Abstract
The coronary collateral circulation is critically important as an adaptation of the heart to prevent the damage from ischemic insults. In their native state, collaterals in the heart would be classified as part of the microcirculation, existing as arterial-arterial anastomotic connections in the range of 30 to 100 μM in diameter. However, these vessels also show a propensity to remodel into components of the macrocirculation and can become arteries larger than a 1000 μM in diameter. This process of outward remodelling is critically important in the adaptation of the heart to ischemia because the resistance to blood flow is inversely related to the fourth power of the diameter of the vessel. Thus, an expansion of a vessel from 100 to 1000 μM would reduce resistance (in this part of the circuit) to a negligible amount and enable delivery of flow to the region at risk. Our goal in this review is to highlight the voids in understanding this adaptation to ischemia—the growth of the coronary collateral circulation. In doing so we discuss the controversies and unknown aspects of the causal factors that stimulate growth of the collateral circulation, the role of genetics, and the role of endogenous stem and progenitor cells in the context of the normal, physiological situation and under more pathological conditions of ischemic heart disease or with some of the underlying risk factors, e.g., diabetes. The major conclusion of this review is that there are many gaps in our knowledge of coronary collateral growth and this knowledge is critical before the potential of stimulating collateralization in the hearts of patients can be realized.
Introduction
In 1971 Schaper published the classic paper describing DNA synthesis in growing collateral vessels [1]. This observation catalysed research in this area for four decades and in the intervening time between this publication and the present, there has been much knowledge gained with over 6000 publications in this area (Medline search). These extensive contributions have lead to myriad reviews [2–14] and even books published on the collateral circulation of the heart [15–17]. Advances in our understanding of coronary collateral growth even now extend to molecular biomarker analyses, genetic analyses and genome wide association studies [18–23]. Despite this progress, there remain many unknowns and many controversies about coronary collateral growth—a process representing the culmination of a coordinated adaptive strategy of the myocardium to circumvent ischemia. The need to investigate these unknowns is highlighted by the increasing appreciation of the clinical benefit of collateral growth as well as the advances made to use collaterals as avenues for therapy [15].
Our goal in this brief review is to focus on these unknowns rather than what is known, because a short review cannot do justice to a topic that has garnered the attention of so much research since the mid 1960’s [24–27]. In our view there are five major questions that need to be answered in order to better understand the process of coronary collateral growth, which hopefully will engender the potential of stimulating this process in patients. These five questions relate to the title of this review, Coronary Collateral Growth—Back to the Future, in the context that filling the many voids of knowledge will require revisiting some questions that have been unanswered for over four decades, as well as answering some questions that have arisen due to recent progress in emerging areas such as stem cell biology.
Question 1: What are the stimuli eliciting coronary collateral growth and when do they stimulate?
If one searches coronary collaterals/collateralization/arteriogenesis, inevitably a search will lead to generic sources of information such as Wikipedia. Reading excerpts from this material would lead to the conclusion that collateral growth is completely understood—that it is a process driven by shear stress and cytokine stimulated mononuclear cell infiltration in the growing vessels. Although we do not disagree that these processes likely contribute in some form to the growth of coronary collaterals, such a narrow view of the basis of coronary collateral growth will never facilitate a complete understanding of the process and the mechanisms underlying poor collateral growth. As we pointed out many years ago, collateral growth is initiated in the heart by ischemia even in the absence of pressure gradients across collateral vessels (and eliminating the effects of shear stress) [28]. More recently, van den Wijngaard made a similar observation; namely that collaterals develop in the absence of pressure gradients [29]. We also documented bioactivity of growth factors in a model of episodic ischemia, which also implicates tissue ischemia as the initiating event in collateral growth [30]. However, is the ischemic tissue the source of growth factors, or do infiltrating inflammatory cells express the mitogens? We believe both sources probably play a key role, but there is likely different timing of these processes. These results do not dismiss the potential importance of shear stress, because it is clear that as collateral vessels outwardly remodel, they carry more oxygenated blood to the ischemic zone, which progressively ameliorates ischemia—yet growth continues as the ischemic signal wanes. This may be the transition point where shear stress becomes important in the remodelling process; specifically after the process has been initiated. The implication of a multi-factorial scheme for the induction of collateral growth is that a therapy designed to stimulate the process may fail if the inhibitory process is not corrected—and depending on the point of collateral growth (initiation phase, expansion phase), the type of therapeutic rescue may be different. These are critical issues that need to be answered because they could influence the type of therapeutic intervention required to correct the abrogated process, e.g., if the problem relates to impaired responses to shear stress, then interventions designed to restore endothelial function may be corrective; however, if the problem is related to inadequate expression of growth factors, then the corrective action may be best accomplished via delivery of the specific factor or factors via gene therapy or a recombinant protein.
The paradigm described in the preceding paragraph is further complicated when one considers that poor collateral growth could be caused by impaired growth factor signalling that occurs under conditions of oxidative or reductive stresses [30, 31]. Perhaps collaterals start to develop in response to myocardial ischemia, but do not remodel fully because of impaired responses to shear stress, which happens in the microcirculation downstream from atherosclerotic lesions [32]. Alternatively, the central issue in poor collateral growth may be “upstream” from a specific kinase and may reside in mitochondrial dysfunction and impaired energy production. With limitations in energy production, ATP levels may limit the actions of specific kinases, which use ATP as a source of PO4 to activate the target protein. Thus, a comprehensive understanding of the “biology” of coronary collateral growth is critical if stimulation of this process as a treatment for ischemic heart disease will ever be realized. Within this context, understanding the signal transduction for the actions and expression of growth factors, timing when shear stress is critical, sequential changes in transcription factor expression, to name a few, are all imperative. The point we wish to make is disturbed signalling—ranging from that inducing expression of growth factors, signalling of the growth factors, signalling and actions of mechanotransduction—likely contributes to poor collateral growth. For example, we previously found that reactive oxygen species make important contributions to collateral growth in the activation of specific redox sensitive mitogen-activated protein kinases (MAPK), in particular p38 MAPK. We reported ephemeral p38 MAPK activation occurring early during growth (days 1 to 3 in a repetitive ischemia protocol that stimulates collateral growth) [31]. Although p38 MAPK is known to be redox sensitive, excessive production of superoxide or inhibition of superoxide production to produce oxidative or reductive stress, respectively, inhibited collateral growth along with inactivation of p38 MAPK. The signalling of vascular endothelial growth factor (a critical factor for collateral growth) depends in part on the p38 MAPK signalling cascade and this activation is impaired in a preclinical model of the metabolic syndrome with abrogated collateral growth. Thus understanding how the signalling of a growth factor is blocked is, perhaps, even more important than understanding that the overall actions of a growth factor are reduced. This understanding would enable development of a treatment regimen to correct the process. The failed therapeutic angiogenesis trials provide an important perspective in this paradigm. Specifically Vascular endothelial growth factor (VEGF) gene or recombinant protein therapy failed to stimulated collateral growth in patients with ischemic heart disease, although initial outcomes were positive [33–35]. This negative result was unexpected because the trials were founded on numerous observations showing that similar treatments in animal models stimulated collateral growth [36–49]. However the preclinical studies were done in “normal” animal models of collateral growth with proper endothelial function, which is vastly different than the patient with vascular disease and endothelial dysfunction. This view is supported by the observation that collateral growth is retarded in animals with compromised endothelial function [50–54], and that VEGF gene therapy does not stimulate collateral growth in a preclinical model with endothelial dysfunction [36].
