Abstract
Rats are responsive to shock from an early age but eyeblink conditioning to a tone conditioned stimulus (CS) paired with a shock unconditioned stimulus (US) does not emerge until postnatal day 20 (P20). More generalized postural responses such as conditioned freezing can occur at P16. Using the same periorbital shock as both the CS and US in a US-US conditioning paradigm previously shown to be effective in adult animals, we found that shock-shock pairings with a 200-ms trace interval resulted in eyeblink conditioning in younger animals than previously thought. Some rat pups showed conditioned eyeblink responses as early as P12, and by P18, conditioned responses were fully developed in all animals. Unpaired control subjects confirmed that responding in paired subjects was associative. Although many stimuli can act as a CS in adults, the advantage of using US-US pairings is that responses to first US ensure young rat pups are capable of detecting the stimulus – something that may not be true when auditory or visual stimuli are used early in the development of altricial animals. The US-US pairing paradigm could be used to study the ontogeny and neural substrates of learning and memory before other sensory systems mature and evaluate learning and memory in animal models of early developmental disorders.
Keywords: development, eyeblink conditioning, ontogeny, rat pup, trace conditioning
Rats are responsive to shock from an early age (Richardson, Wang, & Campbell, 1995) but information about the ability of rat pups to time responding to shock is limited. Adaptive timing of discrete responses including changes in heart rate (Richardson et al., 1995) and eyeblink (Stanton, Freeman, Jr., & Skelton, 1992) following tone-shock pairings does not emerge reliably until after postnatal day 20 (P20) although more generalized postural responses such as freezing to a tone followed by shock can occur as early as P16 (Campbell & Ampuero, 1985). Some of the delay in the development of eyeblink conditioning is due to sensory limitations because Freeman and colleagues have shown that replacing tone with stimulation of pontine auditory inputs supports moderate levels of delay eyeblink conditioning at P12 (Campolattaro & Freeman, Jr., 2008). The slow development of eyeblink conditioning may also be a function of the paradigm. There is convincing evidence that trace fear conditioning – where there is a temporal gap between tone and shock – normally develops later than delay conditioning - where the stimuli overlap (Barnet & Hunt, 2005; Moye & Rudy, 1987). This may also be true for eyeblink conditioning in older rat pups although the length of the interval between the onset of the tone and shock may also be a factor (Claflin, Garrett, & Buffington, 2005; Ivkovich, Paczkowski, & Stanton, 2000).
Classical conditioning using the pairing of two sequential unconditioned stimuli (USs) has been studied in a number of species (Goddard & Jenkins, 1988; Gruart, Pastor, Armengol, & Delgado-García, 1997; Pavlov, 1927; Rodriguez-Moreno, Dominguez Del, Porras-Garcia, & Delgado-Garcia, 2004; Schreurs & Alkon, 1990; Svensson, Ivarsson, & Hesslow, 2000) and was first described by Pavlov (1927, p. 29–31) in an experiment conducted by Erofeeva who presented shock as a signal for food. With repeated pairings, the shock served as a conditioned stimulus (CS) that came to elicit salivation. Schreurs and Alkon (1990) paired two periorbital shocks of different intensity to adult rabbits and reported the gradual emergence of a conditioned nictitating membrane response, i.e., the shock served as a CS that came to elicit a conditioned response (CR). Explicitly unpaired control presentations of the two shocks ensured responding was associative because there was little nonassociative responding (Gormezano & Kehoe, 1975). Rodriguez-Moreno et al. (2004) showed that pairing two shocks of different intensity supported eyeblink conditioning in the adult mouse and Gruart et al. (1997) showed eyeblink conditioning in adult cats could be induced by presenting the same air puff twice. Although there is evidence rats respond to shock at an early age, there are no studies that have investigated the ontogeny of eyeblink conditioning to the pairing of two shocks.
The present experiment was designed to examine the ontogeny of trace eyeblink conditioning using a US1–US2 pairing paradigm. The presence of an unconditioned response (UR) to both US1 and US2 ensured that very young animals were able to detect the stimuli which allowed us to study the development of eyeblink conditioning before other sensory modalities including the auditory and visual systems normally used for eyeblink conditioning had matured. We present data showing pairings of two shocks of the same intensity but of different durations (US1, 10 ms; US2, 25 ms) in the rat pup support trace eyeblink conditioning as a function of age indexed by the emergence of a second response to US1 that is timed to coincide with where US2 would have occurred. Rat pups given explicitly unpaired US presentations showed low levels of nonassociative responding but did not show the emergence of a second response to US1 confirming the associative nature of rat pup eyeblink conditioning to the pairing of two shocks.
