Abstract
Insufficient sleep over long durations of the lifespan is believed to adversely affect proper development and healthful aging, although how this might become manifested is unknown. In the present study, rats were repeatedly sleep restricted during 72 days to permit maladaptations to evolve, thereby permitting study. Densitometric and histomorphometric analyses were performed on harvested bone. In sleep-restricted rats, bone lined by osteoid was reduced 45-fold and osteoid thickness was decreased, compared with controls. This corresponded to a decrease in osteoblast number and activity. The percentage of bone lined by osteoclasts did not differ from that of controls. Plasma concentrations of an osteoclast marker (TRACP 5b) were increased in sleep-restricted rats, indicating increased bone resorption. The low amount of new bone formation without a reduction in bone resorption is diagnostic of osteopenia. Bone mineral density was decreased in femurs from sleep-restricted rats compared with control, indicating osteoporosis. Red marrow in sleep-restricted rats contained only 37% of the fat and more than twice the number of megakaryocytes compared to that of the control rats. These findings in marrow suggest changed plasticity and increased hematopoiesis. Plasma concentrations of insulin-like growth factor-1, a known, major mediator of osteoblast differentiation and the proliferation of progenitor cells, was decreased by 30% in sleep-restricted rats. Taken together, these findings suggest that chronically inadequate sleep affects bone metabolism and bone marrow composition in ways that have implications for development, aging, bone healing and repair, and blood cell differentiation.
Keywords: Partial sleep deprivation, sleep restriction, bone remodeling, osteopenia, osteoclasts, hematopoiesis, bone marrow adipose tissue, megakaryocytes, insulin-like growth factor-1
INTRODUCTION
Bone remodeling is a life-long process of bone tissue resorption by osteoclasts and new bone formation by osteoblasts. To remain normal, apposition of bone must balance the resorption of bone. This is critical for normal development and for repair following injuries, surgeries, and the micro-damage that occurs during normal activity. Inadequate sleep is widely viewed as a health threat that affects normal development and healthful aging, but specific effects on bone metabolism and bone marrow composition are unknown. Biochemical bone markers, such as osteocalcin, are known to display a diurnal pattern, suggesting increased bone apposition during sleeping hours. However, acutely disrupted sleep, which also disrupts the major growth hormone secretory period, does not alter the osteocalcin diurnal pattern.1 Wolff and Money reported in 1977 that patients with reversible somatotropin deficiency had a threefold higher growth rate during periods when sleep was considered good or restful compared with when sleep was considered poor or disrupted,2 but sleep otherwise was not quantified. However, growth hormone deficient children with short stature do not appreciably differ from controls in the amount or regulation of slow wave sleep.3 Epidemiological evidence points to comorbidity between insufficient sleep and osteoporosis, arthritis, diabetes, obesity, and lung disease,4 reflecting the insidious nature of pathologies related to lack of sleep, but not to specific outcomes on bone health. In our rodent studies, we noticed sleep deprivation resulted in a progressive increase in tissue nonspecific alkaline phosphatase (unpublished), which might indicate altered bone metabolism. Follow-up analysis to identify the likely source of this increased alkaline phosphatase revealed a progressive decline in serum osteocalcin (unpublished) which then led us to investigate bone formation processes directly.
The longitudinal design of the present study employed recurrent sleep loss over a large proportion of early adulthood in the rodent. The logic behind this approach is that recurrent exposure to deficient basic needs, such as food, water, air, warmth, and sleep produce adaptations and maladaptations that do not become manifested during acute deficiencies and which may result in pathology later in life. Eight rats were exposed six times to a 10-day period of reduced and disrupted sleep. Between each sleep restriction cycle was a 2-day period of sleep ad libitum that was intended to be a time of sleep rebound and restoration. This 72-day period of chronic sleep-restriction constituted 10–12% of the rat lifespan. Control conditions, in another group of ten rats, consisted of the same ambulation requirements used to disrupt sleep in sleep-restricted rats, only consolidated to permit undisturbed bouts of sleep. We previously reported that the sleep-restricted subjects became hyperphagic and lost body weight without malabsorption of calories.5 We also reported that, despite calorie deficiency indicated by weight loss, protein and lipid amounts in internal organs were largely spared, while adipose tissue depots appeared nearly depleted.5, 6 To evaluate the effects of chronic sleep restriction on bone health, harvested bones were analyzed for bone mineral density, apposition, and resorption. Changes in marrow fat content and megakaryocyte number were also observed, and therefore these variables were quantified in all subjects.
METHODS
Animals and experimental conditions
Protocols for animal care and use were approved by institutional animal care and use committees at The Medical College of Wisconsin and the Zablocki Veterans Administration Medical Center (2560-02 and -04). Eighteen Sprague-Dawley rats (Harlan, Madison, WI) were 27.6 (1.0 SD) wk old and 448 (35 SD) g in weight at the start of the study. Surgery was performed to implant macro electrodes for recording electroencephalographic signals for the purpose of quantifying wakefulness and specific sleep stages.5 The rats were housed under conditions of constant ambient room light (to minimize circadian rhythm amplitude and phase differences between the treatment conditions) and ambient temperature of 26.9 (1.1 SD)°C, within the thermoneutral zone for rats.7 Rats were fed ad libitum a purified diet (modified AIN-76A; Zeigler Brothers, Garners, PA) composed of 20% protein, 45% carbohydrate, 13% fat, 10% fiber, 3.5% minerals, and 1% vitamins by weight, with 0.26% sodium cholate to increase atherogenic properties for planned analyses of the cardiovascular system. The caloric density of this diet was the same as normal laboratory chow at 3.7 kcal/g with 12% of calories from fat.