Another way of viewing the question posed at the beginning of this section relates to understanding the process of collateral growth from the perspective of what occurs. Collateral growth, sometimes termed arteriogenesis (which is a misnomer because the arterial connection is pre-existing, but used frequently in a colloquial manner), represents an expansion of a pre-existing arterial-arterial anastomosis. We termed this abluminal expansion of a vessel as “outward remodelling.” The conditions and stimuli that provoke a vessel to remodel outward, as opposed to the pathological inward remodelling observed during the development of an atheroma is a very important, but yet unanswered question. Schaper reported many years ago that during collateral growth, several arterial-arterial anastomotic connections were positive for 3H-thymidine (indicating cell division) but only a few of these showed abluminal expansion; whereas the others underwent intimal hyperplasia and appeared to eventually disappear [15]. Perhaps this process is dictated by a combination of appropriate shear stress along with the correct combination of growth factors, but the resolution of these two very different outcomes on collateralization is unknown. Our hypothesis is that proper endothelial signalling involving the production of nitric oxide is critical because this vasoactive product also has the property of inhibiting smooth muscle migration and proliferation through a cGMP/Protein Kinase G-dependent process [55–57]. Because there is a gradient of NO (at least according to mathematical models) across the wall of the vessel with the highest concentration near the endothelium/intima and progressively lower concentrations towards the adventitia [58], then the effects of NO may direct smooth muscle migration and proliferation outward. However, this is speculation! Resolution of the issue of what determines inward versus outward remodelling remains a critical, but yet unsolved problem.
Clearly the adaptive response of collateral growth in the heart is a coordinated event that likely has different stimuli during different stages of growth. It is critical that future studies clearly define this sequence, determine the distinction between inward and outward remodelling, and elucidate disturbances in signaling, all of which are critical for the eventual goal of stimulating this process in patients with ischemic heart disease.
Question 2: What is the role of endogenous stem cells in collateral growth?
Ten years ago, Orlic et al. observed that mobilization of endogenous bone marrow-derived stem cells by stem cell factor or granulocyte colony-stimulating factor reduced the consequences of ischemic injury in the heart [59]. This and other observations [60–75] lead to an explosion of research aimed at understanding mechanisms of stem cell recruitment to the ischemic myocardium, factors influencing differentiation, mechanisms by which these cells provide a benefit, and viability of different sources of cells to stimulate vascular growth (this is to name only a few key references). Stem cell biology has catalysed several clinical trials using administration of stem cells or a growth factor/cytokine to enhance their migration into tissue to treat patients with ischemic heart disease. The reader might ask, if clinical trials are already being undertaken, is there a need for more basic research? Our answer would be an unequivocal “yes!” The reason for this resolute affirmative answer relates to the variety of effects reported in clinical trials. Some trials show significant improvements in cardiac function and coronary vasodilator reserve [76–78]; whereas, others show little [79], or at best a marginal benefit [80–82]. It is clear that the type of cells, modes of delivery and timing of delivery are all critical in achieving a clinically relevant result. Furthermore, by defining the relevant mechanisms of action, novel and more direct therapies can be developed that involve less morbidity for the patients, which hopefully will overcome the effects of co-morbidities on stem cell function [80]. Finally, more research is mandated to more fully understand the mechanisms by which the stem cells confer their salubrious effects, e.g., paracrine mechanisms, engraftment [83–86] so that these mechanisms can be amplified to better the effect of the therapy.
We surmise that similar to the previous discussion about the factors stimulating collateral growth and the idea that there is likely a coordinated response involving time-dependent involvement of specific factors for collateralization, there may also be specific times during which stem cell recruitment and/or administrative therapies may or may not work. Moreover, it is imperative that the critical times during which stem cells may exert their paracrine benefit, and even engraft into growing blood vessels, are identified. For example the role of stromal cell-derived factor (SDF-1, CXCL12) has been found to play a critical role in recruiting stem cells to stimulate angiogenesis in the heart [87]. Whether or not this factor would result in a similar effect on collateral growth remains an open question. However, what is now clear with the release of the negative results of the LATETIME trial in which patients were dosed with bone marrow derived stem cells at 3 weeks post-acute myocardial infarction, a time when SDF-1 expression pre-clinically is known to be down-regulated, is that designing clinical trials in a vacuum of knowledge will not move the field forward [87–89]. Furthermore, as it relates to collateral growth and cardiac remodelling in general, it is important to note the consistency of observations between preclinical and clinical studies [89].
Question 3: What are the genetic links/causes to the presence or absence of collateral growth?
The genetic basis of coronary collateral growth is summarized in another review in this series on the coronary circulation; however we are raising the question because of its importance. Currently investigations about genetic links of collateral growth have found associations with VEGF [90, 91], hypoxia inducible factor [92], endothelial nitric-oxide synthase (eNOS) [20, 21], haptoglobin [18, 22], angiotensin-converting enzyme [93], and galectin [94]. The reason we mention this small list is that if one considers the complexity of the process, ranging from cell proliferation, migration, to death of tissue surrounding blood vessels to allow them to expand, this presented list is a beginning of realizing genetic links to coronary collateral growth, or the compromised promise.
Question 4: What is the role for growth inhibitors in attenuating collateral growth under pathological conditions?
The impact offered by growth inhibitors on vascular growth has been appreciated for several years, but the contribution of these inhibitory factors to poor collateral growth in patients remains an open question. One of the earliest reports on growth inhibitors, thrombospondin, was over two decades ago [95–98]. Thrombospondin was found to inhibit angiogenesis under some conditions and endothelial cell proliferation. While the effects of thrombospondin have not been defined in humans regarding collateral growth, the concept is of interest given the findings of the GENEQUEST study [99]. This study enrolled sibling pairs with previous myocardial infarction. A candidate gene approach was performed to define single nucleotide polymorphisms (SNP’s) associated with early myocardial infarction. Of interest the study defined three genetic SNP’s all associated with thrombospondins [100]. The two loss of function SNP’s were associated with significantly elevated risks for early acute myocardial infarction, whereas the one gain in function SNP was associated with decreased risk for acute myocardial infarction [101].
More recently, in the area of cancer biology, the effects of growth inhibitors have been appreciated for many years on modulating tumor angiogenesis [102–106]. This work was founded on many observations concerning the fact that metastatic cancer often was a consequence of removal of a primary tumor [102]. Based on this observation, the idea that tumors produce growth inhibitors was developed. Several years ago we observed in a preclinical model of collateral growth with pharmacologically induced endothelial dysfunction (NO synthase inhibition), an increase in the amount of the growth inhibitor, angiostatin [107]. We determined that the levels of angiostatin in interstitial fluid were sufficient to be biologically active. We also found higher levels of angiostatin in pericardial fluid obtained from patients with angiographically poor collaterals versus those with well-developed collaterals [108]. Also providing support for this concept that growth inhibitors may compromise collateral growth in the heart were observations that the production of endostatin by the myocardium was increased in patients with poor collaterals compared to those with well developed coronary collaterals [109]. Interestingly, the potential complicating effects of growth inhibitors on collateral growth has not been systematically pursued, but we believe that it should be as this negative influence could be one of the contributing reasons to the failed “therapeutic angiogenesis” trials of several years ago [33].
