Abstract
Acetaminophen (APAP) overdose causes severe and occasionally fatal liver injury. Numerous drugs that attenuate APAP toxicity have been described. However these compounds frequently protect by cytochrome P450 inhibition, thereby preventing the initiating step of toxicity. We have previously shown that pretreatment with allopurinol can effectively protect against APAP toxicity, but the mechanism remains unclear. In the current study, C3HeB/FeJ mice were administered allopurinol 18h or 1h prior to an APAP overdose. Administration of allopurinol 18h prior to APAP overdose resulted in an 88% reduction in liver injury (serum ALT) 6h after APAP; however, 1h pretreatment offered no protection. APAP-cysteine adducts and glutathione depletion kinetics were similar with or without allopurinol pretreatment. The phosphorylation and mitochondrial translocation of c-jun-N-terminal-kinase (JNK) has been implicated in the progression of APAP toxicity. In our study we showed equivalent early JNK activation (2h) however late JNK activation (6h) was attenuated in allopurinol treated mice, which suggests that later JNK activation is more critical for the toxicity. Additional mice were administered oxypurinol (primary metabolite of allopurinol) 18h or 1h pre-APAP, but neither treatment protected. This finding implicated an aldehyde oxidase (AO)-mediated metabolism of allopurinol, so mice were treated with hydralazine to inhibit AO prior to allopurinol/APAP administration, which eliminated the protective effects of allopurinol. We evaluated potential targets of AO-mediated preconditioning and found increased hepatic metallothionein 18h post-allopurinol. These data show metabolism of allopurinol occurring independent of P450 isoenzymes preconditions the liver and renders the animal less susceptible to an APAP overdose.
Keywords: acetaminophen hepatotoxicity, allopurinol, oxypurinol, aldehyde oxidase, c-jun-N-terminal kinase, metallothionein
INTRODUCTION
Acetaminophen (APAP) is one of the most commonly used drugs and is very safe if used as directed. However, upon overdose APAP is toxic to the liver and as a result is the most frequent cause of acute liver failure in the US (Larson, 2007). The only approved therapeutic intervention for the treatment of APAP overdose is N-acetylcysteine (Prescott et al., 1977). Many of the mechanisms of toxicity are known in mice (Hinson et al., 2004; Jaeschke and Bajt, 2006) and in humans (McGill et al., 2012). Toxicity is initiated with cytochrome P450-mediated metabolism of APAP to the reactive metabolite N-acetyl-p-benzoquinone imine (NAPQI) (Dahlin et al., 1984). The electrophile NAPQI readily reacts with glutathione (GSH) and other sulfhydryl groups (Mitchell et al., 1973). This results in the rapid depletion of hepatic GSH and the adduction of cellular proteins (Cohen et al., 1997). Of particular concern is the binding of NAPQI to mitochondrial proteins resulting in impaired mitochondrial function (Jaeschke and Bajt, 2006). Mitochondrial dysfunction is believed to be the propagating event of toxicity resulting in loss of ATP production, mitochondrial swelling, generation of reactive oxygen species, formation of the mitochondrial permeability transition pore and the release of mitochondrial contents, all of which ultimately result in hepatic necrosis (Cover et al., 2005; Gujral et al., 2002; Kon et al., 2004).
Over the past several decades various drugs or herbal remedies have been proposed to attenuate APAP-induced liver injury (Jaeschke et al., 2013). In animal models, these drugs are always given as a pretreatment or very near the time of APAP administration to be efficacious (Jaeschke et al., 2013). Although rarely investigated, in most cases the protection is likely caused by inhibition of cytochrome P450 enzymes resulting in the elimination of the initiation event of toxicity. However, these types of approaches have limited clinical utility.
More than three decades ago allopurinol, a well-established drug used for the treatment of gout and hyperuricemia, was shown to protect first against intestinal and later also hepatic ischemia-reperfusion injury (Parks et al., 1982; Nordstrom et al., 1985). It was hypothesized that inhibition of xanthine oxidase by allopurinol, which is a hypoxanthine analogue, inhibits the post-ischemic oxidant stress and attenuates reperfusion injury. Although this mechanism of protection has been questioned (Jaeschke, 2002), the fact remained that allopurinol effectively protected against reperfusion injury (Peglow et al., 2011). In addition to ischemia-reperfusion models, allopurinol also protected against acetaminophen hepatotoxicity (Jaeschke, 1990; Tirmenstein and Nelson, 1990). These early studies demonstrated that the dose of allopurinol necessary to inhibit drug toxicity was 5 to 10-fold higher than the dose needed to inhibit xanthine oxidase (Jaeschke, 1990). In addition, based on GSH depletion kinetics after APAP overdose, allopurinol did not seem to inhibit reactive metabolite formation (Jaeschke, 1990). These conclusions were supported by direct evidence that allopurinol is a poor P450 substrate and is primarily metabolized to oxypurinol in the liver by aldehyde oxidase (Breithaupt and Tittel, 1982). Because allopurinol inhibited the mitochondrial oxidant stress and peroxynitrite formation after APAP overdose (Jaeschke, 1990; Knight et al., 2001; Knight and Jaeschke, 2002), it was hypothesized that allopurinol acted as a radical scavenger. However, this hypothesis was not confirmed (Zimmermann et al., 1988). Obviously from these findings the mechanism of protection is not simply the ability of allopurinol to inhibit xanthine oxidase or scavenge reactive oxygen. Therefore, we hypothesized that allopurinol must alter intracellular signaling pathways or up-regulate the expression of cytoprotective genes. To test this hypothesis we used the in vivo mouse model of APAP overdose with and without allopurinol pretreatment to investigate the early events in liver injury.
MATERIALS AND METHODS
Animals
Male C3HeB/FeJ mice (8–12 weeks old) purchased from Jackson Laboratories (Bar Harbor, ME) were used in our experiments. The mice were kept in an environmentally controlled room with a 12 h light/dark cycle and free access to food and water. All experimental protocols were approved by the Institutional Animal Care and Use Committee of the University of Kansas Medical Center and followed the criteria of the National Research Council for the care and use of laboratory animals.
Experiment design
All chemicals were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise noted. Mice were treated with allopurinol or oxypurinol (100 mg/kg in water, p.o.) 18h or 1h prior to APAP (300 mg/kg in warm saline, i.p.) administration. Mice were fasted overnight and APAP was always administered the following morning. Some mice were treated with hydralazine (0.1 mg/mL) in 5mM potassium phosphate buffered drinking water, pH 6.0, which is approximately 30 mg hydralazine/kg/day. Mice were euthanized at 0h, 1h, 2h, 4h or 6h after APAP injection and then blood and livers were harvested. Blood was drawn into a heparinized syringe to determine alanine aminotransferase (ALT) activity (ALT Reagent Kit, Pointe Scientific, MI). The liver was removed and pieces were fixed in phosphate-buffered formalin or used for mitochondrial isolation. The rest of the liver was snap-frozen in liquid nitrogen and subsequently stored at −80°C.
Isolation of subcellular fractions
The right and caudate lobes of the liver were minced and mechanically disrupted in ice cold isolation buffer (pH 7.4, containing 22 mM mannitol, 70 mM sucrose, 2.5 mM HEPES, 10 mM EDTA, 1 mM EGTA, and 0.1% BSA) with 15 strokes of a tight-fitting motorized Teflon pestle. Cell debris was removed by spinning the homogenate at 2,500 x g for 10 min. The resulting supernatant was then centrifuged at 20,000 x g for 10 min to pellet mostly mitochondria. This supernatant was saved as the cytosolic fraction. The mitochondria pellet was washed with isolation buffer, re-pelleted and flash frozen in liquid nitrogen. Both the cytosolic and the mitochondrial fractions were stored at −80 °C.
Histology
Formalin-fixed tissue samples were embedded in paraffin and 5 μm sections were cut and stained with hematoxylin and eosin (H&E) for evaluation of liver necrosis.
