Abstract
Objectives
Dichloroacetate (DCA) is a highly bioavailable small molecule that inhibits pyruvate dehydrogenase kinase, promoting glucose oxidation and reversing the glycolytic phenotype in preclinical cancer studies. We designed this open label phase II trial to determine the response rate, safety, and tolerability of oral DCA in patients with metastatic breast cancer and advanced stage NSCLC.
Materials and Methods
This trial was conducted with DCA 6.25 mg/kg orally twice daily in previously treated stage IIIB/IV non-small cell lung cancer (NSCLC) or stage IV breast cancer. Growth inhibition by DCA was also evaluated in a panel of 54 NSCLC cell lines with and without cytotoxic chemotherapeutics (cisplatin and docetaxel) in normoxic and hypoxic conditions.
Results and Conclusions
Under normoxic conditions in vitro, single agent IC50 was > 2 mM for all evaluated cell lines. Synergy with cisplatin was seen in some cell lines under hypoxic conditions. In the clinical trial, after seven patients were enrolled, the study was closed based on safety concerns. The only breast cancer patient had stable disease after 8 weeks, quickly followed by progression in the brain. Two patients withdrew consent within a week of enrollment. Two patients had disease progression prior to the first scheduled scans. Within one week of initiating DCA, one patient died suddenly of unknown cause, and one experienced a fatal pulmonary embolism. We conclude that patients with previously treated advanced NSCLC did not benefit from oral DCA. In the absence of a larger controlled trial, firm conclusions regarding the association between these adverse events and DCA are unclear. Further development of DCA should be in patients with longer life expectancy, in whom sustained therapeutic levels can be achieved, and potentially in combination with cisplatin.
Keywords: dichloroacetate, non-small cell lung cancer, cisplatin
Introduction
Cancer is the leading cause of death worldwide, accounting for 7.6 million deaths in 2008, and cancer deaths are projected to continue to rise to an estimated 13.1 million deaths in 2030 [1]. In the US, lung cancer is the leading cause of cancer-related death, in both men and women, with an estimated 160,340 deaths in 2012 [2]. Most lung cancer cases are non-small cell lung cancer (NSCLC), which comprise over 85% of all lung cancers diagnosed in the US [2]. Breast cancer remains the second leading cause of cancer-related death in American women with an estimated 39,510 deaths for 2012 [2]. Therefore, the development of new treatment strategies is essential to improve outcomes for patients with metastatic breast cancer and advanced NSCLC.
Breast cancer and NSCLC, along with many other cancers, share a common aberration in cellular metabolism [3–5]. In normal cells, energy production from glucose most commonly occurs via oxidative phosphorylation in the mitochondria, a highly efficient pathway for energy production, providing up to 36 ATPs from one glucose molecule [6]. Cancer cells, however, exhibit elevated glucose uptake and preferentially produce energy via increased fermentation in the cytoplasm, which occurs at a more rapid rate but produces only 2 ATPs per glucose molecule [4, 7, 8]. Cancer cells may rely on this increased glycolytic pathway flux to provide more biosynthetic precursors important for macromolecule biosynthesis and cell growth [8]. This high glycolytic activity observed in cancer cells is known as the Warburg Effect [4].
Many studies have found that mutations in oncogenes and tumor suppressor genes promote the Warburg Effect [9–13], however, cellular adaptation to hypoxic environments likely also contributes to this phenomenon in vivo [14]. Premalignant lesions develop in a microenvironment that is low in oxygen [15]. Cells which survive in hypoxic settings continue to multiply, and continued growth brings dividing cells further away from the oxygen source, increasing this selective pressure [15]. Increased levels of hypoxia-inducible factor-1α (HIF-1α), a ubiquitously expressed oxygen-sensitive transcription factor that triggers multiple responses to hypoxic conditions, is one adaptive mechanism used by premalignancies [15]. HIF-1α increases expression of pyruvate dehydrogenase kinase (PDHK) [17–18], which inhibits pyruvate dehydrogenase (PDH), thereby inhibiting the conversion of pyruvate to acetyl-CoA, the substrate oxidized in the mitochondrial TCA cycle to produce ATP via the electron transport chain [6–7]. Therefore, in hypoxic environments, PDH (the gatekeeper of mitochondrial glucose oxidation) activity is inhibited, glucose metabolism shifts from oxidative phosphorylation in the mitochondria to fermentation in the cytoplasm, and glycolytic activity is increased to maintain sufficient ATP production via the quicker but less efficient aerobic glycolysis [6, 7, 18]. This metabolic adaptation leads to suppression of apoptosis, providing a proliferative advantage in cancer cells [19–21].
Switching of energy metabolism in cancer cells to oxidative phosphorylation can promote apoptosis and increase sensitivity to chemotherapy and radiation [22, 23]. This switch can be accomplished by activating PDH [6, 7], which results in increases in the following: pyruvate entry to the mitochondria, acetyl-CoA levels, TCA cycle activity, and reactive oxygen species (ROS) production.
