Abstract
Summary
The effects of bariatric surgery on skeletal health are poorly understood. We found that bariatric surgery patients are more prone to fracture when compared to the general population. While further studies of fracture risk in this population are needed, bone health should be discussed in bariatric surgery clinics.
Introduction
Bariatric surgery is an increasingly common treatment for medically complicated obesity. Adverse skeletal changes after bariatric surgery have been reported, but their clinical importance remains unknown. We hypothesized that bariatric surgery patients are at increased risk of fracture.
Methods
We conducted a historical cohort study of fracture incidence among 258 Olmsted County, Minnesota, residents who underwent a first bariatric surgery in 1985–2004. Relative fracture risk was expressed as standardized incidence ratios (SIRs), while potential risk factors were evaluated by hazard ratios (HR) obtained from a time-to-fracture regression model.
Results
The mean (±SD) body mass index at bariatric surgery was 49.0±8.4 kg/m2, with an average age of 44±10 years and 82 % (212) females. Gastric bypass surgery was performed in 94 % of cases. Median follow-up was 7.7 years (range, 6 days to 25 years), during which 79 subjects experienced 132 fractures. Relative risk for any fracture was increased 2.3-fold (95 % confidence interval (CI), 1.8–2.8) and was elevated for a first fracture at the hip, spine, wrist, or humerus (SIR, 1.9; 95 % CI, 1.1–2.9), as well as for a first fracture at any other site (SIR, 2.5; 95 % CI, 2.0–3.2). Better preoperative activity status was associated with a lower age-adjusted risk (HR, 0.4; 95 % CI, 0.2–0.8) while prior fracture history was not associated with postoperative fracture risk.
Conclusions
Bariatric surgery, which is accompanied by substantial biochemical, hormonal, and mechanical changes, is associated with an increased risk of fracture.
Keywords: Bariatric surgery, Cohort study, Fractures, Obesity, Population based
Introduction
A dramatic increase in the prevalence of obesity (body mass index [BMI] >30 kg/m2) in the USA over the past 20 years has been accompanied by increased rates of severe, or class 3, obesity (BMI >40 kg/m2) [1]. Long-term outcome studies have shown that operations such as Roux-en-Y gastric bypass (RYGB) not only result in significant and sustained weight loss but also improve or resolve many comorbid conditions [2] and reduce mortality [3]. As a result, bariatric surgery has become the gold-standard treatment for individuals with BMI ≥40 or ≥35 kg/m2 with obesity-related morbidity such as type 2 diabetes mellitus. This evidence base, combined with improved surgical technique, expanding insurance coverage, and growing patient acceptance if not enthusiasm, led to 220,000 bariatric surgeries being performed in the USA in 2009, representing a >10-fold increase in utilization over the course of a decade [4]. However, many long-term outcomes of bariatric procedures remain poorly defined. Of specific concern for an aging population is the impact of bariatric surgery on skeletal health.
Although two early reports suggested a link between bariatric surgery and fracture [5, 6], a large population-based cohort study in the UK found no evidence that bariatric surgery increased the risk of fracture [7]. However, 60 % of the operated patients in that study had undergone laparoscopic adjustable gastric banding. This purely restrictive procedure produces less extensive and slower weight loss than RYGB [8], is not associated with significant loss of bone mass relative to medical weight loss [9], and is generally associated with less change in bone mass and metabolism than observed after RYGB [10]. Moreover, the bariatric surgery patients and their controls had been matched on BMI in order to focus on the fracture incidence due to surgery rather than emerging skeletal risk factors for fracture associated with severe obesity [11]. Thus, the study suggested that bariatric surgery per se did not have a deleterious effect, but it did not provide guidance about long-term fracture outcomes following RYGB in comparison with the general population.
Concern arises from evidence of calcium and vitamin D deficiency [12, 13] and osteomalacia [14, 15] among these patients. Moreover, even when provided with what is thought to be adequate calcium and vitamin D, bariatric surgery patients appear to have elevated bone turnover markers [10, 16–18] and reduced bone mineral density (BMD) [16, 17, 19, 20]. Consequently, there may be subclinical or latent metabolic bone disease in this population that would eventually lead to skeletal fragility, but indices of skeletal health have not been included in large-scale prospective studies of bariatric surgery outcomes [21]. The aim of the present study was to determine the incidence of fractures in residents of Olmsted County, MN, who have undergone a bariatric surgery, as compared to the general population. In addition, we evaluated potential risk factors for fracture following bariatric surgery.
Methods
The Rochester Epidemiology Project links comprehensive (inpatient and outpatient) medical records for all residents of Olmsted County, enabling the completion of robust disease association studies such as this [22]. Pertinent records can be identified through a centralized index to the diagnoses and surgical procedures recorded by local medical care providers. Following approval by the institutional review boards of Mayo Clinic and the Olmsted Medical Center, we used the Mayo Clinic bariatric surgery practice database and the Rochester Epidemiology Project to identify local residents who underwent bariatric surgery at Mayo Clinic, Rochester, in 1985–September 2004, the only facility in the county offering this procedure during that time. Of 1,508 bariatric surgeries performed at the Mayo Clinic during this interval, 295 cases were identified as potential subjects based on reported local residency status. Of these, 3 declined to authorize the use of their medical records for research [23], 19 were not actually county residents at the time of surgery, and 15 had undergone more than one bariatric surgical procedure.
