Abstract
Hairy roots have the potential to produce a variety of valuable small and large molecules. The mist reactor is a gas phase bioreactor that has shown promise for low-cost culture of hairy roots. Using a newer, disposable culture bag, mist reactor performance was studied with two species, Artemisia annua L. and Arachis hypogaea (peanut), at scales from 1 to 20 L. Both species of hairy roots when grown at 1 L in the mist reactor showed growth rates that surpassed that in shake flasks. From the information gleaned at 1 L, Arachis was scaled further to 4 and then 20 L. Misting duty cycle, culture medium flow rate, and timing of when flow rate was increased were varied. In a mist reactor increasing the misting cycle or increasing the medium flow rate are the two alternatives for increased delivery of liquid nutrients to the root bed. Longer misting cycles beyond 2–3 min were generally deemed detrimental to growth. On the other hand, increasing the medium flow rate to the sonic nozzle especially during the exponential phase of root growth (weeks 2–3) was the most important factor for increasing growth rates and biomass yields in the 20 L reactors. A. hypogaea growth in 1 L reactors was μ = 0.173 day−1 with biomass yield of 12.75 g DWL−1. This exceeded that in shake flasks at μ = 0.166 day−1 and 11.10 g DWL−1. Best growth rate and biomass yield at 20 L was μ = 0.147 and 7.77 g DWL−1, which was mainly achieved when medium flow rate delivery was increased. The mist deposition model was further evaluated using this newer reactor design and when the apparent thickness of roots (+hairs) was taken into account, the empirical data correlated with model predictions. Together these results establish the most important conditions to explore for future optimization of the mist bioreactor for culture of hairy roots.
Keywords: peanut, A. hypogaea, Artemisia annua, mist reactor, scale-up, hairy roots
Introduction
Transformed (hairy) roots can produce high amounts of often unique compounds not found in the whole plant or in untransformed roots (Flores and Medina-Bolivar, 1995; Guillon et al., 2006; Wysokinska and Chmiel, 1997). They also offer advantages for production of engineered proteins (Guillon et al., 2006; Liu et al., 2009; Shanks and Morgan, 1999; Zhang et al., 2005). A real bottleneck, however, exists in the production of useful compounds from hairy roots, especially small molecules, for two main reasons: (1) many of the relevant biochemical pathways and their regulation are inadequately understood; and (2) cost-effective scalable production systems are not yet well developed. Development of low-cost scalable reactors for hairy roots would address this latter problem and could enable large-scale culture and production of many important and valuable chemicals (Guillon et al., 2006). Furthermore, concerns about release of transgenic plants warrants use of controlled production technologies including low-cost bioreactors (Guillon et al., 2006).
We have focused on gas-phase (mist) reactors, because they significantly improve gas-exchange while limiting shear conditions usually found in liquid-phase reactors. Even at high-bed densities (Kim, 2001) roots grown in mist reactors are not oxygen limited (Weathers et al., 1999), and small molecule production is often greater in mist reactors than in liquid-phase reactors (Bais et al., 2002; Kim et al., 2001). For an economically viable reactor system, root biomass density should be high, which depends on the delivery of oxygen and other nutrients into the dense root matrix (Curtis, 2000). Indeed roots are more able to compensate for poor liquid dispersion than for poor gas dispersion in a reactor (Curtis, 1993; McKelvey et al., 1993). When Suresh et al. (2005) compared a variety of reactors, the mist bioreactor was deemed superior to other reactor designs for hairy root growth.
In contrast to liquid-phase reactors, we have shown that in the mist reactor high-density beds of roots grow faster than low-density beds, because high-density beds capture media droplets more efficiently (Towler et al., 2007). This poses a challenge to reactor scale-up because the inoculum levels required to create a dense bed in a reactor quickly become impractical as the reactor size increases. To address this challenge we incorporated a flexible wall growth chamber (a constricted plastic bag) into the mist reactor in order to increase the apparent density of the inoculum root bed early in the growth of the culture to facilitate nutrient droplet capture (Liu et al., 2009). As the roots grow, they can expand the bag and thus maintain a reasonable bed density. The capital costs of this system are anticipated to be at least an order of magnitude less than conventional stirred tank reactors and even less than the latest plant tissue bioreactor designs being tested by others (Ramakrishnan and Curtis, 2004). The culture bag is disposable, a desirable feature being developed for plant-produced therapeutics by others (Weathers et al., 2010), and builds upon the reactor bag liner described by Hsiao et al. (1999). Furthermore, by using a commercial mister (Sono-Tek, NY) droplets can be delivered in the desired size range at much higher medium flow rates (>2 orders of magnitude) than prior mist reactor designs, thus making the system scalable. Although the misting head cannot be autoclaved, it can be sterilized using ethylene oxide. We have also developed several methods for sterilization that can be used to prevent contamination in the reactor (Liu et al., 2009; Sharaf-Eldin and Weathers, 2006). The plastic bag growth chamber and the durable high-throughput sonic mister now make the mist reactor quite scalable, easy to use, and when scaled to 20 L, low in capital costs.
Here, we report on studies of two hairy root cultures, Artemisia annua and Arachis hypogaea and include an analysis of the scale-up of A. hypogaea to 20 L. We chose these two cultures because their morphological diversity represents the extremes: A. hypogaea has thick, effectively hairless roots with little branching, while A. annua has thin, highly branched roots with substantial root hairs. In light of this study, the data are further analyzed with respect to our mist deposition model (Wyslouzil et al., 1997).
Materials and Methods
Clone Origins and Culture Conditions
Artemisia annua L. clone YUT16 (Weathers et al., 1994) was maintained and subcultured every 14 days into 250 mL Erlenmeyer flasks containing 50 mL Gamborg’s B5 medium (Gamborg et al., 1968) with 3% (w/v) sucrose, pH 5.8, on a rotary shaker at 100 rpm under continuous cool white fluorescent room lights at <2 µmol m−2 s−1, and 23–25°C. All reactor experiments and concurrent shake flasks culture controls were run using these conditions with two changes: sucrose was increased to 5% (w/v), and 0.1% (v/v) PPM™ (filter-sterilized Plant Preservative Mixture™ biocide; Plant Cell Technology, Washington, DC) was added. Inoculum was 8 g fresh weight (FW) L−1. Arachis hypogaea Hull III line hairy roots were established using the method of Condori et al. (2010) and maintained and subcultured every 14–15 days into 250 mL Erlenmeyer flasks containing 50 mL modified MS medium (Murashige and Skoog, 1962) with 3% (w/v) sucrose, pH 6.0, on a rotary shaker at 100 rpm in the dark at 23–25°C. Inoculum was 6 g FWL−1. Only healthy root tissue was used as inoculum for experiments.
Mist Reactor Conditions
Design and fabrication of the mist reactor (Fig. 1) has already been described in detail by Liu et al. (2009). Sterile-filtered, humidified air was supplied by an aquarium pump that ran continuously at 0.1 vvm. The vvm was based on the total volume of the growth chamber, which was 1, 4, or 20 L in this study. The peristaltic pump that fed the misting nozzle, the misting nozzle, and associated controllers were all on a timer to regulate the misting duty cycle. All experiments were conducted in a temperature-controlled room (23–25° C).
Figure 1.
The mist reactor used in these studies. Left, a group of 4 L reactors with A. annua; middle, 20 L reactor with A. hypogaea roots; right, A. hypogaea roots harvested from 20 L reactor (ruler = 30 cm).
