Abstract
Corticotropin-releasing factor (CRF) and glutamate are critical signaling molecules in the central nucleus of the amygdala (CeA). Central amygdala CRF, acting via the CRF type 1 receptor (CRF-R1), plays an integral role in stress responses and emotional learning, processes that are generally known to involve functional NMDA-type glutamate receptors. There is also evidence that CRF expressing CeA projection neurons to the bed nucleus of the stria terminalis (BNST) play an important role in stress related behaviors. Despite the potentially significant interactions between CRF and NMDA receptors in the CeA, the synaptic organization of these systems is largely unknown. Using dual labeling high resolution immunocytochemical electron microscopy, it was found that individual somata and dendrites displayed immunoreactivity for CRF and the NMDA-NR1 (NR1) subunit in the mouse CeA. In addition, CRF-containing axon terminals contacted postsynaptic targets in the CeA, some of which also expressed NR1. Neuronal profiles expressing the CRF type 1 receptor (CRF-R1), identified by the expression of green fluorescent protein (GFP) in bacterial artificial chromosome (BAC) transgenic mice, also contained NR1, and GFP immunoreactive terminals formed synapses with NR1 containing dendrites. Although CRF and GFP were only occasionally co-expressed in individual somata and dendritic profiles, contacts between labeled axon terminals and dendrites were frequently observed. A combination of tract tracing and immunocytochemistry revealed that a population of CeA CRF neurons projected to the BNST. It was also found that CRF, or GFP expressing terminals directly contacted CeA-BNST projection neurons. These results indicate that the NMDA receptor is positioned for the postsynaptic regulation of CRF expressing CeA neurons and the modulation of signals conveyed by CRF inputs. Interactions between CRF and NMDA receptor mediated signaling in CeA neurons, including those projecting to the BNST, may provide the synaptic basis for integrating the experience of stress and relevant environmental stimuli with behaviors that may be of particular relevance to stress-related learning and the emergence of psychiatric disorders, including drug addiction.
Keywords: Addiction, Affect, Glutamate, Learning, Stress, Synaptic Plasticity
INTRODUCTION
The central nucleus of the amygdala (CeA) is a key component of neural systems that coordinate sensory experience, psychological states, and memory with physiological responses, relevant behaviors, and learning (Davis et al. 2010; Koob 2009a; LeDoux 2000; Van de Kar and Blair 1999). Consistent with its critical role in stress responses, as well as emotional learning and memory, the CeA has been implicated in several abnormal psychiatric states including anxiety disorders (Davis et al. 2010) and drug addiction (Glass 2010; Koob 2009b).
The CeA contains diverse populations of neuropeptide expressing neurons (Cassell and Gray 1989; Gray et al. 1984), and is a prominent member of the extra-hypothalamic corticotropin-releasing factor (CRF) system (Cummings et al. 1983; Pilcher and Joseph 1984; Sakanaka et al. 1986; Swanson et al. 1983). The CeA CRF system is highly sensitive to the experience of several types of stress (Hand et al. 2002; Makino et al. 1999; Merali et al. 2008), and is implicated in a broad spectrum of physiological and behavioral processes, including cardiovascular function (Brown and Gray 1988; Wiersma et al. 1993), pain related behaviors (Fu and Neugebauer 2008), body weight regulation (Arvaniti et al. 2001), feeding behavior (Cottone et al. 2009), the regulation of affect (Regev et al. 2011), and emotional learning (Pitts and Takahashi 2011; Pitts et al. 2009). The CeA CRF system is also sensitive to the exposure to various drugs of abuse, as well as withdrawal from many of these agents (Lowery-Gionta et al. 2012; Zorrilla et al. 2012).
The brain glutamate system also plays an important role in normal behaviors, neurobehavioral plasticity, and psychiatric disease states. Like CRF, glutamate signaling in the CeA has been implicated in cardiovascular (Salome et al. 2001), nociceptive (Li and Neugebauer 2004), stress-related (Skorzewska et al. 2009), and ingestive (Andrzejewski et al. 2004) processes. The CeA receives a diverse compliment of glutamate afferents involved in cognitive function, learning and memory, as well as arousal and sensory processing. These glutamate projections include inputs from limbic cortices (McDonald 1998), the hippocampal formation (Canteras and Swanson 1992), thalamic nuclei (Li and Kirouac 2008; Turner and Herkenham 1991), and other regions of the amygdala (Pitkanen et al. 1997); some of these glutamatergic inputs have been shown to terminate in areas of the CeA that contain CRF expressing neurons (Li and Kirouac 2008). Fibers entering the CeA express immunoreactivity for CRF and/or the CRF type 1 receptor (CRF-R1), the major local receptor for CRF in the CeA, which also has been strongly implicated in several stress-related disorders (Kehne and Cain 2010). In addition, many of the aforementioned glutamate afferents originate in brain regions that express both CRF or CRF-R1 (Justice et al. 2008; Van Pett et al. 2000).
Ionotropic N-methyl-D-aspartate (NMDA) type glutamate receptors are expressed in CeA neurons (Monaghan and Cotman 1985; Petralia et al. 1994; Sato et al. 1995) and play an important role in local neural signaling (Samson and Paré 2005), as well as cellular plasticity (Pollandt et al. 2006). Functional CeA NMDA receptors are also required for aversive learning, including conditioned fear (Goosens and Maren 2003) and aversive context conditioning in response to opioid withdrawal (Glass et al. 2008), implicating CeA NMDA-dependent plasticity in behaviors relevant to emotional and addictive disorders.
There is evidence for interactions between CRF and NMDA receptors in both stress reactions and drug-related plasticity. For example, it has been reported that in vivo administration of CRF in the CeA results in elevated local presynaptic glutamate release in response to novel as well as conditioned stressors (Skorzewska et al. 2009), whereas in vitro application of CRF results in an NMDA receptor-dependent long-term potentiation of amygdala inputs to the CeA, which is heightened by withdrawal from drugs of abuse via a postsynaptic process (Pollandt et al. 2006). These findings suggest that diverse presynaptic and/or postsynaptic interactions may underlie signaling involving activation of the NMDA receptor, release of CRF, and activation of CeA CRF neurons. Despite the significant roles that CRF and the NDMA receptor play in local signaling, neural plasticity, and stress-related behaviors, the ultrastructural relationship of these molecules within the CeA is unknown.
Experience dependent neural plasticity involving inputs to, and the output of, CeA CRF neurons, is likely to contribute to stress-related neurobehavioral adaptability (Walker and Davis 2008). The bed nucleus of the stria terminalis (BNST) is believed to be a major target of CRF CeA neurons. This pathway is implicated in neurobehavioral responses to stress (Jasnow et al. 2004), as well as stress-induced reinstatement of drug self-administration (Erb et al. 2001), processes that critically involve NMDA receptor activation. Although it has been shown that NMDA receptors are prominently expressed in CeA neurons that project to the BNST (Beckerman and Glass, 2012), aside from previous indirect light microscopic approaches (Sakanaka et al. 1986), there is no direct ultrastructural data characterizing the expression of CRF in somata and dendrites of CeA-BNST projection neurons, as well as CRF expressing axons that contact them.
Given the ability of high resolution immunoelectron microscopy to identify cellular and synaptic sites of peptide and protein localization, a combination of dual labeling immunochemical electron microscopy (EM) and tract tracing were used to characterize the synaptic organization of the NMDA-NR1 (NR1) receptor subunit and CRF in the CeA, as well as the localization of CRF in the CeA-BNST pathway in wild-type mice. The ultrastructural distribution of NR1 in CRF-R1 expressing neurons was also examined using CRF-R1 transgenic bacterial artificial chromosome (BAC) mice, which are useful in identifying proteins that are difficult to detect via conventional immunohistochemical methods (Justice et al. 2008).
METHODS
Animals
Experimental protocols involving animals and their care were approved by the Institutional Animal Care and Use Committee at Weill Cornell Medical College and conformed to the 2011 Eighth Edition of the NIH Guide for the Care and Use of Laboratory Animals. Two strains of mice were used in these studies. To investigate the ultrastructural relationships between NMDA receptors, CRF, and the latter peptide's distribution in CeA-BNST projections neurons, adult (25-30 grams) male C57BL/6 mice were used. In studies requiring identification of CRF-R1 expression, we used a transgenic mouse line generated by BAC technology, where the reporter green fluorescent protein (GFP) is expressed by the CRF-R1 promoter, as previously described (Justice et al. 2008). Adult male (25-30 grams) CRF-R1 BAC mice were maintained on the C57BL/6 background, and were bred and genotyped as previously described (Justice et al. 2008).
Tracer Microinjections
Tracers were administered as previously described (Beckerman and Glass 2012). Under deep isoflurane anesthesia, either fluorogold (FG [Fluorochrome, Denver, CO], 2% in phosphate buffer [PB]) or BDA ([Invitrogen, Carlsbad, CA]; 10% in PB) were unilaterally microinjected in the BNST or CeA, respectively. Approximately 50 nl of each tracer was injected over a 10 minute interval at 1.1 mm posterior and 2.5 mm lateral to bregma, at a depth of 4.8 mm ventral to the skull surface for the CeA, or 0.3 mm anterior and 0.8 mm mediolateral to bregma, at a depth of 4.3 mm for the BNST. Microinjections were made by interfacing a picospritzer (Picospritzer II, General Valve Corp., Fairfield, NJ) to a glass pipette (WPI, Sarasota, FL), whose tip was pulled to a diameter of ~50 μm, via a pipette holder and plastic tubing. In all cases, to prevent leakage of solution, the pipette was left in place for an additional 10 minutes. Bone wax was used to cover the bore-hole, and the mice were allowed to recover in their home cages. For tracer studies, animals were sacrificed at least 7 days post-injection.
Antisera
A guinea pig anti-CRF antiserum (Bachem Peninsula, San Carlos, CA) was used to label CRF. A mouse (Pharmingen, San Diego, CA) anti-NR1 antibody was used to label NR1. The specificity of these antisera have been described in recent publications (Glass et al. 2009; Jaferi and Pickel 2009). The retrograde tracer FG was labeled with an antiserum generated in rabbit (Chemicon, Temecula, CA), and its specificity was shown in FG injected animals by the lack of labeling in brain areas without direct contacts to the BNST. A chicken anti-GFP antibody (Aves Labs, Tigard, OR) was used to label the reporter protein. The specificity of this antiserum was verified by the lack of immunoreactivity in GFP negative BAC mice.
Tissue preparation
Mice were anesthetized with pentobarbital (150 mg/kg, i.p.), and their brains were fixed by aortic arch perfusion sequentially with: (a) 15 ml of normal saline (0.9%) containing 1000 units/ml of heparin, (b) 40 ml of 3.75% acrolein in 2% paraformaldehyde (PFA) in 0.1 M PB (pH 7.4), and (c) 100 ml of 2% paraformaldehyde in PB, all delivered at a flow rate of 25 ml/minute. The brains were removed and post-fixed for 90-120 minutes in 2% paraformaldehyde in PB. Coronal sections (40 μm) from the forebrain at the level of the CeA, or, for animals receiving the tracer administration, the BNST, were cut with a vibrating microtome. Tissue sections were treated with 1.0% sodium borohydride in PB and then washed in PB. To enhance tissue permeability, sections were immersed in a cryoprotectant solution (20% sucrose and 8% glycerol in 0.05M PB) at room temperature followed by 15 minutes in −80° C.
