Abstract
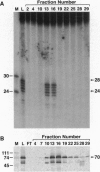
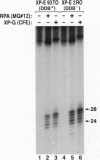
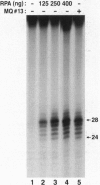
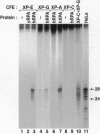
Xeroderma pigmentosum (XP) is caused by a defect in nucleotide excision repair. Patients in the complementation group E (XP-E) have the mildest form of the disease and the highest level of residual repair activity. About 20% of the cell strains derived from XP-E patients lack a damaged DNA-binding protein (DDB) activity that binds to ultraviolet-induced (6-4) photoproducts with high affinity. We report here that cell-free extracts prepared from XP-E cell strains that either lacked or contained DDB activity were severely defective in excising DNA damage including (6-4) photoproducts. However, this excision activity defect was not restored by addition of purified DDB that, in fact, inhibited removal of (6-4) photoproducts by the human excision nuclease reconstituted from purified proteins. Extensive purification of correcting activity from HeLa cells revealed that the correcting activity is inseparable from the human replication/repair protein A [RPA (also known as human single stranded DNA binding protein, HSSB)]. Indeed, supplementing XP-E extracts with recombinant human RPA purified from Escherichia coli restored excision activity. However, no mutation was found in the genes encoding the three subunits of RPA in an XP-E (DDB-) cell line. It is concluded that RPA functionally complements XP-E extracts in vitro, but it is not genetically altered in XP-E patients.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chu G., Chang E. Xeroderma pigmentosum group E cells lack a nuclear factor that binds to damaged DNA. Science. 1988 Oct 28;242(4878):564–567. doi: 10.1126/science.3175673. [DOI] [PubMed] [Google Scholar]
- Coverley D., Kenny M. K., Lane D. P., Wood R. D. A role for the human single-stranded DNA binding protein HSSB/RPA in an early stage of nucleotide excision repair. Nucleic Acids Res. 1992 Aug 11;20(15):3873–3880. doi: 10.1093/nar/20.15.3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coverley D., Kenny M. K., Munn M., Rupp W. D., Lane D. P., Wood R. D. Requirement for the replication protein SSB in human DNA excision repair. Nature. 1991 Feb 7;349(6309):538–541. doi: 10.1038/349538a0. [DOI] [PubMed] [Google Scholar]
- Dualan R., Brody T., Keeney S., Nichols A. F., Admon A., Linn S. Chromosomal localization and cDNA cloning of the genes (DDB1 and DDB2) for the p127 and p48 subunits of a human damage-specific DNA binding protein. Genomics. 1995 Sep 1;29(1):62–69. doi: 10.1006/geno.1995.1215. [DOI] [PubMed] [Google Scholar]
- Erdile L. F., Heyer W. D., Kolodner R., Kelly T. J. Characterization of a cDNA encoding the 70-kDa single-stranded DNA-binding subunit of human replication protein A and the role of the protein in DNA replication. J Biol Chem. 1991 Jun 25;266(18):12090–12098. [PubMed] [Google Scholar]
- Erdile L. F., Wold M. S., Kelly T. J. The primary structure of the 32-kDa subunit of human replication protein A. J Biol Chem. 1990 Feb 25;265(6):3177–3182. [PubMed] [Google Scholar]
- Fairman M. P., Stillman B. Cellular factors required for multiple stages of SV40 DNA replication in vitro. EMBO J. 1988 Apr;7(4):1211–1218. doi: 10.1002/j.1460-2075.1988.tb02933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzder S. N., Habraken Y., Sung P., Prakash L., Prakash S. Reconstitution of yeast nucleotide excision repair with purified Rad proteins, replication protein A, and transcription factor TFIIH. J Biol Chem. 1995 Jun 2;270(22):12973–12976. doi: 10.1074/jbc.270.22.12973. [DOI] [PubMed] [Google Scholar]
