Abstract
Background
Prediction of clinical outcome in the acute stage of ischaemic stroke can be difficult when based on patient characteristics, clinical findings and on non-contrast CT. CT perfusion and CT angiography may provide additional prognostic information and guide treatment in the early stage. We present the study protocol of the Dutch acute Stroke Trial (DUST). The DUST aims to assess the prognostic value of CT perfusion and CT angiography in predicting stroke outcome, in addition to patient characteristics and non-contrast CT. For this purpose, individualised prediction models for clinical outcome after stroke based on the best predictors from patient characteristics and CT imaging will be developed and validated.
Methods/design
The DUST is a prospective multi-centre cohort study in 1500 patients with suspected acute ischaemic stroke. All patients undergo non-contrast CT, CT perfusion and CT angiography within 9 hours after onset of the neurological deficits, and, if possible, follow-up imaging after 3 days. The primary outcome is a dichotomised score on the modified Rankin Scale, assessed at 90 days. A score of 0–2 represents good outcome, and a score of 3–6 represents poor outcome. Three logistic regression models will be developed, including patient characteristics and non-contrast CT (model A), with addition of CT angiography (model B), and CT perfusion parameters (model C). Model derivation will be performed in 60% of the study population, and model validation in the remaining 40% of the patients. Additional prognostic value of the models will be determined with the area under the curve (AUC) from the receiver operating characteristic (ROC) curve, calibration plots, assessment of goodness-of-fit, and likelihood ratio tests.
Discussion
This study will provide insight in the added prognostic value of CTP and CTA parameters in outcome prediction of acute stroke patients. The prediction models that will be developed in this study may help guide future treatment decisions in the acute stage of ischaemic stroke.
Keywords: Stroke, Ischaemia, Infarct, Prediction, CT perfusion, CT angiography
Background
Stroke is the fourth leading cause of death and the most common cause of long term disability [1]. Around 20% of stroke patients die within one year after the stroke event [1]. Prediction of long-term clinical outcome in acute ischaemic stroke is important, but not easy when based on patient characteristics, clinical findings and non-contrast CT (NCCT). Improving outcome prediction may help determine the optimal treatment strategy in the acute stage, when clinicians need to weigh possible benefit and harmful side effects of treatment. For treatment with intravenous thrombolysis with recombinant tissue type plasminogen activator (IV-rtPA) for example, time since stroke onset is the main safety criterion for patient selection. Additional imaging techniques might improve risk stratification.
CT imaging plays a critical role in the acute stage of stroke assessment as it is faster and more feasible than MRI and available 24/7 in stroke clinics worldwide. The NCCT can easily be extended with CT perfusion (CTP) and CT angiography (CTA) within a 5- to 10-minute examination [2] and has been successfully incorporated into acute stroke imaging protocols [3-5]. CTP and CTA may offer important parameters for predicting stroke outcome. CTP offers the possibility to differentiate reversible from irreversible ischaemic brain tissue and can give information on the permeability of the blood brain barrier [2,6-11]. On CTA, the location and extent of the arterial occlusion, collateral circulation, extracranial stenosis, and ischaemic changes within brain tissue can be visualised [12-17]. These parameters, including patient characteristics and NCCT findings, have been described separately in studies with MRI and/or CT, but have not been combined in one study to assess their combined predictive value.
The Dutch acute Stroke Trial (DUST) aims to improve prediction of outcome in patients with acute ischaemic stroke. For this purpose, we will assess the additional predictive value of CTP and CTA to the conventional determinants of patient characteristics and NCCT findings. We will develop an individualised prediction model for clinical outcome after stroke based on patient characteristics and CT parameters. This prediction model may help guide clinicians in making better treatment decisions.
Methods/design
Study design
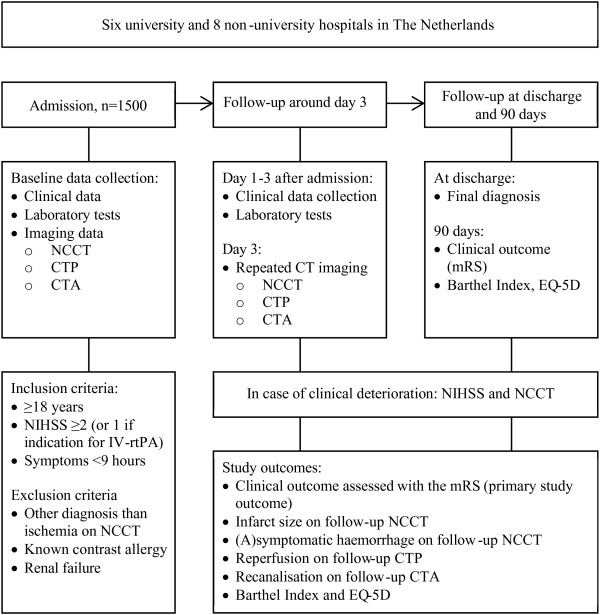
The DUST is a prospective multicentre observational cohort study with the aim to include 1500 patients with a suspected acute ischaemic stroke. Six university and 8 non-university hospitals in The Netherlands participate in this study. On admission, baseline characteristics are collected, laboratory testing is performed, and patients undergo CT imaging consisting of NCCT, CTP and CTA (see Figure 1). The following 3 days, additional patient characteristics and results of laboratory tests are collected. When possible, follow-up imaging is performed around 3 days after admission to evaluate if there is an infarct and if other admission imaging findings have changed. Information on the final diagnosis (or death) is collected at discharge. Clinical outcome, as primary study outcome, is assessed by the modified Rankin Scale (mRS) at 90 days.
