people in the US alone and 7 million people at risk [1]. AD is the most common dementia, doubling every 6 years after the age of 65. In Western countries, AD affects 1–3% of people aged 60–64 years, and 3–12% of people aged 70–80 years. It is estimated that by the mid-century (2050) as much as 13,2 million people will be affected by AD in the US alone [2]. At the molecular level the pathognostic feature of AD is the accumulation of the 39-4 amino acid long β-Amyloid (Aβ) peptide with more that 50% of autopsy cases showing positive correlation. Aβ is also deposited in Drusen in AMD [3]. A prospective population-based Rotterdam Study found that the neuronal degeneration occurring in AMD and AD may, to some extent, represent an evidence of a possible epidemiological connection between the two diseases, but with different origin as for genetic risks [4]. Interestingly, there is a slight prevalence of the female gender towards AMD [5] and AD [6,7]. AMD and AD appear linked because the retina is part of the brain [8], deriving from the neural tube which is the precursor of CNS development; moreover, both have blood–tissue barriers. At present, chronic oxidative stress, inflammation and altered fatty acid metabolism are strongly linked to AMD [9,10] and also to AD pathogenesis [11,12,13,14].
Aging and the mitochondrion
CNS and retina critically depend on oxygen (O2) supply [15], [16 ] and are sensitive to mitochondrial dysfunction [17 ]. However, mitochondrial disorders, human diseases characterized by genetic defects of the oxidative phosphorylation (OXPHOS), affect the visual and the nervous system, even though these display a relative scarcity of mitochondria [17] . Mitochondrial dysfunctions are involved in pathologies associated with many diseases, such as, cancer, neurodegenerative diseases and aging. Aging is an incompletely understood process, in which a decline in mitochondrial function seems to be involved [18].
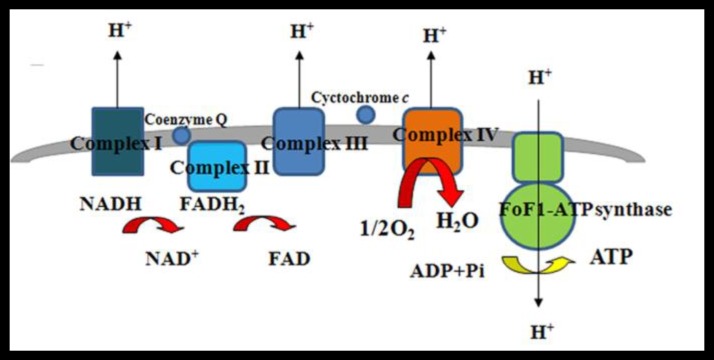
Mitochondria display two membranes, the outer membrane allowing the passage of low molecular-weight substances thanks to porin expression, [19], and the inner membrane (IM) housing the electron transfer chain (ETC) and providing a highly efficient barrier to the ion flow. The IM forms invaginations called cristae where the ETC complexes I-IV are embedded. These enable the transfer of electrons from NADH and FADH synthesized by glycolysis, fatty acid oxidation and Tricarboxylic acid cycle (TCA) to reduce molecular O2 to water [20]. During electron transfer, energy is used to pump protons in the intra membrane space, which promotes ATP generation via OXPHOS, thanks to the nanomotor ATP synthase (complex V). Proton gradient generates a chemiosmotic proton potential driving ADP phosphorylation of ADP to ATP (Figure 1).
Figure 1.
A schematic of the electron transport chain occurring in the inner mitochondrial membrane
In yeast, oligomeric organization of ATP synthase was reported to be essential to the maintenance of the mitochondrial cristae architecture and to correlate with maximum energy conversion capability [21], with an age-associated decline in ATP synthase oligomers. Prior data [20,22,23,24] suggest that the electron transport chain (ETC) and F1Fo-ATP synthase are functionally expressed in extramitochondrial locations of the central nervous system, i.e: rod outer segment (OS) disks and isolated myelin vesicles [ 22, 23, 24]. While the mitochondrial proteome consists of more than 1,000 different proteins, many proteomic analyses of cellular membranes have found the exclusive expression of proteins from the five respiratory complexes [ (reviewed in Panfoli e al. (25)]. Moreover, the enzymes of the Tricarboxylic Acid (TCA) Cycle are catalytically active in the rod outer segment [20 ], in keeping with the knowledge that many mitochondrial proteins possess dual or multiple localization [26] and that mitochondria are dynamic organelles, [27] . Ex vivo staining of the optic nerve and retina [22,23] with MitoTracker (MT), a fluorescent mitochondrial probe sensitive to proton potential , showed that a proton potential is present in rod OS [28]. Mitochondria are currently believed to be central to a both life and death processes, such as energy production, and generation of reactive oxygen intermediates (ROI). However ROI would also be generated by the ectopic ETC coupled to ATP synthase. In fact an ETC not adequately coupled may generate ROI in turn oxidizing the polyunsaturated fatty acids of which the rod OS is rich.
Aging is consistently related to oxidative damage of cellular macromolecules due to ROI production [26, 29]. Impairment in mitochondrial OXPHOS functioning, alterations in mitochondrial DNA (mtDNA), increased production of ROI, with disorganization of mitochondrial structure have been reported with aging [30 ]. During the electron transfer 0.4% to 5% of ETC participate in the formation of superoxide radicals (O2•-) [31], therefore ROI are a physiological by-product of the ETC. However, an increase in O2•- production can activate the mitochondrial permeability transition pore [32], ultimately committing cell to death by apoptosis. A study by Ghosh et al [.] showed that a redox shift precedes ROI changes in 3xTg-AD mice, i.e. a more oxidized redox state and a lower antioxidant GSH defense precedes neuronal damage, and the onset of cognitive defects. This means that even before cells accumulate harmful free radicals, they have changes in their reduction-oxidation reactions (redox). These results would explain why synapses go haywire long before people with Alzheimer’s disease experience any problems with memory [13]. The findings that the “ redox shift precedes ROI changes‘’ in AD mice directly points mostly to our hypothesis showing that the melatonin-Aβaxis may alter mitochondrial energy balance during aging leading to AMD and or AD. Mitochondrial DNA polymorphisms that augment ATP production can reduce Aβ load in mice [34]. It was reported that mitochondrial DNA (mtDNA) mutations can promote aging also independently of enhanced ROI production [35] accumulation of mutations in mtDNA [35]. These were in turn associated to reduced life span, and to aging signs.
In aging the ETC enzyme activity decrease, along with mitochondrial membrane potential. Parallely mitochondrial proteins and mtDNA are oxidatively damaged and there is a quantitative increase in mtDNA mutations. For example, Liang FQ et al., 2003 [36] showed that, when exposed to H2O2, human retinal pigmented epithelium (RPE) cells or rod outer segments display mtDNA but not nDNA damage. Authors concluded that the susceptibility of mtDNA to oxidative damage, and decreased anti-oxidant system capability provides a rationale for mitochondria based model of AMD [37, 38]. Using the same rationale Liang FQ et a., 2004 [37] observed that RPE cells pretreated with melatonin show a significant decrease in mtDNA damage. Another pathway to mitochondrial damage is through the action of oligomeric Aβ to induce alterations of intracellular Ca(2+) levels and to promote the excess accumulation of intracellular Ca(2+) into mitochondria, thus inducing the mitochondrial permeability transition pore opening [31]. Increasing evidence suggests that the amyloid precursor protein (APP) and Aβ accumulate in mitochondrial membranes, cause mitochondrial structural and functional damage by generating ROI, hindering normal neuronal functioning [39,40]. Inhibition of ATP synthase inhibits the electron transport and OXPHOS. Such inhibition can be induced by Aβ [41]. Rhein et al, 2009 [42] reported that Aβ also lead to impaired functions of the mitochondria in human neuroblastoma cells.
