Summary
Borrelia burgdorferi spirochetes cause Lyme Disease, which can result in severe clinical symptoms such as multiple joint inflammation and neurological disorders. IFN-γ and IL-17 have been suggested to play an important role in the host defense against Borrelia, and in the immunopathology of Lyme Disease. The caspase-1 dependent cytokine IL-1β has been linked to the generation of IL-17-producing T cells, whereas caspase-1-mediated IL-18 is crucial for IFN-γ production. In this study, we show by using knockout mice the role of inflammasome-activated caspase-1 for the regulation of cytokine responses by B. burgdorferi. Caspase-1 deficient cells showed significantly less IFN-γ and IL-17 production after Borrelia stimulation. A lack of IL-1β was responsible for the defective IL-17 production, whereas IL-18 was crucial for the IFN-γ production. Caspase-1 dependent IL-33 played no role in the Borrelia-induced production of IL-1β, IFN-γ or IL-17. In conclusion, we describe for the first time the role of the inflammasome-dependent caspase-1 activation of cytokines for the regulation of IL-17 production induced by Borrelia spp. As IL-17 has been implicated in the pathogenesis of chronic Lyme disease, these data suggests that caspase-1 targeting may represent a new immunomodulatory strategy for the treatment of complications of late stage Lyme disease.
Keywords: Borrelia, caspase-1, IL-17
Introduction
Lyme Disease is caused by spirochetes of the genus Borrelia, of which B. burgdorferi sensu stricto is causing disease mainly in the United States, and B. afzelii and B. garinii mainly cause disease in Europe and Asia [1;2]. Clinical Lyme disease can be divided into early localized infection that is often characterized by skin manifestations, and in either the early or late disseminated stage of the disease joint and skin inflammation, as well as neurologic disorders can be seen [3]. The various Borrelia strains appear to cause different clinical symptoms in Europe. B. burgdorferi sensu stricto is the main cause of Lyme arthritis, B. garinii most often induces neurologic manifestations, while B. afzelii is mainly responsible for skin disorders [4;5].
Cytokines play an important role in the pathogenesis of Lyme disease by regulating the immune responses against Borrelia. [6]. It has been reported that Borrelia is able to induce a pro- inflammatory cytokine response, characterized especially by production of IL-1β [7]. In patients diagnosed with a typical skin disorder near the location of the tick bite, called an erythema migrans (EM), high amounts of both IL-1β and IFN-γ were found [8]. Furthermore, the recently described IL-17-producing T-cells, called Th17 cells, are capable of producing high amounts of IL-17 after exposure to Borrelia-derived stimuli [9]. Burchill et al. [10] proposed an important role for IL-17 in the chronic stage of murine Lyme disease. In a mouse model of Borrelia infection, severe destructive arthritis could be induced in IFN-γ knock-out mice after challenge with Borrelia spirochetes. When mice were given antibodies against IL-17, the development of Lyme arthritis was strongly reduced, with the diminished severity of joint swelling [10].
Caspase-1 is an enzyme involved in processing of the cytokines IL-1β, IL-18, and is activated by a protein platform called the inflammasome [11;12]. Host defense against several pathogens have been linked to the proper activation of the inflammasome, including Francisella [13], Salmonella [14], Listeria [15], and Legionella [16]. Interestingly, IL-1β has been implicated in Th17 development [17–20], while IL-18 that was first called IGIF (IFN-γ inducing factor) is associated with the induction of Th1 cells [21].
In the present study we investigated the role of caspase-1 in the host defense against Borrelia. Caspase-1 deficient cells were unable to induce a Th1 or Th17 response upon challenge with Borrelia. Importantly, IL-1β was responsible for the induction of the IL-17 pathway induced by Borrelia, while IL-18 was crucial for the induction of IFN-γ. In contrast, IL-18 has an inhibitory effect on IL-17 production, providing further evidence for counter-regulatory regulation between Th1 and Th17 responses.
Results
Borrelia induces inflammasome activation and bioactive IL-1β
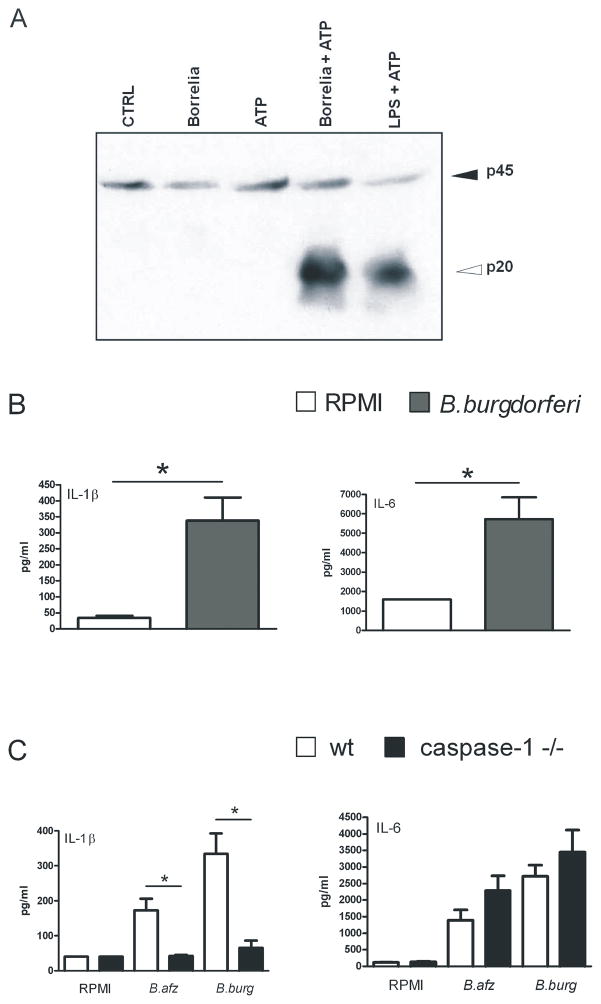
It has been previously reported that caspase-1 is activated by several different microorganisms [14–16]. Here we demonstrate for the first time that caspase-1 is also activated by Borrelia in bone marrow derived macrophages (BMDM) from wild-type C57BL/6 mice. After stimulation for 4 hours with 1×106 /ml heat-killed spirochetes, with the last 30 minutes in the presence of ATP, cleaved caspase-1 was clearly induced (Figure 1A). As a control for caspase-1 activation, BMDMs were stimulated with LPS plus ATP, which also resulted in cleaved caspase-1 (Figure 1A). Since we found strong caspase-1 activation, we next examined whether IL-1β production by murine macrophages could be induced by Borrelia burgdorferi. Peritoneal macrophages from wild-type mice stimulated for 24 hours with 1×106/ml heat-killed spirochetes. Borrelia exposure induced IL-1β production in peritoneal macrophages (Figure 1B). In addition, IL-6 was strongly produced in peritoneal macrophages (Figure 1B).
Figure 1. Borrelia induces caspase-1-dependent IL-1β production.