Method
Subjects
A total of twenty four male and twenty five female Long–Evans rat pups (Harlan) from twelve litters were trained beginning on postnatal day 12 (8 males, 9 females), 15 (7 males, 9 females) or 18 (9 males, 7 females) and were designated P12, P15 or P18. Because of equipment and timing constraints, we typically sampled one or two different ages per litter. Rat pups were randomly selected for each group. Implanted rat pups were housed in a cage with their littermates and mother and maintained on a 12 hour light-dark cycle and given ad libitum access to food and water. Rat pups were maintained in accordance with guidelines issued by the National Institutes of Health and the research was approved by the West Virginia University Animal Care and Use Committee.
Apparatus
The conditioning apparatus consisted of a sound-attenuating, ventilated chamber (Coulborn Instruments) containing a 21-cm diameter Plexiglas cylinder with a padded floor. The chamber was equipped with a small light and camera making it possible to monitor the subject throughout the session. The chamber was maintained at an ambient temperature of 22 ± 1° C. The electromyography (EMG) connector on the subject's head was attached to a lightweight cable that fed through a commutator (Plastics One Inc.) allowing the rats to move freely during the behavioral procedures. The cable separated and terminated as the input to an AC/DC differential EMG amplifier (A-M Systems) and the output of a stimulus isolator (World Precision Instruments) for shock delivery (3.0–3.5 mA). LabVIEW software (National Instruments) controlled the delivery of stimuli and the recording of eyelid EMG activity.
Procedures
Two days prior to stimulus presentations, rat pups were implanted with bipolar stimulating and differential EMG recording electrodes. The rat pups were anesthetized with isoflurane (induction dose 5%), prepared for surgery, and positioned in an infant rodent stereotaxic head holder, Bupivicaine, a local anesthetic (2 mg/kg), was injected subcutaneously at the site of incision. Isoflurane (1.5–2%) was delivered intranasally throughout the procedure. A midline incision through the scalp, anterior to posterior was made in order to expose the skull. A custom-made connector containing a differential EMG electrode, bipolar stimulating electrode and silver ground wire (Plastics One Inc.) was attached to the skull with cement (Jet Repair Acrylic) and anchored with a fabricated skull hook. The ground wire was placed subcutaneously at the back of the neck. The EMG electrodes were implanted by inserting a 27-gauge needle through the skin and orbicularis oculi muscle of the left eye and threading the electrode wires through the needle tip. The recording electrode was implanted in the center of orbicularis oculi muscle, and the reference electrode was implanted rostral to the muscle. The tip of the bipolar stimulating electrode (used to deliver periorbital shock) was placed subdermally immediately caudal to the left eye assuring good contact with the surrounding skin and muscle. Insulation from the recording and reference electrodes was removed and wires trimmed so that there was 2 mm of exposed wire in the eyelid. The incision was closed with surgical sutures and animals received a subcutaneous injection of acetaminophen (50 mg/kg) as an analgesic. Animals recovered on a heated surgery table and upon regaining sternal recumbency were returned to their littermates and mother.
Following a two-day recovery from surgery, training sessions began at P12, P15 or P18 and continued the following day. Three sessions occurred per day and were separated by 4 hours, yielding a total of six training sessions. Rat pups at each age were assigned to Paired and Unpaired groups. At the beginning of each paired and unpaired session there was an adaptation period that lasted 5 minutes during which no stimuli were presented. For the remainder of each session, Paired groups received 100 trials consisting of 90 paired periorbital shock-shock trials (US1–US2) with a US1-alone probe trial occurring every 10th trial. US1 occurred at 200 ms after the beginning of the trial and lasted for 10 ms, and US2 occurred at 410 ms after the beginning of the trial and lasted for 25 ms (200-ms trace between US1 and US2). Paired trials occurred with an average ITI of 30 s. Following five minutes of adaptation, the Unpaired groups received 190 trials (100 US1 and 90 US2) and the US1 and US2 were presented separately in an explicitly unpaired manner with an average ITI of 15 s. Every 10th US1 trial was designated a probe trial for comparison with probe trials from the Paired groups. For each subject, the intensity of US1 and US2 was always the same (3.0–3.5 mA). With a single behavioral system we were able to implant and train a maximum of four pups per week – three training sessions for the four pups took a total of twelve hours per day.