Figure 1.
Study procedures and outcomes.
Inclusion and exclusion criteria
All patients with a clinical diagnosis of acute ischaemic stroke (both anterior and posterior circulation) are included if the following criteria are met: 1) older than 18 years, 2) National Institutes of Health Stroke Scale (NIHSS) ≥2, or 1 if an indication for IV-rtPA is present (e.g. isolated aphasia), 3) acute neurological deficit of less than nine hours of duration. Patients with an unknown time of symptom onset, i.e. patients who wake up or are found with stroke symptoms, can be included if the time from going to sleep or being last seen without symptoms, until imaging is less than 9 hours. Patients are excluded from the study if another diagnosis on admission NCCT explains the neurological deficits (e.g. intracerebral haemorrhage, subarachnoid haemorrhage, or tumour). Furthermore, patients cannot be included if they have a known contrast allergy or previously known renal failure at the time of admission.
Baseline clinical and laboratory data collection
Clinical data collection at baseline
After inclusion, details on stroke symptoms are collected, including time of symptom onset, time of arrival at the hospital, and stroke severity determined by the NIHSS [18]. As patients with an already poor functional status prior to the stroke symptoms are less likely to have a good outcome, pre-existing functional status is summarised in an mRS score [19]. Patient characteristics including blood pressure, pulse rate, length, weight, temperature, any medication use, and medical history are also collected. Regarding medical history, information is recorded about the presence of cardiovascular risk factors such as hypertension, diabetes mellitus, hyperlipidemia, prior vascular disease (including stroke and myocardial infarction), alcohol intake, smoking, and family history of vascular disease in first grade relatives. Furthermore, data on whether or not the patient is treated with IV-rtPA, intra-arterial thrombolysis (IAT), or mechanical thrombectomy, and time from stroke onset to treatment is collected.
Laboratory data collection at baseline
Laboratory tests performed at baseline include serum creatinine, glucose, hemoglobin, thrombocytes, C-reactive protein, and INR.
Baseline CT acquisition and post-processing
Acquisition
On admission to the hospital, all patients undergo NCCT, CTP and CTA. All imaging is performed on 40- to 320-detector CT scanners (Philips, Siemens, GE, Toshiba). NCCT parameters are 120 kV, 300–375 mAs, and a 5 mm reconstructed slice thickness.
CTP scans are generally performed with 80 kV and 150 mAs, and are also reconstructed as 5 mm contiguous axial slices. Forty ml of non-ionic contrast material is injected intravenously with a flow of 6 ml/s followed by 40 ml of saline with a flow of 6 ml/s. Images are acquired every 2 seconds for 50 seconds after initiation of contrast injection, followed by 1 image every 30 seconds from 60 to 210 seconds. CTP coverage ranges from 40 mm to full brain coverage and covers at least the basal ganglia up to the lateral ventricles to ensure that both Alberta Stroke Program Early CT Score (ASPECTS) levels are included [20-22]. CTP coverage can be lowered to cover the cerebellum to the occipital lobes if a stroke in the posterior circulation is expected, covering all three levels of the posterior circulation ASPECTS (pcASPECTS) [17].
For the CTA, from aortic arch to cranium vertex, 50–70 ml of non-ionic contrast material is injected intravenously with a flow of 6 ml/s followed by 40 ml of saline with a flow of 6 ml/s. The CTA scan delay after intravenous contrast injection is calculated for each patient individually from time to peak arterial enhancement on CTP, or by a trigger based on Hounsfield unit threshold measurement of contrast enhancement in the aortic arch.
Post-processing
The scan data from all participating hospitals are collected centrally in the University Medical Center Utrecht. Post-processing of the CTP and CTA scans is performed using commercially available software on an Extended Brilliance Workstation (version 4.5, Philips Healthcare).
The software for CT perfusion post-processing has been validated previously, and applies Gaussian curve fitting by least mean squares to obtain mathematical descriptions of the time-enhancement curves [4,23-25]. After calculation of the cerebral blood volume (CBV) from the area under the time attenuation curve, the mean transit time (MTT) is computed by using a deconvolution operation [26]. Subsequently, cerebral blood flow (CBF) is calculated according to the central volume principle (CBF = CBV/MTT). Based upon previously reported MTT and CBV thresholds, threshold-defined penumbra and infarct core maps are calculated as well as the penumbra/infarct core index [4]. The total ischaemic area is defined as a relative measure of MTT ≥145% compared to the contralateral (unaffected) hemisphere. Within this ischaemic area, the infarct core is separated from the penumbra by an absolute threshold value of CBV <2.0 ml/100 g.
For the CTA, thin slice data is reviewed, with interactive maximum intensity projection (MIP) and multiplanar reconstruction (MPR) possibilities, to assess the anatomy and pathology of the extracranial and intracranial vessels. Additional automated vessel segmentation is performed for the carotid and vertebral arteries to assess the degree of stenosis.
Baseline CT imaging data collection
All admission scans are assessed by an observer with at least five years of experience in neurovascular imaging (from a pool of three observers). Except for the side of symptoms, observers are blinded for all clinical information, including follow-up scans and clinical outcome.
