Abstract
Objective
Massive perivillous fibrin deposition (MPFD) is associated with serious complications of pregnancy including recurrent spontaneous abortion, fetal growth restriction and fetal demise. The aim of the study was to determine if maternal plasma concentrations of angiogenic/anti-angiogenic factors in MPFD differ from uncomplicated pregnancies.
Study Design
This retrospective longitudinal case-control study included MPFD cases (n=10) and control patients (n=175) with uncomplicated pregnancies who were enrolled in a longitudinal study and delivered at term. Serial plasma concentrations of placental growth factor (PlGF), soluble endoglin (sEng), and soluble vascular endothelial growth factor receptor (sVEGFR) -1 and -2 were determined by ELISA (cases, n=28 samples; controls, n=751 samples). Individual analyte concentrations were averaged across gestational length at specimen collection intervals. Linear mixed models were used to test for differences in log transformed mean analyte concentrations both overall and as a function of time.
Results
1) Patients with MPFD had a lower mean plasma PlGF concentration (p=0.03) and higher mean plasma concentrations of sVEGFR-1 and sEng (both p<0.01) than controls, adjusted for potential confounders; 2) the mean plasma concentration of PlGF differed further among cases and controls as a function of gestational age interval (p<0.0001); however, mean sVEGFR-1 and sEng group differences as a function of gestational age interval approached but did not reach significance (p=0.09, p=0.11, respectively); 3) patients with MPFD had lower mean plasma concentrations of PlGF/sVEGFR-1 (p<0.0001) and PlGF/sEng (p<0.001); both of these relationships differed further as a function of gestational age interval (both p<0.0001); and 4) differences in mean sVEGFR-1, sEng, and the ratios of PlGF/sVEGFR-1 and PlGF/sEng were observed before 20 weeks of gestation.
Conclusion
An imbalance of angiogenic/anti-angiogenic factors is present in patients with MPFD prior to the diagnosis. We propose that these changes participate in the mechanisms responsible for adverse pregnancy outcomes in patients with MPFD.
Keywords: maternal floor infarction, recurrent pregnancy loss, flt-1, soluble vascular endothelial growth factor, placental growth factor, soluble endoglin
Introduction
Massive perivillous fibrin deposition (MPFD), also known as “maternal floor infarction” (MFI), is characterized by obliteration of the villous trophoblast with extensive deposition of fibrinoid material in the intervillous space.1 This condition was first described by Benirschke and Driscoll in 1967 and its frequency is 0.09-0.5%.1-4 MPFD is associated with recurrent serious adverse pregnancy outcomes including spontaneous abortion,1, 5 fetal growth restriction,1, 2, 4, 6-12 and fetal death.2, 4-7, 10, 11, 13-16 The proposed etiologies include autoimmunity (such as anti-phospholipid6, 10, 11 or anti-urokinase antibodies)6 and cytotoxicity due to proliferation of “X-cells” which are extravillous trophoblasts that can produce major basic protein similar to that of eosinophil granules.12 However, the precise mechanisms leading to MPFD are unknown.
Angiogenesis, the development of new blood vessels from preexisting vasculature, is crucial for fetal growth and placental development.17-19 Successful pregnancy requires a balance between angiogenic and anti-angiogenic factors.20-25. A growing body of evidence suggests that an imbalance of angiogenic/anti-angiogenic factors is involved in the pathophysiology of preeclampsia (PE),26-70 pregnancies with small-for-gestational-age neonates (SGA),28, 30, 33, 41, 71-77 spontaneous preterm labor and delivery,78-80 stillbirth,81-83 mirror syndrome,84-88 twin to twin transfusion syndrome,89, 90 and molar pregnancies.91,92 Moreover, changes in the concentrations of the angiogenic factor, placental growth factor (PlGF) and anti-angiogenic factors, soluble vascular endothelial growth factor receptor (sVEGFR)-1 and soluble endoglin (sEng) in maternal circulation, precede the clinical diagnosis of PE,30, 33, 34, 41, 43, 48, 55, 58, 62, 93 SGA30, 41, 72 and stillbirth.83 Since the clinical presentation of MPFD includes conditions associated with derangements of angiogenic and anti-angiogenic factors, it is possible that an anti-angiogenic state may play a role in the genesis of MPFD.
The objective of this study was to determine if pregnancies with MPFD have alterations in maternal plasma concentrations of PlGF, sEng, sVEGFR-1 and sVEGFR-2 before the diagnosis of the condition.
Materials and Methods
Study Design and Patient Selection
A longitudinal retrospective case-control study was conducted by reviewing placenta pathology records in our institution from 2006 to 2011. Cases consisted of patients with placental pathology meeting the diagnostic requirements for MPFD, which was defined as a placenta with perivillous fibrinoid material (either limited to the maternal floor of the placenta or extending from maternal to fetal surfaces) encasing at least 50% of the villi on a minimum of one slide. Controls were women without MPFD in the placenta, who had uncomplicated pregnancies, delivered a term neonate whose birth weight was appropriate for gestational age (10th - 90th percentiles)94 and had plasma samples available for at least five of the following gestational age intervals: 6-9.9, 10-14.9, 15-19.9, 20-23.9, 24-27.9, 28-31.9, 32.-36.9 and ≥37 weeks. These patients were enrolled in a longitudinal protocol to identify biological markers for the prediction of PE, SGA, and stillbirth. Venous samples were collected every 4 weeks until 24 weeks and every 2 weeks thereafter until delivery. Exclusion criteria were 1) multiple gestations and 2) congenital fetal anomaly.
All women provided written informed consent before participating in the study and the use of clinical data and collection and utilization of biological samples for research purposes were approved by the Institutional Review Boards of Wayne State University and the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of health, U.S. Department of Health and Human Services.
Sample Collection and Immunoassays
Venipuncture was performed serially at regular prenatal visits and admissions to the hospital for all normal and MPFD affected pregnancies. Blood was collected into tubes containing EDTA. Samples were centrifuged and stored at -70° C until used for assay. Sensitive and specific immunoassays (R&D systems, Minneapolis, MN) were used to determine maternal plasma concentrations of PlGF, sEng, sVEGFR-1 and -2. All immunoassays utilized the quantitative sandwich enzyme immunoassay technique, and their concentrations in maternal plasma were determined by interpolation from the standard curves. The inter- and intra-assay coefficients of variation (CV) obtained in our laboratory were as follows: PlGF, 6.02 and 4.8%, respectively; s-Eng, 2.3 and 4.6%, respectively; sVEGFR-1, 1.4 and 3.9%, respectively; and sVEGFR-2, 2 and 4%, respectively. The sensitivity of the assays was as follows: PlGF, 9.52 pg/mL; s-Eng, 0.08 ng/mL; sVEGFR-1, 16.97 pg/mL; and sVEGFR-2, 19.01 pg/mL.
Statistical Analysis
Demographic and obstetrical characteristics
Comparisons between continuous variables were performed by Mann-Whitney U tests. Proportions were compared using either Fisher exact or Chi-square tests as appropriate. A p-value <0.05 was considered statistically significant. Descriptive analysis was performed using SPSS Version 15.0 (SPSS, Inc., Chicago, IL, USA).
Longitudinal analysis of angiogenic/anti-angiogenic factor concentrations
Individual analyte concentrations (PlGF, sEng, sVEGFR-1, and sVEGFR2) and their ratios (PlGF/sEng and PlGF/sVEGFR-1) were averaged across four intervals defined by gestational length at venipuncture (<14 weeks, 14-16 weeks, 17-19 weeks, and 20-30 weeks). Linear mixed models were used to test for differences in log10 transformed mean analyte concentrations overall and as a function of time using a robust covariance matrix estimator. Covariables included in adjusted models were selected based on clinical knowledge and factors associated with MPFD and/or analyte concentrations. These included gestational age at venipuncture, body mass index (BMI), maternal age, African American ethnicity and nulliparity. Model reduction was additionally performed based on the plausibility of regression coefficients, association with independent/dependent varbles, magnitude of change in the main effect parameter estimates95 and model fit as indicated by the Bayesian Information Criteria (BIC)95. Linear combinations of model parameters comparing differences between cases and controls at each gestational age interval were used to determine the timing of changes in angiogenic/anti-angiogenic factors. Longitudinal analyses were performed using SAS version 9.3 (SAS Institute Inc, Cary, NC, USA).
Results
Clinical Characteristics
During the study period, 10 pregnancies with MPFD and 175 controls were identified. Table 1 describes the clinical and demographic characteristics of the study population. As expected, the median gestational age at delivery and the median birthweights were lower in the MPFD affected pregnancies than those in uncomplicated pregnancies (each p < 0.001; see Table 1). Pregnancy complications in cases with MPFD included miscarriage in the second trimester (n= 4), fetal growth restriction (n=4) with abnormal umbilical artery Doppler velocimetry (n=3), second and third trimester fetal demise (in-utero: n=5; intrapartum: n=1), and abruptio placentae (n=2). With the exception of one patient who delivered at term, all MPFD cases delivered before 31 weeks of gestation and only two had viable neonates (see Table 2). Three pregnancies have been evaluated for the presence of anticardiolipin antibody and lupus anticoagulant and all of these tests were negative.