Another issue regarding growth inhibition pertains to regression of the coronary collateral circulation. This phenomenon has been related to the time it takes for collaterals to open after the stimulus to provoke collateral growth is removed (for example repetitive occlusion) even after full development of the collateral circulation [110, 111]. If the stimulus is applied soon after the end of the protocol, the increase in collateral flow occurs within seconds; however when the stimulus is applied a week after the end of the procedure, the increase in flow takes several minutes. The basis for this “regression” is unknown. Moreover it is not known whether the regression is first functional, perhaps constriction or spasm, and then anatomical, with a rarefaction of coronary collateral vessels.
Question 5: Will therapeutic angiogenesis/collateral growth lead to patients growing their own bypasses?
There have been 11 trials using VEGF gene or recombinant protein therapy and 9 trials using basic fibroblast growth factor (bFGF) directed at stimulating growth of the coronary collateral circulation [112]. Despite some promising results in the early stage trials, the final results were disappointing and did not produce collateral growth in the patients. From a certain point of view, the failure of therapeutic angiogenesis trials was predictable. The basis for some of the trials was founded on the observation that VEGF gene therapy significantly promoted collateral growth in animal models. However, the failing of the pre-clinical observations was that collateral growth and the effects of VEGF were studied in normal animals in the absence of vascular disease. The actions of VEGF are retarded in vascular disease and the associated endothelial dysfunction [53]. Although bFGF gene is reported to have its actions amplified by mild oxidative stress [113], it is likely severe oxidative stress would confer an inhibitory effect as it does with other redox sensitive signalling events [31].
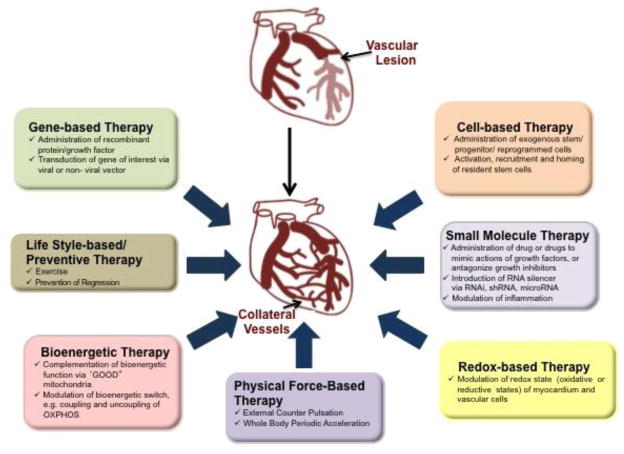
Figure 1 illustrates the complexity of stimulating coronary collateral growth in patients. We have categorized different treatment regimens into 7 strategies, any of which could be potentially combined. As we discussed previously the trials directed at stimulating coronary collateral growth using gene therapy—adenovirus, plasmid, recombinant protein—have failed. However, perhaps these therapies used in conjunction with those directed at restoring redox balance to the heart may rescue the signalling pathways rendered dysfunctional by oxidative stress and eventually succeed. We also included a treatment strategy invoking exercise. The effects of exercise on collateral growth are controversial [114–118], but it could be the repetitive increases in myocardial oxygen demands may provide signals that prevent regression of the collaterals even after full development. We have also included bioenergetics therapy as our approach. Recently we have found that in a preclinical model of the metabolic syndrome, the Zucker obese fatty rat, collateral growth is severely impaired and that this model has mitochondrial dysfunction. We have been able to correct mitochondrial dysfunction by treatment with mitochondrial directed free radical scavengers, which also results in restoration of collateral growth. Physical force based therapies also have been recently applied. External counter pulsation has produced an increased in collateral growth [119, 120] through a yet unresolved mechanism. The authors of these studies inferred that shear stress was a likely factor resulting in the increase in collateral growth. This may be correct but without measurements of pressure and flow during the counterpulsation interventions the precise mechanisms underlying the benefit are unresolved. Likewise, whole body acceleration has been used as a treatment regimen, but its effects on collateral growth seem inconsistent [121]. Cell based therapies are still in their infancy. Animal studies demonstrate “proof of concept” by the use of reprogrammed cells or bone marrow-derived stem cells to amplify coronary collateral growth [122, 123]. Also, G-CSF (granulocyte-colony stimulating factor) that also mobilizes bone marrow cells has a salubrious effect on collateral growth even in the absence of ischemia [124]. Also the use of cytokines such as SDF-1 to promote neovascularization may also have a stimulatory effect on coronary collateral vessels in the heart [69, 125, 126]. Yet important questions, such as the “best” cell type to stimulate collateral growth via paracrine mechanisms or engraftment in the growing collateral remain unresolved. Small molecule therapies to target key pathways for stimulation and inhibition are also on the horizon. Recent work has revealed the important interactions between ERK1/2 and Akt signalling [127], and manipulation of these pathways via small molecule activators may fill a void in the event of compromised signalling. One of the points of figure 1 is to show that there are many possibilities, which at times seems daunting because there seem to be too many approaches. We opine that the specific therapies may be unique to patients, and that one solution may not work for all.
Fig. 1.
Therapeutic Strategies for Coronary Collateral Growth
Conclusions—Back to the Future
We would like to again mention that coronary collateral growth is a complex process stimulated and regulated by myriad signals. The growth of the coronary collateral circulation has tremendous impact on patients’ ability to recover from the morbidity and mortality of ischemic heart disease. Therapeutic collateral growth has enormous potential as a prophylactic therapy to prevent the consequences of the disease, or as a treatment for patients with intractable disease who are not candidates for medical or surgical therapies. We would like to emphasize “back to the future” because if stimulation of coronary collateral growth will ever be realized, a fundamental understanding of the biology of the process is mandatory. Moreover, an understanding of how disease abrogates collateral growth is equally essential. Many of these critical issues have been neglected and this may be a reason why therapeutic coronary collateral growth still remains a “potential” rather than a reality. Finally, thorough interrogation of mechanisms and answers to the first four questions will bring us “back to the future,” by providing an answer to the last question, Will therapeutic angiogenesis/collateral growth lead to patients growing their own bypasses?
Highlights.
This review focuses on what is not known about coronary collateral growth.
This review raises fundamental questions about the regulation of coronary collateral growth.
We present a schematic that we hope will facilitate the goal of therapeutic collateral growth.
Acknowledgments
We wish to acknowledge support from the NHLBI: HL100828Z, HL32788, HL83366 (WMC); Austen BioInnovation Institute in Akron: Collaborative Research and Development Projects (LY); Summa Foundation and Skirball Foundation (MSP), and the American Heart Association, Postdoctoral Fellowship (VO).
