SUMMARY
The CACNA1A gene, encoding the voltage-gated calcium channel subunit α1A, is involved in pre- and postsynaptic Ca2+ signaling, gene expression, and several genetic neurological disorders. We found that CACNA1A employs a novel strategy to directly coordinate a gene expression program, using a bicistronic mRNA bearing a cryptic internal ribosomal entry site (IRES). The first cistron encodes the well-characterized α1A subunit. The second expresses a newly-recognized transcription factor, α1ACT, that coordinates expression of a program of genes involved in neural and Purkinje cell development. α1ACT also contains the polyglutamine (polyQ) tract that, when expanded, causes spinocerebellar ataxia type 6 (SCA6). When expressed as an independent polypeptide, α1ACT, bearing an expanded polyQ tract, lacks transcription factor function and neurite outgrowth properties, causes cell death in culture, and leads to ataxia and cerebellar atrophy in transgenic mice. Suppression of CACNA1A IRES function in SCA6 may be a potential therapeutic strategy.
INTRODUCTION
Voltage-gated calcium channel genes encode a large family of channel proteins (α1 subunits) that play critical roles in neuronal excitability, transmitter release, muscle contractility and gene expression (Catterall, 2011). Genetic defects of these channels have been implicated in a variety of neurological, cardiac and skeletal muscle disorders (Cain and Snutch, 2011). Diverse mutations in the α1A subunit, CACNA1A gene, causing either loss or gain of P/Q-type channel function, have been associated with dominantly inherited conditions of migraine, epilepsy, and episodic and progressive ataxia (Rajakulendran et al., 2012). The recognition that spinocerebellar ataxia type 6 (SCA6) is due to expansion of a polyglutamine (polyQ) tract encoded by a newly identified 47th exon in CACNA1A, from a normal range of Q4-Q17 to pathological range of Q19-Q33, added further complexity to modeling both channel function and disease pathogenesis (Zhuchenko et al., 1997).
Attempts to attribute SCA6 to a disturbance in P/Q-type channel function associated with the expanded polyQ in the α1A subunit in heterologous expression systems have led to contradictory results, while two knockin studies showed no effect of expanded polyQ tracts on P/Q channel gating (Saegusa et al., 2007; Watase et al., 2008). Several laboratories have shown that the C terminus of the α1A subunit (α1ACT), which contains the polyQ tract, is present as a stable fragment in cultured cells or cerebellar tissues (Ishiguro et al., 2010; Kordasiewicz et al., 2006; Kubodera et al., 2003; Marqueze-Pouey et al., 2008; Scott et al., 1998) and enriched in cerebellar nuclei, based on nuclear localization signals in the α1ACT sequence (Kordasiewicz et al., 2006). This finding is similar to that of the C terminal fragment of the L-type subunit that is translocated to the nucleus and functions as a transcription factor (Gomez-Ospina et al., 2006). Finally, the α1ACT fragment bearing SCA6-expanded polyQs is toxic to cultured cells and primary neurons (Ishiguro et al., 2010; Kordasiewicz et al., 2006; Kubodera et al., 2003; Marqueze-Pouey et al., 2008).
Here, we explored the origin and function of the α1ACT polypeptide in physiology and disease. We demonstrate that α1ACT is generated from the full-length α1A transcript by means of a cellular internal ribosomal entry site (IRES) located within the α1A mRNA, i.e., that the CACNA1A gene is bicistronic. The α1ACT protein containing the normal polyQ tract is a transcription factor that binds and enhances expression of several Purkinje cell (PC)-expressed genes, promotes neurite outgrowth, and partially rescues the CACNA1A knockout phenotype. α1ACT with expanded polyQ has altered function, reduces viability of cells in vitro, and causes gait impairment and cerebellar cortical atrophy in vivo. This is the first report of a truly bicistronic, dual-function, cellular gene encoding two proteins with completely distinct functions, in this case an ion channel and a transcription factor. This gene expression strategy demonstrates a novel role for an IRES in coordinating gene expression, as well as a potential therapeutic target for disease modifying therapy.
RESULTS
Identification of the C terminal fragment of CACNA1A-encoded α1A subunit (α1ACT)
To identify the N terminal sequence of the α1ACT fragment, we tagged the full-length human α1A subunit cDNA bearing normal polyQ (Q11), α1AWT, at its 3’ end with a 3xFLAG epitope and established HEK293 cell lines stably expressing this ~220kD α1A-FLAG fusion protein. The cell line grew normally and stably expressed the 75 kD α1ACT-FLAG fusion protein. We affinity purified α1ACT-FLAG from the whole-cell lysate in a two-step procedure. Peak elution fractions (Figure 1A) collected from anion exchange chromatography (HiTrap™ DEAE FF) were subjected to affinity purification using anti-FLAG M2 magnetic beads. The isolated α1ACT fragment protein was seen as a unique, 75kD band (arrowhead) above the heavy chain IgG band on a Coomassie stained SDS-PAGE gel (Figure 1B and 1C). LC-MS/MS analysis of in-gel digest protein (The Rockefeller University Protein Resource Center, NY) revealed that the amino acid sequence of N terminus of α1ACT fragment was Met Ile Met Glu Tyr (amino acids 1960-1964, nucleotides 6114-6128, Genbank access GI: 187828892, NM_001127222,) (Figure 1D and Figure S1). This sequence, which begins within the IQ-like domain of the full length α1A subunit and does not overlap with any known protease cleavage site, is a highly homologous sequence in all vertebrate species (Wilkins et al., 1999).
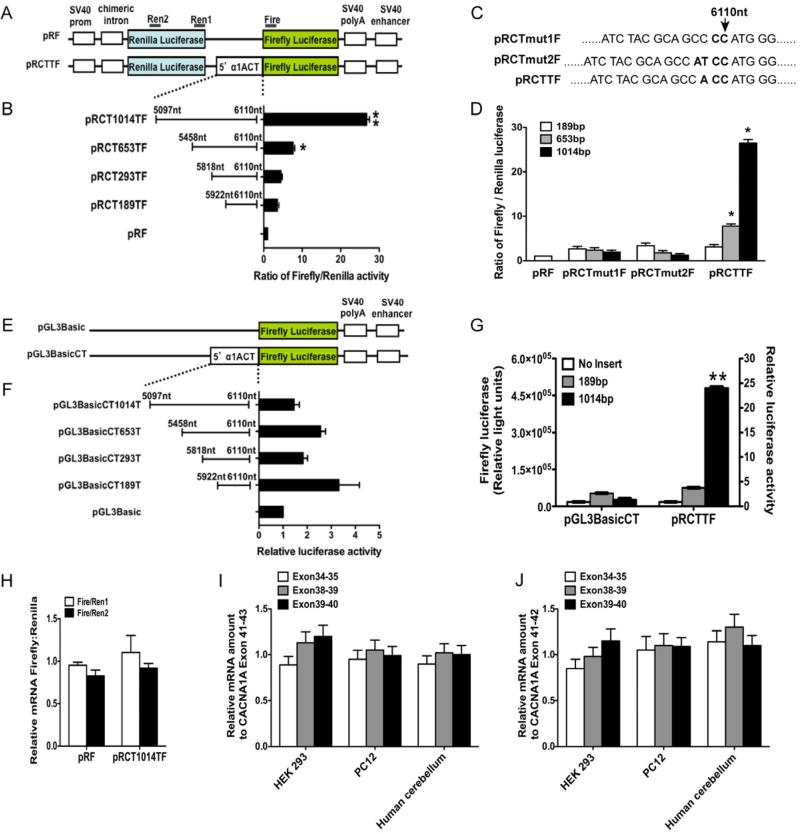
Figure 1. The C terminal fragment of α1A subunit initiates at MIMEY (amino acids 1960-1964, nucleotide 6114-6128).
(A) Western blot analysis of fractions collected from HiTrap™ DEAE FF anion exchange chromatography. 3xFLAG-tagged α1A subunit is used as positive control. (B) Coomassie blue staining of the peak α1ACT-containing fraction after two-step purification. The arrowhead indicates the 75 kD band identified by mass spectrometry as α1ACT. (C) Western blot analysis of lysate from α1A overexpressing cells with anti-FLAG antibody confirms the identity of α1ACT. (D) LC-MS/MS analysis of in-gel digest of proprionylated protein reveals that the starting amino acid sequence of N terminus of the α1ACT fragment is Met Ile Met Glu Tyr. (E) Schematic representation of the constructs with a series of mutations or deletions. (F) In-frame deletion of the start site of α1ACT does not abolish the expression of the 75kD C terminal portion of the FLAG-tagged α1A protein bearing either normal range (Q11) or pathological range (Q33) of polyQ. (G) Expression of the 75kD α1ACT C terminal fragment persists after insertion of termination codons at T1937 or P1847 in the FLAG-tagged α1A subunit, upstream of the start site. (H) Deletion of a 534bp fragment (α1Adel534Q11), but not deletion of a 185bp fragment (α1Adel185Q11) from the α1A coding region upstream of the α1ACT eliminates α1ACT expression, while maintaining expression of full-length α1A-FLAG. Deletions using encoded Q33 repeat expansions constructs (α1Adel185Q33 and α1Adel534Q33) behave similar to the Q11 constructs (see also Figure S1).