For growth kinetics studies of A. annua hairy roots, we harvested root biomass and measured medium pH and conductivity after 6, 14, or 21 days in shake flasks and 1 L mist reactors. The rate of medium delivery was 25 mL min−1. For A. annua the misting cycle variations are shown in Table I. Growth kinetics of A. annua in shake flasks with media analyses were previously published (Kim et al., 2003; Towler et al., 2007) and were not repeated here. A fed batch reactor was also run wherein we replaced the nutrients that were depleted from the medium after 6 days. For A. hypogaea hairy roots kinetic studies, 1 L mist reactors were run with misting 2 min on/28 min off for the entire growth period. The rate of medium delivery was 25 mL min−1 during misting, for a total feed of 100 mL h−1. Root biomass was harvested and medium pH and conductivity were measured at 4 day intervals from shake flasks and 1 L mist reactors starting at inoculation through 28 days. Specific growth rate was calculated, and nutrient analyses of the medium were performed as later described.
Table I.
Growth of A. annua after 1 L mist reactor runs of different durations and misting cycles.
| Misting cycle (min-on/min-off) | Harvest time (days) |
Fresh weight (g L−1) |
Dry weight (g L−1) |
µ (day−1) |
|---|---|---|---|---|
| 0:30/29:30 (0–6 days) | 6 | 14.67 ± 0.17 | 1.40 ± 0.01 | 0.171 ± 0.002 |
| 0:30/29:30 (0–14 days) | 14 | 30.79 ± 2.67 | 3.00 ± 0.20 | 0.128 ± 0.005 |
| 0:30/29:30 (0–6 days) then 2:00/28:00 (7–14 days) | 14 | 34.80 ± 0.29 | 3.34 ± 0.07 | 0.136 ± 0.002 |
| 0:30/29:30 (0–6 days) then 1:00/29:00 (7–21 days) | 21 | 67.42 ± 3.68 | 5.67 ± 0.16 | 0.116 ± 0.002 |
| 0:30/29:30 (0–6 days) then 1:00/29:00 (7–14 days) then 2:00/28:00 (15–21 days) | 21 | 58.36 ± 1.54 | 5.23 ± 0.11 | 0.112 ± 0.001 |
| Fed batch | ||||
| 0:30/29:30 (0–6 days) then 2:00/28:00 (7–14 days) | 14 | 42.72 ± 0.75 | 3.93 ± 0.06 | 0.147 ± 0.001 |
Larger-Scale Mist Reactor Experiments
Using A. hypogaea hairy roots, mist reactors were also run at both 4 and 20 L scale (see Fig. 1), and constructed and inoculated as follows. The same reactor was used for 4 L runs as was used for the 1 L run because the upper working volume of that reactor was 4 L. Inoculum was increased proportionately to 24 g FW. The 4 L reactor bag had a cylindrical volume of 1 L for each 5 cm of vertical bed depth (approx. 16 cm diameter), while the 20 L bag had a volume of 1 L for each 1 cm bed depth (approx. 35 cm diameter), which was within the maximum spray diameter operating limitations of this mist delivery system (unpublished data). For scale-up studies in the 4 L mist reactor, we tested three different misting cycles in which the liquid flow rate, misting time, or both were varied over 3 weeks. Table II summarizes the variations in misting cycle and liquid medium flow rates for both the 4 and 20 L reactor runs.
Table II.
A. hypogaea growth in shake flasks and at 4 and 20 L in the mist reactor* after 20 days.
| Week #, mist cycle min mist on:min mist off |
Media flow rate (mL min−1) |
Hourly feed vol (mL) |
vvma | Inoculum dry mass (g) |
Final dry mass (g) |
Final conductivity (mS cm−1) |
Final pH | Final µ (day−1) |
|---|---|---|---|---|---|---|---|---|
| Shake flasks | — | — | — | 0.02 | 0.556 | 2.18 | 5.24 | 0.166 |
| 1 L working volume | ||||||||
| 2:28 all 3 weeks | 25 | 100 | 0.1 | 0.40 | 12.75 | 1.75 | 4.83 | 0.173 |
| 4 L working volume | ||||||||
| A | ||||||||
| Week 1, 2:28 | 25 all 3 weeksb,c | 100 | 0.1 | 1.6 | 25.2 | 2.38 | 4.39 | 0.127 |
| Week 2, 4:26 | 200 | |||||||
| Week 3, 6:24 | 300 | |||||||
| B | ||||||||
| 2:28 all 3 weeks | Week 1, 25 | 100 | 0.1 | 1.6 | 41.0 | 1.61 | 4.95 | 0.154 |
| Week 2, 50 | 200 | |||||||
| Week 3, 75 | 300 | |||||||
| C | ||||||||
| Week 1, 2:28 | Week 1, 25 | 100 | 0.1 | 1.6 | 37.8 | 1.81 | 4.55 | 0.150 |
| Week 2, 3:27 | Week 2, 50 | 300 | ||||||
| Week 3, 4:26 | Week 3, 75 | 600 | ||||||
| 20 L working volume | ||||||||
| D | ||||||||
| Week 1, 2:28 | Week 1, 25 | 100 | 0.1 | 8.17 | 81.2 | 3.00 | 6.01 | 0.114 |
| Week 2, 4:26 | Week 2, 35 | 280 | ||||||
| Week 3, 8:22 | Week 3, 40 | 640 | ||||||
| E | ||||||||
| Week 1, 2:28 | Week 1, 60 | 240 | 0.1 | 8.17 | 146.2 | 1.39 | 5.80 | 0.144 |
| Week 2, 3:27 | Week 2, 60 | 360 | ||||||
| Week 3, 4:26 | Week 3, 75 | 600 | ||||||
| F | ||||||||
| Week 1, 2:28 | Week 1, 60 | 240 | 0.1 | 8.17 | 148.1 | 1.29 | 5.82 | 0.145 |
| Week 2, 3:27 | Week 2, 60 | 360 | ||||||
| Week 3, 3:27 | Week 3, 100 | 600 | ||||||
| G | ||||||||
| Week 1, 3:27 | Week 1, 100 | 600 | 0.1 | 8.17 | 127.1 | 2.51 | 4.89 | 0.136 |
| Week 2, 4:16 | Week 2, 100 | 1,200 | ||||||
| Week 3, 4:16 | Week 3, 100 | 1,200 | ||||||
| H | ||||||||
| Week 1, 2:28 | Week 1, 60 | 240 | 0.1 | 8.17 | 155.36 | 1.02 | 5.83 | 0.147 |
| Week 2, 3:27 | Week 2, 100 | 600 | ||||||
| Week 3, 3:27 | Week 3, 100 | 600 |
Data are an average of three shake flask experiments and 2–3 reactor runs, except those at 20 L where only one reactor was run each time.
vvm is based on 1, 4, or 20 L total working volume of reactor.
Although in Run A the flow rate was constant, the increased misting time from 2 to 6 min was equivalent in flow rate to the total volume of medium fed to roots per hour in B. For runs D and E volumetric throughput was also nearly equivalent, but misting duty cycle was altered.
For runs A–E, the sonic mister input power was kept constant at 2.5 W; for runs F–H, the power input was raised to 3.0 and 6.0W for 60 and 100mL min−1 flow rates, respectively.
Biomass Measurement
The average specific growth rate (μ), referred to here as the growth rate, was calculated as:
| (1) |
DW is the root dry weight (g), the subscripts f and i are the final and initial states, respectively, and time (t) is in days.