The remaining 258 patients were then followed forward in time through their community medical records (historical cohort study) until death or the most recent clinical contact. The records for every inpatient hospitalization, outpatient office or clinic visit, all emergency room and nursing home care, as well as all laboratory results, radiographic and pathology reports, including autopsies, and all correspondence with each patient were manually searched. Information was collated on patient demographics and behaviors, BMI (in kilogram per square meter) at the time of surgery, the bariatric surgical procedure, surgical or nutritional complications of bariatric surgery, and fracture events. Fractures were identified from the original clinical history and radiologist’s report of each fracture, but radiographs were not individually reviewed. Thus, the diagnosis of vertebral fracture was based on a radiologist’s report of compression or collapse of one or more thoracic or lumbar vertebrae. Ascertainment of clinically evident fractures is believed to be complete [24]. By convention, fractures due to daily activities and falls from standing height or less were considered moderate trauma, while motor vehicle accidents and falls from a height greater than standing were deemed severe trauma. Fractures attributed by attending physicians to a specific bone lesion, mainly metastatic malignancy (pathologic fractures), were excluded. Physical activity was assessed on a 6-point scale, with subjects in the highest two categories (sedentary—normal activities but no exercise, active—walks at a brisk pace) classified as physically active and those in the lower four categories (limitation to walking distance or worse) classified as inactive. Calcium and/or vitamin D deficiency was noted when listed as a diagnosis in the documentation of any medical provider.
The potential influence of bariatric surgery on subsequent fracture risk was evaluated using three basic analyses, all carried out in SAS (SAS Institute Inc., Cary, NC). The primary analysis compared the fractures observed at each site (based on the first fracture of a given type per person) to the number expected in this cohort during their follow-up in the community, i.e., standardized incidence ratios (SIR). As described in detail elsewhere [25], expected numbers were derived by applying local calendar year-, age-, and sex-specific incidence rates for these fractures to the calendar year-, age-, and sex-specific person-years of follow-up in the cohort and summing over the strata. Except for fractures due to a specific pathology (e.g., metastatic malignancy), all fractures were included, recognizing that low bone density predicts fractures due to severe trauma as well as those attributed to moderate trauma [26]. Ninety-five percent confidence intervals (95 % CI) for the SIRs were calculated, assuming the expected rates are fixed and the observed fractures follow a Poisson distribution [27].
In the second analysis, the cumulative incidence of fracture was estimated for up to 15 years following surgery using the Kaplan–Meier method [28]. Kaplan–Meier methods were also used to assess survival, with expected death rates from the Minnesota white population. Observed and expected cumulative incidence estimates, as well as observed and expected survival curves, were compared using the log-rank test [29].
Finally, Andersen–Gill time-to-fracture regression models [30] assessed the impact of gender, physical activity status, clinical diagnosis of calcium/vitamin D deficiency or general malnutrition, smoking, alcohol use, alcoholism, or fracture prior to bariatric surgery on relative fracture risk (hazard ratio [HR]). These models allow for multiple fractures per subject, while accounting for the correlation structure. Univariate relations between the risk of specific fractures and each clinical characteristic were first assessed, and stepwise methods with forward selection and backward elimination were then used to choose independent variables for the final models. The independent variables were age and the various clinical characteristics. When fracture counts were low for a particular model, and coefficient estimates thereby unstable, Firth’s penalized maximum likelihood estimation was used [31].
Results
In the period 1985–2004, 258 Olmsted County residents had a first bariatric surgery at Mayo Clinic, Rochester (Table 1). Their mean (±SD) age at bariatric surgery was 44±10 years, and 82 % were female. Their mean BMI at the time of surgery was 49.0±8.4 kg/m2. RYGB surgery was the most common procedure, performed with a Roux limb length of 150 cm or less in 193 (75 %) and a “very, very long limb” (>300 cm) in 50 (19 %) patients. Reflecting the surgical practice over this time period, 13 subjects had vertical-banded gastroplasty, one had biliopancreatic diversion with duodenal switch, and one had a partial pancreatobiliary diversion. A history of any prior fracture was documented in 94 (36 %) subjects. Altogether, 130 of 258 (50 %) subjects had ever smoked, and 155 of 222 (70 %) had ever used alcohol; alcoholism was documented in 35 of 188 (19 %) subjects with adequate data. Also, 222 of 241 (92 %) subjects were considered physically active. A clinical diagnosis of calcium deficiency was documented in 32 of 237 (14 %) and vitamin D deficiency in 111 of 216 (51 %) subjects for whom data were available.
Table 1.
Characteristics of 258 Olmsted County, Minnesota, residents with mean follow-up of 8.9 (±4.8)years after bariatric surgery in 1985–2004
| Characteristic | All subjects (N=258) |
|---|---|
| Female, N (%) | 212 (82.2 %) |
| Caucasian race (%) | 236 (97 %) |
| Age at surgery (years)a, mean ± SD | 43.6±9.9 |
| Weight at surgery (kg)a, mean ± SD | 137.2±27.9 |
| BMI at surgery (kg/m2)a, mean ± SD | 49.0±8.4 |
| RYGB surgerya, N (%) | 243 (94.2 %) |
| History of any prior fracturea, N (%) | 94 (36.4 %) |
| Ever smoker, N (%) | 130 (50.4 %) |
| Ever used alcohol, N (%) | 155 (69.8 %) |
| Alcoholism, N (%) | 35 (18.6 %) |
| Physically activea, N (%) | 222 (92.1 %) |
| Ever calcium deficiency, N (%) | 32 (13.5 %) |
| Ever vitamin D deficiency, N (%) | 111 (51.4 %) |
Baseline variable; others could occur at any time point
Survival was not reduced following bariatric surgery, as 97 % were alive 15 years later compared to 94 % expected (p=0.998). These patients were observed for 2,286 person-years (mean, 8.9±4.8 years; median, 7.7 years; range, 6 days to 25 years per subject following surgery), during which time 79 individuals experienced 132 fractures. Forty-four (56 %) patients had a single fracture, whereas 24 had two fractures, 6 had three fractures, and 5 had four or more fractures. As delineated in Table 2, the most common mechanism of fracture was a fall from standing height or less, accounting for over half of the fractures, whereas 13 fractures occurred “spontaneously” (e.g., stress fractures). A minority of fractures was due to severe trauma, including 13 motor vehicle accidents and 3 falls from a height greater than standing. Twenty-nine fractures were due to miscellaneous causes (i.e., hitting foot on furniture), and the etiology of the remaining six fractures was unknown.