To investigate the origin of α1ACT protein fragment, we generated a series of constructs bearing different mutations of the α1A cDNA, as defined in Figure 1E, and transfected them into HEK293 cells. Deletion of 534 bps (α1Adel534bp) abolished the α1ACT fragment expression without affecting the expression of the full-length α1A subunit (Figure 1H), whereas other mutations of α1A failed to do so (Figure 1E-1H). This indicates that the 534 bps fragment upstream of the α1ACT start site in the α1A cDNA contains sequences essential for translation of α1ACT. These effects on expression were seen to an equivalent degree with both the α1AWT and the human α1A subunit cDNA bearing the pathological polyQ tract (Q33, α1ASCA6).
CACNA1A mRNA contains an internal ribosome entry site (IRES)
We hypothesized that expression of α1ACT fragment may be mediated by an IRES present within the CACNA1A coding sequence. 2-d structure analyses of the complete α1A mRNA sequence using an M-fold-based algorithm (Palmenberg and Sgro, 1997; Zuker, 2003) did not identify any canonical type I or type II IRES structures in this region (Baird et al., 2006). However, the region containing nucleotides 5096-6110 sequence was predicted to form a highly complex, stable conformation possessing several stem-loop structures that could represent an area of functional significance for ribosomal binding and interaction with trans-activating factors (Figure S2). This region is highly conserved, from 89.4% in Bos taurus to 76.7% in Danio rerio (data not shown).
To test for IRES activity within this region we inserted DNA segments of different lengths (Figure 2) from the region 5’ to the α1ACT start site into the bicistronic (Renilla luciferase, R-Luc, and firefly luciferase, F-Luc) reporter vector, pRF (Figure 2A) (Spriggs et al., 2009). Because the coding region for the R-Luc is followed by a stop codon, an increase in F-Luc activity indicates the presence of an upstream IRES that enables re-binding of the dual luciferase transcript to the ribosomal machinery. Expression of pRCT653TF or pRCT1014TF, but not pRCT189TF and pRCT293TF, in HEK293 cells enhanced the activity of the F-Luc approximately 9- and 26-fold (Figure 2A and 2B). Moreover, insertion of 1 or 2 nucleotides immediately 5’ to the ATG codon prior to the F-Luc coding region within the pRF vector eliminated the accumulation of F-Luc (Figure 2C and 2D). Therefore, the structure of the CACNA1A IRES is highly dependent on initiating translation of the second cistron at a specific codon, ATG 1960, as is typical of most IRES activation (Fitzgerald and Semler, 2009; Wilson et al., 2000). To help exclude the possibility that the increased F-Luc activity was due to a change at the RNA level, we inserted the same segments into the promoter-less reporter vector, pGL3Basic. Transfection into HEK293 cells yielded no significant increase of luciferase activity (Figure 2E-2G), arguing against the presence of a cryptic promoter in these segments. Subsequently, we performed a quantitative real-time PCR (qRT-PCR) on two amplicons within the Renilla ORF and one near the initiation codon of firefly ORF (Figure 2A). The observed ratio of 1:1 Renilla: Firefly mRNA (Figure 2H) favors the presence of an IRES, rather than increased transcription of the second reporter via a cryptic promoter, a splicing event or any other increase in mRNA stability. In addition, the qRT-PCR expression ratios for gene fragments (5’ to or 3’ to the α1ACT start site) within the α1A subunit were approximately 1 in untransfected HEK293 or PC12 cells. Lastly, we found equivalent signals from before and after the α1ACT start site using endogenous α1A mRNA isolated from human cerebellum (Figure 2I and 2J). These results suggest that expression of α1ACT is driven by the presence of a cellular IRES within the α1A coding region rather than by a cryptic promoter or splicing event.
Figure 2. CACNA1A mRNA contains an IRES.
(A) Schematic representation of the constructs pRF and pRCTTF. (B) IRES activity is demonstrated using bicistronic vectors. The ratio of Renilla luciferase and firefly luciferase activities was determined and normalized to β-galactosidase activities. (C) Sequence of CACNA1A IRES nucleotide inserts CTT, CTmut1 and CTmut2, inserting of 1-2 nucleotides. (D) The luciferase activities of bicistronic vectors bearing nucleotide insertions are determined as in (B). (E) Schematic representation of the constructs pGL3Basic and pGL3BasicCT. The same DNA fragments as inserted into pRF were subcloned into the promoter-less pGL3Basic construct. (F) Luciferase activities are determined as in (B). (G) The raw firefly luciferase activities of two fragments are compared between promoter-less vector pGL3BasicCT and bicistronic vector pRCTTF, which suggests 1014bp fragment contains an IRES. (H) Quantitative, real-time reverse transcription-PCR (qRT-PCR) performed using RNA from cells transfected with bicistronic vectors as amplified using primers, Ren1, Ren2 and Fire demonstrates no difference in abundance of R-Luc and F-Luc mRNA. (I and J) qRT-PCR performed using RNA extracted from PC12 and HEK293 cells, transfected with α1A or human cerebellum to compare abundance of amplicons Ex34-35, Ex38-39 and Ex39-40, upstream of the α1ACT start site relative to amplicons Ex41-42 (I) and Ex41-43 (J), from within the α1ACT coding region. Data are mean ± SEM, n ≥ 3 (each involving triplicate assays, *p<0.05, **p<0.01). (see also Figure S2).
The translocated α1ACT fragment binds to non-coding regions of genes expressed in Purkinje cells and activates transcription
Earlier studies indicated that the 75 kD α1ACT, bearing the normal polyQ tract, was enriched in the nucleus (Ishiguro et al., 2010; Kordasiewicz et al., 2006). To investigate whether α1ACTWT (Q11) plays a role in gene expression by binding to genomic DNA, we performed chromatin immunoprecipitation-based cloning (ChIP-based cloning) from PC12 cells transfected with α1ACT-FLAG fusion protein (Methods). Potential target genes identified via ChIP-based cloning and sequencing include GRN (granulin), BTG1 (B-cell translation gene 1), PMCA2 (Ca2+ ATPase, plasma membrane 2), ITGA8 (integrin alpha-8 precursor) and TAF1 (TATA box binding protein-associated factor of RNA pol II) (Table S2). Putative interaction between α1ACT-FLAG fusion protein and cloned DNA segments within the BTG1, PMCA2 and GRN genes was further confirmed by ChIP-qRT-PCR, (Figure 3A and 3B). Although these genes are not uniquely expressed in PCs, the abundant expression of α1ACT in PCs suggests that they are part of a PC-specific developmental program.
Figure 3. α1ACT is a transcription factor that regulates neural gene expression through an AT-rich element.
(A) ChIP and quantitative real-time PCR verification of DNA sequences identified by ChIP-cloning. PC12 cells were transfected with empty vector or FLAG-tagged α1ACTWT. (B) Relative enrichment was calculated as the ratio between the net intensity of each bound sample normalized to its input sample, and the vehicle control sample normalized to vehicle control input sample (n ≥ 3). (C) Enhancer activity of BTG1 gene fragments. Positions of the fragments are indicated. (D) Promoter activity of GRN gene fragments. Positions of the fragments are indicated. (E) Consensus sequence analyzed by CLC main workbench Version 6.5 among the α1ACT ChIP-targeted sequences. Consensus sequence was predicted and labeled in red. (F) EMSA demonstrates the formation of displaceable nucleoprotein complex with the α1ACT and BTG1 WT (517-630nt) element. Lane 1 is biotin-labeled BTG1 WT probe. Lanes 2 and 5 show the three major complexes formed between BTG1 probe and α1ACTWT nuclear protein. Lane 8 shows the fourth complex formed between BTG1 probe and α1ACTSCA6 nuclear protein. (G) EMSA shows that the AT-rich probe (531-553nt) forms three complexes in the absence of competitor. These complexes were displaced by excess unlabeled AT-rich sequence and partially abolished by AT-rich Mut1 and Mut3. The super-shifted bands were only seen in α1ACTWT–FLAG nuclear extracts treated with FLAG-M2 antibodies, but not in lane of pCDNA3 nuclear extracts. (H) EMSA shows that the TTATAA region is critical for the formation of nucleoprotein complexes with AT-rich element. (I) α1ACTWT significantly increases BTG1 enhancer activity through intact TTATAA region. Plasmid pRL-TK is used as transfection efficiency control. Data are mean ± SEM, n ≥ 3 (each involving triplicate assays, *p<0.05 vs. control construct) (see also Figure S3).