Aldehyde oxidase (AO) activity assay
Liver tissue AO activity was determined spectrophotometrically by using dimethylaminocinnamaldehyde (DMAC) as a substrate at 398nm. The method was performed as previously described (Swenson and Casida, 2013).
APAP-cysteine adduct measurement
APAP-protein adducts in liver tissue was measured by high-pressure liquid chromatography with electrochemical detection (HPLC-ECD) according to the method of Muldrew et al. (2002) with modifications (Ni et al., 2012).
Western blotting
Western blotting was performed using mouse anti-metallothionein (Dako, Carpinteria, CA), rabbit anti-JNK and rabbit anti-phospho-JNK antibodies (Cell Signaling Technology, Danvers, MA) with horseradish peroxidase-coupled anti-mouse or anti-rabbit IgG (Santa Cruz Biotechnology, Santa Cruz, CA), as described in detail (Bajt et al., 2000).
Measurement of glutathione
Glutathione (GSH) and glutathione disulfide (GSSG) levels in liver tissue were determined using a modified Tietze assay (Jaeschke and Mitchell, 1990). In brief, tissue was homogenized in 3% sulfosalicylic acid and centrifuged to remove precipitated proteins. After further dilution with potassium phosphate buffer, the samples were then assayed with a cycling reaction utilizing glutathione reductase and dithionitrobenzoic acid. To determine GSSG, reduced GSH was first trapped and removed with N-ethylmaleimide and then assayed similarly.
Real-time PCR
Quantification of mRNA expression was performed by real-time PCR (RT-PCR) analysis. cDNA was generated by reverse transcription of total RNA by M-MLV reverse transcriptase in the presence of random primers (Invitrogen, Carlsbad, CA). Forward and reverse primers for the genes were designed using Primer Express software (Applied Biosystems, Foster City, CA). After normalization of cDNA concentrations, SYBR green PCR Master Mix (Bio-Rad, Hercules, CA) was used for real-time PCR analysis. The relative differences in expression between groups were expressed using cycle time (Ct) values generated by the CFX384 instrument (Bio-Rad). All genes evaluated were first normalized to Gapdh and then expressed as a fold increase relative to control which was arbitrarily set as 1.0. Calculations are made by assuming one cycle is equivalent to a two-fold difference in copy number which is the 2^(−ddCt) formula.
Statistics
All data were expressed as mean ± SEM. For normally distributed data, statistical significance was evaluated by one-way analysis of variance (ANOVA), followed by Student Newman-Keul’s test for multiple comparisons. For non-normally distributed data, ANOVA was performed on ranks, followed by Dunn’s multiple comparisons. P < 0.05 was considered significant.
RESULTS
Allopurinol protects against APAP-induced hepatic injury
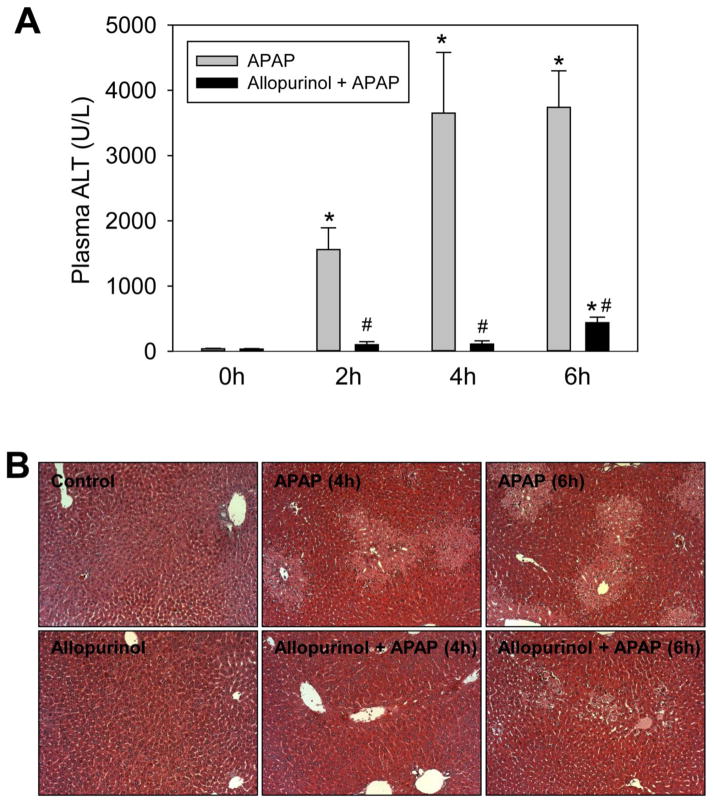
To demonstrate the protective effect of allopurinol on APAP-induced liver injury, mice were pretreated with allopurinol (100 mg/kg, p.o.) either 18h or 1h prior to APAP (300 mg/kg, i.p.) administration after overnight fasting. Previous studies utilized a dual 18h and 1h pretreatment regimen (Jaeschke, 1990; Knight et al., 2001; Knight and Jaeschke, 2002), however, we determined that dual pretreatment is not required to confer protection. The single 18h pretreatment was capable of preventing nearly 90% of the liver injury at 6h after APAP overdose. The time-course of this protection can be seen in Figure 1. In APAP-treated mice an increase in plasma ALT activities was observed at 2h post-APAP, which continued to increase at 4h and 6h (Fig. 1A). With the 18h allopurinol pretreatment no significant increase in plasma ALT levels over controls could be seen until 6h, and at this time the injury was attenuated by 88%. Confirming the plasma ALT data, liver histology showed greatly reduced centrilobular necrosis in the 18h allopurinol treated mice (Fig. 1B). Interestingly, a single 1h allopurinol pretreatment did not protect against injury as determined by plasma ALT and area of necrosis (data not shown).
Figure 1. Eighteen hour allopurinol pretreatment protects mice from APAP induced liver injury.
Mice were treated with APAP (300 mg/kg, i.p.) alone or APAP preceded by an 18h allopurinol pretreatment (100 mg/kg, p.o.). Control mice received either allopurinol or vehicle (16 mL/kg water). All mice were fasted overnight beginning at the time of allopurinol treatment. Mice were sacrificed at 0, 2, 4 and 6 h post-APAP for determination of plasma ALT (A) and liver histology by H&E staining (B). Data are expressed as means ± SE, n=4–7 mice per group. *P<0.05 (compared to control). #P<0.05 (compared to APAP-only time point).