(A) 1 × 106 Bone marrow derived macrophages per ml from 5 WT C57/BL6 mice were incubated for 24 hours with or without 1 × 105 spirochetes/ml heat-killed B. burgdorferi or LPS (10 μg/ml) with or without ATP (3 mM) for 30 minutes. Cleaved caspase-1 was detected by Western blot using antibodies to detect the inactive caspase-1 (p45) or cleaved or active caspase-1 (p20). (B, C) Peritoneal macrophages (1 × 105/well) from 5 WT C57BL/6 mice and 5 caspase-1 knock-out mice were incubated for 24 hours with (B) 3 × 106 spirochetes/ml heat-killed B. burgdorferi or (C) 1×106/ml B. afzelii (B. afz) or B. burgdorferi (B. burg) or medium alone. Supernatant cytokine levels were determined by RIA (IL-1β) or ELISA (IL-6). Data are mean ± SEM (pg/ml, 5 animals per group); *p<0.05, Mann-Whitney U test, two-tailed.
To confirm that caspase-1 is specifically involved in the induction of the cytokines IL-1β and IL-6, these stimulation experiments were repeated with caspase-1 deficient peritoneal macrophages. Peritoneal macrophages of caspase-1 knock-out mice were stimulated for 24 hours with either B. afzelii or B. burgdorferi. Both strains were able to induce IL-1β and IL-6 in peritoneal macrophages of wild-type mice. Macrophages from caspase-1 deficient mice showed significantly decreased levels of IL-1β, while the production of IL-6 by Borrelia was not affected in caspase-1 deficient cells. Although a slight increase in IL-6 in caspase-1 mice was found, this difference was not statistically significant (Figure 1C).
Interleukin-17 and Interferon-γ induction by Borrelia is dependent on caspase-1
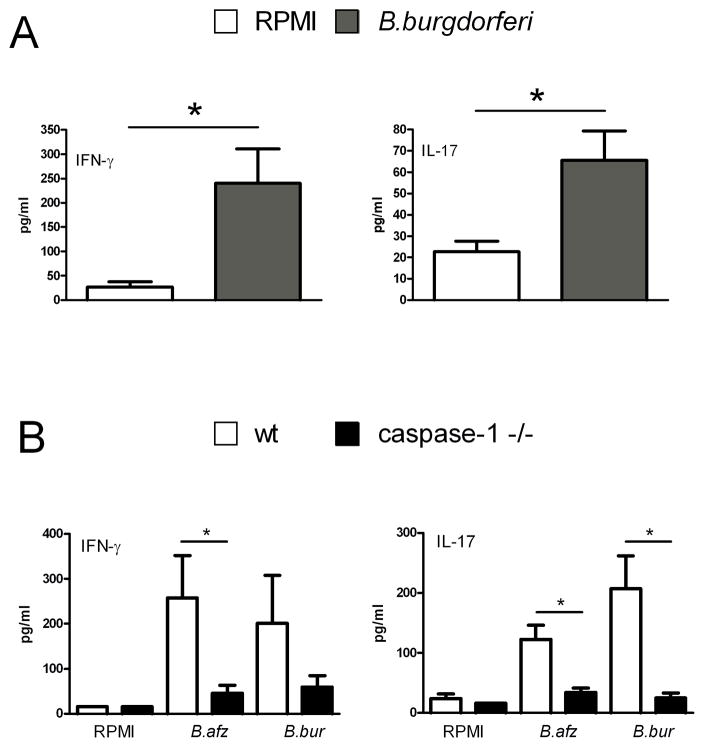
Borrelia is able to elicit IL-β and IL-6 production, cytokines which are often associated with inflammatory processes. In addition, production of IL-17 and IFN-γ by Th17 and Th1 subsets, respectively, have been suggested to play a role in the immune response against Borrelia [9;22]. To investigate whether spleen cells of naive mice are able to produce IL-17 and IFN-γ after Borrelia exposure, spleen cells of wild-type mice were stimulated for five days with 1×106/ml spirochetes. A significant amount of IL-17 production after Borrelia stimulation could be detected (Figure 2A). In addition, IFN-γ production was also potently induced after exposure to Borrelia (Figure 2A). Since it was shown that Borrelia activates caspase-1, the contribution of caspase-1 in the induction of IFN-γ and IL-17 was investigated. A significant decrease in both IL-17 and IFN-γ production was detected in spleen cells of caspase-1 gene deficient mice stimulated with Borrelia spp. (Figure 2B).
Figure 2. Induction of IL-17 and IFN-γ by Borrelia is caspase-1-dependent.
(A, B) Spleen cells (5×106/well) from 5 WT C57BL/6 mice or 5 caspase-1 KO mice were incubated with 1×106/ml B. afzelii or B. burgdorferi species or medium alone (RPMI). Supernatants were collected after 5 days for measurement of cytokines by ELISA. Data are mean ± SEM of 5 mice per group; *p<0.05, two-tailed Mann-Whitney U-test.
Borrelia-induced joint inflammation depends on caspase-1
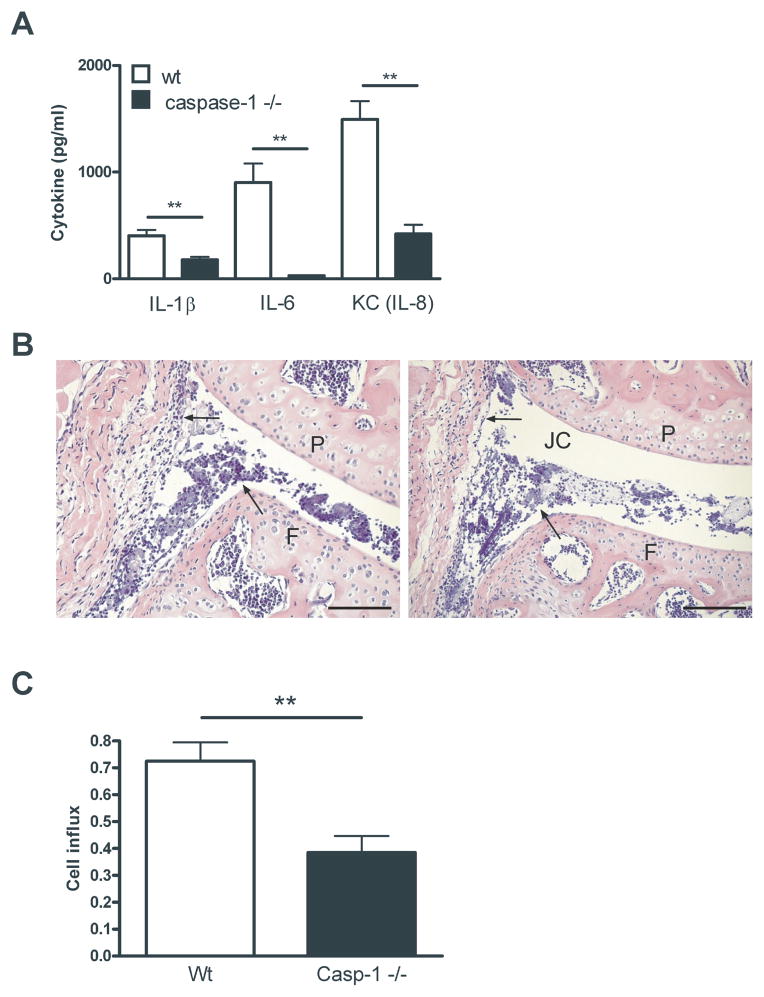
Since we know that caspase-1 plays an important role in the induction of cytokines, we examined the role of caspase-1 in vivo. Borrelia spirochetes were injected directly into knee joints of naive (Wt) and caspase-1 knockout mice. After 4 hours, patellae were collected and cytokine levels were measured in patella washouts. Highly significant differences in IL-1β, IL-6 and KC production could be detected when wild-type patellae were compared with caspase-1 gene deficient patellae (Figure 3A). In addition, the influx inflammatory cells into the joint cavity of caspase-1ko mice was decreased as compared to wild-type mice. Lower amounts of polymorphonuclear cells (PMNs) could be seen in caspase-1 −/− mice as well as less thickening of the synovial lining (Figure 3B). When we counted the cell influx, we were able to see approximately 30% reduction in cell influx in all examined joints (n=10) of the caspase-1 deficient animals in comparison to the wild-type animals (n=10), which was found to be significant (Figure 3C).
