Abstract
Purpose of review
With highly active anti-retroviral therapy (HAART), HIV-1 infection has become a manageable lifelong disease. Developing optimal treatment regimens requires understanding how to best measure anti-HIV activity in vitro and how drug dose response curves generated in vitro correlate with in vivo efficacy.
Recent findings
Several recent studies have indicated that conventional multi-round infectivity assays are inferior to single cycle assays at both low and high levels of inhibition. Multi-round infectivity assays can fail to detect subtle but clinically significant anti-HIV activity. The discoveries of the anti-HIV activity of the hepatitis B drug entecavir and the herpes simplex drug acyclovir were facilitated by single round infectivity assays. Recent studies using a single round infectivity assay have shown that a previously neglected parameter, the dose response curve slope, is an extremely important determinant of antiviral activity. Some antiretroviral drugs have steep slopes that result in extraordinary levels of antiviral activity. The instantaneous inhibitory potential (IIP), the log reduction in infectivity in a single round assay at clinical drug concentrations, has been proposed as a novel index for comparing antiviral activity.
Summary
Among in vitro measures of antiviral activity, single round infection assays have the advantage of measure instantaneous inhibition by a drug. Re-evaluating the antiviral activity of approved HIV-1 drugs has shown that the slope parameter is an important factor in drug activity. Determining the IIP by using a single round infectivity assay may provide important insights that can predict the in vivo efficacy of anti-HIV-1 drugs.
Keywords: HIV-1, highly active anti-retroviral therapy (HAART), slope, Instantaneous Inhibitory Potential (IIP), phenotypic assay, single round infectivity assay
Introduction
For the 33 million people infected with HIV-1, highly active anti-retroviral therapy (HAART) is currently the only treatment that can stop disease progression. HAART consists of selected combinations of three different antiretroviral drugs [1]. In the majority of patients who take the drugs correctly, HAART is successful in reducing the level of viremia to below 50 copies of HIV-1 RNA per ml of plasma, the limit of detection of clinical assays [2, 3, 4]. Despite optimistic early predictions [4], HAART does not cure HIV-1 infection, due in part to persistence of a population of resting memory CD4+ T cells that harbor quiescent viral genomes and that are capable of producing infectious virus upon cellular activation [5, 6, 7]. Nevertheless, in patients who maintain levels of viremia below 50 copies per ml, disease progression is halted and partially reversed. Although these patients do have trace levels of viremia which is detectable with special assays [8, 9], a recent study demonstrated that addition of a fourth antiretroviral drug to their regimens does not further reduce this residual viremia [10]. The implication is that residual viremia originates from stable reservoirs rather than ongoing cycles of replication. Thus current HAART regimens appear to have largely reached their theoretical potential of halting ongoing viral replication.
Despite the success of HAART, a clear understanding of the quantitative aspects of antiviral drug activity is still lacking. In particular, it is still not clear how in vitro measures of antiviral drug activity relate to the performance of the drugs in patients. Antiviral activity is typically expressed in terms of the IC50, the concentration of drug that inhibits viral replication by 50%, or the inhibitory quotient (IQ), the ratio of the plasma drug concentration (D) achieved during standard dosing to the IC50. Recent research has highlighted problems with the conventional approaches for the in vitro analysis of anti-HIV activity. For example, it has been shown that conventional assays can miss subtle but clinically significant anti-HIV activity of drugs used to treat other viral infections. In addition, it has become clear that standard measures like IC50 and IQ are inadequate because they fail to consider the dose response curve slope, which is an extremely important determinant of the inherent inhibitory potential of antiviral drugs. As a result, these measures do not accurately predict the drug classes with the greatest in vivo efficacy. In this review, we discuss the ways in which antiviral activity has been traditionally measured and consider new approaches for measuring and describing anti-HIV activity that more accurately capture the in vivo activity of antiretroviral drugs.
In vitro Assays for Antiretroviral Drugs
The in vitro assessment of anti-HIV activity is typically accomplished by measuring infection in the presence of increasing concentrations of drug in conventional multi-round assays or newer single round assays. A summary of the major features of multi-round and single round assays is presented in Table 1.
Table 1. Comparison Between Multiple and Single Round Infectivity Assays.
| Multiple Round Assays | Single Round Assays | |
|---|---|---|
| Cell Type Infected | Cell lines or in vitro activated PBMC | Cell lines or CD4+T cells isolated from in vitro activated PBMC |
| Assay Read-Out | p24 antigen, RT activity, cell viability | Luciferase or GFP |
| Read-Out Sensitivity | Population level | Population level or single cell level (GFP assay) |
| Time to Reach End-Point | > 5 days | ≤ 7 days |
| Dynamic Range | < 4 logs | ≥ 3 logs |
| High Throughput Capacity | Poor | Good |
| Reproducibility | Variable | Good |
| Viral Evolution During Assay | Possible | No |
| Type of Drug Inhibition Measured | Cumulative | Instantaneous |
| Ability to Measure Slope | No | Yes |
| Ability to Measure IIP | No | Yes |
Multi-round assays
In multi-round assays, cell lines or mitogen-activated peripheral blood mononuclear cells (PBMC) isolated from healthy individuals are infected with replication-competent, cell free virus and cultured for a period of several days to weeks [11, 12, 13]. Quantification of viral antigen (p24) or reverse transcriptase activity in culture supernatants is used to determine the amount of virus produced. Some multi-round infection assays use cell viability as a readout [14]. Viral cytopathic effects on host cells can be quantified using a soluble tetrazolium reagent that is metabolically reduced to a colored formazan product in viable cells. Although these measurements can easily be made by common colorimetric techniques, this assay is indirect. Regardless of the readout used, multi-round assays are time consuming and not always reproducible between laboratories [15].