Data Analysis
The EMG signal was filtered (300–3,000 Hz), amplified (5 K), and stored (raw EMG) in addition to being rectified and integrated (20-ms time constant). Baseline activity was averaged during the first 200 ms from trial onset during US1-alone probe trials. If the integrated EMG activity 100 ms prior to US1 onset exceeded 4 standard deviations from baseline, the trial was omitted from analysis (<1% of total). We characterized URs to US1 and US2 as integrated EMG activity that exceeded the average baseline value by 8 standard deviations. CRs were assessed as EMG activity of 8 standard deviations above the baseline during the period of 350–400 ms from trial onset. This window ensured that movement artifacts, which were common in all three age groups, did not artificially inflate levels of responding. URs to US1 on probe trails were assessed from 245 to 350 ms from trial onset and to US2 on non-probe trials from 470 to 525 ms from trial onset. In addition to response frequency, the magnitude of each response was calculated as the average EMG signal during the baseline period subtracted from the maximum EMG signal during the response period.
Results
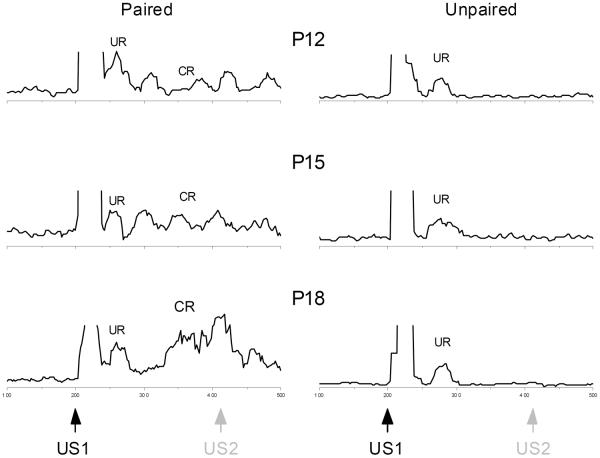
Figure 1 shows sample integrated EMG activity to US1-alone probe trials for individual rat pups given paired (Paired) or explicitly unpaired (Unpaired) US1 and US2 presentations from the P12, P15, and P18 groups. The figure shows that following the shock artifact, rat pups exhibited a UR to US1 and, only in the case of the rat pups receiving paired presentations of US1 and US2, there was responding following the UR to US1 that grew more pronounced as a function of age and overlapped with the point at which US2 would have occurred on paired trials.
Figure 1.
Development of eyeblink trace conditioning to shock-shock pairings. Sample integrated EMG activity in the upper eyelid muscle to a shock-alone unconditioned stimulus (US1) probe trial for individual rat pups given paired (Paired, left) or explicitly unpaired (Unpaired, right) US1 and US2 presentations beginning at postnatal day 12, 15, and 18 (P12, P15, and P18). The black arrow indicates the onset of the first shock unconditioned stimulus (US1) which is followed by an unconditioned response (UR). The gray arrow indicates where the second shock unconditioned stimulus (US2) would have occurred on US1-US2 paired trials for rat pups in the Paired groups. The left samples of integrated EMG activity show the occurrence of a conditioned response (CR) that begins in anticipation of and overlaps with the point at which US2 would have occurred on a paired US1-US2 trial particularly at P18 and the magnitude of the CR increases with age. The right samples show there was no evidence of CRs in the sample integrated EMG activity from rat pups in any age group that received explicitly unpaired presentations of US1 and US2.
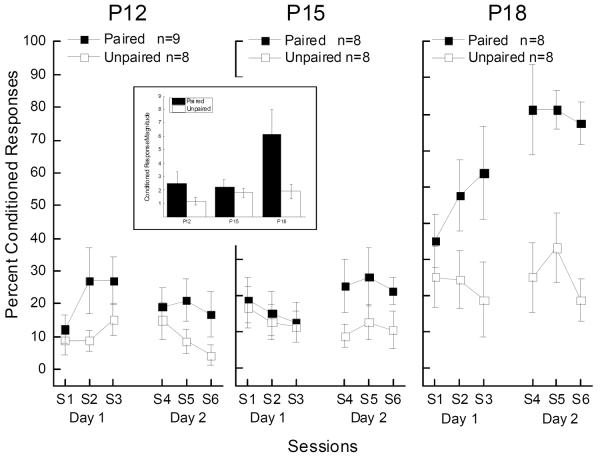
Figure 2 shows differences in mean CR levels between Paired and Unpaired groups developed as a function of age with the strongest differences at P18 and very modest differences at P12 and P15. An analysis of variance (ANOVA) of percent CRs to US1 on probe trials with factors of pairings, age, days, and sessions yielded significant main effects of pairings (F(1,43) = 19.00, p < .001) and age (F(2,43) = 17.82, p < .001), and an interaction of pairings and age (F(2,43) = 4.45, p < .05) confirming paired rat pups acquired CRs to US1 and the acquisition of those CRs improved with age. Analysis also revealed a main effect of days (F(1,43) = 11.08, p < .01), and interactions of days with pairings (F(1,43) = 12.93, p < .01), age (F(2,43) = 13.08, p < .001), and with pairings and age (F(2,43) = 5.15, p < .05) suggesting conditioned responding to US1 improved from Day1 to Day 2 as a function of pairings and age. There were no significant effects of sessions (F's < 1.8).