Drusen and Amyloid plaques, different but the same?
Extracellular protein deposits called drusen, accumulating between the RPE and photoreceptors, are a typical feature of non-neovascular AMD [43]. Drusen area and size positively correlate to risk of AMD progression [44]. Drusen are composed of acute phase proteins, complement components, proteglycans, apolipoproteins, metal ions (Fe, Zn, Cu), proteases ,cholinesterases, lipids [16,17,22], polysaccharides and ATP synthase subunit β [45] Some of these components are made by the eye itself, i.e. retina, RPE and/or choroid [46]. Wang and Wang [47] showed that the most abundant molecules in Drusen where esterified cholesterol and phosphatidine choline which suggest abnormalities in the metabolism of cholesterol, a risk factor also in AD [48]. Isas et al, 2010 [49], found that among the amyloid forms (oligomers, protofibrils, fibrils) the non-fibrilar oligomers where the most abundant form of amyloid in Drusen. Recently, amyloid vesicles as forms pervading in Drusen have also been reported in brains [50] of transgenic mice expressing human APP, suggesting the importance of APP processing in both eye and brain. Aβ accumulation has also been demonstrated in association with drusen in eyes from AMD patients [51, 52, 53] mice models for AMD [50] and in RPE [3]. Recently, Barrett et al., 2012 [54] showed that cholesterol directly binds to the C99 fragment of APP. This fragment, the result of β-secretase cleavage, is important for AD pathology because it is cleaved by γ-secretase to release Aβ.
A causative role of oxidative stress and light exposure in the pathogenesis of AMD and other retinal degeneration has also been proposed [55 ] [56 ] [57] [58] [59] [60]. A critical role of SOD1 in protecting from AMD has been reported [61]. The choroid and retina are the highest oxygen-consuming tissues in the human body. The OS expressing oxygen-absorbing cytochrome c oxidase [22], would be at risk of oxidative stress oxidizing disk membranes, that contain high levels of polyunsaturated fatty acids. ROI are in fact a by-product of the ETC [17] [62] [63]. The result may be photoreceptor loss and visual impairment [64]. Inflammatory responses secondary to oxidative stress have been involved in age-related degenerative diseases. Oxidative stress induces the assembly of inflammatory protein complexes, the so-called inflammasomes, involving nod-like receptor protein 3 (NLRP3) [12]. The inflammasomes recognize danger signals, such as metabolic stress from ROI production, triggering inflammatory responses [12]. It was reported that misfolded protein aggregates such as amyloid-β can trigger NLRP3 inflammasome representing a pathogenetic mechanism in AD. Damaged mitochondria undergo digestion through mitophagy, a specialized form of autophagy, whose impairment may cause aging [65]. Autophagic capacity seems to be compromised in AD [66] and AMD [67]. Melatonin exerting its activity on Aβ in inflammation was presented by the work of Zhou et al. [68.] who found that microglia. i.e. the phagocytes of the nervous system, decrease superoxide anion production by impairing NADPH oxidase assembly in cultures of microglia.
APP/Aβ metabolism in the Eye and Brain
Characteristic pathological features of AD are cerebral plaques with β-amyloid peptide and neurofibrillary tangles. However, as Aβ and tangles appear a normal finding in brains of non-demented individuals, these may be related to brain aging, independently of AD, suggesting their wider hypometabolic origin. The 2011 AD criteria proposes the presence of low CSF Aβ and decreased glucose utilization as AD biomarkers. Aβ is in small amount deposited in the brain [69] and in normal retina [51, 70] and the levels of these deposits increase during aging [71]. Johnson et al. [51] where the first to propose the pathogenic role of Aβ in AMD. Activated component complement component of RPE deposits where co localized to Aβ detected by using immunohistochemical technique. It was shown that Aβ can be detected in sub RPE basal deposits and neurovascular lesions in murine model of AMD [72]. Accumulation of Aβ in the eye occurs primarily among the photoreceptor OS and in the interphase between the RPE and Bruch’s membrane. Indeed, an origin of drusen in OS has never been supposed, but considering their ability to manipulate O2 should be taken into consideration. Such accumulation of Aβ on photoreceptor outer segments with age was confirmed in human retina using immunohistochemistry [71]. This implies that the accumulation of Aβ is associated with efficiency of RPE phagocytic process [3], but also through APP metabolism [73]. Sarangarajan and Apte [74] showed that signaling pathways that upregulate melanization in the RPE may be implicated in down-regulation of the rod OS phagocytosis by RPE, maintaining a balance between ingestion and degradation/recycling lowering metabolic load, suggesting a possible Aβ vs melatonin/melanin interaction in the balance of mitochondrial energy metabolism. Yoshida et al. [75] showed that human RPE expresses constitutively all of the genes that regulate Aβ production ,e.g., APP,α ,β,γ secretase and neprylisin.
Melanization activating pathways may also modulate O2 consumption by the photoreceptors, and the rate of photoisomerization events such that the net effect may be a reduction in drusen and/or lipofuscin accumulation. This interaction may play a role in decreasing choroidal neovascularization. The hormone melatonin may have regulatory effects on APP metabolism. Interestingly, melatonin plays a fundamental role in retinogensis through APP metabolism [10,73,75]. Cultured RPE cells exposed to Aβ increase the expression of VEGF and decrease Pigment Epithelium-derived factor (PEDF, a potent antiangiogenic factor). Balance between these two molecules are important for healthy retina [76].
Melatonin treatment inhibited normal levels of secretion of soluble APP (sAPP) in different cell lines by interfering with APP full maturation [77]. Melatonin also affects the mRNA level of APP in a cell type-specific manner. Additionally, administration of melatonin efficiently reduced Aβ generation and deposition both in vivo [78, 79] and in vitro [77]. Moreover, it has been reported that mitochondrial dysfunction is characteristic of Aβ-induced neuronal toxicity in AD. A mitochondrial cascade hypothesis was proposed postulating that Aβ production, and tau phosphorylation, are consequences of impaired mitochondrial function and hypometabolism. Interestingly, the activity of mitochondrial enzymes (such as pyruvate- and ketoglutarate-dehydrogenase) as well as of some respiratory complexes (NADH:ubiquinone oxidoreductase, complex I, and cytochrome oxidase; complex IV, both partly coded by mitochondrial DNA) are reduced in mitochondria from AD subjects.
HYPOTHESIS
Considerng the findings of Panfoli et al., [25, 60] and others [42, 31, 41, 80], the present paper proposes the hypothesis of a role for melatonin-Aβ axis in mitochondria, and that the interaction of melatonin vs Aβ are intertwined to the balance of the inter and extra mitochondrial energy production. This balance would be deregulated by the ageing process and other environmental/genetic factors, in turn leading to hypometabolism and neurodegenerative diseases characterized by protein deposition, such as AD and /or AMD.
Evaluation and Disscussion of the Hypothesis
Cumulative oxidative status plays a critical role to AMD and AD, both age related disorders [61]. A large gradient of oxygen towards the inner retina [81] fits with an extra mitochondrial respiration [22]. Panfoli et al. [60] proposed a bioenergetic hypothesis drusen, which may originate through hypometabolism , in turn imbalancing clearance of proteins causing aggregation of peptides that accumulate [60]. In fact the OS, that contains high levels of polyunsaturated fatty acids and expresses oxygen-absorbing OXPHOS machinery [82] , outside mitochondria, is at risk of oxidative stress. ROI are in fact a by-product of the ETC [17] [62] [63]. ROI in turn may cause damage to RPE, increase the production of VEGF (Vascular Endothelial Growth Factor). Interestingly, Biochemical and histochemical analyses demonstrated that the labeled protein accumulating in the cytosol of Alzheimer degenerating neurons is the α-chain of the ATP synthase [83 ]. It is specifically observed in degenerating neurons, either alone or tightly associated with aggregates of tau proteins, suggesting that it is a new molecular event related to neurodegeneration. This may be the initiating factor in retinal degenerative diseases, but also in AD, both characterized by extracellular deposits of proteins. Extensive literature demonstrate melatonin antioxidant capacity [84 and refs. therein] both in vivo and in vitro. Its major action is maintenance of mitochondrial protein homeostasis.