A more significant problem with multi-round assays was pointed out by Ferguson and colleagues [16]. They noted that multi-round assays measure cumulative outcomes of infection after drug has been added rather than instantaneous inhibition by the drug. Factors such as target cell proliferation or death, virus decay, and viral cytopathic effects can influence the outcome. Their modeling suggested that IC50 values and the steepness of dose response curves could vary depending on endpoint chosen [16], making it difficult to compare results from different studies. Thus multi-round assays may over or underestimate a drug effect. The fundamental problem with such assays is that the outcome depends on multiple factors in addition to the intrinsic antiviral activity of the drug being tested. As pointed out by Ferguson et al.[16], any drug that reduces the basic reproductive ratio (R0, the number of newly infected cells arising from virus produced by a single infected cell) to <1 can cause the infection to extinguish. Thus multi-round assays may not distinguish drugs that cause an immediate halt to all infectious cycles from drugs that merely reduce R0 to a value below 1. In cases where cell lines are used, there is the additional problem that the outcome may be affected by cell-specific factors not representative of the CD4+ T lymphoblasts which are the primary target cells for HIV-1 in vivo.
Depending on the length of the assay, multi-round infection assays may have the added complication of the production of viral variants during sequential rounds of replication. Although a culture may begin with a clonal virus population, each new round of infection will generate variants that may have altered susceptibility to the drug being tested. In some cases, one point mutation in the viral genome may confer high level resistance to a drug. For example, the K103N or M184V mutations in reverse transcriptase dramatically decrease virus susceptibility to the non-nucleoside reverse transcriptase inhibitor (NNRTI) efavirenz and the nucleoside reverse transcriptase inhibitor (NRTI) lamivudine, respectively [17, 18, 19, 20]. Thus, dose response curves may not reflect a true drug effect if there is evolution of resistant variants.
Multi-round infection assays have been used to compare the fitness of viral variants in a single culture. An excellent summary of these methods has recently been published by Dykes and Demeter [21]. Typically, two viral variants are mixed at a known ratio and used to infect cells in the presence or absence of drug. After several weeks in culture, the relative percentage of each variant can be measured using various assays including clonal analysis or flow cytometry as described by Martinez-Picado et al. [22]. Although informative, these types of assays generally take up to several weeks to complete, are labor-intensive, and the data interpretation is still controversial.
Single round infectivity assays
A more rapid alternative to multi-round infection assays are single round infectivity assays exemplified by the commercial systems used to measure HIV-1 drug resistance [15, 23, 24]. One such assay [23] relies on production of virus particles in the readily transfectable human embryonic kidney (HEK) cell line 293T and infection of the same cell line. Two plasmids are co-transfected into HEK 293T cells: one containing the HIV-1 genome with part of the envelope gene deleted and replaced with a luciferase reporter gene and the second plasmid containing the murine leukemia virus (MLV) envelope gene. Culture supernatants containing pseudovirions are collected and used to infect a new culture of HEK 293T cells in the presence of increasing drug concentrations. Patient-derived partial pol sequences can be incorporated into the parent HIV-1 vector to test patient virus susceptibility to individual antiretroviral drugs. Typically, uncloned patient sequences amplified from plasma are used. Phenotypic analysis is especially useful for patients who are HAART experienced and who have complicated genotypes. Dose response curves of pseudovirus populations containing patient-derived pol sequences are generated, and the IC50 values obtained are compared to those of wild-type virus under the same conditions.
An alternative single-round phenotypic assay [24, 25, 26] replaces the luciferase indicator gene with the enhanced green fluorescent protein (GFP) gene and uses the HIV-1 CXCR4 or CCR5 tropic envelope instead of MLV envelope (Figure 1A). Production of virus is done similarly in HEK 293T cells, but infection is done in primary CD4+ T cells, the main target cells of HIV-1 in vivo, rather than transformed cell lines. Infecting primary CD4+ T cells has the advantage of allowing measurement of drug inhibition by HIV-1 entry inhibitors such as enfuvirtide whereas cell lines such as 293T must be transfected with the CD4 and the CXCR4 or CCR5 co-receptor genes to allow HIV-1 envelope mediated entry. Because infectivity is measured by flow cytometric quantitation of the number of GFP expressing cells, this assay has the sensitivity to detect individual infection events and a wide dynamic range (Figure 1A), with the main limitation being the number of cells infected. Because of the wide dynamic range, measurements of drug inhibition at clinically relevant concentrations can be made for some drugs. The single-round phenotypic assay can be performed in a high throughput fashion using 96 well plates (Figure 1A), making it a useful tool for drug screening.
Figure 1. A single round infectivity assay[24].