Glutathione depletion and adduct formation
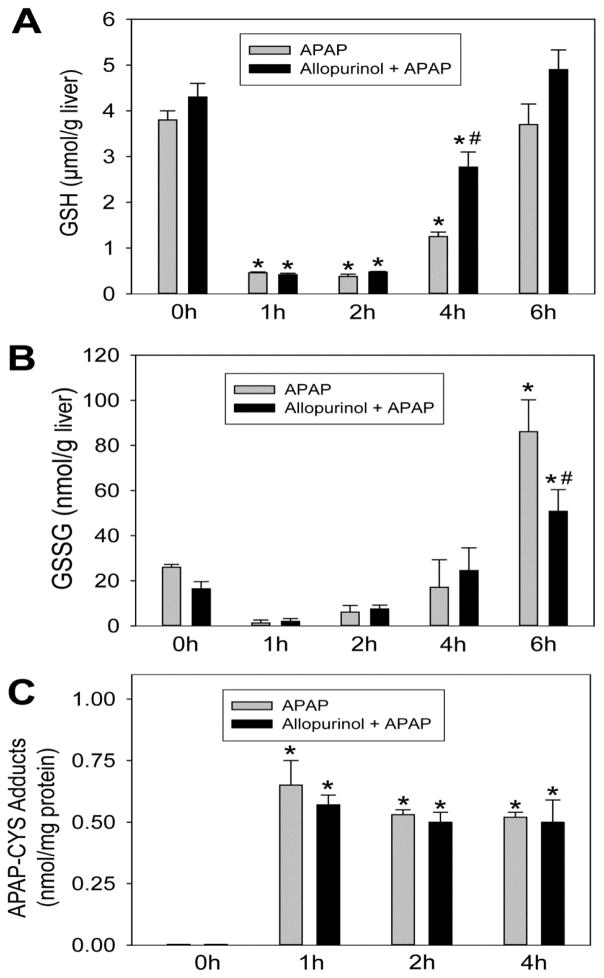
Allopurinol is metabolized predominantly in the liver by aldehyde oxidase (AO) and is a poor substrate for cytochrome P450 mediated reactions (Breithaupt and Tittel, 1982). Previous glutathione depletion kinetics studies showed that allopurinol does not appear to inhibit APAP metabolism in ICR mice (Jaeschke, 1990). To confirm this finding in C3HeB/FeJ mice, liver GSH levels were measured at 0, 1, 2, 4 and 6h post-APAP with and without a single 18h allopurinol pretreatment. Consistent with the previous report, allopurinol did not alter basal GSH levels or the rate of GSH depletion (Fig. 2A). Recovery of GSH occured sooner (4h) in allopurinol treated mice but no difference could be observed 6h post-APAP (Fig. 2A). Glutathione disulfide (GSSG) is a marker of oxidant stress; allopurinol pretreatment significantly attenuated the increase of GSSG levels at 6h (Fig. 2B), which correlated with the reduced injury in these mice. To further confirm that allopurinol did not inhibit NAPQI formation, APAP-cysteine (APAP-CYS) adducts were measured by HPLC-ECD. We have previously shown that adduct levels in mouse liver peak at 1h to 2h after APAP administration (McGill et al., 2013). Allopurinol pretreatment did not alter the adduct formation at 1h, 2h or 4h (Fig. 2C), and from this we can conclude that the NAPQI produced was equivalent with and without allopurinol.
Figure 2. Allopurinol does not alter glutathione depletion kinetics or formation of APAP-cysteine adducts.
Mice were treated with 300 mg/kg APAP with or without 18h allopurinol pretreatment. Total liver glutathione depletion and recovery are shown (A). Oxidant stress was evaluated by the formation of glutathione disulfide (GSSG) (B). The generation of reactive metabolite results in the formation of APAP-cysteine (APAP-CYS) protein adducts which were quantified by HPLC-ECD using total liver homogenate (C). Data are expressed as means ± SE, n=4–7 mice per group. *P<0.05 (compared to control). #P<0.05 (compared to APAP-only time point).
JNK phosphorylation and mitochondrial translocation
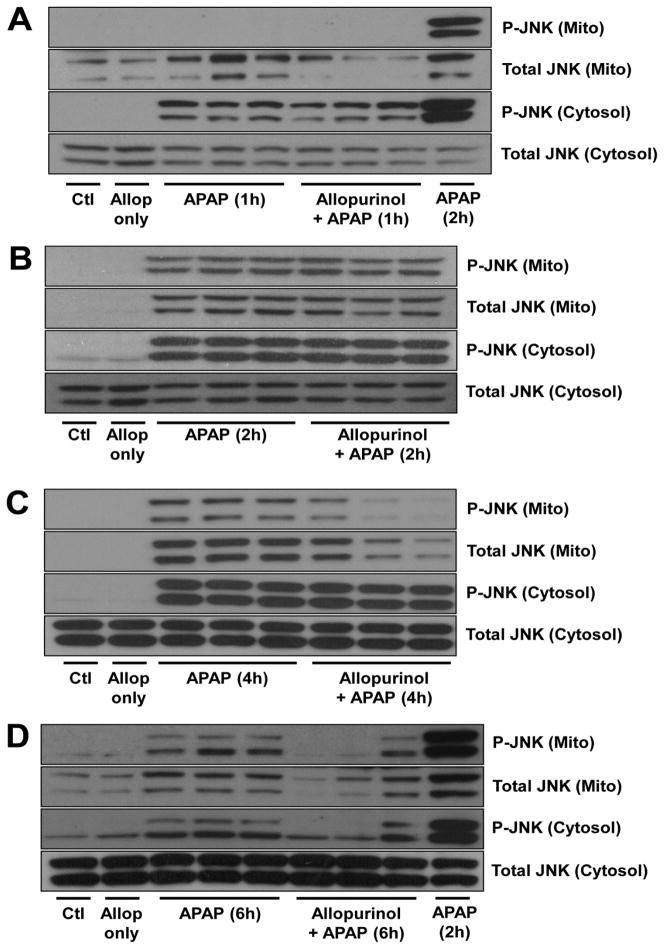
It is well-known that the phosphorylation and mitochondrial translocation of the MAP kinase c-jun-N-terminal kinase (JNK) are critical components of APAP toxicity (Gunawan et al., 2006; Henderson et al., 2007; Latchoumycandane et al., 2007; Hanawa et al., 2008). Evidence exists that APAP-protein adduct formation and mitochondrial oxidant stress may result in the downstream phosphorylation of JNK (Saito et al., 2010a). To investigate how allopurinol could potentially modulate JNK activation mitochondrial and cytosolic fractions were evaluated for phosphorylated (p-JNK) and total JNK (Fig. 3). At 1h post APAP, p-JNK could be detected only in the cytosol, however, there was no difference between the APAP and the 18h allopurinol pretreated APAP groups (Fig. 3A). At two hours post APAP, substantial p-JNK mitochondrial translocation had occurred and interestingly there was no difference between the APAP and allopurinol/APAP groups (Fig. 3B). Four hours after APAP administration the allopurinol pretreated mice showed reduced mitochondrial p-JNK compared to APAP only mice despite equivalent cytosolic p-JNK (Fig. 3C). Six hours after APAP the allopurinol pretreated mice showed both reduced mitochondrial and cytosolic p-JNK (Fig. 3D). These findings were interesting because allopurinol, which prevented a majority of the liver injury, still allowed for equivalent adduct formation and JNK activation at early time points (1h and 2h). This indicated that adduct formation can initiate JNK activation, however, this early activation does not necessarily result in severe downstream injury. Later JNK activation correlates more closely with injury.
Figure 3. Allopurinol does not alter early JNK phosphorylation and mitochondrial translocation but decreases JNK activation at later times.
Mice were treated with 300 mg/kg APAP with or without 18h allopurinol pretreatment. Mitochondrial and cytosolic fractions were used to determine the activation and translocation of JNK-1 (46 kDa) and JNK-2 (54 kDa) by western blotting. The time course shows similar JNK activation at 1h (A) and 2h (B) however allopurinol pretreatment results in decreased mitochondrial p-JNK at 4h (C) and decreased mitochondrial and cytosolic p-JNK by 6h (D).
Oxypurinol has no effect on APAP-induced injury
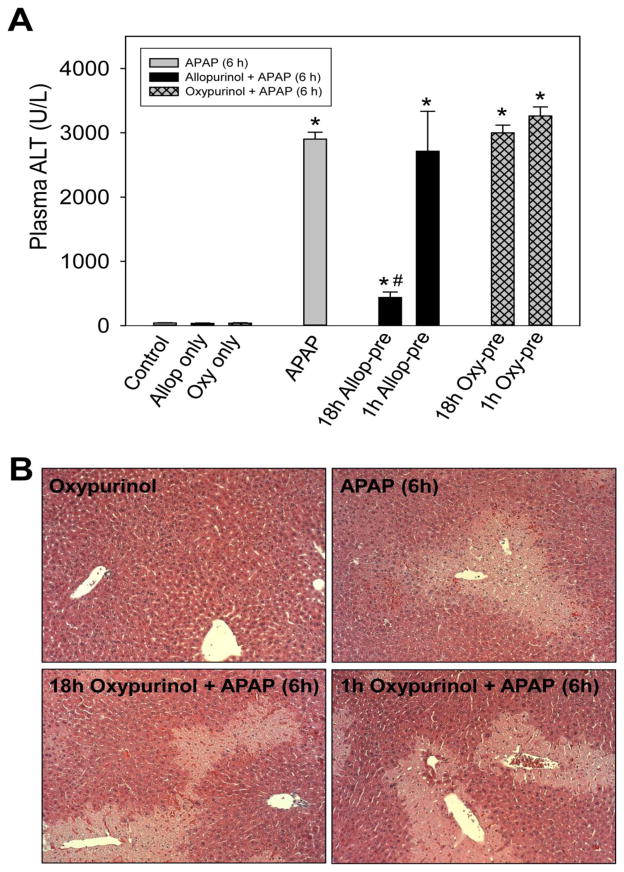
Allopurinol itself has a relatively short half-life (~1.5h) and about 80% of the dose is metabolized to the principle metabolite oxypurinol in vivo while the remaining drug is excreted in urine as the parent drug or allopurinol-1-riboside (Breithaupt and Tittel, 1982). Oxypurinol has a much longer half-life (~20h) than allopurinol, therefore we hypothesized that the protective effect may be due to oxypurinol because the short (1h) pretreatment did not protect. To test this hypothesis, mice were pretreated with oxypurinol (100 mg/kg, p.o.) 18h or 1h prior to APAP. Oxypurinol offered no protection against APAP toxicity with either the 18h or the 1h pretreatment. Neither oxypurinol pretreatment, nor the 1h allopurinol pretreatment, reduced plasma ALT 6h post-APAP (Fig. 4A). Similarly, no protection was observed histologically by H&E stained liver sections (Fig. 4B).