We tested whether α1ACT regulates gene expression through the target sequences identified in ChIP-based cloning. Within BTG1 3’ UTR, we identified an enhancer within the 113 bp sequence that obtained from α1ACT ChIP-based cloning, using reporter assays with segments spanning three lengths (beginning at nt 517) from the 3’ UTR inserted into the pGL3 promoter vector (Du et al., 2009), as shown in Figure 3C. Importantly, over-expression of α1ACTWT significantly increased luciferase activity in the BTG1-630 reporter construct, suggesting that BTG1 3’UTR contains an α1ACT-regulated enhancer element. However, α1ACTSCA6, bearing the pathological polyQ tract (Q33) failed to increase BTG1 3’UTR-enhanced luciferase expression (Figure 3C). We obtained similar results from two segments of 5’ flanking region in the progranulin gene using the pGL3-basic vector. The α1ACTWT fragment, but not the α1ACTSCA6 fragment, increased expression of the reporters bearing the 1662 bp fragment by 2-fold and the 638 bp fragment by 3.2-fold, respectively (Figure 3D). Thus, α1ACTWT has direct gene regulatory effects on at least two target genes, and these are lacking with α1ACTSCA6.
AT-rich element of α1ACT binding site is essential for the BTG1 enhancer activity
Alignments of ChIP-identified sequences predict two distinct motifs, 1) an AT-rich element, TTATAAAA, and 2) a CA-rich element, CCAA, as potential α1ACT binding sites (Figure 3E). The 113bp BTG1 3’UTR sequence (517-630bp BTG1 WT, Table S3) contains both consensus sequences. As shown in Figure 3F by electrophoretic mobility shift assays (EMSA) using the BTG1 WT as probe and nuclear extracts from untreated PC12 cells or from those transfected with α1ACTWT and α1ACTSCA6, we observed a component in PC12 cells, caused a significant gel shift (Complex I, II and III). This indicates that the BTG1 3’UTR contains cis-elements reacting with neuron-specific nuclear protein, present in both untransfected and α1ACT-transfected cells. The three protein complexes with the BTG1 WT probe could be abolished by excess unlabeled BTG1 WT probe, indicating that the binding was specific (Lanes 3 and 4, Figure 3F). Interestingly, nuclear extracts from α1ACTSCA6-transfected PC12 cells (Lane 8, Figure 3F) led to a different migration and pattern of DNA-protein complexes compared to α1ACTWT-expressing cells (Lane 5, Figure 3F), with a greater abundance of complex IV in complexes from the α1ACTSCA6-transfected PC12 cells. This suggests that α1ACTSCA6 has different protein binding pattern from α1ACTWT.
To further identify α1ACT consensus sequences, we used the AT-rich consensus element as a probe in EMSA (Table S3 and Figure 3G). The AT-rich consensus sequence greatly reduced the formation of the complexes (Lane 7, Figure 3G), while mutant AT-rich elements only partially reduced the complex formation (Lanes 5 and 6, Figure 3G). Anti-FLAG antibodies generated a super-shifted complex with nuclear extracts from α1ACTWT-FLAG expressing cells (Lanes 8 and 9, Figure 3G). These studies demonstrate that α1ACT binds to AT-rich element in the BTG1 3’UTR. Finally, we performed EMSA with a series of 3-bp mutations of the TTATAAGAT sequence (Table S3). As shown in Figure 3H, Mut1 abolished complex II and Mut2 abolished complex I. Mut4, combining Mut1 and Mut2, abolished both complex I and II (Figure S3). We also mutated the element, TTATAAGT, within the BTG1 630reporter construct (517-630bp). Constructs 630mut1 and 630mut2 contain 3bp mutation, while 630mut4 contains both Mut1 and Mut2 substitutions. All three constructs had significantly reduced luciferase activities, both basally and in response to over-expressing of α1ACTWT compared to BTG1 630 construct (Figure 3I). These results suggest that TTATAA is the core sequence of AT-rich element that is required to maintain the α1ACT-regulated BTG1 expression.
The α1ACTWT (but not α1ACTSCA6) increases the physiological expression of target genes and enhances neurite outgrowth of differentiating PC12 cells
To test for an effect of α1ACT on endogenous expression of ChIP-identified genes we examined the mRNA and protein levels of TAF, BTG1, PMCA2 and GRN in both cell lines and human cerebellum. Over-expression of normal α1AWT or α1ACTWT, but not α1ACTSCA6, significantly increased the expression of these genes (1.6- to 2.7-fold) in PC12 cells, either compared to empty vector-transfected or α1ACTSCA6-transfected PC12 cells (Figure 4A). As shown in Figure 4B and 4C, BTG1 and PMCA2 protein expression levels were also increased in α1AWT- and α1ACTWT-transfected PC12 cells. Finally, using cerebellar tissues from two SCA6 patients (Q22) and normalizing transcript levels to a PC specific mRNA, Pcp2, we found that BTG1 gene mRNA expression was decreased compared to the normal control (Figure 4D).
Figure 4. α1ACT enhances neurite outgrowth by regulating BTG1 expression.
(A) Relative mRNA expression levels of BTG1, PMCA2, TAF and GRN in PC12 cells transfected with α1AWT, α1ACTWT or α1ACTSCA6 (n ≥ 3). (B and C) Western blot (B) and quantitation of protein expression levels of BTG1 and PMCA2 in PC12 cells transfected with α1AWT, α1ACTWT and α1ACTSCA6 (C). (D) Relative levels of BTG1 mRNA in the cerebellum from two SCA6 patients, normalized to Pcp2. (E and F) α1ACTWT enhances neurite outgrowth. Representative low- and high-magnification images of PC12 cells with transiently transfected pcDNA3-FLAG, α1AWT-FLAG, α1ACTWT-FLAG and α1ACTSCA6-FLAG at 24 hr (E) and 72 hr after NGF treatment (F). Cells were labeled for GAP-43 (green) to visualize PC12 cell body and neurites. (G and H) Quantitation of average neurite length and percentage of neuritis per cell (n = 200; *p<0.05 versus pcDNA3-FLAG). (I) α1ACTWT up-regulates BTG1 gene and increases PRMT1/BTG1 protein interaction. (J and K) Silencing of BTG1 expression inhibits α1ACTWT-enhanced neurite outgrowth. Anti-FLAG staining is shown in red. (L) Quantitation of neurite outgrowth by siBTG1 in transfected cells (n = 3, *p<0.05). The blunted effect by α1ACTSCA6-FLAG was also diminished by BTG1 silencing. Data are mean ± SEM (see also Figure S4).
Several of the genes regulated by α1ACT are known to play a role in differentiation of the neuronal phenotype (Baker et al., 2006). We established PC12 cell lines stably expressing 3xFLAG-tagged versions of either the full-length α1A or the α1ACT fragment. Upon induction with nerve growth factor (NGF) for 24 hrs, we observed that both α1AWT-FLAG and α1ACTWT-FLAG enhanced neurite outgrowth (fraction of cells with neurites) when compared with PC12 cells expressing empty vector (Figure 4E-4H and Figure S4A-S4D). Importantly, the P/Q channel blocker, ω-agatoxin, had no effect on α1ACTWT-induced neurite outgrowth (Figure S4E). Furthermore, blocking the expression of endogenous or transfected full-length α1A by siRNA, had no effect on α1ACTWT-enhanced neurite outgrowth in PC12 cells transfected with α1ACTWT-FLAG (Figure S4E-S4K). These findings suggest that enhanced neurite outgrowth does not depend on functioning channels or the presence of the full channel protein. There was a significant increase in the percentage of cells bearing neurites in cells transfected with α1AWT-FLAG and α1ACTWT-FLAG compared to controls after NGF stimulation for 24, 48 and 72 hrs (Figure 4E, 4F and 4H). Total neurite lengths were also increased 2 fold in α1ACTWT-expressing cells compared with controls at each time point (Figure 4G and Figure S4A-S4D). However, cells expressing α1ACTSCA6-FLAG had significantly lower percentage of cells with neurites and total neurite length/cell compared with cells expressing α1ACTWT-FLAG (Figure 4E-4H and Figure S4A-S4D). Thus, α1ACTSCA6 lacks the normal function of α1ACTWT in potentiating neurite outgrowth in neuronal cells.