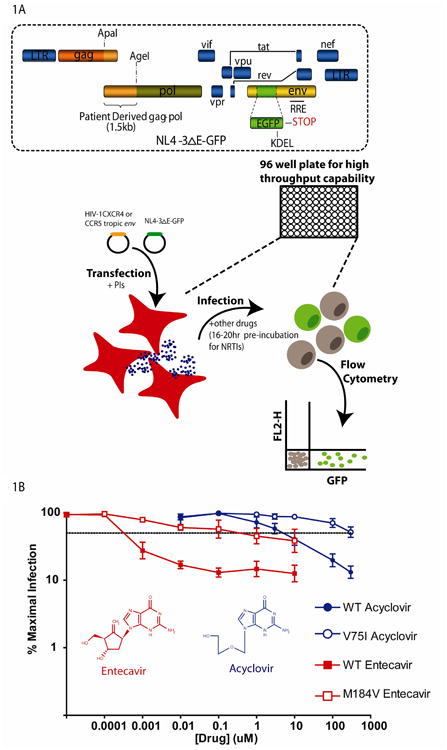
(A) HEK 293T cells are transfected with two constructs. One construct, NL4-3ΔE-GFP contains the HIV-1 genome with part of the envelope gene deleted and replaced with GFP. The second construct contains either an HIV-1 CXCR4 or CCR5 tropic envelope gene. Virus is collected from culture supernatants and used to infect primary activated CD4+ T cells isolated from healthy blood donors. GFP expressing cells are quantified by flow cytometry 3 days post infection. Drugs are added to cells at the time of transfection or 16-20 hours prior to infection. (B) Dose response curves for the anti-Hepatitis B drug entecavir and the anti-Herpes drug acyclovir against wild type (WT (closed symbols) and mutant (open symbols) HIV-1 using the phenotypic assay. Dose response curves represent the percentage of cells infected in the presence of drug divided by the percentage of infected cells with no drug effect. The dashed line represents 50% inhibition. The mutants M184V and V75I were selected for by entecavir monotherapy (in vivo) or acyclovir (in vitro), respectively, and have shifted dose response curves and increased IC50 values compared to wild-type virus.
The most significant advantage of the single round infectivity assays is that it measures instantaneous inhibition by the drug rather than cumulative effects in long term culture. Because of the defective envelope gene in the HIV-1 vectors used, the pseudovirus particles generated in such assays are capable of only a single round of infection. As is discussed below, the degree to which the drug inhibits this single round infection is a simple and accurate reflection of the intrinsic antiviral activity of the drug. Several recent studies have highlighted the advantages of this approach to measuring anti-HIV activity.
Anti-HIV activity of entecavir and acyclovir
The single-round phenotypic assay was able to detect the potent but partial inhibition of HIV-1 replication by the Hepatitis B drug entecavir [26]. Entecavir is a deoxyguanosine analogue that can be incorporated by Hepatitis B virus polymerase, leading to chain termination [27]. Interestingly, initial studies with a multi-round assay in cell lines did not detect any effect of this drug on HIV-1 replication [27]. However, a single round assay detected anti-HIV activity at concentrations as low as 1 nM and confirmed the entecavir resistance of the M184V reverse transcriptase mutant (Figure 1B) identified in a HIV-Hepatitis B co-infected patient taking entecavir monotherapy. In this study, a ∼0.5 log10 decline in HIV-1 RNA was seen in 3 coinfected patients taking entecavir, although only 1 of the 3 patients selected the M184V mutant HIV-1 variant [26]. This result was confirmed independently in a fourth HIV-Hepatitis B co-infected patient [28] and in a retrospective analysis of HAART naïve and experienced co-infected patients taking entecavir monotherapy [29]. These observations were initially challenged by Soriano et al [30] who did not observe a decline in plasma HIV-1 RNA levels in a HIV-Hepatitis delta co-infected patient on entecavir. However, the M184V mutation was later identified in this patient after an extended duration of entecavir monotherapy [31]. Thus it appears clear that entecavir can inhibit HIV-1 in vivo and select for the M184V mutation in HIV-1 reverse transcriptase, a mutation that also confers high level resistance to the widely used HIV-1 drugs lamivudine and emtricitabine [17-19]. The use of entecavir monotherapy in coinfected patients is now contraindicated [29].
In addition to these clinical and functional studies, direct inhibition of HIV-1 reverse transcriptase by entecavir triphosphate has been demonstrated at the biochemical level [32, 33] in support of the single-round phenotypic assay data. Interestingly, using single round infectivity assays with a luciferase reporter, Lin et al [34] found that the sensitivity of HIV-1 to inhibition by entecavir was dependent on the multiplicity of infection (MOI). In this study, lower MOIs gave lower IC50 values, although entecavir was generally not as potent as observed in the GFP reporter assay [26]. It is likely that low MOI assays more closely mimic in vivo infection. In addition, when measuring the instantaneous inhibition of viral replication by nucleoside analogues, it is important to allow time for the drug to become activated to its triphosphate form. In the entecavir studies done with the GFP reporter system [26], the cells were pre-incubated with drug for 16-20 hrs prior to infection. This has been shown to be the optimal time for activation of the NRTIs [24]. Thus in the study by Lin et al, the potency of entecavir may be underestimated since the drug was added at the time of infection or shortly after in some cases.
Another interesting recent example of the usefulness of single round assays is the discovery of the anti-HIV activity of the acyclovir, a nucleoside analogue used to treat Herpes Simplex Virus (HSV) infection. In HSV-infected cells, phosphorylation of acyclovir by HSV thymidine kinase allows the generation of the active triphosphate form of the drug which is a substrate for HSV DNA polymerase, resulting in chain termination. Using a single round infectivity assay with a GFP-expressing HIV-1 pseudovirus, McMahon et al. screened a clinical compound library containing over 3,000 drugs and drug-like molecules [35, 36] (Figure 1B). Surprisingly, acyclovir showed significant anti-HIV activity in CD4+ T cells from normal donors. In addition, the single-round assay confirmed the acyclovir resistance of the V75I variant in HIV-1 reverse transcriptase that was selected for in vitro using a multi-round infection assay [35]. The anti-HIV activity of acyclovir was also identified by Lisco et al. [37, 38] using a replication competent virus in a human tonsil tissue culture system. These authors attributed the acyclovir effect to the presence of occult infections by various human herpes viruses in the tonsil samples. Although a direct anti-HIV effect by acyclovir was surprising, it is consistent with the results of several recent studies showing a reduction in plasma HIV-1 RNA levels in HSV-2/HIV-1 co-infected patients on acyclovir monotherapy [39, 40]. Thus, in two different cases, single round infectivity assays have detected clinically significant anti-HIV activity of nucleoside analogues used to treat other viruses.