Procedure for producing repeated cycles of sleep restriction and ambulation control conditions
The Bergmann-Rechtschaffen apparatus, illustrated elsewhere,8 consisted of a large round platform (45 cm diameter) that could be rotated and which was divided in half by a high Plexiglas wall. Two rats were housed in the apparatus on the platform, one on each side of the Plexiglas wall. The floor area on each side of the platform was sufficient to permit a rat to eat, groom, explore, and lie down fully to sleep. Beneath each side of the platform was a pan of shallow water approximately 2 cm deep that encouraged the rat to stay on the platform. The perimeter of the platform and pans was enclosed by high Plexiglas walls, open at the top. A slow (3.3 rpm) and short (6-s) rotation of the housing platform induced each rat to move because the rat was displaced, typically from a comfortable spot at the widest radius of the platform to a narrow spot closer to the shallow water. The experimental schedule consisted of platform rotations once per hour during a 7-day baseline period and then according to a schedule validated for sleep disruption and reduction during the experimental period.5 The schedule was derived from electronic data capture from previous experiments that had reliably produced highly fragmented and reduced amounts of sleep under acute conditions.9, 10 These pattern files were concatenated into a master disk rotation program. Disk rotations each lasted 6 s and composed a daily average of 26% of total time. The efficacy of the programmed rotation was verified by electroencephalographic recordings matched to behavior, as previously reported.5 Each rat was free to step down into, sit, and walk around in the pans; however, they almost always remained on the platform after initial exploration; this was presumably due to habituation during the baseline period. Each of the 10-day periods of sleep restriction was followed by a 2-day period of ad libitum conditions except for once-hourly, 6-s disk rotations. The ambulation requirements were matched in control rats (ambulation controls), except that the platform rotations were consolidated to permit lengthy opportunities to obtain uninterrupted sleep.5 Ambulation control rats otherwise were maintained under the same experimental conditions of repeated exposure to 10-day periods of control ambulation requirements, consisting of a 90-min period, during which the platform was rotated for 150 s and then was stationary for 30 s, followed by 198 min without platform rotations. Identical to the sleep-restriction schedule, each 10-day period was separated by 2-day periods of ad libitum conditions during 72 days. The 10-day periods of sleep restriction were characterized by highly disrupted sleep. During any given 10-day period, the amount of non-rapid eye movement (NREM) sleep was reduced by 26–37%, and the amount of paradoxical sleep (PS; a.k.a., rapid eye movement sleep, REM sleep) was reduced 53–67% from baseline. The 10-day periods of ambulation control conditions were characterized by consolidated sleep. The amount of NREM sleep in ambulation controls was reduced by 13–16%, and the amount of PS was unchanged from baseline.5 The 2-day sleep ad libitum periods after sleep restriction were marked by a predominant rebound of PS to 150–167% of baseline, while there were no changes in NREM sleep or PS from baseline after ambulation control conditions.5
Bone harvesting and processing
After the planned duration of study, each rat was gently and deeply anesthetized and exsanguinated by cardiac puncture. Plasma specimens and tissues were harvested as previously described.5 Femurs typically were dissected below the third trochanter with intact condyles. Tibias typically were dissected above the medial malleolus, again with intact condyles. Bone specimens were dissected free of soft tissue and fixed in 10% neutral buffered formalin. A set of tibia and femurs was transferred to 70% EtOH for storage. Another set was rinsed free of formalin, scraped clean of fascia, imaged by x-ray, decalcified in EDTA, and embedded in paraffin. Tibias were studied for the length of bone, the thickness of the growth plate and cortical bone, the number and thicknesses of trabeculae, and the number of megakaryocytes. Femurs were studied for the amount of bone lined by osteoid, the number and activity of osteoblasts, the amount of bone lined by osteoclasts, the percentage of marrow fat, and bone mineral density.
Bone mineral density
Bone mineral density (BMD in g/cm2) measurements on calcified bone were obtained by dual energy x-ray absorptiometry, according to established methodology (DEXA; GE Lunar PIXImus).
Bone length
The lengths of tibia having intact articular surfaces were measured from standard x-ray films from the most anterior condyles to the most posterior malleolus.
Bone histomorphometry
Paraffin-embedded femurs and tibias were sectioned longitudinally in 4–6-µm thickness, and stained with the following: Hematoxylin and Eosin, Masson’s Trichrome, Mallory Aniline Blue connective tissue stain, or tartrate-resistant acid phosphatase (TRAP). Slides were coded to keep the examiner blind to the experimental conditions. Histomorphometry was completed by brightfield microscopy (Olympus BX51 microscope and DP71 camera, Image Pro Plus image analysis software; Media cybernetics, Bethesda, MD).
Thicknesses of the growth plate, trabeculae, and cortical bone
The thickness of the superior growth plate of the tibia was measured at 12 sites located at 1/3 and 2/3 of the side-toside width of the growth plate on each of 6 micrographs at 100×. Measurement started at the top of the proliferating zone of chondrocytes and extended to the bottom of the hypertrophic zone of enlarged chondrocytes along the columnar organization of the chondrocytes. To measure the number and thicknesses of trabeculae and cortical bone, a line was drawn on each microscope slide perpendicular to the cortical bone at a distance of 1 mm from the longest extension of the growth plate into the metaphysis. The thicknesses of bone structures were measured at the line crossings. Cortical bone thickness additionally was measured in the narrowest part of the midshaft of the tibia.
Bone apposition
Osteoid thickness and length of bone perimeter covered were measured in the primary center of ossification in the femur of sections stained with Masson’s Trichrome. Representative micrographs are shown in Figure 1. The measurement region was bordered laterally by the cortex in a 2 mm band of interest in the metaphysis, starting 0.5 mm from the longest outcrop of chondrocytes. The thickness of the osteoid was measured at two equidistant points in each adjacent digital image at 400× that together covered the entire region of interest.
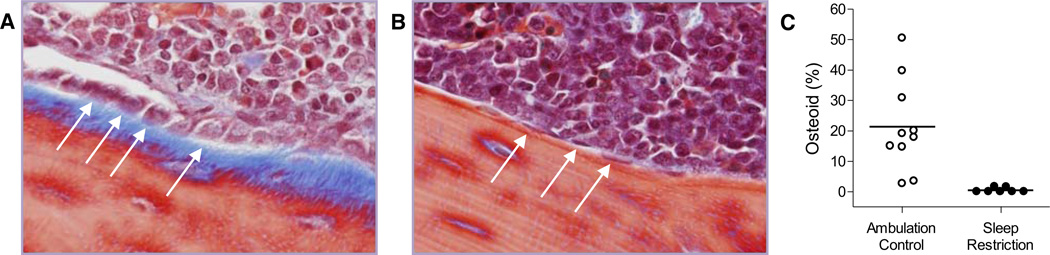
Fig. 1. Bone formation is greatly diminished in sleep-restricted rats.
Representative micrograph (400×) of metaphyseal femur stained with Masson’s Trichrome in an ambulation control (A) and a sleep-restricted (B) rat. Osteoid is stained blue. Osteoblasts are basophilic bone-lining cells, shown here as either plump (A), considered activated, or flattened (B), considered quiescent. A subset of osteoblasts is designated by arrows. The proportion of metaphyseal bone perimeter lined by osteoid in individual ambulation control (○) and sleep-restricted (●) rats is shown by dot plot (C). Group averages are indicated by a horizontal bar. The group comparison is statistically significant at P = 0.002.
Osteoblast number and activity
The proportion of bone bordered by osteoblasts was measured in the same region as was bone apposition. Individual osteoblasts were counted, and the length and height of each was measured to calculate the ellipsoid surface area. Osteoblast activity was considered the product of osteoblast number and ellipsoid area per specimen.
Osteoclast activity
The proportion of cortical and trabecular bone perimeter lined by osteoclasts was measured at 400× in a 2-mm-wide frame in the metaphysis of the distal portion of the femur, as defined above. Osteoclast identification was facilitated by a pale mauve tint imparted by the TRAP stain, as shown by a representative micrograph in Figure 2.