DCA is a small compound with good oral bioavailability that promotes a metabolic shift from cytoplasmic glycolysis to mitochondrial oxidative phosphorylation by inhibiting PDHK [24–27]. DCA has been used for over 25 years to treat children and adults with mitochondrial disorders. It has shown relatively modest toxicities, mostly limited to neurotoxocity (non-demyelinating, dose-dependent and reversible peripheral neuropathy after prolonged use) [28]. Several preclinical studies have successfully demonstrated DCA’s anti-tumor activity [25, 26, 29–31]. A recent clinical trial performed at the University of Alberta found that DCA (6.25 mg/kg po bid) could be administered safely and effectively after debulking surgery, temozolamide, and radiation in the management of glioblastoma multiforme (GBM) [27]. DCA achieves 100% bioavailability with excellent central nervous system (CNS) penetration, increasing its potential in cancers involving the CNS [24, 26, 27]. The half-life of DCA after an oral dose is under an hour, but there is evidence that DCA inhibits its own metabolism, leading to sustained trough levels in the low mM range. We designed this phase II clinical trial to determine the response rate of oral dichloroacetate in patients with previously treated and/or metastatic breast cancer and NSCLC. In addition, we studied the effects of DCA across a panel of 54 NSCLC cell lines.
Patients, Materials and Methods
Patients
Eligible patients were ≥18 years old with pathologically confirmed stage IV breast cancer or stage IIIB/IV NSCLC (by AJCC Staging 6th Edition) with radiographically measurable disease by RECIST 1.0 [32]. Eligible lung cancer patients either demonstrated disease progression despite receiving platinum-based chemotherapy, or refused recommended chemotherapy. Eligible metastatic breast cancer patients received prior anthracycline and taxane-based chemotherapy, hormonal therapy if the tumor was ER positive, and trastuzumab if immunohistochemistry (IHC) or fluorescent in-situ hybridization (FISH) demonstrated HER2 amplification. Patients were required to have an Eastern Cooperative Oncology Group Performance Status (ECOG PS) of 0–2 (this was changed to 0–1 during the course of the study) [33]. Life expectancy of at least 12 weeks, and adequate bone marrow, hepatic, and renal function were required. Ejection fractions were evaluated using multi-gated acquisition (MUGA) scan, and only those with normal ejection fractions were included in this study. Patients were excluded for peripheral neuropathy > grade 1 at time of study initiation, pregnancy, other invasive cancers within the last two years (with the exception of non-melanoma skin cancer), cancer treatment within 28 days of first DCA dose (with the exception of palliative radiation to the bone and trastuzumab in HER-2 positive patients), active CNS metastases, malabsorption syndromes, or medications that may interact with DCA.
This study was approved by the Institutional Review Board at the University of California Los Angeles (UCLA), and all patients provided written informed consent documentation prior to enrollment. The study was monitored by the Jonsson Comprehensive Cancer Center (JCCC) Data Safety and Monitoring Board (DSMB).
Study design
This single arm, phase II, open-labeled study was designed to observe the role of oral DCA therapy in the management of stage IIIB/IV NSCLC and metastatic breast cancer. 29 patients were planned for the NSCLC cohort. As part of a Simon 2-Stage design, the breast cancer cohort was to enroll 18 patients with expansion to 43 patients if 3 responses were seen among the first 18 patients. The primary endpoint was response rate. Secondary endpoints included safety/tolerability, progression free survival (PFS) and overall survival (OS). The analysis described in the manuscript is descriptive based on the low number of patients enrolled.
Treatment
Based on dosage data determined by a glioblastoma trial performed by Michelakis et al in 2010 [27], as well as trials published with DCA as a therapy for patients with congenital mitochondria diseases [34], a dose of 6.25 mg/kg orally twice daily was chosen. DCA was purchased from a commercial chemical supplier (TCI America, Eugene OR). Each dose was administered with food. Patients were continued on treatment until radiographic progression, clinical progression, unacceptable toxicity, withdrawal of consent, or death. One dose reduction to 3.25 mg/kg twice daily was allowed in the setting of grade ≥2 adverse events after resolution to grade ≤1.
Study Assessments
Safety
Safety assessments occurred within 28 days of therapy and on day 1, 8 (added during the study), 15, 29, and every 28 days thereafter as well as at the end of study. Assessments included: history and physical examination (including a focused neurological examination), vital signs, blood counts with differential, adverse events (AEs) graded according to NCI-CTCAE version 3.0, concomitant medications, and standard hematology and chemistry tests. [35] During the study, a requirement for a baseline MRI of the brain with contrast was added. CT scan of the chest and upper abdomen with contrast was required within 28 days of enrollment, and tumor response was evaluated radiographically using the RECIST 1.0 [32] every 8 weeks. An optional PET scan was offered at baseline, after 14 days and 3 months of therapy.
Cell lines, cell cultures and reagents
DCA, cisplatin and docetaxel were studied in 54 NSCLC cell lines in vitro, all of which were obtained from American Type Culture Collection (ATCC, Manassas VA). Cell lines were: A427, A549, Calu-1, Calu-3, Calu-6, H23, H226, H358, H441, H460, H520, H522, H596, H661, H810, H838, H1155, H1299, H1385, H1435, H1437, H1563, H1568, H1581, H1623, H1651, H1666, H1703, H1734, H1755, H1793, H1836, H1838, H1869, H1944, H1975, H2023, H2030, H2073, H2106, H2110, H2122, H2126, H2135, H2172, H2228, H2286, H2291, H2342, H2405, H2444, HCC-827, SHP-77, SK-LU-1.