BTG1 belongs to the BTG/TOB protein family of anti-proliferative genes. Both BTG1 and BTG2 proteins interact with the protein arginine N-methyltransferase (i.e. PRMT1) and positively modulate its activity. The PRMT1/BTG methylation pathway is involved in maintaining neuronal cells in a differentiated state (Berthet et al., 2002). We used BTG1 antibody to immunoprecipitate PRMT1 in PC12 cells stably transfected with α1ACTWT and α1ACTSCA6. In α1ACTWT-expressing cells the quantity of precipitated PRMT1 was increased, while in α1ACTSCA6-expressing cells precipitated PRMT1 was decreased, consistent with the expression of BTG1 in these two cell types (Figure 4I). Meanwhile, silencing of BTG1 by small interference RNA (siRNA) blocked the NGF-induced α1ACT-regulated neurite outgrowth (Figure 4J-4L and Figure S4L). These results demonstrate that α1ACT with normal range polyQ maintains the physiological expression of the BTG1 gene, leading to subsequent interaction of the PRMT1/BTG pathway to mediate neuronal differentiation. Together these observations suggest that the normal α1ACT acts as a transcription factor through target genes to enhance the neuronal phenotype, and that one effect of the SCA6 polyQ expansion in α1ACT is to disrupt the properties of the α1ACT transcription factor.
α1ACT partially rescues the phenotype of α1A−/− mice
α1A−/− mice, with targeted disruption of mouse CACNA1A, develop a gross neurological phenotype of seizures, dystonia, and ataxia soon after birth, and die by P18-21 (Jun et al., 1999). To investigate the importance of expression of the α1ACT fragment in PCs we used the Pcp2 promoter and Tet-off system (Zu et al., 2004) to generate two double transgenic mouse lines, Pcp2-tTA/TRE-α1ACT (abbreviated, PC-α1ACT), expressing at comparable levels either α1ACTWT (WT=Q4, the smallest α1ACT polyQ seen in humans) or α1ACTSCA6 (SCA6=Q33, the largest α1ACT polyQ seen in SCA6) fragments tagged with an N-terminal myc epitope (see Methods). These mice appeared to grow and develop normally and live a full life span. α1ACTSCA6 mice, however, had mild progressive motor problems that were evident using the treadmill (see below).
To test the role of α1ACT in cerebellar PC development in the absence of α1A channels, we bred PC-α1ACT mice with α1A+/− heterozygous knockout mice and subsequently crossed these offspring to generate α1A−/− mice with PC-targeted α1ACT expression (α1A−/−/PC-α1ACT). Curiously we did not identify any mice with α1A−/−/PC-α1ACTSCA6 genotype, suggesting an impaired viability (Figure 5A). As expected, α1A−/− exhibited severe neurological impairment (Jun et al., 1999). Surprisingly, α1A−/− mice expressing α1ACTWT, i.e., α1A−/−/PC-α1ACTWT mice, had an improved behavioral phenotype relative to α1A−/− mice. Although still neurologically impaired, they gained more weight during the first two weeks of postnatal life compared to α1A−/− mice (Figure 5B and 5C), had improved in-cage mobility (Supplementary video 1), and survived approximately 1 week longer than α1A−/− mice (Figure 5D). We reasoned that the improved phenotype of α1A−/−/PC-α1ACTWT mouse might be mediated through improved dendritic and synaptic development. Immunofluorescent staining of cerebellar cortex of P16 mice from three groups, WT, α1A−/− and α1A−/−/PC-α1ACTWT mice showed that, as noted previously, PCs of α1A−/− mice had shortened primary dendrites with premature branching and an immature pattern of parallel fiber (PF) and climbing fiber (CF) synaptic contacts on PC soma and proximal dendrites (Hashimoto et al., 2011) (Figure 5E-5L). In contrast, PC morphology and afferent innervation of α1A−/−/PC-α1ACTWT cells resembled the pattern in WT mice (Figure 5E-5L). In α1A−/−/PC-α1ACTWT mice the thickness of molecular layer (ML), the relative height and density of the dendritic tree were significantly increased, compared to those of α1A−/− mice (p<0.05) (Figure 5F-5H). This finding is consistent with our in vitro studies showing that α1ACT enhances neurite outgrowth.
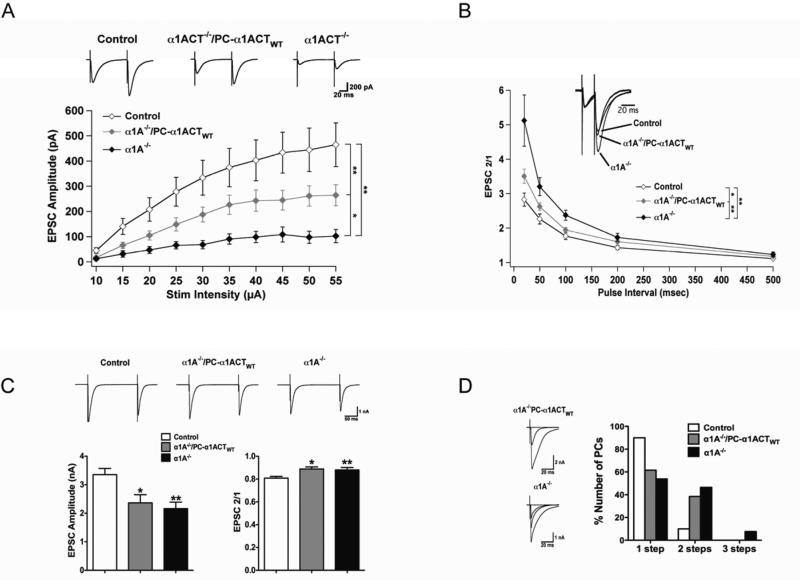
Figure 5. α1A−/−/PC-α1ACTWT transgenic mice have improved phenotype and development of cerebellar cortex compared to α1A−/− mice.
(A and B) The genotype (A) and appearance (B) of α1A−/−/PC-α1ACTWT mice. (C) α1A−/−/PC-α1ACTWT mice had slightly greater body weight compared with α1A−/− mice at age of P14 (*p<0.05). (D) The lifespan of α1A−/−/PC-α1ACTWT mice was significantly improved compared to α1A−/− (*p<0.05). Some pups survived until age of P30 (n = 2, not included in the Figure), while all α1A−/− pups died before P20. (E-H) α1ACT expression improves cerebellar cortex and PC dendrites. Low power images of cerebellar ML (E). PC dendrites are labeled for calbindin-28 kDa (green). The thickness of the ML (F), the relative height of dendritic tree (G), and the density (as defined in Methods) of the PC dendritic tree (H) were reduced in α1A−/− mice and partially corrected in α1A−/−/PC-α1ACTWT mice (100 dendritic trees from 5 mice at each group. Control is set as 1, *p<0.05). (I) Immunolabeling of PFs and PC dendrites using anti-vGlut1 (red) and anti-calbindin (green) antibodies. (J) Immunolabeling of CFs and PC dendrites using anti-vGlut2 (red) and anti-calbindin (green) antibodies. (K and L) Quantitation of CF reach (K) and relative height of dendritic tree (L) (100 CFs, *p<0.05). CF height was measured from the apical pole of PC somata to the tips of vGluT2 labeled CFs. Data are mean ± SEM (see also Figure S5).
We also compared the height of CF innervation between the three mouse groups (Figure 5J-5L) (Hashimoto and Kano, 2005; Hashimoto et al., 2011). In α1A−/− mice, most PCs were multi-innervated, and the CF height only reached 65.2 ± 5.3% of the ML at P16. We found that the CF height was improved in α1A−/−/PC-α1ACTWT mice over that of α1A−/− mice, reaching to 80.7 ± 4.1% of the ML at P16 (p<0.05). However, α1A−/−/PC-α1ACTWT mice exhibited the same three patterns of CF contacts seen with α1A−/− mice, mono CF on PC dendrites, multiple CF contacts on proximal dendrites, and multiple CF contacts on PC soma (Figure 5). These results demonstrate that α1ACT, as a transcription factor, plays an important role in establishing normal dendritic tree morphology in PCs in vivo, but that synapse elimination or selection of dominant innervation requires either P/Q channel function or α1ACT under its endogenous expression pattern.
Finally, we examined mRNA levels of TAF, BTG1, PMCA2 and GRN in the three mouse groups. Figure S5 demonstrates that the expression of these genes was decreased in cerebellar tissue of α1A−/− mice when compared to normal mice, but was increased 1.5 to 3 fold in α1A−/−/PC-α1ACTWT transgenic mice when compared to α1A−/− mice (p<0.05), relative to either β-actin or Pcp2 gene expression (Figure S5A-S5D). These results indicate that expression of the α1ACT target genes is impaired in α1A−/− mice and is corrected by localized expression of α1ACTWT in PCs of transgenic mice.
α1ACT partially restores parallel fiber EPSC amplitude but not climbing fiber mono-innervation
To further assess the effect of α1ACT expression on Purkinje cell synaptic properties, we measured PF excitatory postsynaptic current (PF EPSC) amplitude as a function of stimulus intensity in WT, α1A−/−, and α1A−/−/PC-α1ACTWT mice (P16-18) in cerebellar slice preparations. While PF EPSCs from WT were significantly larger than those from both α1A−/− (p < 0.01, stimulus intensities 20-55 μA) and α1A−/−/PC-α1ACTWT mice (p<0.05), EPSCs from α1A−/−/PC-α1ACTWT mice were greater than those from α1A−/− mice (p<0.05), indicating that α1ACT expression improves PF synaptic connections (Figure 6A).