Dual labeling immunocytochemical procedures for EM
Sections were processed for dual immunoperoxidase (ABC) and immunogold-silver (IGS) immunocytochemistry as previously described (Milner et al. 2011). Briefly, tissue was rinsed in Tris-buffered saline (TBS; pH 7.6) and then blocked with 0.5% bovine serum albumin (BSA) in TBS for 30 min. Tissue then was incubated for 24 (GFP)-48 (other antisera) hours in one of several cocktails of primary antisera as follows: 1). NR1 (1:100 [ABC], 1:50 [IGS]) and CRF (1:10,000 [ABC], 1:5000 [IGS]); 2). NR1 (as above) and GFP (1:10,000 [ABC], 1:5000 [IGS]); 3). CRF (as above) and GFP (as above); 4). CRF (as above) and FG (1:1000 [ABC], 1:500 [IGS]); 5). GFP (as above) and FG (as above). Primary antisera were diluted in 0.1% BSA in TBS. As a control for potential antisera cross-reactivity, adjacent brain sections were processed for ABC and IGS, except that one of the respective primary antisera was omitted during primary incubation. Following primary antisera labeling, sections were washed in TBS, placed in their respective biotin conjugated antisera in 0.1% BSA (1:400, 30 minutes), and then rinsed in TBS. Tissue sections were then incubated for 30 minutes in ABC in TBS. The bound peroxidase was visualized by reaction for 6 minutes in 0.2% solution of 3, 3’-diaminobenzidine (DAB) and 0.003% hydrogen peroxide in TBS. For immunogold labeling, sections were rinsed in 0.01 M phosphate buffered saline (PBS, pH 7.4), and blocked for 10 minutes in 0.8% BSA and 0.1% gelatin in PBS to reduce non-specific binding of gold particles. Sections then were incubated for 2 hours in their respective secondary antisera, which were conjugated with 0.6 nm gold particles (1:50, AuroProbeOne, Amersham, Arlington Heights, IL), and rinsed in 0.5% BSA and 0.1% gelatin in PBS, followed rinses in PBS. Next, sections were incubated for 10 minutes in 2% glutaraldehyde in PBS, and then rinsed in PBS. The bound gold particles were enlarged by a 5-7 minute silver intensification using an IntenSE-M kit (Amersham, Arlington Heights, IL). For dual labeling of the anterograde tracer and CRF in the BNST, tissue sections were processed for dual BDA staining and CRF IGS labeling as above, except with primary antisera only for CRF.
Tissue preparation and procedures for light microscopic identification of tracer injection sites
Tissue processing for identification of tracer injections was performed as described for the ABC procedure above, except as noted below. Sections processed for immunolabeling of FG were incubated one night in primary rabbit anti-FG antisera. Brain sections from BDA injected mice were incubated for 30 minutes in ABC. Sections were mounted in 0.05 M PB on glass slides, dehydrated, and coverslipped with DPX (Aldrich-Sigma). Sections were examined and photographed on a Nikon 81a light microscope.
Electron Microscopy
For EM processing, tissue was postfixed in 2% osmium tetroxide in PB for one hour, and dehydrated in a series of alcohols, through propylene oxide, and flat embedded in EM BED 812 (EMS, Fort Washington, PA) between 2 sheets of Aclar plastic. Ultrathin sections (60-80 nm) from the surface of flat-embedded sections containing the CeA or BNST were cut with a diamond knife using a Leica Ultracut UCT ultramicrotome, and sections were collected on 400 mesh thin-bar copper grids (EMS). Electron microscopic images of this tissue were obtained using a digital camera (Advanced Microscopy Techniques, Danvers, MA) interfaced with a transmission electron microscope (Tecnai 12 BioTwin, FEI, Hillsboro, OR). For preparation of figures, images were adjusted for contrast and brightness using Photoshop 11 software, and imported into PowerPoint X to add lettering.
Ultrastructural analysis
To control for potential labeling artifacts due to penetration of cytological reagents, sampling was performed at the tissue surface as determined by proximity to the epon-tissue interface (Milner et al. 2011). This was achieved by collecting electron micrographs exclusively in the transition zone where one edge of the sampling area was in contact with epon in a field of at least three grid squares. Digital images were captured and analyzed to determine the number of single, dual, and triple labeled neuronal and glial profiles. The classification of labeled profiles was based upon standard morphological descriptions (Peters et al. 1991). Somata were distinguished by the presence of a nucleus. Dendrites were identified by the presence of postsynaptic densities, as well as ribosomes and both rough and smooth endoplasmic reticulum. However, profiles were also considered dendritic whenever postsynaptic densities were observed, independent of endoplasmic reticulum. Axon terminals were identified by size (at least 0.2 μm diameter) and the presence of synaptic vesicles. Astrocytes were identified by their irregular shape, the presence of filamentous membranes apposing dendrites or axons, or the presence of gap junctions. Synapses were defined as either symmetric or asymmetric, according to the presence of either thin or thick postsynaptic specializations, respectively. Appositions were distinguished by closely spaced plasma membranes that lacked recognizable specializations, or interposing astrocytic processes. Immunoperoxidase labeling is identified by a diffuse brown/black precipitate, while IGS labeling is characterized by dense uniformly black granules. Both markers are readily distinguishable by visual inspection. With peroxidase and immunogold methods, non-specific labeling is usually along the membranes of damaged profiles. Background usually contributes 3% or less of silver-enhanced gold particles (Wang et al. 2003).
RESULTS
CRF is co-expressed with NR1 in somata and dendrites of CeA neurons, and is also present in axon terminals contacting NR1 labeled postsynaptic profiles
In forebrain sections taken from six wild-type C57BL/6 mice and processed for dual labeling of NR1 and CRF, a total of 29,520 μm2 of tissue from the CeA was analyzed by electron microscopy. A total of 23,200 μm2 was analyzed from sections processed for detection of CRF by the ABC approach and NR1 by the IGS method, whereas 6,320 μm2 of tissue was analyzed with markers reversed.
Diverse populations of labeled neuronal profiles were observed in the CeA, including those solely expressing CRF or NR1, as well as processes dually labeled for both. In dual labeled somata, labeling for CRF was often diffusely distributed throughout the cytoplasm, and labeling for NR1 was typically found near Golgi complexes and small vesicular organelles (Fig. 1).
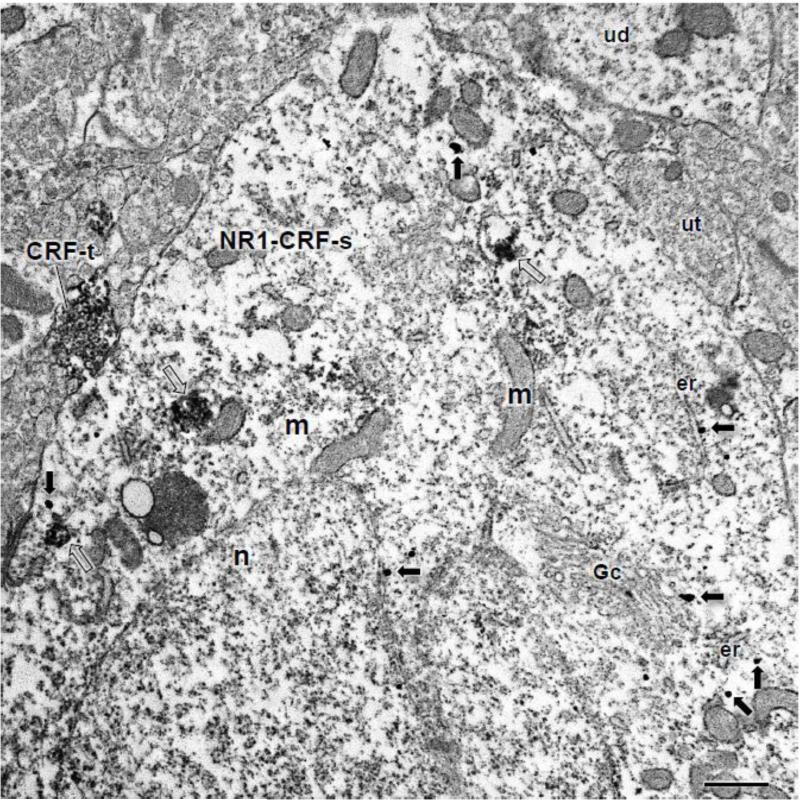
Figure 1. The soma of a CeA neuron shows co-labeling for CRF and NR1.
The cell body of a CeA neuron (NR1+CRF-s) expresses IGS labeling for NR1 (arrows) and ABC labeling for CRF (unfilled arrows). This soma contains an irregularly shaped nucleus (n), numerous mitochondria (m), and endoplasmic reticula (er). Immunogold-silver particles for NR1 are present throughout the cell body, including near the outer membrane of the nucleus (n), Golgi complex (Gc), and endomembranes. This soma is contacted by a CRF labeled axon terminal (CRF-t). Unlabeled dendrites (ud) and axon terminals (ut) are present in the adjacent neuropil. Scale Bar: 1 μm.
Labeling for CRF also extended outside the cell body and was found in dendrites without or with NR1 immunoreactivity. Although dual labeled dendritic profiles were frequently contacted by axon terminals that did not form synaptic specializations in the plane of section analyzed (Fig. 2A), many did receive synapses, which were commonly asymmetric junctions (Fig. 2B). In addition to dendritic profiles, small unmyelinated axons, and, more commonly, axon terminals also showed labeling for CRF (Fig. 2C). When labeled with immunoperoxidase, reaction product was diffusely distributed throughout the axon terminal or aggregated in large dense core vesicles. Axon terminals expressing CRF were frequently in direct contact with NR1 labeled soma and/or dendritic profiles (Fig. 2C).
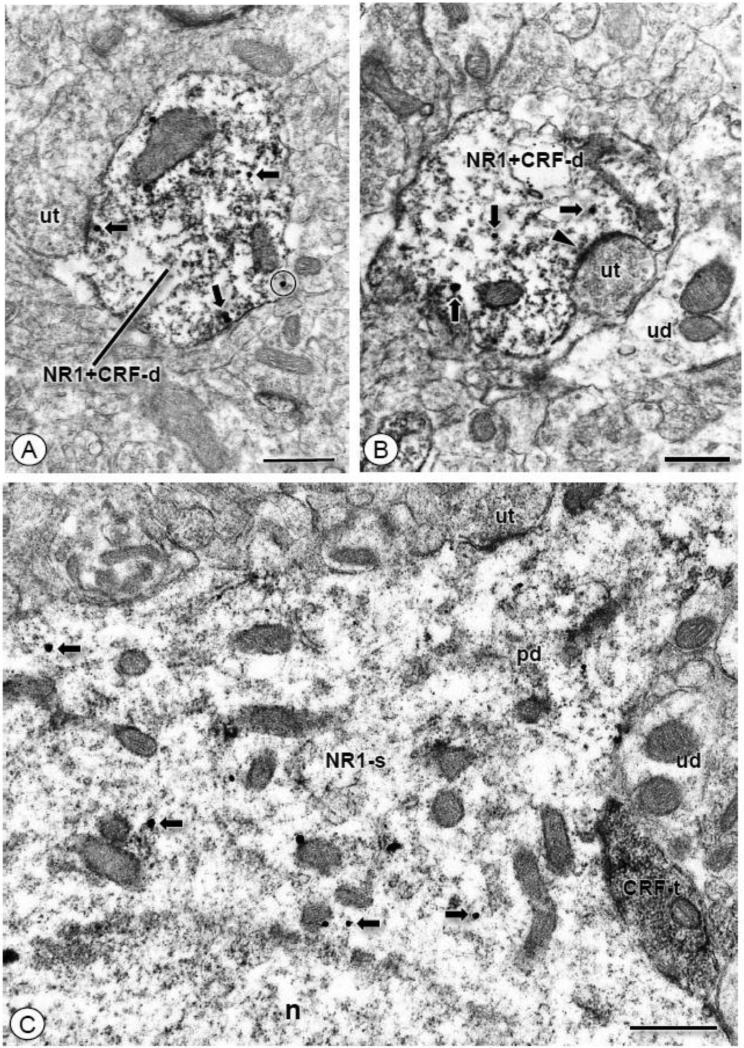
Figure 2. CRF immunolabeled dendritic or axonal profiles co-express NR1 or contact NR1 labeled somatodendrites, respectively.