- He Z., Henricksen L. A., Wold M. S., Ingles C. J. RPA involvement in the damage-recognition and incision steps of nucleotide excision repair. Nature. 1995 Apr 6;374(6522):566–569. doi: 10.1038/374566a0. [DOI] [PubMed] [Google Scholar]
- Henricksen L. A., Umbricht C. B., Wold M. S. Recombinant replication protein A: expression, complex formation, and functional characterization. J Biol Chem. 1994 Apr 15;269(15):11121–11132. [PubMed] [Google Scholar]
- Huang J. C., Sancar A. Determination of minimum substrate size for human excinuclease. J Biol Chem. 1994 Jul 22;269(29):19034–19040. [PubMed] [Google Scholar]
- Huang J. C., Svoboda D. L., Reardon J. T., Sancar A. Human nucleotide excision nuclease removes thymine dimers from DNA by incising the 22nd phosphodiester bond 5' and the 6th phosphodiester bond 3' to the photodimer. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3664–3668. doi: 10.1073/pnas.89.8.3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka H., Fujiwara Y. UV damage-specific DNA-binding protein in xeroderma pigmentosum complementation group E. Biochem Biophys Res Commun. 1991 Mar 29;175(3):1139–1143. doi: 10.1016/0006-291x(91)91684-5. [DOI] [PubMed] [Google Scholar]
- Keeney S., Chang G. J., Linn S. Characterization of a human DNA damage binding protein implicated in xeroderma pigmentosum E. J Biol Chem. 1993 Oct 5;268(28):21293–21300. [PubMed] [Google Scholar]
- Keeney S., Eker A. P., Brody T., Vermeulen W., Bootsma D., Hoeijmakers J. H., Linn S. Correction of the DNA repair defect in xeroderma pigmentosum group E by injection of a DNA damage-binding protein. Proc Natl Acad Sci U S A. 1994 Apr 26;91(9):4053–4056. doi: 10.1073/pnas.91.9.4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeney S., Wein H., Linn S. Biochemical heterogeneity in xeroderma pigmentosum complementation group E. Mutat Res. 1992 Jan;273(1):49–56. doi: 10.1016/0921-8777(92)90049-9. [DOI] [PubMed] [Google Scholar]
- Kenny M. K., Lee S. H., Hurwitz J. Multiple functions of human single-stranded-DNA binding protein in simian virus 40 DNA replication: single-strand stabilization and stimulation of DNA polymerases alpha and delta. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9757–9761. doi: 10.1073/pnas.86.24.9757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T. H., Elledge S. J., Butel J. S. Hepatitis B virus X protein interacts with a probable cellular DNA repair protein. J Virol. 1995 Feb;69(2):1107–1114. doi: 10.1128/jvi.69.2.1107-1114.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Lu X., Peterson C. A., Legerski R. J. An interaction between the DNA repair factor XPA and replication protein A appears essential for nucleotide excision repair. Mol Cell Biol. 1995 Oct;15(10):5396–5402. doi: 10.1128/mcb.15.10.5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manley J. L., Fire A., Cano A., Sharp P. A., Gefter M. L. DNA-dependent transcription of adenovirus genes in a soluble whole-cell extract. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3855–3859. doi: 10.1073/pnas.77.7.3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda T., Saijo M., Kuraoka I., Kobayashi T., Nakatsu Y., Nagai A., Enjoji T., Masutani C., Sugasawa K., Hanaoka F. DNA repair protein XPA binds replication protein A (RPA). J Biol Chem. 1995 Feb 24;270(8):4152–4157. doi: 10.1074/jbc.270.8.4152. [DOI] [PubMed] [Google Scholar]
- Matsunaga T., Mu D., Park C. H., Reardon J. T., Sancar A. Human DNA repair excision nuclease. Analysis of the roles of the subunits involved in dual incisions by using anti-XPG and anti-ERCC1 antibodies. J Biol Chem. 1995 Sep 1;270(35):20862–20869. doi: 10.1074/jbc.270.35.20862. [DOI] [PubMed] [Google Scholar]
- Mu D., Hsu D. S., Sancar A. Reaction mechanism of human DNA repair excision nuclease. J Biol Chem. 1996 Apr 5;271(14):8285–8294. doi: 10.1074/jbc.271.14.8285. [DOI] [PubMed] [Google Scholar]