Figure 4. Oxypurinol does not protect from APAP induced liver injury.
Mice were treated with 300 mg/kg APAP with or without pretreatment. Pretreatments were 100 mg/kg allopurinol or oxypurinol given by oral gavage either 18h or 1h prior to APAP administration following overnight fasting. Mice were sacrificed at 6 h post-APAP for determination of plasma ALT (A) and liver histology by H&E staining (B). Data are expressed as means ± SE, n=3–4 mice per group. *P<0.05 (compared to control). #P<0.05 (compared to APAP-only treatment).
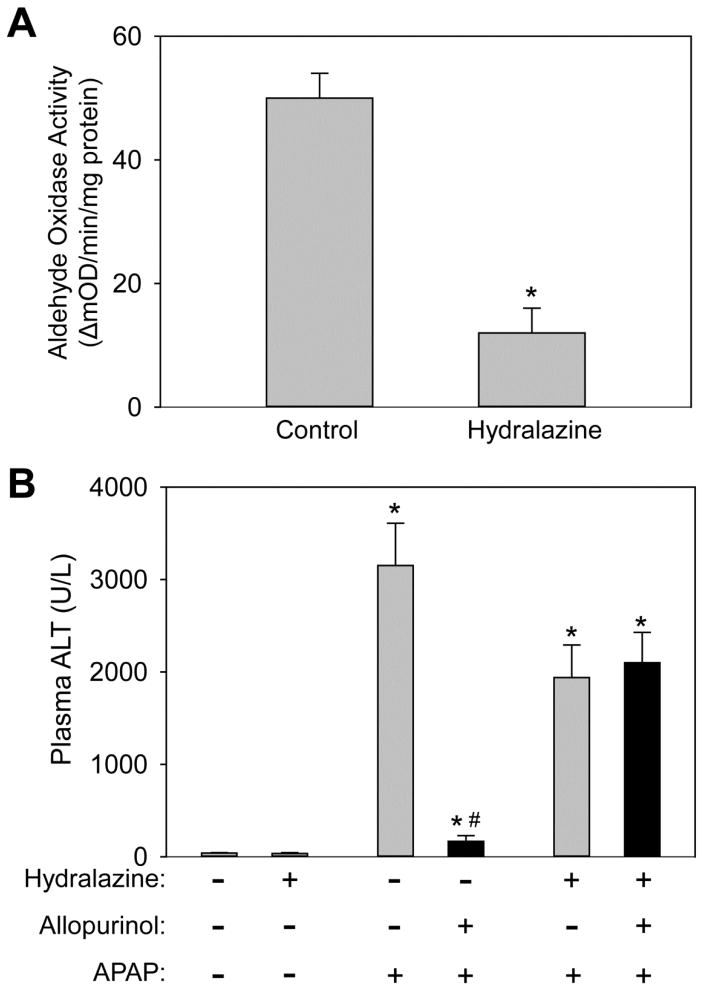
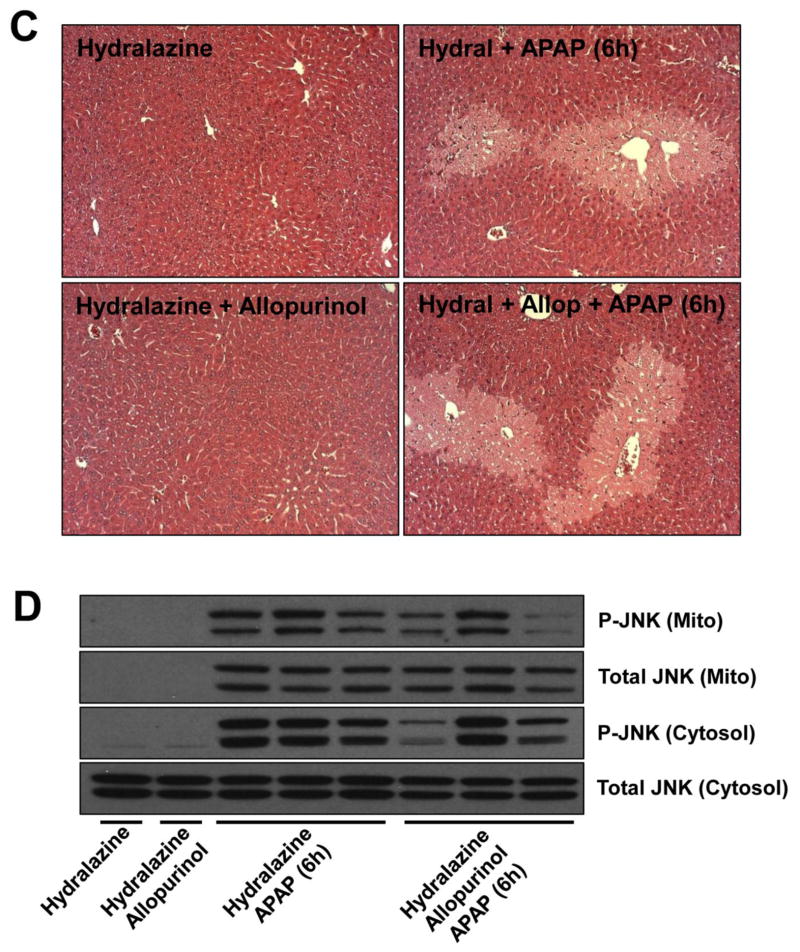
Inhibition of allopurinol metabolism ablates its protective effect
Our previous findings showed that 18h allopurinol but not oxypurinol pretreatment protected against APAP-induced liver injury. Because the half-life of allopurinol is short while the half-life of oxypurinol is substantially longer, we revised our hypothesis to say that allopurinol and oxypurinol themselves were not protective, per se, but rather the metabolic conversion of allopurinol to oxypurinol by aldehyde oxidase (AO) conferred protection. AO-mediated metabolism can produce reactive oxygen species (ROS), which may lead to a pre-conditioning effect through the up-regulation of antioxidant response genes thereby altering the hepatocytes’ ability to respond to additional stressors. To test this hypothesis, AO was inhibited in vivo before treatment with allopurinol. It was reported that treatment of mice with hydralazine-supplemented drinking water substantially reduces AO activity in vivo without altering P450 activity (Johnson et al., 1985; Swenson and Cassida, 2013). Mice were given hydralazine for 7 days and the liver AO activity was compared to vehicle-treated mice. Similar to previous reports the hydralazine-treated mice had a 76% reduction in hepatic AO activity (Fig. 5A). To confirm that the AO-mediated metabolism was responsible for protection, mice were given allopurinol or vehicle 18h prior to APAP with or without hydralazine pretreatment. Confirming our hypothesis, at 6h post-APAP the protective effect of allopurinol was lost if AO activity was inhibited. The hydralazine-treated mice had a trend toward reduced APAP-induced injury but the reduction did not achieve statistical significance. These findings were shown by plasma ALT (Fig. 5B) and histology (Fig. 5C). To confirm details of the injury mechanism in the treatment groups, JNK activation was compared. No differences in JNK phosphorylation or mitochondrial translocation could be seen with or without allopurinol if AO activity was inhibited by hydralazine (Fig. 5D).