A549 cells were cultured in tissue culture medium F12-K (ATCC) supplemented with 10% heat-inactivated fetal bovine serum (FBS), 1% penicillin G-streptomycin-fungizone solution (PSF, Irvine Scientific, Santa Ana, CA). A427, Calu-6, Calu-3 and SK-LU-1 were grown in EMEM (ATCC) supplemented with 10% heat-inactivated FBS and PSF. H1155, H1581, H1693, H1435, H1623, H1651, H1666, H2085 and H1869, were grown in ACL-4 supplemented with 10% heat-inactivated FBS 5 g/L BSA, 0.02% Insulin, 0.01 mg/ml Transferrin, 25 nM Sodium Selenite, 50 nM Hydrocortisone, 1 ng/ml EGF, 0.01 mM Ethanolamine, 0.01 mM O-phophorylethanolamine, 100 pM 3,3′,5-Triiolo-L-Thyronine (T3), 0.5 mM Sodium Pyruvate, 10 mM HEPES, and 2 mM L-glutamine. H2286, H2073 and H2405 were grown in complete ACL-4 with 2 mg/L BSA (instead of 5 g/L). H1793, H1836, H2342, H2106, H2023, H2126, H2135 and H810 were grown in HITES supplemented with 5% heat-inactivated FBS. The remaining cell lines were cultured in RPMI 1640 (Cellgro, Manassas, VA) supplemented with 10% heat-inactivated FBS, 2mmol/L glutamine (Invitrogen, Carlsbad, CA), and PSF. For all cell lines reported, retesting by genomic DNA, with comparison to the ATCC database, occurred within a year of the described experiments.
In vitro proliferation assays
For single-agent experiments evaluating DCA, cisplatin, and docetaxel, cells were seeded in duplicate at 5,000 to 10,000 cells per well in a 24-well plate. On day 1, drug was added starting from 10 M with 2-fold dilutions over 12 concentrations for all drugs except DCA, for which the starting concentration was 10 mM. Data were compared to untreated controls. Cells were counted using a Z1 Coulter Particle Counter (Beckman Coulter) on day 1 (only controls) and day 6 with percent growth inhibition defined as 100 × (1 − [generations in treated wells/generations in untreated controls]). Experiments were performed in duplicate. Error bars represent standard error for each experiment. Nonlinear curve fitting to data points was performed using the Proc NLIN function in SAS for Windows version 9.2 (SAS Institute, Inc., Cary, NC) using a basic four-parameter sigmoid model. IC50 (concentration needed to prevent 50% of cell population doublings) were interpolated from the resulting curves. [39]
Measurement of oxygen consumption rates post 48hr DCA treatment
Cellular oxygen consumption rates were measured in 4 cell lines using a water-jacketed (37°C) anaerobic chamber fitted with a fiber optic oxygen sensor (INSTECH Model 110 Optic Oxygen Monitor). The fiber optic probe was calibrated with 15mM sodium hydrosulfite (Sigma) corresponding to 0% oxygen, and cell culture media corresponding to 20.9% oxygen.
In vitro combination proliferation assays
For combination in vitro proliferation assays, the same techniques were performed as described above, but on day 1, a combination of DCA and cisplatin or docetaxel starting at 2 M with 2-fold dilutions over 6 concentrations were added. Combination indices were then calculated as described elsewhere (Calcusyn, Biosoft). [36] This evaluation was performed under both normoxic and hypoxic conditions of 0.5% oxygen, 5% CO2 using a c-chamber and ProOx model C2 gas oxygen and CO2 controller from BioSpherex.
Results
Patient characteristics
7 patients (6 with NSCLC and 1 with breast cancer) were enrolled in this open label, phase II clinical trial. All patients were previously treated, most with multiple previous therapies. A summary of patient demographics, tumor characteristics, and previous treatments received are summarized in table 1.
Table 1. Baseline characteristics of patients treated with DCA.
Baseline characteristics, including age, gender, race, tumor type, histologic grade, and ECOG performance status.
| Subject | Gender | Stage at diagnosis | Histologic grade | Race | Age | ECOG status |
|---|---|---|---|---|---|---|
| Breast cancer 1 | F | II | G3 | White | 44 | 0 |
| NSCLC 1 | M | IV | G3 | White | 77 | 1 |
| NSCLC 2 | F | IV | GX | White | 69 | 2 |
| NSCLC 3 | F | IV | GX | White | 78 | 0 |
| NSCLC 4 | M | IV | GX | Asian | 57 | 1 |
| NSCLC 5 | M | IV | G3 | White | 42 | 1 |
| NSCLC 6 | F | IV | GX | Asian | 38 | 2 |
Clinical outcomes
Two patients died suddenly within one week of starting DCA, one from a fatal pulmonary embolism (PE) and the other of unknown cause, presumed to be a cerebrovascular accident based on the available medical information. The relation of these deaths to DCA was uncertain. The median number of days the patients stayed on the trial was 12 (range 4 to 72 days). The one breast cancer patient experienced stable disease for 2 months before developing brain metastases, and two patients experienced disease progression prior to their first set of scans (scheduled for 8 weeks). Two patients withdrew consent, one after experiencing a PE and the other to enroll in hospice care. Outcome data is summarized in Table 2. Due to the lack of clinical benefit observed along with the two deaths, the JCCC DSMB closed the study.
Table 2. Clinical Activity of DCA.