Liquid Retention Ratio
Sections of 10 roots were excised, dipped in water, briefly allowed to drain, and weighed. Roots were then gently blotted and reweighed to determine the weight of the water coating. Liquid retention ratio was calculated as water weight divided by blotted fresh weight. A. hypogaea root segments taken from mist reactor runs at harvest were all 5 cm long from the tip and unbranched, but A. annua root length varied due to the higher branching frequency. That is, the distance from the tip of an A. annua root to a branching point was less than 5 cm. Straight unbranched sections were used in order to eliminate liquid retention from meniscus formation at branch junctions, and aerial portions from roots grown on semi-solid medium were chosen since they exhibit the greatest proliferation of root hairs.
Assays
Fresh mass was washed, blotted with absorbent paper and measured immediately after root harvest; dry mass was obtained after drying the roots at 60°C for at least 48 h. Conductivity and pH were measured in media from harvested cultures using a Seveneasy conductivity (Mettler-Toledo GmbH, Switzerland) and pH (Oakton Instruments, IL) meters. Nitrate, ammonia, phosphate, and residual sugars were assayed using the methods described by Dubois et al. (1956), Kim (2001), Kim et al. (2003), and Towler et al. (2007), respectively.
Statistical Analysis
Experiments and assays were run at least three times except for the 4 L bioreactors that were each run at least twice, and the fed-batch and 20 L reactors that were each run once per condition tested. Where there were at least two replicated reactor runs, data were averaged with experimental variation (or range) shown as standard error. Student’s t-test was used to show statistical significance.
Results and Discussion
Before large-scale analysis can be done on a reactor, it is important to compare small-scale data from both shake flask cultures and small volume reactors. In this study, we first compared growth in shake flasks to that in 1 L (working volume) mist reactors for both plant species. Using those results, we subsequently measured the growth of only A. hypogaea at 4 and 20 L working volumes. The latter is the maximum capacity of the current version of this newly designed mist reactor that could be accommodated in our lab. The data in this study were further analyzed and compared to data from our prior studies with respect to the mist deposition model previously reported (Wyslouzil et al., 1997), which treats dense beds of roots like fibrous filters.
Comparison of the Kinetics of Growth of A. annua in Shake Flasks With That in the 1 L Mist Reactor
In the studies by Towler et al. (2007), A. annua root growth in a mist reactor was found to depend on two main factors: the nutrient (sugar) concentration, CS (g L−1), in the medium, and the packing fraction, α, the volume fraction of the roots in the growth chamber, where α = [root FW (g L−1) × 0.001] since the mass density of roots is 1 g mL−1. In the mist reactor, dense beds of roots function like a fibrous filter and the mist deposition model reasonably describes the qualitative trends of droplet deposition onto the bed as long as the Reynolds number (Re) is <10 (Wyslouzil et al., 1997). The Reynolds number characterizes the relation between inertial and viscous forces during fluid flow, and for filtration problems Re is defined as
| (2) |
where ρ and μg are the density and viscosity of the carrier gas, DR is the diameter of the root, and Uo is the gas velocity in the root bed (volumetric flow rate per cross sectional area). It was previously demonstrated that predictions of the mist deposition model deviate from experimental measurements for Re>10 (Wyslouzil et al., 1997). In these experiments, the Reynolds number was always <2.0.
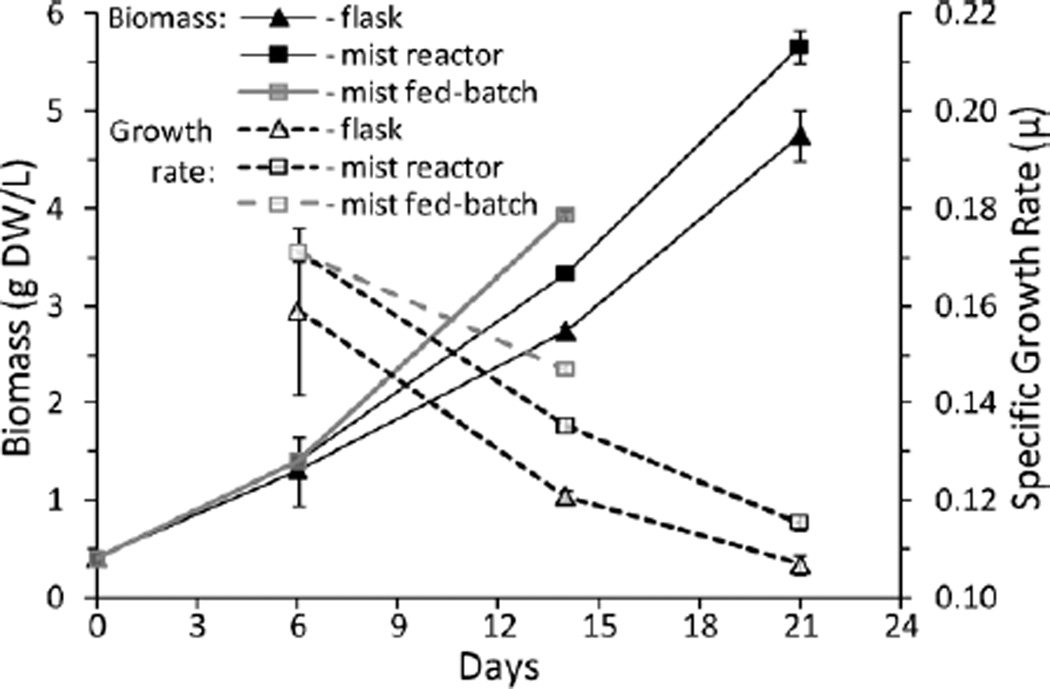
In earlier experiments (Kim et al., 2002, 2003) the growth rate, μ, of A. annua roots in shake flasks always exceeded that of roots grown in a mist reactor unless α ≥ 0.19 and CS = 50 g L−1. Under these extreme conditions growth was at least equal to that in shake flasks (Towler et al., 2007). In all of those studies, mist delivery to the roots was entirely dependent upon the flowing gas. In this study, where the gas and liquid flow rates are independent of each other, A. annua growth in the mist reactor was greater than in shake flasks using the same culture medium (Fig. 2). Similar to observations by Towler et al. (2007), the growth rate for days 0–6 in the mist reactor used in this study and in shake flasks was highest at 0.17 and 0.16 day−1, respectively (Fig. 2). Growth in the mist reactor, however, continued at a higher rate than in the shake flasks. Compared to data from the 1.5 L working volume mist reactor used by Kim et al. (2003), A. annua growth rates increased 1.66-fold in the new reactor from 0.07 (Kim et al., 2003) to 0.116 day−1 after 21 days (Table I). We subsequently hypothesized that replenishing the nutrients on day 7 with those that had been depleted from the culture medium by day 6, when μ was highest (Fig. 2), should further increase growth. Roots harvested from the fed-batch experiment yielded 23% more fresh mass and 18% more dry mass than the corresponding batch culture, but the increased growth rate (0.147 vs. 0.136 day−1) still did not achieve the original high-growth levels (0.171 day−1) measured during week 1 (Table I). During fed-batch mode, Kim et al. (2003) observed a slight increase in growth rate from 0.07 to 0.08 day−1, and this study also showed an increase (0.136–0.147 day−1; Table I). However, we replaced only the nutrients that were depleted from the medium after 6 days, while Kim et al. (2003) completely replaced the medium. Taken together these data demonstrate for the first time that at least at a 1 L working volume, early root growth in the mist reactor can be sustained at a higher rate than in shake flasks (Fig. 2; Table I). This suggests that the concept of the flexible wall growth chamber is functioning as anticipated and that this reactor can enhance the long-term growth rate of roots.
Figure 2.
Kinetics of A. annua hairy roots grown for 14–21 days in shake flasks and 1 L mist reactors.
