Abstract
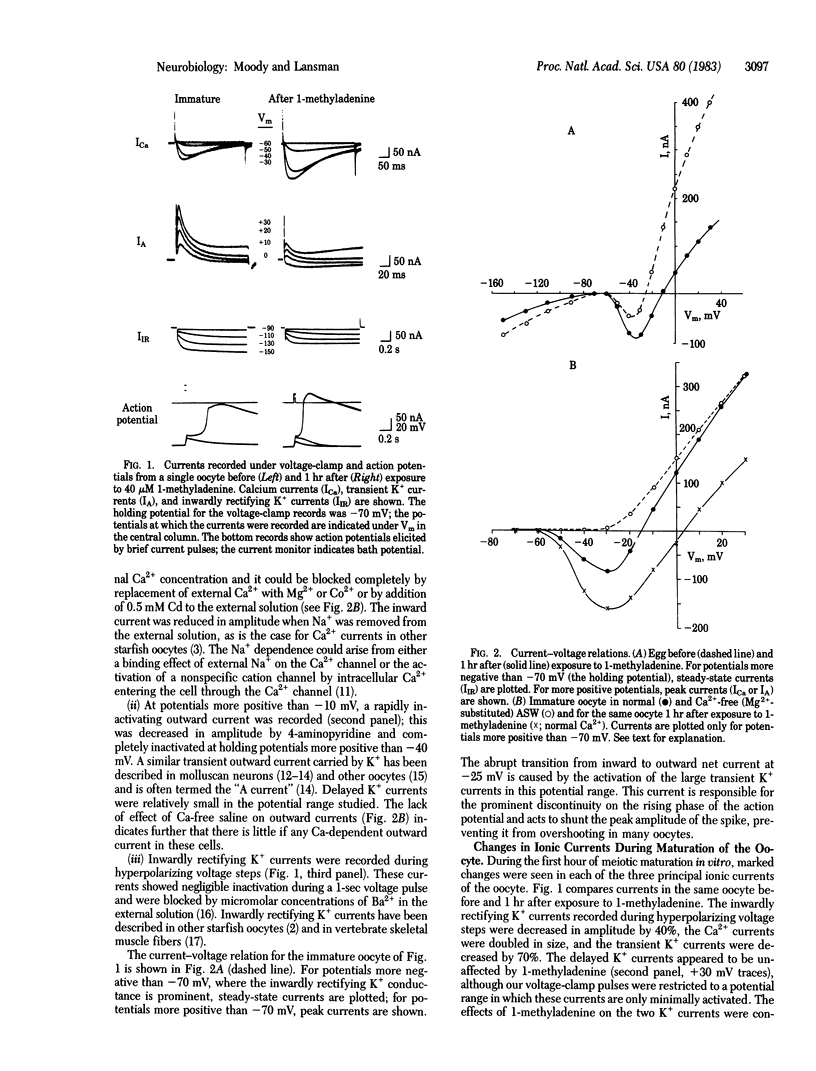
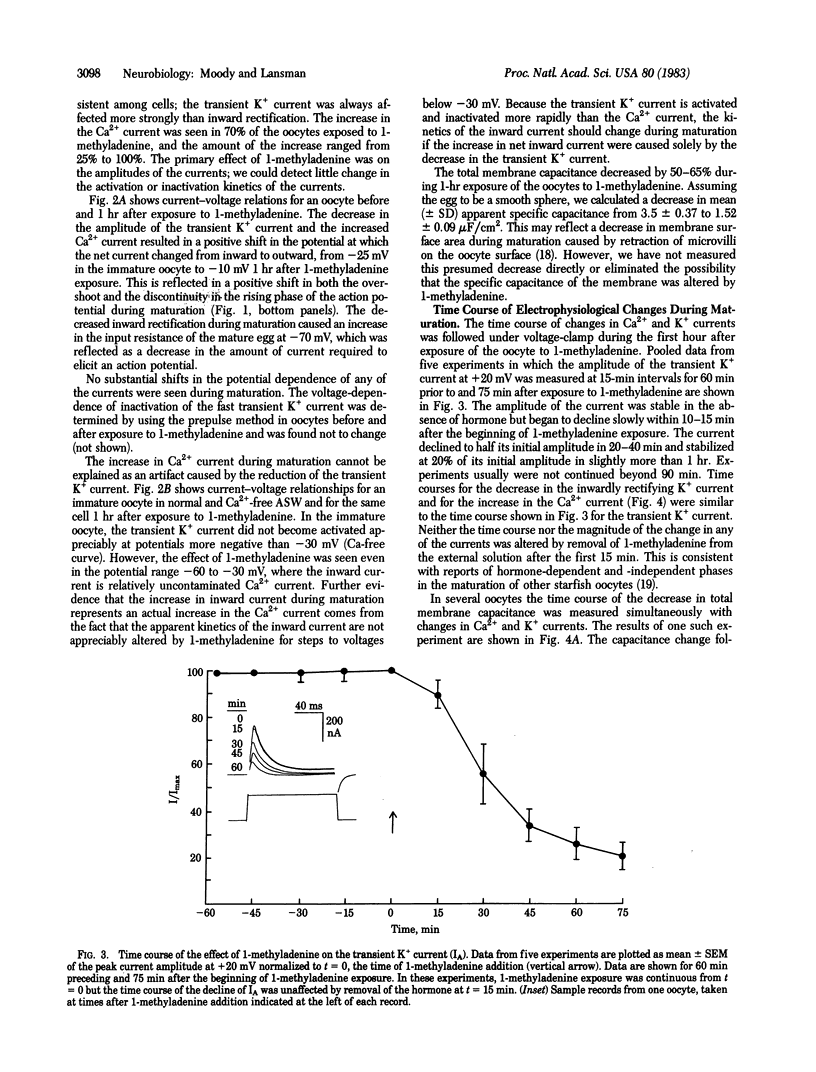
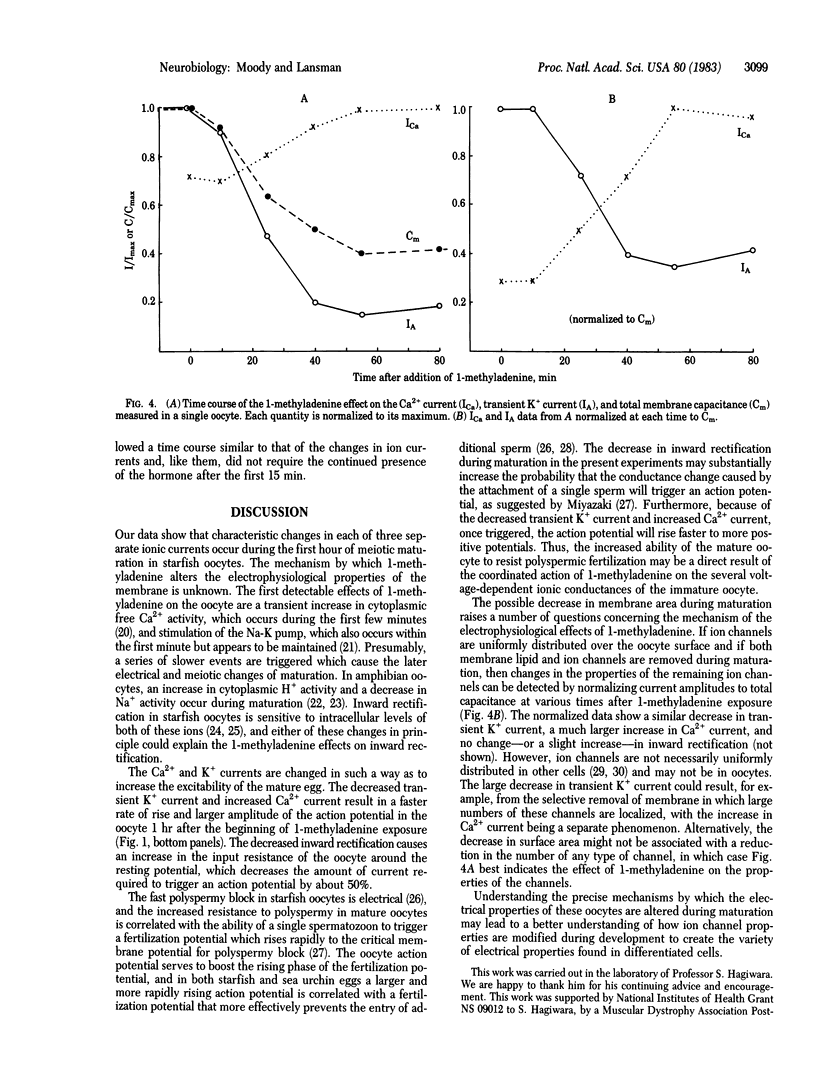
Changes in the electrical properties of starfish oocytes during hormone-induced maturation (the reinitiation of meiosis prior to fertilization) were studied by using the voltage-clamp technique. Three voltage-dependent ionic currents dominate the current-voltage relation of the immature oocyte: an inward Ca2+ current, a fast transient K+ current similar to the "A current" of molluscan neurons, and an inwardly rectifying K+ current. During in vitro maturation stimulated by the natural maturing hormone 1-methyladenine, gradual changes in the amplitudes of all three currents were seen: the Ca2+ currents became larger, and both K+ currents became smaller. The kinetics of the currents were not significantly altered during maturation. As a result of these changes, action potentials in the mature egg had lower thresholds, faster rates of rise, and larger overshoots than those of the immature oocyte. We also found that the total membrane capacitance decreased substantially during maturation, perhaps indicating a decrease in membrane surface area triggered by the hormone. The significance of these results is discussed in terms of the preparation of the immature oocyte for fertilization and the mechanisms of modification of ion channel properties during development.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian R. H., Chandler W. K., Hodgkin A. L. Slow changes in potassium permeability in skeletal muscle. J Physiol. 1970 Jul;208(3):645–668. doi: 10.1113/jphysiol.1970.sp009140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Neher E., Reuter H., Stevens C. F. Inward current channels activated by intracellular Ca in cultured cardiac cells. Nature. 1981 Dec 24;294(5843):752–754. doi: 10.1038/294752a0. [DOI] [PubMed] [Google Scholar]