Table 2.
Distribution of fractures among 258 Olmsted County, Minnesota, residents with mean follow-up of 8.9 (±4.8)years after bariatric surgery in 1985–2004, by fracture site and immediate cause
| Fracture site | Fracture cause
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Severe trauma
|
Fall from standing height or less
|
Spontaneous
|
Other
|
Uncertain
|
All causes
|
|||||||
| n | %a | n | %a | n | %a | n | %a | n | %a | n | %b | |
| Skull/face | 1 | 20.0 | 3 | 60.0 | 0 | 0.0 | 1 | 20.0 | 0 | 0.0 | 5 | 3.8 |
| Hands/fingers | 0 | 0.0 | 11 | 55.0 | 0 | 0.0 | 8 | 40.0 | 1 | 5.0 | 20 | 15.2 |
| Distal forearm | 2 | 16.7 | 9 | 75.0 | 0 | 0.0 | 0 | 0.0 | 1 | 8.3 | 12 | 9.1 |
| Proximal humerus | 2 | 22.2 | 7 | 77.8 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 9 | 6.8 |
| Other arm | 0 | 0.0 | 4 | 80.0 | 0 | 0.0 | 1 | 20.0 | 0 | 0.0 | 5 | 3.8 |
| Clavicle/scapula/sternum | 1 | 100 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.8 |
| Ribs | 3 | 30.0 | 4 | 40.0 | 0 | 0.0 | 3 | 30.0 | 0 | 0.0 | 10 | 7.6 |
| Thoracic/lumbar vertebrae | 6 | 50.0 | 1 | 8.3 | 5 | 41.7 | 0 | 0.0 | 0 | 0.0 | 12 | 9.1 |
| Cervical vertebrae | 0 | 0.0 | 1 | 50. | 0 | 0.0 | 1 | 50.0 | 0 | 0.0 | 2 | 1.5 |
| Pelvis | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 100 | 1 | 0.8 |
| Proximal femur | 0 | 0.0 | 4 | 100 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 4 | 3.0 |
| Other leg | 1 | 4.5 | 15 | 68.2 | 2 | 9.1 | 3 | 13.6 | 1 | 4.5 | 22 | 16.7 |
| Feet/toes | 0 | 0.0 | 9 | 31.0 | 6 | 20.7 | 12 | 41.4 | 2 | 6.9 | 29 | 22.0 |
| All sites | 16 | 12.1 | 68 | 51.5 | 13 | 9.8 | 29 | 22.0 | 6 | 4.5 | 132 | 100 |
Percentage (%) of each type of fracture
Percentage (%) of total
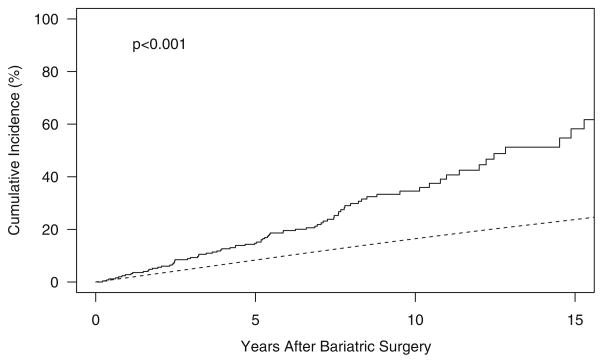
After 15 years of follow-up, the cumulative incidence of any new fracture was 58 %, compared to an expected 24 % (p<0.001) for men and women of like age. The median time to first fracture was 13 years. An early and sustained increase in the cumulative fracture incidence was observed in the bariatric surgery group compared to what was expected (Fig. 1). However, the relative risk of fracture was somewhat greater in the follow-up years 5 to <10 (SIR, 2.9; 95 % CI, 2.0–4.1) and 10+ (SIR, 2.9; 95 % CI, 1.5–5.0) than it was within the first 5 years of observation following surgery (SIR, 1.8; 95 % CI, 1.3–2.5). Compared to expected incidence rates among community men and women, generally, the overall fracture rate was increased more than twofold (SIR, 2.3; 95 % CI, 1.8–2.8) in the bariatric cohort, although the risk of a fracture due to no more than moderate trauma was elevated (SIR, 3.2; 95 % CI, 2.4–4.1) even more. The risks of a first fracture at traditional osteoporotic sites (hip, wrist, spine, and humerus) and at nonosteoporotic sites (all other sites) were both increased (SIR—2.0, 95 % CI, 1.3–3.0, and 2.4, 95 % CI, 1.8–3.0, respectively). Half of all fractures occurred in the foot, leg, or hand, and SIRs at all three sites were statistically significantly increased (Table 3).
Fig. 1.
Cumulative incidence of fracture among Olmsted County, Minnesota, residents following bariatric surgery in 1985–2004 (solid line) vs. expected incidence among community men and women (dashed line)
Table 3.