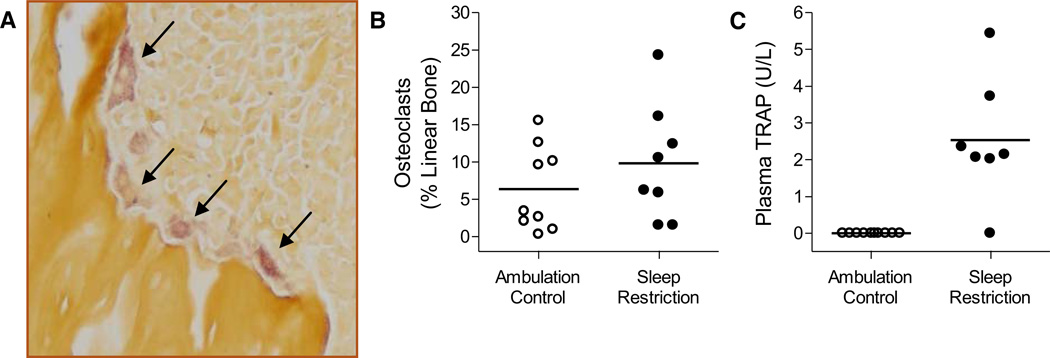
Fig. 2. Osteoclast activity is not diminished in sleep-restricted rats.
Representative micrograph (A, 200×) of metaphyseal trabeculae stained with tartrate-resistant acid phosphatase. Osteoclasts are the mauve-tinted, amorphous cells with a foamy appearance typically found in resorption pits, indicated by arrows. The proportion of metaphyseal bone perimeter lined by osteoclasts (B) and the plasma values for TRACP 5b (C) in individual ambulation control (○) and sleep-restricted (●) rats are shown in dot plots. Group averages are indicated by a horizontal bar. The morphometric measurement of osteoclasts (B) was not statistically significant, while the biochemical measurement of TRACP 5b (C) was significant at P = 0.007.
Plasma markers of bone metabolism
Bone-resorbing osteoclasts express a high amount of TRAP form 5b (TRACP 5b), and its amount in the circulation correlates with osteoclast number and activity, with little diurnal variability.11, 12 TRACP 5b was measured in EDTA-treated plasma, processed and stored at −80°C at the time of cardiac puncture during tissue harvest procedures. The determinations were made by using a solid-phase immunofixed enzyme activity assay (IDS Immunodiagnostic Systems, Forest Hills, AZ). The range of detection was 0.47 to 10 U/l and the coefficient of variation (CV) was <5%. Insulin-like growth factor-I (IGF-1) was measured post hoc as marker of bone formation instead of osteocalcin, which is unstable and subject to divergent results due, in part, to multiple osteocalcin peptides and biological variability.13 Determinations of IGF-1 in stored EDTA-treated plasma were made by immunoenzymometric assay (Immunodiagnostics, Fountain Hills, AZ). The range of detection was 222 to 3881 ng/ml and the CV was <5%.
Marrow fat and megakaryocyte number
The proportion of marrow containing fat was determined in the distal portion of the femur in an area of 0.56 mm2, at a distance of not more than 1 mm from the longest outcrop of the growth plate in the metaphysis. Fat cells were identified by well-circumscribed, rounded vacuoles. Irregular voids and trabeculae were excluded from the measurement area. The size of fat cells was determined by measuring the area of each of 100 cells, except in two specimens in which fewer than 100 cells were present. Marrow was insufficient in one specimen in the intended region of measurement and therefore fat cells were measured further into the diaphysis.
Megakaryocytes were identified morphologically by their large size, lobulated nucleus and basophilic cytoplasm. Megakaryocyte number was counted in H&E-stained slides at 200× within a region starting 1 mm from the longest outcrop of the growth plate and extending 325 µm into the metaphysis between the lateral sides of compact bone of the upper extremity of the tibia.
Data analysis
Data were compared by means of Welch’s t-tests that do not assume equal variances. The family-wise type I error was set at P < 0.05 for all comparisons. Holm’s adjustment was applied to correct for multiple comparisons, in which each null hypothesis is tested for rejection according to the sequential decreasing strength of ordered P values. Values are means ± standard deviation (SD). P values considered nonsignificant after the Holm’s adjustment are designated, NS.
RESULTS
Bone apposition, resorption, and density
Sleep restriction resulted in a dramatically decreased bone formation compared with ambulation control conditions. The proportion of bone lined by osteoid in the primary center of ossification (within the diaphysis) was 21.4% [15.1% SD] in ambulation control rats and only 0.5% (0.8% SD) in sleep-restricted rats, a 45-fold difference (P < 0.002). The decrease in osteoid in sleep-restricted rats corresponded with few osteoblasts in the measured region (ambulation control 28.3 [18.4 SD] cells; sleep restricted: 0.4 [0.8 SD] cells, P = 0.001), and 10-fold lower osteoblast activity compared with that in ambulation controls (P = 0.002). The individual values for the proportion of bone lined by osteoid are provided in Figure 1 with a micrograph showing the reduced distribution of osteoid and diminished osteoblast activity in a sleep-restricted rat compared to that of an ambulation control rat. Altered bone metabolism observed in histology was further manifested as a statistically significant reduction in bone mineral density in sleep-restricted rats. Values for these and other histomorphometric measurements are shown in Table 1. IGF-1 values reflected the indices of decreased bone formation. IGF-1 averaged 881 (85 SD) ng/ml in ambulation controls, compared with 617 (157 SD) ng/ml in sleep-restricted rats (P < 0.004).
Table 1.
Bone mineral density and bone measurements in chronically sleep-restricted and ambulation control rats.
| Bone Indicesa | Ambulation Control |
Chronic Sleep Restriction |
P Value* | |
|---|---|---|---|---|
| Osteoid thickness† | 4.0 ± 1.6 | 0.6 ± 1.1 | 0.0001* | |
| Bone Mineral Density (g/cm2) | 0.213 ± 0.009 | 0.189 ± 0.010 | 0.0004* | |
| Osteoid (% bone perimeter)† | 21.4 ± 15.1 | 0.5 ± 0.8 | 0.002* | |
| Osteoblasts (number)† | 28.3 ± 18.4 | 0.4 ± 0.8 | 0.001* | |
| Osteoblast activity (µm3)† | 1033 ± 766 | 11 ± 23 | 0.002* | |
| Growth plate thickness (µm) | 130 ± 9 | 114 ± 15 | 0.055 | |
| Perimeter of metaphyseal bone (µm) | 10594 ± 4248 | 7069 ± 3702 | 0.091 | |
| Tibia length (mm) | 44.5 ± 0.7 | 43.8 ± 0.4 | 0.159 | |
| Cortical bone thickness (µm) | ||||
| Near growth plate | 339 ± 72 | 308 ± 38 | 0.354 | |
| In the diaphysis | 391 ± 35 | 358 ± 62 | 0.280 | |
| Resorption surface (osteoclasts;% of bone perimeter) | 6.4 ± 5.6 | 9.8 ± 7.8 | 0.317 | |
Values are means ± standard deviation.