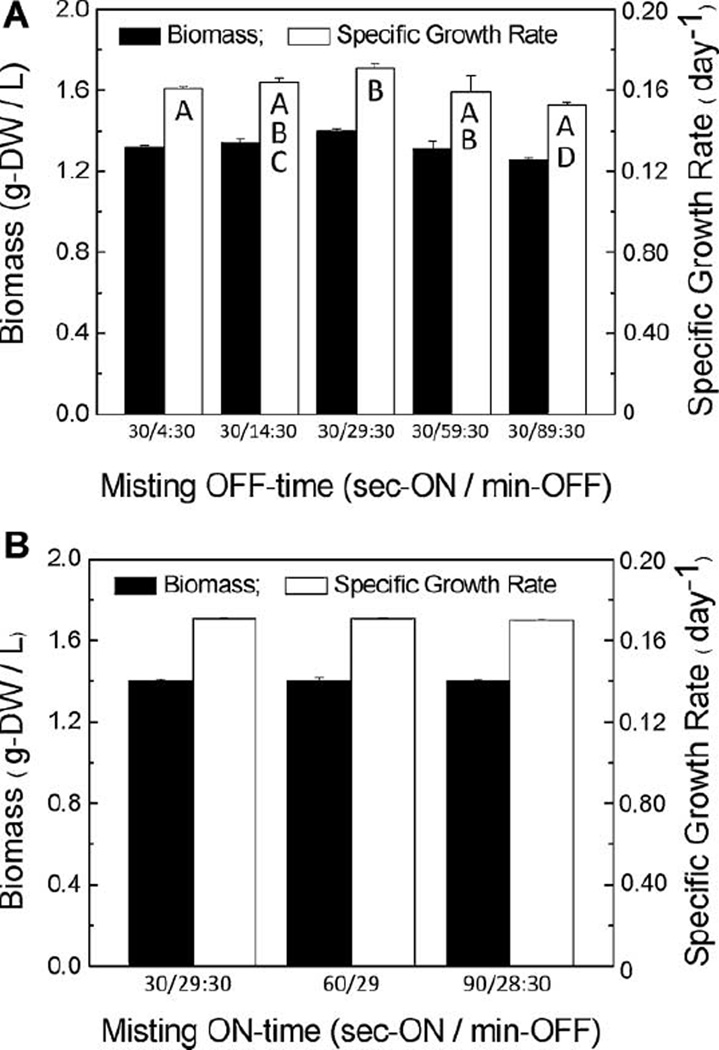
Although in previous studies the misting duty cycle (min on vs. min off) did play a role in root growth, this parameter primarily affected root quality without significantly increasing growth rate (DiIorio et al., 1992; Liu et al., 1999; Towler et al., 2007; Wyslouzil et al., 2000). Since one significant feature in the newly designed mist reactor is independent control of the gas and liquid flow rates, we reexamined the effect of the misting duty cycle on growth of A. annua roots, and observed that the mist off time still appeared to be more important than the mist on time. The highest growth rate during the first 6 days of culture was with a duty cycle of 30 s on during a 30 min cycle (Fig. 3A). Of importance to our scaling study was that increasing the misting time from 30 to 90 s in the first week had no effect on growth (Fig. 3B). At larger working volumes with more inoculum, liquid nutrient delivery must correspondingly increase, meaning either higher volumetric feed, increased misting times, or both. Indeed, even at the 1 L scale when the misting cycle was increased from 30 s on every 30 min for 2 weeks of culture to 2 min on every 30 min during the 2nd week of culture, FW increased 13%, DW 11%, and overall growth rate μ by 6%. However, as substantiated by earlier reports (DiIorio et al., 1992; Liu et al., 1999; Towler et al., 2007; Wyslouzil et al., 2000), it appeared that even in this version of the mist reactor, feeding too much media or too frequently in the early stages of growth was not beneficial. Together these data demonstrate that to ensure adequate delivery of nutrients to the roots, the mist duty cycle must be increased to accommodate increased growth, but a premature increase may be ineffective or detrimental. The importance of these parameters became more apparent when scaling the reactors from 1 L to 4 and 20 L.
Figure 3.
Effect of duty cycle on A. annua hairy roots grown for 6 days in 1 L mist reactors. A: Varied misting off time; B: varied misting on time. In (A), letters indicate statistical significance at P ≤ 0.05.
Comparison of the Kinetics of Growth of A. hypogaea in Shake Flasks With That in the 1 L Mist Reactor
All of our earlier studies with the mist reactor used the YUT16 clone of A. annua. For this reactor to be useful, it must facilitate the culture of other hairy root species. We chose A. hypogaea for two reasons: it is morphologically very different from A. annua, and when elicited, it produces high amounts of resveratrol and other stilbenes and, thus, may be of eventual commercial interest (Condori et al., 2010).
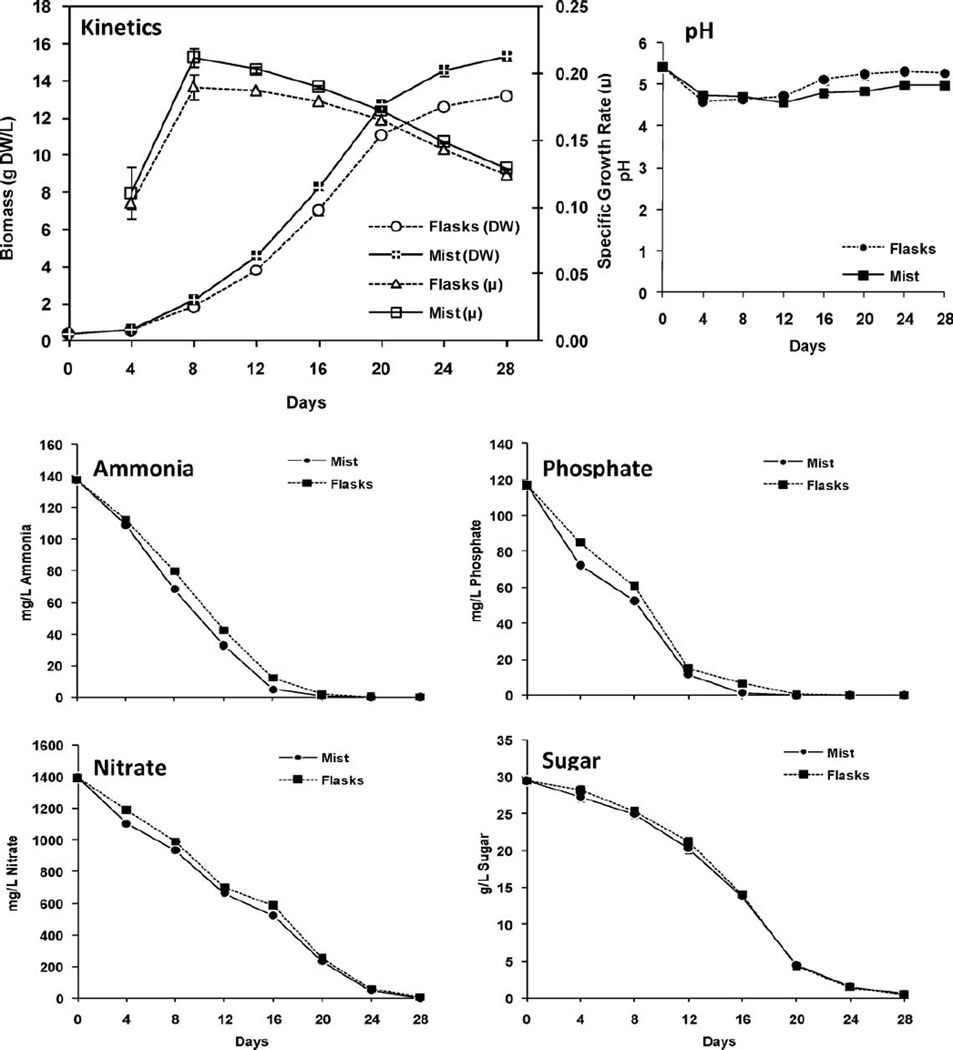
When compared to growth in shake flasks, A. hypogaea hairy root cultures also grew faster in the 1 L mist reactors with growth rates of 0.212 and 0.190 day−1, respectively, after 8 days of culture (Fig. 4). Similar to A. annua, A. hypogaea also showed its highest growth rates early in the culture period in both flasks and the mist reactor, but with high growth rates (μ ≈ 0.2 day−1) extending much longer than Artemisia and through the 16th day (Fig. 4). Changes in medium pH for A. hypogaea cultures were somewhat different between shake flasks and mist reactors. In shake flasks, the pH initially dropped from 5.4 to 4.5, and then gradually increased to end at 5.3 at 28 days. In the mist reactors, however, pH initially dropped from 5.4 to 4.6 ending at 5.0 at 28 days (Fig. 4). The lower pH at the end of mist reactor runs may be the result of increased CO2 provided by the added air at 0.1 vvm, suggesting that the low levels of CO2 inherently present in the poorly ventilated shake flasks results in their higher end pH compared to the reactors.