Fractures observed among 258 Olmsted County, Minnesota, residents with mean follow-up of 8.9 (±4.8)years after bariatric surgery in 1985–2004 compared with the numbers expected and SIR with 95 % CI [25]
| Fracture site | Number of observed fractures | Number of expected fractures | SIR (95 % CI) |
|---|---|---|---|
| Skull/face | 5 | 1.49 | 3.4 (1.1–7.8) |
| Hands/fingers | 18 | 5.26 | 3.4 (2.0–5.4) |
| Distal forearm | 11 | 5.65 | 2.0 (0.97–3.5) |
| Proximal humerus | 8 | 1.59 | 5.0 (2.2–9.9) |
| Other arm | 4 | 3.10 | 1.3 (0.4–3.3) |
| Clavicle/scapula/sternum | 1 | 1.34 | 0.8 (0.02–4.1) |
| Ribs | 10 | 3.93 | 2.5 (1.2–4.7) |
| Thoracic/lumbar vertebrae | 9 | 2.92 | 3.1 (1.4–5.9) |
| Cervical vertebrae | 2 | 0.48 | 4.2 (0.5–15) |
| Pelvis | 1 | 0.97 | 1.0 (0.03–5.7) |
| Proximal femur | 4 | 0.72 | 5.5 (1.5–14) |
| Other leg | 20 | 8.33 | 2.4 (1.5–3.7) |
| Feet/toes | 29 | 9.11 | 3.2 (2.1–4.6) |
| Any site | 79 | 34.7 | 2.3 (1.8–2.8) |
Note that the number of fractures observed at specific skeletal sites may differ from those reported in Table 2 because only the first fracture of each type per patient was counted in this analysis
In a univariate analysis (Table 4), age was a risk factor for any fracture (HR per 10-year increase, 1.3; 95 % CI, 1.02–1.6). After adjustment for age, reported vitamin D deficiency was also a predictor of fractures (HR, 2.0; 95 % CI, 1.2–3.2), but an increased age-adjusted risk due to calcium deficiency was not statistically significant (HR, 1.9; 95 % CI, 0.9–4.3). Higher activity status before surgery was protective against fracture after adjustment for age (HR, 0.4; 95 % CI, 0.2–0.8). There was no age-adjusted association of fracture risk with gender, cigarette or alcohol use, alcoholism, year of surgery, type of surgery (including limb length in RYGB procedures), height, weight, or BMI prior to surgery. Likewise, the 94 subjects (36 % of the total) with a history of fracture before undergoing bariatric surgery were not at significantly increased risk of fracture after surgery (HR, 1.3; 95 % CI, 0.9–2.0).
Table 4.
Predictors of fracture risk among 258 Olmsted County, Minnesota, residents with mean follow-up of 8.9 (±4.8)years after bariatric surgery in 1985–2004, after adjustment for age at surgery
| Risk factor | Any fracture (n=132) HR (95 % CI) |
|---|---|
| Female | 1.3 (0.8–2.2) |
| Weight at surgery (per 20 kg increase) | 1.1 (0.9–1.3) |
| BMI (kg/m2) at surgery (per 10 unit increase) | 1.2 (0.9–1.5) |
| RYBG surgery | 1.2 (0.6–2.2) |
| History of any prior fracture | 1.3 (0.8–2.0) |
| Ever smoker | 0.8 (0.5–1.3) |
| Ever used alcohol | 1.8 (0.9–3.3) |
| Alcoholism | 1.4 (0.8–2.5) |
| Physically active | 0.4 (0.2–0.8) |
| Calcium deficiency | 1.9 (0.9–4.3) |
| Vitamin D deficiency | 2.0 (1.2–3.2) |
Discussion
We found an increased risk of fracture in our population-based bariatric surgery cohort. Site-specific analysis revealed a twofold increased risk of fracture at the traditional osteoporotic sites (hip, spine, wrist), as well as a 2.3-fold increased risk at nonosteoporotic skeletal sites. This appears to conflict with results from a large cohort study in the UK, where 2,079 bariatric surgery patients were compared to 10,442 controls [7]. In that investigation, bariatric surgery was not associated with an increased risk of any fracture, any osteoporotic fracture, or any nonosteoporotic fracture. However, those investigators matched patients and controls on BMI in order to isolate the effects of bariatric surgery per se (quasi clinical trial). By contrast, we compared fracture outcomes following bariatric surgery to those expected in the population generally, as this provides a better basis for counseling obese patients about the risk of fracture that might be expected after bariatric surgery. Moreover, follow-up in the UK study averaged only 2.2 years, while over half of the subsequent fractures that we observed did not occur until 5 years following bariatric surgery.
It is also important to point out that the subjects in our study were much heavier (mean BMI, 49.0 kg/m2) compared to the bariatric surgery patients followed by Lalmohamed and colleagues (43.2 kg/m2) [7]. Thus, any adverse effects of morbid obesity are likely accentuated in this report. While low BMI is an established risk factor for fracture, increased BMI has traditionally been considered protective against fractures [32]. However, doubts about the protective effects of obesity are emerging [33]. For example, a study among women presenting with low-trauma fractures found that many of the subjects with class I or II obesity had largely normal BMD, although BMD was lower than women of similar BMI without fracture [34]. Likewise, a study of older men found that obesity was associated with an increased risk of fracture after adjustment for BMD, but this effect was not statistically significant after additional adjustment for physical function [35]. In addition, we also found that the majority of fractures postbariatric surgery occurred in the hand, leg, and foot, which are not traditional sites for osteoporotic fractures.