Outcome data is limited by small numbers of patients. Data regarding days on study, dose modifications or delays and best response to therapy are shown.
| Subject | # Days on Study | Dose Reduction Required? | Best Response on DCA | Dose Delay (Reason) |
|---|---|---|---|---|
| Breast Cancer 1 | 72 Days | No | stable disease | No |
| NSCLC 1 | 7 Days | No | patient withdrew consent | Yes (Grade 3 Right Leg Swelling*) |
| NSCLC 2 | 46 Days | No | disease progression | Yes (Grade 3 Elevated AST**) |
| NSCLC 3 | 12 Days | No | patient withdrew consent | No |
| NSCLC 4 | 57 Days | No | disease progression | Yes (Grade 2 Tremors) |
| NSCLC 5 | 4 Days | No | death | No |
| NSCLC 6 | 7 Days | No | death | No |
| Mean # Days on Study | 29.3 Days | |||
| Median # Days on Study | 12 Days |
Leg swelling not likely related to DCA
Elevated AST likely due to progression of liver metastases and not DCA
Safety and tolerability
All patients experienced AEs, most low grade (Table 3). The most frequent AEs were lower extremity edema and abdominal pain, both of which were seen in 3 patients. Grades ≥ 3 AEs were: abdominal pain (1), lower extremity edema (1), elevated AST (1), pulmonary embolism (2), hyponatremia (1), volume depletion (1), and sudden death (1). Four out of the 7 patients experienced grade >3 AEs. No patients received a dose reduction, but 3 patients required dose delay for grade ≥ 2 AEs. There were two instances of grade 1 disequilibrium that were attributed to DCA and one seizure that was attributed to a new brain metastasis, but no other neurological AEs.
Table 3. List of adverse events.
Each adverse event is listed, including number of patients experiencing that event and grade.
| AE Description | Grade 1 | Grade 2 | Grade 3 | Grade 4/5 | Total # with serious AE (grades 3–5) |
|---|---|---|---|---|---|
| Abdominal Pain | 2 | 1 | 1 | ||
| Fever | 1 | 0 | |||
| Fatigue | 2 | 1 | 0 | ||
| Lower Extremity Edema | 2 | 1 | 1 | ||
| Urinary Retention | 1 | 0 | |||
| Elevated AST | 1 | 1 | |||
| Dyspnea | 2 | 0 | |||
| Pain (Back, Pelvis, Leg) | 1 | 0 | |||
| Constipation | 1 | 0 | |||
| Xerostomia | 1 | 0 | |||
| Vomiting | 1 | 0 | |||
| Tremor | 1 | 1 | 0 | ||
| Pulmonary Embolus | 1 | 1 | 2 | ||
| Nausea | 1 | 0 | |||
| Candidiasis | 2 | 0 | |||
| Disequilibrium | 2 | 0 | |||
| Lower Extremity Coldness | 1 | 0 | |||
| Tumor Pain | 1 | 0 | |||
| Seizures* | 1 | 0 | |||
| Hyponatremia | 1 | 1 | |||
| Volume Depletion | 1 | 1 | |||
| Sudden Death | 1 | 1 |
Seizures likely due to new brain metastases and not DCA
Sensitivity to DCA, cisplatin, and docetaxel in human NSCLC cell lines in vitro
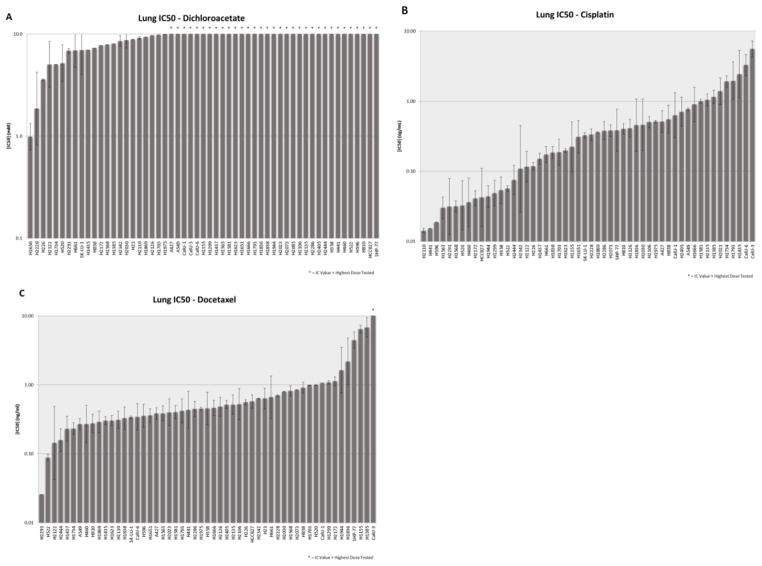
We studied several well-characterized NSCLC cell lines in order to determine the in vitro effects of DCA by exposure to serial dilutions. In addition, we sought to determine the possibility of synergy with commonly used cytotoxic agents. Limited sensitivity was seen across the cell line panel with DCA, with no lines demonstrating an IC50 < 2 mM (Figure 1A). Sensitivity of the cell lines to the standard chemotherapeutics cisplatin (Figure 1B) and docetaxel (Figure 1C) was determined so that they could be evaluated as part of combination assays with DCA.
Figure 1. In vitro sensitivity to DCA with cisplatin or docetaxel.
54 NSCLC cell lines with IC50 for DCA represented in mM (A). The NSCLC cell lines were also evaluated for IC50 for cisplatin (B) and docetaxel (C) represented in M. Error bars indicate the standard error based on multiple experiments. Asterisks indicate cell lines for which the IC50 exceeded the highest dose evaluated.