Figure 5. Inhibition of aldehyde oxidase eliminates the protective effect of allopurinol.
To inhibit aldehyde oxidase mice were given drinking water containing hydralazine (0.1 mg/mL) for seven days. On the seventh day mice were given allopurinol or vehicle (18h), fasted overnight and then treated with APAP the following morning for 6h. To verify the hydralazine treatment inhibited aldehyde oxidase liver homogenate was assayed using a spectrophotometric assay with DMAC substrate (A). Plasma ALT of mice pretreated with allopurinol was not different than APAP alone (B). Confirming these data the area of necrosis was not different between these groups (C). The JNK phosphorylation and mitochondrial translocation was evaluated by western blotting (D). Data are expressed as means ± SE, n = 6–10 mice per group. *P<0.05 (compared to control). #P<0.05 (compared to APAP-only, hydralazine with APAP, and hydralazine with APAP with allopurinol).
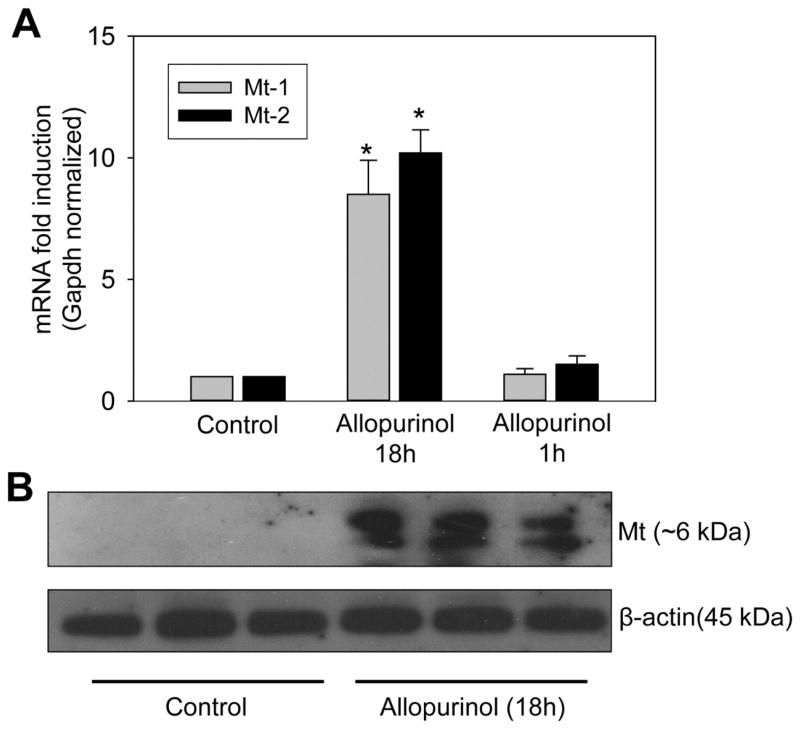
Hepatic preconditioning and metallothionein induction
Clearly, the AO-mediated conversion of allopurinol to oxypurinol, if given sufficient time prior to APAP, altered the liver in a way that made it resistant to APAP toxicity. However, the mechanism(s) of this preconditioning effect remained unknown. Our hypothesis was that the metabolic conversion of this relatively large dose of allopurinol activated hepatoprotective genes, thereby preconditioning the liver to toxicity. To investigate this, multiple genes known to be hepatoprotective were evaluated by real-time PCR including a number of Nrf-2 target genes as well as heat shock proteins (Aleksunes et al., 2008; Chiu et al., 2002; Enomoto et al, 2001; Masubuchi et al., 2003; Ni et al., 2012). Surprisingly there was very little to no induction (less than two-fold increase) of most genes evaluated with either 18h or 1h allopurinol treatments; these genes include catalase, superoxide dismutase-1 and -2, glutamate-cysteine ligase, glutathione peroxidase, glutathione s-transferases, heme oxygenase-1, inducible Hsp70 and others (data not shown). One notable exception was observed. Metallothionein (Mt)-1 and -2 were both substantially induced with the 18h allopurinol pretreatment, but the induction had yet to occur at 1h after allopurinol administration (Fig. 6A). Confirming the mRNA data, total liver homogenate showed a strong and consistent Mt protein induction by western blotting (Fig. 6B).
Figure 6. Allopurinol pretreatment induces metallothionein.
Mice were given water, allopurinol for 18h or allopurinol for 1h by oral gavage and fasted overnight to mimic the previously described allopurinol pretreatments. The 18h pretreatment causes the mRNA induction of metallothionein-1 (Mt-1) and metallothionein-2 (Mt-2) genes (A). Confirming the mRNA data, metallothionein protein was increased by the 18h pretreatment as determined by western blotting and compared to β-actin (B). Data are expressed as means ± SE, n=3 mice per group. *P<0.05 (compared to control).
DISCUSSION
The main objective of this study was to determine the mechanism of protection of the xanthine oxidase (XO) inhibitor allopurinol from APAP-induced liver injury. Early dose-response experiments with allopurinol demonstrated that 10 mg/kg or less is effective in completely inhibiting XO and XDH activities in the liver but it requires at least 50 mg/kg or more to protect against APAP toxicity (Jaeschke, 1990). In addition, in the current investigation, we demonstrated that oxypurinol, which is also an effective XO inhibitor, did not protect even at 100 mg/kg. Together, these findings strongly suggest that the protective effect of allopurinol is independent of XO and its capacity to generate reactive oxygen species.
Metabolic activation of APAP after allopurinol pretreatment
Consistent with our previous findings, we verified via GSH depletion kinetics as well as APAP-CYS adduct formation that allopurinol does not alter reactive metabolite formation. Frequently other interventional studies alter toxicity simply through competitive inhibition for cytochrome P450 metabolizing enzymes (Xie et al., 2013; Du et al., 2013). Allopurinol, however, is a poor substrate for P450-mediated reactions and does not alter P450 activity in vivo (Swenson and Casida, 2013); therefore allopurinol pretreatment does not impair the initiation of toxicity. While this initiating event is equivalent with or without allopurinol, the downstream liver toxicity is clearly different. This is an interesting finding and confirms the currently accepted understanding that protein adduction initiates toxicity but downstream events propagate injury (Jaeschke and Bajt, 2006).