in vitro Measures of Antiviral Activity
Regardless of which in vitro assay is used, the classic method to analyze antiviral drug effects is to obtain dose-response curves by plotting the fraction of infection events unaffected by the drug (fu) vs. the log of the drug concentration (Figure 2A). The IC50, which can be readily determined from such plots, is widely used to compare the potency of individual drugs. However, plasma concentrations of antiretroviral drugs achieved with standard dosing are typically well above the IC50. Thus IQ, the ratio of plasma drug concentration (D) to the IC50, has been introduced as a second measure of drug activity. IQ takes into account two important dimensions of antiviral activity and appears to be a predictor of virological responses in patients on some protease inhibitor (PI) based regimens [41]. However, all widely applicable theoretical models for drug activity, including the sigmoidal Emax model and the median effect model, state that drug activity depends on three parameters: D, IC50, and a third parameter, the dose-response curve slope (m) [42]. A recent study has shown that this third parameter is a critical determinant of the inhibitory activity of antiviral drugs [25]. The importance of the slope parameter is revealed in a dramatic fashion when the traditional semi-log dose response curves (fu vs. log D) are transformed to log-log plots (log fu vs. log D). On the traditional semi-log plots, drugs with different values of the slope parameter appear to be equally efficient at inhibiting replication at clinically relevant drug concentrations (Figure 2A). However, transformation of these plots into log-log plots reveals that the dose-response curve slope dramatically affects the level of inhibition that can be achieved at clinically relevant drug concentrations (Figure 2B). Using a linear scale for inhibition obscures the difference between modest inhibition (99%) and very high level inhibition (99.9999%). Because viruses replicate exponentially, the inhibition of viral replication is best considered on a logarithmic scale. When this is done, the importance of the slope parameter as a determinant of antiviral activity becomes immediately obvious.
Figure 2. Effect of slope (m) on inhibitory potential of antiretroviral drugs.
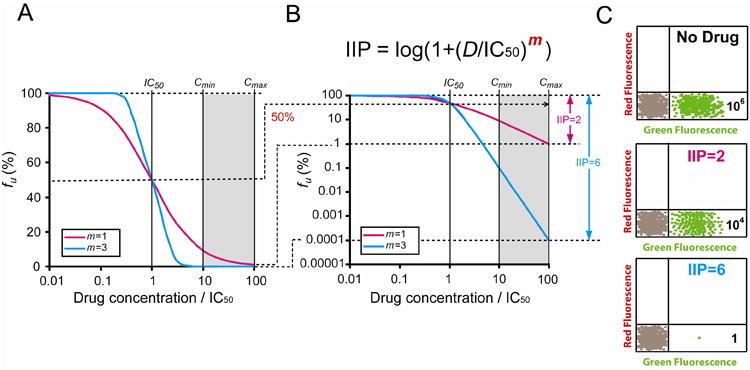
(A)The fraction of viruses remaining unaffected (fu) by a drug is plotted vs. drug concentration (normalized by IC50) on a linear-log scale using the median-effect model with m = 1 or 3. A typical in vivo concentration range 10-100 fold above the IC50 is indicated in the shaded area. Antiviral activity for drugs with different m values appears similar at Cmax in this kind of plot. (B) A log-log plot of the same curves revealing the dramatic impact of slope on the inhibitory potential of antiretroviral drugs. Instantaneous inhibitory potential (IIP) is the number of logs of reduction of single round infectivity at a clinically relevant drug concentration. At Cmax, a drug with a slope of 3 can achieve 6 logs of inhibition (IIP = 6), which is 10,000 fold greater than that of a drug with a slope of 1. This dramatic influence of slope on antiviral activity is only evident in the log-log plot because the difference between 2 logs (99%) and 6 logs (99.9999%) of inhibition is small in a linear-log plot. The equation for calculation of IIP is also shown. (C) Hypothetical flow cytometry plots for infections in the presence and absence of antiviral drugs. Expression of GFP is an indicator of successful infection. In this example, 106 GFP+ cells are detected in cultures without antiviral drugs. In cultures treated with an antiviral drug with IIP = 2, 104 infection events are still readily detectable at D = Cmax. However, in the presence of a drug with IIP=6, only 1 GFP+ cell on average may be detected at D = Cmax.
The slope parameter is analogous to the Hill coefficient [43], a measure of cooperativity in the binding of multiple ligand molecules to a multivalent receptor. Because there is only a single binding site for NRTIs, NNRTIs, and PIs on the relevant HIV-1 enzymes, the slope parameter has largely been ignored in the analysis of anti-HIV drugs. In fact, prior to the study by Shen et al. [25], the values of the slope parameter had not been measured for antiretroviral drugs using an appropriate methodology. As mentioned above, the slope parameter cannot be accurately obtained using multi-round assays. Shen et al. used a single round assay with a pseudovirus carrying the gene for GFP to measure the slope parameter for antiretroviral drugs in current use. This assay detects individual infection events and has the sensitivity and dynamic range necessary for accurate measurement of m. Interestingly, they discovered that slope values are different for different classes of antiretroviral drugs and seem to correlate with the mechanism of drug action [25]. The NRTIs and integrase inhibitors have slopes of ∼1, whereas the NNRTIs, PIs and entry inhibitors have slopes >1. Thus the slope parameter has to be considered for accurate evaluation of antiviral activity.