For NCCT, presence of a hyperdense vessel sign is determined and early ischaemic changes are assessed with ASPECTS or pcASPECTS [12,20-22]. An area is considered ischaemic on NCCT if there is parenchymal hypoattenuation with or without swelling of the brain [21,22]. Areas with isolated cortical swelling, but without hypoattenuation, are not considered ischaemic [22]. Presence of haemorrhage, atrophy, white matter lesions, or old infarct is also noted. On CTA source images (CTA-SI), areas with diminished contrast enhancement are considered ischaemic and are assessed with (pc)ASPECTS [12]. Next, the CTA is evaluated for location and extent of arterial occlusion, leptomeningeal collateral circulation, circle of Willis configuration, presence of dissection or sinus thrombosis, as well as presence and degree of carotid or vertebrobasilar stenosis, and presence of intracranial atherosclerosis. Measurement of carotid or vertebrobasilar stenosis is performed according to the NASCET method [27]. For CTP, presence of a perfusion deficit is visually assessed on CBV, CBF, MTT, time to peak (TTP), and penumbra/infarct core maps, and evaluated with (pc)ASPECTS [11,28]. Reliability of ASPECTS has been shown to be fair for NCCT, moderate for CTA-SI and excellent for CTP [29], and inter-observer agreement is excellent for assessment of site of occlusion, good for collateral circulation, and good to excellent for carotid stenosis [15,30].
Follow-up clinical and laboratory data collection
Follow-up clinical data collection
Temperature data is collected on day 1 to 3 after admission, and blood pressure and pulse rate on day 3. In case of clinical deterioration, severity of the worsening symptoms is determined by a new NIHSS assessment (plus an additional NCCT). At discharge, information on the final diagnosis, flow territory, and cause of ischaemia (such as large vessel disease, cardiac origin, dissection, etc.) is collected. Clinical outcome is assessed with the mRS at 90 days [31]. At 90 days, the Barthel Index and EQ-5D are also collected [32,33].
Follow-up laboratory data collection
Laboratory tests performed on day 1 include (fasting) glucose, HbA1c, HDL- and LDL-cholesterol, and triglycerides. On day 3, data on serum creatinine, glucose (non-fasting), and C-reactive protein are collected.
Follow-up imaging data collection
When possible, patients undergo follow-up CT imaging 3 days after admission, or earlier if patients are discharged. Standard follow-up imaging consists of NCCT, CTP and CTA with an imaging protocol identical to the baseline imaging protocol. Radiological evidence of an infarct is determined on the follow-up NCCT. If an infarct is present, final infarct volume is calculated and (pc)ASPECTS is allocated. Any visible haemorrhage is classified according to the ECASS classification [34]. Reperfusion is evaluated on the CTP and recanalisation on the CTA. NCCT suffices if there is any reason not to administer intravenous contrast material. MRI can also serve as follow-up imaging if clinically indicated (preferably with diffusion-weighted, T2-FLAIR and T2-weighted images, and MR angiography). In case of clinical deterioration, a NCCT is performed to detect intracranial haemorrhage (plus an additional NIHSS).
Study outcome measures
The primary outcome measure of this study is poor clinical outcome assessed by the mRS at 90 days [31]. Collection of the 90 day mRS is performed by a trained research nurse or neurologist by telephone interview [35]. A score of 0–2 represents good outcome, and a score of 3–6 represents poor outcome [36]. Secondary outcome measures include infarct size, symptomatic and/or asymptomatic haemorrhage on follow-up NCCT, reperfusion on follow-up CTP, recanalisation on follow-up CTA, and Barthel Index and EQ-5D at 90 days.
Data entry and monitoring
Individual clinical and laboratory patient data is collected by the local investigators and supporting staff in the participating hospitals, and entered in a password-protected electronic online case report form. Participating centres can only access patient data from patients included in their own specific hospital. Prior to access to the online case report form, all investigators are trained in its use. To minimise missing or wrong (implausible) data, investigators in the coordinating centre check the database and missing values are retrieved where possible.
Analysis
Candidate predictors
Prediction models will be developed to investigate the primary study outcome. In these models, we only include variables with an expected strong predictive value for the outcome based on prior literature. Candidate predictors that will be included in the prediction models are divided into 3 groups: (1) patient characteristics and NCCT, (2) CTA, and (3) CTP predictors.
1) Patient characteristics include age [37-39], stroke severity (NIHSS) [38-40], time from symptom onset to imaging, mRS prior to current stroke symptoms [19], admission glucose level [41-43], and treatment (yes/no for IV-rtPA/IAT/mechanical thrombectomy) [44,45]. NCCT predictors are early ischaemic changes (ASPECTS or pcASPECTS) [17,20,21], and presence of a hyperdense vessel sign [19,46].
2) CTA predictors include ischaemic changes on CTA-SI (ASPECTS or pcASPECTS) [12,13,17], location and extent of arterial occlusion [16], leptomeningeal collaterals [15,47], and presence of severe ipsilateral carotid or vertebrobasilar artery stenosis (>70%) or occlusion [48].
3) CTP predictors include perfusion deficits on CBV and MTT maps (ASPECTS or pcASPECTS) [11], and penumbra/infarct core index [4].