Figure 2.
Trace conditioning frequency and magnitude (inset) to shock-shock pairings develops as a function of age. Mean (± SEM) percent conditioned responding (%CRs) to the shock-alone unconditioned stimulus (US1) trials for rat pups given paired (Paired) or explicitly unpaired (Unpaired) US1 and US2 presentations beginning at postnatal day 12, 15, and 18 (P12, P15, and P18) across three sessions of stimulus presentations on Day 1 and three sessions of stimulus presentations on Day 2. The inset shows mean conditioned response (CR) magnitude to the shock-alone unconditioned stimulus (US1) trials for rat pups given Paired or explicitly Unpaired US1 and US2 presentations beginning at postnatal day 12, 15, and 18 (P12, P15, and P18) collapsed across sessions and days.
Given the main effect of age in the overall analysis, separate ANOVAs of percent CRs to US1 were conducted for the Paired and Unpaired at P12, P15 and P18 with factors of pairings, age, days, and sessions. There were significant main effects of pairings (F(1,14) = 15.57, p < .01) and days (F(1,14) = 23.93, p < .001), and an interaction of pairings and days (F(2,28) = 12.55, p < .01) at P18 confirming the strong differences in CRs to US1 as a function of US1-US2 pairings that increased from Day 1 to Day 2. There was a significant interaction of pairings and days at P15 (F(1,14) = 6.52, p < .05) but not of sessions suggesting the increase in CRs to US1 in the Paired P15 group on Day 2 which was confirmed by a post hoc comparison (F (1,14) = 4.76 p < .05). There was no significant effects of pairings or days at P12 although there was an interaction of pairings and sessions at P12 (F(2,28) = 3.65, p < .05). Post hoc comparisons yielded no significant group differences. Nevertheless, an examination of individual response levels at P12 revealed that three of eight rat pups showed levels of conditioning of at least 70% CRs on at least one session and seven of eight rat pups showed conditioning of at least 50% CRs on at least one session. As a group, however, these rats did not show consistent conditioning across sessions. Finally, an analysis of responding to US1 in the Unpaired groups found no significant changes in nonassociative responding as a function of days, sessions or age (F's < 2.65).
Examination of Figure 1 suggests that in addition to the level of CRs, the size of CRs grew larger as a function of age in the Paired groups. The low mean frequency of CRs shown by the Paired P12 and P15 groups and by all of the Unpaired groups precluded a standard analysis of CR amplitude because of the large amount of missing data (empty cells with no CRs). As a result, we calculated magnitude of all responses within the CR window (Garcia, Mauk, Weidemann, & Kehoe, 2003; Schreurs, Smith-Bell, & Burhans, 2011) to analyze changes in response size as a function of age for rat pups from Paired and Unpaired groups. The inset in Figure 2 shows CR magnitude as a function of pairings and age and an ANOVA of CR magnitude revealed significant main effects of pairings (F(1,43) = 11.93, p < .001), age (F(2,43) = 5.82, p < .01), and an interaction of pairings and age (F(2,43) = 3.93, p < .05) confirming the increase in response size in the Paired groups as a function of age. The analysis also revealed interactions of days with age (F(2,43) = 7.64, p < .01), and days with pairings and age (F(2,43) = 5.92, p < .01) suggesting magnitude of CRs to US1, as well as frequency, improved from Day1 to Day 2 as a function of pairings and age.