Footnotes
Disclosures: None declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schaper W, De Brabander M, Lewi P. DNA synthesis and mitoses in coronary collateral vessels of the dog. Circ Res. 1971;28:671–9. doi: 10.1161/01.res.28.6.671. [DOI] [PubMed] [Google Scholar]
- 2.Schaper W. Therapeutic arteriogenesis has arrived. Circulation. 2001;104:1994–5. [PubMed] [Google Scholar]
- 3.Schaper W, Gorge G, Winkler B, Schaper J. The collateral circulation of the heart. ProgCardDis. 1988;31:57–77. doi: 10.1016/0033-0620(88)90011-4. [DOI] [PubMed] [Google Scholar]
- 4.Schaper W, Ito WD. Molecular mechanisms of coronary collateral vessel growth. Circ Res. 1996;79:911–9. doi: 10.1161/01.res.79.5.911. [DOI] [PubMed] [Google Scholar]
- 5.Schaper W, Scholz D. Factors regulating arteriogenesis. Arterioscler Thromb Vasc Biol. 2003;23:1143–51. doi: 10.1161/01.ATV.0000069625.11230.96. [DOI] [PubMed] [Google Scholar]
- 6.Schaper W. Coronary collaterals. IntSocApplCardiovascBiol. 1991;2:25–30. [Google Scholar]
- 7.Schaper W. Collateral circulation: past and present. Basic Res Cardiol. 2009;104:5–21. doi: 10.1007/s00395-008-0760-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simons M. Angiogenesis, arteriogenesis, and diabetes: paradigm reassessed? J Am Coll Cardiol. 2005;46:835–7. doi: 10.1016/j.jacc.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 9.de Muinck ED, Simons M. Re-evaluating therapeutic neovascularization. J Mol Cell Cardiol. 2004;36:25–32. doi: 10.1016/j.yjmcc.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 10.Baklanov D, Simons M. Arteriogenesis: lessons learned from clinical trials. Endothelium. 2003;10:217–23. doi: 10.1080/10623320390246397. [DOI] [PubMed] [Google Scholar]
- 11.Post MJ, Simons M. The rational phase of therapeutic angiogenesis. Minerva cardioangiologica. 2003;51:421–32. [PubMed] [Google Scholar]
- 12.Laham RJ, Simons M, Sellke F. Gene transfer for angiogenesis in coronary artery disease. Annual review of medicine. 2001;52:485–502. doi: 10.1146/annurev.med.52.1.485. [DOI] [PubMed] [Google Scholar]
- 13.Pung YF, Chilian WM. Corruption of coronary collateral growth in metabolic syndrome: Role of oxidative stress. World J Cardiol. 2010;2:421–7. doi: 10.4330/wjc.v2.i12.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yun J, Rocic P, Pung YF, Belmadani S, Carrao AC, Ohanyan V, et al. Redox-dependent mechanisms in coronary collateral growth: the “redox window” hypothesis. Antioxid Redox Signal. 2009;11:1961–74. doi: 10.1089/ars.2009.2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schaper W. The Collateral Circulation of the Heart. New York, N. Y: Elsevier; 1971. [Google Scholar]
- 16.Schaper W, Schaper J. Arteriogenesis. Boston: Kluwer Academic Publishers; 2004. [Google Scholar]
- 17.Schaper W, Schaper J. Collateral circulation: heart, brain, kidney, limbs. Boston: Kluwer Academic Publishers; 1993. [Google Scholar]
- 18.Hochberg I, Roguin A, Nikolsky E, Chanderashekhar PV, Cohen S, Levy AP. Haptoglobin phenotype and coronary artery collaterals in diabetic patients. Atherosclerosis. 2002;161:441–6. doi: 10.1016/s0021-9150(01)00657-8. [DOI] [PubMed] [Google Scholar]
- 19.Roguin A, Resar JR. Genetics and susceptibility of coronary collateral formation. Circulation. 2003;108:e149. doi: 10.1161/01.CIR.0000101951.57939.7E. author reply e. [DOI] [PubMed] [Google Scholar]
- 20.Lamblin N, Cuilleret FJ, Helbecque N, Dallongeville J, Lablanche JM, Amouyel P, et al. A common variant of endothelial nitric oxide synthase (Glu298Asp) is associated with collateral development in patients with chronic coronary occlusions. BMC cardiovascular disorders. 2005;5:27. doi: 10.1186/1471-2261-5-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gulec S, Karabulut H, Ozdemir AO, Ozdol C, Turhan S, Altin T, et al. Glu298Asp polymorphism of the eNOS gene is associated with coronary collateral development. Atherosclerosis. 2008;198:354–9. doi: 10.1016/j.atherosclerosis.2007.09.037. [DOI] [PubMed] [Google Scholar]
- 22.Zhang J, Regieli JJ, Schipper M, Entius MM, Liang F, Koerselman J, et al. Inflammatory gene haplotype-interaction networks involved in coronary collateral formation. Hum Hered. 2008;66:252–64. doi: 10.1159/000143407. [DOI] [PubMed] [Google Scholar]
- 23.Meier P, Antonov J, Zbinden R, Kuhn A, Zbinden S, Gloekler S, et al. Non-invasive gene-expression-based detection of well-developed collateral function in individuals with and without coronary artery disease. Heart. 2009;95:900–8. doi: 10.1136/hrt.2008.145383. [DOI] [PubMed] [Google Scholar]
- 24.Schaper W. Tangential wall stress as a molding force in the development of collateral vessels in the canine heart. Experientia. 1967;23:595–6. doi: 10.1007/BF02137994. [DOI] [PubMed] [Google Scholar]
- 25.Schaper W, Jageneau A, Xhonneux R. The development of collateral circulation in the pig and dog heart. Cardiologia. 1967;51:321–35. doi: 10.1159/000165875. [DOI] [PubMed] [Google Scholar]
- 26.Schaper W. Influence of physical factors on the radial growth of collateral vessels in coronary circulation. Verhandlungen der Deutschen Gesellschaft fur Kreislaufforschung. 1966;32:282–6. [PubMed] [Google Scholar]
- 27.Schaper WK, Xhonneux R, Jageneau AH. Stimulation of the coronary collateral circulation by lidoflazine (R 7904) Naunyn-Schmiedebergs Archiv fur experimentelle Pathologie und Pharmakologie. 1965;252:1–8. doi: 10.1007/BF00246424. [DOI] [PubMed] [Google Scholar]
- 28.Chilian WM, Mass HJ, Williams SE, Layne SM, Smith EE, Scheel KW. Microvascular occlusions promote coronary collateral growth. Am J Physiol. 1990;258:H1103–11. doi: 10.1152/ajpheart.1990.258.4.H1103. [DOI] [PubMed] [Google Scholar]
- 29.van den Wijngaard JP, Schulten H, van Horssen P, Ter Wee RD, Siebes M, Post MJ, et al. Porcine coronary collateral formation in the absence of a pressure gradient remote of the ischemic border zone. Am J Physiol Heart Circ Physiol. 2011;300:H1930–7. doi: 10.1152/ajpheart.00403.2010. [DOI] [PubMed] [Google Scholar]
- 30.Weihrauch D, Tessmer J, Warltier DC, Chilian WM. Repetitive coronary artery occlusions induce release of growth factors into the myocardial interstitium. Am J Physiol. 1998;275:H969–76. doi: 10.1152/ajpheart.1998.275.3.H969. [DOI] [PubMed] [Google Scholar]
- 31.Rocic P, Kolz C, Reed R, Potter B, Chilian WM. Optimal reactive oxygen species concentration and p38 MAP kinase are required for coronary collateral growth. Am J Physiol Heart Circ Physiol. 2007;292:H2729–36. doi: 10.1152/ajpheart.01330.2006. [DOI] [PubMed] [Google Scholar]