Figure 4.
Kinetics of growth and changes in culture media of A. hypogaea hairy roots grown for 28 days in shake flasks and 1 L mist reactors. Mist cycle was 2 min on/28 min off, with 0.1 vvm and 25 mL min−1 medium flow rate when mist was on.
Nutrients in the A. hypogaea culture media were depleted at different rates. Ammonia was exhausted by day 24 in mist reactors versus day 28 in shake flasks (Fig. 4). Phosphate was depleted earlier at day 20 in the reactors versus day 24 in flasks (Fig. 4). This suggested that phosphate and ammonia were most likely the nutrients limiting the growth of A. hypogaea. On the other hand, there was still some nitrate and sugar on day 20 in both flask and reactor cultures (Fig. 4).
The Role of Root Morphology and Packing Density on Growth in the Mist Reactor
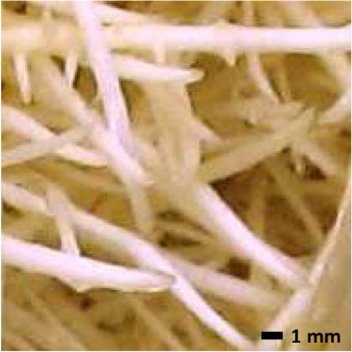
The higher growth rate of A. hypogaea was sustained longer than observed for A. annua, which decreased from 0.171 day−1 at 6 days to 0.136 day−1 at 14 days (Fig. 2). Indeed, Towler et al. (2007) showed that the maximum growth rate for A. annua was obtained at 4 days (μ = 0.20 day−1), dropping to 0.18 day−1 at 6 days even in densely packed small reactors that are more efficient at capturing nutrient droplets. The difference in sustained high growth rates in the mist reactor may be related to the large differences in root morphology between these two species (Table III). At the same packing density (α) thinner roots will capture more droplets than thicker ones and this may lead to a densely packed root bed accumulating media, and, thus, shifting to more of a liquid phase instead of a gas-phase reactor (Wyslouzil et al., 1997).
Table III.
Comparative morphologies of A. annua and A. hypogaea Hull III hairy roots.
| A. annua | |||
|---|---|---|---|
| Root feature | −root hairs | +root hairs | A. hypogaea |
| Primary root diameter (µm) | 500 | 1,000–3,500 | 1,200 |
| Lateral root diameter (µm)a | 300 | 750 | |
| Root hairs | Profuse in elongation zone | None | |
| Liquid retention ratiob | 0.570 | 0.333 | |
 |
 |
||
Does not include any root hair extension.
Weight of water coating divided by blotted fresh weight of root.
The volume of medium captured by the roots (Vdep) depends on the duty cycle, medium flow rate, and mass deposition efficiency. The volume of medium required to support root growth (Vreq) in mL day−1 depends on the amount of biomass present, its growth rate, μ (day−1), the apparent biomass yield of the growth-limiting nutrient, and the concentration of the limiting nutrient in the medium. Vdep must equal or exceed Vreq to maintain a desired growth rate. See Wyslouzil et al. (1997) for a detailed explanation and equations that explain the model for mist droplet deposition in the mist reactor.
Kim et al. (2002) proposed first and Towler et al. (2007) subsequently showed that a lack of nutrients was limiting A. annua hairy root growth in the mist bioreactor especially for low values of α. Vdep is a strong nonlinear function of α, so increasing α rapidly increases Vdep thereby supporting a higher growth rate by allowing more nutrients to be captured by the roots. Higher values of Vdep can be achieved by increasing the duty cycle or the flow rate. As mentioned previously and further discussed below, altering the misting cycle is not a trivial matter and can also negatively affect growth. Vreq is impacted by another function, CS, the limiting nutrient concentration in the medium (here taken to be the carbon source, sucrose). Increasing CS decreases Vreq, assuming no adverse effects on growth. Indeed, Towler et al. (2007) verified that increasing the sucrose level in the medium from 30 to 50 g L−1 significantly increased root growth in the mist reactor at a constant α. In the case of A. hypogaea, however, studies in shake flasks showed that roots of this species did not tolerate sucrose concentrations of 50 g L−1 (data not shown), so 30 g L−1 was used; maximum growth in shake flasks was achieved with 3 g L−1 sucrose (unpublished data).