Model derivation
For the development of the prediction rule, three logistic regression models will be developed in the derivation cohort consisting of 60% of the study population. Selection of patients for the derivation cohort based on date of inclusion. The first model will include patient characteristics and NCCT parameters (Model A), reflecting the situation in which CTP and CTA are not performed as part of standard stroke workup. The second model will consist of Model A + CTA predictors (Model B). The third model will include Model B + CTP predictors (Model C). Model selection and shrinkage of the model coefficients will be performed [49]. The optimal penalty factor will be determined with bootstrapping. Single imputation will be used to decrease missing values. In addition to true missing values, biologically improbable values are also considered missing values. To minimise the effect of outliers, continuous predictors will be truncated at the 1st and 99th percentile [49].
Model validation
Model performance will be validated in the remaining 40% of the study population. Discrimination of the models, i.e. the ability of the models to discriminate between patients with good and patients with poor outcome, will be assessed with receiver operator characteristics (ROC) analysis and corresponding area under the curve (AUC) [50]. Calibration of the models, i.e. agreement between predicted outcomes and observed outcomes, will be evaluated with calibration plots. In these plots, patients are divided into quantiles according to their predicted risk. For each quantile, the mean predicted probability is compared to the mean observed outcome. Goodness-of-fit will be tested with Hosmer-Lemeshow tests. Likelihood ratio tests will be used to compare the difference in the models for statistical significance. Superiority of the models will be determined by using a combination of the results of the ROC analyses, calibration plots and Hosmer-Lemeshow tests, and likelihood ratio tests. A nomogram or score chart will be developed for the best model to facilitate use by clinicians.
Sample size
Based on previously published data on acute stroke in The Netherlands, we expect approximately half of the 1500 patients to have a poor clinical outcome [51]. Hence, in the derivation cohort consisting of 900 patients, we anticipate a poor outcome in about 450 patients. In total, we plan to include 15 variables in the most extensive model (model C). We are able to reliably estimate the weight of the predictors, as we have more than the (arbitrary) amount of 10 events per predictor, allowing for enough power for unexpected findings [52]. As we expect about half of the patients to undergo some form of treatment (IV-rtPA/IAT/mechanical thrombectomy) we still have enough power to assess outcome in both the treatment and non-treatment group [53]. The large study size also ensures sufficient numbers for subgroup analyses, such as stroke subtype, posterior circulation stroke, and stroke complications.
Ethical considerations
The medical ethics committee of the University Medical Center Utrecht approved the DUST. The study is conducted according to the principles of the Declaration of Helsinki and in accordance with the Dutch Medical Research Involving Human Subjects Act. Patients are only included in the DUST after written informed consent is obtained from the patient or his/her legal representative. Clinical data collection, laboratory testing, and CT imaging at admission is performed as part of the routine clinical work-up of stroke patients. Informed consent is obtained before any further study related tests or procedures are performed. Because stroke patients may have a compromised ability to consent, the capacity to consent is established first. In patients with a compromised ability to consent, written informed consent is obtained from their legal representative. Patients who die before informed consent can be obtained are an exception. Because it is undesirable to burden the relatives with this request, the medical ethics committee waived the need for informed consent in these patients.
Discussion
Prediction of clinical outcome in patients with symptoms of acute ischaemic stroke is not easy when based on patient characteristics and NCCT alone. To the best of our knowledge, the combined prognostic value of CTP and CTA parameters in addition to clinical and NCCT parameters has never been investigated in one single study.
In our study design we aim to resemble clinical practice as much as possible for the sake of clinical applicability and generalizability. Our study domain consists of patients with a suspected acute ischaemic stroke at the time of admission to the hospital, and therefore patients with an unknown time of symptom onset, such as wake-up strokes, and patients who turn out to have a stroke mimic, are also included. As treatment decisions are made in the acute stage when time is critical, prediction of outcome as guideline for further treatment is needed for all patients who are suspected of acute ischaemic stroke, and not just for selected patients with middle cerebral artery stroke who are eligible for treatment. The large study population enables analysis of sufficient candidate predictors, and also permits further division, such as between anterior and posterior circulation strokes and whether treatment is given or not. The candidate predictors selected for our prediction models can be determined quickly in the clinical setting, enabling their use in the acute stage. For this multicentre study CT imaging is performed on scanners from different vendors. While this may result in more variation in the imaging results, it improves generalizability to other hospitals, which is of major importance for prediction studies. Imaging assessment is performed by a single experienced observer, which reflects the clinical practice of most hospitals. Lastly, while many studies use surrogate endpoints such as infarct volume or recanalization, using clinical outcome at 90 days remains the most relevant outcome for stroke patients.
In conclusion, this study will provide insight in the added prognostic value of CTP and CTA parameters in outcome prediction of acute stroke patients. The prediction models that are developed in this study may guide future treatment decisions in the acute stage.