Figure 3. Borrelia-induced cytokine production and cell influx is dependent on caspase-1.
(A) Four hours after intra-articular injection of 1×105 heat-inactivated Borrelia species in 10 μl of PBS, patellae from 5 WT C57BL/6 mice or 5 caspase-1 knock-out were cultured for 1 hour and IL-1β, IL-6 and KC levels were measured using Luminex. Data are mean ± SEM; 5 animals in each group; **p<0.01; Mann-Whitney U-test, two-tailed. (B) Histology (H&E staining) 1 day after i.a. injection of heat-inactivated Borrelia. Left panel, WT; right panel, caspase 1 knockout mice. Arrows highlight areas of cell influx and the synovial lining; 200x magnification; P, patella; F, femur; JC, joint cavity; Scale bar represents 50 μM. (C) Scored cell influx 1 day after i.a. injection of heat-inactivated Borrelia. Data are mean ± SEM from 10 animals in each group; **p<0.01; Mann-Whitney U test, two-sided.
Borrelia-induced IL-17 production is dependent on IL-1β
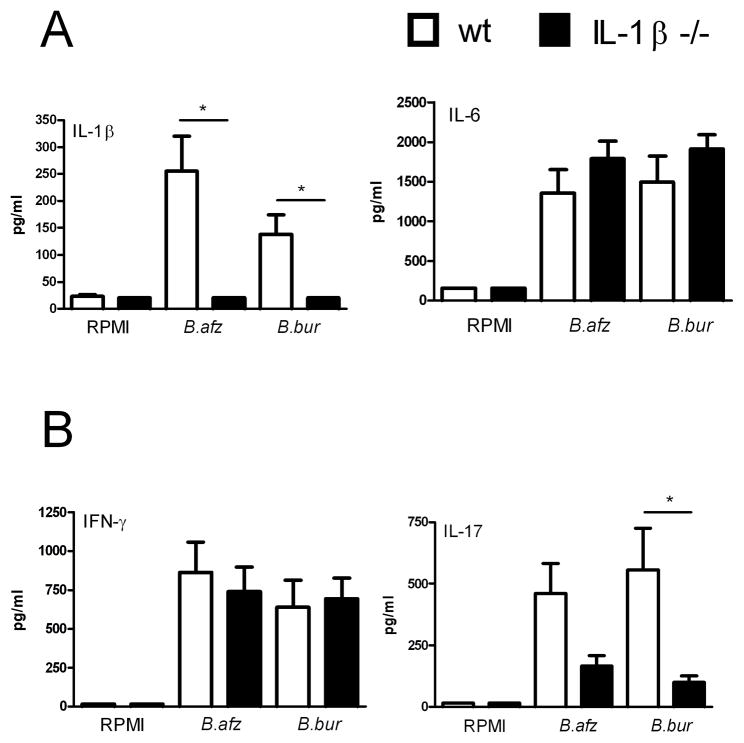
We explored whether IL-1β might play a role in the induction of IL-17 during Borrelia host defense. Peritoneal macrophages and spleen cells of IL-1β gene deficient mice were stimulated with 1×106/ml B. afzelii and B. burgdorferi for 24 hours or 5 days, respectively. No differences in IL-6 production could be observed between wild-type and IL-1β deficient cells (Figure 4A). Significant reduced IL-17 concentrations were detected in spleen cells from mice lacking IL-1β that were stimulated with Borrelia, while IFN-γ production by IL-1β deficient cells did not differ from that of wild-type cells (Figure 4B).
Figure 4. IL-1β is critical for induction of IL-17 by Borrelia.
(A) Peritoneal macrophages (1 × 105 / well) and (B) spleen cells (5 × 106 / well) from 4 WT 129SvBl/6 mice and 4 IL-1β knock-out mice were incubated with 3 × 106 B. burgdorferi spirochetes/ml for 24h and 5 days, respectively. Supernatants were collected for measurement of cytokines by ELISA (IL-6, IFN-γ and IL-17) or RIA (IL-1β). Data (n = 4 per group) are mean ± SEM; *p<0.05; Mann-Whitney U-test, two-tailed.
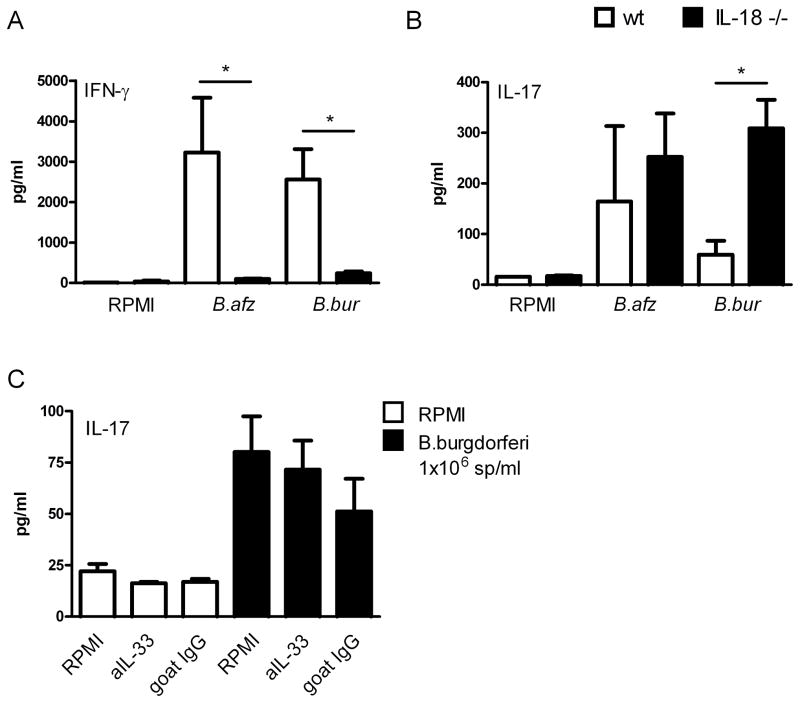
IL-18 suppresses the induction of IL-17 by Borrelia species
As demonstrated in figure 4B, IL-1β is not important in the regulation of IFN-γ production after Borrelia exposure. Since caspase-1 is still functional in IL-1β deficient cells, it will still be able to process pro-IL-18. To determine whether IL-18 was responsible for the induction of IFN-γ by Borrelia, spleen cells of wild-type and IL-18 deficient mice were exposed to Borrelia. IFN-γ levels were significantly reduced in the IL-18 gene deficient cells stimulated with Borrelia (Figure 5A). Of high interest, IL-17 concentrations were significantly enhanced in IL-18 deficient spleen cells after stimulation with B. burgdorferi when compared to wild-type spleen cells (Figure 5B). Stimulation of cells with B. afzelii led to similar results, but this difference was not found to be statistically significant.