N = 6–10 rats per group after unsatisfactory specimens were excluded, except for tibia length for which N = 3–5.
Statistical significance after Holm’s correction for multiple comparisons.
Includes zero values.
Osteoclasts lined 6.4 [5.6 SD]% of cortical and trabecular bone in ambulation controls and 9.8 [7.8 SD]% in sleep-restricted rats (NS). The individual variability in the amount of bone lined by osteoclasts is shown in Figure 2, along with that of plasma TRACP 5b, which was detected in 6 of 7 sleep-restricted rats but none of the ambulation controls (P = 0.007). The percentage of bone lined by osteoclasts and the highly significant amount of TRACP 5b measured in plasma were not correlated.
Bone marrow fat cell and megakaryocyte number
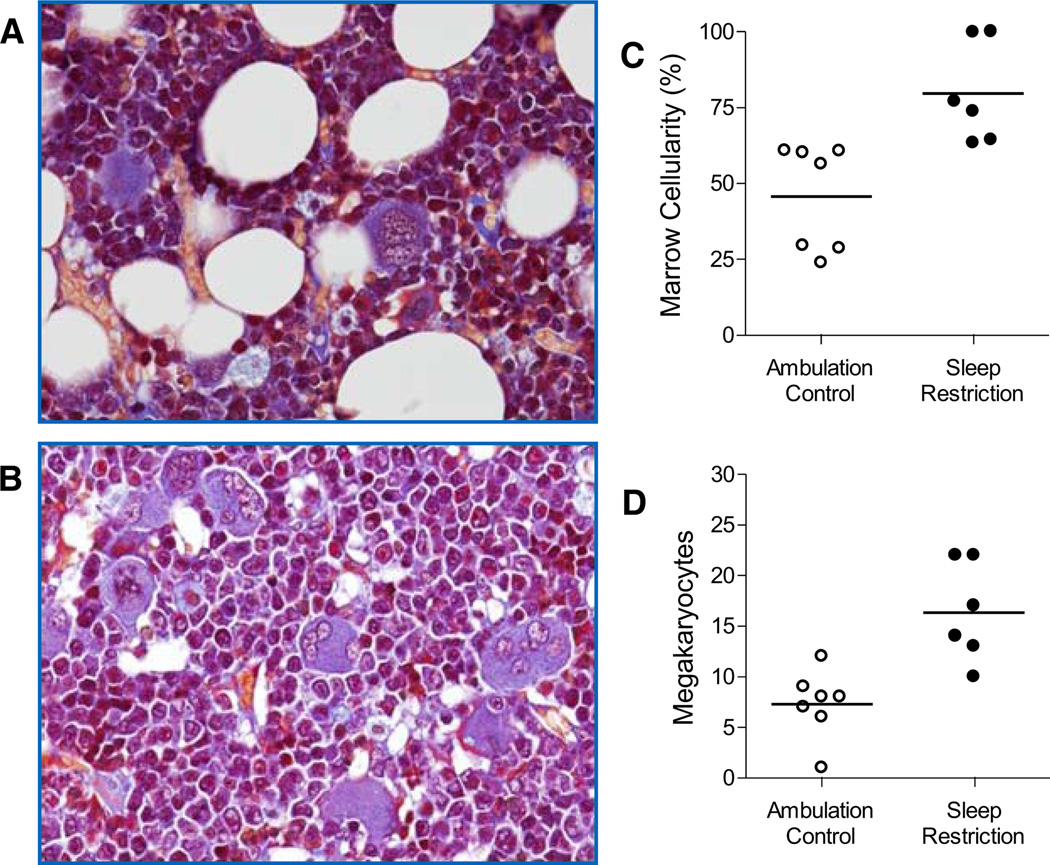
As can be seen in Figure 3, megakaryocyte number was doubled in the marrow of sleep-restricted rats compared with that of ambulation controls (P = 0.004). Also shown in Figure 3, the proportion of red marrow comprised of fat cells was decreased by 63% in sleep-restricted rats compared with that of ambulation controls (P = 0.004). The average sizes of fat cell vacuoles were not significantly different between groups.
Fig. 3. Sleep restriction increased the numbers of megakaryocytes and decreased the volume of adipose in red marrow.
Micrographs (400×) indicate fat cells and megakaryocytes composition in red marrow in ambulation control (A) and sleep-restricted (B) rat. Fat cells are denoted by well-circumscribed vacuoles. Megakaryocytes are identified by their large size, large lobulated nucleus and basophilic cytoplasm. The marrow cellularity (C) and the number of megakaryocytes (D) in individual ambulation control (○) and sleep-restricted (●) rats are shown in dot plots. Group averages are indicated by a horizontal bar. Both group comparison were each statistically significant at P = 0.004.
DISCUSSION
Bone apposition in sleep-restricted rats was marked by greatly diminished amounts of osteoid in association with a dramatic decrease in osteoblast number and activity. Bone resorption appeared undiminished, indicated by amounts of bone lined by osteoclasts that did not differ from controls and by detection of the biochemical marker, TRACP 5b, in the plasma of only the sleep-restricted rats, indicating increased osteoclast activity. The decrease in new bone formation without a decrease in bone resorption is diagnostic of osteopenia. Moreover, bone mineral density in the femurs was decreased in sleep-restricted rats, indicating osteoporosis. Other histomorphometric determinations of bone formation, such as growth plate or cortical bone thicknesses, were not statistically significantly different in sleep-restricted rats compared with controls. The statistically significant findings demonstrate altered cancellous bone remodeling and bone volume controlled by intramembranous ossification, rather than changes in endochondral ossification at the growth plate, as indicated by nonsignificant group differences in growth plate thickness and tibia length (see Table 1). The overall trend of the average values was lower in the sleep-restricted rats, except for the amount of bone lined by osteoclasts. In the marrow, metaphyseal fat in sleep-restricted rats was substantially decreased. Decreased marrow fat would be expected to affect adaptive plasticity because of its secretion of a spectrum of hormones, cytokines, and growth factors important in the stromal and hematopoietic microenvironment.14 The decrease in marrow fat and a doubling of megakaryocytes in the marrow of sleep-restricted rats indicates increased hematopoiesis.