Table 1. Demographics and clinical characteristics of the study population.
| Uncomplicated pregnancies (n=175) | MPFD (n=10) | p-value | |
|---|---|---|---|
| Maternal Age (years) | 23 (20-26) | 31 (26-35) | <0.001 |
| African American | 151 (86%) | 10 (100%) | 0.4 |
| Nulliparity | 63 (35%) | 0 (0%) | 0.03 |
| BMI (kg/m2) | 27 (23-32) | 29 (28-35) | 0.04 |
| Gestational Age at Delivery (weeks) | 39 (39-40) | 23 (17-29) | <0.001 |
| Birth weight (grams) | 3330 (3150-3555) | 277 (175-605) | <0.001 |
| Stillbirth (> 20 weeks) | 0 | 4 (40%) | --- |
| Miscarriage in the Second Trimester (<20 weeks) | 0 | 4 (40%) | --- |
| Fetal Growth Restriction | 0 | 4 (40%) | --- |
| Placental Abruption | 0 | 2 (20%) | --- |
Data are expressed as median (interquartile range) or number (percent).
MPFD: Massive perivillous fibrin deposition
BMI: Body mass index
Table 2. Clinical and obstetrical characteristics of patients with massive perivillous fibrin deposition.
| Case Number | Age | Gravida, Parity | Gestational age at delivery (weeks+days) | Clinical Description | Birth Weight (grams, percentile for GA) | Prelabor Rupture of Membranes | Fetal Growth Restriction | Fetal Demise | Second Trimester Miscarriage |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 24 | G 4 P 2-0-1-2 | 15+6 | Presented with ruptured membranes and was induced for inevitable abortion. | 150 | Yes | No | No | Yes |
| 2 | 27 | G 3 P 0-0-2-0 | 30+0 | Presented with fetal growth restriction, heavy vaginal bleeding/clinical placental abruption and emergent cesarean delivery was performed. | 755(1%) | no | Yes | No | No |
| 3 | 22 | G 2 P 0-0-1-0 | 22+3 | Short cervix was noted at 20 weeks; membranes ruptured with spontaneous labor at 22 weeks and delivery of a stillborn infant. | 448 (34%) | Yes | No | Yes | No |
| 4 | 28 | G 11 P 0-1-9-1 | 23+6 | Fetus noted to have thickened placenta, multiple placental lacunae, and oligohydramnios at 18 weeks; abnormal Doppler parameters, fetal demise was diagnosed and the patient was induced | 277 (1%) | No | Yes | Yes | No |
| 5 | 43 | G 13 P 3-3-6-4 | 16+4 | Presented with ruptured membranes and fetal demise. | Unknown | Yes | No | Yes | Yes |
| 6 | 35 | G 7 P 0-0-6-0 | 17+3 | Cervical length of 0 mm on routine scan; A rescue cerclage was placed but membranes ruptured shortly afterwards. Induction for inevitable abortion. | 160 | Yes | No | No | Yes |
| 7 | 29 | G 3 P 1-1-0-1 | 17+2 | Presented with abdominal pain and vaginal bleeding. Fetal demise was diagnosed and the patient was induced. | 190 | No | No | Yes | Yes |
| 8 | 35 | G 12 P 8-2-1-8 | 23+1 | Fetus noted to have decreased growth and progressive deterioration of Doppler parameters starting at 20 weeks gestation. Fetal demise diagnosed at 23 weeks. | 274 (1%) | No | Yes | Yes | No |
| 9 | 34 | G 11 P 8-1-1-8 | 28+2 | Fetus noted to have growth restriction and progressive deterioration of Doppler parameters starting at 20 weeks. Fetal demise diagnosed and the patient was induced. | 454 (1%) | No | Yes | Yes | No |
| 10 | 33 | G 10 P 7-1-1-7 | 38+1 | Spontaneous labor at term. | 3285 (51.5%) | No | No | No | No |
Cases #8-10 are pregnancies from the same patient; GA=gestational age; G=gravida; P=parity
Longitudinal analysis of plasma sVEGFR-1, sVEGFR-2, sEng, and PlGF concentrations
Patients with MPFD had a significantly lower mean plasma PlGF concentration (p=0.03), but significantly higher mean plasma concentrations of sVEGFR-1 (p<0.01) and sEng (p<0.01) than controls after adjusting for potential confounders (see Figures 1 and 2). The mean maternal plasma concentrations of PlGF differed further among patients who had MPFD and the control group as a function of gestational age interval (p<0.0001). However, the magnitude of the differences in mean plasma concentrations of sVEGFR-1 and sEng did not change significantly with gestational age interval (p=0.09, Figure 1; p=0.11, Figure 2). There were no significant differences in plasma concentrations of sVEGFR-2 observed overall (p=0.97), or as a function of time (p=0.17) among cases and controls (see Figure 3).
Figure 1. Estimated mean +/- standard error of plasma concentrations (log10) of PlGF (1A) and sEng (1B) in MPFD and uncomplicated pregnancies by gestational age interval.
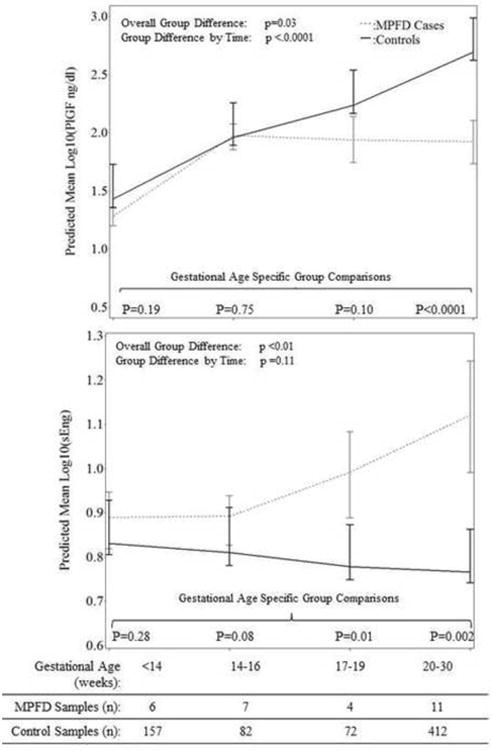
Estimated mean PlGF concentrations over time are adjusted by gestational age at venipuncture and body mass index; Estimated mean sEng concentrations over time are adjusted by gestational age at venipuncture, African American ethnicity and nulliparity; P-values reflect the group differences in estimated mean concentrations overall, as a function of gestational age interval, and at each gestational age interval determined by the linear mixed effects model.
Figure 2. Estimated mean +/- standard error of plasma concentrations (log10) of sVEGFR-1 in MPFD and uncomplicated pregnancies by gestational age interval.
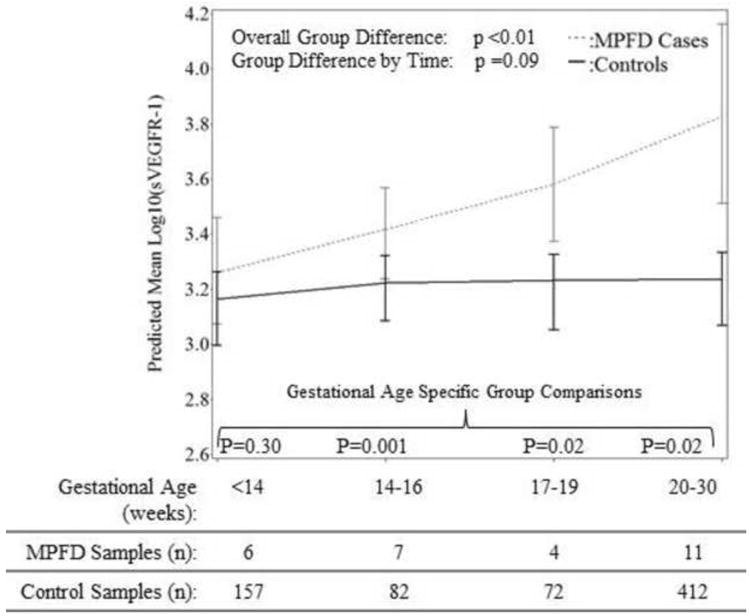
Estimated mean sVEGFR-1 concents over time are adjusted by gestational age at venipuncture and maternal age; P-values reflect the group differences in estimated mean concentrations overall, as a function of gestational age interval, and at each gestational age interval determined by the linear mixed effects model.