Figure 5. (A) IL-18 is crucial for Borrelia induced IFN-γ but not IL-33 production.
(A, B) 5 × 106 /well spleen cells of 5 WT C57BL/6 mice and 5 IL-18 knock-out mice were incubated with 1 × 106 spirochetes/ml B. burgdorferi or B. afzelii or medium alone for 5 days. Supernatants were collected for measurement of (A) IFN-γ or (B) IL-17 by ELISA. Data are mean ± SEM in pg/ml; 5 mice per group *p<0.05 two-tailed Mann-Whitney U test. (C) Spleen cells (5 × 106 /well) of 3 wild-type Balb/c mice were cultured for 5 days in RPMI alone or with 1 × 106/mL B. burgdorferi in the presence/absence of 10 μg/ml IL-33 antibody or 10 μg/ml control goat IgG antibody. Supernatants were collected and IL-17 was measured by ELISA. Data are mean ± SEM; pg/ml; 3 animals per group.
IL-33 is not involved in Borrelia-induced production of IL-17
It has been suggested by an earlier study that apart from IL-1β and IL-18, also IL-33 is cleaved by caspase-1 [23]. To examine the contribution of this novel cytokine in anti-Borrelia host defense, spleen cells from wild-type mice were stimulated with Borrelia spirochetes with or without the presence of a neutralizing anti-murine IL-33 antibody. The neutralizing activity of the anti-IL-33 antibody was confirmed in an IL-33 bioassay, in which the IL-33-induced IL-5 production was inhibited (data not shown). When spleen cells were stimulated with heat-killed Borrelia, a slight decrease in IL-17 levels could be observed after blockade of IL-33, but this difference was not found to be significant (Figure 5C). Also, Borrelia-induced IL-1β, IL-6, and IFN-γ production did not reveal any differences after blockade of endogenous IL-33 (data not shown).
Discussion
Activation of caspase-1 and subsequently IL-1β and IL-18 by the inflammasome has been suggested to represent an important host defense mechanism. In the present study we demonstrate that Borrelia spp. are strong inducers of inflammasome activation. Other research groups demonstrated already the role of inflammasome components in sensing pathogens, for example Listeria monocytogenes [24]. In addition, our data also show that inflammasome/caspase-1 activation by Borrelia is a crucial event in the modulation of cytokine responses by the spirochete. This immune response is crucial for both host defense and immunopathogenesis. Borrelia spirochetes are able to induce IL-1β, IL-6, IL-17 and IFN-γ. The production of IL-17 after Borrelia infection is regulated by both caspase-1 and IL-1β, but not via IL-18 or IL-33. IFN-γ induction is regulated through caspase-1-dependent IL-18-production. Furthermore, there is an important counter-regulatory mechanism between IFN-γ and IL-17 responses during anti-Borrelia host defense. In addition, caspase-1 plays an important role in Borrelia-induced arthritis.
Recently, it has been suggested that caspase-1 plays a minimal role in a murine Borrelia infection model [25]. However, in this study caspase-1 knock-out mice were significantly more susceptible to acute infection with Borrelia, when acute joint inflammation seen in knock-out mice was compared to wild-type mice. It was already known that caspase was necessary for the activation of T cells after recognition of Borrelia species by PRRs [26], which is in line with our results. The induction of pro-inflammatory cytokines IL-1β and IL-17 by Borrelia was caspase-1-dependent, and both cytokines have been shown already to play a role in the pathogenesis caused by Borrelia [27–29]. In line with this, we have demonstrated that stimulation of macrophages and spleen cells by Borrelia resulted in production of IL-1β, IL-6, IL-17, and IFN-γ (Fig. 1). In addition, after intra articular injection with Borrelia we observed less cell influx and cytokine production in caspase-1 deficient animals as compared to the wild-type animals (Fig. 3). We observed differences in IL-6 production after Borrelia stimulation between caspase-1 deficient peritoneal macrophages and PMNs isolated from the knee of caspase-1 knockout animals. This difference can be explained by the fact that different type of cells are involved and different time points were used in these assays. In the patella washouts assays, the main cell types that could produce IL-6 are granulocytes (PMN) and synovial fibroblasts. These cells may respond different after exposure to Borrelia when compared to peritoneal macrophages. On other explanation could be that the synovial cells were only 4 hours exposed to Borrelia whereas the peritoneal macrophages were treated for 24 hours with Borrelia.
We also describe that Borrelia-induced IL-1β, is the main inducer of IL-17 production after stimulation with Borrelia (Fig. 4). Furthermore, caspase-1-cleaved IL-18 is responsible for induction of IFN-γ by Borrelia species (Fig. 5A).
Caspase-1 is crucial for Borrelia-induced IFN-γ production, as caspase-1 deficient mice produced almost no IFN-γ. The exact role of IFN-γ in the host defense against Borrelia has not yet been elucidated. On the one hand, the induction of Borrelia-induced arthritis does not seem dependent on IFN-γ [30–32], and it has been reported that mice with a disrupted IFN-γ gene are more susceptible to autoimmune disorders such as experimental autoimmune encephalomyelitis (EAE) and collagen-induced arthritis [33;34]. On the other hand, several groups have proposed a role for IFN-γ-producing T cells in Lyme arthritis [34;35]. In patients infected with Borrelia, high levels of IFN-γ were measured [36]. In line with this, we found that IFN-γ is produced in large amounts by spleen cells after stimulation with Borrelia spirochetes. Dame et. al [37] described that IFN-γ in combination with B. burgdorferi cooperatively induced upregulation of endothelial cell genes, causing more T cell infiltration.
It has been known that IFN-γ modulates other T cell cytokines. It has been described before that IFN-γ controls or modulates Th17 responses [38;39], but until now this has not been demonstrated for Borrelia-induced Th17 responses.