Finally, analyses of UR1 frequency and amplitude to US1 on probe trials revealed no significant main effects or interactions of pairings (F's < 2.5) suggesting URs to US1 did not change as a function US1–US2 pairings. Analyses of the frequency of URs to US2 on non-probe trials revealed a significant main effect of pairing (F (1, 43) = 5.12, p < .05), suggesting that the presence of CRs on paired trials increased the likelihood of a response to US2. There was also a main effect of age (F (1, 43) = 3.92, p < .05) which, surprisingly, was due to higher levels of responding at P12 (68.54 ± 6.16) than at P15 (51.43 ± 6.31) or P18 (59.40 ± 6.14). Analysis of the amplitude of URs to US2 revealed no significant effects of pairings or age (F's < 2.70).
Discussion
The principal findings of the present experiment were that rat pups showed strong evidence of trace conditioning to pairings of two shocks by P18 and began to show evidence of trace conditioning as early as P12. The emergence of trace conditioning to the first shock (i.e., the “conditioned stimulus”) was associative, grew larger as a function of age, and was timed in anticipation of and to overlap with the occurrence of the second shock (i.e., the unconditioned stimulus). The results suggest rat pups as young as P12 that do show learning could make an ideal preparation for studying the neural substrates of trace conditioning. The preparation may also be important for studying the effects of pre- and perinatal events on learning and memory – events such maternal alcohol consumption, malnutrition, drug abuse, or deprivation that may lead to any number of disorders including, for example, fetal alcohol syndrome (Green, Johnson, Goodlett, & Steinmetz, 2004; Green, Tran, Steinmetz, & Goodlett, 2002; Jacobson et al., 2011).
There is convincing evidence trace conditioning in animal models normally develops later than delay conditioning. During fear conditioning, acquisition of trace conditioning begins between P18 and P21 whereas delay conditioning begins as early as P15 (Barnet & Hunt, 2005; Moye & Rudy, 1987). During eyeblink conditioning, rat pups show trace conditioning beginning at between P20 and P23 whereas delay conditioning begins to emerge at P17 (Ivkovich et al., 2000; Stanton et al., 1992). The fact that delay conditioning begins before trace conditioning suggests rat pups can detect the stimuli at an early age but the ability to bridge the temporal gap between those stimuli develops later presumably because of postnatal development of the hippocampus and prefrontal cortex - structures required for the acquisition and retention of trace conditioning (Quinn, Ma, Tinsley, Koch, & Fanselow, 2008; Weible, McEchron, & Disterhoft, 2000; Woodruff-Pak & Disterhoft, 2008). This is certainly true for eyeblink conditioning with trace intervals of 500-ms or more, but for shorter traces including the 200 ms used in the present experiment, the hippocampus and prefrontal cortex may not be necessary and the cerebellum may suffice (Takehara, Kawahara, & Kirino, 2003; Weiss & Disterhoft, 2011; Woodruff-Pak & Disterhoft, 2008). The role of the cerebellum in the ontogeny of rat eyeblink conditioning has been explored extensively by Freeman and colleagues (Freeman, Jr. & Nicholson, 2000; Nicholson & Freeman, Jr., 2003) who have shown delay conditioning can begin as early as P12 if the immature auditory system is bypassed by direct stimulation of the pontine nuclei (Campolattaro & Freeman, Jr., 2008; Freeman, Jr., Rabinak, & Campolattaro, 2005) and inactivation of the cerebellum prevents the expression of conditioning to pontine stimulation (Campolattaro & Freeman, Jr., 2008). The present results are consistent with the Freeman pontine stimulation delay conditioning data and extend them to trace conditioning with peripheral sensory stimulation. Moreover, the elicitation of a reliable UR to US1 ensured that all rat pups detected the shock CS - something that cannot be ensured in young animals if presented with other CSs such as tones and lights. The higher levels of responding to US2 by P12 rat pups also suggest that any potential effects of ambient temperature on sensory or motor systems in young animals did not contribute to the low levels of learning although potential temperature effects on associative processes cannot be ruled out (Blumberg & Sokoloff, 1998). A question of some significance for the present pairing paradigm is whether US-US conditioning is fundamentally different from CS-US conditioning using a tone or light CS. Shock sensory inputs reach the cerebellum via mossy fibers and climbing fibers although shock is traditionally thought to only be conveyed via climbing fibers that project from the inferior olive (Meng, Hu, Benetti, & Bereiter, 1997; Schreurs, 1988). If one assumes that US-US conditioning is a case of simply stimulating climbing fiber inputs to the cerebellum twice then it would be different from CS-US pairings where the CS activates mossy fiber inputs and the US activates climbing fiber inputs. In that case, the US-US paradigm would be a new form of associative plasticity mediated by the cerebellum that might have features in common with in vivo and in vitro paired pulse phenomena (Johnson, Goel, & Buonomano, 2010). If, on the other hand, US-US conditioning activates mossy fibers as well as climbing fibers as a good deal of evidence suggests (Cody & Richardson, 1978; Ikeda, 1979; Jorntell & Ekerot, 2011; Richardson, Cody, Paul, & Thomas, 1978; Watson & Switzer, III, 1978), it would contain the essential elements of CS-US conditioning and would be consistent with the eyeblink conditioning that occurs with direct electrical stimulation of mossy fiber and climbing fiber pathways (Gould, Sears, & Steinmetz, 1993; Steinmetz, Lavond, & Thompson, 1989). There is good evidence that a shock US does activate mossy fibers particularly from the work of Jorntell and Ekerot who have shown that shock to the skin approximates natural stimulation as mossy fiber activity (Jorntell & Ekerot, 2011). Of course, the complication from a mechanistic point of view is that in the current US-US paradigm both mossy fibers and climbing fibers are activated at essentially the same time by the US and both inputs are activated twice.