(A). A dendritic profile (NR1+CRF-d) contains IGS particles for NR1 (arrows) and immunoperoxidase reaction product for CRF. Immunogold-silver particles are affiliated with the plasma membrane (circle), and are also present in the intracellular compartment (arrows). This dendrite is contacted by an unlabeled axon terminal (ut). (B). A dendritic profile (NR1+CRF-d) of a CeA neuron contains labeling for NR1 (arrows) and CRF. Immunogold-silver particles for NR1 are present intracellularly, and immunoperoxidase reaction product is diffusely distributed throughout this profile. This dendrite is contacted by an unlabeled axon terminal (ut) forming an excitatory-type asymmetric synapse (filled arrow head). (C). A CRF labeled axon terminal (CRF-t) contacts the region bordering the soma (NR1-s) and emerging proximal dendritic shaft (pd) of a neuron expressing IGS labeling for NR1 (arrows). Unlabeled axon terminals (ut) and dendrites (ud) are also apposed to this soma. Scale Bars: 0.5 μm.
Of the numerous neuronal profiles exhibiting labeling for both CRF and NR1, these were most commonly somata (21-23%) or dendrites (71-79%; Table 1). From a total of 133 CRF labeled axons or axon terminals that were apposed to dendritic profiles, 30 formed direct appositions with single (85% of contacts) or dual (15% of contacts) labeled dendritic profiles. Among all the CRF labeled axon terminals that formed identifiable synapses, 69% (11/16) formed symmetric contacts and 31% (5/16) made asymmetric junctions.
Table 1.
Distribution of CRF and NR1 in CeA neuronal profiles
| NR1 (gold) CRF (ABC) | NR1 (ABC) CRF (gold) | |
|---|---|---|
| CRF-somata | 2% (13/807) | 5% (7/148) |
| CRF-dendrites | 47% (382/807) | 55% (81/148) |
| CRF axons | 51% (412/807) | 40% (60/148) |
| NR1-somata | 11% (24/219) | 1% (1/157) |
| NR1-dendrite | 65% (143/219) | 89% (140/157) |
| NR1-axons | 23% (52/219) | 10% (16/157) |
| Dual-somata | 23% (47/204) | 21% (10/48) |
| Dual-dendrites | 71% (145/204) | 79% (38/48) |
| Dual-axons | 6% (12/204) | 0% (0/48) |
In the CeA of CRF-R1 reporter mice, GFP is found in dendritic or axonal structures that contain NR1, or contact NR1 expressing profiles, respectively
To determine if NR1 was present in somata or dendritic profiles of CRF-R1 expressing neurons, or if NR1 labeled profiles were contacted by axons or axon terminals of CRF-R1 expressing neurons, we employed dual labeling for NR1 and the GFP reporter protein that is expressed by the CRF-R1 promoter in BAC transgenic mice. A total of 25,200 μm2 of tissue from the CeA of four transgenic mice was analyzed from forebrain sections processed for dual labeling of GFP and NR1. Electron microscopic analysis was performed on a total of 15,120 μm2 of CeA samples obtained from forebrain sections processed for detection of GFP by IGS and NR1 by the ABC approach, whereas 10,080 μm2 of tissue was analyzed with markers reversed.
Single NR1 labeled and dual labeled dendritic profiles were found in neuropil populated by unlabeled profiles. Many single GFP labeled dendritic profiles were contacted by unlabeled axon terminals forming asymmetric excitatory type synapses (Fig. 3A). Dendritic profiles expressing labeling for both GFP and NR1 also received symmetric type contacts from unlabeled terminals (Fig. 3B). In addition to dendrites, axon terminals also expressed exclusive labeling for GFP (Fig. 3C). These presynaptic structures were frequently small in size, typically expressed exclusive labeling for GFP, and contacted unlabeled or NR1 labeled dendritic profiles (Fig. 3C).
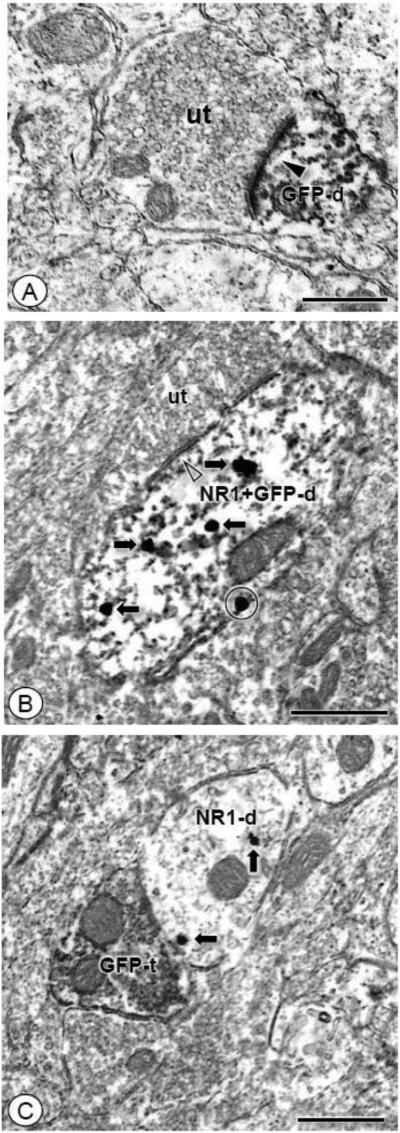
Figure 3. GFP immunolabeling is found in dendritic profiles, including those that co-express NR1, as well as in axon terminals contacting NR1 labeled dendritic profiles.
(A). A small caliber GFP labeled dendritic profile (GFP-d) receives an asymmetric excitatory-type synapse (arrow head) from an unlabeled axon terminal (ut). (B). Both GFP and NR1 are expressed in an obliquely sectioned dendritic profile (NR1+GFP-d). Immunogold-silver particles are present near the plasmalemma (circle) and intracellularly (arrows). This profile is contacted by an unlabeled axon terminal (ut). (C). A GFP labeled axon terminal (GFP-t) is apposed to a dendritic profile (NR1-d) that expresses IGS particles for NR1 (arrows). Scale bars: 0.5 μm.
Of the numerous neuronal profiles exhibiting labeling for both GFP and NR1, most were dendrites (Table 2). The majority of all identifiable synapses involving single GFP labeled and dual labeled dendritic profiles were formed by unlabeled axon terminals that made asymmetric synapses (93%; 50/54). Of all GFP labeled axon terminals forming identifiable synapses, 74% (20/27) formed asymmetric type junctions and 26% (7/27) formed symmetric type contacts. Among GFP labeled axons contacting single or dual labeled profiles, 64% (9/14) of GFP labeled axons contacted NR1 labeled dendritic profiles and 36% (5/14) contacted dual labeled dendrites.
Table 2.
Distribution of GFP and NR1 in CeA neuronal profiles
| NR1 (gold) GFP (ABC) | NR1 (gold) GFP (ABC) | |
|---|---|---|
| GFP-somata | 2% (3/174) | 3% (6/239) |
| GFP-dendrites | 70% (122/174) | 79% (189/239) |
| GFP-axons | 28% (49/174) | 18% (44/239) |
| NR1-somata | 5% (31/620) | 3% (19/663) |
| NR1-dendrites | 88% (547/620) | 91% (606/663) |
| NR1-axons | 7% (42/620) | 6% (38/663) |
| Dual-somata | 6% (6/101) | 5% (12/247) |
| Dual-dendrites | 93% (94/101) | 93% (231/247) |
| Dual-axons | 1% (1/101) | 2% (4/247) |
GFP labeling is found in dendritic or axonal structures that contain CRF, or contact CRF expressing profiles, respectively, in the CeA of CRF-R1 reporter mice
Tissue sections containing the CeA from CRF-R1 GFP mice were processed for dual detection of GFP and CRF by alternate immunoperoxidase and immunogold labeling. Immunolabeling for each antisera was most commonly found in seperate neuronal profiles, although dual labeled structures were found. Somata and dendrites were most commonly found to exhibit dual labeling. Dual labeled dendrites were typically large proximal structures, or intermediate profiles, including those exhibiting intense immunoperoxidase reaction product for GFP and scattered IGS labeling for CRF (Fig. 4A). Appositions between CRF and GFP labeled profiles were also observed. Contacts were typically formed by single labeled axon terminals and single labeled dendrites. These included axon terminals labeled for CRF, which formed synapses with GFP labeled dendrites (Fig. 4B). A total of 475 profiles were counted, of which 5% (23/475) were dual labeled. Among dual labeld profiles, 91% (21/23) were somata or dendrites, and 9% (2/23) were axon terminals. Contacts between GFP and CRF labeled profiles were also observed. Among 26 observed conacts, 32% (9/28) involved CRF labeled axons apposed to GFP labeled dendrites, and 68% (19/28) of appositions were between GFP labeled axon terminals and CRF labeled somata or dendrites.
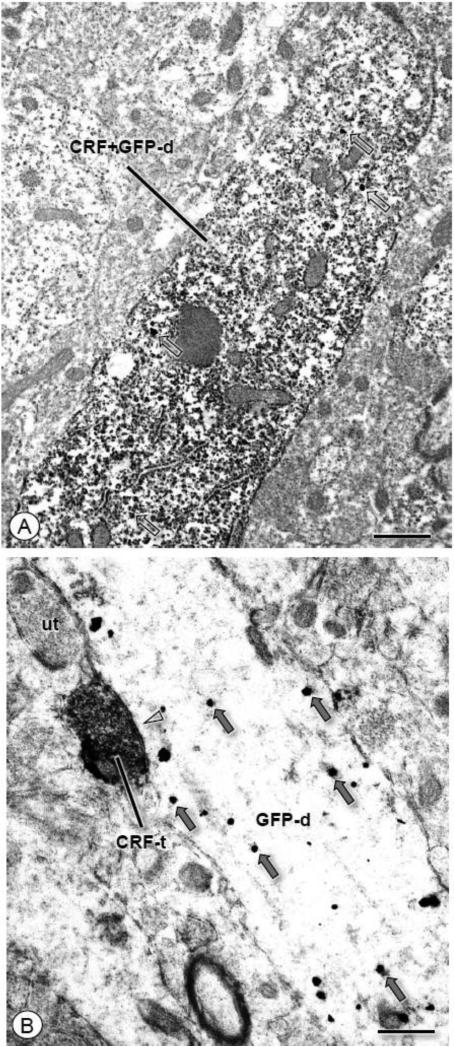
Figure 4. CRF and GFP are co-expressed in CeA neurons as well as apposed axon terminals and dendrites.