Elevated levels of IL-1β were induced by cells stimulated with Borrelia, and it was shown before that IL-1β is needed for the induction of Th17 and IL-17 production [40]. We also now formally demonstrate activation of the inflammasome by Borrelia. When spleen cells of mice lacking IL-1β were stimulated with Borrelia, IL-17 production was significantly diminished, which also raises the hypothesis the IL-1β is also involved in induction of Th17 cells by Borrelia species. IL-17 is associated with more severe disease progression in several autoimmune disorders, such as rheumatoid arthritis (RA) or multiple sclerosis [41]. In patients diagnosed with RA, elevated levels of IL-17 were found in synovial fluid [42;43]. Since several clinical symptoms between RA and Lyme arthritis are similar, it has been proposed that IL-17 might be involved in the development of Lyme arthritis [10]. In line with this hypothesis, it has been demonstrated that blockade of endogenous IL-17 in IFN-γ deficient mice results in complete protection against development of arthritis after infection by Borrelia [44]. These data indicate that controlling the IL-17 response by IFN-γ plays an important role in chronic Lyme Disease.
IL-33 is a member of the IL-1 family and is mainly involved in induction of T-helper 2-like cytokines, such as IL-4 and IL-5 [23]. Although it was shown that IL-33 is cleaved by caspase-1, the activity of the mature protein has never been assessed. IL-33 can be secreted from cells after caspase-1 stimulation [45], very recent data suggest that IL-33 activity is independent of caspase-1 [46;47]. More recently it was shown that IL-33 can be functionally active and bind to its receptor ST2 without being cleaved by caspase-1, and that this cytokine is more related to IL-1α than to IL-1β or IL-18 [48]. It was also described that IL-33/ST2 binding results in the regulation of mainly Th2 responses, which is in line with our results. IL-33 seems not to be involved in either IL-17 or IFN- production by Borrelia species [23]. In the present study we also demonstrate that IL-33 does not play a role in the regulation of pro-inflammatory cytokines such as IL-1β and IL-6 induced after Borrelia exposure.
The present study demonstrates modulation of IFN-γ/IL-17 responses by Borrelia species through inflammasome and caspase-1 activity. These findings are the first to demonstrate the existence of a counter-regulatory mechanism of Th1 versus Th17 cytokines during stimulation with Borrelia species. As shown in this study, IL-18 is crucial for the Borrelia-induced IFN-γ production, and IFN-γ has been suggested to be essential for induction of Th1 cells. Th1 cells drive cell-mediated immune responses and support the fight against invading pathogens. Induction of Th1 cells after recognition of Borrelia might be very important in the early immune response against spirochetes. Induction of large amounts of Th1 cells will represent effective killing of invaded spirochetes by the host immune responses already in the first stage of disease which might prevent the development of chronic Borrelia-infection. On the other hand, when IL-1β is highly produced by host cells after Borrelia recognition, high levels of Th17 cells may be produced. Borrelia-primed Th17 cells might facilitate development of a chronic stage of Lyme disease, as already described in other diseases, such as RA [41]. At this moment, it is still unknown which specific T cell population is responsible for the induction of IL-17 (CD4+, γδT cells, NK T cells, CD4−/CD8). One of our future plans is to detect which specific T cell population is responsible for the induction of IL-17 by Borrelia species.
In summary, Borrelia is a strong inducer of inflammasome activation and caspase-1-mediated IL-1β induction amplifies the production of IL-17 after Borrelia exposure. The Borrelia-induced IL-17 production is modulated by the IL-18-driven IFN-γ. These data indicate that caspase-1-dependent cytokines IL-1β and IL-18 determine the development and clinical outcome of Lyme disease which was also demonstrated by our in-vivo data. This findings give more insight in the pathogenesis of Lyme disease and may provide useful information for the development of new therapeutic strategies targeting the inflammasome.
Materials and Methods
Borrelia burgdorferi and B. afzelii cultures
B. burgdorferi pKo strain and B. afzelii, patient isolate, were cultured at 33°C in Barbour-Stoenner-Kelley (BSK)-H medium (Sigma-Aldrich) supplemented with 6% rabbit serum. Spirochetes were grown to late-logarithmic phase and examined for motility by dark-field microscopy. Organisms were quantitated by fluorescence microscopy after mixing 10 μl aliquots of culture material with 10 μl of an acridine orange solution to concentrations. Bacteria were harvested by centrifugation of the culture at 7000 × g for 15 min., washed twice with sterile PBS (pH 7.4), and diluted in the specified medium to required concentrations between 1–3 × 106 spirochetes per ml. Heat-killed B. burgdorferi and B. afzelii were prepared as described above except for heating at 52°C for 30 min. before dilution. Heat-inactivated bacteria were used according to Wang et al [6].
Animals
C57BL/6 and Balb/c mice were obtained from Charles River Wiga (Sulzfeld, Germany). IL-1β gene deficient mice were kindly provided by J. Mudgett, Merck (Rahway, NJ, USA). Caspase-1 deficient mice were originally obtained from R.A. Flavell, New Haven, CT, USA and generation of these mice were previously described [49;50]. The generation of IL-18 knock-out mice were previously described [51]. Male wild-type and knock-out mice between 6 and 8 weeks of age were used. The mice were fed sterilized laboratory chow (Hope Farms, Woerden, The Netherlands) and water ad libitum. The experiments were approved by the Ethics Committee on Animal Experiments of the Radboud University Nijmegen.
Preperation of BMDM and Western blot
Bone marrow from mice (age between 8–20 weeks) was flushed out after dissecting mouse legs. Differentiation into macrophages occurred in 5 days at 37°C (5% CO2) in the presence of Iscove’s modified Dulbecco’s medium (IMDM) supplemented with 30% of L929 supernatant containing 10% fetal bovine serum (heat-inactivated, Invitrogen), 100 U / mL penicillin and 100 mg/mL streptomycin. Ultrapure LPS was purchased from Invitrogen and used in a concentration of 10 μg/ml. ATP was from Sigma and used in a final concentration of 3 mM. For immunoblotting, cells were washed twice with sterile phosphate-buffered saline and lysed in buffer (150 mM NaCl, 10 mM Tris, pH 7.4, 5 mM EDTA, 1 mM EGTA, 0.1% Nonidet P-40) which was supplemented with a Roche protease inhibitor cocktail tablet. After clarification and denaturation with SDS buffer, samples were boiled for 5 minutes. Separation of the proteins was done by using SDS-PAGE and thereafter transferred to a nitrocellulose membrane. These membranes were coated with primary antibodies and active caspase-1 was detected using secondary anti-rabbit antibody conjugated to horseradish peroxidase followed by enhanced chemiluminiscence.