Interestingly, a modified model of the mitochondrial hypothesis for AD has been proposed, in which Aβ would cause neurotoxicity by interacting with mitochondrial targets or being itself intramitochondrial [85 ]. To strengthen the extramitochondrial idea, Schmidt et al. [86] showed in vitro that ATP synthase subunit α is a binding partner for APP and Aβ on the surface of cultured hippocampal neurons and astrocytes indicating regulation of extracellular ATP levels in the brain. Human drusen were found to contain Aβ and this was interpreted as an indication that the pathogenic pathways giving rise to drusen and AMD may be common in neurodegenerative diseases characterized by misfolded protein aggregation [53]. San Li Xing et al., 2012 [41] showed in amyloid precursor protein/presennillin-1 transgenic mice that the α-subunit of ATP synthase is associated with aggregates of Aβ proteins in amyloid plaques and when extracellular ATP generation was analyzed a inhibition pattern was observed by the aggregating Aβ peptide but not the level of ATP synthase subunit alpha on neurons. Chronic exposure to soluble Aβ may result in an impairment of energy homeostasis due to a decreased respiratory capacity of mitochondrial electron transport chain which, in turn, may accelerate neurons demise [41].
We have addressed that Aβ is a pathological component in AD and AMD and that Aβ and APP can be addressed to the mitochondrion. In respect to new insights of the extramitochondrial role in energy production for eyesight [20,25] in the OS of rods and that Aβ directly binds to theα subunit of the ATP synthase at the neuronal membranes and the demonstration of a number of complexes to capture and direct electrons and protons in the cell, melatonin shows probably a primary constituent in balancing the energy production in mitochondrial by acting upon the production of Aβ.
In the introduction we showed that melatonin regulates APP metabolsim and can efficiantly protect cells against Aβ toxicity, oxidative damage and cell death in vitro and in vivo [47]. A recent study showed that, chronic melatonin therapy in old Tg2576 mice initiated at 14 months of age failed to remove existing plaques, but also to prevent additional Aβ deposition [87]. Data on a diminished Aβ in melatonin-treated wild type mice [88] and reduced Aβ and protein nitration in melatonin treated Tg2576 mice also exist [89]. However, both studies concur in finding little evidence of the potent antioxidant effects of melatonin in the oldest mice. These findings indicate that melatonin has the ability to regulate APP metabolism and prevent Aβ pathology, but fails to exert anti-amyloid or antioxidant effects when initiated after the age of Aβ deposition. Although consistent conclusions were achieved, none of the related studies further explained how melatonin exerts its inhibitory effect on Aβ generation. One explanation of why aged mice are immune to melatonin might be in the process of melanogenesis, i.e. a failure in light/melanin/water system would be a cause rather than effect of AD has been proposed [90]. The decrease in melanins ability to dissociate water (human photosynthesis) in AMD [91] and or AD has been proposed to be a cause of these diseases is a simplistic overview of the bioenegetic mechanism related to these diseases. In our view hypometabolism, likely due to decline in both intra- and extra-mitochondrial OXPHOS functioning, are indeed fundamental to the understanding of pathological processes in these related diseases and that there is a homeostatic mechanism of energy balance related to relationship of melatonin versus Aβ through the regulation of mitochondrial fidelity. Melatonin protective role in AMD and AD may be a result of its action on mitochondrial physiology as suggested by its presence in mitochondrian circadian and seasonal variations in the brain and retina [92]. Locally produced melatonin in the surrounding of photoreceptors protects these cells thanks to its anti oxidant capacity or by activation of melatonin receptors [93]. Melatonin can increase membrane fluidity, as well as the activity of the ETC and ATP production, mitochondria membrane potential, while reducing oxidative stress [94]. Important pathological properties of Aβ, such as neurotoxicity and resistance to proteolytic degradation, depend on the ability of peptides to form β-sheet structures and/or amyloid fibrils [47]. Intervention in the Aβ aggregation process can be considered an approach to stopping or slowing the progression of AD and new investigation AMD. Melatonin can interact with Aβ40 and Aβ42 and inhibit the progressive formation of β-sheet and/or amyloid fibrils[95,96]. Melatonin could promote the conversion of β-sheets into random coils by disrupting the imidazole-carboxylate salt bridges and thus prevent Aβ fibrillogenesis and aggregation. It is therefore possible that by blocking the formation of the secondary β-sheet conformation, melatonin may not only reduce neurotoxicity but also facilitate clearance of the peptide via increased proteolytic degradation.
However, it is difficult to determine the extent of the contribution from each of these properties to the overall effects of melatonin treatment in vivo. In mammals melatonin exerts some of its functions through two specific high-affinity membrane receptors belonging to the superfamily of G-protein-coupled receptors: MT1 and MT2. Decreased MT2 immunoreactivity and increased MT1 immunoreactivity have been reported in the hippocampus of AD patients [97]. Contrary to these findings, a study by Pappolla et al. [98] demonstrated that melatonin protective activities against Aβ toxicity does not require its binding to membrane receptors, which strongly suggests that protection is a result of its antioxidant and anti amyloidegenic features. Melatonin receptors have been found to modulate the visual function in mouse retina [99]. Numerous relationships are shown between melatonin and mitochondria in which protection of ETC proteins are crucial [94]. The hypothesis herein exposed has concentrated on the melatonin-Aβ axis in mitochondrial age related processes leading to AD and AMD. Still, there is a more complex view of this axis which is not in the scope of this paper, i.e. first, melatonin functions exceeds its role as hormone that mediates signal ‘’darkness’’, second melanocytes are viewed as ‘’neurons of the skin’’ with sensory and regulatory properties which can detect and transform external and internal signals/energy into organized regulatory networks for the maintenance of skin homeostasis [100] and melanogenesis and its product melanin is by itself an pigment that has extraordinary properties [101]. The most important property is melanin participation in electron transfer reactions, reducing and oxidizing other molecules. Also, its key monomer, indolequinone, exhibits photodriven proton transfer cycles [102]. Melanin has showed radiotropism, melanized fungi are stimulated to grow in environments with high ionizing radiation, suggesting melanin may function as a broad-band radiation energy harvester, similiar to chlorophyll [103].
In summary, the mitochondrion is the prime cross road enabling electron transfer for all these transfer, and it is reasonable that proton flow may represent a fundamental physical force that sustains, drives, and informs all biological organization and dynamics, Nevertheless, electron driven transport of protons would not be confined to the mitochondrion but it seems to be a fundamental properties of many cell membranes.
CONCLUSION
Both AMD and AD are age-related neurodegenerative diseases. They share similar environmental risk factors thereby comprising smoking, hypertension, hypercholesterolemia, atherosclerosis, obesity, and unhealthy diet [104]. The pathogenesis is associated with increased oxidative stress, and hypometabolism with impaired proteasomal and lysosomal function that evoke formation of intra- and extracellular deposits, drusen, lipofuscin and amyloid plaques, features of both AMD and AD, even though with a different genetic background. These facts imply a role for intra but also for extra-mitochondial OXPHOS.