- 32.Kuo L, Davis MJ, Cannon S, Chilian WM. Pathophysiological consequences of atherosclerosis extend into the coronary microcirculation. Restoration of endothelium-dependent responses by L-argine. Circ Research. 1992;70:465–76. doi: 10.1161/01.res.70.3.465. [DOI] [PubMed] [Google Scholar]
- 33.Simons M, Bonow RO, Chronos NA, Cohen DJ, Giordano FJ, Hammond HK, et al. Clinical trials in coronary angiogenesis: issues, problems, consensus: An expert panel summary. Circulation. 2000;102:E73–86. doi: 10.1161/01.cir.102.11.e73. [DOI] [PubMed] [Google Scholar]
- 34.Emanueli C, Madeddu P. Angiogenesis gene therapy to rescue ischaemic tissues: achievements and future directions. British journal of pharmacology. 2001;133:951–8. doi: 10.1038/sj.bjp.0704155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Losordo DW, Vale PR, Isner JM. Gene therapy for myocardial angiogenesis. Am Heart J. 1999;138:S132–41. doi: 10.1016/s0002-8703(99)70333-9. [DOI] [PubMed] [Google Scholar]
- 36.Hattan N, Warltier D, Gu W, Kolz C, Chilian WM, Weihrauch D. Autologous vascular smooth muscle cell-based myocardial gene therapy to induce coronary collateral growth. Am Journal Physiology Heart Circ Physiol. 2004;287:H488–93. doi: 10.1152/ajpheart.00145.2004. [DOI] [PubMed] [Google Scholar]
- 37.Bauters C, Asahara T, Zheng LP, Takeshita S, Bunting S, Ferrara N, et al. Site-specific therapeutic angiogenesis after systemic administration of vascular endothelial growth factor. Journal of vascular surgery: official publication, the Society for Vascular Surgery [and] International Society for Cardiovascular Surgery, North American Chapter. 1995;21:314–24. doi: 10.1016/s0741-5214(95)70272-5. discussion 24–5. [DOI] [PubMed] [Google Scholar]
- 38.Takeshita S, Pu LQ, Stein LA, Sniderman AD, Bunting S, Ferrara N, et al. Intramuscular administration of vascular endothelial growth factor induces dose-dependent collateral artery augmentation in a rabbit model of chronic limb ischemia. Circulation. 1994;90:II228–34. [PubMed] [Google Scholar]
- 39.Takeshita S, Weir L, Chen D, Zheng LP, Riessen R, Bauters C, et al. Therapeutic angiogenesis following arterial gene transfer of vascular endothelial growth factor in a rabbit model of hindlimb ischemia. Biochem Biophys Res Commun. 1996;227:628–35. doi: 10.1006/bbrc.1996.1556. [DOI] [PubMed] [Google Scholar]
- 40.Takeshita S, Zheng LP, Brogi E, Kearney M, Pu LQ, Bunting S, et al. Therapeutic angiogenesis. A single intraarterial bolus of vascular endothelial growth factor augments revascularization in a rabbit ischemic hind limb model. J Clin Invest. 1994;93:662–70. doi: 10.1172/JCI117018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsurumi Y, Takeshita S, Chen D, Kearney M, Rossow ST, Passeri J, et al. Direct intramuscular gene transfer of naked DNA encoding vascular endothelial growth factor augments collateral development and tissue perfusion. Circulation. 1996;94:3281–90. doi: 10.1161/01.cir.94.12.3281. [DOI] [PubMed] [Google Scholar]
- 42.van der Zee R, Murohara T, Luo Z, Zollmann F, Passeri J, Lekutat C, et al. Vascular endothelial growth factor/vascular permeability factor augments nitric oxide release from quiescent rabbit and human vascular endothelium. Circulation. 1997;95:1030–7. doi: 10.1161/01.cir.95.4.1030. [DOI] [PubMed] [Google Scholar]
- 43.Srivastava S, Terjung RL, Yang HT. Basic fibroblast growth factor increases collateral blood flow in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2003;285:H1190–7. doi: 10.1152/ajpheart.00280.2003. [DOI] [PubMed] [Google Scholar]
- 44.Yang HT, Yan Z, Abraham JA, Terjung RL. VEGF(121)- and bFGF-induced increase in collateral blood flow requires normal nitric oxide production. Am J Physiol Heart Circ Physiol. 2001;280:H1097–104. doi: 10.1152/ajpheart.2001.280.3.H1097. [DOI] [PubMed] [Google Scholar]
- 45.Lopez JJ, Edelman ER, Stamler A, Hibberd MG, Prasad P, Thomas KA, et al. Angiogenic potential of perivascularly delivered aFGF in a porcine model of chronic myocardial ischemia. Am J Physiol. 1998;274:H930–6. doi: 10.1152/ajpheart.1998.274.3.H930. [DOI] [PubMed] [Google Scholar]
- 46.Lopez JJ, Edelman ER, Stamler A, Morgan JP, Sellke FW, Simons M. Local perivascular administration of basic fibroblast growth factor: drug delivery and toxicological evaluation. Drug metabolism and disposition: the biological fate of chemicals. 1996;24:922–4. [PubMed] [Google Scholar]
- 47.Sellke FW, Li J, Stamler A, Lopez JJ, Thomas KA, Simons M. Angiogenesis induced by acidic fibroblast growth factor as an alternative method of revascularization for chronic myocardial ischemia. Surgery. 1996;120:182–8. doi: 10.1016/s0039-6060(96)80286-8. [DOI] [PubMed] [Google Scholar]
- 48.Banai S, Jaklitsch MT, Shou M, Lazarous DF, Scheinowitz M, Biro S, et al. Angiogenic-induced enhancement of collateral blood flow to ischemic myocardium by vascular endothelial growth factor in dogs. Circulation. 1994;89:2183–9. doi: 10.1161/01.cir.89.5.2183. [DOI] [PubMed] [Google Scholar]
- 49.Unger EF, Banai S, Shou M, Lazarous DF, Jaklitsch MT, Scheinowitz M, et al. Basic fibroblast growth factor enhances myocardial collateral flow in a canine model. Am J Physiol. 1994;266:H1588–95. doi: 10.1152/ajpheart.1994.266.4.H1588. [DOI] [PubMed] [Google Scholar]
- 50.Tirziu D, Moodie KL, Zhuang ZW, Singer K, Helisch A, Dunn JF, et al. Delayed arteriogenesis in hypercholesterolemic mice. Circulation. 2005;112:2501–9. doi: 10.1161/CIRCULATIONAHA.105.542829. [DOI] [PubMed] [Google Scholar]
- 51.Matsunaga TDCW, Moniz M, Tessmmer J, Weihrauch D, Chilian WM. Role of nitric oxide and vascular endothelial growth factor in coronary collateral growth. Circulation. 2000;102:3098–103. doi: 10.1161/01.cir.102.25.3098. [DOI] [PubMed] [Google Scholar]
- 52.Reed R, Kolz C, Potter B, Rocic P. The mechanistic basis for the disparate effects of angiotensin II on coronary collateral growth. Arterioscler Thromb Vasc Biol. 2008;28:61–7. doi: 10.1161/ATVBAHA.107.154294. [DOI] [PubMed] [Google Scholar]
- 53.Hattan N, Chilian WM, Park F, Rocic P. Restoration of coronary collateral growth in the Zucker obese rat:: Impact of VEGF and ecSOD. Basic Res Cardiol. 2007;102:217–23. doi: 10.1007/s00395-007-0646-3. [DOI] [PubMed] [Google Scholar]