(A). A longitudinally sectioned dendritic profile (CRF+GFP-d) expresses diffuse immunoperoxidase immunoreaction product for GFP. Immunogold particles for CRF (unfilled arrows) are also present in this profile. (B). An axon terminal (CRF-t) labeled for CRF forms a symmetric inhibitory type synapse (open arrow head) with a dendritic profile (GFP-d) containing IGS labeling for GFP (gray arrows). Scale bars: 0.5 μm.
CRF is expressed in CeA somata and dendrites retrogradely labeled from the commisural BNST and in axon terminals that contact these projection neurons
As shown by light microscopy, injection of FG in the BNST of wild-type C57BL/6 mice (Fig. 5A) resulted in retrogradely labeled neurons in the CeA (Fig. 5B). For electron microscopic analysis, forebrain sections containing the CeA from three mice were processed for dual immunocytochemical labeling of FG and CRF. From this, a total of 15,200 μm2 of tissue from the lateral CeA, the main area containing retrogradely labeled neurons, was analyzed.
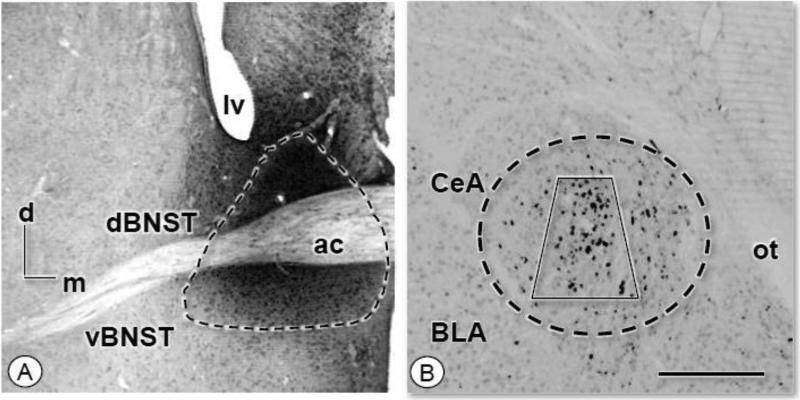
Figure 5. Microinjection of FG in the commissural BNST results in retrogradely labeled neurons in the CeA.
(A-B). Light micrographs illustrating a representative example of the site of FG injection in the BNST (enclosed area) and retrogradely labeled neurons in the CeA (enclosed area). Retrogradely labeled neurons are found primarily in the lateral division of the CeA. Electron microscopic analysis of FG and receptor protein labeling was performed in samples taken from the region of the CeA represented by the area bound by the trapezoid. ac: anterior commissure; d: dorsal; lv: lateral ventricle; m: medial; ot: optic tract. Scale Bar: 1 mm.
Single and dual labeled somata and dendrites were found throughout the lateral CeA. Dual labeled somata contained numerous intracellular organelles such as mitochondria and endomembraneous structures, and immunoreactivity for both CRF and FG was diffusely distributed throughout the cytoplasm (Fig. 6). In addition to somata, numerous dual labeled dendritic profiles were also seen. These included larger proximal dendrites, as well as smaller distal processes. Immunoreactivity for CRF was typically characterized by punctate granules present in the intracellular compartment that also contained diffuse labeling for FG (Fig. 7A). In addition, distal dendritic profiles exclusively labeled for FG were contacted by CRF containing axon terminals (Fig. 7B). These presynaptic structures typically formed non-synaptic appositions with retrogradely labeled dendrites, but when synaptic contacts were made these were mostly symmetric inhibitory type junctions (Fig. 7C).
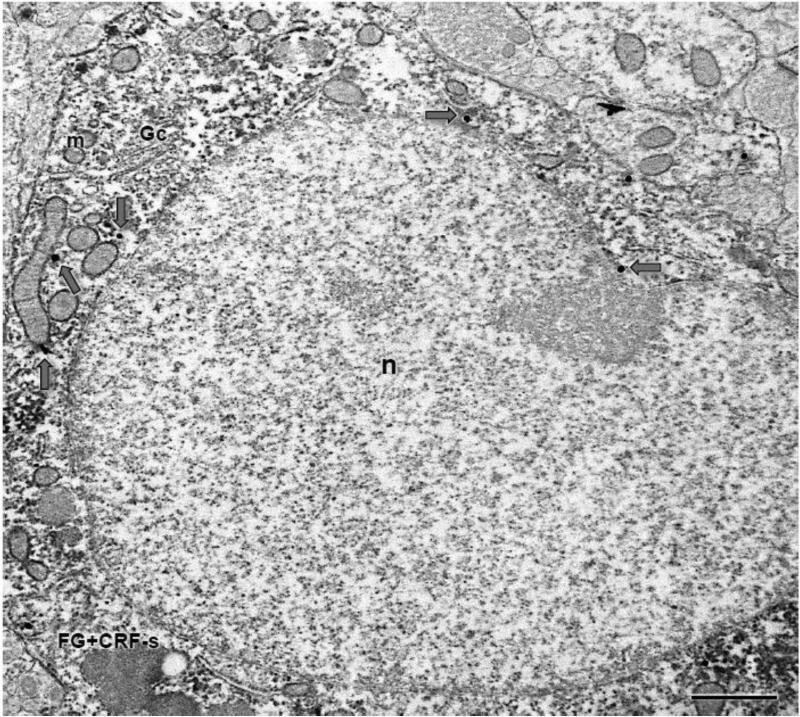
Figure 6. A CRF labeled CeA soma expresses FG immunoreactivity after administration of the tracer in the BNST.
A cell body (FG+CRF-s) shows diffuse immunoperoxidase labeling for CRF and IGS labeling for FG (gray arrows). A large nucleus (n), as well as a Golgi complex (Gc) and numerous mitochondria (m) are present in this profile. Scale bar: 1 μm
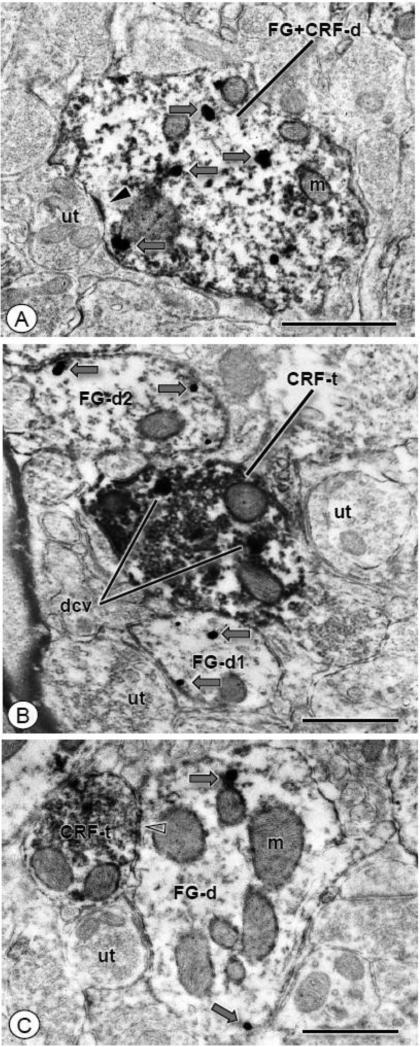
Figure 7. CRF immunoreactivity is found in dendritc profiles that express FG, as well as in axon terminals that contact retrogradely labeled dendrites.
(A). A dendritic profile of a CeA neuron (FG+CRF-d) shows individual intracellular (gray arrows) IGS particles for FG and diffuse ABC labeling for CRF (open arrow). An unlabeled axon terminal (ut) forms an asymmetric synapse (arrow head) with this dual labeled dendrite. (B). An axon terminal (CRF-t) and dendritic profiles (FG-d1-2) show exclusive labeling for CRF or FG, respectively. The CRF labeled axon terminal shows diffuse immunoperoxidase reaction product that is especially concentrated in structures characteristic of large dense core vesicles (dcv). This terminal contacts a dendritic profile (FG-d1) showing immunogold-silver particles for FG (gray arrows). Another FG labeled dendritic profile (FG-d2), as well as unlabeled axon terminals (ut), are present nearby. (C). A dendritic profile (FG-d) showing IGS labeling for FG (gray arrows) receives a symmetric type synapse (open arrow head) from an axon terminal labeled for CRF (CRF-t). Scale bars: 0.5 μm
Quantitative analysis revealed that over 51% (203/394) of CRF labeled somatodendritic profiles also contained FG, whereas over 36% (203/566) of FG labeled somata and dendritic profiles also contained CRF immunoreactivity. Of 42 CRF labeled axon terminals that contacted single or dual labeled somata or dendritic profiles, 76% (32/42) contacted single FG labeled somatodendritic processes, while 24% (10/42) contacted single CRF or dual CRF and FG labeled somata or dendritic profiles.
To verify that CRF was transported in axons of CeA neurons projecting to the BNST, the anterograde tracer BDA was injected into the CeA. In tissue processed for light microscopy, BDA labeled fibers were seen in the BNST following CeA administration of the tracer (Fig. 8A-B). Forebrain sections from three mice were processed for dual detection of BDA and CRF, and samples from the BNST were examined by electron microscopy. Immunoperoxidase reaction product for BDA was present in axons without CRF labeling (Fig. 8C) as well as axon terminals that also contained CRF (Fig. 8D).
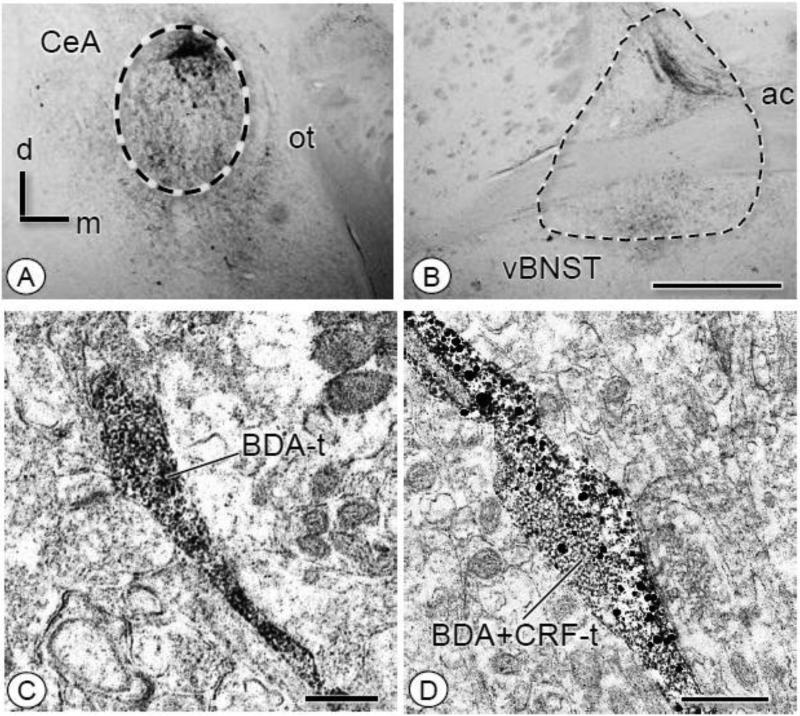
Figure 8. Microinjection of BDA in the CeA results in anterogradely labeled fibers in the BNST whose axon terminals express CRF.