- Connor J. A., Stevens C. F. Voltage clamp studies of a transient outward membrane current in gastropod neural somata. J Physiol. 1971 Feb;213(1):21–30. doi: 10.1113/jphysiol.1971.sp009365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap K. Localization of calcium channels in Paramecium caudatum. J Physiol. 1977 Sep;271(1):119–133. doi: 10.1113/jphysiol.1977.sp011993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAGIWARA S., KUSANO K., SAITO N. Membrane changes of Onchidium nerve cell in potassium-rich media. J Physiol. 1961 Mar;155:470–489. doi: 10.1113/jphysiol.1961.sp006640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Jaffe L. A. Electrical properties of egg cell membranes. Annu Rev Biophys Bioeng. 1979;8:385–416. doi: 10.1146/annurev.bb.08.060179.002125. [DOI] [PubMed] [Google Scholar]
- Hagiwara S., Miyazaki S., Moody W., Patlak J. Blocking effects of barium and hydrogen ions on the potassium current during anomalous rectification in the starfish egg. J Physiol. 1978 Jun;279:167–185. doi: 10.1113/jphysiol.1978.sp012338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Miyazaki S., Rosenthal N. P. Potassium current and the effect of cesium on this current during anomalous rectification of the egg cell membrane of a starfish. J Gen Physiol. 1976 Jun;67(6):621–638. doi: 10.1085/jgp.67.6.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Ozawa S., Sand O. Voltage clamp analysis of two inward current mechanisms in the egg cell membrane of a starfish. J Gen Physiol. 1975 May;65(5):617–644. doi: 10.1085/jgp.65.5.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Takahashi K. The anomalous rectification and cation selectivity of the membrane of a starfish egg cell. J Membr Biol. 1974;18(1):61–80. doi: 10.1007/BF01870103. [DOI] [PubMed] [Google Scholar]