Figure 3. Estimated mean +/- standard error of plasma concentrations (log10) of sVEGFR-2 in MPFD and uncomplicated pregnancies by gestational age interval.
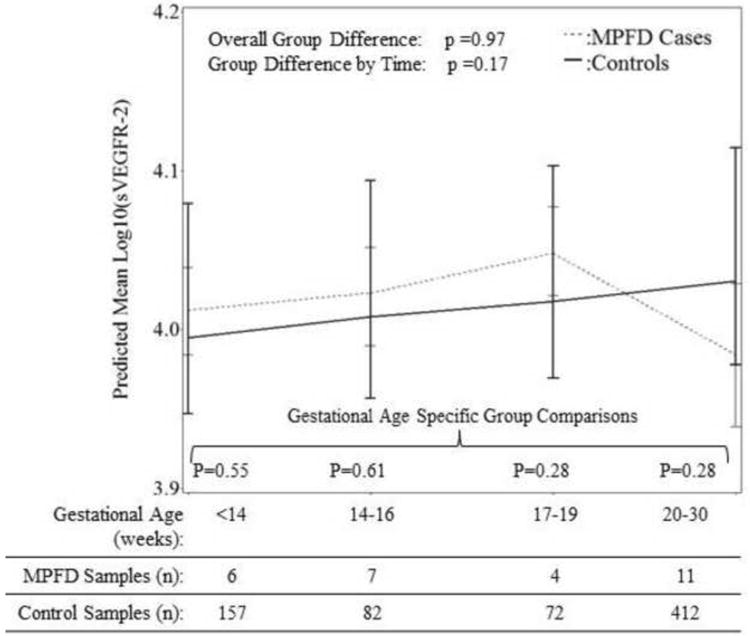
Estimated mean sVEGFR-2 concentrations over time are adjusted for effects of gestational age at venipuncture, body mass index, and African American ethnicity; P-values reflect the group differences in estimated mean concentrations overall, as a function of gestational age interval, and at each gestational age interval determined by the linear mixed effects model.
Patients with MPFD had significantly lower mean ratio concentrations of PlGF/sVEGFR-1 (p<0.0001) and PlGF/sEng (p<0.001; Figure 4) after adjustment for potential confounders; both of these relationships differed significantly as a function of gestational age interval (each p<0.0001; Figure 4).
Figure 4. Estimated mean +/- standard error of plasma concentrations (log10) in the Ratios of PlGF/sVEGFR-1 (1A) and PLGF/sEng (1B) in MPFD and uncomplicated pregnancies by gestational age interval.
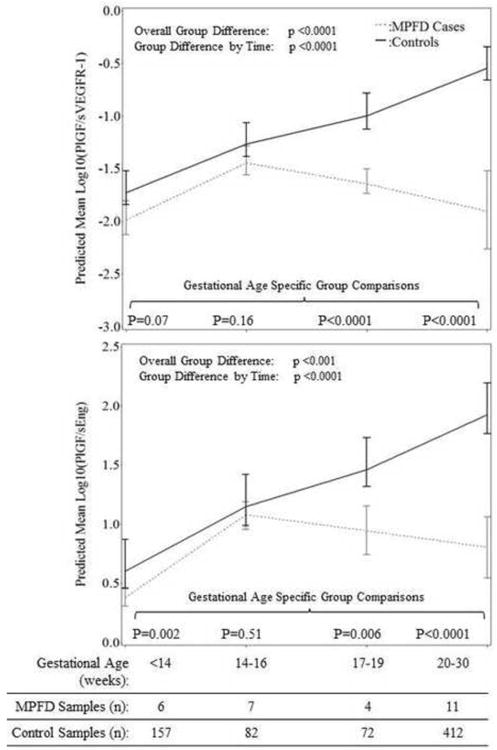
Estimated mean PlGF/sVEGFR-1 concentration ratios over time are adjusted for gestational age at venipuncture, body mass index, and nulliparity; Estimated mean PlGF/sVEGFR-1 concentration ratios over time are adjusted for gestational age at venipuncture, African American ethnicity, and body mass index; P-values reflect the difference in estimated mean concentrations at each gestational age interval determined by the linear mixed effects model.
As shown in Figures 1-4, while the differences in mean plasma PlGF concentration among cases and controls became statistically significant at 20-30 weeks of gestation, differences in mean sEng and the ratios of PlGF/sEng and PlGF/sVEGFR-1 among cases and controls became significant from 17-19 weeks of gestation onwards. Consistent changes in the mean plasma sVEGFR-1 concentration in cases compared to controls appear to begin early, at 14-16 weeks gestation. The mean concentration of each angiogenic and anti-angiogenic factor for each gestational age interval in MPFD patients and controls are shown in Table 3.
Table 3. Mean, standard deviation, median and inter-quartile range plasma analyte concentrations and their ratios by study group and gestational length interval at measurement.
| Analyte | Gestational Length Interval | Study Group | N* | Mean | Std. Dev. | Median | 25th centile | 75th centile |
|---|---|---|---|---|---|---|---|---|
| PlGF(pg/mL) | I: <14 weeks | Case | 4 | 20 | 7 | 22 | 16 | 24 |
| Control | 110 | 40 | 98 | 23 | 16 | 36 | ||
| II: 14-16 weeks | Case | 7 | 106 | 45 | 119 | 76 | 152 | |
| Control | 82 | 106 | 61 | 87 | 59 | 132 | ||
| III: 17-19 weeks | Case | 4 | 110 | 63 | 126 | 73 | 148 | |
| Control | 72 | 201 | 106 | 189 | 125 | 260 | ||
| IV: 20-30 weeks | Case | 6 | 125 | 103 | 100 | 43 | 206 | |
| Control | 172 | 598 | 409 | 516 | 320 | 775 | ||
| sEng (ng/mL) | I: <14 weeks | Case | 4 | 20 | 7 | 22 | 16 | 24 |
| Control | 110 | 40 | 98 | 23 | 16 | 36 | ||
| II: 14-16 weeks | Case | 7 | 106 | 45 | 119 | 76 | 152 | |
| Control | 82 | 106 | 61 | 87 | 59 | 132 | ||
| III: 17-19 weeks | Case | 4 | 110 | 63 | 126 | 73 | 148 | |
| Control | 72 | 201 | 106 | 189 | 125 | 260 | ||
| IV: 20-30 weeks | Case | 6 | 125 | 103 | 100 | 43 | 206 | |
| Control | 172 | 598 | 409 | 516 | 320 | 775 | ||
| sVEGFR-1 (pg/mL | I: <14 weeks | Case | 4 | 2200 | 1165 | 2487 | 1510 | 2891 |
| Control | 110 | 1697 | 1276 | 1513 | 986 | 1930 | ||
| II: 14-16 weeks | Case | 7 | 2972 | 1439 | 3006 | 1305 | 4102 | |
| Control | 82 | 1912 | 1022 | 1661 | 1249 | 2355 | ||
| III: 17-19 weeks | Case | 4 | 4955 | 2961 | 5664 | 2799 | 7111 | |
| Control | 72 | 2276 | 2707 | 1737 | 1295 | 2660 | ||
| IV: 20-30 weeks | Case | 6 | 28526 | 56386 | 4209 | 1695 | 16377 | |
| Control | 172 | 2092 | 1610 | 1727 | 1173 | 2456 | ||
| sVEGFR-2 (ng/mL) | I: <14 weeks | Case | 4 | 10.3 | 1.4 | 10.2 | 9.2 | 11.5 |
| Control | 110 | 10.1 | 2.0 | 9.9 | 8.8 | 11.1 | ||
| II: 14-16 weeks | Case | 7 | 10.7 | 1.7 | 11.1 | 9.7 | 11.8 | |
| Control | 82 | 10.3 | 1.8 | 10.2 | 9.0 | 11.2 | ||
| III: 17-19 weeks | Case | 4 | 11.2 | 1.3 | 11.1 | 10.2 | 12.3 | |
| Control | 72 | 10.6 | 1.9 | 10.3 | 9.3 | 12.0 | ||
| IV: 20-30 weeks | Case | 6 | 9.9 | 2.5 | 10.5 | 7.6 | 11.9 | |
| Control | 172 | 10.9 | 2.0 | 10.7 | 9.7 | 12.2 | ||
| PlGF/sEng (pg/ng) | I: <14 weeks | Case | 4 | 2.4 | 0.7 | 2.4 | 1.8 | 2.9 |
| Control | 110 | 5.9 | 13.7 | 3.7 | 2.6 | 5.2 | ||
| II: 14-16 weeks | Case | 7 | 14.1 | 7.3 | 14.3 | 5.6 | 20.6 | |
| Control | 82 | 16.6 | 9.0 | 14.2 | 9.7 | 22.2 | ||
| III: 17-19 weeks | Case | 4 | 11.8 | 8.2 | 11.7 | 6.1 | 17.6 | |
| Control | 72 | 34.1 | 17.4. | 33.6 | 21.3 | 49.2 | ||
| IV: 20-30 weeks | Case | 6 | 14.1 | 15.2 | 9.8 | 1.2 | 23.7 | |
| Control | 172 | 1024 | 67.8 | 98.1 | 55.3 | 125.4 | ||
| PlGF/sVEGFR-1 | I: <14 weeks | Case | 4 | 0.01 | 0.01 | 0.01 | 0.01 | 0.02 |
| Control | 110 | 0.02 | 0.03 | 0.02 | 0.01 | 0.03 | ||
| II: 14-16 weeks | Case | 7 | 0.04 | 0.03 | 0.03 | 0.03 | 0.03 | |
| Control | 82 | 0.07 | 0.04 | 0.06 | 0.03 | 0.09 | ||
| III: 17-19 weeks | Case | 4 | 0.02 | 0.00 | 0.02 | 0.02 | 0.03 | |
| Control | 72 | 0.13 | 0.11 | 0.10 | 0.07 | 0.15 | ||
| IV: 20-30 weeks | Case | 6 | 0.04 | 0.05 | 0.03 | 0.00 | 0.05 | |
| Control | 172 | 0.36 | 0.30 | 0.29 | 0.19 | 0.42 |
Note:
N= number of patients, not samples; PlGF= placental growth factor, sEng = soluble endoglin, sVEGFR-1= soluble vascular endothelial growth factor receptor-1
Comment
Principal Findings
Patients who developed MPFD had 1) a significantly lower mean plasma concentration of PlGF than controls at 20-30 weeks of gestation; 2) a higher mean plasma concentration of sVEGFR-1 than the control group from 14-16 weeks of gestation; and 3) a higher mean sEng concentration, but a lower mean PlGF/VEGFR-1 ratio and PlGF/sEng ratio concentration, than those with uncomplicated pregnancies starting from 17-19 weeks onwards.