(A-B). By light microscopy, injection of BDA into the CeA resulted in anterograde transport in the BNST. Fibers were present throughout the ventral and dorsal BNST. (C). In the ventral BNST, an axon and varicosity (BDA-t) express immunoperoxidase reaction product for BDA without CRF labeling. (D). An axon terminal (BDA+CRF-t) contains ABC labeling for BDA and IGS labeling for CRF. Particles for CRF are widely dispersed throughout the bouton. ac: anterior commissure; d: dorsal; m: medial; ot: optic tract. Scale Bars: 1.0 mm (A-B); 0.5 μm (C-D)
CeA neurons expressing GFP rarely show retrograde labeling after FG injection in the commisural BNST, whereas GFP containing terminals contact CeA-BNST projection neurons
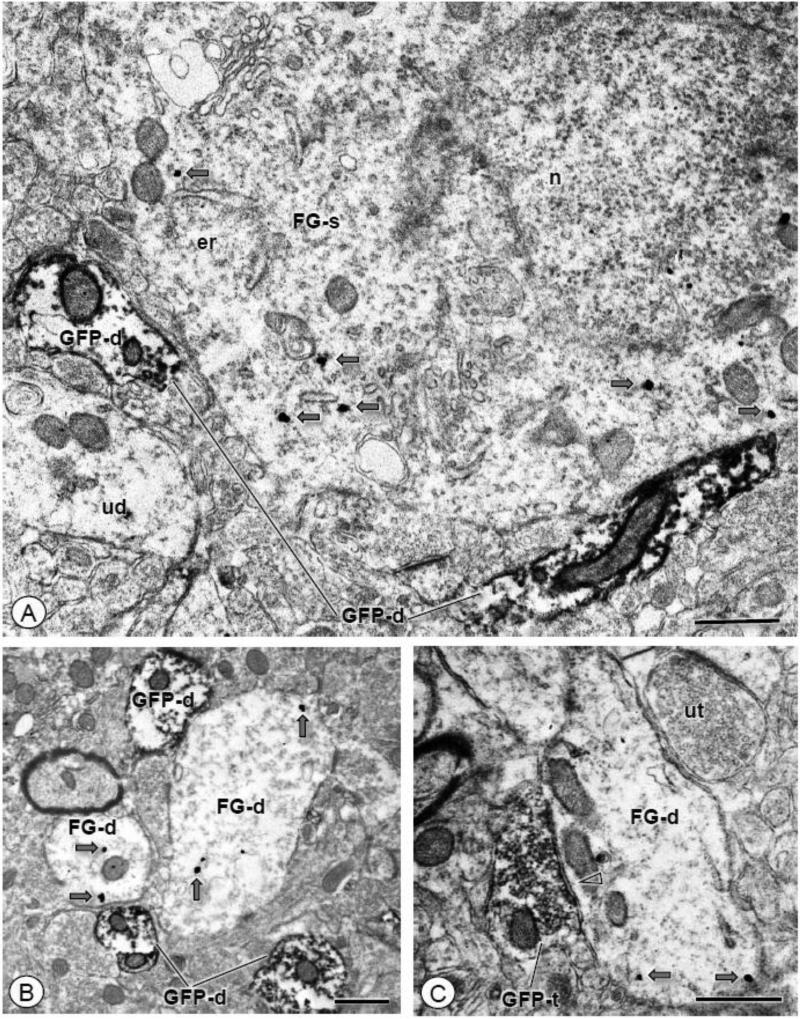
To determine if CeA-BNST projection neurons expressed CRF-R1, FG was injected in the commissural BNST of CRF-R1 GFP reporter mice (N = 3). In these mice, a total of 17,625 μm2 of tissue was sampled by EM in the lateral CeA, the area of the CeA showing prominent FG labeling by light microscopy. Immunoreactivity for FG was seen in somata typically devoid of GFP, although the surrounding neuropil was populated by GFP labeled dendritic profiles (Fig. 9A), as well as scattered dual labeled dendritic profiles. In addition, single GFP labeled profiles and FG labeled dendrites were frequently located in close proximity to each other (Fig. 9B), and GFP labeled axon terminals often contacted FG labeled dendritic profiles (Fig. 9C).
Figure 9. GFP and FG are typically present in distinct neuronal profiles in the CeA.
(A). A single labeled soma (FG-s) shows IGS labeling for the retrograde tracer (gray arrows). Neuronal profiles can be seen in the surrounding neuropil, including unlabeled dendritic profiles (ud), as well as those showing single ABC labeling for GFP (GFP-d). (B). Dendritic profiles (FG-d) show IGS labeling for FG (gray arrows). These dendrites are in close proximity to small dendritic profiles (GFP-d) showing exclusive immunoperoxidase reaction product for GFP. (C). A dendritic profile (FG-d) shows IGS labeling for FG (gray arrows). This profile is apposed to an axon terminal expressing diffuse immunoperoxidase labeling for GFP. This terminal forms a symmetric inhibitory type synapse (open arrow head) with the FG labeled dendrite. Gc: Golgi complex; er: endoplasmic reticula; n: nucleus; ut: unlabeled terminal. Scale bars: 1.0 μm (A); 0.5 μm (B-C)
A total of 526 neuronal profiles showing single labeling for GFP or FG, or dual labeling for GFP and FG were counted in the sampled region. A total of 347 somatodendritic profiles showed immunoreactivity for GFP, 33 (9%) of which were co-labeled for FG, and most of these were dendrites. Of the 76 GFP labeled axons, 22% contacted somata or dendritic profiles that showed FG and/or GFP immunoreactivity.
DISCUSSION
Our dual labeling electron microscopic studies revealed extensive expression of NR1 within CRF containing somata and dendrites in the CeA (see Figure 10 for a summary of the major ultrastructural findings). In addition, CRF containing axon terminals frequently contacted NR1 labeled postsynaptic structures. Somata and dendrites of GFP expressing neurons also expressed NR1, and GFP labeled axon terminals contacted NR1 immunoreactive dendritic profiles in the CeA of mice expressing GFP under the control of the CRF-R1 promoter. Ultrastructural tract tracing revealed that many somata and dendrites of CeA neurons projecting to the BNST expressed CRF or were contacted by CRF expressing axon terminals. However, GFP was found in few projection neurons, although axon terminals with GFP frequently contacted CeA-BNST projection neurons. These results indicate that the NMDA receptor is positioned for the postsynaptic regulation of CRF expressing neurons in the CeA, and the modulation of signaling induced by presynaptic CRF release from CRF inputs. In addition, the NMDA receptor is also positioned for the postsynaptic modulation of CRF-R1 expressing neurons, or inputs from CRF-R1 expressing neurons.
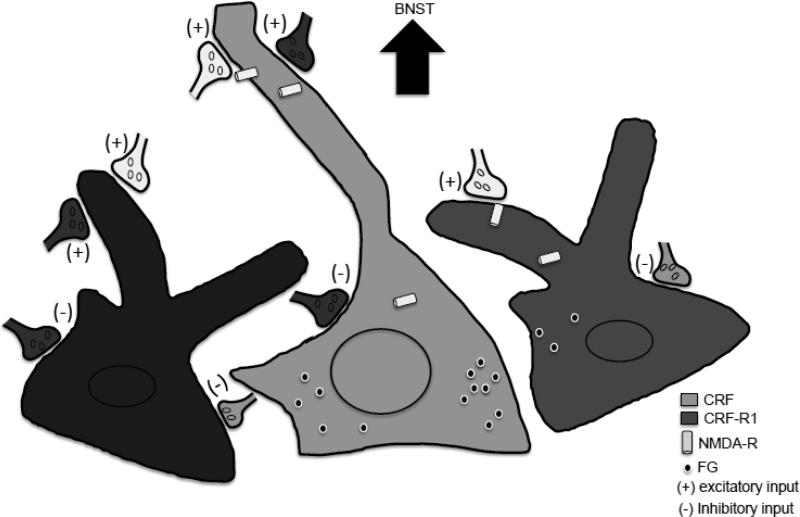
Figure 10. Schematic overview of CRF, NR1, and CRF-R1 distributions in CeA neurons including those projecting to the BNST.
Numerous CRF expressing neurons (light gray) were labeled for NR1, indicating the presence of NMDA receptors (light gray cylinders). Dendrites and somata of these neurons received extensive contacts from unlabeled (no fill) excitatory (+) and inhibitory (−) inputs. Axon terminals showing CRF immunoreactivity (light gray) contacted unlabelled (not shown) as well as NR1 labeled neurons, with or without (not shown) co-expression of CRF. Axon terminals labeled for CRF were also shown to contact GFP labeled somata and dendrites (medium gray) in the CRF-R1 reporter mouse. Dendrites of CRF-R1 expressing neurons also showed extensive NR1 labeling, indicating that CRF-R1 neurons also express NMDA receptors. Individual somata and dendrites also co-expressed CRF and GFP (black) in the CRF-R1 reporter mouse; although not directly determined, given the high degree of co-expression of NR1 with each of the latter, it is highly likely that CRF and CRF-R1 neurons also express NMDA receptors. Neurons expressing CRF, as well as those devoid of CRF labeling (not shown), were labeled for the retrograde tracer FG (black circles) after administration into the BNST. A small number of GFP labeled neurons were found to project to the BNST in the CRF-R1 reporter mouse. Axon terminals labeled for CRF or GFP were observed to form synapses with FG labeled dendrites. The relative numbers of projection neurons are indicated by the density of symbols for FG.
Methodological considerations
Somata, dendrites, and axons originating from CRF-R1 neurons were identified by use of a transgenic BAC mouse line that expresses GFP under the control of CRF-R1 promoter activation (Justice et al. 2008). The BAC-based approach provides for the inclusion of most promoter and enhancer sequences that regulate receptor expression in a manner that, to a large degree, parallels endogenous gene expression patterns. In this mouse line, the expression of GFP closely corresponds to CRF-R1 mRNA expression. Moreover, GFP extensively fills somata, dendrites, and axons of neuronal profiles and can readily provide a qualitative marker of cells with an active CRF-R1 promoter. However, mismatches in the turnover or cellular distribution of CRF-R1 protein and GFP would be expected to contribute to under- or overestimation of the actual content of CRF-R1 in any particular cellular profile. Thus, although the presence of functional CRF-R1 cannot be inferred by GFP labeling alone, expression of the reporter does indicate that labeled pre- and postsynaptic structures originate from neurons that actively express the CRF receptor. Despite these caveats, we believe the latter possibilities may not be serious concerns given the close correspondence between the pattern of labeling seen in the GFP reporter mouse and prior results seen with a low avidity CRF-R1 antisera (Jaferi and Pickel 2009; Treweek et al. 2009). Thus, it is likely that many GFP labeled profiles do contain the CRF-R1.