- Hagiwara S., Yoshida S., Yoshii M. Transient and delayed potassium currents in the egg cell membrane of the coelenterate, Renilla koellikeri. J Physiol. 1981 Sep;318:123–141. doi: 10.1113/jphysiol.1981.sp013854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Yoshii M. Effects of internal potassium and sodium on the anomalous rectification of the starfish egg as examined by internal perfusion. J Physiol. 1979 Jul;292:251–265. doi: 10.1113/jphysiol.1979.sp012849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe L. A. Fast block to polyspermy in sea urchin eggs is electrically mediated. Nature. 1976 May 6;261(5555):68–71. doi: 10.1038/261068a0. [DOI] [PubMed] [Google Scholar]
- Kanatani H. Maturation-inducing substance in starfishes. Int Rev Cytol. 1973;35:253–298. doi: 10.1016/s0074-7696(08)60356-3. [DOI] [PubMed] [Google Scholar]
- Lee S. C., Steinhardt R. A. pH changes associated with meiotic maturation in oocytes of Xenopus laevis. Dev Biol. 1981 Jul 30;85(2):358–369. doi: 10.1016/0012-1606(81)90267-0. [DOI] [PubMed] [Google Scholar]
- Masui Y., Clarke H. J. Oocyte maturation. Int Rev Cytol. 1979;57:185–282. doi: 10.1016/s0074-7696(08)61464-3. [DOI] [PubMed] [Google Scholar]
- Miyazaki S. I., Ohmori H., Sasaki S. Potassium rectifications of the starfish oocyte membrane and their changes during oocyte maturation. J Physiol. 1975 Mar;246(1):55–78. doi: 10.1113/jphysiol.1975.sp010880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki S. Fast polyspermy block and activation potential. Electrophysiological bases for their changes during oocyte maturation of a starfish. Dev Biol. 1979 Jun;70(2):341–354. doi: 10.1016/0012-1606(79)90032-0. [DOI] [PubMed] [Google Scholar]
- Miyazaki S., Hirai S. Fast polyspermy block and activation potential. Correlated changes during oocyte maturation of a starfish. Dev Biol. 1979 Jun;70(2):327–340. doi: 10.1016/0012-1606(79)90031-9. [DOI] [PubMed] [Google Scholar]