We have addressed that Aβ is a pathological component in both AD and AMD and that both Aβ and APP can be addressed to the mitochondrion. Moreover, ATP synthase α-subunit was found to be a component of AMD drusen that in turn contain Aβ. New insights on the role of extramitochondrial energy production suggest that it may support visual process [5, 8] in the rod OS and neuronal conduction in myelin vesicles [23, 105] and are consistent with the finding that the α-subunit of ATP synthase is associated with Aβ in Alzheimer's disease [35]. Melatonin seems to be a primary constituent in balancing the energy production in mitochondrial by acting upon the production of Aβ. In fact, melatonin can regulate APP metabolism and efficiently protect cells against Aβ toxicity, oxidative damage and cell death, by interacting with Aβ40 and Aβ42 and inhibit the progressive formation of β-sheet and/or amyloid fibrils [47].
Our hypothesis does to some extent comprise an epigenetic paradigm coupling aging as an underling mechanism of AD and AMD.A genetic background would be a “blue print’’ in which environmental, genetic and bioenergetic factors (intra- and extra-mitochondrial energy production) tending to act upon them, thus leading to AMD and /or AD. There is a direct link between perturbed energy states in neurons and the retina [60] and creatinin and ATP metabolism. Also, there is a direct interaction between APP and the precursor of ubiquitous mitochondrial creatin kinase supporting a relationship between AD, cellular energy levels and mitochondrial function [106]. The same principle is allied to the retina and occurrence of AMD [60]. An understanding of the processes related to extra-mitochondrial and intra-mitochondrial regulation of metabolism in the brain and in retina and their balance by a melatonin-Aβ axis may emerge as new therapeutic pathway for the therapy of both AMD and AD.
DISCLOSURE
The authors report no conflicts of interest in this work.
ACKNOWLEDGMENT
This work is supported by the Ministry of Education and Science of the Republic of Serbia (Grant 173034) for VB, and Athenaeum Research Projects from University of Genoa (Grant CUP D31J11001610005) for IP. The authors declare no conflict of interest.
References
- 1.Gehrs KM, Anderson DH, Johnson LV, Hageman GS. Age-related macular degeneration--emerging pathogenetic and therapeutic concepts. Ann Med. 2006;38(7):450–71. doi: 10.1080/07853890600946724. PMID: 17101537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hebert LE, Scherr PA, Bienias JL, Bennett DA, Evans DA. Alzheimer disease in the US population: prevalence estimates using the 2000 census. Arch Neurol. 2003 Aug;60(8):1119–22. doi: 10.1001/archneur.60.8.1119. PMID: 12925369. [DOI] [PubMed] [Google Scholar]
- 3.Ohno-Matsui K. Parallel findings in age-related macular degeneration and Alzheimer's disease. Prog Retin Eye Res. 2011 Jul;30(4):217–38. doi: 10.1016/j.preteyeres.2011.02.004. PMID: 21440663. [DOI] [PubMed] [Google Scholar]
- 4.Klaver CC, Ott A, Hofman A, Assink JJ, Breteler MM, de Jong PT. Is age-related maculopathy associated with Alzheimer's Disease? The Rotterdam Study. Am J Epidemiol. 1999 Nov;150(9):963–8. doi: 10.1093/oxfordjournals.aje.a010105. PMID: 10547142. [DOI] [PubMed] [Google Scholar]
- 5.Rudnicka AR, Jarrar Z, Wormald R, Cook DG, Fletcher A, Owen CG. Age and gender variations in age-related macular degeneration prevalence in populations of European ancestry: a meta-analysis. Ophthalmology. 2012 Mar;119(3):571–80. doi: 10.1016/j.ophtha.2011.09.027. PMID: 22176800. [DOI] [PubMed] [Google Scholar]
- 6.Bajić VP, Spremo-Potparević B, Zivković L, Bonda DJ, Siedlak SL, Casadesus G, Lee HG, Smith MA. The X-chromosome instability phenotype in Alzheimer's disease: a clinical sign of accelerating aging? Med Hypotheses. 2009 Dec;73(6):917–20. doi: 10.1016/j.mehy.2009.06.046. PMID: 19647374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Damoiseaux JS, Seeley WW, Zhou J, Shirer WR, Coppola G, Karydas A, Rosen HJ, Miller BL, Kramer JH, Greicius MD. Alzheimer's Disease Neuroimaging Initiative. Gender modulates the APOE ε4 effect in healthy older adults: convergent evidence from functional brain connectivity and spinal fluid tau levels. J Neurosci. 2012 Jun;32(24):8254–62. doi: 10.1523/JNEUROSCI.0305-12.2012. PMID: 22699906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dowling JE. The Retina: An Approachable Part of the Brain. Cambridge, MA: Harvard University Press; 1987. pp. 1–282. [Google Scholar]
- 9.Tuppo EE, Arias HR. The role of inflammation in Alzheimer's disease. Int J Biochem Cell Biol. 2005 Feb;37(2):289–305. doi: 10.1016/j.biocel.2004.07.009. PMID: 15474976. [DOI] [PubMed] [Google Scholar]
- 10.Ho T, Vessey KA, Cappai R, Dinet V, Mascarelli F, Ciccotosto GD, Fletcher EL. Amyloid precursor protein is required for normal function of the rod and cone pathways in the mouse retina. PLoS One. 2012;7(1):e29892. doi: 10.1371/journal.pone.0029892. PMID: 22279552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dowling JK, O'Neill LA. Biochemical regulation of the inflammasome. Crit Rev Biochem Mol Biol. 2012 Jun 11; doi: 10.3109/10409238.2012.694844. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 12.Salminen A, Ojala J, Kaarniranta K, Kauppinen A. Mitochondrial dysfunction and oxidative stress activate inflammasomes: impact on the aging process and age-related diseases. Cell Mol Life Sci. 2012 Mar 25; doi: 10.1007/s00018-012-0962-0. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee SH, Kim KR, Ryu SY, Son S, Hong HS, Mook-Jung I, Lee SH, Ho WK. Impaired short-term plasticity in mossy fiber synapses caused by mitochondrial dysfunction of dentate granule cells is the earliest synaptic deficit in a mouse model of Alzheimer's disease. J Neurosci. 2012 Apr;32(17):5953–63. doi: 10.1523/JNEUROSCI.0465-12.2012. PMID: 22539855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ho GJ, Drego R, Hakimian E, Masliah E. Mechanisms of cell signaling and inflammation in Alzheimer's disease. Curr Drug Targets Inflamm Allergy. 2005 Apr;4(2):247–56. doi: 10.2174/1568010053586237. PMID: 15853747. [DOI] [PubMed] [Google Scholar]
- 15.Kann O, Kovács R. Mitochondria and neuronal activity. Am J Physiol Cell Physiol. 2007 Feb;292(2):C641–57. doi: 10.1152/ajpcell.00222.2006. PMID: 17092996. [DOI] [PubMed] [Google Scholar]