In vitro cytokine production
Peritoneal macrophages were isolated by injecting 5 ml of ice-cold sterile PBS (pH 7.4) in the peritoneal cavity. After centrifugation and washing, cells were resuspended in RPMI 1640 containing 1 mM pyruvate, 2 mM L-glutamine and 100 μg/ml gentamycin (culture medium). Cells were counted using a Z1 Coulter Particle Counter (Beckman Coulter, Woerden, The Netherlands) and adjusted to 1 × 106 cells/ml. Cells were cultured in 96-well round-bottom microtiter plates (Costar, Corning, The Netherlands) at 1 × 105 cells/well, in a final volume of 200 μl. The cells were stimulated with RPMI or two different heat-killed Borrelia strains. After 24 hours of incubation at 37°C in air and 5% CO2, the plates were centrifuged at 500×g for 10 min, and the supernatant was collected and stored at −80°C until cytokine assays were performed.
Investigating the role of IL-33 was performed by incubation of peritoneal macrophages which were stimulated with RPMI or Borrelia in the presence or absence of 10 μg/ml anti-mouse IL-33 antibody (R&D Systems). After 48 hours of incubation, IL-4 and IL-5 levels were measured using ELISA kits (eBioscience). ELISA’s were performed according to the manufacturer’s instructions. Spleen cells were isolated by gently squeezing spleens in a sterile 200 μm filter chamber. After washing with sterile PBS and centrifugation at 4°C (1200 rpm 5 min), cells were resuspended in 4 ml RPMI 1640 in presence of 20% FCS. Cells were counted and concentrations were adjusted to 1 × 107 cells/ml. Cells were cultured in 24-wells plates (Greiner, Alphen a/d Rijn, The Netherlands) at 5 × 106 cells/well, in a final volume of 1000 μl. After 5 days of incubation, supernatant was collected and stored at −80°C until cytokine assays were performed.
Cytokine measurements
Concentrations of mouse IL-1β were determined by specific radioimmunoassay (RIA; detection limit is 20 pg/ml) as described by Netea et. al [52]. Mouse IL-6, IL-17, and IFN-γ, concentrations were measured by a commercial ELISA kit (Biosource, Camarillo, CA; detection limits 16 pg/ml), according to the instructions of the manufacturer. IL-4 and IL-5 levels were measured using mouse ELISA Ready-SET-Go! Kits (eBioscience, San Diego, California, USA; detection limits 4 pg/ml), according to the instructions of the manufacturer.
In brief, IL-4 and IL-5 were detected using biotinylated monoclonal antibodies, which are able to bind to avidin-conjugated horseradish peroxidase followed by TMB-substrate incubation. After stopping the reaction with 0.1M acid, reactions were measured in an ELISA reader.
Induction of Borrelia-induced joint inflammation
Joint inflammation was induced by intra articular injection (i.a.) of 1×105 heat-inactivated B. burgdorferi in 10 μl of PBS into the right knee joint of naive or knock-out mice. 4 hour after i.a. injection, synovial specimens were isolated. After one day, knee joints were removed for histology.
Patella washouts and cytokine measurements
Protein levels of murine IL-1β, IL-6 or KC were measured in patellae washouts. 4h after injection of 1×105 sp B. burgdorferi, patellae were isolated from inflamed knee joints and cultured 1 hour at RT in RPMI 1640 medium containing 0.1% bovine serum albumin (200 μl / patella). Thereafter supernatant was harvested and centrifugated for 5 minutes at 1000 × g. For intracellular IL-1β levels, patellae were frozen directly after isolation. After repeated freeze-thawing IL-1β was determined. Mouse cytokines were determined by Luminex technology, kits for IL-1β, IL-6 and KC were obtained from Bio-Rad (Hercules, CA, USA).
Histological analysis
Mice were sacrificed by cervical dislocation. Whole knee joints were removed and fixed in 4% formaldehyde for 7 days before decalcification in 5% formic acid and processing for paraffin embedding. Tissue sections (7 μm) were stained with Haematoxylin/Eosin. Histopathological changes in the knee joints were scored in the patella/femur region on 5 semi-serial sections, spaced 140 μm apart. Scoring was performed on decoded slides by two separate observers, using the following parameters: in the haematoxylin/eosin stained slides the amount of cells infiltrating the synovial lining and the joint cavity was scored from 0–3 [53;54].
Statistical Analysis
The data are expressed as mean ± SEM unless mentioned otherwise. Differences between experimental groups were tested using the two-tailed Mann-Whitney U test (95% confidence interval) performed on GraphPad Prism 4.0 software (GraphPad). P values of ≤0.05 were considered significant.
Acknowledgments
We thank P. Vandenabeele (Ghent University, Ghent, Belgium). for the generous supply of Rabbit anti-mouse caspase-1 antibody. M.M. Helsen is acknowledged for histology.
M.G. Netea was supported by a VIDI grant of the Netherlands Organization for Scientific Research. This work was also supported by grants from the National Institutes of Health grant number AR056296 and by the American Lebanese and Syrian Associated Charities to T-D.K.
Footnotes
Conflict of interest
No conflict of interest.
References
- 1.Guerau-de-Arellano M, Huber BT. Chemokines and Toll-like receptors in Lyme disease pathogenesis. Trends Mol Med. 2005;11:114–120. doi: 10.1016/j.molmed.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 2.Steere AC, Coburn J, Glickstein L. The emergence of Lyme disease. J Clin Invest. 2004;113:1093–1101. doi: 10.1172/JCI21681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steere AC. Lyme disease. N Engl J Med. 2001;345:115–125. doi: 10.1056/NEJM200107123450207. [DOI] [PubMed] [Google Scholar]
- 4.Balmelli T, Piffaretti JC. Association between different clinical manifestations of Lyme disease and different species of Borrelia burgdorferi sensu lato. Res Microbiol. 1995;146:329–340. doi: 10.1016/0923-2508(96)81056-4. [DOI] [PubMed] [Google Scholar]