Abbreviations
ASPECTS: Alberta stroke program early CT score; AUC: Area under the curve; CBF: Cerebral blood flow; CBV: Cerebral blood volume; CTA: CT angiography; CTA-SI: CTA source images; CTP: CT perfusion; DUST: Dutch acute stroke trial; IAT: Intra-arterial thrombolysis; IV-rtPA: Intravenous recombinant tissue type plasminogen activator; MIP: Maximum intensity projection; mRS: Modified Rankin scale; MTT: Mean transit time; MPR: Multiplanar reconstruction; NCCT: Non-contrast computed tomography; NIHSS: National institutes of health stroke scale; pcASPECTS: Posterior circulation ASPECTS; ROC: Receiver operator characteristics; TTP: Time to peak.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
BV, LJK, WM, and YvdG designed the study with BV as the principal investigator. AH, BV, GJB, IvdS, JN, JWD, ML and TvS are responsible for data collection and analysis. All authors have read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Contributor Information
Tom van Seeters, Email: T.vanSeeters@umcutrecht.nl.
Geert Jan Biessels, Email: G.J.Biessels@umcutrecht.nl.
Irene C van der Schaaf, Email: I.vanderSchaaf@umcutrecht.nl.
Jan Willem Dankbaar, Email: J.W.Dankbaar@umcutrecht.nl.
Alexander D Horsch, Email: A.Horsch@umcutrecht.nl.
Merel JA Luitse, Email: M.Luitse-2@umcutrecht.nl.
Joris M Niesten, Email: J.Niesten@umcutrecht.nl.
Willem PTM Mali, Email: W.Mali@umcutrecht.nl.
L Jaap Kappelle, Email: L.Kappelle@umcutrecht.nl.
Yolanda van der Graaf, Email: Y.vanderGraaf@umcutrecht.nl.
Birgitta K Velthuis, Email: B.K.Velthuis@umcutrecht.nl.
Acknowledgements
The Dutch acute stroke trial (DUST) investigators are: Academic Medical Center, Amsterdam, The Netherlands (Majoie CB, Roos YB); Catharina Hospital, Eindhoven, The Netherlands (Duijm LE, Keizer K); Erasmus Medical Center, Rotterdam, The Netherlands (van der Lugt A, Dippel DW); Gelre Hospitals, Apeldoorn, The Netherlands (Droogh - de Greeve KE, Bienfait HP); Leiden University Medical Center, Leiden, The Netherlands (van Walderveen MA, Wermer MJ); Medical Center Haaglanden, The Hague, The Netherlands (Lycklama à Nijeholt GJ, Boiten J); Onze Lieve Vrouwe Gasthuis, Amsterdam, The Netherlands (Duyndam D, Kwa VI); Radboud University Nijmegen Medical Centre, Nijmegen, The Netherlands (Meijer FJ, van Dijk EJ); Rijnstate Hospital, Arnhem, The Netherlands (Kesselring FO, Hofmeijer J); St. Antonius Hospital, Nieuwegein, The Netherlands (Vos JA, Schonewille WJ); St. Elisabeth Hospital, Tilburg, The Netherlands (van Rooij WJ, de Kort PL); St. Franciscus Hospital, Rotterdam, The Netherlands (Pleiter CC, Bakker SL); VU Medical Center, Amsterdam, The Netherlands (Bot J, Visser MC); University Medical Center Utrecht, Utrecht, The Netherlands (Velthuis BK, van der Schaaf IC, Dankbaar JW, Mali WP, van Seeters T, Horsch AD, Niesten JM, Biessels GJ, Kappelle LJ, Luitse MJ, van der Graaf Y).
Funding
This study was supported by a grant from the Dutch Heart Foundation (grant number 2008 T034) and the Nuts Ohra Foundation (grant number 0903–012). The funders had no role in study design, in the collection, analysis and interpretation of data, in the writing of the manuscript, and in the decision to submit the manuscript for publication.
References
- Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Magid D, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME. et al. Heart disease and stroke statistics–2013 update: a report from the american heart association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schramm P, Schellinger PD, Klotz E, Kallenberg K, Fiebach JB, Kulkens S, Heiland S, Knauth M, Sartor K. Comparison of perfusion computed tomography and computed tomography angiography source images with perfusion-weighted imaging and diffusion-weighted imaging in patients with acute stroke of less than 6 hours’ duration. Stroke. 2004;35:1652–1658. doi: 10.1161/01.STR.0000131271.54098.22. [DOI] [PubMed] [Google Scholar]
- Smith WS, Roberts HC, Chuang NA, Ong KC, Lee TJ, Johnston SC, Dillon WP. Safety and feasibility of a CT protocol for acute stroke: combined CT, CT angiography, and CT perfusion imaging in 53 consecutive patients. AJNR Am J Neuroradiol. 2003;24:688–690. [PMC free article] [PubMed] [Google Scholar]
- Wintermark M, Flanders AE, Velthuis B, Meuli R, van Leeuwen M, Goldsher D, Pineda C, Serena J, van der Schaaf I, Waaijer A, Anderson J, Nesbit G, Gabriely I, Medina V, Quiles A, Pohlman S, Quist M, Schnyder P, Bogousslavsky J, Dillon WP, Pedraza S. Perfusion-CT assessment of infarct core and penumbra: receiver operating characteristic curve analysis in 130 patients suspected of acute hemispheric stroke. Stroke. 2006;37:979–985. doi: 10.1161/01.STR.0000209238.61459.39. [DOI] [PubMed] [Google Scholar]