MPFD was originally described as a pathologic finding characterized by increased fibrinoid material surrounding trophoblastic villi.3, 14 Subsequently, Katzman and Genest proposed to subdivide this pathologic condition into 3 categories: 1) maternal floor infarction (MFI); 2) transmural MPFD; and 3) borderline MPFD.1 The diagnosis of MPFD in the current study required the identification of fibrinoid material encasing at least 50% of the villi on a minimum of one slide, and included either cases with fibrinoid material only on the maternal floor of the placenta or cases with transplacental fibrinoid deposition (extending from maternal to fetal surfaces). Therefore, MPFD cases included in the current study would have been classified as either classic MFI or transmural MPFD, but we did not include cases that would be in the borderline MPFD category, as described by Katzman and Genest1.
The largest pathological review of MPFD was based upon data collected from the Collaborative Perinatal Project.4 Naeye et al. described an association between MPFD and stillbirth as well as fetal growth restriction.4 Subsequently, additional case reports and case series have reported this pathologic finding in pregnancies with spontaneous abortion,1, 5 spontaneous preterm delivery,2, 5 and early-onset fetal growth restriction.2 In a case-series reported by Andres et al, among 60 pregnancies with MPFD, the prevalence of fetal death was 40% and among the live-born infants, 40% delivered preterm and 58% had birthweights below 10th percentile for gestational age.2 MPFD is also associated with adverse neurodevelopmental outcome in neonates who survive.96, 97 Consistent with previous observations, 9 patients with MPFD in the current study resulted in severe fetal growth restriction, placental abruption or fetal death. Specifically, we observed 4 cases of MPFD that presented with spontaneous rupture of membranes and miscarriage in the second trimester.
Risk factors for MPFD include anti-phospholipid antibody syndrome,6, 10, 11, 98 elevated maternal serum alpha-feto-protein,99 activated protein C resistance,100 and long-chain-3 hydroxyacyl-CoA Dehydrogenase deficiency.101 The composition of the deposited material has been evaluated and includes fibrin, extracellular matrix proteins (such as fibronectin, laminin, or collagen) and other proteins which are part of the coagulation system.102-104 MPFD, however, is unlikely the result of a true infarction such as would occur in thrombosis or ischemic necrosis of the villi.1, 4 The villi in MPFD are hypoplastic, sclerotic and avascular.4, 99 Occasionally, atherosis in the decidual arteries, foci of decidual necrosis and histologic evidence of low uteroplacental blood flow such as small villi and increased syncytial knots similar to those in patients with preeclampsia can be observed.4 Placenta from patients with preeclampsia occasionally shows increased fibrinoid material deposition along the basal plate and in the intervillous space.105 However, the degree of fibrinoid material deposition (as judged by the percentage of villi covered by this material) in the placenta of preeclampsia is generally less than that observed in MPFD cases and does not meet the criteria to diagnose MPFD.106 It is noteworthy that fibrinoid material deposition has been demonstrated within the glomeruli of patients with preeclampsia.106-109 Some investigators propose that a prolonged state of slow intravascular coagulation resulting from placental release of unknown factors causes glomerular damage and specific glomerular lesion in preeclampsia.110 The mechanisms leading to adverse pregnancy outcomes in MPFD are unknown. However, it has been proposed that fibrin and/or fibrinoid material deposition interferes with perfusion of the intervillous space and gas/nutrient exchange in the intervillous space resulting in “chronic placental insufficiency”.6, 12, 99
Angiogenic Profile of MPFD Compared to Other Pregnancy Complications
In MPFD, maternal plasma concentrations of sVEGFR-1 were higher across gestational age intervals (marginal difference as a function of time, p=0.09) while that of PlGF was significantly lower than uncomplicated pregnancies after 20 weeks of gestation. The early elevation of sVEGFR-1 in the second trimester especially from 14-16 weeks of gestation without a change in PlGF has never been observed in other pregnancy complications, and appears to be characteristic of MPFD thus far.
Three conclusions could be drawn from these findings. First, an elevation of plasma concentration of sVEGFR-1 is not specific to preeclampsia since none of the patients with MPFD developed new-onset hypertension and proteinuria. Second, although an imbalance of angiogenic/anti-angiogenic factors has been observed in several obstetrical syndromes, the clinical presentation of the disorders may differ depending on the gestational age at which this perturbation occurs. For example, patients destined to develop preterm and term preeclampsia have higher plasma concentrations of sVEGFR-1 starting from 26 and 30 weeks of gestation, respectively, and lower plasma concentrations of PlGF starting from 10-11 weeks of gestation compared with those in uncomplicated pregnancies.29 Different profiles of angiogenic/anti-angiogenic factors have also been reported in pregnancies with spontaneous preterm labor,78 small-for-gestational-age neonates30, 41 and stillbirth.83 Third, the change in plasma concentrations of angiogenic/anti-angiogenic factors in MPFD was observed prior to the diagnosis of an abnormal pregnancy outcome, and thus, provides an opportunity for the diagnosis and enrollment of these patients for interventional trials especially in patients with a history of MPFD. Sonography may also assist in the prenatal diagnosis of MPFD.7
Prevention of Recurrence of MPFD
Several studies suggest that MPFD is a recurrent condition.2-4, 7, 10, 13, 99. Andres et al reported that among 33 pregnancies that occurred after a MPFD, 13 (39%) placentas showed gross and microscopic evidence of MPFD.2 Similarly, in many cases included in the current study, patients had prior poor pregnancy outcomes, although placental pathology was not available for review. The strong association between MPFD and serious adverse pregnancy outcomes such as fetal death,2, 4-7, 10, 11, 13-16 fetal growth restriction1, 2, 4, 6-12 or recurrent miscarriage4, 5, 10 strengthens the value of placental pathologic examination in such cases. If MPFD is diagnosed, subsequent pregnancies are also at risk for these complications. The novel identification of abnormal concentrations of angiogenic and anti-angiogenic factors in the current study indicates that an anti-angiogenic state may be a mechanism of disease in MPFD. This has implications because recent observations suggest that there may be therapeutic interventions to reverse an anti-angiogenic state during pregnancy including the administration of pravastatin,111-114 VEGF 121115-117 or extracorporeal removal of sVEGFR-1. 118
Strengths and Limitations
This is the first study demonstrating an association between an imbalance of angiogenic/anti-angiogenic factors and MPFD. Moreover, the longitudinal nature of our study allows us to demonstrate that these changes could be detected prior to the diagnosis of MPFD and occurrence of adverse pregnancy outcomes. A limitation of the study was the number of cases, which is a reflection of the low prevalence of the condition. However, the magnitude of the differences in plasma concentrations of angiogenic/anti-angiogenic factors between cases and controls observed herein was remarkable despite this limitation.