Finally, some may consider the US-US paradigm unusual and not relevant to more traditional forms of CS-US pairings. However, presenting the same pulsed stimulus twice has been shown to produce plastic changes in preparations as disparate as cortical brain slices in the rat (Johnson et al., 2010) to motor cortex in human using transcranial magnetic stimulation (Valls-Sole, Pascual-Leone, Wasserman, & Hallett, 1992). Perhaps more importantly, the present data add support to the observation that almost any stimulus to which the organism is sensitive (Hilgard & Marquis, 1940) can act as a CS including shock to the skin (Pavlov, 1927).
Acknowledgments
We thank Jimena Gonzalez-Joekes for technical assistance. This research was supported in part by NIH grant NS061103, a WVU Bridge Grant, and internal funds from BRNI.
Reference List
- Barnet RC, Hunt PS. Trace and long-delay fear conditioning in the developing rat. Learning & Behavior. 2005;33:437–443. doi: 10.3758/bf03193182. [DOI] [PubMed] [Google Scholar]
- Blumberg MS, Sokoloff G. Thermoregulatory competence and behavioral expression in the young of altricial species - revisited. Developmental Psychobiology. 1998;33:107–123. doi: 10.1002/(sici)1098-2302(199809)33:2<107::aid-dev2>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Campbell BA, Ampuero MX. Dissociation of autonomic and behavioral components of conditioned fear during development in the rat. Behavioral Neuroscience. 1985;99:1089–1102. doi: 10.1037//0735-7044.99.6.1089. [DOI] [PubMed] [Google Scholar]
- Campolattaro MM, Freeman JH., Jr. Eyeblink conditioning in 12-day-old rats using pontine stimulation as the conditioned stimulus. Proceedings of the National Academy of Sciences. 2008;105:8120–8123. doi: 10.1073/pnas.0712006105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claflin DI, Garrett T, Buffington ML. A developmental comparison of trace and delay eyeblink conditioning in rats using matching interstimulus intervals. Developmental Psychobiology. 2005;47:77–88. doi: 10.1002/dev.20068. [DOI] [PubMed] [Google Scholar]
- Cody FWJ, Richardson HC. Mossy and climbing fibre projections of trigeminal inputs to the cerebellar cortex in the cat. Brain Research. 1978;153:352–356. doi: 10.1016/0006-8993(78)90414-6. [DOI] [PubMed] [Google Scholar]
- Freeman JH, Jr., Nicholson DA. Developmental changes in eye-blink conditioning and neuronal activity in the cerebellar interpositus nucleus. Journal of Neuroscience. 2000;20:813–819. doi: 10.1523/JNEUROSCI.20-02-00813.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman JH, Jr., Rabinak CA, Campolattaro MM. Pontine stimulation overcomes developmental limitations in the neural mechanisms of eyeblink conditioning. Learning & Memory. 2005;12:255–259. doi: 10.1101/lm.91105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia KS, Mauk MD, Weidemann G, Kehoe EJ. Covariation of alternative measures of responding in rabbit (Oryctolagus cuniculus) eyeblink conditioning during acquisition training and tone generalization. Behavioral Neuroscience. 2003;117:292–303. doi: 10.1037/0735-7044.117.2.292. [DOI] [PubMed] [Google Scholar]
- Goddard MJ, Jenkins HM. Blocking of a CS-US association by a US-US association. Journal of Experimental Psychology: Animal Behavior Processes. 1988;14:177–186. [Google Scholar]
- Gormezano I, Kehoe EJ. Classical conditioning: Some methodological-conceptual issues. In: Estes WK, editor. Handbook of learning and cognitive processes. 2 ed. Erlbaum; Hillsdale, NJ: 1975. pp. 143–179. [Google Scholar]
- Gould TJ, Sears LL, Steinmetz JE. Possible CS and US pathways for rabbit classical eyelid conditioning: electrophysiological evidence for projections from the pontine nuclei and inferior olive to cerebellar cortex and nuclei. Behavioral and Neural Biology. 1993;60:172–185. doi: 10.1016/0163-1047(93)90285-p. [DOI] [PubMed] [Google Scholar]