Figure 6. α1ACT partially restores PF EPSC amplitude but does not affect CF innervation or EPSC properties.
(A) PF-EPSC amplitude as a function of stimulus intensity for α1A−/− (n = 9, N = 4), α1A−/−/PC-α1ACT (n = 16, N = 6), and WT (n = 11, N = 3) mice. Top: Typical PF-EPSCs at a stimulus intensity of 45 μA. (B) Paired-pulse ratios as a function of stimulus interval in α1A−/−, α1A−/−/PC-α1ACT, and WT mice. Inset shows an overlay of representative traces from all three groups of mice with an interstimulus interval of 20 ms. EPSC1 from α1A−/−/PC-α1ACT and WT mice were scaled to match the amplitude of EPSC1 from the α1A−/− mouse to facilitate comparison. (C) Top: Representative CF-EPSCs elicited while holding at -30 mV. Bottom left: CF-EPSC amplitudes for α1A−/− (n = 10, N = 3), α1A−/−/PC-α1ACT (n = 11, N = 3), and WT (n = 8, N = 3) mice. Bottom right: Paired-pulse depression of CF-EPSCs with 200 ms stimulus interval. (D) Left: representative traces from PCs in α1A−/− and α1A−/−/PC-α1ACT mice exhibiting multiple CF innervation. Right: Percentage of PCs exhibiting either one, two, or three discrete CF steps in α1A−/− (2 steps: 6/13, 3 steps: 1/13, N = 3), α1A−/−/PC-α1ACT (2 steps: 5/13, N = 3), and WT (2 Steps: 1/9, N = 3) mice. All mice were age P16-18. *p<0.05, **p<0.01. Data are mean ± SEM.
The inability of α1ACT to completely restore EPSC amplitudes to WT levels could be explained by the absence of P/Q-type Ca2+ channels in PF presynaptic terminals of α1A−/−/PC-α1ACTWT mice, as these channels play a critical role in neurotransmitter release at PF-Purkinje cell (PF-PC) synapses (Matsushita et al., 2002; Mintz et al., 1995). We therefore examined the presynaptic release properties of PF-PC synapses in α1A−/− and α1A−/−/PC-α1ACTWT mice by measuring the paired-pulse ratios (PPRs) of PF EPSCs. Large PPRs are indicative of low initial release probabilities, and are a sign of impaired presynaptic function (Zucker and Regehr, 2002). In accordance with observations from mouse models harboring loss-of-function mutations in the CACNA1A gene, α1A−/− mice exhibited PPRs significantly greater than WT at several stimulus intervals (WT to α1A−/− mice: p<0.01 for stimulus intervals of 20-100 ms, Figure 6B) (Liu and Friel, 2008; Matsushita et al., 2002). While α1ACT expression did lower PPRs closer to WT levels (α1A−/−/PC-α1ACTWT to α1A−/− mice: p<0.01 for stimulus intervals of 20 and 100 ms, p<0.05 for stimulus interval of 50 ms), the measured values were still elevated (WT to α1A−/−/PC-α1ACTWT: p<0.05 for stimulus intervals of 20 and 50 ms) (Figure 6B), suggesting a residual presynaptic deficit.
We also examined properties of the CF synapse onto Purkinje cells. We observed a reduction in CF EPSC amplitudes and a decrease in the degree of paired-pulse depression in both α1A−/− and α1A−/−/PC-α1ACTWT mice with respect to WT, both of which could also be due to the absence of P/Q-type channels presynaptically (Figure 6C). Additionally, we investigated whether α1ACT expression affects the process of CF maturation. During development, Purkinje cells undergo a competitive and activity-dependent elimination of superfluous CF inputs until only one remains. Impairments to this process results in the persistent innervation of Purkinje cells by multiple climbing fibers into adulthood. We found the proportion of Purkinje cells with multiple CF innervations to be increased in both α1A−/− and α1A−/−/PC-α1ACTWT mice compared to WT, a result indicating that actual P/Q-type Ca2+ channel function, and not just expression of the C terminus, may be essential for proper CF maturation (Watanabe and Kano, 2011) (Figure 6D). Our results provide evidence that α1ACT expression improves the synaptic connections of PFs, but not CFs, onto Purkinje cells.
α1ACTSCA6 causes cell death in vitro and mediates ataxia and cerebellar cortical atrophy in transgenic mice
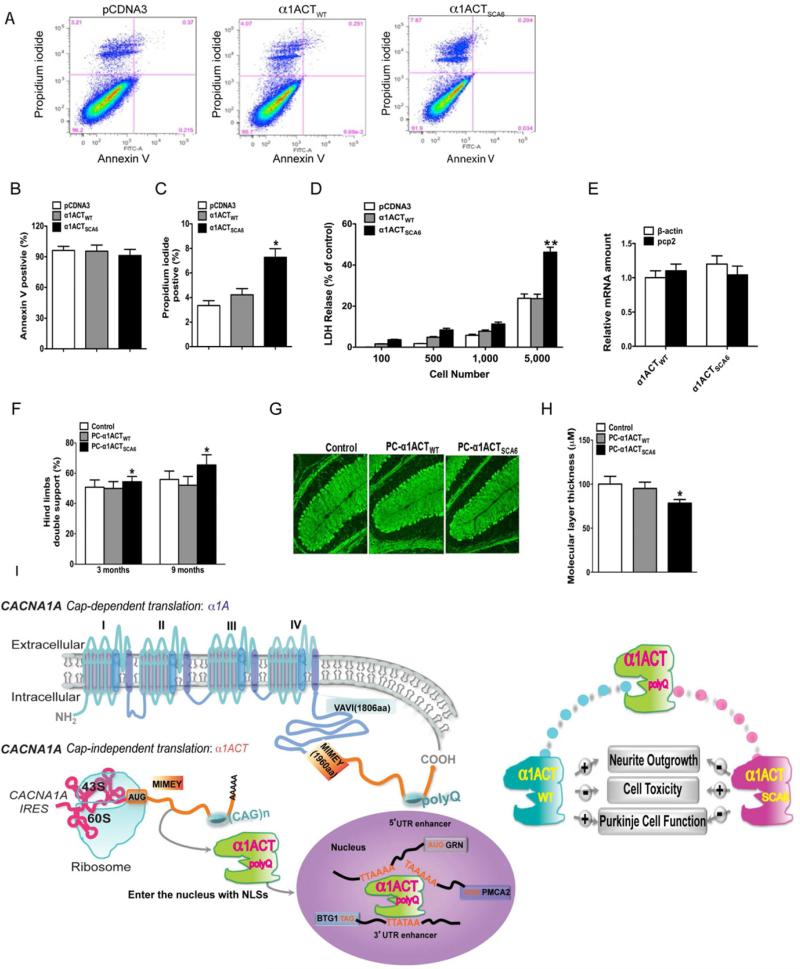
Previous studies have shown that the α1A C terminus bearing an expanded polyQ tract is toxic relative to WT α1A C terminus when transiently over-expressed in cultured mammalian cells (Ishiguro et al., 2010; Kubodera et al., 2003). Our flow cytometry results showed that PC12 cells stably expressing α1ACTWT exhibited equivalent viability to vector control cells, while cells expressing α1ACTSCA6 had approximately a 2-fold increase in cell death (p<0.05, Figure 7A-7C) (Koopman et al., 1994). Also, LDH release was significantly higher in cells expressing the α1ACTSCA6 compared with that of PC12 cells expressing only pcDNA3 or α1ACTWT (Figure 7D). These findings indicate that over-expression of α1ACT bearing pathological size of polyQ reduces cell viability in cultured mammalian cells.
Figure 7. α1ACTSCA6 is a pathogenic fragment.
(A) Representative fluorescence dot blots of FITC–Annexin V and propidium iodide (PI) stained PC12 cells with stably-transfected pcDNA3-FLAG, α1ACTWT-FLAG and α1ACTSCA6-FLAG. (B and C) Quantitation of Annexin V and PI positive cells (*p<0.05). (D) Cell death as measured by LDH release assay (*p<0.05). (E) Expression levels of α1ACTWT and α1ACTSCA6 in cerebellar homogenates by qRT-PCR. (F) Double support of Hind paw was impaired in PC-α1ACTSCA6 transgenic mice compared with PC-α1ACTWT transgenic mice at age 3-month, 9-month old (**p<0.01). (G and H) Cerebellar cortical atrophy in PC-α1ACTSCA6 transgenic mice. Low power images of cerebellar ML in mice at ages of 20-26 months (G) and Quantitation of ML thickness (H) (*p<0.05). PCs dendrites are labeled for calbindin (green). (I) Schematic illustration of expression regulation and function of α1ACT. Data are mean ± SEM(see also Figure S6).