Conclusions
An imbalance of angiogenic/anti-angiogenic factors is present in patients with MPFD. We propose that these changes participate in the mechanism of disease responsible for adverse pregnancy outcomes in patients with this placental lesion.
Acknowledgments
Financial support: This research was supported, in part, by the Perinatology Research Branch, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, DHHS.
Footnotes
Disclosure: The authors report no conflict of interest.
Presented at the 58th Annual Meeting of the Society for Gynecologic Investigation, March 21-24, 2012, San Diego, CA
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Katzman PJ, Genest DR. Maternal floor infarction and massive perivillous fibrin deposition: histological definitions, association with intrauterine fetal growth restriction, and risk of recurrence. Pediatr Dev Pathol. 2002;5:159–64. doi: 10.1007/s10024001-0195-y. [DOI] [PubMed] [Google Scholar]
- 2.Andres RL, Kuyper W, Resnik R, Piacquadio KM, Benirschke K. The association of maternal floor infarction of the placenta with adverse perinatal outcome. Am J Obstet Gynecol. 1990;163:935–8. doi: 10.1016/0002-9378(90)91100-q. [DOI] [PubMed] [Google Scholar]
- 3.Benirschke K, Hommel AB. The Pathology of the Human Placenta. New York: Springer-Verlag; Number of pages. [Google Scholar]
- 4.Naeye RL. Maternal floor infarction. Hum Pathol. 1985;16:823–8. doi: 10.1016/s0046-8177(85)80254-9. [DOI] [PubMed] [Google Scholar]
- 5.Bane AL, Gillan JE. Massive perivillous fibrinoid causing recurrent placental failure. BJOG. 2003;110:292–5. [PubMed] [Google Scholar]
- 6.Bendon RW, Hommel AB. Maternal floor infarction in autoimmune disease: two cases. Pediatr Pathol Lab Med. 1996;16:293–7. [PubMed] [Google Scholar]
- 7.Mandsager NT, Bendon R, Mostello D, Rosenn B, Miodovnik M, Siddiqi TA. Maternal floor infarction of the placenta: prenatal diagnosis and clinical significance. Obstet Gynecol. 1994;83:750–4. [PubMed] [Google Scholar]
- 8.Nickel RE. Maternal floor infarction: an unusual cause of intrauterine growth retardation. Am J Dis Child. 1988;142:1270–1. doi: 10.1001/archpedi.1988.02150120024020. [DOI] [PubMed] [Google Scholar]
- 9.Redline RW, Jiang JG, Shah D. Discordancy for maternal floor infarction in dizygotic twin placentas. Hum Pathol. 2003;34:822–4. doi: 10.1016/s0046-8177(03)00288-0. [DOI] [PubMed] [Google Scholar]
- 10.Sebire NJ, Backos M, El Gaddal S, Goldin RD, Regan L. Placental pathology, antiphospholipid antibodies, and pregnancy outcome in recurrent miscarriage patients. Obstet Gynecol. 2003;101:258–63. doi: 10.1016/s0029-7844(02)02385-2. [DOI] [PubMed] [Google Scholar]
- 11.Sebire NJ, Backos M, Goldin RD, Regan L. Placental massive perivillous fibrin deposition associated with antiphospholipid antibody syndrome. BJOG. 2002;109:570–3. doi: 10.1111/j.1471-0528.2002.00077.x. [DOI] [PubMed] [Google Scholar]
- 12.Vernof KK, Benirschke K, Kephart GM, Wasmoen TL, Gleich GJ. Maternal floor infarction: relationship to X cells, major basic protein, and adverse perinatal outcome. Am J Obstet Gynecol. 1992;167:1355–63. doi: 10.1016/s0002-9378(11)91716-5. [DOI] [PubMed] [Google Scholar]
- 13.Clewell WH, Manchester DK. Recurrent maternal floor infarction: a preventable cause of fetal death. Am J Obstet Gynecol. 1983;147:346–7. doi: 10.1016/0002-9378(83)91130-4. [DOI] [PubMed] [Google Scholar]
- 14.Fox H, Elston CW. Pathology of the placenta. Major Probl Pathol. 1978;7:1–491. [PubMed] [Google Scholar]
- 15.Al-Adnani M, Kiho L, Scheimberg I. Recurrent placental massive perivillous fibrin deposition associated with polymyositis: a case report and review of the literature. Pediatr Dev Pathol. 2008;11:226–9. doi: 10.2350/07-06-0306.1. [DOI] [PubMed] [Google Scholar]
- 16.Hung NA, Jackson C, Nicholson M, Highton J. Pregnancy-related polymyositis and massive perivillous fibrin deposition in the placenta: are they pathogenetically related? Arthritis Rheum. 2006;55:154–6. doi: 10.1002/art.21710. [DOI] [PubMed] [Google Scholar]
- 17.Poole TJ, Coffin JD. Vasculogenesis and angiogenesis: two distinct morphogenetic mechanisms establish embryonic vascular pattern. J Exp Zool. 1989;251:224–31. doi: 10.1002/jez.1402510210. [DOI] [PubMed] [Google Scholar]
- 18.Zygmunt M, Herr F, Munstedt K, Lang U, Liang OD. Angiogenesis and vasculogenesis in pregnancy. Eur J Obstet Gynecol Reprod Biol. 2003;110(1):S10–8. doi: 10.1016/s0301-2115(03)00168-4. [DOI] [PubMed] [Google Scholar]
- 19.Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–4. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- 20.Arthur HM, Ure J, Smith AJ, et al. Endoglin, an ancillary TGFbeta receptor, is required for extraembryonic angiogenesis and plays a key role in heart development. Dev Biol. 2000;217:42–53. doi: 10.1006/dbio.1999.9534. [DOI] [PubMed] [Google Scholar]
- 21.Bourdeau A, Dumont DJ, Letarte M. A murine model of hereditary hemorrhagic telangiectasia. J Clin Invest. 1999;104:1343–51. doi: 10.1172/JCI8088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carmeliet P, Ferreira V, Breier G, et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380:435–9. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- 23.Ferrara N, Carver-Moore K, Chen H, et al. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996;380:439–42. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- 24.Li DY, Sorensen LK, Brooke BS, et al. Defective angiogenesis in mice lacking endoglin. Science. 1999;284:1534–7. doi: 10.1126/science.284.5419.1534. [DOI] [PubMed] [Google Scholar]
- 25.Shibuya M. Vascular endothelial growth factor-dependent and -independent regulation of angiogenesis. BMB Rep. 2008;41:278–86. doi: 10.5483/bmbrep.2008.41.4.278. [DOI] [PubMed] [Google Scholar]
- 26.Bujold E, Romero R, Chaiworapongsa T, et al. Evidence supporting that the excess of the sVEGFR-1 concentration in maternal plasma in preeclampsia has a uterine origin. J Matern Fetal Neonatal Med. 2005;18:9–16. doi: 10.1080/14767050500202493. [DOI] [PubMed] [Google Scholar]
- 27.Chaiworapongsa T, Romero R, Espinoza J, et al. Evidence supporting a role for blockade of the vascular endothelial growth factor system in the pathophysiology of preeclampsia. Young Investigator Award. Am J Obstet Gynecol. 2004;190:1541–7. doi: 10.1016/j.ajog.2004.03.043. discussion 47-50. [DOI] [PubMed] [Google Scholar]
- 28.Chaiworapongsa T, Romero R, Gotsch F, et al. Low maternal concentrations of soluble vascular endothelial growth factor receptor-2 in preeclampsia and small for gestational age. J Matern Fetal Neonatal Med. 2008;21:41–52. doi: 10.1080/14767050701831397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chaiworapongsa T, Romero R, Kim YM, et al. Plasma soluble vascular endothelial growth factor receptor-1 concentration is elevated prior to the clinical diagnosis of preeclampsia. J Matern Fetal Neonatal Med. 2005;17:3–18. doi: 10.1080/14767050400028816. [DOI] [PubMed] [Google Scholar]
- 30.Erez O, Romero R, Espinoza J, et al. The change in concentrations of angiogenic and anti-angiogenic factors in maternal plasma between the first and second trimesters in risk assessment for the subsequent development of preeclampsia and small-for-gestational age. J Matern Fetal Neonatal Med. 2008;21:279–87. doi: 10.1080/14767050802034545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koga K, Osuga Y, Yoshino O, et al. Elevated serum soluble vascular endothelial growth factor receptor 1 (sVEGFR-1) levels in women with preeclampsia. J Clin Endocrinol Metab. 2003;88:2348–51. doi: 10.1210/jc.2002-021942. [DOI] [PubMed] [Google Scholar]
- 32.Kupferminc MJ, Daniel Y, Englender T, et al. Vascular endothelial growth factor is increased in patients with preeclampsia. Am J Reprod Immunol. 1997;38:302–6. doi: 10.1111/j.1600-0897.1997.tb00519.x. [DOI] [PubMed] [Google Scholar]