We previously reported that these same chronically sleep-restricted rats developed a progressive negative energy balance, manifested by 2–3-fold increases in food intake and a 15% loss of body weight.5 We established that these animals were neither dehydrated nor suffering from malabsorption of calories. On one hand, this deep negative energy balance may provide a parsimonious explanation for arrested bone formation. This is the case for the lactating rat that has zero apposition of bone and high demand for minerals,15 despite a 3-fold increase in food consumption.16 On the other hand, many physical signs of sleep restriction do not resemble primary calorie deficiency, suggesting that a unidirectional effect of increased energy expenditure on bone cannot be assumed a priori. As a primary example, most vital organ masses in sleep-restricted rats are increased relative to body mass instead of markedly decreased to compensate for the negative energy balance. Protein and lipid content in the vital organs are largely spared.6 In contrast, food restriction resulting in a 17.5% weight loss, comparable to that observed in sleep-restricted rats, is associated with severe organ weight losses; such as 60% in the liver and 55% in the small intestine.17 Not only are the effects of calorie restriction opposite to those of sleep restriction with regard to vital organ masses, but mild calorie restriction alone has been shown to have a beneficial effect on metaphyseal strength, as determined in sedentary, aging rats.18 Comparisons of the sleep-deprived state with other examples of hypermetabolism also do not provide enough similarities to conclude that energy expenditure during sleep restriction has only a unidirectional effect of on bone formation. For example, hypermetabolism induced by thyroid hormone administration results not only in increased osteoclast surface areas and bone loss, but in increased osteoid and osteoblast surface areas.19 Chronic cold exposure results in stunting of growth, but this is manifested by shorter, thicker bones due to osteoblast proliferation in the periosteum and resulting in intramembranous growth.20 Therefore, energy deficiency during sleep loss might mirror not only the demands of cellular functions of visceral organs, but perhaps also those of the bone and the bone marrow, as suggested by increased hematopoiesis, discussed below. Current views on bone and energy metabolism stress their reciprocal interactions.21, 22
Circadian rhythm disruption, which is a well-known consequence of sleep deprivation, would be expected to contribute to poor bone health. Sleep disruption results in a decrease in the amplitude, and possibly the phase, of the circadian rhythm.23, 24 To balance these influences in the present study, the ambulation control rats were housed under the same constant, normal ambient room illumination as were sleep-restricted rats; a condition well known to decrease circadian rhythm amplitude and entrainment.25 Furthermore, the schedule of platform rotations under the ambulation control treatment was a constant routine and would not have provided diurnal clues for entrainment. With these comparables in place, the present outcomes suggest that sleep restriction, and the physiological disruption it entails, is the principle state responsible for bone and marrow pathology, rather than independent effects of circadian rhythm alterations.
Physical activity is another factor expected to contribute to bone health. While the forced ambulation requirements were matched for sleep-restricted and ambulation control rats, differences in physical activity incidental to the disk rotation schedules or to treatment-induced changes in voluntary ambulation are conceivable. However, it is limb disuse that is associated with decreased bone formation, whereas weight bearing physical activity nearly always is considered beneficial to bone formation. Even strenuous dynamic loading promotes bone apposition and higher bone mineral density.26, 27 Static components of mechanical loading have been shown to suppress appositional growth in growing rats, but not in a model of skeletal maturity28, and perhaps not if there also is exposure to dynamic loading.27 Furthermore, suppression of appositional growth by static loading appears limited to the distal growth plate, which becomes thicker,27 instead of tending toward thinner as in the sleep-restricted rats. Therefore, without other changes in sensitivity of the skeleton to loading induced by sleep loss, the bones of sleep-restricted rats should have had the capacity to adapt to meet the mechanical loading requirements of these experiments.
The diminished lipid in the marrow of sleep-restricted rats is not likely due to either the weight loss or the calorie deficiency, but to altered differentiation of common progenitor cells. Bone marrow adipose stores are regulated differently from peripheral sites.29 For example, Bathija et al. showed that marrow fat cell volume remained essentially unchanged from control values in rabbits starved for 21 days to a weight loss of 34%.30 Both marrow fat cells and osteoblasts are derived from the mesenchymal stem cells. There generally exists a competitive balance between osteoblast activity and fat cell volume because stromal cell differentiation is regulated in a reciprocal manner,29 as reviewed elsewhere.14, 31, 32 This relationship is manifested clinically in both diabetes and aging, which are marked by decreased bone formation and increased bone marrow fat.29, 33 The reductions in both osteoblast number and marrow fat volume under conditions of sleep restriction imply upstream effects on stimulating factors for stromal cell differentiation.
Marrow fat is functionally related to hematopoiesis. Red marrow fat readily gives way to hematopoietic expansion during an increased demand to produce more blood cells.34 All hematopoietic cells arise from the same multipotent hematopoietic stem cells that then become differentiated into erythrocytes, granulocytes, megakaryocytes, and lymphocytes. While this initial survey of bone and marrow in sleep-restricted rats does not permit us to discern the exact profile of hematopoietic changes, two components were revealed. First, 2-fold more megakaryocytes were counted in the marrow of sleep-restricted rats compared with those of controls; this implies a change to the commitment of megakaryocyte-erythroid progenitor cells. Second, the prominence of osteoclasts in sleep-restricted rat bones implies changes in hematopoietic factors that guide the localization and recruitment of osteoclast precursors to sites for bone resorption. Other indirect evidence of increased hematopoiesis during sleep loss includes long-standing findings of mild leukocytosis in both sleep-deprived humans and laboratory rats.35 This leukocytosis has been shown in rats to result in phagocyte migration into tissues, which is an inflammatory response, as opposed to a “fight or flight” or corticosterone-mediated neutrophilia.10 Prior studies also have revealed a mild increase in the number of immature erythrocytes in the bloodstream of rats under total sleep deprivation conditions in association with anemia,36 which suggests increased red blood cell destruction potentially exists. Other evidence of changes to hematopoietic stem cells during sleep loss is limited. Rolls et al. have reported that a short, acute period of sleep deprivation in mice decreases the migration of hematopoietic stem cells from marrow to blood.37 Guaniniello et al. reported that sleep restriction in mice decreases hematopoietic stem/progenitor cells and colony-forming units in the marrow.38 The present results provide evidence of hematopoietic expansion, which implies marked changes to hematopoietic stem cell differentiation, and that this is consistent with evidence of cell damage and inflammatory processes.
The potential functional significance of increased megakaryocyte production during sleep loss is at least two-fold: cell engulfment and platelet production. Megakaryocytes may engulf other hematopoietic cells and debris by the process of emperipolesis, which does not damage the engulfed cells which then travel to the blood circulation within the megakaryocyte. Megakaryocte emperipolesis of cells such as granulocytes is observed under conditions such as neoplasms, irradiation, and blood loss, during high demand for cell delivery to the circulation. This prediction would be consistent with signs that sleep deprivation is a state of cell injury and inflammatory responses.9, 10 Platelet production by megakaryocytes is a highly coordinated process that involves pseudopodial projections of their cytoplasm through endothelial cells and delivery of platelet precursors into the marrow sinusoids. In night shift workers, there are reports of increases in platelets, as well as circulating granulocytes.39 Scheer et al. (2011) have demonstrated a strong circadian component to the appearance of platelet activation markers in blood specimens associated with the morning hours in humans, which is a time that coincides with a peak in adverse cardiovascular events.40 Megakaryocyte numbers in the present data reflect a change in hematopoiesis that may reflect demands for cell delivery to the circulation and the promotion of thrombocytosis.