Regarding the retrograde labeling study, tracer was injected at the commissural level of the BNST, generally diffusing into the dorsal medial, as well as the ventral medial and lateral regions, resulting in preferential, although not exclusive, labeling in the lateral CeA. However, the existence of subregional differences in the pattern of CeA-BNST projections has also been described; more medial regions of the CeA have been previously shown to innervate the anterior BNST (Sun et al. 1991), an area typically outside the boundaries of tracer diffusion seen in the present study. Thus, in this context, it should be stressed that the projection patterns of CRF and CRF-R1-GFP expressing neurons described here are necessarily limited to the specific area of the BNST where tracer was administered.
In the present study, the general pattern of CRF, as well retrograde labeling, in the mouse was found to be in agreement with prior studies in this species (Beckerman and Glass 2012; Treweek et al. 2009), and in the rat as well (Cassell and Gray 1989; Jaferi and Pickel 2009; Justice et al. 2008; Treweek et al. 2009). These broad anatomical similarities correspond to generally similar responses of the CeA CRF system to relevant behaviors, including exposure to, or withdrawal from, drugs of abuse (McNally and Akil 2002; Nie et al. 2004; Roberto et al. 2010; Shaw-Lutchman et al. 2002), in addition to conditioned fear behaviors (Kolber et al. 2008; Thompson et al. 2004).
NR1 containing somata and dendrites co-express CRF and are contacted by CRF-labeled axon terminals
Separate populations of neuronal cell bodies in the CeA showed exclusive labeling for either CRF or NR1, however, another group of somata showed co-labeling for both. In dual labeled neurons, immunoreactivity for NR1 was often found near sites of protein synthesis, packaging, and transport, including Golgi complexes and endoplasmic reticula in cell bodies containing smooth and rough nuclei. In addition, NR1 also was present in both intracellular and plasmalemmal compartments in proximal and distal dendritic profiles that showed labeling for CRF. Dual labeled dendritic profiles were frequently contacted by unlabeled axon terminals, and although these frequently formed non-synaptic associations, when synaptic junctions were apparent these were mainly asymmetric excitatory-type specializations characteristic of glutamatergic inputs. The present results suggest that CRF neurons express the NMDA receptor, and that this protein is transported to intracellular locations in proximal and distal dendrites. Moreover, some of these proteins reach the plasma membrane where they are, presumably, positioned for functional activation by released glutamate. These characteristics are consistent with prior descriptions of CeA dendrites that expressed NR1 and the mu-opioid receptor (Glass et al. 2009). The latter protein also has been shown to be expressed in CRF containing somata and dendritic profiles (Jaferi and Pickel 2009). Together, these results suggest that a population of CeA CRF neurons may be capable of integrating signaling by NMDA receptor and mu-opioid receptor ligands.
In addition to somata and dendritic profiles, axon terminals also showed CRF labeling. These axon terminals were heterogeneous in size and typically devoid of NR1 labeling. Axonal profiles labeled for CRF formed appositions or synapses with unlabeled, as well as single (i.e. NR1, CRF) or dual labeled somata and dendritic profiles. When synapses were apparent, these were most commonly symmetric inhibitory-type junctions characteristic of those containing GABA. The presence of CRF within inhibitory-type axon terminals is consistent with interactions between this peptide and the inhibitory transmitter GABA (Skorzewska et al. 2009) in fear related behaviors (Pitts et al. 2009). It is also consistent with the significant enhancement of GABA mediated transmission in the CeA by ethanol and CRF in mice (Nie et al. 2004) and rats (Cruz et al. 2012; Roberto et al. 2010), as well as the potentiation of these effects in ethanol dependent rats (Cruz et al. 2012; Roberto et al. 2010). Moreover, the presence of the CRF-R1 GFP reporter protein in symmetric type synapses is also consistent with the essential role of functional presynaptic CRF-R1 in the elevation in GABA transmission by ethanol and CRF (Nie et al. 2004). Although the origin of CRF inputs to the CeA were not identified in the present study, previously identified CRF projections into the CeA include functionally relevant contacts from the dorsomedial serotonergic dorsal raphe nucleus, an area implicated in stress responses and anxiety disorders (Commons et al. 2003).
GFP is found in somata and dendrites containing NR1 and in axons contacting NR1 labeled dendrites
In the CRF-R1 BAC mouse, immunoreactivity for GFP was expressed primarily within dendrites, as well as axons and axon terminals, however, it was expressed in only a small population of neuronal cell bodies. This is consistent with a prior light microscopic report in this CRF-R1 GFP expressing mouse line, and also is consistent with low levels of CRF-R1 mRNA in the lateral CeA (Justice et al. 2008). Some of the larger proximal GFP labeled dendritic processes likely arise from the scattered somata in the lateral CeA, while the larger population of smaller distal profiles are most likely derived from encroaching dendrites originating from GFP expressing neurons in the medial CeA, or from areas adjacent to the CeA (Justice et al. 2008). Proximal and distal dendrites expressing GFP also frequently contained NR1, the latter of which was found in intracellular locations, as well as near the plasmalemma.
In addition to dendrites, GFP was often found in axons and axons terminals, including a subpopulation that contacted dendritic profiles expressing NR1. Many GFP labeled axon terminals formed asymmetric junctions suggesting that they from excitatory contacts. This finding is consistent with prior in vivo microdialysis (Skorzewska et al. 2009) and in vitro electrophysiological analysis of CeA slices (Liu et al. 2004) indicating that CRF stimulation is associated with an increase in CeA glutamate release and neuronal activity, respectively. In addition, the current finding of extensive co-labeling of GFP and NR1, as well as the expression of CRF in axon terminals contacting NR1 labeled dendrites, is in agreement with the ability of CRF to heighten long-term potentiation in the CeA in a model of psychostimulant withdrawal via postsynaptic processes that involve activation of NMDA receptors (Fu et al. 2007; Pollandt et al. 2006). Thus, the current ultrastructural results provide a synaptic basis for elucidating the relationship between CRF and NMDA receptor activated glutamate systems in the CeA that are involved in stress and addiction.
CeA-BNST projection neurons commonly express CRF and are contacted by axon terminals expressing CRF or GFP
In mice injected with FG in the BNST, retrograde labeled neurons in the lateral CeA also contained CRF as determined by EM analysis. The presence of CRF within axonal projections of CeA neurons that innervate the BNST also was verified in animals receiving the anterograde tracer BDA in the CeA. The BNST receives extensive CRF input, and this peptide has been shown to modulate the local actions of GABA and glutamate (Kash et al. 2008; Kash and Winder 2006). In addition, BNST CRF has also been show to modulate the interaction between glutamate and monoamine signaling (Kash et al. 2008; Nobis et al. 2011), which may be critical in addiction related behaviors (Dumont et al. 2005). The present results provide definitive fine structural data showing direct symmetric inhibitory type synapses between axon terminals of CRF CeA neurons and dendrites of dorsal and ventral BSNT neurons, and strengthen previous empirical and theoretical work positing that CRF is a critical signaling molecule in the monosynaptic pathway from the CeA to the BNST that is critically involved in stress related behaviors (Walker and Davis 2008) implicated in addiction and other psychiatric disorders.
Somata of CeA neurons expressing labeling for FG and CRF contained large nuclei and a dense endomembrane system, both cytological features similar to CeA-BNST projection neurons that express NR1 and mu-opioid receptor as previously shown by triple labeling immunocytochemistry (Beckerman and Glass 2012). In addition to co-expression in somata and dendrites, both single FG labeled, and dual FG and CRF labeled somata and dendrites were contacted by CRF expressing axon terminals. When identifiable synapses were observed, CRF labeled axon terminals commonly formed symmetric inhibitory type synapses typically in the perisomatic and proximal dendritic regions of CeA BNST projection neurons. These results demonstrate that CeA-BNST projection neurons expressing CRF are contacted by CRF axon terminals, indicating that the output of, at least a population of these projection neurons, is regulated by CRF.
Unlike CRF, few GFP somata contained retrogradely transported FG following an injection in the BNST, although dual labeled dendritic profiles were found. However, many GFP expressing axon terminals formed appositions with retrogradely labeled somata and dendrites. In addition, GFP expressing axon terminals made synapses with CeA-BNST projection neurons. These contacts included symmetric inhibitory junctions with somata and proximal dendrites, as well as asymmetric excitatory-type synapses with smaller distal dendritic processes. The latter results suggest that the activity of CeA-BNST projection neurons is influenced by GABA or glutamate release from axon terminals of neurons that express CRF-R1.
Functional implications
The CeA CRF system is highly sensitive to the experience of diverse types of stress (Hatalski et al. 1998; Hsu et al. 1998; Makino et al. 1999), including withdrawal from most drugs of abuse (Koob 2010). Significantly, the sensitivity of the CeA CRF system to stress exposure can be persistently influenced by prior stress history (McCormick et al. 2007; Plotsky et al. 2005), suggesting that stress-induced experience dependent plasticity can modulate the response of the CeA CRF system to subsequent encounters with stressors. In the context of the present ultrastructural data, the experience dependent plasticity of the CeA CRF system is consistent with the known involvement of local NMDA receptors in cellular models of synaptic plasticity (Samson and Paré 2005), a phenomenon that is itself subject to drug withdrawal-induced adaptations (Pollandt et al. 2006). The latter findings also comport with reports that functional CeA NMDA receptors are required for affective learning and memory (Goosens and Maren 2003), including context learning associated with drug withdrawal (Glass et al. 2008). Therefore, the extensive presence of NR1 in dendrites of CeA CRF containing neurons, as well as postsynaptic structures contacted by axon terminals expressing CRF, may provide a cellular and synaptic framework for understanding how the experience of stressors elicits behavioral responses, which can, under the proper conditions, be translated into learned behaviors.
The experience of stress can significantly modulate the behavioral impact of addictive drugs (Lu et al. 2003). The pathway between the CeA and BNST has been implicated in stress-related behaviors and the neural response to acute opiate exposure (Beckerman and Glass 2012) and opiate withdrawal (Nakagawa et al. 2005). In addition, within this pathway, CRF has been implicated in neurobehavioral responses to stress (Jasnow et al. 2004) and models of stress-induced relapse of drug self-administration (Erb et al. 2001). Therefore, CRF CeA-BNST projection neurons, and their CRF, CRF-R1, and/or glutamate expressing inputs may be essential integrators of the experience of stressors, addictive drugs, and learned behaviors implicated in addiction. In addition, the presence of CRF in GABA-type synapses, may provide a synaptic substrate for emerging GABA-based therapeutics for addictive disease (Addolorato et al. 2012).
Highlights.