To incorporate all three dimensions affecting antiviral activity - IC50, D and m – into one single measure of antiviral activity, a new index termed instantaneous inhibitory potential (IIP) was developed [25]. IIP is the number of the logs by which single round infectivity is reduced at clinically relevant drug concentrations and can be calculated based on the median effect model (Figure 2B). The biological meaning of IIP can also be understood from an experimental perspective. Consider an experiment in which 106 infected (GFP+) cells are detected by flow cytometry in the absence of drug in the single round infectivity assay described above (Figure 1A). In the presence of a drug with an IIP value of 2, a 2 log reduction can be achieved but 104 infection events will still be observed. However, in the presence of a drug with an IIP value >6, such as the NNRTI efavirenz and some PIs, it may be difficult to detect even a single infected cell (Figure 2C).
Notably, IIP is strongly dependent upon the slope parameter and varies by > 8 logs for current available anti-HIV drugs. The NRTIs and integrase inhibitors can only produce 1-3.5 logs of inhibition of single round replication, largely because of the intrinsic limitation on antiviral activity imposed by their low slope values. Some NNRTIs and PIs, however, have high slope values and can achieve IIP values of 5-10. The extraordinary ability of these drugs to inhibit viral replication is apparent when using IIP as a measure for antiviral activity, but is not evident if IC50 or IQ values are used for comparison. Interestingly, the drugs that can maintain IIP >5 for 24 hours after the last dose, including the NNRTI efavirenz, and boosted forms of the PIs darunavir and atazanavir, have not been beaten in head to head comparative clinical trials and are recommended as the mainstays of initial HAART regimens [1]. Therefore, IIP may provide a valuable new tool for evaluating antiviral activity more accurately in vitro. The high inhibitory potential of certain antiretroviral drugs also helps to explain the results of HAART intensification studies which suggest that current regimens stop ongoing replication [10].
in vivo Measures of Antiviral Activity
Since antiretroviral drugs must generally be used in combinations of three or more to achieve optimal suppression of HIV-1 replication [1], it is much more difficult to evaluate the relative efficacy of individual drugs in vivo. Because monotherapy can result in the rapid emergence of resistance [44,45,46], clinical trials involving monotherapy can only be carried out under certain situations and over a short period of time. In cases where monotherapy data are available, the rate of decay of viremia and the magnitude of the drop in viremia have both been used to assess the in vivo antiviral activity of new drugs [47]. The initial rate of decay of viremia has also been used as a measure of the antiviral activity of combination therapy regimens [48]. One important question is whether newer in vitro measures of antiviral activity such as IIP can be directly related to the initial decay rate of viremia or the magnitude of the drop in viremia. Interest in this issue has been heightened by a study [49] showing that the rate of decay of viremia is faster in patients initiating therapy with regimens containing the integrase inhibitor raltegravir than in patients initiating standard efavirenz-based regimens. However, a recent modeling study has shown that the rate of decay of viremia is influenced by where in the life cycle an antiretroviral drug acts [50]. Drugs that act later in the viral life cycle (such as integrase inhibitors) will produce a more rapid decay in viremia since the observed decay reflects the turnover of cells that are still capable of producing virus in the presence of the drugs. The rapid decay in viremia in the presence of integrase inhibitors reflects the fact that only cells past the integration step are capable of producing virus. In contrast, in patients on efavirenz-based regimens, all cells past the reverse transcription step can produce virus. This is a larger population of cells and hence the decay in viremia is slower. This simple concept may explain why a very rapid drop in viremia has been observed with raltegravir in a 10 day monotherapy study [51] and as part of a HAART regimen for naïve patients [49]. Thus the rapid decay of viremia in patients on raltegravir is not necessarily indicative of unique efficacy. Rather, it is an expected consequence of the fact that raltegravir acts later in the virus life cycle compared to NNRTIs and PIs. Furthermore, the magnitude of the drop in viremia caused by a single agent is also influenced by the rate at which resistance develops [46]. Therefore, more complex models are needed to correlate in vivo parameters, such as the rate and extent of the decay of viremia, with drug efficacy.