- 5.Demaerschalck I, Ben MA, De KM, Hoyois B, Lobet Y, Hoet P, Bigaignon G, Bollen A, Godfroid E. Simultaneous presence of different Borrelia burgdorferi genospecies in biological fluids of Lyme disease patients. J Clin Microbiol. 1995;33:602–608. doi: 10.1128/jcm.33.3.602-608.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang G, Petzke MM, Iyer R, Wu H, Schwartz I. Pattern of proinflammatory cytokine induction in RAW264.7 mouse macrophages is identical for virulent and attenuated Borrelia burgdorferi. J Immunol. 2008;180:8306–8315. doi: 10.4049/jimmunol.180.12.8306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bernardino AL, Myers TA, Alvarez X, Hasegawa A, Philipp MT. Toll-like receptors: insights into their possible role in the pathogenesis of lyme neuroborreliosis. Infect Immun. 2008;76:4385–4395. doi: 10.1128/IAI.00394-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones KL, Muellegger RR, Means TK, Lee M, Glickstein LJ, Damle N, Sikand VK, Luster AD, Steere AC. Higher mRNA levels of chemokines and cytokines associated with macrophage activation in erythema migrans skin lesions in patients from the United States than in patients from Austria with Lyme borreliosis. Clin Infect Dis. 2008;46:85–92. doi: 10.1086/524022. [DOI] [PubMed] [Google Scholar]
- 9.Infante-Duarte C, Horton HF, Byrne MC, Kamradt T. Microbial lipopeptides induce the production of IL-17 in Th cells. J Immunol. 2000;165:6107–6115. doi: 10.4049/jimmunol.165.11.6107. [DOI] [PubMed] [Google Scholar]
- 10.Burchill MA, Nardelli DT, England DM, DeCoster DJ, Christopherson JA, Callister SM, Schell RF. Inhibition of interleukin-17 prevents the development of arthritis in vaccinated mice challenged with Borrelia burgdorferi. Infect Immun. 2003;71:3437–3442. doi: 10.1128/IAI.71.6.3437-3442.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mariathasan S, Monack DM. Inflammasome adaptors and sensors: intracellular regulators of infection and inflammation. Nat Rev Immunol. 2007;7:31–40. doi: 10.1038/nri1997. [DOI] [PubMed] [Google Scholar]
- 12.Eder C. Mechanisms of interleukin-1beta release. Immunobiology. 2009 doi: 10.1016/j.imbio.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 13.Gavrilin MA, Mitra S, Seshadri S, Nateri J, Berhe F, Hall MW, Wewers MD. Pyrin critical to macrophage IL-1beta response to Francisella challenge. J Immunol. 2009;182:7982–7989. doi: 10.4049/jimmunol.0803073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mariathasan S, Newton K, Monack DM, Vucic D, French DM, Lee WP, Roose-Girma M, Erickson S, Dixit VM. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature. 2004;430:213–218. doi: 10.1038/nature02664. [DOI] [PubMed] [Google Scholar]
- 15.Ozoren N, Masumoto J, Franchi L, Kanneganti TD, Body-Malapel M, Erturk I, Jagirdar R, Zhu L, Inohara N, Bertin J, Coyle A, Grant EP, Nunez G. Distinct roles of TLR2 and the adaptor ASC in IL-1beta/IL-18 secretion in response to Listeria monocytogenes. J Immunol. 2006;176:4337–4342. doi: 10.4049/jimmunol.176.7.4337. [DOI] [PubMed] [Google Scholar]
- 16.Zamboni DS, Kobayashi KS, Kohlsdorf T, Ogura Y, Long EM, Vance RE, Kuida K, Mariathasan S, Dixit VM, Flavell RA, Dietrich WF, Roy CR. The Birc1e cytosolic pattern-recognition receptor contributes to the detection and control of Legionella pneumophila infection. Nat Immunol. 2006;7:318–325. doi: 10.1038/ni1305. [DOI] [PubMed] [Google Scholar]
- 17.costa-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007;8:942–949. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- 18.Bettelli E, Oukka M, Kuchroo VK. T(H)-17 cells in the circle of immunity and autoimmunity. Nat Immunol. 2007;8:345–350. doi: 10.1038/ni0407-345. [DOI] [PubMed] [Google Scholar]
- 19.Joosten LA, bdollahi-Roodsaz S, Heuvelmans-Jacobs M, Helsen MM, van den Bersselaar LA, Oppers-Walgreen B, Koenders MI, van den Berg WB. T cell dependence of chronic destructive murine arthritis induced by repeated local activation of Toll-like receptor-driven pathways: crucial role of both interleukin-1beta and interleukin-17. Arthritis Rheum. 2008;58:98–108. doi: 10.1002/art.23152. [DOI] [PubMed] [Google Scholar]
- 20.Sutton C, Brereton C, Keogh B, Mills KH, Lavelle EC. A crucial role for interleukin (IL)-1 in the induction of IL-17-producing T cells that mediate autoimmune encephalomyelitis. J Exp Med. 2006;203:1685–1691. doi: 10.1084/jem.20060285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dinarello CA, Novick D, Puren AJ, Fantuzzi G, Shapiro L, Muhl H, Yoon DY, Reznikov LL, Kim SH, Rubinstein M. Overview of interleukin-18: more than an interferon-gamma inducing factor. J Leukoc Biol. 1998;63:658–664. [PubMed] [Google Scholar]
- 22.Jones KL, Muellegger RR, Means TK, Lee M, Glickstein LJ, Damle N, Sikand VK, Luster AD, Steere AC. Higher mRNA levels of chemokines and cytokines associated with macrophage activation in erythema migrans skin lesions in patients from the United States than in patients from Austria with Lyme borreliosis. Clin Infect Dis. 2008;46:85–92. doi: 10.1086/524022. [DOI] [PubMed] [Google Scholar]
- 23.Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X, Gorman DM, Bazan JF, Kastelein RA. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 24.Kim S, Bauernfeind F, Ablasser A, Hartmann G, Fitzgerald KA, Latz E, Hornung V. Listeria monocytogenes is sensed by the NLRP3 and AIM2 inflammasome. Eur J Immunol. 2010;40:1545–1551. doi: 10.1002/eji.201040425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu N, Belperron AA, Booth CJ, Bockenstedt LK. The Caspase 1 Inflammasome is Not Required for Control of Murine Lyme Borreliosis. Infect Immun. 2009 doi: 10.1128/IAI.00100-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Collins C, Shi C, Russell JQ, Fortner KA, Budd RC. Activation of gamma delta T cells by Borrelia burgdorferi is indirect via a TLR- and caspase-dependent pathway. J Immunol. 2008;181:2392–2398. doi: 10.4049/jimmunol.181.4.2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Montgomery RR, Wang XM, Malawista SE. Murine Lyme disease: no evidence for active immune down-regulation in resolving or subclinical infection. J Infect Dis. 2001;183:1631–1637. doi: 10.1086/320703. [DOI] [PubMed] [Google Scholar]
- 28.Potter MR, Rittling SR, Denhardt DT, Roper RJ, Weis JH, Teuscher C, Weis JJ. Role of osteopontin in murine Lyme arthritis and host defense against Borrelia burgdorferi. Infect Immun. 2002;70:1372–1381. doi: 10.1128/IAI.70.3.1372-1381.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wooten RM, Ma Y, Yoder RA, Brown JP, Weis JH, Zachary JF, Kirschning CJ, Weis JJ. Toll-like receptor 2 is required for innate, but not acquired, host defense to Borrelia burgdorferi. J Immunol. 2002;168:348–355. doi: 10.4049/jimmunol.168.1.348. [DOI] [PubMed] [Google Scholar]