Finally, we tested whether over-expression of the SCA6-expanded α1ACTSCA6 fragment in mice would lead to clinical or pathological features resembling SCA6, compared with over-expression of the fragment with normal allele size, α1ACTWT. Both α1ACTWT and α1ACTSCA6 can be detected in the nucleus of PCs in the corresponding PC-α1ACT lines at comparable levels by western blot, RT-PCR and immunostaining (Figure 7E, Figure S6A and Figure S6B). There were no obvious clinical differences between these two lines in young adults, and lifespan was not affected. However, we found that PC-α1ACTSCA6 mice demonstrated significant abnormalities in several gait parameters compared with PC-α1ACTWT mice using a video-assisted computerized treadmill for gait analysis (DigiGait, Mouse Specifics, Inc) (p<0.05, after post hoc correction). Most importantly, they exhibited an age-dependent increase in shared stance (double support time) of hind limbs, between ages 3 and 9 months, indicating progressive instability during walking (p<0.05, Figure 7F and Supplementary video 2 and 3) (Matsukawa et al., 2003; Stolze et al., 2002). The gait disturbance never progressed to the level of severe ataxia, although natural changes in weight gain in older mice may have obscured an impact on normal cage activity. The lack of severe ataxia even in 2-year-old mice is not surprising for a model of SCA6, with an age of onset of 43-52 years. Although we found no obvious sign of cell loss in the cerebellum in α1ACTSCA6 mice at approximately 2 years of age, measurement of the ML thickness showed that α1ACTSCA6-expressing mice have significant thinning of the ML, compared with age-matched α1ACTWT and WT mice (Figure 7G and 7H). Lastly, using RT-PCR to examine the expression levels of ChIP-identified genes TAF, GRN, BTG1 and PMCA2 in cerebellar tissues of 2-year-old PC-α1ACTSCA6 mice compared with age-matched WT mice, we found that the transcript levels of each of the α1ACT-regulated genes were decreased by between 11% and 34% (Figure S6C-6F). These findings are the first to demonstrate clinical and pathological changes in an animal model of SCA6 expressing appropriate-sized pathological alleles within a CACNA1A protein.
DISCUSSION
CACNA1A contains a cellular IRES
Cellular IRESs play an increasingly recognized role in the control of eukaryote gene expression where in many 5’ UTRs they provide alternatives to cap-dependent translation initiation during times of cellular stress (Coldwell et al., 2001; Spriggs et al., 2008). IRES sequences have also been detected within the coding regions of some cellular mRNAs, leading to the expression of isoforms or distinct protein products (Cornelis et al., 2000; Ul-Hussain et al., 2008). Together with the present report, these observations suggest that expression of bi-functional genes, particularly those encoding separate transcription factor proteins in the second cistron, may be a newly-recognized strategy for coordinating gene expression programs tied to individual gene products. This could enable the timely expression of a set of genes coincident with the appearance of key proteins during differentiation.
α1ACT, a CACNA1A-encoded transcription factor promotes a neurite outgrowth program
As a transcription factor, the normal α1ACT enhances the expression of at least three genes, GRN, PMCA2 and BTG1, in PC12 cells and cerebellar tissue, potentiates NGF-mediated neurite outgrowth in PC12 cells, and partially rescues the CACNA1A knockout phenotype. GRN is involved in neurite outgrowth and is critical in maintaining neuronal survival, since loss-of-function mutations of the GRN gene lead to cell death in the frontal and temporal lobes (Baker et al., 2006; Cruts et al., 2006; Van Damme et al., 2008). PMCA2 is highly expressed in the cerebellum, particularly in PCs and throughout the ML and granule cell layer (Zacharias and Kappen, 1999). PMCA2 knock-out mice exhibit vestibular and gait abnormalities and reduced thickness of the cerebellar ML (Kozel et al., 1998). Lack of PMCA2 also dramatically alters PC morphology. PMCA2 is a component of mGluR1-IP3R1 signaling complex, which has been implicated in plasticity at the PF-PC synapse (Kurnellas et al., 2007).
The PRMT1/BTG methylation pathway is involved in neurogenesis or in maintaining neuronal cells in a differentiated state. The 3’ UTR of BTG1 is highly conserved throughout evolution and plays a key role in this pathway (Rouault et al., 1993), consistent with our finding that α1ACT activates a novel 3’ enhancer element of BTG1. Enhanced BTG1/PRMT1-driven arginine methylation partly accounts for the essential role of protein methylation during PC12 differentiation (Cimato et al., 1997), which is in line with findings here that BTG1 knockdown blocks neurite outgrowth. Together these findings suggest that α1ACTWT is essential for maintenance of neurite outgrowth through gene-specific signaling pathways. It will be of interest to extend our ChIP-based cloning approach using RNA-seq methodology to more completely characterize the normal repertoire of α1ACT-regulated genes, as well as those bound by α1ACTSCA6.
Except for the in vivo studies, the properties of α1ACT are similar to those demonstrated for the 70 kD fragment, termed CCAT, derived from the Cav1.2 channel, α1C subunit, which may arise from a similar translational mechanism (Gomez-Ospina et al., 2006). In that case, as well as in ours, the set of genes regulated by these novel transcription factors does not conform to an obvious functional class of proteins, but appears to be involved in elaborating key components of the neuronal phenotype, and in neurogenesis or neurodegeneration, timed with the appearance of Ca2+ channel activity.
Phenotype rescue of α1A−/− mice by α1ACT
The improved phenotype at behavioral, histological, and electrophysiological levels in α1A−/−/PC-α1ACTWT mice is remarkable, particularly because α1ACTWT expression, under the control of PC specific promoter, Pcp2, was only restored in PCs. Consistent with these findings and our in vitro results, ChIP-identified genes TAF, BTG1, PMCA2 and GRN were down-regulated in α1A−/− mice, but were increased 1.5 to 3 fold in α1A−/−/PC-α1ACTWT transgenic mice. These results suggest that α1ACT, as a second gene product from CACNA1A, plays an important role in establishing normal morphology and function of PCs.
α1ACT expression improves PF-PC connections
PF EPSC amplitudes measured in α1A−/−/PC-α1ACTWT were significantly greater than those observed in α1A−/− mice, though they were not completely restored to WT levels, presumably due to the importance of P/Q-type Ca2+ channel function to neurotransmitter release at PF-PC synapses (Mintz et al., 1995). α1ACT expression also resulted in a partial reduction of PPRs at this synapse, which was surprising as PPRs generally reflect presynaptic release probabilities, and α1ACT is present only postsynaptically in α1A−/−/PC-α1ACTWT mice, indicating that factors either directly or indirectly related to the postsynaptic target cell can also influence this parameter. Regardless, the residual elevation of PPRs in α1A−/−/PC-α1ACTWT mice suggests that the incomplete restoration of PF EPSC amplitude is due to a presynaptic deficit in these mice.
The absence of any discernable effect of α1ACT expression on CF-EPSCs indicates that actual P/Q-type Ca2+ channel function is required for the process of CF maturation, as has been suggested previously (Watanabe and Kano, 2011). Thus, it appears that the phenotypic benefits of α1ACT expression are due predominantly to the improvement in PF synaptic transmission.
α1ACTSCA6 abolishes the normal function of α1ACT in gene expression regulation and is pathogenic in vitro and in vivo
Compared the properties of the normal α1ACT protein, α1ACTWT, bearing 4 or 11 glutamines, the α1ACTSCA6 polypeptide had altered binding to the BTG1 enhancer, showing additional DNA-protein complexes, and lacked the capacity to mediate expression via BTG1 and GRN luciferase reporters and impaired expression of these genes in PC12 cells. α1ACTSCA6 also failed to mediate neurite outgrowth when stably expressed in PC12 cells and caused increased cell death. This may explain why we did not obtain any mice with α1A−/−/PC-α1ACTSCA6 genotype. Mice over-expressing α1ACTSCA6 on a normal background exhibited subtle, but clearly measurable defects in motor functioning. Computerized treadmill gait analysis demonstrated progressive gait impairment with an increase in double limb support, a compensatory reaction to instability during walking. With advanced age α1ACTSCA6 was associated with thinning of the cerebellar cortex. These mice have reduced levels of expression of TAF1, GRN, BTG1 and PMCA2, the targets of α1ACT, relative to endogenous PC transcripts. These studies suggest that, rather than arising from ion channel dysfunction, the pathogenesis of SCA6 more closely resembles the toxic gain-of-function mechanism of the polyQ disorders (La Spada et al., 1991; Palhan et al., 2005; Sopher et al., 2004). This is supported by the lack of disturbed Ca2+ channel function in two mouse knockin studies of the SCA6 mutation (Saegusa et al., 2007; Watase et al., 2008). If additional studies further support this Ca2+ channel-independent mechanism, the demonstration of selective translation based on an IRES may pave the way for therapies targeted at suppressing the IRES function.