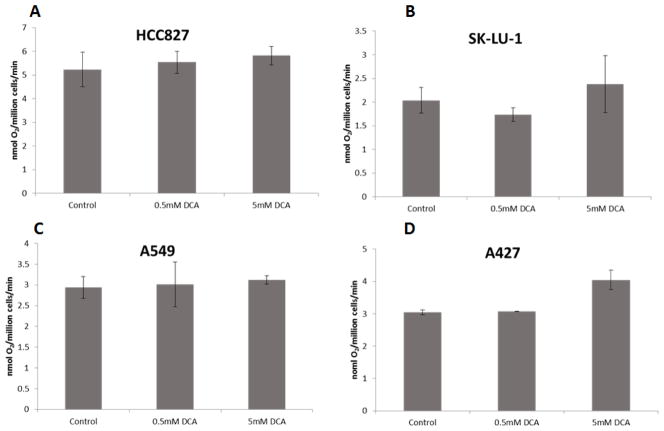
Oxygen consumption
We observed a trend towards increased oxygen consumption in cell lines treated with DCA, with 5 mM DCA (Figure 2). This increase in oxygen consumption reached statistical significance (p = 0.005) for the A427 cell line after exposure to 5 mM DCA (Figure 2D).
Figure 2. Oxygen consumption rates of NSCLC cell lines exposed to DCA.
Oxygen consumption rates of HCC-827 (A), SKLU-1 (B), A549 (C) or A427 (D) cells treated with either vehicle (H2O), 0.5 mM DCA or 5 mM DCA for 48hrs. Error bars indicate the standard error based on multiple experiments.
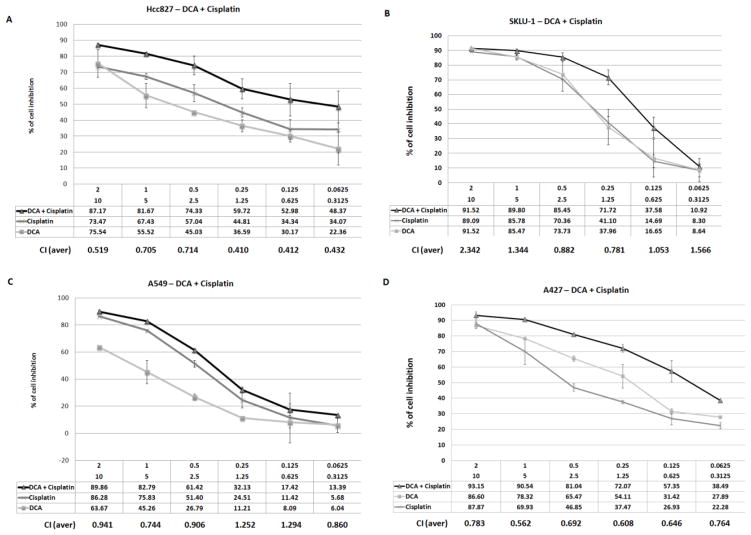
Combination of DCA and cisplatin or docetaxel in selected cell lines
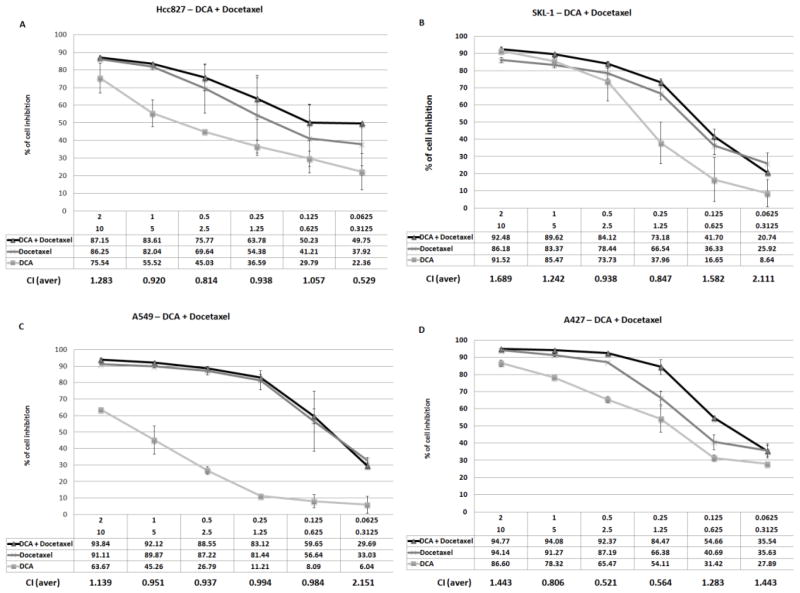
The in vitro response to DCA in combination with cisplatin and docetaxel was compared to cisplatin and docetaxel alone. Under normoxic conditions, DCA did not enhance the anti-proliferative action of cisplatin or docetaxel (data not shown). However, under hypoxic conditions, a condition that is known to induce the expression of DCA’s target enzyme PDHK, the combination of DCA and cisplatin showed evidence of synergy in two of the evaluated cell lines, A427 and HCC-827 (Figure 3A and B). The combination of DCA and cisplatin in SKLU-1 and A549 did not show evidence of synergy at relevant doses (Figure 3C and D). Experiments combining DCA and docetaxel did not demonstrate a synergistic effect (Figure 4).
Figure 3. In vitro combination data evaluating DCA and cisplatin under hypoxic conditions.
The sensitivity of four cell lines, HCC-827 (A), SKLU-1 (B), A549 (C) and A427 (D), were evaluated by exposing cells to decreasing concentrations of DCA starting at 10 mM and cisplatin starting at 2 M. Error bars indicate the standard error based on multiple experiments.