Corticotropin-releasing factor (CRF) is expressed in central amygdala (CeA) neurons
NMDA-NR1 is present in CRF containing somatodendrites of CeA neurons
NMDA-NR1 is found in dendrites of CRF type 1 receptor expressing neurons
CeA neurons projecting to the bed nucleus of the stria terminalis (BNST) express CRF
Axon terminals containing CRF synapse with CeA-BNST projection neurons
Acknowledgments
Supported by: NIH grants HL098351 (MJG and TAM), DA016735, DA024030 (MJG), HL09571, DA08259 (TAM) and T32 DA007274 (TAV)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Addolorato G, Leggio L, Hopf FW, Diana M, Bonci A. Novel therapeutic strategies for alcohol and drug addiction: focus on GABA, ion channels and transcranial magnetic stimulation. Neuropsychopharmacol. 2012;37:163–77. doi: 10.1038/npp.2011.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrzejewski ME, Sadeghian K, Kelley AE. Central amygdalar and dorsal striatal NMDA receptor involvement in instrumental learning and spontaneous behavior. Behav. Neurosci. 2004;118:715–29. doi: 10.1037/0735-7044.118.4.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvaniti K, Huang Q, Richard D. Effects of leptin and corticosterone on the expression of corticotropin-releasing hormone, agouti-related protein, and proopiomelanocortin in the brain of ob/ob mouse. Neuroendocrinol. 2001;73:227–36. doi: 10.1159/000054639. [DOI] [PubMed] [Google Scholar]
- Beckerman MA, Glass MJ. The NMDA-NR1 receptor subunit and the mu-opioid receptor are expressed in somatodendritic compartments of central nucleus of the amygdala neurons projecting to the bed nucleus of the stria terminalis. Exp. Neurol. 2012;234:112–126. doi: 10.1016/j.expneurol.2011.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MR, Gray TS. Peptide injections into the amygdala of conscious rats: effects on blood pressure, heart rate, and plasma catecholamines. Reg. Peptides. 1988;21:95–106. doi: 10.1016/0167-0115(88)90094-8. [DOI] [PubMed] [Google Scholar]
- Canteras NS, Swanson LW. Projections of the ventral subiculum to the amygdala, septum, and hypothalamus: a PHAL anterograde tract-tracing study in the rat. J. Comp. Neurol. 1992;324:180–94. doi: 10.1002/cne.903240204. [DOI] [PubMed] [Google Scholar]
- Cassell MD, Gray TS. Morphology of peptide-immunoreactive neurons in the rat central nucleus of the amygdala. J. Comp. Neurol. 1989;281:320–33. doi: 10.1002/cne.902810212. [DOI] [PubMed] [Google Scholar]
- Commons KG, Connolley KR, Valentino RJ. A neurochemically distinct dorsal raphe limbic circuit with a potential role in affective disorders. Neuropsychopharmacol. 2003;28:206–15. doi: 10.1038/sj.npp.1300045. [DOI] [PubMed] [Google Scholar]
- Cottone P, Sabino V, Roberto M, Bajo M, Pockros L, Frihauf JB, Fekete EM, Steardo L, Rice KC, Grigoriadis DE, Conti B, Koob GF, Zorrilla EP. CRF system recruitment mediates dark side of compulsive eating. PNAS. 2009;106:20016–20. doi: 10.1073/pnas.0908789106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz MT, Herman MA, Kallupi M, Roberto M. Nociceptin/orphanin FQ blockade of corticotropin-releasing factor-induced gamma-aminobutyric acid release in central amygdala is enhanced after chronic ethanol exposure. Biol. Psychiatry. 2012;71:666–76. doi: 10.1016/j.biopsych.2011.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings S, Elde R, Ells J, Lindall A. Corticotropin-releasing factor immunoreactivity is widely distributed within the central nervous system of the rat: an immunohistochemical study. J. Neurosci. 1983;3:1355–68. doi: 10.1523/JNEUROSCI.03-07-01355.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Walker DL, Miles L, Grillon C. Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacol. 2010;35:105–35. doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont EC, Mark GP, Mader S, Williams JT. Self-administration enhances excitatory synaptic transmission in the bed nucleus of the stria terminalis. Nature Neurosci. 2005;8:413–414. doi: 10.1038/nn1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb S, Salmaso N, Rodaros D, Stewart J. A role for the CRF-containing pathway from central nucleus of the amygdala to bed nucleus of the stria terminalis in the stress-induced reinstatement of cocaine seeking in rats. Psychopharmacol. 2001;158:360–5. doi: 10.1007/s002130000642. [DOI] [PubMed] [Google Scholar]
- Fu Y, Neugebauer V. Differential mechanisms of CRF1 and CRF2 receptor functions in the amygdala in pain-related synaptic facilitation and behavior. J. Neurosci. 2008;28:3861–76. doi: 10.1523/JNEUROSCI.0227-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Pollandt S, Liu J, Krishnan B, Genzer K, Orozco-Cabal L, Gallagher JP, Shinnick-Gallagher P. Long-term potentiation (LTP) in the central amygdala (CeA) is enhanced after prolonged withdrawal from chronic cocaine and requires CRF1 receptors. J. Neurophysiol. 2007;97:937–41. doi: 10.1152/jn.00349.2006. [DOI] [PubMed] [Google Scholar]
- Glass MJ. The role of functional postsynaptic NMDA receptors in the central nucleus of the amygdala in opioid dependence. Vitam. Horm. 2010;82:145–66. doi: 10.1016/S0083-6729(10)82008-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass MJ, Hegarty DM, Oselkin M, Quimson L, S.M. S, Xu Q, Pickel VM, Inturrisi CE. Conditional deletion of the NMDA-NR1 receptor subunit gene in the central nucleus of the amygdala inhibits naloxone-induced conditioned place aversion in morphine dependent mice. Exp. Neurol. 2008;213:57–70. doi: 10.1016/j.expneurol.2008.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass MJ, Vanyo L, Quimson L, Pickel VM. Ultrastructural relationship between NMDA NR1 and mu-opioid receptor in the mouse central nucleus of the amygdala. Neurosci. 2009;163:857–67. doi: 10.1016/j.neuroscience.2009.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goosens KA, Maren S. Pretraining NMDA receptor blockade in the basolateral complex, but not the central nucleus, of the amygdala prevents savings of conditional fear. Behav. Neurosci. 2003;117:738–50. doi: 10.1037/0735-7044.117.4.738. [DOI] [PubMed] [Google Scholar]
- Gray TS, Cassell MD, Kiss JZ. Distribution of pro-opiomelanocortin-derived peptides and enkephalins in the rat central nucleus of the amygdala. Brain Res. 1984;306:354–8. doi: 10.1016/0006-8993(84)90386-x. [DOI] [PubMed] [Google Scholar]
- Hand GA, Hewitt CB, Fulk LJ, Stock HS, Carson JA, Davis JM, Wilson MA. Differential release of corticotropin-releasing hormone (CRH) in the amygdala during different types of stressors. Brain Res. 2002;949:122–30. doi: 10.1016/s0006-8993(02)02972-4. [DOI] [PubMed] [Google Scholar]
- Hatalski CG, Guirguis C, Baram TZ. Corticotropin releasing factor mRNA expression in the hypothalamic paraventricular nucleus and the central nucleus of the amygdala is modulated by repeated acute stress in the immature rat. J. Neuroendocrinol. 1998;10:663–9. doi: 10.1046/j.1365-2826.1998.00246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu DT, Chen FL, Takahashi LK, Kalin NH. Rapid stress-induced elevations in corticotropin-releasing hormone mRNA in rat central amygdala nucleus and hypothalamic paraventricular nucleus: an in situ hybridization analysis. Brain Res. 1998;788:305–10. doi: 10.1016/s0006-8993(98)00032-8. [DOI] [PubMed] [Google Scholar]
- Jaferi A, Pickel VM. Mu-opioid and corticotropin-releasing-factor receptors show largely postsynaptic co-expression,and separate presynaptic distributions, in the mouse central amygdala and bed nucleus of the stria terminalis. Neurosci. 2009;159:526–39. doi: 10.1016/j.neuroscience.2008.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasnow AM, Davis M, Huhman KL. Involvement of central amygdalar and bed nucleus of the stria terminalis corticotropin-releasing factor in behavioral responses to social defeat. Behav. Neurosci. 2004;118:1052–61. doi: 10.1037/0735-7044.118.5.1052. [DOI] [PubMed] [Google Scholar]
- Justice NJ, Yuan ZF, Sawchenko PE, Vale W. Type 1 corticotropin-releasing factor receptor expression reported in BAC transgenic mice: implications for reconciling ligand-receptor mismatch in the central corticotropin-releasing factor system. J. Comp. Neurol. 2008;511:479–96. doi: 10.1002/cne.21848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kash TL, Nobis WP, Matthews RT, Winder DG. Dopamine enhances fast excitatory synaptic transmission in the extended amygdala by a CRF-R1-dependent process. J. Neurosci. 2008;28:13856–65. doi: 10.1523/JNEUROSCI.4715-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kash TL, Winder DG. Neuropeptide Y and corticotropin-releasing factor bi-directionally modulate inhibitory synaptic transmission in the bed nucleus of the stria terminalis. Neuropharmacol. 2006;51:1013–22. doi: 10.1016/j.neuropharm.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Kehne JH, Cain CK. Therapeutic utility of non-peptidic CRF1 receptor antagonists in anxiety, depression, and stress-related disorders: evidence from animal models. Pharmacol. Therap. 2010;128:460–87. doi: 10.1016/j.pharmthera.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolber BJ, Roberts MS, Howell MP, Wozniak DF, Sands MS, Muglia LJ. Central amygdala glucocorticoid receptor action promotes fear-associated CRH activation and conditioning. PNAS. 2008;105:12004–9. doi: 10.1073/pnas.0803216105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Brain stress systems in the amygdala and addiction. Brain Res. 2009a;1293:61–75. doi: 10.1016/j.brainres.2009.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Neurobiological substrates for the dark side of compulsivity in addiction. Neuropharmacol. 56 Suppl. 2009b;1:18–31. doi: 10.1016/j.neuropharm.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. The role of CRF and CRF-related peptides in the dark side of addiction. Brain Res. 2010;1314:3–14. doi: 10.1016/j.brainres.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Ann. Rev. Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Li S, Kirouac GJ. Projections from the paraventricular nucleus of the thalamus to the forebrain, with special emphasis on the extended amygdala. J. Comp. Neurol. 2008;506:263–87. doi: 10.1002/cne.21502. [DOI] [PubMed] [Google Scholar]
- Li W, Neugebauer V. Block of NMDA and non-NMDA receptor activation results in reduced background and evoked activity of central amygdala neurons in a model of arthritic pain. Pain. 2004;110:112–22. doi: 10.1016/j.pain.2004.03.015. [DOI] [PubMed] [Google Scholar]