- 16.Ames A 3rd. CNS energy metabolism as related to function. Brain Res Brain Res Rev. 2000 Nov;34(1-2):42–68. doi: 10.1016/s0165-0173(00)00038-2. PMID: 11086186. [DOI] [PubMed] [Google Scholar]
- 17.Zeviani M, Di Donato S. Mitochondrial disorders. Brain. 2004 Oct;127(Pt 10):2153–72. doi: 10.1093/brain/awh259. Erratum in: Brain. 2004 Dec;127(Pt 12):2783. PMID: 15358637. [DOI] [PubMed] [Google Scholar]
- 18.Bratic I, Trifunovic A. Mitochondrial energy metabolism and ageing. Biochim Biophys Acta. 2010 Jun-Jul;1797(6-7):961–7. doi: 10.1016/j.bbabio.2010.01.004. PMID: 20064485. [DOI] [PubMed] [Google Scholar]
- 19.Zeth K, Thein M. Porins in prokaryotes and eukaryotes: common themes and variations. Biochem J. 2010 Oct;431(1):13–22. doi: 10.1042/BJ20100371. PMID: 20836765. [DOI] [PubMed] [Google Scholar]
- 20.Panfoli I, Calzia D, Ravera S, Bruschi M, Tacchetti C, Candiani S, Morelli A, Candiano G. Extramitochondrial tricarboxylic acid cycle in retinal rod outer segments. Biochimie. 2011 Sep;93(9):1565–75. doi: 10.1016/j.biochi.2011.05.020. PMID: 21683117. [DOI] [PubMed] [Google Scholar]
- 21.Habersetzer J, Ziani W, Larrieu I, Stines-Chaumeil C, Giraud MF, Brèthes D, Dautant A, Paumard P. ATP synthase oligomerization: from the enzyme models to the mitochondrial morphology. Int J Biochem Cell Biol. 2013 Jan;45(1):99–105. doi: 10.1016/j.biocel.2012.05.017. PMID: 22664329. [DOI] [PubMed] [Google Scholar]
- 22.Panfoli I, Calzia D, Bianchini P, Ravera S, Diaspro A, Candiano G, Bachi A, Monticone M, Aluigi MG, Barabino S, Calabria G, Rolando M, Tacchetti C, Morelli A, Pepe IM. Evidence for aerobic metabolism in retinal rod outer segment disks. Int J Biochem Cell Biol. 2009 Dec;41(12):2555–65. doi: 10.1016/j.biocel.2009.08.013. PMID: 19715769. [DOI] [PubMed] [Google Scholar]
- 23.Ravera S, Panfoli I, Calzia D, Aluigi MG, Bianchini P, Diaspro A, Mancardi G, Morelli A. Evidence for aerobic ATP synthesis in isolated myelin vesicles. Int J Biochem Cell Biol. 2009 Jul;41(7):1581–91. doi: 10.1016/j.biocel.2009.01.009. PMID: 19401152. [DOI] [PubMed] [Google Scholar]
- 24.Adriano E, Perasso L, Panfoli I, Ravera S, Gandolfo C, Mancardi G, Morelli A, Balestrino M. A novel hypothesis about mechanisms affecting conduction velocity of central myelinated fibers. Neurochem Res. 2011 Oct;36(10):1732–9. doi: 10.1007/s11064-011-0488-0. PMID: 21553257. [DOI] [PubMed] [Google Scholar]
- 25.Panfoli I, Ravera S, Bruschi M, Candiano G, Morelli A. Proteomics unravels the exportability of mitochondrial respiratory chains. Expert Rev Proteomics. 2011 Apr;8(2):231–9. doi: 10.1586/epr.11.1. PMID: 21501016. [DOI] [PubMed] [Google Scholar]
- 26.Gregersen N, Hansen J, Palmfeldt J. Mitochondrial proteomics-a tool for the study of metabolic disorders. J Inherit Metab Dis. 2012 doi: 10.1007/s10545-012-9480-3. (in press) [DOI] [PubMed] [Google Scholar]
- 27.McBride HM, Neuspiel M, Wasiak S. Mitochondria: more than just a powerhouse. Curr Biol. 2006 Jul;16(14):R551–60. doi: 10.1016/j.cub.2006.06.054. PMID: 16860735. [DOI] [PubMed] [Google Scholar]
- 28.Bianchini P, Calzia D, Ravera S, Candiano G, Bachi A, Morelli A, Bruschi M, Pepe IM, Diaspro A, Panfoli I. Live imaging of mammalian retina: rod outer segments are stained by conventional mitochondrial dyes. J Biomed Opt. 2008 Sep-Oct;13(5):054017. doi: 10.1117/1.2982528. PMID: 19021397. [DOI] [PubMed] [Google Scholar]
- 29.HARMAN D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956 Jul;11(3):298–300. doi: 10.1093/geronj/11.3.298. PMID: 13332224. [DOI] [PubMed] [Google Scholar]
- 30.Lee HC, Wei YH. Mitochondria and aging. Adv Exp Med Biol. 2012;942:311–27. doi: 10.1007/978-94-007-2869-1_14. PMID: 22399429. [DOI] [PubMed] [Google Scholar]
- 31.Reddy PH. Amyloid beta, mitochondrial structural and functional dynamics in Alzheimer's disease. Exp Neurol. 2009 Aug;218(2):286–92. doi: 10.1016/j.expneurol.2009.03.042. PMID: 19358844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chinopoulos C, Starkov AA, Fiskum G. Cyclosporin A-insensitive permeability transition in brain mitochondria: inhibition by 2-aminoethoxydiphenyl borate. J Biol Chem. 2003 Jul;278(30):27382–9. doi: 10.1074/jbc.M303808200. PMID: 12750371. [DOI] [PubMed] [Google Scholar]
- 33.Ghosh D, LeVault KR, Barnett AJ, Brewer GJ. A reversible early oxidized redox state that precedes macromolecular ROS damage in aging nontransgenic and 3xTg-AD mouse neurons. J Neurosci. 2012 Apr 25;32(17):5821–32. doi: 10.1523/JNEUROSCI.6192-11.2012. PMID: 22539844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scheffler K, Krohn M, Dunkelmann T, Stenzel J, Miroux B, Ibrahim S, von Bohlen Und Halbach O, Heinze HJ, Walker LC, Gsponer JA, Pahnke J. Mitochondrial DNA polymorphisms specifically modify cerebral β-amyloid proteostasis. Acta Neuropathol. 2012 Apr 18; doi: 10.1007/s00401-012-0980-x. Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trifunovic A, Hansson A, Wredenberg A, Rovio AT, Dufour E, Khvorostov I, Spelbrink JN, Wibom R, Jacobs HT, Larsson NG. Somatic mtDNA mutations cause aging phenotypes without affecting reactive oxygen species production. Proc Natl Acad Sci U S A. 2005 Dec 13;102(50):17993–8. doi: 10.1073/pnas.0508886102. PMID: 16332961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liang FQ, Godley BF. Oxidative stress-induced mitochondrial DNA damage in human retinal pigment epithelial cells: a possible mechanism for RPE aging and age-related macular degeneration. Exp Eye Res. 2003 Apr;76(4):397–403. doi: 10.1016/s0014-4835(03)00023-x. PMID: 12634104. [DOI] [PubMed] [Google Scholar]
- 37.Liang FQ, Green L, Wang C, Alssadi R, Godley BF. Melatonin protects human retinal pigment epithelial (RPE) cells against oxidative stress. Exp Eye Res. 2004 Jun;78(6):1069–75. doi: 10.1016/j.exer.2004.02.003. PMID: 15109913. [DOI] [PubMed] [Google Scholar]
- 38.Ding X, Patel M, Chan CC. Molecular pathology of age-related macular degeneration. Prog Retin Eye Res. 2009 Jan;28(1):1–18. doi: 10.1016/j.preteyeres.2008.10.001. PMID: 19026761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Du H, Guo L, Fang F, Chen D, Sosunov AA, McKhann GM, Yan Y, Wang C, Zhang H, Molkentin JD, Gunn-Moore FJ, Vonsattel JP, Arancio O, Chen JX, Yan SD. Cyclophilin D deficiency attenuates mitochondrial and neuronal perturbation and ameliorates learning and memory in Alzheimer's disease. Nat Med. 2008 Oct;14(10):1097–105. doi: 10.1038/nm.1868. PMID: 18806802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Manczak M, Park BS, Jung Y, Reddy PH. Differential expression of oxidative phosphorylation genes in patients with Alzheimer's disease: implications for early mitochondrial dysfunction and oxidative damage. Neuromolecular Med. 2004;5(2):147–62. doi: 10.1385/NMM:5:2:147. PMID: 15075441. [DOI] [PubMed] [Google Scholar]