Bone metabolism and hematopoiesis are considered largely under the control of hormonal influences. Increased circulating corticosterone or cortisol in rats and humans, respectively, are associated with low bone formation.41, 42 However, plasma corticosterone was decreased in these sleep-restricted rats,6 indicating that it is not the candidate mediator for either low bone formation or hypercatabolism. Comparably, corticosterone has been found unchanged in rats acutely sleep restricted under the Bergmann-Rechtschaffen paradigm, on which the present method is based,10, 43–45 Either unchanged or decreased cortisol has been found in nearly every human study of sleep restriction, as reviewed previously.6 Minor increases in cortisol concentrations have sometimes been detected in humans, but only at certain times of day.46 Findings of increased corticosterone in association with sleep disruption in other rodent models may be methodology based.47, 48
The present results point to IGF-1 as one strong candidate mechanism that links abnormal bone turnover and hematopoiesis in the sleep-restricted state. Systemic IGF-1 was significantly decreased by chronic sleep-restriction in the present study, consistent with the close association of low IGF-1 and diminished bone mass in clinical conditions.49 IGF-1 is a principal mediator of skeletal growth that stimulates osteoblast differentiation and plays important autocrine, paracrine, and/or endocrine roles in the proliferation and differentiation of progenitor cells.49, 50 IGF-1 has both stimulatory and inhibitory effects on a large variety of parameters involved in bone turnover and hematopoiesis, and mediates most of the anabolic and growth-promoting actions of growth hormone.51 The IGF-1 levels in sleep-restricted rats is consistent with the emerging profile of chronic inflammatory disease, which is known to lead to decreased IGF-1 production.52, 53 Another candidate hormone, leptin, is also low in these sleep-restricted rats.5 This decrease is consistent with hyperphagia and loss of adipose tissue, from which it is produced, but inconsistent with the increase in bone mass that occurs in leptin-deficient mice if they are hyperphagic.54 Other hormones of pituitary, thyroidal, and gonadal origin also participate in bone and marrow health and their roles in abnormal bone and marrow during sleep restriction are yet to be evaluated.
The laboratory rat is the most common model for evaluation of developmental and osteoporotic processes in humans because of comparable histomorphometric changes, biochemical markers, and methodology for bone densitometry.55, 56 Implications of sleep loss on bone turnover and marrow hematopoiesis are potentially far-reaching. If the same phenomenon occurs in humans, chronic sleep restriction could cause osteopenic bone leading to increased fracture risk, decreased bone healing after surgery, and decreased bone development resulting in short stature. Decreased bone formation would be expected to alter healthful aging and exacerbate abnormalities in bone due to endocrine, nutritional, hematologic, and metabolic disorders. Changed functional demands on mesenchymal and hematopoietic stem cell lineages and cell differentiation would be expected to affect disease predisposition and disease resistance. For instance, alterations in regulation of the bone marrow microenvironment due to sleep disruption would be expected to affect the success of cell transplantation, which has been an expressed therapeutic concern.57 Changes to regulatory factors and progenitor cells would be expected to be influential in regenerative medicine and in outcomes of prevention and interventional therapies. Hematopoietic compensation during sleep loss may point to one of the properties that account for sleep restoration, which still is ill-defined. Future studies will be needed to determine the extent to which the effects of chronic lack of sleep on bone formation and hematopoeisis can be generalized beyond adult male rats to females, and beyond adulthood to development, aging, and declines in reproductive hormones. The extent to which there are long-term effects on bone and marrow health after lengthy recuperation is not yet known, but enduring effects are expected. This is because chronic sleep restriction results in persistent abnormalities in energy expenditure, nutrient intake, and leptin concentrations, suggesting a potential lifetime burden of physiological effects.6
Acknowledgements
We express our appreciation to Amy Rizzo MT (ASCP), Nicholas Kampa, and Christopher Henchen for technical assistance; to Patsy Ruegg and IHCtech at the University of Colorado Health Sciences Center for staining of paraffin-embedded decalcified bone with TRAP and Masson’s Trichrome; and to Aniko Szabo in the Department of Population Health at the Medical College of Wisconsin, for consultation on statistical procedures. Facilities were provided by the Medical College of Wisconsin and the Department of Veteran Affairs.
Research support was provided by the National Heart, Lung and Blood Institute, Grants HL-086447 and HL-080744.
Footnotes
Statement of Author Contributions
All authors participated in the design, interpretation of the studies and analysis of the data and review of the manuscript: CAE and JMT designed the studies; CAE and AEF carried out the studies and assembled the data; CAE, AEF, and JMT interpreted the results; CE prepared the manuscript, and AEF and JMT edited the manuscript.
A portion of this work was presented at a joint meeting of the American Academy of Sleep Medicine and the Sleep Research Society in 2011, and to the Marquette University School of Dentistry in 2011.
Contributor Information
Carol A. Everson, Email: ceverson@mcw.edu.
Anne E. Folley, Email: afolley@gwmail.gwu.edu.
Jeffrey M. Toth, Email: jtoth@mcw.edu.