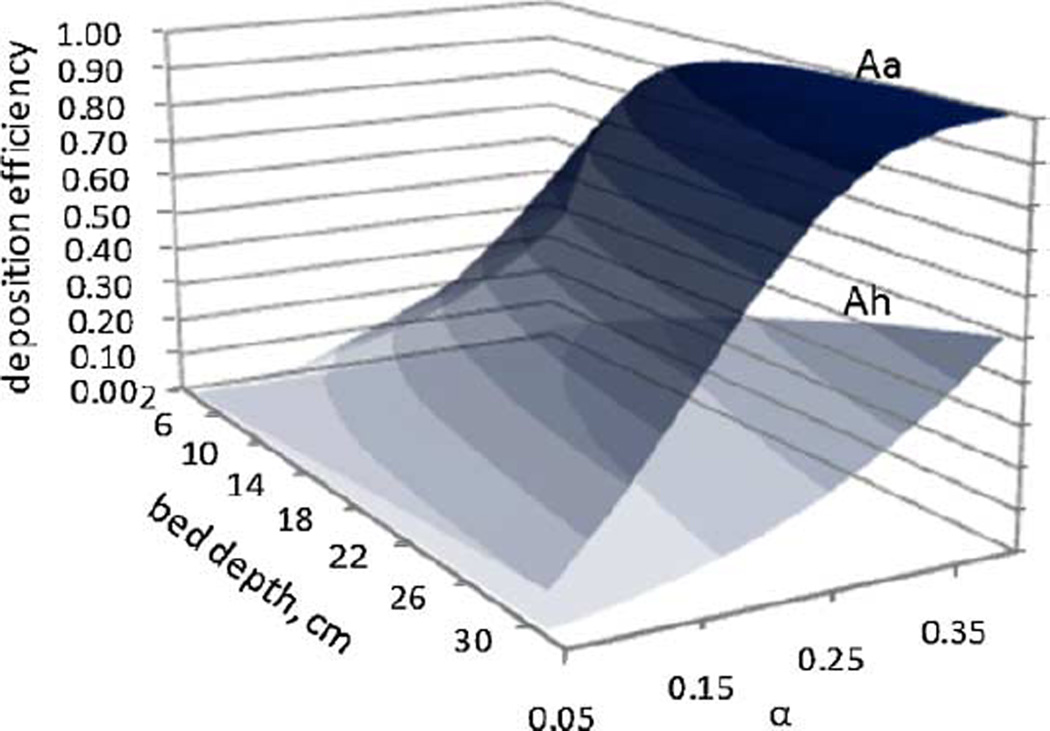
The mist deposition model suggests that at low values of α, thicker roots will not capture adequate nutrient droplets compared to thinner roots at the same α, and that can slow growth. This calculation implies that at the same α, thinner roots should be more efficient at capturing droplets than thicker roots and, therefore, grow faster; that is, A. annua should grow faster than A. hypogaea. This prediction is illustrated in Figure 5, which shows the mist deposition efficiencies predicted by the mist deposition model (Wyslouzil et al., 1997) for A. annua versus A. hypogaea hairy roots (i.e., thinner vs. thicker roots) at the same α and gas velocity, for different bed depths. The 1 L data shown in Figures 2 and 4, however, do not support this model prediction; at the same α, A. hypogaea grew better than A. annua. In root cultures, liquid plays two roles: it coats the roots (and hairs) thereby increasing the effective root diameter and it provides a resistance to effective gas exchange. Root hairs on A. annua may play a role by capturing excess liquid. We measured how much liquid the roots of each species retained (Table III); the liquid retention ratio was 1.7 times greater for hairy A. annua vs. smooth A. hypogaea roots. We further measured the effective diameter of A. annua when hairs are present, and found that, in contrast to smooth A. hypogaea roots, hairs increased the effective diameter of A. annua roots, approaching that of A. hypogaea (Table III). This would imply that the expected deposition efficiency of mist droplets onto the two species might indeed be similar. As implied by Payne et al. (1991) and demonstrated by Jørgensen and Revsbech (1985), liquid boundary layer thickness increases with surface roughness. In the presence of root hairs, the relative thickness of the boundary layer on A. annua roots is greater than that on A. hypogaea roots, so mass transport into the root likely decreases. Taken together, this would suggest that although A. annua would capture about the same number of droplets as A. hypogaea, based on the effective root diameter, growth actually may be lower due to increased mass transfer resistance. The more rapid decline in growth rate for A. annua versus A. hypogaea may also be indicative of this.
Figure 5.
Predicted mist deposition efficiencies for A. annua (Aa, 500 µm diameter) and A. hypogaea (Ah, 1,200 µm diameter) at different packing densities and root bed depths; gas velocity was 0.034 cm s−1. Maximum bed depth attained in the 20 L reactor after 20 days was about 30 cm.
It is also important to acknowledge that an interspecies comparison is difficult. Biomass yield (DW produced per amount of nutrient consumed) may be different. Therefore, even if roots capture less mist, if they are more efficient at converting the nutrients into biomass, they would show higher growth than roots that capture more mist but use it less efficiently. Ideally, one should compare growth of hairy root clones with identical biomass yields but with and without root hairs; this is not, however, a trivial endeavor.
Scaling the Mist Reactor to 4 and 20 L
To evaluate the potential of the mist reactor for larger-scale use, the 4 L reactor used for the 1 L A. hypogaea root studies was inoculated with four times the inoculum used in the 1 L reactors and provided with 4 L of media. In a series of replicated reactor runs, the misting cycle and medium flow rates were varied as shown in Table II, reactor conditions A–C. Runs A and B varied the misting cycle and flow rate to yield equal hourly delivery of media (Table II). These results showed that to increase the growth rate it is more effective to alter the flow rate than to alter the misting cycle. When the misting cycle was kept short, but flow rate increased (Table II, Run B), the root dry mass increased by 65% and the growth rate increased by 21%. Further increasing the flow rate in weeks 2 and 3 of growth together with increasing the misting cycle slightly (Table II, Run C) only decreased the growth rate and biomass yield from 0.154 to 0.150 day−1, and from 41.0 to 37.8 g DW, respectively, compared to reactor Run B.
When we scaled from 4 to 20 L, the cross-sectional area of the culture chamber was increased approximately 5×; this allowed us to take advantage of the larger potential spray diameter of the misting nozzle. At 20 L, only a single reactor was tested at each condition because of the large amounts of inoculum required as well as the difficulty involved in preparing and sterilizing the large quantities of culture medium and the reactors using our smaller-scale lab facilities (e.g., autoclave, transfer hoods, etc.). In an attempt to obtain growth rates equal to or better than those in the 4 L runs (Table II, Run B), the following parameters were varied further: medium flow rate through the nozzle, timing of flow rate increases, and slight variations in the misting cycle. Run D (Table II) verified that even in the 20 L reactors, increasing the misting cycle was detrimental to growth rate (μ = 0.114 day−1), while increasing the medium flow rate through the sonic nozzle increased the growth rate by 26% to 0.144 day−1 (Run E, Table II). For runs F–H, we also increased the power input at flow rates ≥60 mL min−1 to provide sufficient energy for consistent media atomization. The growth rates for Runs E and F were nearly identical. In these experiments the hourly feed rates were the same, but there were slight changes in the liquid flow rate and misting cycle in week 3. We examined the conditions that yielded the highest growth at the 4 L scale and extrapolated to the 20 L scale as follows. Since only start and end biomass amounts were known, we calculated the amount of feed per hour per g FW at harvest. The range for 4 L reactors was 0.6–0.8 mL h−1 g FW−1; for Runs E and F, it was ~0.35 mL h−1 g FW−1. For Run G, the feed rate was increased to 100 mL min−1 for all 3 weeks, along with increased misting cycles (Run G, Table II; ~0.75 mL h−1 g FW−1 at harvest); however, growth rate declined 6% compared to Runs E and F. Similar to A. annua (Fig. 3), it appeared that feeding too much, too often, and too early, is not beneficial for A. hypogaea roots. Considering that A. hypogaea growth increases exponentially especially in week 2 (Fig. 4), it was hypothesized that this time may be the most critical because of the high demand for nutrients during rapid root growth. Medium flow rate in week 2, therefore, was increased from 60 to 100 mL min−1 (Run H), but misting cycles were the same as for Run F. The growth rate subsequently increased slightly from 0.145 to 0.147, with a biomass yield of 1,877.8 g DW (7.77 g DWL−1; Table II, Run H). Although this increased growth generally supported our hypothesis, biomass yields did not reach those obtained in the 4 L reactors.
Although there have been substantial increases in scale of some reactors for culturing roots there have been few in depth studies on scaling up hairy root cultures using mist or spray reactors. In the earliest example, Wilson (1997) used a series of hanging hooks to immobilize Datura roots grown for 40 days at 500 L, but productivity was low (1.3 g DWL−1 day−1), as was the final packing density (α = 0.08). Using gas-phase reactors, others (Huang and Chou, 2006; Ramakrishnan and Curtis, 2004; Williams and Doran, 2000) showed more promising results for working volumes up to 14 L. While a very high productivity of Hyoscyamus muticus biomass was obtained in a 14 L reactor by Ramakrishnan and Curtis (2004), they had to increase the vvm and considerably enrich the aeration gas to 37% oxygen to achieve high-growth rates. They provided 648 L day−1 of liquid media to the roots (752 g FW at harvest) and noted a drop in respiration at α ≈ 0.5, which is indicative of mass transfer limitation due to liquid accumulation. Their final α = 0.75 and the growth rate was 0.21 day−1 with oxygen enrichment; without oxygen enrichment growth rate dropped to 0.13 day−1. Similar to our studies, they also observed their highest growth rates of (0.35–0.40 day−1) for the first 4 days of culture. In contrast, the mist reactor used by Towler et al. (2007), although much smaller (0.05 L), yielded a final α = 0.71 and overall growth rate of 0.11 day−1, which was comparable to that obtained by Ramakrishnan and Curtis’ (2004) nonoxygen supplemented reactor run.