- Green JT, Johnson TB, Goodlett CR, Steinmetz JE. Eyeblink classical conditioning and interpositus nucleus activity are disrupted in adult rats exposed to ethanol as neonates. Learning & Memory. 2004;9:304–320. doi: 10.1101/lm.47602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green JT, Tran T, Steinmetz JE, Goodlett CR. Neonatal ethanol produces cerebellar deep nuclear cell loss and correlated disruption of eyeblink conditioning in adult rats. Brain Research. 2002;956:302–311. doi: 10.1016/s0006-8993(02)03561-8. [DOI] [PubMed] [Google Scholar]
- Gruart A, Pastor AM, Armengol JA, Delgado-García JM. Involvement of cerebellar cortex and nuclei in the genesis and control of unconditioned eyelid motor responses. Progress in Brain Research. 1997;114:511–528. doi: 10.1016/s0079-6123(08)63383-x. [DOI] [PubMed] [Google Scholar]
- Hilgard ER, Marquis DG. Conditioning and learning. Wiley; New York: 1940. [Google Scholar]
- Ikeda M. Projections from the spinal and the principal sensory nuclei of the trigeminal nerve to the cerebellar cortex in the cat, as studied by retrograde transport of horseradish peroxidase. Journal of Comparative Neurology. 1979;184:567–586. doi: 10.1002/cne.901840309. [DOI] [PubMed] [Google Scholar]
- Ivkovich D, Paczkowski C, Stanton ME. Ontogeny of delay versus trace eyeblink conditioning in the rat. Developmental Psychobiology. 2000;36:148–160. doi: 10.1002/(sici)1098-2302(200003)36:2<148::aid-dev6>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Jacobson SW, Stanton ME, Dodge NC, Pienaar M, Fuller DS, Molteno CD, et al. Impaired delay and trace eyeblink conditioning in school-age children with fetal alcohol syndrome. Alcoholism, Clinical and Experimental Research. 2011;35:250–264. doi: 10.1111/j.1530-0277.2010.01341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson HA, Goel A, Buonomano DV. Neural dynamics of in vitro cortical networks reflects experienced termporal patterns. Nature Neuroscience. 2010;13:917–919. doi: 10.1038/nn.2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorntell H, Ekerot C-F. Receptive field remodeling induced by skin stimulation in cerebellar neurons in vivo. Frontiers in Neural Circuits. 2011;5:1–10. doi: 10.3389/fncir.2011.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng ID, Hu JW, Benetti AP, Bereiter DA. Encoding of corneal input in two distinct regions of the spinal trigeminal nucleus in the rat: cutaneous receptive field properties, responses to thermal and chemical stimulation, modulation by diffuse noxious inhibitory controls, and projections to the parabrachial area. Journal of Neurophysiology. 1997;77:43–56. doi: 10.1152/jn.1997.77.1.43. [DOI] [PubMed] [Google Scholar]
- Moye TB, Rudy JW. Ontogenesis of trace conditioning in young rats: dissociation of associative and memory processes. Developmental Psychobiology. 1987;20:405–414. doi: 10.1002/dev.420200405. [DOI] [PubMed] [Google Scholar]
- Nicholson DA, Freeman JH., Jr. Developmental changes in eyeblink conditioning and simple spike activity in the cerebellar cortex. Developmental Psychobiology. 2003;44:45–57. doi: 10.1002/dev.10149. [DOI] [PubMed] [Google Scholar]
- Pavlov IP. Conditioned reflexes: An investigation of the physiological activity of the cerebral cortex. (G. V. Anrep, Trans.) Oxford University Press; London: 1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn JJ, Ma QD, Tinsley MR, Koch C, Fanselow MS. Inverse temporal contributions of the dorsal hippocampus and medial prefrontal cortex to the expression of long-term fear memories. Learning & Memory. 2008;15:368–372. doi: 10.1101/lm.813608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson HC, Cody FWJ, Paul VE, Thomas AG. Convergence of trigeminal and limb inputs onto cerebellar interpositus nuclear neurones in the cat. Brain Research. 1978;156:355–359. doi: 10.1016/0006-8993(78)90518-8. [DOI] [PubMed] [Google Scholar]