- Moody W. J., Hagiwara S. Block of inward rectification by intracellular H+ in immature oocytes of the starfish Mediaster aequalis. J Gen Physiol. 1982 Jan;79(1):115–130. doi: 10.1085/jgp.79.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau M., Guerrier P., Doree M., Ashley C. C. Hormone-induced release of intracellular Ca2+ triggers meiosis in starfish oocytes. Nature. 1978 Mar 16;272(5650):251–253. doi: 10.1038/272251a0. [DOI] [PubMed] [Google Scholar]
- Moreau M., Guerrier P., Dorée M. Hormonal control of meiosis reinitation in starfish oocytes. New evidence for the absence of efficient intracellular receptors for l-methyladenine recognition. Exp Cell Res. 1978 Sep;115(2):245–249. doi: 10.1016/0014-4827(78)90278-1. [DOI] [PubMed] [Google Scholar]
- Neher E. Two fast transient current components during voltage clamp on snail neurons. J Gen Physiol. 1971 Jul;58(1):36–53. doi: 10.1085/jgp.58.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer L. G., Century T. J., Civan M. M. Activity coefficients of intracellular Na+ and K+ during development of frog oocytes. J Membr Biol. 1978 Apr 20;40(1):25–38. doi: 10.1007/BF01909737. [DOI] [PubMed] [Google Scholar]
- Shen S., Steinhardt R. A. An electrophysiological study of the membrane properties of the immature and mature oocyte of the batstar, Patiria miniata. Dev Biol. 1976 Jan;48(1):148–162. doi: 10.1016/0012-1606(76)90053-1. [DOI] [PubMed] [Google Scholar]
- Stevens M. Procedures for induction of spawning and meiotic maturation of starfish oocytes by treatment with 1-methyladenine. Exp Cell Res. 1970 Mar;59(3):482–484. doi: 10.1016/0014-4827(70)90658-0. [DOI] [PubMed] [Google Scholar]
- Stühmer W., Almers W. Photobleaching through glass micropipettes: sodium channels without lateral mobility in the sarcolemma of frog skeletal muscle. Proc Natl Acad Sci U S A. 1982 Feb;79(3):946–950. doi: 10.1073/pnas.79.3.946. [DOI] [PMC free article] [PubMed] [Google Scholar]