The mechanism(s) driving the increase in fractures following bariatric surgery appears to be active early, and sustained long term, given the relatively uniform divergence in cumulative fracture incidence observed in the bariatric surgery patients compared to that expected in the general population. Impaired calcium metabolism would appear to be relevant [10, 16–18, 36, 37]. Our observation that calcium deficiency was not a significant independent determinant of fracture risk was, therefore, unexpected given that calcium consumption is reduced, co-intake of absorption-enhancing protein is reduced, and calcium-avid duodenal and proximal jejunal mucosa is bypassed [35, 36]. This apparently negative result may have been due to our limited sample size and to incomplete detection using clinical judgment to define deficiency. Noting that these limitations also apply to the interpretation of vitamin D status, it might not be surprising that reported vitamin D deficiency prior to bariatric surgery increased fracture risk subsequently, particularly since vitamin D malabsorption and secondary hyperparathyroidism after surgical weight loss can be significant [17]. However, failure of vitamin D deficiency to fully explain [38] or vitamin D supplementation to suppress [39] elevated parathyroid hormone concentrations after bariatric surgery suggests that the impact of serum 25(OH) vitamin D on calcium homeostasis and fracture risk may be different than that observed in subjects with normal fat mass or normal gastrointestinal anatomy. Furthermore, the observation that decrease in weight, fat mass, BMI, and percent excess weight loss at 1-year status postbariatric surgery was associated with increased serum 25(OH) vitamin D concentrations would portend a salutary effect of bariatric surgery on fracture risk if vitamin D deficiency were operative [40]. Although a trial of high-dose vitamin D supplementation in vitamin D-deficient women reported an attenuation of BMD decline following bariatric surgery [41], the impact of vitamin D on fracture risk after bariatric surgery remains uncertain.
Indirect evidence supporting a negative effect of bariatric surgery on fracture risk is provided by studies on bone turnover and BMD. Prospective studies consistently observed changes in turnover markers as early as 1 [37] and up to 18 months [36] after surgery. Reported are increased markers of bone resorption (e.g., urinary pyridinoline, deoxypyridinoline, and N-telopeptide cross-linked collagen type 1), which would be expected to enhance fracture risk, while changes in markers of bone formation (e.g., osteocalcin and bone-specific alkaline phosphatase) have been inconsistent [10, 16–18]. In addition, while acknowledging technical challenges to accurate measurement of BMD by dual-energy x-ray absorptiometry in severely obese subjects, it is generally accepted that BMD decreases after bariatric surgery [16, 17, 19, 20]. There is consistent bone loss at the total hip and femoral neck and mixed results at other sites such as the lumbar spine and distal radius. Moreover, even when patients are provided with what is thought to be adequate calcium and vitamin D supplementation following bariatric surgery, they appear to have increased bone turnover markers [10, 16–18] and reduced BMD [16, 17, 19, 20].
Changes in bone turnover and BMD are also seen in intentional nonsurgical weight loss [42]; therefore, it is unclear whether this reflects normal physiological remodeling due to weight loss itself. In a 1-year prospective study of postoperative bariatric subjects, there was a strong positive correlation between reduction in BMD and weight loss per se [17]. A larger 2-year study observed that bone loss was greatest in the first postoperative year and then stabilized, in parallel with the weight loss, after bariatric surgery [19]. However, persistent elevation in bone resorption markers after weight loss has plateaued [36], and that initial weight, weight loss, fat mass loss and adiponectin levels only account for one third of BMD changes, suggest other factors play a role. This is probably not due solely to mechanical unloading of bone from significant, rapid weight loss following surgery, but our data do suggest that increased preoperative activity levels were protective against fractures. This, in turn, raises the possibility that loss of muscle strength and balance and subsequent falls are potential mechanisms for an increase in fracture risk after bariatric surgery. However, a history of fracture prior to bariatric surgery did not influence postoperative risk so the increase in fractures was not simply due to fractures in individuals with a prior history of fracturing.
It is worth noting that energy restriction, including reduced protein intake, is also observed following bariatric surgery [20], and many of the hormones involved in energy metabolism also impact on bone health. For example, osteoblasts and adipocytes are derived from common progenitor cells in bone marrow, and endocrine products of each tissue impact the other, e.g., leptin regulates bone mass while osteocalcin affects insulin secretion [43]. The peripheral effects of leptin are thought to dominate, especially in obesity, when leptin resistance develops in the central nervous system [44]. After bariatric surgery, falling leptin levels are associated with elevated markers of bone resorption [36]. Adiponectin, another adipocyte hormone, exerts a negative effect on bone by inducing osteoclast formation [45]. Adiponectin levels are reduced in obesity [46] and rise after bariatric surgery in association with weight loss [20]. Conversely, insulin directly stimulates osteoblast proliferation in vitro and is positively associated with bone density in clinical studies [44]. Amylin and preptin, hormones which are cosecreted with insulin, further stimulate osteoblast proliferation [44]. Hyperinsulinemia, which is common among obese subjects due to insulin resistance, resolves after bariatric surgery [47]. Therefore, lower levels of osteogenic factors such as leptin, insulin, estrogen, and insulin-like growth factor, and a higher level of inhibitory factors such as adiponectin and cortisol after bariatric surgery, would be consistent with the increase in fracture risk observed following bariatric surgery.
Strengths of our study were the long duration of follow-up and the population-based nature of the study cohort. There are also several limitations. First, as a retrospective cohort study based on review of contemporary medical records, it is limited by nonstandardized data and incomplete follow-up. Although most Olmsted County residents receive all of their medical care in the community, a few may have sought medical care elsewhere, thereby underestimating the true incidence of fracture in this population. More importantly, the potential mechanisms examined were limited to those with a reasonable expectation for complete ascertainment in a retrospective review of medical records. For example, a recent randomized controlled trial among postmenopausal women found that a high-protein diet attenuated bone loss [48], but we were unable to evaluate protein malnutrition as a risk factor since only two subjects had a documented diagnosis of protein malnutrition, and there was often no assessment of protein intake in the clinical record. In addition, our control population was age and sex matched but not weight matched. Therefore, we cannot discern the relative effects of obesity, weight loss, and bariatric surgical procedures on fracture risk. We also did not evaluate several important determinants of skeletal health such as lifetime estrogen exposure in women, other causes of primary or secondary bone disease, and use of medications that affect bone health. In addition, our findings may not reflect recent changes in surgical practice and improvements in medical management. Finally, our ability to generalize these results nationally and internationally is limited by the demographics of Olmsted County, a relatively homogenous Caucasian population [49].