- 33.Levine RJ, Lam C, Qian C, et al. Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N Engl J Med. 2006;355:992–1005. doi: 10.1056/NEJMoa055352. [DOI] [PubMed] [Google Scholar]
- 34.Levine RJ, Maynard SE, Qian C, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672–83. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 35.Lyall F, Greer IA, Boswell F, Fleming R. Suppression of serum vascular endothelial growth factor immunoreactivity in normal pregnancy and in pre-eclampsia. Br J Obstet Gynaecol. 1997;104:223–8. doi: 10.1111/j.1471-0528.1997.tb11050.x. [DOI] [PubMed] [Google Scholar]
- 36.Maynard SE, Min JY, Merchan J, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111:649–58. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McKeeman GC, Ardill JE, Caldwell CM, Hunter AJ, McClure N. Soluble vascular endothelial growth factor receptor-1 (sFlt-1) is increased throughout gestation in patients who have preeclampsia develop. Am J Obstet Gynecol. 2004;191:1240–6. doi: 10.1016/j.ajog.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 38.Park CW, Park JS, Shim SS, Jun JK, Yoon BH, Romero R. An elevated maternal plasma, but not amniotic fluid, soluble fms-like tyrosine kinase-1 (sFlt-1) at the time of mid-trimester genetic amniocentesis is a risk factor for preeclampsia. Am J Obstet Gynecol. 2005;193:984–9. doi: 10.1016/j.ajog.2005.06.033. [DOI] [PubMed] [Google Scholar]
- 39.Reuvekamp A, Velsing-Aarts FV, Poulina IE, Capello JJ, Duits AJ. Selective deficit of angiogenic growth factors characterises pregnancies complicated by preeclampsia. Br J Obstet Gynaecol. 1999;106:1019–22. doi: 10.1111/j.1471-0528.1999.tb08107.x. [DOI] [PubMed] [Google Scholar]
- 40.Robinson CJ, Johnson DD, Chang EY, Armstrong DM, Wang W. Evaluation of placenta growth factor and soluble Fms-like tyrosine kinase 1 receptor levels in mild and severe preeclampsia. Am J Obstet Gynecol. 2006;195:255–9. doi: 10.1016/j.ajog.2005.12.049. [DOI] [PubMed] [Google Scholar]
- 41.Romero R, Nien JK, Espinoza J, et al. A longitudinal study of angiogenic (placental growth factor) and anti-angiogenic (soluble endoglin and soluble vascular endothelial growth factor receptor-1) factors in normal pregnancy and patients destined to develop preeclampsia and deliver a small for gestational age neonate. J Matern Fetal Neonatal Med. 2008;21:9–23. doi: 10.1080/14767050701830480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Staff AC, Braekke K, Johnsen GM, Karumanchi SA, Harsem NK. Circulating concentrations of soluble endoglin (CD105) in fetal and maternal serum and in amniotic fluid in preeclampsia. Am J Obstet Gynecol. 2007;197:176 e1–6. doi: 10.1016/j.ajog.2007.03.036. [DOI] [PubMed] [Google Scholar]
- 43.Stepan H, Unversucht A, Wessel N, Faber R. Predictive value of maternal angiogenic factors in second trimester pregnancies with abnormal uterine perfusion. Hypertension. 2007;49:818–24. doi: 10.1161/01.HYP.0000258404.21552.a3. [DOI] [PubMed] [Google Scholar]
- 44.Taylor RN, Grimwood J, Taylor RS, McMaster MT, Fisher SJ, North RA. Longitudinal serum concentrations of placental growth factor: evidence for abnormal placental angiogenesis in pathologic pregnancies. Am J Obstet Gynecol. 2003;188:177–82. doi: 10.1067/mob.2003.111. [DOI] [PubMed] [Google Scholar]
- 45.Tidwell SC, Ho HN, Chiu WH, Torry RJ, Torry DS. Low maternal serum levels of placenta growth factor as an antecedent of clinical preeclampsia. Am J Obstet Gynecol. 2001;184:1267–72. doi: 10.1067/mob.2001.113129. [DOI] [PubMed] [Google Scholar]
- 46.Torry DS, Wang HS, Wang TH, Caudle MR, Torry RJ. Preeclampsia is associated with reduced serum levels of placenta growth factor. Am J Obstet Gynecol. 1998;179:1539–44. doi: 10.1016/s0002-9378(98)70021-3. [DOI] [PubMed] [Google Scholar]
- 47.Tsatsaris V, Goffin F, Munaut C, et al. Overexpression of the soluble vascular endothelial growth factor receptor in preeclamptic patients: pathophysiological consequences. J Clin Endocrinol Metab. 2003;88:5555–63. doi: 10.1210/jc.2003-030528. [DOI] [PubMed] [Google Scholar]
- 48.Vatten LJ, Eskild A, Nilsen TI, Jeansson S, Jenum PA, Staff AC. Changes in circulating level of angiogenic factors from the first to second trimester as predictors of preeclampsia. Am J Obstet Gynecol. 2007;196:239 e1–6. doi: 10.1016/j.ajog.2006.10.909. [DOI] [PubMed] [Google Scholar]
- 49.Venkatesha S, Toporsian M, Lam C, et al. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med. 2006;12:642–9. doi: 10.1038/nm1429. [DOI] [PubMed] [Google Scholar]
- 50.Wikstrom AK, Larsson A, Eriksson UJ, Nash P, Norden-Lindeberg S, Olovsson M. Placental growth factor and soluble FMS-like tyrosine kinase-1 in early-onset and late-onset preeclampsia. Obstet Gynecol. 2007;109:1368–74. doi: 10.1097/01.AOG.0000264552.85436.a1. [DOI] [PubMed] [Google Scholar]
- 51.Benton SJ, Hu Y, Xie F, et al. Angiogenic factors as diagnostic tests for preeclampsia: a performance comparison between two commercial immunoassays. Am J Obstet Gynecol. 205:469 e1–8. doi: 10.1016/j.ajog.2011.06.058. [DOI] [PubMed] [Google Scholar]
- 52.Bosio PM, Wheeler T, Anthony F, Conroy R, O'Herlihy C, McKenna P. Maternal plasma vascular endothelial growth factor concentrations in normal and hypertensive pregnancies and their relationship to peripheral vascular resistance. Am J Obstet Gynecol. 2001;184:146–52. doi: 10.1067/mob.2001.108342. [DOI] [PubMed] [Google Scholar]
- 53.Chaiworapongsa T, Romero R, Kusanovic JP, et al. Plasma soluble endoglin concentration in pre-eclampsia is associated with an increased impedance to flow in the maternal and fetal circulations. Ultrasound Obstet Gynecol. 2010;35:155–62. doi: 10.1002/uog.7491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chaiworapongsa T, Romero R, Savasan ZA, et al. Maternal plasma concentrations of angiogenic/anti-angiogenic factors are of prognostic value in patients presenting to the obstetrical triage area with the suspicion of preeclampsia. J Matern Fetal Neonatal Med. 2011;24:1187–207. doi: 10.3109/14767058.2011.589932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chaiworapongsa T, Romero R, Tarca AL, et al. A decrease in maternal plasma concentrations of sVEGFR-2 precedes the clinical diagnosis of preeclampsia. Am J Obstet Gynecol. 2010;202:550 e1–10. doi: 10.1016/j.ajog.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Crispi F, Dominguez C, Llurba E, Martin-Gallan P, Cabero L, Gratacos E. Placental angiogenic growth factors and uterine artery Doppler findings for characterization of different subsets in preeclampsia and in isolated intrauterine growth restriction. Am J Obstet Gynecol. 2006;195:201–7. doi: 10.1016/j.ajog.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 57.Furuya M, Kurasawa K, Nagahama K, et al. Disrupted balance of angiogenic and antiangiogenic signalings in preeclampsia. J Pregnancy. 2011;2011:123717. doi: 10.1155/2011/123717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ghosh SK, Raheja S, Tuli A, Raghunandan C, Agarwal S. Serum PLGF as a potential biomarker for predicting the onset of preeclampsia. Arch Gynecol Obstet. 2012;285:417–22. doi: 10.1007/s00404-011-1960-4. [DOI] [PubMed] [Google Scholar]
- 59.Levine RJ, Qian C, Maynard SE, Yu KF, Epstein FH, Karumanchi SA. Serum sFlt1 concentration during preeclampsia and mid trimester blood pressure in healthy nulliparous women. Am J Obstet Gynecol. 2006;194:1034–41. doi: 10.1016/j.ajog.2005.10.192. [DOI] [PubMed] [Google Scholar]
- 60.Masuyama H, Suwaki N, Nakatsukasa H, Masumoto A, Tateishi Y, Hiramatrsu Y. Circulating angiogenic factors in preeclampsia, gestational proteinuria, and preeclampsia superimposed on chronic glomerulonephritis. Am J Obstet Gynecol. 2006;194:551–6. doi: 10.1016/j.ajog.2005.08.034. [DOI] [PubMed] [Google Scholar]