Role of early and late JNK activation during APAP overdose
It is well established that prolonged JNK activation (phosphorylation) plays a critical role in the pathophysiology of APAP hepatotoxicity (Gunawan et al., 2006; Henderson et al., 2007; Latchoumycandane et al., 2007). It is thought that the early oxidant stress induced by disturbances of the mitochondrial electron transport chain by protein adduct formation initiates JNK activation (Hanawa et al., 2008; Saito et al., 2010a); P-JNK subsequently translocates to the mitochondria and amplifies the mitochondrial oxidant stress (Hanawa et al., 2008; Saito et al., 2010a), which triggers the opening of the mitochondrial membrane permeability transition pore and collapse of the membrane potential leading to cell necrosis (Kon et al., 2004; Ramachandran et al., 2011; LoGuidice and Boelsterli, 2011). The protein adduct formation seen in this study appears to be a direct link to early JNK activation and mitochondrial JNK translocation, as was previously proposed (Hanawa et al., 2008; Saito et al., 2010a), but the early JNK activation (~2h) does not directly correlate with initial injury or later downstream injury. We have shown that cytosolic p-JNK could be seen as early as 1h (Fig. 3A) and substantial p-JNK translocation to the mitochondria occurs at 2h post-APAP (Fig. 3B). At these early time points allopurinol does not modulate JNK activation but a substantial reduction in injury can be seen. Clearly this is a disconnection between injury and early JNK activation. At later times (4h and 6h), mitochondrial JNK begins to disappear faster in allopurinol-treated mice and this disappearance correlates with the attenuated injury. Generally the disappearance of total JNK in the mitochondria is very similar to the disappearance of p-JNK. It is not entirely clear if the dephosphorylation of JNK results in loss of its interaction with the mitochondria or if p-JNK is degraded thereby decreasing the total amount of mitochondrial JNK. Recent studies showed that mitogen-activated protein kinase phosphatase-1 (Mkp-1) attenuates JNK phosphorylation during APAP hepatotoxicity (Wancket et al., 2012). This suggests that in order to maintain p-JNK levels in the cytosol and subsequently in mitochondria, JNK needs to be continuously phosphorylated. This activation occurs through upstream kinases. Apoptosis signal-regulating kinase 1 (ASK1) has been identified as a kinase responsible mainly for the later phase of JNK activation (4–6h) during APAP-induced liver injury (Nakagawa et al., 2008). On the other hand, mixed-lineage protein kinase 3 (MLK3) is involved in early JNK activation (Sharma et al., 2012). However, other kinases such as glycogen synthase kinase-3beta (GSK-3β) (Shinohara et al., 2010) and receptor interacting protein kinase-1 and -3 (Sharma et al., 2012; Ramachandran et al., 2013) may also play a role in JNK activation after APAP overdose. More work is needed to clearly identify the upstream kinases and phosphatases that are responsible for prolonged JNK activation, which appears to be most important for cell death. Based on our data, allopurinol can affect this prolonged JNK activation. Future studies will have to evaluate if allopurinol inhibits any of the upstream kinases or activates phosphatases.
Role of aldehyde oxidase metabolism in liver preconditioning
There are several misconceptions regarding the use of allopurinol to inhibit xanthine dehydrogenase (XDH) and more importantly xanthine oxidase (XO). Both allopurinol and oxypurinol are capable of inhibiting XO, however, the half-lives of these drugs are quite different. In patients, allopurinol is given once per day and this is efficacious despite a relatively short half-life (~1.5h). Oxypurinol, however, has a much longer half-life (~20h), which would make it the more likely candidate responsible for XO inhibition (Stockert and Stechschulte, 2010). While the conversion of allopurinol to oxypurinol can be catalyzed by XO, it has been shown that AO is most likely the principle metabolizing enzyme and responsible for the majority of oxypurinol produced after allopurinol administration. In patients lacking all XDH/XO activity normal levels of oxypurinol can still be generated from therapeutic doses of allopurinol (Reiter et al., 1990; Shibutani et al., 1999). Aldehyde oxidase is distributed in many tissues, however, it is primarily found in the liver (Pryde et al., 2010).
As the name implies, AO is an oxidase and uses molecular oxygen as an electron acceptor ultimately resulting in the generation of hydrogen peroxide (Pryde et al, 2010) and superoxide (Kundu et al., 2007). We speculate that the high dose of allopurinol used (100 mg/kg) results in a stress response possibly mediated through ROS production thereby triggering a compensatory hepatoprotective response. The dose of allopurinol that confers protection is high and it was previously shown that a dose of only 10 mg/kg can inhibit XO but offers no protection against APAP toxicity (Jaeschke, 1990; Knight et al., 2001). Agreeing with this finding, we showed that inhibiting ~75% of AO activity with hydralazine eliminates the protective effect of allopurinol by preventing the liver preconditioning. Further confirming that this occurs independently of XO, it has been shown that hydralazine in vivo is a very poor inhibitor of XO (Johnson et al., 1985).
Role of metallothionein in allopurinol preconditioning
It has been demonstrated that Mt induction can attenuate APAP-induced liver injury (Liu et al., 1999; Saito et al., 2010b). The high levels of sulfhydryl groups in Mt are able to scavenge NAPQI and reactive oxygen species (Liu et al., 1999; Saito et al., 2010b) and therefore can effectively attenuate JNK activation (Saito et al., 2010a). These properties of Mt induction during allopurinol preconditioning could explain its protective effect. However, it is unlikely that Mt induction alone could account for the degree of protection observed in these mice. Zinc pretreatment in vivo, which is a very strong inducer and stabilizer of Mt, reduced APAP-induced injury by 60–70% (Saito et al., 2010b), while allopurinol reduced the injury at 6h by nearly 90%. In addition, part of the protection by Zinc treatment may have also been caused by elevated basal GSH levels not just Mt induction (Saito et al., 201b). Thus, there is a strong possibility that additional hepatoprotective genes are induced by allopurinol preconditioning, which need to be identified. We assessed the mRNA levels of catalase, superoxide dismutase-1 and -2, glutamate-cysteine ligase, glutathione peroxidase, glutathione s-transferases, heme oxygenase-1, inducible Hsp70 and others after 1h and 18h allopurinol treatment and did not find relevant changes. However, changes in gene expression may have peaked and waned between the two pretreatments, so these changes were undetected by our study design.
Summary
Our study demonstrates several key points: 1) Although adduct formation is required for APAP induced injury, equivalent early adduct formation can still result in different hepatic injury at later time points. These observations strongly support the concept that reactive metabolite and protein adduct formation are initiating events, which require propagation mechanisms to cause cell death. Allopurinol pretreated animals have the same GSH depletion and adduct formation but downstream injury is greatly attenuated. 2) JNK phosphorylation and mitochondrial translocation are equivalent with and without allopurinol at 1h and 2h despite major differences in later injury. 3) Allopurinol itself is not protective (1h pretreatment), oxypurinol is not protective (18h and 1h pretreatments) and the actual mechanism of protection is dependent on the AO-mediated conversion of allopurinol, which preconditions the liver at least in part by metallothionein induction. Our findings do not only increase the insight into the protective mechanism of allopurinol against APAP hepatotoxicity but also suggest that the interpretation and conclusions of hundreds of studies using high doses of allopurinol as xanthine oxidase inhibitor should be revisited.
Highlights.
18h allopurinol pretreatment protects against acetaminophen-induced liver injury
1h allopurinol pretreatment does not protect from APAP-induced injury
18h or 1h oxypurinol pretreatment does not alter APAP-induced injury
Inhibiting aldehyde oxidase eliminates protection from 18h allopurinol pretreatment
18h allopurinol induces metallothionein which protects the liver against APAP injury
Acknowledgments
This work was supported in part by the National Institutes of Health grants R01 DK070195 and R01 AA12916, and by grants from the National Center for Research Resources (5P20RR021940-07) and the National Institute of General Medical Sciences (8 P20 GM103549-07) of the National Institutes of Health. Additional support came from an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number P20 GM12345 and, from the “Training Program in Environmental Toxicology” T32 ES007079-26A2 from the National Institute of Environmental Health Sciences.