References
- 1.Nielsen HK, Brixen K, Kassem M, Christensen SE, Mosekilde L. Diurnal rhythm in serum osteocalcin: relation with sleep, growth hormone, and PTH(1–84) Calcif Tissue Int. 1991;49:373–377. doi: 10.1007/BF02555845. [DOI] [PubMed] [Google Scholar]
- 2.Wolff G, Money J. Relationship between sleep and growth in patients with reversible somatotropin deficiency (psychosocial dwarfism) Psychol Med. 1973;3:18–27. doi: 10.1017/s0033291700046316. [DOI] [PubMed] [Google Scholar]
- 3.Orr WC, Vogel GW, Stahl ML, Griffiths WJ, Seely JR. Sleep patterns in growth hormone deficient children and age-matched controls: developmental considerations. Neuroendocrinology. 1977;24:347–352. doi: 10.1159/000122721. [DOI] [PubMed] [Google Scholar]
- 4.Foley D, Ancoli-Israel S, Britz P, Walsh J. Sleep disturbances and chronic disease in older adults: results of the 2003 National Sleep Foundation Sleep in America Survey. J Psychosom Res. 2004;56:497–502. doi: 10.1016/j.jpsychores.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 5.Everson CA, Szabo A. Recurrent restriction of sleep and inadequate recuperation induce both adaptive changes and pathological outcomes. Am J Physiol Regul Integr Comp Physiol. 2009;297:1430–1440. doi: 10.1152/ajpregu.00230.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Everson CA, Szabo A. Repeated exposure to severely limited sleep results in distinctive and persistent physiological imbalances in rats. PLoS ONE. 2011;6:e22987. doi: 10.1371/journal.pone.0022987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Szymusiak R, Satinoff E. Maximal REM sleep time defines a narrower thermoneutral zone than does minimal metabolic rate. Physiol Behav. 1981;26:687–690. doi: 10.1016/0031-9384(81)90145-1. [DOI] [PubMed] [Google Scholar]
- 8.Everson CA, Wehr TA. Nutritional and metabolic adaptations to prolonged sleep deprivation in the rat. Am J Physiol Regul Integr Comp Physiol. 1993;264:376–387. doi: 10.1152/ajpregu.1993.264.2.R376. [DOI] [PubMed] [Google Scholar]
- 9.Everson CA, Laatsch CD, Hogg N. Antioxidant defense responses to sleep loss and sleep recovery. Am J Physiol Regul Integr Comp Physiol. 2004;288:374–383. doi: 10.1152/ajpregu.00565.2004. [DOI] [PubMed] [Google Scholar]
- 10.Everson CA, Thalacker CD, Hogg N. Phagocyte migration and cellular stress induced in liver, lung, and intestine during sleep loss and sleep recovery. Am J Physiol Regul Integr Comp Physiol. 2008;295:2067–2074. doi: 10.1152/ajpregu.90623.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Halleen JM, Alatalo SL, Suominen H, Cheng S, Janckila AJ, Vaananen HK. Tartrate-resistant acid phosphatase 5b: a novel serum marker of bone resorption. J Bone Miner Res. 2000;15:1337–1345. doi: 10.1359/jbmr.2000.15.7.1337. [DOI] [PubMed] [Google Scholar]
- 12.Rissanen JP, Suominen MI, Peng Z, Halleen JM. Secreted tartrate-resistant acid phosphatase 5b is a marker of osteoclast number in human osteoclast cultures and the rat ovariectomy model. Calcif Tissue Int. 2008;82:108–115. doi: 10.1007/s00223-007-9091-4. [DOI] [PubMed] [Google Scholar]
- 13.Naylor KE, Eastell R. Measurement of biochemical markers of bone formation. In: Seibel MJ, Robins SP, Bilezikian JP, editors. Dynamics of Bone and Cartilage Metabolism. New York: Academic Press; 1999. pp. 401–426. [Google Scholar]
- 14.Laharrague P, Casteilla L. Bone marrow adipose tissue. In: Fantuzzi G, Mazzone T, editors. Nutrition and Health: Adipose Tissue and Adipokines in Health and Disease. Totowa, NJ: Humana Press; 2007. pp. 159–180. [Google Scholar]
- 15.Miller SC, Bowman BM. Rapid improvements in cortical bone dynamics and structure after lactation in established breeder rats. Anat Rec. 2004;Part A:143–149. doi: 10.1002/ar.a.10138. [DOI] [PubMed] [Google Scholar]
- 16.Cripps AW, Williams VJ. The effect of pregnancy and lactation on food intake, gastrointestinal anatomy and the absorptive capacity of the small intestine in the albino rat. Br J Nutr. 1975;33:17–32. doi: 10.1079/bjn19750005. [DOI] [PubMed] [Google Scholar]
- 17.Goodman MN, Lowell B, Belur E, Ruderman NB. Sites of protein conservation and loss during starvation: influence of adiposity. Am J Physiol Endocrinol Metab. 1984;246:383–390. doi: 10.1152/ajpendo.1984.246.5.E383. [DOI] [PubMed] [Google Scholar]
- 18.Thomsen JS, Skalicky M, Viidik A. Influence of physical exercise and food restriction on the biomechanical properties of the femur of ageing male rats. Gerontology. 2008;54:32–39. doi: 10.1159/000113502. [DOI] [PubMed] [Google Scholar]
- 19.Yamamoto M, Markatos A, Seedor JG, Masarachia P, Gentile M, Rodan GA, Balena R. The effects of the aminobisphosphonate alendronate on thyroid hormone-induced osteopenia in rats. Calcif Tissue Int. 1993;53:278–282. doi: 10.1007/BF01320914. [DOI] [PubMed] [Google Scholar]
- 20.Lee MMC, Chu PC, Chan HC. Effects of cold on the skeletal growth of albino rats. Am J Anat. 1969;124:239–250. doi: 10.1002/aja.1001240207. [DOI] [PubMed] [Google Scholar]
- 21.Lee NK, Karsenty G. Reciprocal regulation of bone and energy metabolism. Trends Endocrinol Metab. 2008;19:161–166. doi: 10.1016/j.tem.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 22.Lieben L, Callewaert F, Bouillon R. Bone and metabolism: a complex crosstalk. Horm Res. 2009;71(Suppl 1):134–138. doi: 10.1159/000178056. [DOI] [PubMed] [Google Scholar]
- 23.Mistlberger RE, Belcourt J, Antle MC. Circadian clock resetting by sleep deprivation without exercise in Syrian hamsters: dark pulses revisited. J Biol Rhythms. 2002;17:227–237. doi: 10.1177/07430402017003006. [DOI] [PubMed] [Google Scholar]
- 24.Tsai LL, Bergmann BM, Rechtschaffen A. Sleep deprivation in the rat: XVI. Effects in a light-dark cycle. Sleep. 1992;15:537–544. doi: 10.1093/sleep/15.6.537. [DOI] [PubMed] [Google Scholar]
- 25.Eastman C, Rechtschaffen A. Circadian temperature and wake rhythms of rats exposed to prolonged continuous illumination. Physiol Behav. 1983;31:417–427. doi: 10.1016/0031-9384(83)90061-6. [DOI] [PubMed] [Google Scholar]
- 26.Forwood MR. Physical activity and bone development during childhood: insights from animal models. J Appl Physiol. 2008;105:334–341. doi: 10.1152/japplphysiol.00040.2008. [DOI] [PubMed] [Google Scholar]
- 27.Robling AG, Duijvelaar KM, Geevers JV, Ohashi N, Turner CH. Modulation of appositional and longitudinal bone growth in the rat ulna by applied static and dynamic force. Bone. 2001;29:105–113. doi: 10.1016/s8756-3282(01)00488-4. [DOI] [PubMed] [Google Scholar]
- 28.Lanyon LE, Rubin CT. Static vs dynamic loads as an influence on bone remodelling. J Biomech. 1984;17:897–905. doi: 10.1016/0021-9290(84)90003-4. [DOI] [PubMed] [Google Scholar]