- Richardson R, Wang P, Campbell BA. Delayed development of conditioned heart rate responses to auditory stimuli in the rat. Developmental Psychobiology. 1995;28:221–238. doi: 10.1002/dev.420280404. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Moreno A, Dominguez Del TE, Porras-Garcia E, Delgado-Garcia JM. The use of alert behaving mice in the study of learning and memory processes. Neurotoxicity Research. 2004;6:225–232. doi: 10.1007/BF03033224. [DOI] [PubMed] [Google Scholar]
- Schreurs BG. Stimulation of the spinal trigeminal nucleus supports classical conditioning of the rabbit's nictitating membrane response. Behavioral Neuroscience. 1988;102:163–172. doi: 10.1037//0735-7044.102.1.163. [DOI] [PubMed] [Google Scholar]
- Schreurs BG, Alkon DL. US-US conditioning of the rabbit's nictitating membrane response: emergence of a conditioned response without alpha conditioning. Psychobiology. 1990;18:312–320. [Google Scholar]
- Schreurs BG, Smith-Bell CA, Burhans LB. Unpaired extinction: implications for treating post-traumatic stress disorder. Journal of Psychiatric Research. 2011;45:638–649. doi: 10.1016/j.jpsychires.2010.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton ME, Freeman JH, Jr., Skelton RW. Eyeblink conditioning in the developing rat. Behavioral Neuroscience. 1992;106:657–665. doi: 10.1037//0735-7044.106.4.657. [DOI] [PubMed] [Google Scholar]
- Steinmetz JE, Lavond DG, Thompson RF. Classical conditioning in rabbits using pontine nucleus stimulation as a conditioned stimulus and inferior olive stimulation as an unconditioned stimulus. Synapse. 1989;3:225–233. doi: 10.1002/syn.890030308. [DOI] [PubMed] [Google Scholar]
- Svensson P, Ivarsson M, Hesslow G. Involvement of the cerebellum in a new temporal property of the conditioned eyeblink response. Progress in Brain Research. 2000;124:317–323. doi: 10.1016/S0079-6123(00)24026-0. [DOI] [PubMed] [Google Scholar]
- Takehara K, Kawahara S, Kirino Y. Time-dependent reorganization of the brain components underlying memory retention in trace eyeblink conditioning. The Journal of Neuroscience. 2003;23:9897–9905. doi: 10.1523/JNEUROSCI.23-30-09897.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valls-Sole J, Pascual-Leone A, Wasserman EM, Hallett M. Human motor evoked responses to paired transcranial magnetic stimuli. Electroencephalography and Clinical Neurophysiology. 1992;85:355–364. doi: 10.1016/0168-5597(92)90048-g. [DOI] [PubMed] [Google Scholar]
- Watson CRR, Switzer RC., III Trigeminal projections to cerebellar tactile areas in the rat - origin mainly from N. interpolaris and N. principalis. Neuroscience Letters. 1978;10:77–82. doi: 10.1016/0304-3940(78)90015-0. [DOI] [PubMed] [Google Scholar]
- Weible AP, McEchron MD, Disterhoft JF. Cortical involvement in acquisition and extinction of trace eyeblink conditioning. Behavioral Neuroscience. 2000;114:1058–1067. doi: 10.1037//0735-7044.114.6.1058. [DOI] [PubMed] [Google Scholar]
- Weiss C, Disterhoft JF. Exploring prefrontal cortical memory mechanisms with eyeblink conditioning. Behavioral Neuroscience. 2011;125:318–326. doi: 10.1037/a0023520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff-Pak DS, Disterhoft JF. Where is the trace in trace conditioning? Trends in Neurosciences. 2008;31:105–112. doi: 10.1016/j.tins.2007.11.006. [DOI] [PubMed] [Google Scholar]