- 61.Moore Simas TA, Crawford SL, Solitro MJ, Frost SC, Meyer BA, Maynard SE. Angiogenic factors for the prediction of preeclampsia in high-risk women. Am J Obstet Gynecol. 2007;197:244 e1–8. doi: 10.1016/j.ajog.2007.06.030. [DOI] [PubMed] [Google Scholar]
- 62.Perni U, Sison C, Sharma V, et al. Angiogenic factors in superimposed preeclampsia: a longitudinal study of women with chronic hypertension during pregnancy. Hypertension. 2012;59:740–6. doi: 10.1161/HYPERTENSIONAHA.111.181735. [DOI] [PubMed] [Google Scholar]
- 63.Robinson CJ, Johnson DD. Soluble endoglin as a second-trimester marker for preeclampsia. Am J Obstet Gynecol. 2007;197:174 e1–5. doi: 10.1016/j.ajog.2007.03.058. [DOI] [PubMed] [Google Scholar]
- 64.Soto E, Romero R, Kusanovic JP, et al. Late-onset preeclampsia is associated with an imbalance of angiogenic and anti-angiogenic factors in patients with and without placental lesions consistent with maternal underperfusion. J Matern Fetal Neonatal Med. 2012;25:498–507. doi: 10.3109/14767058.2011.591461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Srinivas SK, Morrison AC, Andrela CM, Elovitz MA. Allelic variations in angiogenic pathway genes are associated with preeclampsia. Am J Obstet Gynecol. 202:445 e1–11. doi: 10.1016/j.ajog.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 66.Uddin MN, Allen SR, Jones RO, Zawieja DC, Kuehl TJ. Pathogenesis of preeclampsia: marinobufagenin and angiogenic imbalance as biomarkers of the syndrome. Transl Res. 2012 doi: 10.1016/j.trsl.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 67.Unal ER, Robinson CJ, Johnson DD, Chang EY. Second-trimester angiogenic factors as biomarkers for future-onset preeclampsia. Am J Obstet Gynecol. 2007;197:211 e1–4. doi: 10.1016/j.ajog.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 68.Verlohren S, Galindo A, Schlembach D, et al. An automated method for the determination of the sFlt-1/PIGF ratio in the assessment of preeclampsia. Am J Obstet Gynecol. 202:161 e1–61 e11. doi: 10.1016/j.ajog.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 69.Verlohren S, Herraiz I, Lapaire O, et al. The sFlt-1/PlGF ratio in different types of hypertensive pregnancy disorders and its prognostic potential in preeclamptic patients. Am J Obstet Gynecol. 206:58 e1–8. doi: 10.1016/j.ajog.2011.07.037. [DOI] [PubMed] [Google Scholar]
- 70.Weissgerber TL, Roberts JM, Jeyabalan A, et al. Haptoglobin phenotype, angiogenic factors, and preeclampsia risk. Am J Obstet Gynecol. 2012;206:358 e10–8. doi: 10.1016/j.ajog.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chaiworapongsa T, Espinoza J, Gotsch F, et al. The maternal plasma soluble vascular endothelial growth factor receptor-1 concentration is elevated in SGA and the magnitude of the increase relates to Doppler abnormalities in the maternal and fetal circulation. J Matern Fetal Neonatal Med. 2008;21:25–40. doi: 10.1080/14767050701832833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Coolman M, Timmermans S, de Groot CJ, et al. Angiogenic and fibrinolytic factors in blood during the first half of pregnancy and adverse pregnancy outcomes. Obstet Gynecol. 2012;119:1190–200. doi: 10.1097/AOG.0b013e318256187f. [DOI] [PubMed] [Google Scholar]
- 73.Gotsch F, Romero R, Kusanovic JP, et al. Preeclampsia and small-for-gestational age are associated with decreased concentrations of a factor involved in angiogenesis: soluble Tie-2. J Matern Fetal Neonatal Med. 2008;21:389–402. doi: 10.1080/14767050802046069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jeyabalan A, McGonigal S, Gilmour C, Hubel CA, Rajakumar A. Circulating and placental endoglin concentrations in pregnancies complicated by intrauterine growth restriction and preeclampsia. Placenta. 2008;29:555–63. doi: 10.1016/j.placenta.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nishizawa H, Ota S, Suzuki M, et al. Comparative gene expression profiling of placentas from patients with severe pre-eclampsia and unexplained fetal growth restriction. Reprod Biol Endocrinol. 2011;9:107. doi: 10.1186/1477-7827-9-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schlembach D, Wallner W, Sengenberger R, et al. Angiogenic growth factor levels in maternal and fetal blood: correlation with Doppler ultrasound parameters in pregnancies complicated by pre-eclampsia and intrauterine growth restriction. Ultrasound Obstet Gynecol. 2007;29:407–13. doi: 10.1002/uog.3930. [DOI] [PubMed] [Google Scholar]
- 77.Wallner W, Sengenberger R, Strick R, et al. Angiogenic growth factors in maternal and fetal serum in pregnancies complicated by intrauterine growth restriction. Clin Sci (Lond) 2007;112:51–7. doi: 10.1042/CS20060161. [DOI] [PubMed] [Google Scholar]
- 78.Chaiworapongsa T, Romero R, Tarca A, et al. A subset of patients destined to develop spontaneous preterm labor has an abnormal angiogenic/anti-angiogenic profile in maternal plasma: evidence in support of pathophysiologic heterogeneity of preterm labor derived from a longitudinal study. J Matern Fetal Neonatal Med. 2009;22:1122–39. doi: 10.3109/14767050902994838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Savasan ZA, Romero R, Chaiworapongsa T, et al. Evidence in support of a role for anti-angiogenic factors in preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med. 2010;23:828–41. doi: 10.3109/14767050903440471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Smith GC, Crossley JA, Aitken DA, et al. Circulating angiogenic factors in early pregnancy and the risk of preeclampsia, intrauterine growth restriction, spontaneous preterm birth, and stillbirth. Obstet Gynecol. 2007;109:1316–24. doi: 10.1097/01.AOG.0000265804.09161.0d. [DOI] [PubMed] [Google Scholar]
- 81.Chaiworapongsa T, Romero R, Kusanovic JP, et al. Unexplained fetal death is associated with increased concentrations of anti-angiogenic factors in amniotic fluid. J Matern Fetal Neonatal Med. 2010;23:794–805. doi: 10.3109/14767050903443467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Espinoza J, Chaiworapongsa T, Romero R, et al. Unexplained fetal death: another anti-angiogenic state. J Matern Fetal Neonatal Med. 2007;20:495–507. doi: 10.1080/14767050701413022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Romero R, Chaiworapongsa T, Erez O, et al. An imbalance between angiogenic and anti-angiogenic factors precedes fetal death in a subset of patients: results of a longitudinal study. J Matern Fetal Neonatal Med. 2010;23:1384–99. doi: 10.3109/14767051003681121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bixel K, Silasi M, Zelop CM, et al. Placental origins of angiogenic dysfunction in mirror syndrome. Hypertens Pregnancy. 2012;31:211–7. doi: 10.3109/10641955.2011.638959. [DOI] [PubMed] [Google Scholar]
- 85.Espinoza J, Romero R, Nien JK, et al. A role of the anti-angiogenic factor sVEGFR-1 in the ‘mirror syndrome’ (Ballantyne's syndrome) J Matern Fetal Neonatal Med. 2006;19:607–13. doi: 10.1080/14767050600922677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Llurba E, Marsal G, Sanchez O, et al. Angiogenic and anti-angiogenic factors before and after resolution of maternal mirror syndrome. Ultrasound Obstet Gynecol. 2011 doi: 10.1002/uog.10136. [DOI] [PubMed] [Google Scholar]
- 87.Rana S, Venkatesha S, DePaepe M, Chien EK, Paglia M, Karumanchi SA. Cytomegalovirus-induced mirror syndrome associated with elevated levels of circulating antiangiogenic factors. Obstet Gynecol. 2007;109:549–52. doi: 10.1097/01.AOG.0000248538.03280.cf. [DOI] [PubMed] [Google Scholar]
- 88.Stepan H, Faber R. Cytomegalovirus-induced mirror syndrome associated with elevated levels of angiogenic factors. Obstet Gynecol. 2007;109:1205–6. doi: 10.1097/01.AOG.0000263776.46071.d1. author reply 06. [DOI] [PubMed] [Google Scholar]