- 30.Brown CR, Reiner SL. Experimental lyme arthritis in the absence of interleukin-4 or gamma interferon. Infect Immun. 1999;67:3329–3333. doi: 10.1128/iai.67.7.3329-3333.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Glickstein L, Edelstein M, Dong JZ. Gamma interferon is not required for arthritis resistance in the murine Lyme disease model. Infect Immun. 2001;69:3737–3743. doi: 10.1128/IAI.69.6.3737-3743.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jones KL, Muellegger RR, Means TK, Lee M, Glickstein LJ, Damle N, Sikand VK, Luster AD, Steere AC. Higher mRNA levels of chemokines and cytokines associated with macrophage activation in erythema migrans skin lesions in patients from the United States than in patients from Austria with Lyme borreliosis. Clin Infect Dis. 2008;46:85–92. doi: 10.1086/524022. [DOI] [PubMed] [Google Scholar]
- 33.Ferber IA, Brocke S, Taylor-Edwards C, Ridgway W, Dinisco C, Steinman L, Dalton D, Fathman CG. Mice with a disrupted IFN-gamma gene are susceptible to the induction of experimental autoimmune encephalomyelitis (EAE) J Immunol. 1996;156:5–7. [PubMed] [Google Scholar]
- 34.McKisic MD, Redmond WL, Barthold SW. Cutting edge: T cell-mediated pathology in murine Lyme borreliosis. J Immunol. 2000;164:6096–6099. doi: 10.4049/jimmunol.164.12.6096. [DOI] [PubMed] [Google Scholar]
- 35.Yssel H, Shanafelt MC, Soderberg C, Schneider PV, Anzola J, Peltz G. Borrelia burgdorferi activates a T helper type 1-like T cell subset in Lyme arthritis. J Exp Med. 1991;174:593–601. doi: 10.1084/jem.174.3.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jones KL, Muellegger RR, Means TK, Lee M, Glickstein LJ, Damle N, Sikand VK, Luster AD, Steere AC. Higher mRNA levels of chemokines and cytokines associated with macrophage activation in erythema migrans skin lesions in patients from the United States than in patients from Austria with Lyme borreliosis. Clin Infect Dis. 2008;46:85–92. doi: 10.1086/524022. [DOI] [PubMed] [Google Scholar]
- 37.Dame TM, Orenzoff BL, Palmer LE, Furie MB. IFN-gamma alters the response of Borrelia burgdorferi-activated endothelium to favor chronic inflammation. J Immunol. 2007;178:1172–1179. doi: 10.4049/jimmunol.178.2.1172. [DOI] [PubMed] [Google Scholar]
- 38.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 40.Chung Y, Chang SH, Martinez GJ, Yang XO, Nurieva R, Kang HS, Ma L, Watowich SS, Jetten AM, Tian Q, Dong C. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity. 2009;30:576–587. doi: 10.1016/j.immuni.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shahrara S, Huang Q, Mandelin AM, Pope RM. TH-17 cells in rheumatoid arthritis. Arthritis Res Ther. 2008;10:R93. doi: 10.1186/ar2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chabaud M, Garnero P, Dayer JM, Guerne PA, Fossiez F, Miossec P. Contribution of interleukin 17 to synovium matrix destruction in rheumatoid arthritis. Cytokine. 2000;12:1092–1099. doi: 10.1006/cyto.2000.0681. [DOI] [PubMed] [Google Scholar]
- 43.Codolo G, Amedei A, Steere AC, Papinutto E, Cappon A, Polenghi A, Benagiano M, Paccani SR, Sambri V, Del PG, Baldari CT, Zanotti G, Montecucco C, D’Elios MM, de BM. Borrelia burgdorferi NapA-driven Th17 cell inflammation in lyme arthritis. Arthritis Rheum. 2008;58:3609–3617. doi: 10.1002/art.23972. [DOI] [PubMed] [Google Scholar]
- 44.Nardelli DT, Burchill MA, England DM, Torrealba J, Callister SM, Schell RF. Association of CD4+ CD25+ T cells with prevention of severe destructive arthritis in Borrelia burgdorferi-vaccinated and challenged gamma interferon-deficient mice treated with anti-interleukin-17 antibody. Clin Diagn Lab Immunol. 2004;11:1075–1084. doi: 10.1128/CDLI.11.6.1075-1084.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hudson CA, Christophi GP, Gruber RC, Wilmore JR, Lawrence DA, Massa PT. Induction of IL-33 expression and activity in central nervous system glia. J Leukoc Biol. 2008;84:631–643. doi: 10.1189/jlb.1207830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cayrol C, Girard JP. The IL-1-like cytokine IL-33 is inactivated after maturation by caspase-1. Proc Natl Acad Sci USA. 2009;106:9021–9026. doi: 10.1073/pnas.0812690106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Talabot-Ayer D, Lamacchia C, Gabay C, Palmer G. Interleukin-33 is biologically active independently of caspase-1 cleavage. J Biol Chem. 2009 doi: 10.1074/jbc.M901744200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ali S, Nguyen DQ, Falk W, Martin MU. Caspase 3 inactivates biologically active full length interleukin-33 as a classical cytokine but does not prohibit nuclear translocation. Biochem Biophys Res Commun. 2010;391:1512–1516. doi: 10.1016/j.bbrc.2009.12.107. [DOI] [PubMed] [Google Scholar]
- 49.Fantuzzi G, Ku G, Harding MW, Livingston DJ, Sipe JD, Kuida K, Flavell RA, Dinarello CA. Response to local inflammation of IL-1 beta-converting enzyme- deficient mice. J Immunol. 1997;158:1818–1824. [PubMed] [Google Scholar]
- 50.Kuida K, Lippke JA, Ku G, Harding MW, Livingston DJ, Su MS, Flavell RA. Altered cytokine export and apoptosis in mice deficient in interleukin-1 beta converting enzyme. Science. 1995;267:2000–2003. doi: 10.1126/science.7535475. [DOI] [PubMed] [Google Scholar]
- 51.Takeda K, Tsutsui H, Yoshimoto T, Adachi O, Yoshida N, Kishimoto T, Okamura H, Nakanishi K, Akira S. Defective NK cell activity and Th1 response in IL-18-deficient mice. Immunity. 1998;8:383–390. doi: 10.1016/s1074-7613(00)80543-9. [DOI] [PubMed] [Google Scholar]
- 52.Netea MG, Demacker PN, Kullberg BJ, Boerman OC, Verschueren I, Stalenhoef AF, van der Meer JW. Low-density lipoprotein receptor-deficient mice are protected against lethal endotoxemia and severe gram-negative infections. J Clin Invest. 1996;97:1366–1372. doi: 10.1172/JCI118556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Joosten LA, Heinhuis B, bdollahi-Roodsaz S, Ferwerda G, Lebourhis L, Philpott DJ, Nahori MA, Popa C, Morre SA, van der Meer JW, Girardin SE, Netea MG, van den Berg WB. Differential function of the NACHT-LRR (NLR) members Nod1 and Nod2 in arthritis. Proc Natl Acad Sci USA. 2008;105:9017–9022. doi: 10.1073/pnas.0710445105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Joosten LA, Netea MG, Fantuzzi G, Koenders MI, Helsen MM, Sparrer H, Pham CT, van der Meer JW, Dinarello CA, van den Berg WB. Inflammatory arthritis in caspase 1 gene-deficient mice: contribution of proteinase 3 to caspase 1-independent production of bioactive interleukin-1beta. Arthritis Rheum. 2009;60:3651–3662. doi: 10.1002/art.25006. [DOI] [PMC free article] [PubMed] [Google Scholar]