We have shown that the CACNA1A gene is bicistronic, encoding a newly identified transcription factor, α1ACT, involved with neurite outgrowth and PC maturation, within the α1A mRNA. α1ACT also bears the expanded polyQ tract in SCA6, which interrupts transcription factor function, impairs viability of cultured cells and is pathogenic in transgenic mice. These findings are summarized in the diagram in Figure 7I.
EXPERIMENTAL PROCEDURES
Materials and Standard Protocols
Cells, animals/mouse strains, antibodies, plasmids, and other materials were described in the Extended Experimental Procedures. The detailed protocols used for ChIP, ChIP-based cloning, ChIP-qRT-PCR, EMSA, Luciferase Assays, immunoprecipitation, immunohistochemistry, immunoblotting, quantitative RT-PCR, electrophysiology and LDH assay are outlined in the Extended Experimental Procedures.
Protein Purification and Mass Spectrometry
Protein extracts from HEK-293 cells stably transfected with α1A subunit bearing a Q11 allele were separated via anion exchange chromatography (His Trap FF column) and further purified by ANTI-FLAG M2 affinity purification. α1ACTWT fragment protein in SDS-PAGE gel was digested in-gel with trypsin and the resulting peptides were then analyzed via LC-MS/MS on an LTQ-Velos-Orbitrap mass spectrometer. See details in the Extended Experimental Procedures.
Immunohistochemistry and Microscopy
For detailed procedures of immunohistochemistry, antibodies and dilutions were described in the Extended Experimental Procedures. PC12 cells were imaged 0 hr, 24 hrs, 48 hrs and 72 hrs after NGF 24 hrs treatment. Neurites were defined as processes longer than the width of the cell body. For each experiment, cells were imaged. Dendrites were analyzed by employing ImageJ and NeuroJ programs.
Supplementary Material
ACKNOWLEDGMENTS
We thank Professors Sangram S. Sisodia, Brian Popko, and Bert L. Semler for helpful comments on the manuscript; Dr. Raymond Roos for helpful discussions; Dr. Xiaorong Zhu for protein purification technical assistance; Dr. Jeremy Marks and Dr. Janice Wang for rat granule cell cultures and transfection; Dr. Yuanxin Hu for assembling the vectors for PC-α1ACTWT and PC-α1ACTSCA6 mice and performing pilot immunoblot and immunohistochemical studies; Devon Collins and Kareisha Robinson for technical support in mouse breeding, genotyping, and DigiGait testing; Tom Hampton for DigiGait assistance on data analysis. We also thank Professor Anne Willis for providing pRF vector, Dr. Suneil Malik for providing pGL3GRN vector and Dr. Haiteng Deng for performing LC-MS/MS. We thank the Floyd family for support of SCA6 research. This work was also supported by funding from the National Ataxia Foundation (NAF), the National Organization of Rare Diseases (NORD), and the National Institute of Neurological Disorders and Stroke (NS-062771 to CH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
SUPPLEMENTAL INFORMATION
Supplemental Information includes Extended Experimental Procedures, 6 figures, 4 tables and 3 videos.
REFERENCES
- Baird SD, Turcotte M, Korneluk RG, Holcik M. Searching for IRES. RNA. 2006;12:1755–1785. doi: 10.1261/rna.157806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker M, Mackenzie IR, Pickering-Brown SM, Gass J, Rademakers R, Lindholm C, Snowden J, Adamson J, Sadovnick AD, Rollinson S, et al. Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature. 2006;442:916–919. doi: 10.1038/nature05016. [DOI] [PubMed] [Google Scholar]
- Berthet C, Guehenneux F, Revol V, Samarut C, Lukaszewicz A, Dehay C, Dumontet C, Magaud JP, Rouault JP. Interaction of PRMT1 with BTG/TOB proteins in cell signalling: molecular analysis and functional aspects. Genes to cells : devoted to molecular & cellular mechanisms. 2002;7:29–39. doi: 10.1046/j.1356-9597.2001.00497.x. [DOI] [PubMed] [Google Scholar]
- Cain SM, Snutch TP. Voltage-gated calcium channels and disease. Biofactors. 2011;37:197–205. doi: 10.1002/biof.158. [DOI] [PubMed] [Google Scholar]
- Catterall WA. Voltage-gated calcium channels. Cold Spring Harbor perspectives in biology. 2011;3:a003947. doi: 10.1101/cshperspect.a003947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimato TR, Ettinger MJ, Zhou X, Aletta JM. Nerve growth factor-specific regulation of protein methylation during neuronal differentiation of PC12 cells. The Journal of cell biology. 1997;138:1089–1103. doi: 10.1083/jcb.138.5.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coldwell MJ, deSchoolmeester ML, Fraser GA, Pickering BM, Packham G, Willis AE. The p36 isoform of BAG-1 is translated by internal ribosome entry following heat shock. Oncogene. 2001;20:4095–4100. doi: 10.1038/sj.onc.1204547. [DOI] [PubMed] [Google Scholar]
- Cornelis S, Bruynooghe Y, Denecker G, Van Huffel S, Tinton S, Beyaert R. Identification and characterization of a novel cell cycle-regulated internal ribosome entry site. Molecular cell. 2000;5:597–605. doi: 10.1016/s1097-2765(00)80239-7. [DOI] [PubMed] [Google Scholar]
- Cruts M, Gijselinck I, van der Zee J, Engelborghs S, Wils H, Pirici D, Rademakers R, Vandenberghe R, Dermaut B, Martin JJ, et al. Null mutations in progranulin cause ubiquitin-positive frontotemporal dementia linked to chromosome 17q21. Nature. 2006;442:920–924. doi: 10.1038/nature05017. [DOI] [PubMed] [Google Scholar]
- Du X, Rosenfield RL, Qin K. KLF15 Is a transcriptional regulator of the human 17beta- hydroxysteroid dehydrogenase type 5 gene. A potential link between regulation of testosterone production and fat stores in women. The Journal of clinical endocrinology and metabolism. 2009;94:2594–2601. doi: 10.1210/jc.2009-0139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald KD, Semler BL. Bridging IRES elements in mRNAs to the eukaryotic translation apparatus. Biochimica et biophysica acta. 2009;1789:518–528. doi: 10.1016/j.bbagrm.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Ospina N, Tsuruta F, Barreto-Chang O, Hu L, Dolmetsch R. The C terminus of the L-type voltage-gated calcium channel Ca(V)1.2 encodes a transcription factor. Cell. 2006;127:591–606. doi: 10.1016/j.cell.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K, Kano M. Postnatal development and synapse elimination of climbing fiber to Purkinje cell projection in the cerebellum. Neuroscience research. 2005;53:221–228. doi: 10.1016/j.neures.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Tsujita M, Miyazaki T, Kitamura K, Yamazaki M, Shin HS, Watanabe M, Sakimura K, Kano M. Postsynaptic P/Q-type Ca2+ channel in Purkinje cell mediates synaptic competition and elimination in developing cerebellum. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:9987–9992. doi: 10.1073/pnas.1101488108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiguro T, Ishikawa K, Takahashi M, Obayashi M, Amino T, Sato N, Sakamoto M, Fujigasaki H, Tsuruta F, Dolmetsch R, et al. The carboxy-terminal fragment of alpha(1A) calcium channel preferentially aggregates in the cytoplasm of human spinocerebellar ataxia type 6 Purkinje cells. Acta neuropathologica. 2010;119:447–464. doi: 10.1007/s00401-009-0630-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun K, Piedras-Renteria ES, Smith SM, Wheeler DB, Lee SB, Lee TG, Chin H, Adams ME, Scheller RH, Tsien RW, et al. Ablation of P/Q-type Ca(2+) channel currents, altered synaptic transmission, and progressive ataxia in mice lacking the alpha(1A)-subunit. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:15245–15250. doi: 10.1073/pnas.96.26.15245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopman G, Reutelingsperger CP, Kuijten GA, Keehnen RM, Pals ST, van Oers MH. Annexin V for flow cytometric detection of phosphatidylserine expression on B cells undergoing apoptosis. Blood. 1994;84:1415–1420. [PubMed] [Google Scholar]
- Kordasiewicz HB, Thompson RM, Clark HB, Gomez CM. C-termini of P/Q-type Ca2+ channel alpha1A subunits translocate to nuclei and promote polyglutamine-mediated toxicity. Human molecular genetics. 2006;15:1587–1599. doi: 10.1093/hmg/ddl080. [DOI] [PubMed] [Google Scholar]
- Kozel PJ, Friedman RA, Erway LC, Yamoah EN, Liu LH, Riddle T, Duffy JJ, Doetschman T, Miller ML, Cardell EL, et al. Balance and hearing deficits in mice with a null mutation in the gene encoding plasma membrane Ca2+-ATPase isoform 2. The Journal of biological chemistry. 1998;273:18693–18696. doi: 10.1074/jbc.273.30.18693. [DOI] [PubMed] [Google Scholar]