- 89.Kusanovic JP, Romero R, Espinoza J, et al. Twin-to-twin transfusion syndrome: an antiangiogenic state? Am J Obstet Gynecol. 2008;198:382 e1–8. doi: 10.1016/j.ajog.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fox CE, Lash GE, Pretlove SJ, Chan BC, Holder R, Kilby MD. Maternal plasma and amniotic fluid angiogenic factors and their receptors in monochorionic twin pregnancies complicated by twin-to-twin transfusion syndrome. Ultrasound Obstet Gynecol. 2010;35:695–701. doi: 10.1002/uog.7515. [DOI] [PubMed] [Google Scholar]
- 91.Kanter D, Lindheimer MD, Wang E, et al. Angiogenic dysfunction in molar pregnancy. Am J Obstet Gynecol. 2010;202:184 e1–5. doi: 10.1016/j.ajog.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Koga K, Osuga Y, Tajima T, et al. Elevated serum soluble fms-like tyrosine kinase 1 (sFlt1) level in women with hydatidiform mole. Fertil Steril. 94:305–8. doi: 10.1016/j.fertnstert.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 93.Kusanovic JP, Romero R, Chaiworapongsa T, et al. A prospective cohort study of the value of maternal plasma concentrations of angiogenic and anti-angiogenic factors in early pregnancy and midtrimester in the identification of patients destined to develop preeclampsia. J Matern Fetal Neonatal Med. 2009;22:1021–38. doi: 10.3109/14767050902994754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Alexander GR, Kogan MD, Himes JH. 1994-1996 U.S. singleton birth weight percentiles for gestational age by race, Hispanic origin, and gender. Matern Child Health J. 1999;3:225–31. doi: 10.1023/a:1022381506823. [DOI] [PubMed] [Google Scholar]
- 95.Steyerberg EW, Eijkemans MJC, Harrell FE, Habbema JDF. Prognostic modelling with logistic regression analysis: a comparison of selection and estimation methods in small data sets. Statistics in Medicine. 2000;19:1059–79. doi: 10.1002/(sici)1097-0258(20000430)19:8<1059::aid-sim412>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 96.Adams-Chapman I, Vaucher YE, Bejar RF, Benirschke K, Baergen RN, Moore TR. Maternal floor infarction of the placenta: association with central nervous system injury and adverse neurodevelopmental outcome. J Perinatol. 2002;22:236–41. doi: 10.1038/sj.jp.7210685. [DOI] [PubMed] [Google Scholar]
- 97.Kumazaki K, Nakayama M, Sumida Y, et al. Placental features in preterm infants with periventricular leukomalacia. Pediatrics. 2002;109:650–5. doi: 10.1542/peds.109.4.650. [DOI] [PubMed] [Google Scholar]
- 98.Chang P, Millar D, Tsang P, Lim K, Houlihan E, Stephenson M. Intravenous immunoglobulin in antiphospholipid syndrome and maternal floor infarction when standard treatment fails: a case report. Am J Perinatol. 2006;23:125–9. doi: 10.1055/s-2006-931805. [DOI] [PubMed] [Google Scholar]
- 99.Katz VL, Bowes WA, Jr, Sierkh AE. Maternal floor infarction of the placenta associated with elevated second trimester serum alpha-fetoprotein. Am J Perinatol. 1987;4:225–8. doi: 10.1055/s-2007-999778. [DOI] [PubMed] [Google Scholar]
- 100.Katz VL, DiTomasso J, Farmer R, Carpenter M. Activated protein C resistance associated with maternal floor infarction treated with low-molecular-weight heparin. Am J Perinatol. 2002;19:273–7. doi: 10.1055/s-2002-33085. [DOI] [PubMed] [Google Scholar]
- 101.Matern D, Schehata BM, Shekhawa P, Strauss AW, Bennett MJ, Rinaldo P. Placental floor infarction complicating the pregnancy of a fetus with long-chain 3-hydroxyacyl-CoA dehydrogenase (LCHAD) deficiency. Mol Genet Metab. 2001;72:265–8. doi: 10.1006/mgme.2000.3135. [DOI] [PubMed] [Google Scholar]
- 102.Frank HG, Malekzadeh F, Kertschanska S, et al. Immunohistochemistry of two different types of placental fibrinoid. Acta Anat (Basel) 1994;150:55–68. doi: 10.1159/000147602. [DOI] [PubMed] [Google Scholar]
- 103.Nelson DM, Crouch EC, Curran EM, Farmer DR. Trophoblast interaction with fibrin matrix. Epithelialization of perivillous fibrin deposits as a mechanism for villous repair in the human placenta. Am J Pathol. 1990;136:855–65. [PMC free article] [PubMed] [Google Scholar]
- 104.Nelson DM, Swanson PE, Davison BB, Baskin GB, Enders AC. Ontogenetic and phylogenetic evaluation of the presence of fibrin-type fibrinoid in the villous haemochorial placenta. Placenta. 1997;18:605–8. doi: 10.1016/0143-4004(77)90017-0. [DOI] [PubMed] [Google Scholar]
- 105.Stalker AL. Fibrin deposition in pregnancy. Journal of clinical pathology Supplement (Royal College of Pathologists) 1976;10:70–6. [PMC free article] [PubMed] [Google Scholar]
- 106.Kanfer A, Bruch JF, Nguyen G, et al. Increased placental antifibrinolytic potential and fibrin deposits in pregnancy-induced hypertension and preeclampsia. Laboratory investigation; a journal of technical methods and pathology. 1996;74:253–8. [PubMed] [Google Scholar]
- 107.Correa RR, Gilio DB, Cavellani CL, et al. Placental morphometrical and histopathology changes in the different clinical presentations of hypertensive syndromes in pregnancy. Arch Gynecol Obstet. 2008;277:201–6. doi: 10.1007/s00404-007-0452-z. [DOI] [PubMed] [Google Scholar]
- 108.Hottor B, Bosio P, Waugh J, et al. Variation in composition of the intervillous space lining in term placentas of mothers with pre-eclampsia. Placenta. 2010;31:409–17. doi: 10.1016/j.placenta.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 109.Hustin J, Foidart JM, Lambotte R. Maternal vascular lesions in pre-eclampsia and intrauterine growth retardation: light microscopy and immunofluorescence. Placenta. 4 Spec No:489–98. [PubMed] [Google Scholar]
- 110.Vassalli P, Morris RH, McCluskey RT. The Pathogenic Role of Fibrin Deposition in the Glomerular Lesions of Toxemia of Pregnancy. J Exp Med. 1963;118:467–78. doi: 10.1084/jem.118.3.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cudmore M, Ahmad S, Al-Ani B, et al. Negative regulation of soluble Flt-1 and soluble endoglin release by heme oxygenase-1. Circulation. 2007;115:1789–97. doi: 10.1161/CIRCULATIONAHA.106.660134. [DOI] [PubMed] [Google Scholar]
- 112.Girardi G. Pravastatin prevents miscarriages in antiphospholipid antibody-treated mice. J Reprod Immunol. 2009;82:126–31. doi: 10.1016/j.jri.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 113.Girardi G. Role of tissue factor in pregnancy complications: crosstalk between coagulation and inflammation. Thromb Res. 2011;127(3):S43–6. doi: 10.1016/S0049-3848(11)70012-3. [DOI] [PubMed] [Google Scholar]
- 114.Kumasawa K, Ikawa M, Kidoya H, et al. Pravastatin induces placental growth factor (PGF) and ameliorates preeclampsia in a mouse model. Proc Natl Acad Sci U S A. 2011;108:1451–5. doi: 10.1073/pnas.1011293108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gilbert JS, Verzwyvelt J, Colson D, Arany M, Karumanchi SA, Granger JP. Recombinant vascular endothelial growth factor 121 infusion lowers blood pressure and improves renal function in rats with placentalischemia-induced hypertension. Hypertension. 2010;55:380–5. doi: 10.1161/HYPERTENSIONAHA.109.141937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Li Z, Zhang Y, Ying Ma J, et al. Recombinant vascular endothelial growth factor 121 attenuates hypertension and improves kidney damage in a rat model of preeclampsia. Hypertension. 2007;50:686–92. doi: 10.1161/HYPERTENSIONAHA.107.092098. [DOI] [PubMed] [Google Scholar]
- 117.Woods AK, Hoffmann DS, Weydert CJ, et al. Adenoviral delivery of VEGF121 early in pregnancy prevents spontaneous development of preeclampsia in BPH/5 mice. Hypertension. 2011;57:94–102. doi: 10.1161/HYPERTENSIONAHA.110.160242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Thadhani R, Kisner T, Hagmann H, et al. Pilot study of extracorporeal removal of soluble fms-like tyrosine kinase 1 in preeclampsia. Circulation. 2011;124:940–50. doi: 10.1161/CIRCULATIONAHA.111.034793. [DOI] [PubMed] [Google Scholar]