Despite these limitations, this is the only population-based study to estimate fracture incidence during a long period of follow-up in women and men who have undergone bariatric surgery. Site-specific analyses revealed increased risk at the traditional osteoporotic fracture sites and at nonosteoporotic appendicular sites as well. After adjustment for age, the increased risk of fracture in this bariatric cohort was associated with low preoperative activity status and vitamin D deficiency. These results do suggest that previously reported changes in BMD and bone turnover markers after bariatric surgery are clinically significant [10, 16–20]. Although factors other than bariatric surgery per se may be responsible, we recommend that clinicians discuss bone health with patients who have undergone or are planning to undergo bariatric surgery. This is especially important since the mean age in our cohort was only 44 years, yet our data suggest that an individual’s risk of sustaining any fracture over the subsequent 10 years after bariatric surgery is 35 %, or approximately one in three subjects. We further recommend that skeletal health assessments be included in future prospective studies of bariatric surgery outcomes.
Acknowledgments
We would like to thank Dr. Michael Sarr for providing the database of all patients completing a bariatric surgery procedure at Mayo Clinic between 1985 and 2004. This study was supported in part by the Division of Endocrinology, Mayo Clinic, Rochester, and P01-AG-04875 from the National Institute on Aging and made possible by the Rochester Epidemiology Project (R01-AG-034676 from the National Institute on Aging), US Public Health Service. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflicts of interest None.
Contributor Information
K. M. Nakamura, Johns Hopkins Hospital, Baltimore, MD, USA
E. G. C. Haglind, HealthEast Clinics, St. Paul, MN, USA
J. A. Clowes, Guthrie Clinic, Sayre, PA, USA
S. J. Achenbach, Division of Biomedical Statistics and Informatics, Department of Health Sciences Research, College of Medicine, Mayo Clinic, Rochester, MN, USA
E. J. Atkinson, Division of Biomedical Statistics and Informatics, Department of Health Sciences Research, College of Medicine, Mayo Clinic, Rochester, MN, USA
L. J. Melton, III, Division of Epidemiology, Department of Health Sciences Research, College of Medicine, Mayo Clinic, Rochester, MN, USA. Division of Endocrinology, Diabetes, Metabolism and Nutrition, Department of Internal Medicine, College of Medicine, Mayo Clinic, 200 First Street S.W, Rochester, MN 55905, USA.
K. A. Kennel, Email: kennel.kurt@mayo.edu, Division of Endocrinology, Diabetes, Metabolism and Nutrition, Department of Internal Medicine, College of Medicine, Mayo Clinic, 200 First Street S.W, Rochester, MN 55905, USA
References
- 1.Centers for Disease Control and Prevention. [Accessed 2 Jan 2013];Overweight and obesity. 2011 http://www.cdc.gov/obesity.
- 2.Sjostrom L, Lindroos AK, Peltonen M, Torgerson J, Bouchard C, Carlsson B, Dahlgren S, Larsson B, Narbro K, Sjostrom CD, Sullivan M, Wedel H. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351:2683–2693. doi: 10.1056/NEJMoa035622. [DOI] [PubMed] [Google Scholar]
- 3.Adams TD, Gress RE, Smith SC, Halverson RC, Simper SC, Rosamond WD, Lamonte MJ, Stroup AM, Hunt SC. Long-term mortality after gastric bypass surgery. N Engl J Med. 2007;357:753–761. doi: 10.1056/NEJMoa066603. [DOI] [PubMed] [Google Scholar]
- 4.American Society for Metabolic and Bariatric Surgery. [Accessed November 11 2011];Fact sheet: metabolic and bariatric surgery. 2010 http://asmbs.org/
- 5.Berarducci A, Haines K, Murr MM. Incidence of bone loss, falls, and fractures after Roux-en-Y gastric bypass for morbid obesity. Appl Nurs Res. 2009;22:35–41. doi: 10.1016/j.apnr.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 6.Abu-Lebdeh HS, Paat JJ. Are post bariatric surgery fractures different from fractures in severely obese subjects?. In IOF World Congress on Osteoporosis; Florence, Italy. 2010. [Google Scholar]
- 7.Lalmohamed A, de Vries F, Bazelier MT, Cooper A, van Staa TP, Cooper C, Harvey NC. Risk of fracture after bariatric surgery in the United Kingdom: population based, retrospective cohort study. BMJ. 2012;345:e5085. doi: 10.1136/bmj.e5085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tice JA, Karliner L, Walsh J, Petersen AJ, Feldman MD. Gastric banding or bypass? A systematic review comparing the two most popular bariatric procedures. Am J Med. 2008;121:885–893. doi: 10.1016/j.amjmed.2008.05.036. [DOI] [PubMed] [Google Scholar]
- 9.Dixon JB, Strauss BJ, Laurie C, O’Brien PE. Changes in body composition with weight loss: obese subjects randomized to surgical and medical programs. Obesity (Silver Spring) 2007;15:1187–1198. doi: 10.1038/oby.2007.639. [DOI] [PubMed] [Google Scholar]