Tracking Culture Growth From Small to Large Scale
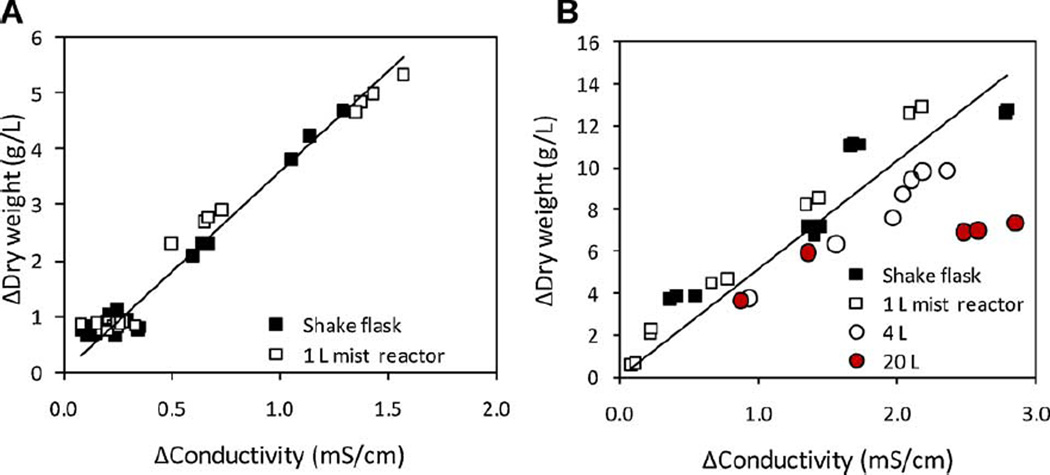
Both A. annua and A. hypogaea showed inverse linear relationships between conductivity measured in the medium reservoir and root DW during exponential growth (Fig. 6). These results showed that even in the 1 L mist reactor, culture growth can be indirectly monitored in order to maximize biomass. We then compared the change in conductivity of the culture medium after the 20 L reactor runs and found that reactors with higher biomass yields (Runs E, F, and H) had conductivity measurements that did not show linear correlation with any of the smaller volume cultures including the 4 L reactors (Fig. 6). There are several possibilities for this anomaly. Roots may have depleted a nutrient; we did not measure the content of the spent medium at harvest, so this cannot be verified. However, considering the much higher growth rates of the lower volume reactors (1 and 4 L) that were fed the same amount of nutrients per unit biomass, this seemed unlikely. Indeed, compared to 1 L reactors that had substantially more biomass L−1 at day 20 (Fig. 4), the 20 L reactors probably still had at least low levels of the major nutrients. Roots at the center of the 20 L root mass showed some necrosis, but that was not proportionately greater than the root mass at harvest in the 1 L reactors. Further, the center of this large root mass felt warmer than the outer regions, indicating there was active respiration. This higher internal temperature of the root mass is also unlikely to have negatively impacted root growth as this A. hypogaea clone grows well at higher temperatures (e.g., 28°C; Condori et al., 2010) than used in this study. To our knowledge, there are no reports of comparisons of conductivity measurements between small- and large-scale reactor cultures of hairy roots. It would be useful to learn if others have reported similar deviations between linear correlations in conductivity and accumulated root biomass. This nonlinear correlation between conductivity and dry mass may not be a phenomenon solely of roots grown in large-scale mist reactors, but rather a general phenomenon of morphologically complex cultures when grown at large scale. Clearly maintaining homogeneity in large, dense, morphologically complex cultures is challenging and further work is required to optimize their growth using this and other larger-scale reactors.
Figure 6.
Relationship between decrease in conductivity and increase in dry mass for A. annua and A. hypogaea hairy roots grown in shake flasks and mist reactors. A: A. annua; B: A. hypogaea; the linear correlation was developed using only the 1 L and shake flask data.
Summary and Conclusions
These scaling studies show that at 1 L both A. annua and A. hypogaea had better growth in the mist reactor than shake flasks. When scaled further, A. hypogaea growth at 4 and 20 L only declined from 1 L runs by 11% and 16%, respectively, and it is likely that with optimization, growth could be substantially improved at 20 L. These studies also show that one of the most important factors to alter in operating the mist reactor to maximize growth is, not surprisingly, medium flow rate to the misting nozzle. Limitations to final volume of scale will probably be most affected by volumetric delivery of misted media; greater throughput may result in delivery of larger droplets thereby obviating the aeration advantages of using a sonic nozzle. Multiple nozzles could be employed, but that would increase capital cost. A second important factor is the timing of the increase in flow rate, particularly during early exponential growth. Finally, increases in misting cycle beyond some as yet undetermined point appears to be generally detrimental, a phenomenon that thus far has not been explained. However, further studies on alteration of the length of the misting off time, especially at larger scales, seem to be warranted. While increasing the power input of the sonic mister was important to ensure complete atomization of media and minimize large droplet deposition onto the growing root bed, it is not clear if that factor had any other impact on root growth. In summary, this gas-phase mist reactor could provide cost-effective growth of root cultures, but at smaller scale than reported for other reactors. By using a series of inexpensive modular mist reactors, however, one could potentially achieve process advantages especially for mid- to high-value products.
Acknowledgments
Thanks to Prof. Fabricio Medina-Bolivar, Arkansas Biosciences Institute, Jonesboro, AR, for providing the Hull III line of A. hypogaea hairy roots, and to Prof. Barbara Wyslouzil, Ohio State University, for providing critical review of the draft manuscript. This study was supported in part by NIH grant 2R15GM069562-02 from the National Institute of General Medical Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of General Medical Sciences or the National Institutes of Health. Additional support was provided by WPI, and the Arkansas Biosciences Institute, the major research component of the Arkansas Tobacco Settlement Proceeds Act of 2000. C.Z. Liu is partially supported by the National Natural Science Foundation of China (No. 20806085) and the National High Technology Research and Development Program of China (863 Program) (No. 2007AA021501).