Figure 4. In vitro combination data evaluating DCA and docetaxel under hypoxic conditions.
The sensitivity of four cell lines, HCC-827 (A), SKLU-1(B), A549 (C) and A427 (D), were evaluated by exposing cell to decreasing concentrations of DCA starting at 10 mM and docetaxel starting at 2 M. Error bars indicate the standard error based on multiple experiments.
Discussion
Several preclinical studies and one recent clinical trial have suggested that DCA holds promise as a novel anti-cancer agent [24, 25, 27, 29–31]. DCA has also received a fair amount of attention in the lay press [37]. Its relatively favorable side effects and low cost make DCA an attractive consideration for patients, though definitive clinical data is lacking.
Therefore, we designed this phase II trial to determine the response rate, safety, and tolerability of oral DCA in patients with metastatic breast cancer and advanced stage NSCLC. Our study demonstrated no clinical improvement in 7 subjects with advanced stage cancers treated with DCA. The study was modified to address safety concerns at multiple time points, including amendments to include only patients with baseline ECOG 0 or 1, require a baseline MRI of the brain, and add an additional blood draw. The lack of a control arm makes it difficult to assess the expected frequency of AEs. However, the JCCC DSMB felt that the expected rate was exceeded and they terminated the study. The rate of AEs also exceeded what has been seen in our studies of similar populations [39].
Oral DCA is rapidly and completely absorbed, but it is also rapidly cleared from the plasma, and repeated dosing is required to obtain sustained plasma levels [40]. The pharmacokinetic clearance of DCA increases as a function of DCA exposure in 20 healthy subjects [40, 41]. This pharmacokinetic profile may be in part due to DCA-mediated inhibition and decrease in expression of GSTz1, the enzyme responsible for dehalogenation of DCA, an important step for its metabolism and excretion [41].
Michelakis et. al. examined activity of DCA in five glioblastoma (GBM) patients, initially managed with surgery, radiation, and temozolomide, after 15 months of DCA therapy and demonstrated radiographic evidence of tumor regression in three of four patients [27]. In the three responsive patients, tissue samples before and after DCA therapy all demonstrated decreased cell proliferation, increased apoptosis, and increased PDH activity [27]. However, therapeutic trough DCA levels were only achieved after several months of consistent dosing [27]. Thus, therapeutic DCA levels may not have been achieved in our patient population in time to prevent disease progression, suggesting that DCA may not be an ideal drug for cancers at risk of rapid progression.
In our in vitro analysis of the effect of DCA on NSCLC, significantly enhanced oxygen consumption was observed with 5 mM DCA in the A427 cell line. With respect to the antiproliferative activity of DCA, no significant single agent inhibition or synergistic activity with docetaxel or cisplatin under normoxic conditions was observed. However, we observed a trend towards enhanced growth inhibition when DCA was combined with cisplatin in two of four evaluated cell lines in hypoxic conditions, suggesting that DCA may be an effective agent when given with cisplatin under hypoxic conditions. Similar results have been seen when lung carcinoid cell lines are treated with platinum-based chemotherapy and DCA [31]. A novel chimeric drug generated by combining 2 molecules of DCA with one of cisplatin (mitaplatin) showed clear synergism in terms of cell death in several cancer cell lines [42], including induction of apoptosis in cell lines resistant to cisplatin [43].
The interest in DCA by the general public has led to unique issues in its development as an antineoplastic. Cancer patients have frequently gone to websites which have touted benefits of DCA that are not clearly supported by medical literature [32]. During the conduct of this trial, the investigators received countless calls and e-mails from cancer patients around the world requesting additional information on DCA. Since DCA is commercially available and accessible, patients can (and often have) obtained DCA for treatment of cancer [37]. We received several calls from outside hospitals (who found our information on clinicaltrials.gov) inquiring about toxicity, particularly neurologic, of DCA. On further investigation, these patients were frequently self-administering doses that are larger than what has been shown to be safe in cancer patients.
Conclusion
We acknowledge the difficulty in drawing solid conclusions about efficacy and safety of DCA in patients with advanced cancer, based on our data. However, we feel it is important to publish our experience and stress the danger of using DCA in patients with advanced and rapidly progressing disease, where DCA may not reach adequate drug levels to have the potential for therapeutic effect. Desperate and unfortunate patients may self-medicate with DCA given its wide availability on the internet and the potential for exploitation by for-profit suppliers. Further studies should focus on DCA as a sensitizing agent in combination therapy, in patients with an expected meaningful survival of at least several months.
Acknowledgments
Financial Support: This work was supported by K23CA149079 (Garon)
Funding for this project came from K23CA149079, NIH CTSI UL1TR000124, the Lincy Foundation, the Aljian Family Trust in Memory of James Aljian, the Hecht Foundation, the Wolfen Family Clinical/Translational Lung Cancer Research Program, the Katz Family and the One Ball Matt Golf Tournament.
Footnotes
Conflicts of Interest:
The authors have no conflicts of interest to report.