- 10.von Mach MA, Stoeckli R, Bilz S, Kraenzlin M, Langer I, Keller U. Changes in bone mineral content after surgical treatment of morbid obesity. Metabolism. 2004;53:918–921. doi: 10.1016/j.metabol.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 11.Shapses SA, Sukumar D. Bone metabolism in obesity and weight loss. Annu Rev Nutr. 2012;32:287–309. doi: 10.1146/annurev.nutr.012809.104655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shah M, Simha V, Garg A. Review: long-term impact of bariatric surgery on body weight, comorbidities, and nutritional status. J Clin Endocrinol Metab. 2006;91:4223–4231. doi: 10.1210/jc.2006-0557. [DOI] [PubMed] [Google Scholar]
- 13.De Prisco C, Levine SN. Metabolic bone disease after gastric bypass surgery for obesity. Am J Med Sci. 2005;329:57–61. doi: 10.1097/00000441-200502000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Mosekilde L, Melsen F, Hessov I, Christensen MS, Lund BJ, Lund BI, Sorensen OH. Low serum levels of 1.25-dihydroxyvitamin D and histomorphometric evidence of osteomalacia after jejunoileal bypass for obesity. Gut. 1980;21:624–631. doi: 10.1136/gut.21.7.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Collazo-Clavell ML, Jimenez A, Hodgson SF, Sarr MG. Osteomalacia after Roux-en-Y gastric bypass. Endocr Pract. 2004;10:195–198. doi: 10.4158/EP.10.3.195. [DOI] [PubMed] [Google Scholar]
- 16.Coates PS, Fernstrom JD, Fernstrom MH, Schauer PR, Greenspan SL. Gastric bypass surgery for morbid obesity leads to an increase in bone turnover and a decrease in bone mass. J Clin Endocrinol Metab. 2004;89:1061–1065. doi: 10.1210/jc.2003-031756. [DOI] [PubMed] [Google Scholar]
- 17.Fleischer J, Stein EM, Bessler M, Della Badia M, Restuccia N, Olivero-Rivera L, McMahon DJ, Silverberg SJ. The decline in hip bone density after gastric bypass surgery is associated with extent of weight loss. J Clin Endocrinol Metab. 2008;93:3735–3740. doi: 10.1210/jc.2008-0481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mahdy T, Atia S, Farid M, Adulatif A. Effect of Roux-en Y gastric bypass on bone metabolism in patients with morbid obesity: Mansoura experiences. Obes Surg. 2008;18:1526–1531. doi: 10.1007/s11695-008-9653-1. [DOI] [PubMed] [Google Scholar]
- 19.Johnson JM, Maher JW, Samuel I, Heitshusen D, Doherty C, Downs RW. Effects of gastric bypass procedures on bone mineral density, calcium, parathyroid hormone, and vitamin D. J Gastrointest Surg. 2005;9:1106–1110. doi: 10.1016/j.gassur.2005.07.012. discussion 1110–1101. [DOI] [PubMed] [Google Scholar]
- 20.Carrasco F, Ruz M, Rojas P, Csendes A, Rebolledo A, Codoceo J, Inostroza J, Basfi-Fer K, Papapietro K, Rojas J, Pizarro F, Olivares M. Changes in bone mineral density, body composition and adiponectin levels in morbidly obese patients after bariatric surgery. Obes Surg. 2009;19:41–46. doi: 10.1007/s11695-008-9638-0. [DOI] [PubMed] [Google Scholar]
- 21.Belle S. The NIDDK bariatric surgery clinical research consortium (LABS) Surg Obes Relat Dis. 2005;1:145–147. doi: 10.1016/j.soard.2005.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rocca WA, Yawn BP, St Sauver JL, Grossardt BR, Melton LJ., 3rd History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc. 2012;87:1202–1213. doi: 10.1016/j.mayocp.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Melton LJ., 3rd The threat to medical-records research. N Engl J Med. 1997;337:1466–1470. doi: 10.1056/NEJM199711133372012. [DOI] [PubMed] [Google Scholar]
- 24.Melton LJ, 3rd, Crowson CS, O’Fallon WM. Fracture incidence in Olmsted County, Minnesota: comparison of urban with rural rates and changes in urban rates over time. Osteoporos Int. 1999;9:29–37. doi: 10.1007/s001980050113. [DOI] [PubMed] [Google Scholar]
- 25.Melton LJ, 3rd, Alothman KI, Khosla S, Achenbach SJ, Oberg AL, Zincke H. Fracture risk following bilateral orchiectomy. J Urol. 2003;169:1747–1750. doi: 10.1097/01.ju.0000059281.67667.97. [DOI] [PubMed] [Google Scholar]
- 26.Mackey DC, Lui LY, Cawthon PM, Bauer DC, Nevitt MC, Cauley JA, Hillier TA, Lewis CE, Barrett-Connor E, Cummings SR. High-trauma fractures and low bone mineral density in older women and men. JAMA. 2007;298:2381–2388. doi: 10.1001/jama.298.20.2381. [DOI] [PubMed] [Google Scholar]
- 27.Cox DR. Some simple approximate tests for Poisson variates. Biometrika. 1953;40:354–360. [Google Scholar]
- 28.Kaplan EL, Meier P. Non-parametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 29.Kalbfleisch JD, Prentice RL, editors. The statistical analysis of failure time data. Wiley; New York: 1980. [Google Scholar]
- 30.Therneau TM, Grambsch PM. Modeling survival data: extending the Cox model. Springer; New York: 2000. [Google Scholar]
- 31.Firth D. Bias reduction of maximum likelihood estimates. Biometrika. 1993;80:27–38. [Google Scholar]
- 32.De Laet C, Kanis JA, Oden A, Johanson H, Johnell O, Delmas P, Eisman JA, Kroger H, Fujiwara S, Garnero P, McCloskey EV, Mellstrom D, Melton LJ, 3rd, Meunier PJ, Pols HA, Reeve J, Silman A, Tenenhouse A. Body mass index as a predictor of fracture risk: a meta-analysis. Osteoporos Int. 2005;16:1330–1338. doi: 10.1007/s00198-005-1863-y. [DOI] [PubMed] [Google Scholar]