- 54.Lassaletta AD, Chu LM, Sellke FW. Therapeutic neovascularization for coronary disease: current state and future prospects. Basic Res Cardiol. 2011;106:897–909. doi: 10.1007/s00395-011-0200-1. [DOI] [PubMed] [Google Scholar]
- 55.Lincoln TM, Dey NB, Boerth NJ, Cornwell TL, Soff GA. Nitric oxide--cyclic GMP pathway regulates vascular smooth muscle cell phenotypic modulation: implications in vascular diseases. Acta physiologica Scandinavica. 1998;164:507–15. doi: 10.1111/j.1365-201x.1998.tb10700.x. [DOI] [PubMed] [Google Scholar]
- 56.Boerth NJ, Dey NB, Cornwell TL, Lincoln TM. Cyclic GMP-dependent protein kinase regulates vascular smooth muscle cell phenotype. Journal of vascular research. 1997;34:245–59. doi: 10.1159/000159231. [DOI] [PubMed] [Google Scholar]
- 57.Lincoln TM, Dey N, Sellak H. Invited review: cGMP-dependent protein kinase signaling mechanisms in smooth muscle: from the regulation of tone to gene expression. J Appl Physiol. 2001;91:1421–30. doi: 10.1152/jappl.2001.91.3.1421. [DOI] [PubMed] [Google Scholar]
- 58.Seraia IP, Nartsissov Ia R, Brown G. Mathematical modeling of non-stationary spatial gradients of nitric oxide in the muscle wall of blood vessels. Biofizika. 2003;48:91–6. [PubMed] [Google Scholar]
- 59.Orlic D, Kajstura J, Chimenti S, Limana F, Jakoniuk I, Quaini F, et al. Mobilized bone marrow cells repair the infarcted heart, improving function and survival. Proc Natl Acad Sci U S A. 2001;98:10344–9. doi: 10.1073/pnas.181177898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Leri A, Hosoda T, Kajstura J, Anversa P, Rota M. Identification of a coronary stem cell in the human heart. J Mol Med (Berl) 2011;89:947–59. doi: 10.1007/s00109-011-0769-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Leri A, Kajstura J, Anversa P. Cardiac stem cells and mechanisms of myocardial regeneration. Physiol Rev. 2005;85:1373–416. doi: 10.1152/physrev.00013.2005. [DOI] [PubMed] [Google Scholar]
- 62.Nadal-Ginard B, Anversa P, Kajstura J, Leri A. Cardiac stem cells and myocardial regeneration. Novartis Foundation symposium. 2005;265:142–54. discussion 55–7, 204–11. [PubMed] [Google Scholar]
- 63.Orlic D, Kajstura J, Chimenti S, Bodine DM, Leri A, Anversa P. Bone marrow stem cells regenerate infarcted myocardium. Pediatric transplantation. 2003;7 (Suppl 3):86–8. doi: 10.1034/j.1399-3046.7.s3.13.x. [DOI] [PubMed] [Google Scholar]
- 64.Penn MS, Zhang M, Deglurkar I, Topol EJ. Role of stem cell homing in myocardial regeneration. Int J Cardiol. 2004;95 (Suppl 1):S23–5. doi: 10.1016/s0167-5273(04)90007-1. [DOI] [PubMed] [Google Scholar]
- 65.Chavakis E, Carmona G, Urbich C, Gottig S, Henschler R, Penninger JM, et al. Phosphatidylinositol-3-kinase-gamma is integral to homing functions of progenitor cells. Circ Res. 2008;102:942–9. doi: 10.1161/CIRCRESAHA.107.164376. [DOI] [PubMed] [Google Scholar]
- 66.Agarwal U, Ghalayini W, Dong F, Weber K, Zou YR, Rabbany SY, et al. Role of cardiac myocyte CXCR4 expression in development and left ventricular remodeling after acute myocardial infarction. Circ Res. 2010;107:667–76. doi: 10.1161/CIRCRESAHA.110.223289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Askari AT, Penn MS. Stromal cell-derived factor-1 mediates stem cell homing and tissue regeneration. Discovery medicine. 2003;3:46–7. [PubMed] [Google Scholar]
- 68.Schenk S, Mal N, Finan A, Zhang M, Kiedrowski M, Popovic Z, et al. Monocyte chemotactic protein-3 is a myocardial mesenchymal stem cell homing factor. Stem Cells. 2007;25:245–51. doi: 10.1634/stemcells.2006-0293. [DOI] [PubMed] [Google Scholar]
- 69.Zhang M, Mal N, Kiedrowski M, Chacko M, Askari AT, Popovic ZB, et al. SDF-1 expression by mesenchymal stem cells results in trophic support of cardiac myocytes after myocardial infarction. Faseb J. 2007;21:3197–207. doi: 10.1096/fj.06-6558com. [DOI] [PubMed] [Google Scholar]
- 70.Dimmeler S, Vasa-Nicotera M. Aging of progenitor cells: limitation for regenerative capacity? J Am Coll Cardiol. 2003;42:2081–2. doi: 10.1016/j.jacc.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 71.Urbich C, Heeschen C, Aicher A, Dernbach E, Zeiher AM, Dimmeler S. Relevance of monocytic features for neovascularization capacity of circulating endothelial progenitor cells. Circulation. 2003;108:2511–6. doi: 10.1161/01.CIR.0000096483.29777.50. [DOI] [PubMed] [Google Scholar]
- 72.Heeschen C, Aicher A, Lehmann R, Fichtlscherer S, Vasa M, Urbich C, et al. Erythropoietin is a potent physiologic stimulus for endothelial progenitor cell mobilization. Blood. 2003;102:1340–6. doi: 10.1182/blood-2003-01-0223. [DOI] [PubMed] [Google Scholar]
- 73.Walter DH, Dimmeler S. Endothelial progenitor cells: regulation and contribution to adult neovascularization. Herz. 2002;27:579–88. doi: 10.1007/s00059-002-2427-y. [DOI] [PubMed] [Google Scholar]
- 74.Rehman J, Traktuev D, Li J, Merfeld-Clauss S, Temm-Grove CJ, Bovenkerk JE, et al. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004;109:1292–8. doi: 10.1161/01.CIR.0000121425.42966.F1. [DOI] [PubMed] [Google Scholar]
- 75.Traktuev DO, Merfeld-Clauss S, Li J, Kolonin M, Arap W, Pasqualini R, et al. A population of multipotent CD34-positive adipose stromal cells share pericyte and mesenchymal surface markers, reside in a periendothelial location, and stabilize endothelial networks. Circ Res. 2008;102:77–85. doi: 10.1161/CIRCRESAHA.107.159475. [DOI] [PubMed] [Google Scholar]
- 76.Schachinger V, Assmus B, Britten MB, Honold J, Lehmann R, Teupe C, et al. Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction: final one-year results of the TOPCARE-AMI Trial. J Am Coll Cardiol. 2004;44:1690–9. doi: 10.1016/j.jacc.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 77.Schachinger V, Assmus B, Honold J, Lehmann R, Hofmann WK, Martin H, et al. Normalization of coronary blood flow in the infarct-related artery after intracoronary progenitor cell therapy: intracoronary Doppler substudy of the TOPCARE-AMI trial. Clinical research in cardiology: official journal of the German Cardiac Society. 2006;95:13–22. doi: 10.1007/s00392-006-0314-x. [DOI] [PubMed] [Google Scholar]
- 78.Heusch G. SCIPIO brings new momentum to cardiac cell therapy. Lancet. 2011;378:1827–8. doi: 10.1016/S0140-6736(11)61648-6. [DOI] [PubMed] [Google Scholar]
- 79.Lunde K, Solheim S, Aakhus S, Arnesen H, Abdelnoor M, Egeland T, et al. Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. N Engl J Med. 2006;355:1199–209. doi: 10.1056/NEJMoa055706. [DOI] [PubMed] [Google Scholar]