Conclusions
Although antiviral activity can be measured by many different ways using multi- or single round infectivity assays, a standardized approach is desirable for comparing the efficacy of currently approved drugs and novel drugs. Sensitive single round infectivity assays offer the best approach for detecting anti-HIV activity at both ends of the inhibition spectrum. Differences in the level of inhibition at clinically relevant drug concentrations cannot be appreciated with traditional log-linear dose response curves. When the slope parameter is considered and the dose response curves are plotted on a logarithmic scale, it becomes evident that some antiretroviral drugs have an extraordinarily high potential to inhibit viral replication in vivo. An understanding of why certain drug classes, like the PIs, can inhibit viral replication by as much as 6-10 logs may provide new insight into designing novel inhibitors and provide a rationale for choosing HAART regimens.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1**.Hammer SM, Eron JJ, Jr, Reiss P, et al. Antiretroviral treatment of adult HIV infection: 2008 recommendations of the International AIDS Society-USA Panel. JAMA. 2008;300:555–570. doi: 10.1001/jama.300.5.555. Representing the recommendations of a panel of experts, this article contains the latest guidelines for the treatment of HIV-1 infection in the US. [DOI] [PubMed] [Google Scholar]
- 2.Hammer SM, Squires KE, Hughes MD, et al. A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimeter or less. AIDS clinical trials group 320 study team. N Engl J Med. 1997;337:725–733. doi: 10.1056/NEJM199709113371101. [DOI] [PubMed] [Google Scholar]
- 3.Gulick RM, Mellors JW, Havlir D, et al. Treatment with indinavir, zidovudine, and lamivudine in adults with human immunodeficiency virus infection and prior antiretroviral therapy. N Engl J Med. 1997;337:734–739. doi: 10.1056/NEJM199709113371102. [DOI] [PubMed] [Google Scholar]
- 4.Perelson AS, Essunger P, Cao Y, et al. Decay characteristics of HIV-1-infected compartments during combination therapy. Nature. 1997;387:188–191. doi: 10.1038/387188a0. [DOI] [PubMed] [Google Scholar]
- 5.Chun TW, Carruth L, Finzi D, et al. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature. 1997;387:183–188. doi: 10.1038/387183a0. [DOI] [PubMed] [Google Scholar]
- 6.Finzi D, Hermankova M, Pierson T, et al. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science. 1997;278:1295–1300. doi: 10.1126/science.278.5341.1295. [DOI] [PubMed] [Google Scholar]
- 7.Wong JK, Hezareh M, Gunthard HF, et al. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science. 1997;278:1291–1295. doi: 10.1126/science.278.5341.1291. [DOI] [PubMed] [Google Scholar]
- 8.Dornadula G, Zhang H, VanUitert B, et al. Residual HIV-1 RNA in blood plasma of patients taking suppressive highly active antiretroviral therapy. JAMA. 1999;282:1627–1632. doi: 10.1001/jama.282.17.1627. [DOI] [PubMed] [Google Scholar]
- 9.Palmer S, Wiegand AP, Maldarelli F, et al. New real-time reverse transcriptase-initiated PCR assay with single-copy sensitivity for human immunodeficiency virus type 1 RNA in plasma. J Clin Microbiol. 2003;41:4531–4536. doi: 10.1128/JCM.41.10.4531-4536.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10**.Dinoso JB, Kim SY, Wiegand AM, et al. Treatment intensification does not reduce residual HIV-1 viremia in patients on highly active antiretroviral therapy. Proc Natl Acad Sci U S A. 2009;106:9403–9408. doi: 10.1073/pnas.0903107106. This recent study demonstrates that the addition of a fourth antiretroviral drug to the regimens of patients who have suppression of viremia to <50 copies of HIV-1 RNA/ml on HAART does not decrease residual viremia. This suggests that residual viremia is due to the release of virus from stable reservoirs and not ongoing viral replication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Japour AJ, Mayers DL, Johnson VA, et al. Standardized peripheral blood mononuclear cell culture assay for determination of drug susceptibilities of clinical human immunodeficiency virus type 1 isolates. the RV-43 study group, the AIDS clinical trials group virology committee resistance working group. Antimicrob Agents Chemother. 1993;37:1095–1101. doi: 10.1128/aac.37.5.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schinazi RF, McMillan A, Cannon D, et al. Selective inhibition of human immunodeficiency viruses by racemates and enantiomers of cis-5-fluoro-1-[2-(hydroxymethyl)-1,3-oxathiolan-5-yl]cytosine. Antimicrob Agents Chemother. 1992;36:2423–2431. doi: 10.1128/aac.36.11.2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schinazi RF, Sommadossi JP, Saalmann V, et al. Activities of 3′-azido-3′-deoxythymidine nucleotide dimers in primary lymphocytes infected with human immunodeficiency virus type 1. Antimicrob Agents Chemother. 1990;34:1061–1067. doi: 10.1128/aac.34.6.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weislow OS, Kiser R, Fine DL, et al. New soluble-formazan assay for HIV-1 cytopathic effects: Application to high-flux screening of synthetic and natural products for AIDS-antiviral activity. J Natl Cancer Inst. 1989;81:577–586. doi: 10.1093/jnci/81.8.577. [DOI] [PubMed] [Google Scholar]
- 15.Kellam P, Larder BA. Recombinant virus assay: A rapid, phenotypic assay for assessment of drug susceptibility of human immunodeficiency virus type 1 isolates. Antimicrob Agents Chemother. 1994;38:23–30. doi: 10.1128/aac.38.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferguson NM, Fraser C, Anderson RM. Viral dynamics and anti-viral pharmacodynamics: Rethinking in vitro measures of drug potency. Trends Pharmacol Sci. 2001;22:97–100. doi: 10.1016/s0165-6147(00)01615-1. [DOI] [PubMed] [Google Scholar]