- 29.Botolin S, Faugere MC, Malluche H, Orth M, Meyer R, McCabe LR. Increased bone adiposity and peroxisomal proliferator-activated receptor-gamma2 expression in type I diabetic mice. Endocrinology. 2005;146:3622–3631. doi: 10.1210/en.2004-1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bathija A, Davis S, Trubowitz S. Bone marrow adipose tissue: response to acute starvation. Am J Hematol. 1979;6:191–198. doi: 10.1002/ajh.2830060303. [DOI] [PubMed] [Google Scholar]
- 31.Gimble JM, Robinson CE, Wu X, Kelly KA. The function of adipocytes in the bone marrow stroma: an update. Bone. 1996;19:421–428. doi: 10.1016/s8756-3282(96)00258-x. [DOI] [PubMed] [Google Scholar]
- 32.Muruganandan S, Roman AA, Sinal CJ. Adipocyte differentiation of bone marrow-derived mesenchymal stem cells: cross talk with the osteoblastogenic program. Cell Mol Life Sci. 2009;66:236–253. doi: 10.1007/s00018-008-8429-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Justesen J, Stenderup K, Ebbesen EN, Mosekilde L, Steiniche T, Kassem M. Adipocyte tissue volume in bone marrow is increased with aging and in patients with osteoporosis. Biogerontology. 2001;2:165–171. doi: 10.1023/a:1011513223894. [DOI] [PubMed] [Google Scholar]
- 34.Bathija A, Davis S, Trubowitz S. Marrow adipose tissue: response to erythropoiesis. Am J Hematol. 1978;5:315–321. doi: 10.1002/ajh.2830050406. [DOI] [PubMed] [Google Scholar]
- 35.Everson CA. Clinical assessment of blood leukocytes, serum cytokines, and serum immunoglobulins as responses to sleep deprivation in laboratory rats. Am J Physiol Regul Integr Comp Physiol. 2005;289:1054–1063. doi: 10.1152/ajpregu.00021.2005. [DOI] [PubMed] [Google Scholar]
- 36.Everson CA, Bergmann BM, Rechtschaffen A. Sleep deprivation in the rat: III. Total sleep deprivation. Sleep. 1989;12:13–21. doi: 10.1093/sleep/12.1.13. [DOI] [PubMed] [Google Scholar]
- 37.Rolls A, Pang W, Bonnavion P, Colas D, Heller CH, Weissman I, De Lecea L. Neuroscience. Washington, DC: Society for Neuroscience; 2011. Sleep regulates haematopoietic stem cell trafficking. [abstract], 2011. [Google Scholar]
- 38.Guariniello LD, Vicari P, Lee KS, de Oliveira AC, Tufik S. Bone marrow and peripheral white blood cells number is affected by sleep deprivation in a murine experimental model. J Cell Physiol. 2012;227:361–366. doi: 10.1002/jcp.22743. [DOI] [PubMed] [Google Scholar]
- 39.Khosro S, Alireza S, Omid A, Forough S. Night work and inflammatory markers. Indian J Occup Environ Med. 2011;15:38–41. doi: 10.4103/0019-5278.82996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scheer FA, Michelson AD, Frelinger AL, 3rd, Evoniuk H, Kelly EE, McCarthy M, Doamekpor LA, Barnard MR, Shea SA. The human endogenous circadian system causes greatest platelet activation during the biological morning independent of behaviors. PLoS ONE. 2011;6:e24549. doi: 10.1371/journal.pone.0024549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ortoft G, Bruel A, Andreassen TT, Oxlund H. Growth hormone is not able to counteract osteopenia of rat cortical bone induced by glucocorticoid with protracted effect. Bone. 1995;17:543–548. doi: 10.1016/8756-3282(95)00386-x. [DOI] [PubMed] [Google Scholar]
- 42.Rosen CJ, Klibanski A. Bone, fat, and body composition: evolving concepts in the pathogenesis of osteoporosis. Am J Med. 2009;122:409–414. doi: 10.1016/j.amjmed.2008.11.027. [DOI] [PubMed] [Google Scholar]
- 43.Everson CA, Crowley WR. Reductions in circulating anabolic hormones induced by sustained sleep deprivation in rats. Am J Physiol Endocrinol Metab. 2004;286:1060–1070. doi: 10.1152/ajpendo.00553.2003. [DOI] [PubMed] [Google Scholar]
- 44.Everson CA, Gilliland MA, Kushida CA, Pilcher JJ, Fang VS, Refetoff S, Bergmann BM, Rechtschaffen A. Sleep deprivation in the rat: IX. Recovery. Sleep. 1989;12:60–67. [PubMed] [Google Scholar]
- 45.Everson CA, Reed HL. Pituitary and peripheral thyroid hormone responses to thyrotropin-releasing hormone during sustained sleep deprivation in freely moving rats. Endocrinology. 1995;136:1426–1434. doi: 10.1210/endo.136.4.7895653. [DOI] [PubMed] [Google Scholar]
- 46.Buxton OM, Pavlova M, Reid EW, Wang W, Simonson DC, Adler GK. Sleep restriction for 1 week reduces insulin sensitivity in healthy men. Diabetes Care. 2010;59:2126–2133. doi: 10.2337/db09-0699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sgoifo A, Buwalda B, Roos M, Costoli T, Merati G, Meerlo P. Effects of sleep deprivation on cardiac autonomic and pituitary-adrenocortical stress reactivity in rats. Psychoneuroendocrinology. 2006;31:197–208. doi: 10.1016/j.psyneuen.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 48.Suchecki D, Tufik S. Social stability attenuates the stress in the modified multiple platform method for paradoxical sleep deprivation in the rat. Physiol Behav. 2000;68:309–316. doi: 10.1016/s0031-9384(99)00181-x. [DOI] [PubMed] [Google Scholar]
- 49.Rosen CJ, Donahue LR. Insulin-like growth factors and bone: the osteoporosis connection revisited. Proc Soc Exp Biol Med. 1998;219:1–7. doi: 10.3181/00379727-219-44310. [DOI] [PubMed] [Google Scholar]
- 50.Zumkeller W. The insulin-like growth factor system in hematopoietic cells. Leuk Lymphoma. 2002;43:487–491. doi: 10.1080/10428190290011958. [DOI] [PubMed] [Google Scholar]
- 51.Giustina A, Mazziotti G, Canalis E. Growth hormone, insulin-like growth factors, and the skeleton. Endocr Rev. 2008;29:535–559. doi: 10.1210/er.2007-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ahmed SF, Savendahl L. Promoting growth in chronic inflammatory disease: lessons from studies of the growth plate. Horm Res. 2009;72:42–47. doi: 10.1159/000229763. [DOI] [PubMed] [Google Scholar]
- 53.Skerry TM. The effects of the inflammatory response on bone growth. Eur J Clin Nutr. 1994;48:190–197. [PubMed] [Google Scholar]
- 54.Coll AP, Farooqi IS, O'Rahilly S. The hormonal control of food intake. Cell. 2007;129:251–262. doi: 10.1016/j.cell.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Frost HM, Jee WS. On the rat model of human osteopenias and osteoporoses. Bone Miner. 1992;18:227–236. doi: 10.1016/0169-6009(92)90809-r. [DOI] [PubMed] [Google Scholar]
- 56.Turner A. Animal models of osteoporosis--necessity and limitations. Eur Cell Mater. 2001:66–81. doi: 10.22203/ecm.v001a08. [DOI] [PubMed] [Google Scholar]
- 57.Yarrington A, Mehta P. Does sleep promote recovery after bone marrow transplantation? - A hypothesis. Pediatr Transplantation. 1998;2:51–55. [PubMed] [Google Scholar]