- 41.Xing SL, Chen B, Shen DZ, Zhu CQ. β-amyloid peptide binds and regulates ectopic ATP synthase α-chain on neural surface. Int J Neurosci. 2012 Jun;122(6):290–7. doi: 10.3109/00207454.2011.649867. PMID: 22185089. [DOI] [PubMed] [Google Scholar]
- 42.Rhein V, Baysang G, Rao S, Meier F, Bonert A, Müller-Spahn F, Eckert A. Amyloid-beta leads to impaired cellular respiration, energy production and mitochondrial electron chain complex activities in human neuroblastoma cells. Cell Mol Neurobiol. 2009 Sep;29(6-7):1063–71. doi: 10.1007/s10571-009-9398-y. PMID: 19350381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hollyfield JG, Bonilha VL, Rayborn ME, Yang X, Shadrach KG, Lu L, Ufret RL, Salomon RG, Perez VL. Oxidative damage-induced inflammation initiates age-related macular degeneration. Nat Med. 2008 Feb;14(2):194–8. doi: 10.1038/nm1709. PMID: 18223656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ramkumar HL, Zhang J, Chan CC. Retinal ultrastructure of murine models of dry age-related macular degeneration (AMD) Prog Retin Eye Res. 2010 May;29(3):169–90. doi: 10.1016/j.preteyeres.2010.02.002. PMID: 20206286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang L, Clark ME, Crossman DK, Kojima K, Messinger JD, Mobley JA, Curcio CA. Abundant lipid and protein components of drusen. PLoS One. 2010 Apr 23;5(4) doi: 10.1371/journal.pone.0010329. PMID: 20428236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mullins RF, Russell SR, Anderson DH, Gregory SH. Drusen associated with aging and age-related macular degeneration contain proteins common to extracellular deposits associated with atherosclerosis, elastosis, amyloidosis, and dense deposit disease. The FASEB Journal. 2000 May;14(7):835–846. [PubMed] [Google Scholar]
- 47.Wang JZ, Wang ZF. Role of melatonin in Alzheimer-like neurodegeneration. Acta Pharmacol Sin. 2006 Jan;27(1):41–9. doi: 10.1111/j.1745-7254.2006.00260.x. PMID: 16364209. [DOI] [PubMed] [Google Scholar]
- 48.Reiss AB, Voloshyna I. Regulation of cerebral cholesterol metabolism in Alzheimer disease. J Investig Med. 2012 Mar;60(3):576–82. doi: 10.231/JIM.0b013e318246d973. PMID: 22367100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Isas JM, Luibl V, Johnson LV, Kayed R, Wetzel R, Glabe CG, Langen R, Chen J. Soluble and mature amyloid fibrils in drusen deposits. Invest Ophthalmol Vis Sci. 2010 Mar;51(3):1304–10. doi: 10.1167/iovs.09-4207. PMID: 19892876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Terai K, Iwai A, Kawabata S, Tasaki Y, Watanabe T, Miyata K, Yamaguchi T. beta-amyloid deposits in transgenic mice expressing human beta-amyloid precursor protein have the same characteristics as those in Alzheimer's disease. Neuroscience. 2001;104(2):299–310. doi: 10.1016/s0306-4522(01)00095-1. PMID: 11377835. [DOI] [PubMed] [Google Scholar]
- 51.Johnson LV, Leitner WP, Rivest AJ, Staples MK, Radeke MJ, Anderson DH. The Alzheimer's A beta -peptide is deposited at sites of complement activation in pathologic deposits associated with aging and age-related macular degeneration. Proc Natl Acad Sci U S A. 2002 Sep 3;99(18):11830–5. doi: 10.1073/pnas.192203399. PMID: 12189211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dentchev T, Milam AH, Lee VM, Trojanowski JQ, Dunaief JL. Amyloid-beta is found in drusen from some age-related macular degeneration retinas, but not in drusen from normal retinas. Mol Vis. 2003 May 14;:9:184–90. PMID: 12764254. [PubMed] [Google Scholar]
- 53.Anderson DH, Talaga KC, Rivest AJ, Barron E, Hageman GS, Johnson LV. Characterization of beta amyloid assemblies in drusen: the deposits associated with aging and age-related macular degeneration. Exp Eye Res. 2004 Feb;78(2):243–56. doi: 10.1016/j.exer.2003.10.011. PMID: 14729357. [DOI] [PubMed] [Google Scholar]
- 54.Barrett PJ, Song Y, Van Horn WD, Hustedt EJ, Schafer JM, Hadziselimovic A, Beel AJ, Sanders CR. The amyloid precursor protein has a flexible transmembrane domain and binds cholesterol. Science. 2012 Jun 1;336(6085):1168–71. doi: 10.1126/science.1219988. PMID: 22654059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Krishnadev N, Meleth AD, Chew EY. Nutritional supplements for age-related macular degeneration. Curr Opin Ophthalmol. 2010 May;21(3):184–9. doi: 10.1097/ICU.0b013e32833866ee. PMID: 20216418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Burstedt MS, Ristoff E, Larsson A, Wachtmeister L. Rod-cone dystrophy with maculopathy in genetic glutathione synthetase deficiency: a morphologic and electrophysiologic study. Ophthalmology. 2009 Feb;116(2):324–31. doi: 10.1016/j.ophtha.2008.09.007. PMID: 19111905. [DOI] [PubMed] [Google Scholar]
- 57.Vingolo EM, Pelaia P, Forte R, Rocco M, Giusti C, Rispoli E. Does hyperbaric oxygen (HBO) delivery rescue retinal photoreceptors in retinitis pigmentosa? Doc Ophthalmol. 1998-1999(1):33–9. doi: 10.1023/a:1002015317479. PMID: 10710240. [DOI] [PubMed] [Google Scholar]
- 58.Berson EL. Light deprivation for early retinitis pigmentosa. A hypothesis. Arch Ophthalmol. 1971 May;85(5):521–9. doi: 10.1001/archopht.1971.00990050523001. PMID: 4996600. [DOI] [PubMed] [Google Scholar]
- 59.Shintani K, Shechtman DL, Gurwood AS. Review and update: current treatment trends for patients with retinitis pigmentosa. Optometry. 2009 Jul;80(7):384–401. doi: 10.1016/j.optm.2008.01.026. PMID: 19545852. [DOI] [PubMed] [Google Scholar]
- 60.Panfoli I, Calzia D, Ravera S, Morelli AM, Traverso CE. Extra-mitochondrial aerobic metabolism in retinal rod outer segments: new perspectives in retinopathies. Med Hypotheses. 2012 Apr;78(4):423–7. doi: 10.1016/j.mehy.2011.12.012. PMID: 22284635. [DOI] [PubMed] [Google Scholar]
- 61.Imamura H, Nhat KP, Togawa H, Saito K, Iino R, Kato-Yamada Y, Nagai T, Noji H. Visualization of ATP levels inside single living cells with fluorescence resonance energy transfer-based genetically encoded indicators. Proc Natl Acad Sci U S A. 2009 Sep 15;106(37):15651–6. doi: 10.1073/pnas.0904764106. PMID: 19720993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Genova ML, Baracca A, Biondi A, Casalena G, Faccioli M, Falasca AI, Formiggini G, Sgarbi G, Solaini G, Lenaz G. Is supercomplex organization of the respiratory chain required for optimal electron transfer activity? Biochim Biophys Acta. 2008 Jul-Aug;1777(7-8):740–6. doi: 10.1016/j.bbabio.2008.04.007. PMID: 18454935. [DOI] [PubMed] [Google Scholar]