Footnotes
CONFLICT OF INTEREST DISCLOSURE
The authors declare no competing financial interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aleksunes LM, Slitt AL, Maher JM, Augustine LM, Goedken MJ, Chan JY, Cherrington NJ, Klaassen CD, Manautou JE. Induction of Mrp3 and Mrp4 transporters during acetaminophen hepatotoxicity is dependent on Nrf2. Toxicol Appl Pharmacol. 2008;226:74–83. doi: 10.1016/j.taap.2007.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajt ML, Lawson JA, Vonderfecht SL, Gujral JS, Jaeschke H. Protection against Fas receptor-mediated apoptosis in hepatocytes and nonparenchymal cells by a caspase-8 inhibitor in vivo: evidence for a postmitochondrial processing of caspase-8. Toxicol Sci. 2000;58:109–117. doi: 10.1093/toxsci/58.1.109. [DOI] [PubMed] [Google Scholar]
- Breithaupt B, Tittel M. Kinetics of allopurinol after single intravenous and oral doses. Noninteraction with benzbromarone and hydrochlorothiazide. Eur J Clin Pharmacol. 1982;22:77–84. doi: 10.1007/BF00606429. [DOI] [PubMed] [Google Scholar]
- Chiu H, Brittingham JA, Laskin DL. Differential induction of heme oxygenase-1 in macrophages and hepatocytes during acetaminophen-induced hepatotoxicity in the rat: effects of hemin and biliverdin. Toxicology and applied pharmacology. 2002;181:106–115. doi: 10.1006/taap.2002.9409. [DOI] [PubMed] [Google Scholar]
- Cohen SD, Pumford NR, Khairallah EA, Boekelheide K, Pohl LR, Amouzadeh HR, Hinson JA. Selective protein covalent binding and target organ toxicity. Toxicol Appl Pharmacol. 1997;143:1–12. doi: 10.1006/taap.1996.8074. [DOI] [PubMed] [Google Scholar]
- Cover C, Mansouri A, Knight TR, Bajt ML, Lemasters JJ, Pessayre D, Jaeschke H. Peroxynitrite-induced mitochondrial and endonuclease-mediated nuclear DNA damage in acetaminophen hepatotoxicity. J Pharmacol Exp Ther. 2005;315:879–887. doi: 10.1124/jpet.105.088898. [DOI] [PubMed] [Google Scholar]
- Dahlin DC, Miwa GT, Lu AY, Nelson SD. N-acetyl-p-benzoquinone imine: a cytochrome P-450-mediated oxidation product of acetaminophen. Proc Natl Acad Sci USA. 1984;81:1327–1331. doi: 10.1073/pnas.81.5.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du K, Williams CD, McGill MR, Xie Y, Farhood A, Vinken M, Jaeschke H. The gap junction inhibitor 2-aminoethoxy-dephenyl-borate protects against acetaminophen hepatotoxicity by inhibiting cytochrome p450 enzymes and c-Jun N-terminal kinase activation. Toxicol Appl Pharmacol. 2013 doi: 10.1016/j.taap.2013.09.010. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enomoto A, Itoh K, Nagayoshi E, Haruta J, Kimura T, O’Connor T, Harada T, Yamamoto M. High sensitivity of Nrf2 knockout mice to acetaminophen hepatotoxicity associated with decreased expression of ARE-regulated drug metabolizing enzymes and antioxidant genes. Toxicol Sci. 2001;59:169–177. doi: 10.1093/toxsci/59.1.169. [DOI] [PubMed] [Google Scholar]
- Gujral JS, Knight TR, Farhood A, Bajt ML, Jaeschke H. Mode of cell death after acetaminophen overdose in mice: apoptosis or oncotic necrosis? Toxicol Sci. 2002;67:322–328. doi: 10.1093/toxsci/67.2.322. [DOI] [PubMed] [Google Scholar]
- Gunawan BK, Liu ZX, Han D, Hanawa N, Gaarde WA, Kaplowitz N. c-Jun N-terminal kinase plays a major role in murine acetaminophen hepatotoxicity. Gastroenterology. 2006;131:165–178. doi: 10.1053/j.gastro.2006.03.045. [DOI] [PubMed] [Google Scholar]
- Hanawa N, Shinohara M, Saberi B, Gaarde WA, Han D, Kaplowitz N. Role of JNK translocation to mitochondria leading to inhibition of mitochondria bioenergetics in acetaminophen-induced liver injury. J Biol Chem. 2008;283:13565–13577. doi: 10.1074/jbc.M708916200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson NC, Pollock KJ, Frew J, Mackinnon AC, Flavell RA, Davis RJ, Sethi T, Simpson KJ. Critical role of c-jun (NH2) terminal kinase in paracetamol-induced acute liver failure. Gut. 2007;56:982–990. doi: 10.1136/gut.2006.104372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinson JA, Reid AB, McCullough SS, James LP. Acetaminophen-induced hepatotoxicity: role of metabolic activation, reactive oxygen/nitrogen species, and mitochondrial permeability transition. Drug Metab Rev. 2004;36:805–822. doi: 10.1081/dmr-200033494. [DOI] [PubMed] [Google Scholar]
- Jaeschke H. Glutathione disulfide formation and oxidant stress during acetaminophen-induced hepatotoxicity in mice in vivo: the protective effect of allopurinol. J Pharmacol Exp Ther. 1990;255:935–941. [PubMed] [Google Scholar]
- Jaeschke H. Xanthine oxidase-induced oxidant stress during hepatic ischemia-reperfusion: are we coming full circle after 20 years? Hepatology. 2002;36:761–763. doi: 10.1053/jhep.2002.36038. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, Bajt ML. Intracellular signaling mechanisms of acetaminophen-induced liver cell death. Toxicol Sci. 2006;89:31–41. doi: 10.1093/toxsci/kfi336. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, Mitchell JR. Use of isolated perfused organs in hypoxia and ischemia/reperfusion oxidant stress. Methods Enzymol. 1990;186:752–759. doi: 10.1016/0076-6879(90)86175-u. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, Williams CD, McGill MR, Xie Y, Ramachandran A. Models of drug-induced liver injury for evaluation of phytotherapeutics and other natural products. Food Chem Toxicol. 2013;55:279–289. doi: 10.1016/j.fct.2012.12.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson C, Stubley-Beedham C, Stell JG. Hydralazine: a potent inhibitor of aldehyde oxidase activity in vitro and in vivo. Biochem Pharmacol. 1985;34:4251–4256. doi: 10.1016/0006-2952(85)90280-1. [DOI] [PubMed] [Google Scholar]
- Knight TR, Kurtz A, Bajt ML, Hinson JA, Jaeschke H. Vascular and hepatocellular peroxynitrite formation during acetaminophen toxicity: role of mitochondrial oxidant stress. Toxicol Sci. 2001;62:212–220. doi: 10.1093/toxsci/62.2.212. [DOI] [PubMed] [Google Scholar]
- Knight TR, Jaeschke H. Acetaminophen-induced inhibition of Fas receptor-mediated liver cell apoptosis: mitochondrial dysfunction versus glutathione depletion. Toxicol Appl Pharmacol. 2002;181:133–141. doi: 10.1006/taap.2002.9407. [DOI] [PubMed] [Google Scholar]
- Kon K, Kim JS, Jaeschke H, Lemasters JJ. Mitochondrial permeability transition in acetaminophen-induced necrosis and apoptosis of cultured mouse hepatocytes. Hepatology. 2004;40:1170–1179. doi: 10.1002/hep.20437. [DOI] [PubMed] [Google Scholar]
- Kundu TK, Hille R, Velayutham M, Zweier JL. Characterization of superoxide production from aldehyde oxidase: an important source of oxidants in biological tissues. Arch Biochem Biophys. 2007;460:113–121. doi: 10.1016/j.abb.2006.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson AM. Acetaminophen hepatotoxicity. Clin Liver Dis. 2007;11:525–548. doi: 10.1016/j.cld.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Latchoumycandane C, Goh CW, Ong MM, Boelsterli UA. Mitochondrial protection by the JNK inhibitor leflunomide rescues mice from acetaminophen-induced liver injury. Hepatology. 2007;45:412–421. doi: 10.1002/hep.21475. [DOI] [PubMed] [Google Scholar]
- Liu J, Liu Y, Hartley D, Klaassen CD, Shehin-Johnson SE, Lucas A, Cohen SD. Metallothionein-I/II knockout mice are sensitive to acetaminophen-induced hepatotoxicity. J Pharmacol Exp Ther. 1999;289:580–586. [PubMed] [Google Scholar]