- Luitse MJ, van Seeters T, Horsch AD, Kool HA, Velthuis BK, Kappelle LJ, Biessels GJ. Admission hyperglycaemia and cerebral perfusion deficits in acute ischaemic stroke. Cerebrovasc Dis. 2013;35:163–167. doi: 10.1159/000346588. [DOI] [PubMed] [Google Scholar]
- Lin K, Kazmi KS, Law M, Babb J, Peccerelli N, Pramanik BK. Measuring elevated microvascular permeability and predicting hemorrhagic transformation in acute ischemic stroke using first-pass dynamic perfusion CT imaging. AJNR Am J Neuroradiol. 2007;28:1292–1298. doi: 10.3174/ajnr.A0539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dankbaar JW, Hom J, Schneider T, Cheng SC, Lau BC, van der Schaaf I, Virmani S, Pohlman S, Dillon WP, Wintermark M. Dynamic perfusion CT assessment of the blood–brain barrier permeability: first pass versus delayed acquisition. AJNR Am J Neuroradiol. 2008;29:1671–1676. doi: 10.3174/ajnr.A1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wintermark M, Reichhart M, Cuisenaire O, Maeder P, Thiran JP, Schnyder P, Bogousslavsky J, Meuli R. Comparison of admission perfusion computed tomography and qualitative diffusion- and perfusion-weighted magnetic resonance imaging in acute stroke patients. Stroke. 2002;33:2025–2031. doi: 10.1161/01.STR.0000023579.61630.AC. [DOI] [PubMed] [Google Scholar]
- Schaefer PW, Barak ER, Kamalian S, Gharai LR, Schwamm L, Gonzalez RG, Lev MH. Quantitative assessment of core/penumbra mismatch in acute stroke: CT and MR perfusion imaging are strongly correlated when sufficient brain volume is imaged. Stroke. 2008;39:2986–2992. doi: 10.1161/STROKEAHA.107.513358. [DOI] [PubMed] [Google Scholar]
- Muir KW, Halbert HM, Baird TA, McCormick M, Teasdale E. Visual evaluation of perfusion computed tomography in acute stroke accurately estimates infarct volume and tissue viability. J Neurol Neurosurg Psychiatry. 2006;77:334–339. doi: 10.1136/jnnp.2005.074179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons MW, Pepper EM, Chan V, Siddique S, Rajaratnam S, Bateman GA, Levi CR. Perfusion computed tomography: prediction of final infarct extent and stroke outcome. Ann Neurol. 2005;58:672–679. doi: 10.1002/ana.20638. [DOI] [PubMed] [Google Scholar]
- Coutts SB, Lev MH, Eliasziw M, Roccatagliata L, Hill MD, Schwamm LH, Pexman JH, Koroshetz WJ, Hudon ME, Buchan AM, Gonzalez RG, Demchuk AM. ASPECTS on CTA source images versus unenhanced CT: added value in predicting final infarct extent and clinical outcome. Stroke. 2004;35:2472–2476. doi: 10.1161/01.STR.0000145330.14928.2a. [DOI] [PubMed] [Google Scholar]
- Camargo EC, Furie KL, Singhal AB, Roccatagliata L, Cunnane ME, Halpern EF, Harris GJ, Smith WS, Gonzalez RG, Koroshetz WJ, Lev MH. Acute brain infarct: detection and delineation with CT angiographic source images versus nonenhanced CT scans. Radiology. 2007;244:541–548. doi: 10.1148/radiol.2442061028. [DOI] [PubMed] [Google Scholar]
- Flint AC, Duckwiler GR, Budzik RF, Liebeskind DS, Smith WS. Merci, Multi MWC. Mechanical thrombectomy of intracranial internal carotid occlusion: pooled results of the MERCI and Multi MERCI Part I trials. Stroke. 2007;38:1274–1280. doi: 10.1161/01.STR.0000260187.33864.a7. [DOI] [PubMed] [Google Scholar]
- Tan JC, Dillon WP, Liu S, Adler F, Smith WS, Wintermark M. Systematic comparison of perfusion-CT and CT-angiography in acute stroke patients. Ann Neurol. 2007;61:533–543. doi: 10.1002/ana.21130. [DOI] [PubMed] [Google Scholar]
- Puetz V, Dzialowski I, Hill MD, Subramaniam S, Sylaja PN, Krol A, O’Reilly C, Hudon ME, Hu WY, Coutts SB, Barber PA, Watson T, Roy J, Demchuk AM. Calgary CTASG. Intracranial thrombus extent predicts clinical outcome, final infarct size and hemorrhagic transformation in ischemic stroke: the clot burden score. Int J Stroke. 2008;3:230–236. doi: 10.1111/j.1747-4949.2008.00221.x. [DOI] [PubMed] [Google Scholar]
- Puetz V, Sylaja PN, Coutts SB, Hill MD, Dzialowski I, Mueller P, Becker U, Urban G, O’Reilly C, Barber PA, Sharma P, Goyal M, Gahn G, von Kummer R, Demchuk AM. Extent of hypoattenuation on CT angiography source images predicts functional outcome in patients with basilar artery occlusion. Stroke. 2008;39:2485–2490. doi: 10.1161/STROKEAHA.107.511162. [DOI] [PubMed] [Google Scholar]