- Kubodera T, Yokota T, Ohwada K, Ishikawa K, Miura H, Matsuoka T, Mizusawa H. Proteolytic cleavage and cellular toxicity of the human alpha1A calcium channel in spinocerebellar ataxia type 6. Neuroscience letters. 2003;341:74–78. doi: 10.1016/s0304-3940(03)00156-3. [DOI] [PubMed] [Google Scholar]
- Kurnellas MP, Lee AK, Szczepanowski K, Elkabes S. Role of plasma membrane calcium ATPase isoform 2 in neuronal function in the cerebellum and spinal cord. Annals of the New York Academy of Sciences. 2007;1099:287–291. doi: 10.1196/annals.1387.025. [DOI] [PubMed] [Google Scholar]
- La Spada AR, Wilson EM, Lubahn DB, Harding AE, Fischbeck KH. Androgen receptor gene mutations in X-linked spinal and bulbar muscular atrophy. Nature. 1991;352:77–79. doi: 10.1038/352077a0. [DOI] [PubMed] [Google Scholar]
- Liu S, Friel DD. Impact of the leaner P/Q-type Ca2+ channel mutation on excitatory synaptic transmission in cerebellar Purkinje cells. The Journal of physiology. 2008;586:4501–4515. doi: 10.1113/jphysiol.2008.156232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marqueze-Pouey B, Martin-Moutot N, Sakkou-Norton M, Leveque C, Ji Y, Cornet V, Hsiao WL, Seagar M. Toxicity and endocytosis of spinocerebellar ataxia type 6 polyglutamine domains: role of myosin IIb. Traffic. 2008;9:1088–1100. doi: 10.1111/j.1600-0854.2008.00743.x. [DOI] [PubMed] [Google Scholar]
- Matsukawa H, Wolf AM, Matsushita S, Joho RH, Knopfel T. Motor dysfunction and altered synaptic transmission at the parallel fiber-Purkinje cell synapse in mice lacking potassium channels Kv3.1 and Kv3.3. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:7677–7684. doi: 10.1523/JNEUROSCI.23-20-07677.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita K, Wakamori M, Rhyu IJ, Arii T, Oda S, Mori Y, Imoto K. Bidirectional alterations in cerebellar synaptic transmission of tottering and rolling Ca2+ channel mutant mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22:4388–4398. doi: 10.1523/JNEUROSCI.22-11-04388.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintz IM, Sabatini BL, Regehr WG. Calcium control of transmitter release at a cerebellar synapse. Neuron. 1995;15:675–688. doi: 10.1016/0896-6273(95)90155-8. [DOI] [PubMed] [Google Scholar]
- Palhan VB, Chen S, Peng GH, Tjernberg A, Gamper AM, Fan Y, Chait BT, La Spada AR, Roeder RG. Polyglutamine-expanded ataxin-7 inhibits STAGA histone acetyltransferase activity to produce retinal degeneration. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:8472–8477. doi: 10.1073/pnas.0503505102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmenberg AC, Sgro JY. Topological organization of picornaviral genomes: Statistical prediction of RNA structural signals. Semin Virol. 1997;8:231–241. [Google Scholar]
- Rajakulendran S, Kaski D, Hanna MG. Neuronal P/Q-type calcium channel dysfunction in inherited disorders of the CNS. Nature reviews Neurology. 2012;8:86–96. doi: 10.1038/nrneurol.2011.228. [DOI] [PubMed] [Google Scholar]
- Rouault JP, Samarut C, Duret L, Tessa C, Samarut J, Magaud JP. Sequence analysis reveals that the BTG1 anti-proliferative gene is conserved throughout evolution in its coding and 3′ non-coding regions. Gene. 1993;129:303–306. doi: 10.1016/0378-1119(93)90284-a. [DOI] [PubMed] [Google Scholar]
- Saegusa H, Wakamori M, Matsuda Y, Wang J, Mori Y, Zong S, Tanabe T. Properties of human Cav2.1 channel with a spinocerebellar ataxia type 6 mutation expressed in Purkinje cells. Molecular and cellular neurosciences. 2007;34:261–270. doi: 10.1016/j.mcn.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Scott VE, Felix R, Arikkath J, Campbell KP. Evidence for a 95 kDa short form of the alpha1A subunit associated with the omega-conotoxin MVIIC receptor of the P/Q-type Ca2+ channels. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1998;18:641–647. doi: 10.1523/JNEUROSCI.18-02-00641.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sopher BL, Thomas PS, Jr., LaFevre-Bernt MA, Holm IE, Wilke SA, Ware CB, Jin LW, Libby RT, Ellerby LM, La Spada AR. Androgen receptor YAC transgenic mice recapitulate SBMA motor neuronopathy and implicate VEGF164 in the motor neuron degeneration. Neuron. 2004;41:687–699. doi: 10.1016/s0896-6273(04)00082-0. [DOI] [PubMed] [Google Scholar]
- Spriggs KA, Cobbold LC, Ridley SH, Coldwell M, Bottley A, Bushell M, Willis AE, Siddle K. The human insulin receptor mRNA contains a functional internal ribosome entry segment. Nucleic acids research. 2009;37:5881–5893. doi: 10.1093/nar/gkp623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spriggs KA, Stoneley M, Bushell M, Willis AE. Re-programming of translation following cell stress allows IRES-mediated translation to predominate. Biology of the cell / under the auspices of the European Cell Biology Organization. 2008;100:27–38. doi: 10.1042/BC20070098. [DOI] [PubMed] [Google Scholar]
- Stolze H, Klebe S, Petersen G, Raethjen J, Wenzelburger R, Witt K, Deuschl G. Typical features of cerebellar ataxic gait. Journal of neurology, neurosurgery, and psychiatry. 2002;73:310–312. doi: 10.1136/jnnp.73.3.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ul-Hussain M, Zoidl G, Klooster J, Kamermans M, Dermietzel R. IRES-mediated translation of the carboxy-terminal domain of the horizontal cell specific connexin Cx55.5 in vivo and in vitro. BMC molecular biology. 2008;9:52. doi: 10.1186/1471-2199-9-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Damme P, Van Hoecke A, Lambrechts D, Vanacker P, Bogaert E, van Swieten J, Carmeliet P, Van Den Bosch L, Robberecht W. Progranulin functions as a neurotrophic factor to regulate neurite outgrowth and enhance neuronal survival. The Journal of cell biology. 2008;181:37–41. doi: 10.1083/jcb.200712039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M, Kano M. Climbing fiber synapse elimination in cerebellar Purkinje cells. The European journal of neuroscience. 2011;34:1697–1710. doi: 10.1111/j.1460-9568.2011.07894.x. [DOI] [PubMed] [Google Scholar]
- Watase K, Barrett CF, Miyazaki T, Ishiguro T, Ishikawa K, Hu Y, Unno T, Sun Y, Kasai S, Watanabe M, et al. Spinocerebellar ataxia type 6 knockin mice develop a progressive neuronal dysfunction with age-dependent accumulation of mutant CaV2.1 channels. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:11987–11992. doi: 10.1073/pnas.0804350105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins MR, Gasteiger E, Bairoch A, Sanchez JC, Williams KL, Appel RD, Hochstrasser DF. Protein identification and analysis tools in the ExPASy server. Methods Mol Biol. 1999;112:531–552. doi: 10.1385/1-59259-584-7:531. [DOI] [PubMed] [Google Scholar]
- Wilson JE, Powell MJ, Hoover SE, Sarnow P. Naturally occurring dicistronic cricket paralysis virus RNA is regulated by two internal ribosome entry sites. Molecular and cellular biology. 2000;20:4990–4999. doi: 10.1128/mcb.20.14.4990-4999.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacharias DA, Kappen C. Developmental expression of the four plasma membrane calcium ATPase (Pmca) genes in the mouse. Biochimica et biophysica acta. 1999;1428:397–405. doi: 10.1016/s0304-4165(99)00058-6. [DOI] [PubMed] [Google Scholar]
- Zhuchenko O, Bailey J, Bonnen P, Ashizawa T, Stockton DW, Amos C, Dobyns WB, Subramony SH, Zoghbi HY, Lee CC. Autosomal dominant cerebellar ataxia (SCA6) associated with small polyglutamine expansions in the alpha 1A-voltage-dependent calcium channel. Nature genetics. 1997;15:62–69. doi: 10.1038/ng0197-62. [DOI] [PubMed] [Google Scholar]
- Zu T, Duvick LA, Kaytor MD, Berlinger MS, Zoghbi HY, Clark HB, Orr HT. Recovery from polyglutamine-induced neurodegeneration in conditional SCA1 transgenic mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:8853–8861. doi: 10.1523/JNEUROSCI.2978-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker RS, Regehr WG. Short-term synaptic plasticity. Annual review of physiology. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]
- Zuker M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic acids research. 2003;31:3406–3415. doi: 10.1093/nar/gkg595. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.