- Mu D., Park C. H., Matsunaga T., Hsu D. S., Reardon J. T., Sancar A. Reconstitution of human DNA repair excision nuclease in a highly defined system. J Biol Chem. 1995 Feb 10;270(6):2415–2418. doi: 10.1074/jbc.270.6.2415. [DOI] [PubMed] [Google Scholar]
- Prakash S., Sung P., Prakash L. DNA repair genes and proteins of Saccharomyces cerevisiae. Annu Rev Genet. 1993;27:33–70. doi: 10.1146/annurev.ge.27.120193.000341. [DOI] [PubMed] [Google Scholar]
- Reardon J. T., Nichols A. F., Keeney S., Smith C. A., Taylor J. S., Linn S., Sancar A. Comparative analysis of binding of human damaged DNA-binding protein (XPE) and Escherichia coli damage recognition protein (UvrA) to the major ultraviolet photoproducts: T[c,s]T, T[t,s]T, T[6-4]T, and T[Dewar]T. J Biol Chem. 1993 Oct 5;268(28):21301–21308. [PubMed] [Google Scholar]
- Reardon J. T., Thompson L. H., Sancar A. Excision repair in man and the molecular basis of xeroderma pigmentosum syndrome. Cold Spring Harb Symp Quant Biol. 1993;58:605–617. doi: 10.1101/sqb.1993.058.01.067. [DOI] [PubMed] [Google Scholar]
- Sancar A. Excision repair in mammalian cells. J Biol Chem. 1995 Jul 7;270(27):15915–15918. doi: 10.1074/jbc.270.27.15915. [DOI] [PubMed] [Google Scholar]
- Sancar A. Mechanisms of DNA excision repair. Science. 1994 Dec 23;266(5193):1954–1956. doi: 10.1126/science.7801120. [DOI] [PubMed] [Google Scholar]
- Sibghatullah, Husain I., Carlton W., Sancar A. Human nucleotide excision repair in vitro: repair of pyrimidine dimers, psoralen and cisplatin adducts by HeLa cell-free extract. Nucleic Acids Res. 1989 Jun 26;17(12):4471–4484. doi: 10.1093/nar/17.12.4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. A., Taylor J. S. Preparation and characterization of a set of deoxyoligonucleotide 49-mers containing site-specific cis-syn, trans-syn-I, (6-4), and Dewar photoproducts of thymidylyl(3'-->5')-thymidine. J Biol Chem. 1993 May 25;268(15):11143–11151. [PubMed] [Google Scholar]
- Svoboda D. L., Taylor J. S., Hearst J. E., Sancar A. DNA repair by eukaryotic nucleotide excision nuclease. Removal of thymine dimer and psoralen monoadduct by HeLa cell-free extract and of thymine dimer by Xenopus laevis oocytes. J Biol Chem. 1993 Jan 25;268(3):1931–1936. [PubMed] [Google Scholar]
- Takao M., Abramic M., Moos M., Jr, Otrin V. R., Wootton J. C., McLenigan M., Levine A. S., Protic M. A 127 kDa component of a UV-damaged DNA-binding complex, which is defective in some xeroderma pigmentosum group E patients, is homologous to a slime mold protein. Nucleic Acids Res. 1993 Aug 25;21(17):4111–4118. doi: 10.1093/nar/21.17.4111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K., Wood R. D. Xeroderma pigmentosum and nucleotide excision repair of DNA. Trends Biochem Sci. 1994 Feb;19(2):83–86. doi: 10.1016/0968-0004(94)90040-X. [DOI] [PubMed] [Google Scholar]
- Thompson L. H., Busch D. B., Brookman K., Mooney C. L., Glaser D. A. Genetic diversity of UV-sensitive DNA repair mutants of Chinese hamster ovary cells. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3734–3737. doi: 10.1073/pnas.78.6.3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umbricht C. B., Erdile L. F., Jabs E. W., Kelly T. J. Cloning, overexpression, and genomic mapping of the 14-kDa subunit of human replication protein A. J Biol Chem. 1993 Mar 25;268(9):6131–6138. [PubMed] [Google Scholar]
- Wold M. S., Kelly T. Purification and characterization of replication protein A, a cellular protein required for in vitro replication of simian virus 40 DNA. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2523–2527. doi: 10.1073/pnas.85.8.2523. [DOI] [PMC free article] [PubMed] [Google Scholar]