Clinical Trial Registration Number: NCT01029925
References
- 1.World Health Organization Cancer Fact Sheet. 2012 Feb; 2012 [cited 2012 September 12]; Available from: http://www.who.int/mediacentre/factsheets/fs297/en/
- 2.Howlader NNA, Krapcho M, Neyman N, et al. Cancer Statistics Review, 1975–2009 (Vintage 2009 Populations) 2012 Aug 20; 2012 [cited 2012 September 14]; Available from: http://seer.cancer.gov/csr/1975_2009_pops09/
- 3.Spira A, Ettinger DS. Multidisciplinary Management of Lung Cancer. New England Journal of Medicine. 2004;350:379–92. doi: 10.1056/NEJMra035536. [DOI] [PubMed] [Google Scholar]
- 4.Warburg O. On the Origin of Cancer Cells. Science. 1956;123:309–14. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 5.Feuerecker B, Pirsig S, Seidl C, et al. Lipoic acid inhibits cell proliferation of tumor cells in vitro and in vivo. Cancer Biology and Tharapy. 2012:13. doi: 10.4161/cbt.22003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mathews CK, Holde Kv, Ahern KG. Biochemistry. 3. San Francisco: Benjamin/Cummings; 2000. Electron transport, oxidative phosphorylation, and oxygen metabolism; pp. 522–57. [Google Scholar]
- 7.Mathews CK, Holde Kv, Ahern KG. Biochemistry. 3. San Francisco: Benjamin/Cummings; 2000. Carbohydrate metabolism I: Anaerobic processes in generating metabolic energy; pp. 446–82. [Google Scholar]
- 8.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg Effect: The Metabolic Requirements of Cell Proliferation. Science. 2009;324:1029–33. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elstrom RL, Bauer DE, Buzzai M, et al. Akt Stimulates Aerobic Glycolysis in Cancer Cells. Cancer Research. 2004;64:3892–9. doi: 10.1158/0008-5472.CAN-03-2904. [DOI] [PubMed] [Google Scholar]
- 10.Buzzai M, Bauer DE, Jones RG, et al. The glucose dependence of Akt-transformed cells can be reversed by pharmacologic activation of fatty acid [beta]-oxidation. Oncogene. 2005;24:4165–73. doi: 10.1038/sj.onc.1208622. [DOI] [PubMed] [Google Scholar]
- 11.Bensaad K, Tsuruta A, Selak MA, et al. TIGAR, a p53-Inducible Regulator of Glycolysis and Apoptosis. Cell. 2006;126:107–20. doi: 10.1016/j.cell.2006.05.036. [DOI] [PubMed] [Google Scholar]
- 12.Hu Y, Lu W, Chen G, et al. K-rasG12V transformation leads to mitochondrial dysfunction and a metabolic switch from oxidative phosphorylation to glycolysis. Cell Res. 2012;22:399–412. doi: 10.1038/cr.2011.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ying H, Kimmelman Alec C, Lyssiotis Costas A, et al. Oncogenic Kras Maintains Pancreatic Tumors through Regulation of Anabolic Glucose Metabolism. Cell. 2012;149:656–70. doi: 10.1016/j.cell.2012.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer. 2004;4:891–9. doi: 10.1038/nrc1478. [DOI] [PubMed] [Google Scholar]
- 15.Gatenby RA, Gillies RJ. Why do cancer cells have high aerobic glycolysis? Nature Reviews Cancer. 2004;4:891–9. doi: 10.1038/nrc1478. [DOI] [PubMed] [Google Scholar]
- 16.Loscalzo J. The cellular response to hypoxia: tuning the system with microRNAs. The Journal of Clinical Investigation. 2010;120:3815–7. doi: 10.1172/JCI45105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ke Q, Costa M. Hypoxia-Inducible Factor-1 (HIF-1) Molecular Pharmacology. 2006;70:1469–80. doi: 10.1124/mol.106.027029. [DOI] [PubMed] [Google Scholar]
- 18.Dakubo GD. Mitochondrial Genetics and Cancer. 1. Heidelberg: Springer-Verlag; 2010. The Warburg Phenomenon and Other Metabolic Alterations of Cancer Cells; pp. 39–62. [Google Scholar]
- 19.Remillard CV, Yuan JXJ. Activation of K+ channels: an essential pathway in programmed cell death. American Journal of Physiology - Lung Cellular and Molecular Physiology. 2004;286:L49–L67. doi: 10.1152/ajplung.00041.2003. [DOI] [PubMed] [Google Scholar]
- 20.Bonnet Sb, Archer SL, Allalunis-Turner J, et al. A Mitochondria-K+ Channel Axis Is Suppressed in Cancer and Its Normalization Promotes Apoptosis and Inhibits Cancer Growth. Cancer Cell. 2007;11:37–51. doi: 10.1016/j.ccr.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 21.Ruckenstuhl C, Büttner S, Carmona-Gutierrez D, et al. The Warburg Effect Suppresses Oxidative Stress Induced Apoptosis in a Yeast Model for Cancer. PLoS ONE. 2009;4:e4592. doi: 10.1371/journal.pone.0004592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brahimi-Horn MC, Ben-Hail D, Ilie M, et al. Expression of a Truncated Active Form of VDAC1 in Lung Cancer Associates with Hypoxic Cell Survival and Correlates with Progression to Chemotherapy Resistance. Cancer Research. 2012;72:2140–50. doi: 10.1158/0008-5472.CAN-11-3940. [DOI] [PubMed] [Google Scholar]