- LoGuidice A, Boelsterli UA. Acetaminophen overdose-induced liver injury in mice is mediated by peroxynitrite independently of the cyclophilin D-regulated permeability transition. Hepatology. 2011;54:969–978. doi: 10.1002/hep.24464. [DOI] [PubMed] [Google Scholar]
- Masubuchi Y, Bourdi M, Reilly TP, Graf ML, George JW, Pohl LR. Role of interleukin-6 in hepatic heat shock protein expression and protection against acetaminophen-induced liver disease. Biochemical and biophysical research communications. 2003;304:207–212. doi: 10.1016/s0006-291x(03)00572-2. [DOI] [PubMed] [Google Scholar]
- McGill MR, Lebofsky M, Norris HR, Slawson MH, Bajt ML, Xie Y, Williams CD, Wilkins DG, Rollins DE, Jaeschke H. Plasma and liver acetaminophen-protein adduct levels in mice after acetaminophen treatment: dose-response, mechanisms, and clinical implications. Toxicol Appl Pharmacol. 2013;269:240–249. doi: 10.1016/j.taap.2013.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill MR, Sharpe MR, Williams CD, Taha M, Curry SC, Jaeschke H. The mechanism underlying acetaminophen-induced hepatotoxicity in humans and mice involves mitochondrial damage and nuclear DNA fragmentation. J Clin Invest. 2012;122:1574–1583. doi: 10.1172/JCI59755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JR, Jollow DJ, Potter WZ, Davis DC, Gillette JR, Brodie BB. Acetaminophen-induced hepatic necrosis. I Role of drug metabolism. J Pharmacol Exp Ther. 1973;187:185–194. [PubMed] [Google Scholar]
- Muldrew KL, James LP, Coop L, McCullough SS, Hendrickson HP, Hinson JA, Mayeux PR. Determination of acetaminophen-protein adducts in mouse liver and serum and human serum after hepatotoxic doses of acetaminophen using high-performance liquid chromatography with electrochemical detection. Drug Metab Dispos. 2002;30:446–451. doi: 10.1124/dmd.30.4.446. [DOI] [PubMed] [Google Scholar]
- Nakagawa H, Maeda S, Hikiba Y, Ohmae T, Shibata W, Yanai A, Sakamoto K, Ogura K, Noguchi T, Karin M, Ichijo H, Omata M. Deletion of apoptosis signal-regulating kinase 1 attenuates acetaminophen-induced liver injury by inhibiting c-Jun N-terminal kinase activation. Gastroenterology. 2008;135:1311–1321. doi: 10.1053/j.gastro.2008.07.006. [DOI] [PubMed] [Google Scholar]
- Ni HM, Boggess N, McGill MR, Lebofsky M, Borude P, Apte U, Jaeschke H, Ding WX. Liver specific loss of Atg5 causes persistent activation of Nrf2 and protects against acetaminophen-induced liver injury. Toxicol Sci. 2012;127:438–450. doi: 10.1093/toxsci/kfs133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordström G, Seeman T, Hasselgren PO. Beneficial effect of allopurinol in liver ischemia. Surgery. 1985;97:679–684. [PubMed] [Google Scholar]
- Parks DA, Bulkley GB, Granger DN, Hamilton SR, McCord JM. Ischemic injury in the cat small intestine: role of superoxide radicals. Gastroenterology. 1982;82:9–15. [PubMed] [Google Scholar]
- Peglow S, Toledo AH, Anaya-Prado R, Lopez-Neblina F, Toledo-Pereyra LH. Allopurinol and xanthine oxidase inhibition in liver ischemia reperfusion. J Hepatobiliary Pancreat Sci. 2011;18:137–146. doi: 10.1007/s00534-010-0328-7. [DOI] [PubMed] [Google Scholar]
- Prescott LF, Park J, Ballantyne A, Adriaenssens P, Proudfoot AT. Treatment of paracetamol (acetaminophen) poisoning with N-acetylcysteine. Lancet. 1977;27:432–434. doi: 10.1016/s0140-6736(77)90612-2. [DOI] [PubMed] [Google Scholar]
- Pryde DC, Dalvie D, Hu Q, Jones P, Obach RS, Tran TD. Aldehyde oxidase: an enzyme of emerging importance in drug discovery. J Med Chem. 2010;53:8441–8460. doi: 10.1021/jm100888d. [DOI] [PubMed] [Google Scholar]
- Ramachandran A, Lebofsky M, Baines CP, Lemasters JJ, Jaeschke H. Cyclophilin D deficiency protects against acetaminophen-induced oxidant stress and liver injury. Free Radic Res. 2011;45:156–164. doi: 10.3109/10715762.2010.520319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran A, McGill MR, Xie Y, Ni HM, Ding WX, Jaeschke H. Receptor interacting protein kinase 3 is a critical early mediator of acetaminophen-induced hepatocyte necrosis in mice. Hepatology. 2013 Jun 6; doi: 10.1002/hep.26547. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter S, Simmonds HA, Zollner N, Braun SL, Knedel M. Demonstration of a combined deficiency of xanthine oxidase and aldehyde oxidase in xanthinuric patients not forming oxipurinol. Clin Chimica Acta. 1990;187:221–234. doi: 10.1016/0009-8981(90)90107-4. [DOI] [PubMed] [Google Scholar]
- Saito C, Lemasters JJ, Jaeschke H. c-Jun N-terminal kinase modulates oxidant stress and peroxynitrite formation independent of inducible nitric oxide synthase in acetaminophen hepatotoxicity. Toxicol Appl Pharmacol. 2010a;246:8–17. doi: 10.1016/j.taap.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito C, Yan HM, Artigues A, Villar MT, Farhood A, Jaeschke H. Mechanism of protection by metallothionein against acetaminophen hepatotoxicity. Toxicol Appl Pharmacol. 2010b;242:182–190. doi: 10.1016/j.taap.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma M, Gadang V, Jaeschke A. Critical role for mixed-lineage kinase 3 in acetaminophen-induced hepatotoxicity. Mol Pharmacol. 2012;82:1001–1007. doi: 10.1124/mol.112.079863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibutani Y, Ueo T, Yamamoto T, Takahashi S, Moriwaki Y, Higashino K. A case of classical xanthinuria (type 1) with diabetes mellitus and Hashimoto’s thyroiditis. Clin Chimica Acta. 1999;285:183–189. doi: 10.1016/s0009-8981(99)00070-4. [DOI] [PubMed] [Google Scholar]
- Shinohara M, Ybanez MD, Win S, Than TA, Jain S, Gaarde WA, Han D, Kaplowitz N. Silencing glycogen synthase kinase-3beta inhibits acetaminophen hepatotoxicity and attenuates JNK activation and loss of glutamate cysteine ligase and myeloid cell leukemia sequence 1. J Biol Chem. 2010;285:8244–8255. doi: 10.1074/jbc.M109.054999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockert A, Stechschulte M. Allopurinol to Febuxostat: How far have we come? Clin Med Insights. 2010;2:927–945. [Google Scholar]
- Swenson TL, Casida JE. Aldehyde oxidase importance in vivo in xenobiotic metabolism: imidacloprid nitroreduction in mice. Toxicol Sci. 2013;133:22–28. doi: 10.1093/toxsci/kft066. [DOI] [PubMed] [Google Scholar]
- Tirmenstein MA, Nelson SD. Acetaminophen-induced oxidation of protein thiols. Contribution of impaired thiol-metabolizing enzymes and the breakdown of adenine nucleotides. J Biol Chem. 1990;265:3059–3065. [PubMed] [Google Scholar]
- Wancket LM, Meng X, Rogers LK, Liu Y. Mitogen-activated protein kinase phosphatase (Mkp)-1 protects mice against acetaminophen-induced hepatic injury. Toxicol Pathol. 2012;40:1095–1105. doi: 10.1177/0192623312447551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, Williams CD, McGill MR, Lebofsky M, Ramachandran A, Jaeschke H. Purinergic receptor antagonist A438079 protects against acetaminophen-induced liver injury by inhibiting p450 isoenzymes, not by inflammasome activation. Toxicol Sci. 2013;131:325–335. doi: 10.1093/toxsci/kfs283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman BJ, Parks DA, Grisham MB, Granger DN. Allopurinol does not enhance antioxidant properties of extracellular fluid. Am J Physiol. 1988;255:H202–206. doi: 10.1152/ajpheart.1988.255.1.H202. [DOI] [PubMed] [Google Scholar]