- 63.Hansford RG, Hogue BA, Mildaziene V. Dependence of H2O2 formation by rat heart mitochondria on substrate availability and donor age. J Bioenerg Biomembr. 1997 Feb;29(1):89–95. doi: 10.1023/a:1022420007908. PMID: 9067806. [DOI] [PubMed] [Google Scholar]
- 64.Jarrett SG, Boulton ME. Consequences of oxidative stress in age-related macular degeneration. Mol Aspects Med. 2012 doi: 10.1016/j.mam.2012.03.009. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Barnett A, Brewer GJ. Autophagy in aging and Alzheimer's disease: pathologic or protective. J Alzheimers Dis. 2011;25(3):385–94. doi: 10.3233/JAD-2011-101989. PMID: 21422527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nixon RA, Yang DS. Autophagy failure in Alzheimer's disease--locating the primary defect. Neurobiol Dis. 2011 Jul;43(1):38–45. doi: 10.1016/j.nbd.2011.01.021. PMID: 21296668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kaarniranta K. Autophagy--hot topic in AMD. Acta Ophthalmol. 2010 Jun;88(4):387–8. doi: 10.1111/j.1755-3768.2009.01840.x. PMID: 20353515. [DOI] [PubMed] [Google Scholar]
- 68.Zhou J, Zhang S, Zhao X, Wei T. Melatonin impairs NADPH oxidase assembly and decreases superoxide anion production in microglia exposed to amyloid-beta1-42. J Pineal Res. 2008 Sep;45(2):157–65. doi: 10.1111/j.1600-079X.2008.00570.x. PMID: 18298462. [DOI] [PubMed] [Google Scholar]
- 69.Mattson MP, Magnus T. Ageing and neuronal vulnerability. Nat Rev Neurosci. 2006 Apr;7(4):278–94. doi: 10.1038/nrn1886. PMID: 16552414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Koronyo Y, Salumbides BC, Black KL, Koronyo-Hamaoui M. Alzheimer's disease in the retina: imaging retinal aβ plaques for early diagnosis and therapy assessment. Neurodegener Dis. 2012;10(1-4):285–93. doi: 10.1159/000335154. PMID: 22343730. [DOI] [PubMed] [Google Scholar]
- 71.Hoh Kam J, Lenassi E, Jeffery G. Viewing ageing eyes: diverse sites of amyloid Beta accumulation in the ageing mouse retina and the up-regulation of macrophages. PLoS One. 2010 Oct 1;5(10):e13127. doi: 10.1371/journal.pone.0013127. PMID: 20957206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ding JD, Johnson LV, Herrmann R, Farsiu S, Smith SG, Groelle M, Mace BE, Sullivan P, Jamison JA, Kelly U, Harrabi O, Bollini SS, Dilley J, Kobayashi D, Kuang B, Li W, Pons J, Lin JC, Bowes Rickman C. Anti-amyloid therapy protects against retinal pigmented epithelium damage and vision loss in a model of age-related macular degeneration. Proc Natl Acad Sci U S A. 2011 Jul 12;108(28):E279–87. doi: 10.1073/pnas.1100901108. PMID: 21690377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dutescu RM, Li QX, Crowston J, Masters CL, Baird PN, Culvenor JG. Amyloid precursor protein processing and retinal pathology in mouse models of Alzheimer's disease. Graefes Arch Clin Exp Ophthalmol. 2009 Sep;247(9):1213–21. doi: 10.1007/s00417-009-1060-3. PMID: 19271231. [DOI] [PubMed] [Google Scholar]
- 74.Sarangarajan R, Apte SP. Melanization and phagocytosis: implications for age related macular degeneration. Mol Vis. 2005 Jul 7;11:482–90. PMID: 16030499. [PubMed] [Google Scholar]
- 75.Yoshida T, Ohno-Matsui K, Ichinose S, Sato T, Iwata N, Saido TC, Hisatomi T, Mochizuki M, Morita I. The potential role of amyloid beta in the pathogenesis of age-related macular degeneration. J Clin Invest. 2005 Oct;115(10):2793–800. doi: 10.1172/JCI24635. PMID: 16167083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ma W, Lee SE, Guo J, Qu W, Hudson BI, Schmidt AM, Barile GR. RAGE ligand upregulation of VEGF secretion in ARPE-19 cells. Invest Ophthalmol Vis Sci. 2007 Mar;48(3):1355–61. doi: 10.1167/iovs.06-0738. PMID: 17325184. [DOI] [PubMed] [Google Scholar]
- 77.Wang XC, Zhang YC, Chatterjie N, Grundke-Iqbal I, Iqbal K, Wang JZ. Effect of melatonin and melatonylvalpromide on beta-amyloid and neurofilaments in N2a cells. Neurochem Res. 2008 Jun;33(6):1138–44. doi: 10.1007/s11064-007-9563-y. PMID: 18231852. [DOI] [PubMed] [Google Scholar]
- 78.Olcese JM, Cao C, Mori T, Mamcarz MB, Maxwell A, Runfeldt MJ, Wang L, Zhang C, Lin X, Zhang G, Arendash GW. Protection against cognitive deficits and markers of neurodegeneration by long-term oral administration of melatonin in a transgenic model of Alzheimer disease. J Pineal Res. 2009 Aug;47(1):82–96. doi: 10.1111/j.1600-079X.2009.00692.x. PMID: 19538338. [DOI] [PubMed] [Google Scholar]
- 79.Dinet V, An N, Ciccotosto GD, Bruban J, Maoui A, Bellingham SA, Hill AF, Andersen OM, Nykjaer A, Jonet L, Cappai R, Mascarelli F. APP involvement in retinogenesis of mice. Acta Neuropathol. 2011 Mar;121(3):351–63. doi: 10.1007/s00401-010-0762-2. PMID: 20978902. [DOI] [PubMed] [Google Scholar]
- 80.Xing SL, Yan J, Yu ZH, Zhu CQ. Neuronal cell surface ATP synthase mediates synthesis of extracellular ATP and regulation of intracellular pH. Cell Biol Int. 2011 Jan;35(1):81–6. doi: 10.1042/CBI20090441. PMID: 20626349. [DOI] [PubMed] [Google Scholar]
- 81.Rattner A, Nathans J. Macular degeneration: recent advances and therapeutic opportunities. Nat Rev Neurosci. 2006 Nov;7(11):860–72. doi: 10.1038/nrn2007. PMID: 17033682. [DOI] [PubMed] [Google Scholar]
- 82.Panfoli I, Musante L, Bachi A, Ravera S, Calzia D, Cattaneo A, Bruschi M, Bianchini P, Diaspro A, Morelli A, Pepe IM, Tacchetti C, Candiano G. Proteomic analysis of the retinal rod outer segment disks. J Proteome Res. 2008 Jul;7(7):2654–69. doi: 10.1021/pr7006939. PMID: 18489131. [DOI] [PubMed] [Google Scholar]
- 83.Sergeant N, Wattez A, Galván-valencia M, Ghestem A, David JP, Lemoine J, Sautiére PE, Dachary J, Mazat JP, Michalski JC, Velours J, Mena-López R, Delacourte A. Association of ATP synthase alpha-chain with neurofibrillary degeneration in Alzheimer's disease. Neuroscience. 2003;117(2):293–303. doi: 10.1016/s0306-4522(02)00747-9. PMID: 12614671. [DOI] [PubMed] [Google Scholar]
- 84.Acuña Castroviejo D, López LC, Escames G, López A, García JA, Reiter RJ. Melatonin-mitochondria interplay in health and disease. Curr Top Med Chem. 2011;11(2):221–40. doi: 10.2174/156802611794863517. Review. [DOI] [PubMed] [Google Scholar]