- Liu J, Yu B, Neugebauer V, Grigoriadis DE, Rivier J, Vale WW, Shinnick-Gallagher P, Gallagher JP. Corticotropin-releasing factor and Urocortin I modulate excitatory glutamatergic synaptic transmission. J. Neurosci. 2004;24:4020–9. doi: 10.1523/JNEUROSCI.5531-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery-Gionta EG, Navarro M, Li C, Pleil KE, Rinker JA, Cox BR, Sprow GM, Kash TL, Thiele TE. Corticotropin releasing factor signaling in the central amygdala is recruited during binge-like ethanol consumption in C57BL/6J mice. J. Neurosci. 2012;32:3405–13. doi: 10.1523/JNEUROSCI.6256-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Shepard JD, Hall FS, Shaham Y. Effect of environmental stressors on opiate and psychostimulant reinforcement, reinstatement and discrimination in rats: a review. Neurosci. Biobehav. Rev. 2003;27:457–91. doi: 10.1016/s0149-7634(03)00073-3. [DOI] [PubMed] [Google Scholar]
- Makino S, Shibasaki T, Yamauchi N, Nishioka T, Mimoto T, Wakabayashi I, Gold PW, Hashimoto K. Psychological stress increased corticotropin-releasing hormone mRNA and content in the central nucleus of the amygdala but not in the hypothalamic paraventricular nucleus in the rat. Brain Res. 1999;850:136–43. doi: 10.1016/s0006-8993(99)02114-9. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Merrick A, Secen J, Helmreich DL. Social instability in adolescence alters the central and peripheral hypothalamic-pituitary-adrenal responses to a repeated homotypic stressor in male and female rats. J. Neuroendocrinol. 2007;19:116–26. doi: 10.1111/j.1365-2826.2006.01515.x. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Cortical pathways to the mammalian amygdala. Prog. Neurobiol. 1998;55:257–332. doi: 10.1016/s0301-0082(98)00003-3. [DOI] [PubMed] [Google Scholar]
- McNally GP, Akil H. Role of corticotropin-releasing hormone in the amygdala and bed nucleus of the stria terminalis in the behavioral, pain modulatory, and endocrine consequences of opiate withdrawal. Neurosci. 2002;112:605–17. doi: 10.1016/s0306-4522(02)00105-7. [DOI] [PubMed] [Google Scholar]
- Merali Z, Anisman H, James JS, Kent P, Schulkin J. Effects of corticosterone on corticotrophin-releasing hormone and gastrin-releasing peptide release in response to an aversive stimulus in two regions of the forebrain (central nucleus of the amygdala and prefrontal cortex). Eur. J. Neurosci. 2008;28:165–72. doi: 10.1111/j.1460-9568.2008.06281.x. [DOI] [PubMed] [Google Scholar]
- Milner TA, Waters EM, Robinson DC, Pierce JP. Degenerating processes identified by electron microscpic immunocytochemical methods. In: Manfredi GKH, editor. Neurodegeneration, Methods and Protocols. Springer; New York: 2011. pp. 23–59. [DOI] [PubMed] [Google Scholar]
- Monaghan DT, Cotman CW. Distribuiton of N-methyl-D-aspartate-sensitive L-[3H]glutamate-binding sites in rat brain. J. Neurosci. 1985;5:2909–2919. doi: 10.1523/JNEUROSCI.05-11-02909.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T, Yamamoto R, Fujio M, Suzuki Y, Minami M, Satoh M, Kaneko S. Involvement of the bed nucleus of the stria terminalis activated by the central nucleus of the amygdala in the negative affective component of morphine withdrawal in rats. Neurosci. 2005;134:9–19. doi: 10.1016/j.neuroscience.2005.03.029. [DOI] [PubMed] [Google Scholar]
- Nie Z, Schweitzer P, Roberts AJ, Madamba SG, Moore SD, Siggins GR. Ethanol augments GABAergic transmission in the central amygdala via CRF1 receptors. Science. 2004;303:1512–4. doi: 10.1126/science.1092550. [DOI] [PubMed] [Google Scholar]
- Nobis WP, Kash TL, Silberman Y, Winder DG. beta-Adrenergic receptors enhance excitatory transmission in the bed nucleus of the stria terminalis through a corticotrophin-releasing factor receptor-dependent and cocaine-regulated mechanism. Biol. Psychiatry. 2011;69:1083–90. doi: 10.1016/j.biopsych.2010.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A, Palay SL, Webster H. The fine structure of the nervous system. Oxford University Press, Oxford University Press; 1991. [Google Scholar]
- Petralia RS, Yokotani N, Wenthold RJ. Light and electron microscope distribution of the NMDA receptor subunit NMDAR1 in the rat nervous system using a selective anti-peptide antibody. J. Neurosci. 1994;14:667–696. doi: 10.1523/JNEUROSCI.14-02-00667.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilcher WH, Joseph SA. Co-localization of CRF-ir perikarya and ACTH-ir fibers in rat brain. Brain Res. 1984;299:91–102. doi: 10.1016/0006-8993(84)90791-1. [DOI] [PubMed] [Google Scholar]
- Pitkanen A, Savander V, LeDoux JE. Organization of intra-amygdaloid circuitries in the rat: an emerging framework for understanding functions of the amygdala. Trends in Neurosci. 1997;20:517–23. doi: 10.1016/s0166-2236(97)01125-9. [DOI] [PubMed] [Google Scholar]
- Pitts MW, Takahashi LK. The central amygdala nucleus via corticotropin-releasing factor is necessary for time-limited consolidation processing but not storage of contextual fear memory. Neurobiol. Learn. Mem. 2011;95:86–91. doi: 10.1016/j.nlm.2010.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitts MW, Todorovic C, Blank T, Takahashi LK. The central nucleus of the amygdala and corticotropin-releasing factor: insights into contextual fear memory. J. Neurosci. 2009;29:7379–88. doi: 10.1523/JNEUROSCI.0740-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotsky PM, Thrivikraman KV, Nemeroff CB, Caldji C, Sharma S, Meaney MJ. Long-term consequences of neonatal rearing on central corticotropin-releasing factor systems in adult male rat offspring. Neuropsychopharmacol. 2005;30:2192–204. doi: 10.1038/sj.npp.1300769. [DOI] [PubMed] [Google Scholar]
- Pollandt S, Liu J, Orozco-Cabal L, Grigoriadis DE, Vale WW, Gallagher JP, Shinnick-Gallagher P. Cocaine withdrawal enhances long-term potentiation induced by corticotropin-releasing factor at central amygdala glutamatergic synapses via CRF, NMDA receptors and PKA. Eur. J. Neurosci. 2006;24:1733–43. doi: 10.1111/j.1460-9568.2006.05049.x. [DOI] [PubMed] [Google Scholar]
- Regev L, Neufeld-Cohen A, Tsoory M, Kuperman Y, Getselter D, Gil S, Chen A. Prolonged and site-specific over-expression of corticotropin-releasing factor reveals differential roles for extended amygdala nuclei in emotional regulation. Mol. Psychiat. 2011;16:714–28. doi: 10.1038/mp.2010.64. [DOI] [PubMed] [Google Scholar]
- Roberto M, Cruz MT, Gilpin NW, Sabino V, Schweitzer P, Bajo M, Cottone P, Madamba SG, Stouffer DG, Zorrilla EP, Koob GF, Siggins GR, Parsons LH. Corticotropin releasing factor-induced amygdala gamma-aminobutyric Acid release plays a key role in alcohol dependence. Biol. Psychiat. 2010;67:831–9. doi: 10.1016/j.biopsych.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakanaka M, Shibasaki T, Lederis K. Distribution and efferent projections of corticotropin-releasing factor-like immunoreactivity in the rat amygdaloid complex. Brain Res. 1986;382:213–38. doi: 10.1016/0006-8993(86)91332-6. [DOI] [PubMed] [Google Scholar]
- Salome N, Viltart O, Leman S, Sequeira H. Activation of ventrolateral medullary neurons projecting to spinal autonomic areas after chemical stimulation of the central nucleus of amygdala: a neuroanatomical study in the rat. Brain Res. 2001;890:287–295. doi: 10.1016/s0006-8993(00)03178-4. [DOI] [PubMed] [Google Scholar]
- Samson RD, Paré D. Activity-dependent synaptic plasticity in the central nucleus of the amygdala. J. Neurosci. 2005;25:1847–55. doi: 10.1523/JNEUROSCI.3713-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K, Mick G, Kiyama H, Tohyama M. Expression patterns of a glutamate-binding protein in the rat central nervous system: comparison with N-methyl-D-aspartate receptor subunit 1 in rat. Neurosci. 1995;64:459–475. doi: 10.1016/0306-4522(94)00335-3. [DOI] [PubMed] [Google Scholar]
- Shaw-Lutchman TZ, Barrot M, Wallace T, Gilden L, Zachariou V, Impey S, Duman RS, Storm D, Nestler EJ. Regional and cellular mapping of cAMP response element-mediated transcription during naltrexone-precipitated morphine withdrawal. J. Neurosci. 2002;22:3663–3672. doi: 10.1523/JNEUROSCI.22-09-03663.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skorzewska A, Bidzinski A, Hamed A, Lehner M, Turzynska D, Sobolewska A, Szyndler J, Maciejak P, Wislowska-Stanek A, Plaznik A. The effect of CRF and alpha-helical CRF((9-41)) on rat fear responses and amino acids release in the central nucleus of the amygdala. Neuropharmacol. 2009;57:148–56. doi: 10.1016/j.neuropharm.2009.04.016. [DOI] [PubMed] [Google Scholar]
- Sun N, Roberts L, Cassell MD. Rat central amygdaloid nucleus projections to the bed nucleus of the stria terminalis. Brain Res. Bull. 1991;27:651–62. doi: 10.1016/0361-9230(91)90041-h. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE, Rivier J, Vale WW. Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinol. 1983;36:165–86. doi: 10.1159/000123454. [DOI] [PubMed] [Google Scholar]
- Thompson BL, Erickson K, Schulkin J, Rosen JB. Corticosterone facilitates retention of contextually conditioned fear and increases CRH mRNA expression in the amygdala. Behav. Brain Res. 2004;149:209–15. doi: 10.1016/s0166-4328(03)00216-x. [DOI] [PubMed] [Google Scholar]
- Treweek JB, Jaferi A, Colago EE, Zhou P, Pickel VM. Electron microscopic localization of corticotropin-releasing factor (CRF) and CRF receptor in rat and mouse central nucleus of the amygdala. J. Comp. Neurol. 2009;512:323–35. doi: 10.1002/cne.21884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner BH, Herkenham M. Thalamoamygdaloid projections in the rat: a test of the amygdala's role in sensory processing. J. Comp. Neurol. 1991;313:295–325. doi: 10.1002/cne.903130208. [DOI] [PubMed] [Google Scholar]
- Van de Kar LD, Blair ML. Forebrain pathways mediating stress-induced hormone secretion. Front. Neuroendocrinol. 1999;20:1–48. doi: 10.1006/frne.1998.0172. [DOI] [PubMed] [Google Scholar]
- Van Pett K, Viau V, Bittencourt JC, Chan RK, Li HY, Arias C, Prins GS, Perrin M, Vale W, Sawchenko PE. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J. Comp. Neurol. 2000;428:191–212. doi: 10.1002/1096-9861(20001211)428:2<191::aid-cne1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Walker DL, Davis M. Role of the extended amygdala in short-duration versus sustained fear: a tribute to Dr. Lennart Heimer. Brain Struct. Funct. 2008;213:29–42. doi: 10.1007/s00429-008-0183-3. [DOI] [PubMed] [Google Scholar]
- Wang H, Cuzon VC, Pickel VM. Postnatal development of mu-opioid receptors in the rat caudate-putamen nucleus parallels asymmetric synapse formation. Neurosci. 2003;118:695–708. doi: 10.1016/s0306-4522(02)00926-0. [DOI] [PubMed] [Google Scholar]
- Wiersma A, Bohus B, Koolhaas JM. Corticotropin-releasing hormone microinfusion in the central amygdala diminishes a cardiac parasympathetic outflow under stress-free conditions. Brain Res. 1993;625:219–27. doi: 10.1016/0006-8993(93)91062-w. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Wee S, Zhao Y, Specio S, Boutrel B, Koob GF, Weiss F. Extended access cocaine self-administration differentially activates dorsal raphe and amygdala corticotropin-releasing factor systems in rats. Addiction Biol. 2012;17:300–8. doi: 10.1111/j.1369-1600.2011.00329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]