- 33.Compston JE, Watts NB, Chapurlat R, Cooper C, Boonen S, Green-span S, Pfeilschifter J, Silverman S, Diez-Perez A, Lindsay R, Saag KG, Netelenbos JC, Gehlbach S, Hooven FH, Flahive J, Adachi JD, Rossini M, LaCroix AZ, Roux C, Sambrook PN, et al. Obesity is not protective against fracture in postmenopausal women: GLOW. Am J Med. 2011;124:1043–1050. doi: 10.1016/j.amjmed.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Premaor MO, Pilbrow L, Tonkin C, Parker RA, Compston J. Obesity and fractures in postmenopausal women. J Bone Miner Res. 2010;25:292–297. doi: 10.1359/jbmr.091004. [DOI] [PubMed] [Google Scholar]
- 35.Nielson CM, Marshall LM, Adams AL, LeBlanc ES, Cawthon PM, Ensrud K, Stefanick ML, Barrett-Connor E, Orwoll ES for the Osteoporotic Fractures in Men Study Research G. BMI and fracture risk in older men: The Osteoporotic Fractures in Men Study (MrOS) J Bone Miner Res. 2011;26:496–502. doi: 10.1002/jbmr.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bruno C, Fulford AD, Potts JR, McClintock R, Jones R, Cacucci BM, Gupta CE, Peacock M, Considine RV. Serum markers of bone turnover are increased at 6 and 18 months after Roux-en-Y bariatric surgery: correlation with the reduction in leptin. J Clin Endocrinol Metab. 2010;95:159–166. doi: 10.1210/jc.2009-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Riedt CS, Brolin RE, Sherrell RM, Field MP, Shapses SA. True fractional calcium absorption is decreased after Roux-en-Y gastric bypass surgery. Obesity (Silver Spring) 2006;14:1940–1948. doi: 10.1038/oby.2006.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hewitt S, Sovik TT, Aasheim ET, Kristinsson J, Jahnsen J, Birketvedt GS, Bohmer T, Eriksen EF, Mala T. Secondary hyperparathyroidism, vitamin D sufficiency, and serum calcium 5 years after gastric bypass and duodenal switch. Obes Surg. 2013;23:384–390. doi: 10.1007/s11695-012-0772-3. [DOI] [PubMed] [Google Scholar]
- 39.Pramyothin P, Biancuzzo RM, Lu Z, Hess DT, Apovian CM, Holick MF. Vitamin D in adipose tissue and serum 25-hydroxyvitamin D after roux-en-Y gastric bypass. Obesity (Silver Spring) 2011;19:2228–2234. doi: 10.1038/oby.2011.170. [DOI] [PubMed] [Google Scholar]
- 40.Beckman LM, Earthman CP, Thomas W, Compher CW, Muniz J, Horst RL, Ikramuddin S, Kellogg TA, Sibley SD. Serum 25(OH) vitamin D concentration changes after roux-en-y gastric bypass surgery. Obesity (Silver Spring) 2013 doi: 10.1002/oby.20464. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carlin AM, Rao DS, Yager KM, Parikh NJ, Kapke A. Treatment of vitamin D depletion after Roux-en-Y gastric bypass: a randomized prospective clinical trial. Surg Obes Relat Dis. 2009;5:444–449. doi: 10.1016/j.soard.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 42.Ricci TA, Heymsfield SB, Pierson RN, Jr, Stahl T, Chowdhury HA, Shapses SA. Moderate energy restriction increases bone resorption in obese postmenopausal women. Am J Clin Nutr. 2001;73:347–352. doi: 10.1093/ajcn/73.2.347. [DOI] [PubMed] [Google Scholar]
- 43.Rosen CJ, Klibanski A. Bone, fat, and body composition: evolving concepts in the pathogenesis of osteoporosis. Am J Med. 2009;122:409–414. doi: 10.1016/j.amjmed.2008.11.027. [DOI] [PubMed] [Google Scholar]
- 44.Reid IR. Relationships between fat and bone. Osteoporos Int. 2008;19:595–606. doi: 10.1007/s00198-007-0492-z. [DOI] [PubMed] [Google Scholar]
- 45.Gomez-Ambrosi J, Rodriguez A, Catalan V, Fruhbeck G. The bone-adipose axis in obesity and weight loss. Obes Surg. 2008;18:1134–1143. doi: 10.1007/s11695-008-9548-1. [DOI] [PubMed] [Google Scholar]
- 46.Weyer C, Funahashi T, Tanaka S, Hotta K, Matsuzawa Y, Pratley RE, Tataranni PA. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab. 2001;86:1930–1935. doi: 10.1210/jcem.86.5.7463. [DOI] [PubMed] [Google Scholar]
- 47.Pories WJ, MacDonald KG, Jr, Flickinger EG, Dohm GL, Sinha MK, Barakat HA, May HJ, Khazanie P, Swanson MS, Morgan E, et al. Is type II diabetes mellitus (NIDDM) a surgical disease? Ann Surg. 1992;215:633–642. doi: 10.1097/00000658-199206000-00010. discussion 643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sukumar D, Ambia-Sobhan H, Zurfluh R, Schlussel Y, Stahl TJ, Gordon CL, Shapses SA. Areal and volumetric bone mineral density and geometry at two levels of protein intake during caloric restriction: a randomized, controlled trial. J Bone Miner Res. 2011;26:1339–1348. doi: 10.1002/jbmr.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.St Sauver JL, Grossardt BR, Leibson CL, Yawn BP, Melton LJ, 3rd, Rocca WA. Generalizability of epidemiological findings and public health decisions: an illustration from the Rochester Epidemiology Project. Mayo Clin Proc. 2012;87:151–160. doi: 10.1016/j.mayocp.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]