- Brott T, Adams HP Jr, Olinger CP, Marler JR, Barsan WG, Biller J, Spilker J, Holleran R, Eberle R, Hertzberg V, Rorick M, Moomaw CJ, Walker M. Measurements of acute cerebral infarction: a clinical examination scale. Stroke. 1989;20:864–870. doi: 10.1161/01.STR.20.7.864. [DOI] [PubMed] [Google Scholar]
- Strbian D, Meretoja A, Ahlhelm FJ, Pitkaniemi J, Lyrer P, Kaste M, Engelter S, Tatlisumak T. Predicting outcome of IV thrombolysis-treated ischemic stroke patients: the DRAGON score. Neurology. 2012;78:427–432. doi: 10.1212/WNL.0b013e318245d2a9. [DOI] [PubMed] [Google Scholar]
- Barber PA, Demchuk AM, Zhang J, Buchan AM. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. Lancet. 2000;355:1670–1674. doi: 10.1016/S0140-6736(00)02237-6. [DOI] [PubMed] [Google Scholar]
- Pexman JH, Barber PA, Hill MD, Sevick RJ, Demchuk AM, Hudon ME, Hu WY, Buchan AM. Use of the alberta stroke program early CT score (ASPECTS) for assessing CT scans in patients with acute stroke. AJNR Am J Neuroradiol. 2001;22:1534–1542. [PMC free article] [PubMed] [Google Scholar]
- Puetz V, Dzialowski I, Hill MD, Demchuk AM. The alberta stroke program early CT score in clinical practice: what have we learned? Int J Stroke. 2009;4:354–364. doi: 10.1111/j.1747-4949.2009.00337.x. [DOI] [PubMed] [Google Scholar]
- Wintermark M, Lau BC, Chien J, Arora S. The anterior cerebral artery is an appropriate arterial input function for perfusion-CT processing in patients with acute stroke. Neuroradiology. 2008;50:227–236. doi: 10.1007/s00234-007-0336-8. [DOI] [PubMed] [Google Scholar]
- Soares BP, Dankbaar JW, Bredno J, Cheng S, Bhogal S, Dillon WP, Wintermark M. Automated versus manual post-processing of perfusion-CT data in patients with acute cerebral ischemia: influence on interobserver variability. Neuroradiology. 2009;51:445–451. doi: 10.1007/s00234-009-0516-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn F, Muenzel D, Meier R, Poppert H, Rummeny EJ, Huber A. Brain perfusion CT for acute stroke using a 256-slice CT: improvement of diagnostic information by large volume coverage. Eur Radiol. 2011;21:1803–1810. doi: 10.1007/s00330-011-2128-0. [DOI] [PubMed] [Google Scholar]
- Axel L. Tissue mean transit time from dynamic computed tomography by a simple deconvolution technique. Invest Radiol. 1983;18:94–99. doi: 10.1097/00004424-198301000-00018. [DOI] [PubMed] [Google Scholar]
- North American Symptomatic Carotid Endarterectomy Trial. Methods, patient characteristics, and progress. Stroke. 1991;22:711–720. doi: 10.1161/01.str.22.6.711. [DOI] [PubMed] [Google Scholar]
- Aviv RI, Mandelcorn J, Chakraborty S, Gladstone D, Malham S, Tomlinson G, Fox AJ, Symons S. Alberta stroke program early CT scoring of CT perfusion in early stroke visualization and assessment. AJNR Am J Neuroradiol. 2007;28:1975–1980. doi: 10.3174/ajnr.A0689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Seeters T, Biessels GJ, Niesten JM, van der Schaaf IC, Dankbaar JW, Horsch AD, Mali WP, Kappelle LJ, van der Graaf Y, Velthuis BK, Dust I. CT angiography source images and CT perfusion in patients with suspected ischemic stroke. PloS One. 2013;8:e75615. doi: 10.1371/journal.pone.0075615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waaijer A, Weber M, van Leeuwen MS, Kardux J, Veldhuis WB, Lo R, Beek FJ, Prokop M. Grading of carotid artery stenosis with multidetector-row CT angiography: visual estimation or caliper measurements? Eur Radiol. 2009;19:2809–2818. doi: 10.1007/s00330-009-1508-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19:604–607. doi: 10.1161/01.STR.19.5.604. [DOI] [PubMed] [Google Scholar]
- Collin C, Wade DT, Davies S, Horne V. The barthel ADL index: a reliability study. Int Disabil Stud. 1988;10:61–63. doi: 10.3109/09638288809164103. [DOI] [PubMed] [Google Scholar]
- Brooks R. EuroQol: the current state of play. Health Policy. 1996;37:53–72. doi: 10.1016/0168-8510(96)00822-6. [DOI] [PubMed] [Google Scholar]
- Berger C, Fiorelli M, Steiner T, Schabitz WR, Bozzao L, Bluhmki E, Hacke W, von Kummer R. Hemorrhagic transformation of ischemic brain tissue: asymptomatic or symptomatic? Stroke. 2001;32:1330–1335. doi: 10.1161/01.STR.32.6.1330. [DOI] [PubMed] [Google Scholar]
- Janssen PM, Visser NA, Dorhout Mees SM, Klijn CJ, Algra A, Rinkel GJ. Comparison of telephone and face-to-face assessment of the modified rankin scale. Cerebrovasc Dis. 2010;29:137–139. doi: 10.1159/000262309. [DOI] [PubMed] [Google Scholar]
- Weisscher N, Vermeulen M, Roos YB, de Haan RJ. What should be defined as good outcome in stroke trials; a modified rankin score of 0–1 or 0-2? J Neurol. 2008;255:867–874. doi: 10.1007/s00415-008-0796-8. [DOI] [PubMed] [Google Scholar]