- 17.Schinazi RF, Lloyd RM, Jr, Nguyen MH, et al. Characterization of human immunodeficiency viruses resistant to oxathiolane-cytosine nucleosides. Antimicrob Agents Chemother. 1993;37:875–881. doi: 10.1128/aac.37.4.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tisdale M, Kemp SD, Parry NR, et al. Rapid in vitro selection of human immunodeficiency virus type 1 resistant to 3′-thiacytidine inhibitors due to a mutation in the YMDD region of reverse transcriptase. Proc Natl Acad Sci U S A. 1993;90:5653–5656. doi: 10.1073/pnas.90.12.5653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gao Q, Gu Z, Parniak MA, et al. The same mutation that encodes low-level human immunodeficiency virus type 1 resistance to 2′,3′-dideoxyinosine and 2′,3′-dideoxycytidine confers high-level resistance to the (-) enantiomer of 2′,3′-dideoxy-3′-thiacytidine. Antimicrob Agents Chemother. 1993;37:1390–1392. doi: 10.1128/aac.37.6.1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bacheler LT, Anton ED, Kudish P, et al. Human immunodeficiency virus type 1 mutations selected in patients failing efavirenz combination therapy. Antimicrob Agents Chemother. 2000;44:2475–2484. doi: 10.1128/aac.44.9.2475-2484.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dykes C, Demeter LM. Clinical significance of human immunodeficiency virus type 1 replication fitness. Clin Microbiol Rev. 2007;20:550–578. doi: 10.1128/CMR.00017-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22**.Martinez-Picado J, Martinez MA. HIV-1 reverse transcriptase inhibitor resistance mutations and fitness: A view from the clinic and ex vivo. Virus Res. 2008;134:104–123. doi: 10.1016/j.virusres.2007.12.021. This is a comprehensive review comparing the different in vitro assays used for measuring viral fitness. [DOI] [PubMed] [Google Scholar]
- 23.Petropoulos CJ, Parkin NT, Limoli KL, et al. A novel phenotypic drug susceptibility assay for human immunodeficiency virus type 1. Antimicrob Agents Chemother. 2000;44:920–928. doi: 10.1128/aac.44.4.920-928.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang H, Zhou Y, Alcock C, et al. Novel single-cell-level phenotypic assay for residual drug susceptibility and reduced replication capacity of drug-resistant human immunodeficiency virus type 1. J Virol. 2004;78:1718–1729. doi: 10.1128/JVI.78.4.1718-1729.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25**.Shen L, Peterson S, Sedaghat AR, et al. Dose-response curve slope sets class-specific limits on inhibitory potential of anti-HIV drugs. Nat Med. 2008;14:762–766. doi: 10.1038/nm1777. A recent study analyzing the dose-response curve slopes for all current available anti-HIV-1 drugs using a single round infectivity assay. The results demonstrated that dose-response curve slope has dramatic effect on the inhibitory potential of anti-HIV-1 drugs. A novel index incorporating the slope parameter, instantaneous inhibitory potential, may be a more accurate pharmacodynamic measure of antiviral activity than IC50 or IQ. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McMahon MA, Jilek BL, Brennan TP, et al. The HBV drug entecavir - effects on HIV-1 replication and resistance. N Engl J Med. 2007;356:2614–2621. doi: 10.1056/NEJMoa067710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bisacchi GS, Chao ST, Bachard C, et al. BMS-200475, a novel carbocyclic 2′-deoxyguanosine analog with potent and selective antihepatitis B virus activity in vitro. Bioorg Med Chem Lit. 1997;7:127–132. [Google Scholar]
- 28.Jain MK, Zoellner CL. Entecavir can select for M184V of HIV-1: A case of an HIV/hepatitis B (HBV) naive patient treated for chronic HBV. AIDS. 2007;21:2365–2366. doi: 10.1097/QAD.0b013e3282f1e769. [DOI] [PubMed] [Google Scholar]
- 29**.Sasadeusz J, Audsley J, Mijch A, et al. The anti-HIV activity of entecavir: A multicentre evaluation of lamivudine-experienced and lamivudine-naive patients. AIDS. 2008;22:947–955. doi: 10.1097/QAD.0b013e3282ffde91. This is a retrospective analysis of Hepatitis B/HIV-1 co-infected patients taking entecavir monotherapy. The analysis shows that the acquisition of the M184V mutation in HIV-1 reverse transcriptase does not depend on prior treatment with lamivudine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soriano V, Sheldon J, Garcia-Gasco P, et al. Lack of anti-HIV activity of entecavir in an HIV patient coinfected with hepatitis B and delta viruses. AIDS. 2007;21:2253–2254. doi: 10.1097/QAD.0b013e3282f08bae. [DOI] [PubMed] [Google Scholar]
- 31*.Soriano V, Vispo E, Labarga P, et al. A low antiretroviral activity of the antihepatitis B drug entecavir may be enough to select for M184V in HIV-1. AIDS. 2008;22:911–912. doi: 10.1097/QAD.0b013e3282f82b96. This report shows the selection of the M184V mutation in HIV reverse transcriptase in a Hepatitis delta/HIV-1 co-infected patient taking entecavir monotherapy. [DOI] [PubMed] [Google Scholar]
- 32*.Tchesnokov EP, Obikhod A, Schinazi RF, et al. Delayed chain termination protects the anti-hepatitis B virus drug entecavir from excision by HIV-1 reverse transcriptase. J Biol Chem. 2008;283:34218–34228. doi: 10.1074/jbc.M806797200. This biochemical study demonstrates that entecavir triphosphate is a substrate of HIV-1 reverse transcriptase and describes three mechanisms of inhibition of reverse transcription. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33**.Domaoal RA, McMahon M, Thio CL, et al. Pre-steady-state kinetic studies establish entecavir 5′-triphosphate as a substrate for HIV-1 reverse transcriptase. J Biol Chem. 2008;283:5452–5459. doi: 10.1074/jbc.M707834200. This biochemical study shows that entecavir triphosphate is a substrate of HIV-1 reverse transcriptase and describes the mechanism of action and mechanism of resistance of the M184V reverse transcriptase mutant. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34*.Lin PF, Nowicka-Sans B, Terry B, et al. Entecavir exhibits inhibitory activity against human immunodeficiency virus under conditions of reduced viral challenge. Antimicrob Agents Chemother. 2008;52:1759–1767. doi: 10.1128/AAC.01313-07. This study determined the susceptibility of multiple HIV-1 isolates to entecavir and examined the effect of MOI on the ability of entecavir to inhibit HIV-1 infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35**.McMahon MA, Siliciano JD, Lai J, et al. The antiherpetic drug acyclovir inhibits HIV replication and selects the V75I reverse transcriptase multidrug resistance mutation. J Biol Chem. 2008;283:31289–31293. doi: 10.1074/jbc.C800188200. This study shows that acyclovir is a direct inhibitor of HIV-1 reverse transcriptase and is the first to show selection of the HIV V75I reverse transcriptase mutant in vitro with acyclovir drug pressure. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chong CR, Chen X, Shi L, et al. A clinical drug library screen identifies astemizole as an antimalarial agent. Nature Chemical Biology. 2006;2:415. doi: 10.1038/nchembio806. [DOI] [PubMed] [Google Scholar]
- 37*.Lisco A, Vanpouille C, Tchesnokov EP, et al. Acyclovir is activated into a HIV-1 reverse transcriptase inhibitor in herpesvirus-infected human tissues. Cell Host Microbe. 2008;4:260–270. doi: 10.1016/j.chom.2008.07.008. This study shows that acyclovir triphosphate is an inhibitor of HIV-1 reverse transcriptase and suggests that in cell culture, co-infection with various herpes viruses is necessary for acyclovir inhibition of HIV-1 replication, presumably because of the requirement for virally encoded kinases to phophorylate acyclovir. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38*.Tchesnokov EP, Obikhod A, Massud I, et al. Mechanisms associated with HIV-1 resistance to acyclovir by the V75I mutation in reverse transcriptase. J Biol Chem. 2009 doi: 10.1074/jbc.M109.024026. [epub ahead of print] This mechanistic study shows that the V75I mutation in reverse transcriptase confers resistance to acyclovir mainly by increasing discrimination between acyclovir triphosphate and dGTP but also to some extent by increasing in excision of incorporated acyclovir monophosphate. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zuckerman RA, Lucchetti A, Whittington WL, et al. Herpes simplex virus (HSV) suppression with valacyclovir reduces rectal and blood plasma HIV-1 levels in HIV-1/HSV-2-seropositive men: A randomized, double-blind, placebo-controlled crossover trial. J Infect Dis. 2007;196:1500–1508. doi: 10.1086/522523. [DOI] [PubMed] [Google Scholar]
- 40.Nagot N, Ouedraogo A, Foulongne V, et al. Reduction of HIV-1 RNA levels with therapy to suppress herpes simplex virus. N Engl J Med. 2007;356:790–799. doi: 10.1056/NEJMoa062607. [DOI] [PubMed] [Google Scholar]
- 41*.la Porte C. Inhibitory quotient in HIV pharmacology. Curr Opin HIV AIDS. 2008;3:283–287. doi: 10.1097/COH.0b013e3282fbaaba. A recent comprehensive review on the clinical utility of the inhibitory quotient of anti-HIV-1 drugs. [DOI] [PubMed] [Google Scholar]
- 42.Chou TC. Derivation and properties of michaelis-menten type and Hill type equations for reference ligands. J Theor Biol. 1976;59:253–276. doi: 10.1016/0022-5193(76)90169-7. [DOI] [PubMed] [Google Scholar]
- 43.Hill AV. The possible effects of the aggregation of the molecules of haemoglobin on its dissociation curves. J Physiol (Lond) 1910;40:iv–vii. [Google Scholar]
- 44.Larder BA, Darby G, Richman DD. HIV with reduced sensitivity to zidovudine (AZT) isolated during prolonged therapy. Science. 1989;243:1731–1734. doi: 10.1126/science.2467383. [DOI] [PubMed] [Google Scholar]
- 45.Coffin JM. HIV population dynamics in vivo: Implications for genetic variation, pathogenesis, and therapy. Science. 1995;267:483–489. doi: 10.1126/science.7824947. [DOI] [PubMed] [Google Scholar]
- 46.Wei X, Ghosh SK, Taylor ME, et al. Viral dynamics in human immunodeficiency virus type 1 infection. Nature. 1995;373:117–122. doi: 10.1038/373117a0. [DOI] [PubMed] [Google Scholar]
- 47.Grossman Z, Polis M, Feinberg MB, et al. Ongoing HIV dissemination during HAART. Nat Med. 1999;5:1099–1104. doi: 10.1038/13410. [DOI] [PubMed] [Google Scholar]
- 48.Kuritzkes DR, Ribaudo HJ, Squires KE, et al. Plasma HIV-1 RNA dynamics in antiretroviral-naive subjects receiving either triple-nucleoside or efavirenz-containing regimens: ACTG A5166s. J Infect Dis. 2007;195:1169–1176. doi: 10.1086/512619. [DOI] [PubMed] [Google Scholar]
- 49.Markowitz M, Nguyen BY, Gotuzzo E, et al. Rapid and durable antiretroviral effect of the HIV-1 integrase inhibitor raltegravir as part of combination therapy in treatment-naive patients with HIV-1 infection: Results of a 48-week controlled study. J Acquir Immune Defic Syndr. 2007;46:125–133. doi: 10.1097/QAI.0b013e318157131c. [DOI] [PubMed] [Google Scholar]
- 50*.Sedaghat AR, Dinoso JB, Shen L, et al. Decay dynamics of HIV-1 depend on the inhibited stages of the viral life cycle. Proc Natl Acad Sci U S A. 2008;105:4832–4837. doi: 10.1073/pnas.0711372105. This study demonstrates that the decay rate of viremia depends on where in the viral life cycle the drug acts. The study supports the notion that the rapid decay of viral load observed in patients treated with regimens containing integrase inhibitors may not necessarily indicate superior antiviral activity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Markowitz M, Morales-Ramirez JO, Nguyen BY, et al. Antiretroviral activity, pharmacokinetics, and tolerability of MK-0518, a novel inhibitor of HIV-1 integrase, dosed as monotherapy for 10 days in treatment-naive HIV-1-infected individuals. J Acquir Immune Defic Syndr. 2006;43:509–515. doi: 10.1097/QAI.0b013e31802b4956. [DOI] [PubMed] [Google Scholar]