- 85.Pagani L, Eckert A. Amyloid-Beta interaction with mitochondria. Int J Alzheimers Dis. 2011 Mar 15;2011:925050. doi: 10.4061/2011/925050. PMID: 21461357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schmidt C, Lepsverdize E, Chi SL, Das AM, Pizzo SV, Dityatev A, Schachner M. Amyloid precursor protein and amyloid beta-peptide bind to ATP synthase and regulate its activity at the surface of neural cells. Mol Psychiatry. 2008 Oct;13(10):953–69. doi: 10.1038/sj.mp.4002077. PMID: 17726461. [DOI] [PubMed] [Google Scholar]
- 87.Quinn J, Kulhanek D, Nowlin J, Jones R, Praticò D, Rokach J, Stackman R. Chronic melatonin therapy fails to alter amyloid burden or oxidative damage in old Tg2576 mice: implications for clinical trials. Brain Res. 2005 Mar 10;1037(1-2):209–13. doi: 10.1016/j.brainres.2005.01.023. PMID: 15777772. [DOI] [PubMed] [Google Scholar]
- 88.Lahiri DK, Chen D, Ge YW, Bondy SC, Sharman EH. Dietary supplementation with melatonin reduces levels of amyloid beta-peptides in the murine cerebral cortex. J Pineal Res. 2004 May;36(4):224–31. doi: 10.1111/j.1600-079X.2004.00121.x. PMID: 15066046. [DOI] [PubMed] [Google Scholar]
- 89.Matsubara E, Bryant-Thomas T, Pacheco Quinto J, Henry TL, Poeggeler B, Herbert D, Cruz-Sanchez F, Chyan YJ, Smith MA, Perry G, Shoji M, Abe K, Leone A, Grundke-Ikbal I, Wilson GL, Ghiso J, Williams C, Refolo LM, Pappolla MA, Chain DG, Neria E. Melatonin increases survival and inhibits oxidative and amyloid pathology in a transgenic model of Alzheimer's disease. J Neurochem. 2003 Jun;85(5):1101–8. doi: 10.1046/j.1471-4159.2003.01654.x. Erratum in: J Neurochem. [DOI] [PubMed] [Google Scholar]
- 90.Arias-Esparza M, Arias R, Arias P, Arias M, Solís-Herrera A. The Unexpected Capability of Melanin to Split the Water Molecule and the Alzheimer’s Disease. Neuroscience & Medicine. 2011;2(3):217–221. [Google Scholar]
- 91.Herrera AS, Esparza MDCA, Esquivel JJA, Landín G, Miranda RISA, Arias PES, Arias MPS. The Pharmacologic Intensification of the Water Dissociation Process, or Human Photosynthesis, and Its Effect over the Recovery Mechanisms in Tissues Affected by Bloodshed of Diverse Etiology. International Journal of Clinical Medicine. 2011;2(3):332–338. [Google Scholar]
- 92.Acuna-Castroviejo D, Escames G, Rodriguez MI, Lopez LC. Melatonin role in the mitochondrial function. Front Biosci. 2007 Jan 1;12:947–63. doi: 10.2741/2116. PMID: 17127351. [DOI] [PubMed] [Google Scholar]
- 93.Rastmanesh R. Potential of melatonin to treat or prevent age-related macular degeneration through stimulation of telomerase activity. Med Hypotheses. 2011 Jan;76(1):79–85. doi: 10.1016/j.mehy.2010.08.036. PMID: 20884126. [DOI] [PubMed] [Google Scholar]
- 94.Hardeland R. Melatonin, Mitochondrial Electron Flux and Leakage: Recent Findings and Resolution of Contradictory Results. Adv Stud Biol. 2009;1(5):207–230. [Google Scholar]
- 95.Pappolla M, Bozner P, Soto C, Shao H, Robakis NK, Zagorski M, Frangione B, Ghiso J. Inhibition of Alzheimer beta-fibrillogenesis by melatonin. J Biol Chem. 1998 Mar 27;273(13):7185–8. doi: 10.1074/jbc.273.13.7185. PMID: 9516407. [DOI] [PubMed] [Google Scholar]
- 96.Poeggeler B, Miravalle L, Zagorski MG, Wisniewski T, Chyan YJ, Zhang Y, Shao H, Bryant-Thomas T, Vidal R, Frangione B, Ghiso J, Pappolla MA. Melatonin reverses the profibrillogenic activity of apolipoprotein E4 on the Alzheimer amyloid Abeta peptide. Biochemistry. 2001 Dec 11;40(49):14995–5001. doi: 10.1021/bi0114269. PMID: 11732920. [DOI] [PubMed] [Google Scholar]
- 97.Savaskan E, Ayoub MA, Ravid R, Angeloni D, Fraschini F, Meier F, Eckert A, Müller-Spahn F, Jockers R. Reduced hippocampal MT2 melatonin receptor expression in Alzheimer's disease. J Pineal Res. 2005 Jan;38(1):10–6. doi: 10.1111/j.1600-079X.2004.00169.x. PMID: 15617532. [DOI] [PubMed] [Google Scholar]
- 98.Pappolla MA, Simovich MJ, Bryant-Thomas T, Chyan YJ, Poeggeler B, Dubocovich M, Bick R, Perry G, Cruz-Sanchez F, Smith MA. The neuroprotective activities of melatonin against the Alzheimer beta-protein are not mediated by melatonin membrane receptors. J Pineal Res. 2002 Apr;32(3):135–42. doi: 10.1034/j.1600-079x.2002.1o838.x. PMID: 12074096. [DOI] [PubMed] [Google Scholar]
- 99.Baba K, Pozdeyev N, Mazzoni F, Contreras-Alcantara S, Liu C, Kasamatsu M, Martinez-Merlos T, Strettoi E, Michael Iuvone P, Tosini G. Melatonin modulates visual function and cell viability in the mouse retina via the MT1 melatonin receptor. Proc Natl Acad Sci U S A. 2009 Jun 30;106(35):15043–8. doi: 10.1073/pnas.0904400106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Slominski A. Neuroendocrine activity of the melanocyte. Exp Dermatol. 2009 Sep;18(9):760–3. doi: 10.1111/j.1600-0625.2009.00892.x. PMID: 19558501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kurakin A. The self-organizing fractal theory as a universal discovery method: the phenomenon of life. Theor Biol Med Model. 2011 Mar 29;8:4. doi: 10.1186/1742-4682-8-4. PMID: 21447162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Olsen S, Riesz J, Mahadevan I, Coutts A, Bothma JP, Powell BJ, McKenzie RH, Smith SC, Meredith P. Convergent Proton-Transfer Photocycles Violate Mirror-Image Symmetry in a Key Melanin Monomer. J Am Chem Soc. 2007;129(21):6672–3. doi: 10.1021/ja069280u. [DOI] [PubMed] [Google Scholar]
- 103.Dadachova E, Casadevall A. Ionizing radiation: how fungi cope, adapt, and exploit with the help of melanin. Curr Opin Microbiol. 2008 Dec;11(6):525–31. doi: 10.1016/j.mib.2008.09.013. PMID: 18848901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Khandhadia S, Lotery A. Oxidation and age-related macular degeneration: insights from molecular biology. Expert Rev Mol Med. 2010 Oct 20;12:e34. doi: 10.1017/S146239941000164X. PMID: 20959033. [DOI] [PubMed] [Google Scholar]
- 105.Morelli A, Ravera S, Panfoli I. Hypothesis of an energetic function for myelin. Cell Biochem Biophys. 2011 Sep;61(1):179–87. doi: 10.1007/s12013-011-9174-8. PMID: 21455684. [DOI] [PubMed] [Google Scholar]
- 106.Bürklen TS, Schlattner U, Homayouni R, Gough K, Rak M, Szeghalmi A, Wallimann T. The creatine kinase/creatine connection to Alzheimer's disease: CK-inactivation, APP-CK complexes and focal creatine deposits. J Biomed Biotechnol. 2006;2006(3):35936. doi: 10.1155/JBB/2006/35936. PMID: 17047305. [DOI] [PMC free article] [PubMed] [Google Scholar]