- 80.Schachinger V, Erbs S, Elsasser A, Haberbosch W, Hambrecht R, Holschermann H, et al. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med. 2006;355:1210–21. doi: 10.1056/NEJMoa060186. [DOI] [PubMed] [Google Scholar]
- 81.Abdel-Latif A, Bolli R, Tleyjeh IM, Montori VM, Perin EC, Hornung CA, et al. Adult bone marrow-derived cells for cardiac repair: a systematic review and meta-analysis. Arch Intern Med. 2007;167:989–97. doi: 10.1001/archinte.167.10.989. [DOI] [PubMed] [Google Scholar]
- 82.Assmus B, Schachinger V, Teupe C, Britten M, Lehmann R, Dobert N, et al. Transplantation of Progenitor Cells and Regeneration Enhancement in Acute Myocardial Infarction (TOPCARE-AMI) Circulation. 2002;106:3009–17. doi: 10.1161/01.cir.0000043246.74879.cd. [DOI] [PubMed] [Google Scholar]
- 83.Marban E, Cheng K. Heart to heart: The elusive mechanism of cell therapy. Circulation. 2010;121:1981–4. doi: 10.1161/CIRCULATIONAHA.110.952580. [DOI] [PubMed] [Google Scholar]
- 84.Yoon CH, Koyanagi M, Iekushi K, Seeger F, Urbich C, Zeiher AM, et al. Mechanism of improved cardiac function after bone marrow mononuclear cell therapy: role of cardiovascular lineage commitment. Circulation. 2010;121:2001–11. doi: 10.1161/CIRCULATIONAHA.109.909291. [DOI] [PubMed] [Google Scholar]
- 85.Ziebart T, Yoon CH, Trepels T, Wietelmann A, Braun T, Kiessling F, et al. Sustained persistence of transplanted proangiogenic cells contributes to neovascularization and cardiac function after ischemia. Circ Res. 2008;103:1327–34. doi: 10.1161/CIRCRESAHA.108.180463. [DOI] [PubMed] [Google Scholar]
- 86.Zaruba MM, Soonpaa M, Reuter S, Field LJ. Cardiomyogenic potential of C-kit(+)-expressing cells derived from neonatal and adult mouse hearts. Circulation. 2010;121:1992–2000. doi: 10.1161/CIRCULATIONAHA.109.909093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sundararaman S, Miller TJ, Pastore JM, Kiedrowski M, Aras R, Penn MS. Plasmid-based transient human stromal cell-derived factor-1 gene transfer improves cardiac function in chronic heart failure. Gene Ther. 2011;18:867–73. doi: 10.1038/gt.2011.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Askari AT, Unzek S, Popovic ZB, Goldman CK, Forudi F, Kiedrowski M, et al. Effect of stromal-cell-derived factor 1 on stem-cell homing and tissue regeneration in ischaemic cardiomyopathy. Lancet. 2003;362:697–703. doi: 10.1016/S0140-6736(03)14232-8. [DOI] [PubMed] [Google Scholar]
- 89.Deglurkar I, Mal N, Mills WR, Popovic ZB, McCarthy P, Blackstone EH, et al. Mechanical and electrical effects of cell-based gene therapy for ischemic cardiomyopathy are independent. Human gene therapy. 2006;17:1144–51. doi: 10.1089/hum.2006.17.1144. [DOI] [PubMed] [Google Scholar]
- 90.Ripa RS, Jorgensen E, Baldazzi F, Frikke-Schmidt R, Wang Y, Tybjaerg-Hansen A, et al. The influence of genotype on vascular endothelial growth factor and regulation of myocardial collateral blood flow in patients with acute and chronic coronary heart disease. Scandinavian journal of clinical and laboratory investigation. 2009;69:722–8. doi: 10.3109/00365510903078803. [DOI] [PubMed] [Google Scholar]
- 91.Lin TH, Wang CL, Su HM, Hsu PC, Juo SH, Voon WC, et al. Functional vascular endothelial growth factor gene polymorphisms and diabetes: effect on coronary collaterals in patients with significant coronary artery disease. Clin Chim Acta. 2010;411:1688–93. doi: 10.1016/j.cca.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 92.Resar JR, Roguin A, Voner J, Nasir K, Hennebry TA, Miller JM, et al. Hypoxia-inducible factor 1alpha polymorphism and coronary collaterals in patients with ischemic heart disease. Chest. 2005;128:787–91. doi: 10.1378/chest.128.2.787. [DOI] [PubMed] [Google Scholar]
- 93.Ceyhan K, Kadi H, Celik A, Burucu T, Koc F, Sogut E, et al. Angiotensin-Converting Enzyme DD Polymorphism Is Associated With Poor Coronary Collateral Circulation in Patients With Coronary Artery Disease. Journal of investigative medicine: the official publication of the American Federation for Clinical Research. 2011 doi: 10.2310/JIM.0b013e31823908e2. [DOI] [PubMed] [Google Scholar]
- 94.van der Laan AM, Schirmer SH, de Vries MR, Koning JJ, Volger OL, Fledderus JO, et al. Galectin-2 expression is dependent on the rs7291467 polymorphism and acts as an inhibitor of arteriogenesis. European heart journal. 2011 doi: 10.1093/eurheartj/ehr220. [DOI] [PubMed] [Google Scholar]
- 95.Bagavandoss P, Wilks JW. Specific inhibition of endothelial cell proliferation by thrombospondin. Biochem Biophys Res Commun. 1990;170:867–72. doi: 10.1016/0006-291x(90)92171-u. [DOI] [PubMed] [Google Scholar]
- 96.Good DJ, Polverini PJ, Rastinejad F, Le Beau MM, Lemons RS, Frazier WA, et al. A tumor suppressor-dependent inhibitor of angiogenesis is immunologically and functionally indistinguishable from a fragment of thrombospondin. Proc Natl Acad Sci U S A. 1990;87:6624–8. doi: 10.1073/pnas.87.17.6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Iruela-Arispe ML, Bornstein P, Sage H. Thrombospondin exerts an antiangiogenic effect on cord formation by endothelial cells in vitro. Proc Natl Acad Sci U S A. 1991;88:5026–30. doi: 10.1073/pnas.88.11.5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dameron KM, Volpert OV, Tainsky MA, Bouck N. Control of angiogenesis in fibroblasts by p53 regulation of thrombospondin-1. Science. 1994;265:1582–4. doi: 10.1126/science.7521539. [DOI] [PubMed] [Google Scholar]
- 99.Stenina OI, Byzova TV, Adams JC, McCarthy JJ, Topol EJ, Plow EF. Coronary artery disease and the thrombospondin single nucleotide polymorphisms. The international journal of biochemistry & cell biology. 2004;36:1013–30. doi: 10.1016/j.biocel.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 100.Topol EJ, McCarthy J, Gabriel S, Moliterno DJ, Rogers WJ, Newby LK, et al. Single nucleotide polymorphisms in multiple novel thrombospondin genes may be associated with familial premature myocardial infarction. Circulation. 2001;104:2641–4. doi: 10.1161/hc4701.100910. [DOI] [PubMed] [Google Scholar]
- 101.Boekholdt SM, Trip MD, Peters RJ, Engelen M, Boer JM, Feskens EJ, et al. Thrombospondin-2 polymorphism is associated with a reduced risk of premature myocardial infarction. Arterioscler Thromb Vasc Biol. 2002;22:e24–7. doi: 10.1161/01.atv.0000046235.22451.66. [DOI] [PubMed] [Google Scholar]