Nomenclature
- DR
diameter of root
- DW
dry weight
- FW
fresh weight
- Re
Reynold’s number
- t
time
- Uo
gas velocity in root bed
- Vdep
volume of medium captured by roots
- Vreq
volume of medium required by roots
Greek Symbols
- α
packing fraction
- μ
average specific growth rate
- μg
viscosity of carrier gas
- ρ
density of carrier gas
References
- Bais HP, Suresh B, Raghavarao KSMS, Ravishankar GA. Performance of hairy root cultures of Cichorium intybus L in bioreactors of different configurations. In Vitro Cell Dev Biol Plant. 2002;38:573–580. [Google Scholar]
- Condori J, Sivakumar G, Hubstenberger J, Dolan MC, Sobolev VS, Medina-Bolivar F. Induced biosynthesis of resveratrol and the prenylated stilbenoids arachidin-1 and arachidin-3 in hairy root cultures of peanut: Effects of culture medium and growth stage. Plant Physiol Biochem. 2010 doi: 10.1016/j.plaphy.2010.01.008. [DOI] [PubMed] [Google Scholar]
- Curtis WR. Cultivation of roots in bioreactors. Curr Opin Biotechnol. 1993;4:205–210. doi: 10.1016/0958-1669(93)90126-h. [DOI] [PubMed] [Google Scholar]
- Curtis WR. Hairy roots, bioreactor growth. In: Spier RE, editor. Encyclopedia of cell biotechnology. New York, NY: John Wiley and Sons; 2000. pp. 827–841. [Google Scholar]
- DiIorio AA, Cheetham RD, Weathers PJ. Growth of transformed roots in a nutrient mist bioreactor: Reactor performance and evaluation. Appl Microbiol Biotechnol. 1992;37:457–462. [Google Scholar]
- Dubois M, Gilles KA, Hamilton JK. Colorimetric methods for the determination of sugar and related substances. Anal Chem. 1956;28:350–357. [Google Scholar]
- Flores HE, Medina-Bolivar F. Root culture and plant natural products: Unearthing the hidden half of plant metabolism. Plant Tiss Cult Biotechnol. 1995;1:59–74. [Google Scholar]
- Gamborg OL, Miller RA, Ojima K. Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res. 1968;50:148–151. doi: 10.1016/0014-4827(68)90403-5. [DOI] [PubMed] [Google Scholar]
- Guillon S, Trémouillaux-Guiller J, Pati PK, Rideau M, Gantet P. Harnessing the potential of hairy roots: Dawn of a new era. Trends Biotechnol. 2006;24:403–409. doi: 10.1016/j.tibtech.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Huang SY, Chou SN. Elucidation of the effects of nitrogen source on proliferation of transformed hairy roots and secondary metabolite productivity in a mist trickling reactor by redox potential measurement. Enz Microbiol Technol. 2006;38:803–813. [Google Scholar]
- Jørgensen BB, Revsbech NP. Diffusive boundary layers and the oxygen uptake of sediments and detritus. Limnol Oceanogr. 1985;30:111–122. [Google Scholar]
- Kim Y, Wyslouzil BE, Weathers PJ. A comparative study of mist and bubble column reactors in the in vitro production of artemisinin. Plant Cell Rep. 2001;20:451–455. [Google Scholar]
- Kim YJ, Weathers PJ, Wyslouzil BE. Growth dynamics of Artemisia annua hairy roots in three culture systems. Biotechnol Bioeng. 2003;83:428–443. doi: 10.1002/bit.10685. [DOI] [PubMed] [Google Scholar]
- Liu CZ, Wang YC, Zhao B, Guo C, Ouyang F, Ye HC, Li GF. Development of a nutrient mist bioreactor for growth of hairy roots. In Vitro Cell Dev Biol Plant. 1999;35:271–274. [Google Scholar]
- Liu CZ, Towler MJ, Medrano G, Cramer CL, Weathers PJ. Production of mouse interleukin-12 is greater in tobacco hairy roots grown in a mist reactor than in an airlift reactor. Biotechnol Bioeng. 2009;102:1074–1086. doi: 10.1002/bit.22154. [DOI] [PubMed] [Google Scholar]
- McKelvey SA, Gehrig JA, Holar KA, Curtis WR. Growth of plant root cultures in liquid- and gas-dispersed reactor environments. Biotechnol Prog. 1993;9:317–322. doi: 10.1021/bp00021a011. [DOI] [PubMed] [Google Scholar]
- Medina-Bolivar F, Condori J, Rimando AM, Hubstenberger J, Shelton K, O’Keefe SF, Bennett S, Dolan MC. Production and secretion of resveratrol in hairy root cultures of peanut. Phytochemistry. 2007;68:1992–2003. doi: 10.1016/j.phytochem.2007.04.039. [DOI] [PubMed] [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant. 1962;15:23–28. [Google Scholar]
- Payne GF, Bringi V, Prince C, Shuler ML. Plant cell and tissue culture in liquid systems. NY: Oxford University Press; 1991. pp. 71–120. [Google Scholar]
- Ramakrishnan D, Curtis WR. Trickle-bed root culture bioreactor design and scale-up: Growth, fluid-dynamics, and oxygen mass transfer. Biotechnol Bioeng. 2004;88:248–260. doi: 10.1002/bit.20231. [DOI] [PubMed] [Google Scholar]
- Sharaf-Eldin M, Weathers PJ. Movement and containment of microbial contamination in the nutrient mist bioreactor. In Vitro Cell Dev Biol Plant. 2006;42:553–557. [Google Scholar]
- Sharp JM, Doran PM. Characteristics of growth and tropane alkaloid synthesis in Atropa belladonna roots transformed by Agrobacterium rhizogenes. J Biotechnol. 1990;16:171–186. [Google Scholar]
- Suresh B, Bais HP, Raghavarao KSMS, Ravishankar GA, Ghildyal NP. Comparative evaluation of bioreactor design using Tagetes patula L hairy roots as a model system. Proc Biochem. 2005;40:1509–1515. [Google Scholar]
- Towler MJ, Wyslouzil BE, Weathers PJ. Using an aerosol deposition model to increase hairy root growth in a mist reactor. Biotechnol Bioeng. 2007;96:881–891. doi: 10.1002/bit.21143. [DOI] [PubMed] [Google Scholar]
- Weathers PJ, Wyslouzil BE, Wobbe KK, Kim YJ, Yigit E. Workshop on bioreactor technology. The biological response of hairy roots to O2 levels in bioreactors. In Vitro Cell Dev Biol Plant. 1999;35:286–289. [Google Scholar]
- Weathers PJ, Liu CZ, Towler MJ, Wyslouzil BE. Mist reactors: Principles, comparison of various systems, case studies. Electr J Integr Biol. 2008;3:29–37. http://clt.astate.edu/electronicjournal/Articles/Weathers-FinalHR-07-010.05.09.08.pdf. [Google Scholar]
- Weathers PJ, Towler MJ, Xu JF. Bench to batch: Advances in plant cell culture for producing useful products. Appl Microbiol Biotechnol. 2010;85:1339–1351. doi: 10.1007/s00253-009-2354-4. [DOI] [PubMed] [Google Scholar]
- Williams GRC, Doran PM. Hairy root culture in a liquid-dispersed bioreactor: Characterization of spatial heterogeneity. Biotechnol Prog. 2000;16:391–401. doi: 10.1021/bp0000306. [DOI] [PubMed] [Google Scholar]
- Wilson DG. The pilot-scale cultivation of transformed roots. In: Doran PM, editor. Hairy roots: Culture and applications. UK: Gordon and Breach/Harwood Academic; 1997. pp. 179–190. [Google Scholar]
- Wyslouzil BE, Whipple M, Chatterjee C, Walcerz DB, Weathers PJ, Hart DP. Mist deposition onto hairy root cultures: Aerosol modeling and experiments. Biotechnol Prog. 1997;13:185–194. doi: 10.1021/bp960093h. [DOI] [PubMed] [Google Scholar]
- Wyslouzil BE, Waterbury RG, Weathers PJ. The growth of single roots of Artemisia annua in nutrient mist reactors. Biotechnol Bioeng. 2000;70:143–150. doi: 10.1002/1097-0290(20001020)70:2<143::aid-bit3>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Wysokinska H, Chmiel A. Transformed root cultures for biotechnology. Acta Biotechnol. 1997;2:131–159. [Google Scholar]
- Zhang C, Medina-Bolivar F, Buswell S, Cramer CL. Purification and stabilization of ricin B from tobacco hairy root culture medium by aqueous two-phase extraction. J Biotechnol. 2005;117:39–48. doi: 10.1016/j.jbiotec.2004.12.015. [DOI] [PubMed] [Google Scholar]