- Knoflach M, Matosevic B, Rucker M, Furtner M, Mair A, Wille G, Zangerle A, Werner P, Ferrari J, Schmidauer C, Seyfang L, Kiechl S, Willeit J. Functional recovery after ischemic stroke–a matter of age: data from the austrian stroke unit registry. Neurology. 2012;78:279–285. doi: 10.1212/WNL.0b013e31824367ab. [DOI] [PubMed] [Google Scholar]
- Weimar C, Konig IR, Kraywinkel K, Ziegler A, Diener HC. Age and national institutes of health stroke scale score within 6 hours after onset are accurate predictors of outcome after cerebral ischemia: development and external validation of prognostic models. Stroke. 2004;35:158–162. doi: 10.1161/01.STR.0000106761.94985.8B. [DOI] [PubMed] [Google Scholar]
- König IR, Ziegler A, Bluhmki E, Hacke W, Bath PM, Sacco RL, Diener HC, Weimar C. Virtual International Stroke Trials Archive I. Predicting long-term outcome after acute ischemic stroke: a simple index works in patients from controlled clinical trials. Stroke. 2008;39:1821–1826. doi: 10.1161/STROKEAHA.107.505867. [DOI] [PubMed] [Google Scholar]
- Adams HP Jr, Davis PH, Leira EC, Chang KC, Bendixen BH, Clarke WR, Woolson RF, Hansen MD. Baseline NIH stroke scale score strongly predicts outcome after stroke: a report of the trial of Org 10172 in acute stroke treatment (TOAST) Neurology. 1999;53:126–131. doi: 10.1212/WNL.53.1.126. [DOI] [PubMed] [Google Scholar]
- Capes SE, Hunt D, Malmberg K, Pathak P, Gerstein HC. Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview. Stroke. 2001;32:2426–2432. doi: 10.1161/hs1001.096194. [DOI] [PubMed] [Google Scholar]
- Luitse MJ, Biessels GJ, Rutten GE, Kappelle LJ. Diabetes, hyperglycaemia, and acute ischaemic stroke. Lancet Neurol. 2012;11:261–271. doi: 10.1016/S1474-4422(12)70005-4. [DOI] [PubMed] [Google Scholar]
- Desilles JP, Meseguer E, Labreuche J, Lapergue B, Sirimarco G, Gonzalez-Valcarcel J, Lavallee P, Cabrejo L, Guidoux C, Klein I, Amarenco P, Mazighi M. Diabetes mellitus, admission glucose, and outcomes after stroke thrombolysis: a registry and systematic review. Stroke. 2013;44:1915–1923. doi: 10.1161/STROKEAHA.111.000813. [DOI] [PubMed] [Google Scholar]
- The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- Hacke W, Kaste M, Bluhmki E, Brozman M, Davalos A, Guidetti D, Larrue V, Lees KR, Medeghri Z, Machnig T, Schneider D, von Kummer R, Wahlgren N, Toni D, Investigators E. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317–1329. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- von Kummer R, Meyding-Lamade U, Forsting M, Rosin L, Rieke K, Hacke W, Sartor K. Sensitivity and prognostic value of early CT in occlusion of the middle cerebral artery trunk. AJNR Am J Neuroradiol. 1994;15:9–15. discussion 16–18. [PMC free article] [PubMed] [Google Scholar]
- Lima FO, Furie KL, Silva GS, Lev MH, Camargo EC, Singhal AB, Harris GJ, Halpern EF, Koroshetz WJ, Smith WS, Yoo AJ, Nogueira RG. The pattern of leptomeningeal collaterals on CT angiography is a strong predictor of long-term functional outcome in stroke patients with large vessel intracranial occlusion. Stroke. 2010;41:2316–2322. doi: 10.1161/STROKEAHA.110.592303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell PM, Eliasziw M, Gutnikov SA, Fox AJ, Taylor DW, Mayberg MR, Warlow CP, Barnett HJ. Analysis of pooled data from the randomised controlled trials of endarterectomy for symptomatic carotid stenosis. Lancet. 2003;361:107–116. doi: 10.1016/S0140-6736(03)12228-3. [DOI] [PubMed] [Google Scholar]
- Steyerberg EW. Clinical Prediction Models: a Practical Approach to Development, Validation, and Updating. New York: Springer; 2009. [Google Scholar]
- Harrell FE Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Lingsma HF, Dippel DW, Hoeks SE, Steyerberg EW, Franke CL, van Oostenbrugge RJ, de Jong G, Simoons ML, Scholte Op Reimer WJ. Variation between hospitals in patient outcome after stroke is only partly explained by differences in quality of care: results from the netherlands stroke survey. J Neurol Neurosurg Psychiatry. 2008;79:888–894. doi: 10.1136/jnnp.2007.137059. [DOI] [PubMed] [Google Scholar]
- Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. 1996;49:1373–1379. doi: 10.1016/S0895-4356(96)00236-3. [DOI] [PubMed] [Google Scholar]
- Lahr MM, Luijckx GJ, Vroomen PC, van der Zee DJ, Buskens E. Proportion of patients treated with thrombolysis in a centralized versus a decentralized acute stroke care setting. Stroke. 2012;43:1336–1340. doi: 10.1161/STROKEAHA.111.641795. [DOI] [PubMed] [Google Scholar]