- 23.Aykin-Burns N, Slane BG, Liu ATY, et al. Sensitivity to Low-Dose/Low-LET Ionizing Radiation in Mammalian Cells Harboring Mutations in Succinate Dehydrogenase Subunit C is Governed by Mitochondria-Derived Reactive Oxygen Species. Radiation Research. 2011;175:150–8. doi: 10.1667/rr2220.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sutendra G, Dromparis P, Kinnaird A, et al. Mitochondrial activation by inhibition of PDKII suppresses HIF1a signaling and angiogenesis in cancer. Oncogene. 2012 doi: 10.1038/onc.2012.198. [DOI] [PubMed] [Google Scholar]
- 25.Kluza J, Corazao-Rozas P, Touil Y, et al. Inactivation of the HIF-1a/PDK3 Signaling Axis Drives Melanoma toward Mitochondrial Oxidative Metabolism and Potentiates the Therapeutic Activity of Pro-Oxidants. Cancer Research. 2012;72:5035–47. doi: 10.1158/0008-5472.CAN-12-0979. [DOI] [PubMed] [Google Scholar]
- 26.Ayyanathan K, Kesaraju S, Dawson-Scully K, Weissbach H. Combination of Sulindac and Dichloroacetate Kills Cancer Cells via Oxidative Damage. PLoS ONE. 2012;7:e39949. doi: 10.1371/journal.pone.0039949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Michelakis ED, Sutendra G, Dromparis P, et al. Metabolic Modulation of Glioblastoma with Dichloroacetate. Science Translational Medicine. 2010;2:31ra4. doi: 10.1126/scitranslmed.3000677. [DOI] [PubMed] [Google Scholar]
- 28.Pfeffer G, Majamaa K, Turnbull DM, et al. Treatment for mitochondrial disorders. The Cochrane Library. 2012;4:CD004426. doi: 10.1002/14651858.CD004426.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morfouace M, Lalier L, Bahut M, et al. Comparison of Spheroids Formed by Rat Glioma Stem Cells and Neural Stem Cells Reveals Differences in Glucose Metabolism and Promising Therapeutic Applications. Journal of Biological Chemistry. 2012;287:33664–74. doi: 10.1074/jbc.M111.320028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saed GM, Fletcher NM, Jiang ZL, et al. Dichloroacetate Induces Apoptosis of Epithelial Ovarian Cancer Cells Through a Mechanism Involving Modulation of Oxidative Stress. Reproductive Sciences. 2011;18:1253–61. doi: 10.1177/1933719111411731. [DOI] [PubMed] [Google Scholar]
- 31.Fiebiger W, Olszewski U, Ulsperger E, et al. In vitro cytotoxicity of novel platinum-based drugs and dichloroacetate against lung carcinoid cell lines. Clinical and Translational Oncology. 2011;13:43–9. doi: 10.1007/s12094-011-0615-z. [DOI] [PubMed] [Google Scholar]
- 32.Lichtenfeld L. DCA: Cancer breakthrough or urban legend? ABC World News. 2007 [cited 2012 Oct 28]. Available from: http://abcnews.go.com/Health/CancerPreventionAndTreatment/story?id=2848454&page=1#.UI6j2m-jz7o.
- 33.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. American Journal of Clinical Oncology. 1982;5:649–56. [PubMed] [Google Scholar]
- 34.Stacpoole PW, Kerr DS, Barnes C, et al. Controlled Clinical Trial of Dichloroacetate for Treatment of Congenital Lactic Acidosis in Children. Pediatrics. 2006;117:1519–31. doi: 10.1542/peds.2005-1226. [DOI] [PubMed] [Google Scholar]
- 35.Common Terminology Criteria for Adverse Events. Bethesda, MD: NCI; Version 3.0. [cited 2012 Oct 19]. Available from: http://ctep.cancer.gov. [Google Scholar]
- 36.Chou TC. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010;70:440–6. doi: 10.1158/0008-5472.CAN-09-1947. [DOI] [PubMed] [Google Scholar]
- 37.The DCA Site. [cited 2012 Oct 19]. Available from: http://www.thedcasite.com/index.html.
- 38.Nichols L, Saunders R, Knollmann FD. Causes of Death of Patients With Lung Cancer. Archives of Pathology & Laboratory Medicine. 2012;136:1552–7. doi: 10.5858/arpa.2011-0521-OA. [DOI] [PubMed] [Google Scholar]
- 39.Garon EB, Siegfried JM, Dubinett SM, et al. Result of TORI L-03, a randomized, multicenter phase II clinical trial of erlotinib (E) or E + fulvestrant (F) in previously treated advanced non-small cell lung cancer (NSCLC) American Association for Cancer Research; Washington, DC: 2013. p. Abstract # 4664. [Google Scholar]
- 40.Curry SH, Chu PI, Baumgartner TG, et al. Plasma concentrations and metabolic effects of intravenous sodium dichloroacetate. Clin Pharm Ther. 1985;37:89–93. doi: 10.1038/clpt.1985.17. [DOI] [PubMed] [Google Scholar]
- 41.Jia M, Coats B, Chadha M, et al. Human Kinetics of Orally and Intravenously Administered Low-Dose 1,2-13C-Dichloroacetate. The Journal of Clinical Pharmacology. 2006;46:1449–59. doi: 10.1177/0091270006292627. [DOI] [PubMed] [Google Scholar]
- 42.Dhar S, Lippard SJ. Mitaplatin, a potent fusion of cisplatin and the orphan drug dichloroacetate. Proceedings of the National Academy of Sciences. 2009;106:22199–204. doi: 10.1073/pnas.0912276106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xue X, You S, Zhang Q, et al. Mitaplatin Increases Sensitivity of Tumor Cells to Cisplatin by Inducing Mitochondrial Dysfunction. Molecular Pharmaceutics. 2012;9:634–44. doi: 10.1021/mp200571k. [DOI] [PMC free article] [PubMed] [Google Scholar]