- 102.Folkman J. Role of angiogenesis in tumor growth and metastasis. Seminars in oncology. 2002;29:15–8. doi: 10.1053/sonc.2002.37263. [DOI] [PubMed] [Google Scholar]
- 103.Kuo CJ, Farnebo F, Yu EY, Christofferson R, Swearingen RA, Carter R, et al. Comparative evaluation of the antitumor activity of antiangiogenic proteins delivered by gene transfer. Proc Natl Acad Sci U S A. 2001;98:4605–10. doi: 10.1073/pnas.081615298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hahnfeldt P, Panigrahy D, Folkman J, Hlatky L. Tumor development under angiogenic signaling: a dynamical theory of tumor growth, treatment response, and postvascular dormancy. Cancer Res. 1999;59:4770–5. [PubMed] [Google Scholar]
- 105.O’Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS, et al. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88:277–85. doi: 10.1016/s0092-8674(00)81848-6. [DOI] [PubMed] [Google Scholar]
- 106.Folkman J. Endogenous inhibitors of angiogenesis. Harvey lectures. 1996;92:65–82. [PubMed] [Google Scholar]
- 107.Matsunaga T, Weihrauch DW, Moniz MC, Tessmer J, Warltier DC, Chilian WM. Angiostatin inhibits coronary angiogenesis during impaired production of nitric oxide. Circulation. 2002;105:2185–91. doi: 10.1161/01.cir.0000015856.84385.e9. [DOI] [PubMed] [Google Scholar]
- 108.Matsunaga T, Chilian WM, March K. Angiostatin is negatively associated with coronary collateral growth in patients with coronary artery disease. Am J Physiol Heart Circ Physiol. 2005;288:H2042–6. doi: 10.1152/ajpheart.00669.2004. [DOI] [PubMed] [Google Scholar]
- 109.Mitsuma W, Kodama M, Hanawa H, Ito M, Ramadan MM, Hirono S, et al. Serum endostatin in the coronary circulation of patients with coronary heart disease and its relation to coronary collateral formation. Am J Cardiol. 2007;99:494–8. doi: 10.1016/j.amjcard.2006.09.095. [DOI] [PubMed] [Google Scholar]
- 110.Fujita M, Nakae I, Kihara Y, Hasegawa K, Nohara R, Ueda K, et al. Determinants of collateral development in patients with acute myocardial infarction. Clin Cardiol. 1999;22:595–9. doi: 10.1002/clc.4960220911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fujita M, McKown DP, McKown MD, Franklin D. Effects of glyceryl trinitrate on functionally regressed newly developed collateral vessels in conscious dogs. Cardiovasc Res. 1988;22:639–47. doi: 10.1093/cvr/22.9.639. [DOI] [PubMed] [Google Scholar]
- 112.Teng CJ, Lachapelle K, Chiu RC. Reappraisal of recent clinical trials of angiogenic therapy in myocardial ischemia. Asian cardiovascular & thoracic annals. 2005;13:90–7. doi: 10.1177/021849230501300124. [DOI] [PubMed] [Google Scholar]
- 113.Herbert JM, Bono F, Savi P. The mitogenic effect of H2O2 for vascular smooth muscle cells is mediated by an increase of the affinity of basic fibroblast growth factor for its receptor. FEBS Lett. 1996;395:43–7. doi: 10.1016/0014-5793(96)00998-2. [DOI] [PubMed] [Google Scholar]
- 114.Denenberg DL. The effects of exercise on the coronary collateral circulation. The Journal of sports medicine and physical fitness. 1972;12:76–81. [PubMed] [Google Scholar]
- 115.Cohen MV, Yipintsoi T, Malhotra A, Penpargkul S, Scheuer J. Effect of exercise on collateral development in dogs with normal coronary arteries. J Appl Physiol. 1978;45:797–805. doi: 10.1152/jappl.1978.45.5.797. [DOI] [PubMed] [Google Scholar]
- 116.Neill WA, Oxendine JM. Exercise can promote coronary collateral development without improving perfusion of ischemic myocardium. Circulation. 1979;60:1513–9. doi: 10.1161/01.cir.60.7.1513. [DOI] [PubMed] [Google Scholar]
- 117.Scheel KW, Ingram LA, Wilson JL. Effects of exercise on the coronary and collateral vasculature of beagles with and without coronary occlusion. Circ Res. 1981;48:523–30. doi: 10.1161/01.res.48.4.523. [DOI] [PubMed] [Google Scholar]
- 118.Roth DM, White FC, Nichols ML, Dobbs SL, Longhurst JC, Bloor CM. Effect of long-term exercise on regional myocardial function and coronary collateral development after gradual coronary artery occlusion in pigs. Circulation. 1990;82:1778–89. doi: 10.1161/01.cir.82.5.1778. [DOI] [PubMed] [Google Scholar]
- 119.Vogel R, Traupe T, Steiger VS, Seiler C. Physical coronary arteriogenesis: a human “model” of collateral growth promotion. Trends in cardiovascular medicine. 2010;20:129–33. doi: 10.1016/j.tcm.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 120.Pagonas N, Utz W, Schulz-Menger J, Busjahn A, Monti J, Thierfelder L, et al. Assessment of the effect of external counterpulsation on myocardial adaptive arteriogenesis by invasive functional measurements--design of the arteriogenesis network trial 2. Int J Cardiol. 2010;145:432–7. doi: 10.1016/j.ijcard.2009.05.050. [DOI] [PubMed] [Google Scholar]
- 121.Fujita M, Sasayama S. Coronary collateral growth and its therapeutic application to coronary artery disease. Circulation journal: official journal of the Japanese Circulation Society. 2010;74:1283–9. doi: 10.1253/circj.cj-10-0376. [DOI] [PubMed] [Google Scholar]
- 122.Yin L, Ohanyan V, Fen Pung Y, Delucia A, Bailey E, Enrick M, et al. Induction of Vascular Progenitor Cells From Endothelial Cells Stimulates Coronary Collateral Growth. Circ Res. 2011 doi: 10.1161/CIRCRESAHA.111.250126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Belmadani S, Matrougui K, Kolz C, Pung YF, Palen D, Prockop DJ, et al. Amplification of coronary arteriogenic capacity of multipotent stromal cells by epidermal growth factor. Arterioscler Thromb Vasc Biol. 2009;29:802–8. doi: 10.1161/ATVBAHA.109.186189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Carrao AC, Chilian WM, Yun J, Kolz C, Rocic P, Lehmann K, et al. Stimulation of coronary collateral growth by granulocyte stimulating factor: role of reactive oxygen species. Arterioscler Thromb Vasc Biol. 2009;29:1817–22. doi: 10.1161/ATVBAHA.109.186445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Penn MS. SDF-1:CXCR4 axis is fundamental for tissue preservation and repair. Am J Pathol. 2010;177:2166–8. doi: 10.2353/ajpath.2010.100803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Penn MS. Importance of the SDF-1:CXCR4 axis in myocardial repair. Circ Res. 2009;104:1133–5. doi: 10.1161/CIRCRESAHA.109.198929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ren B, Deng Y, Mukhopadhyay A, Lanahan AA, Zhuang ZW, Moodie KL, et al. ERK1/2-Akt1 crosstalk regulates arteriogenesis in mice and zebrafish. J Clin Invest. 2010;120:1217–28. doi: 10.1172/JCI39837. [DOI] [PMC free article] [PubMed] [Google Scholar]