Abstract
The new health care buzz words include “personalized or individualized medicine.” Populations such as American Indians and Alaska Natives potentially have much to gain from this new science to overcome the known health disparities in these populations. This will require participation and acceptance of diverse populations. This article reviews the promise and challenges of individualizing cancer care using principles of community-based participatory research.
Keywords: American Indian, CBPR, Genotype, Cancer health disparities
Introduction
The new health care buzz words are “personalized medicine” or “individualized medicine.” Ever since the complete human genome sequence was published 10 years ago, there has been great hope that genomic sciences will provide ways to optimize medical care [1]. Populations such as American Indians and Alaska Natives (AIAN) could have much to gain from this new science to overcome the known health disparities present in these populations. Cancer is certainly a ripe area for this type of research, but so far, there has been little education or inclusion of AIAN patients, clinicians caring for them, nor policy makers within the Indian health system in this effort.
In the past decade, the National Cancer Institute (NCI) has commissioned a specific branch, the Center to Reduce Cancer Health Disparities (CRCHD), dedicated to community-based participatory research and evidence-based medicine to overcome these disparities. The CRCHD coordinates and strengthens the NCI cancer research portfolio in basic, clinical, translational, and population-based research to address cancer health disparities. One program within CRCHD is the Community Networks Program (CNP) which supports outreach to minority and other underserved communities across the country. Many of these CNPs are partnering with NCI on programs such as GMaP and BMaP described below.
GMaP and BMaP
State of the art medicine today acknowledges that “one size doesn’t fit all” and that approximately 30% of all medications either do not work or are too toxic for certain individuals [2]. Potentially, minority populations would have the most to gain from understanding these issues because they are more likely to have individuals with genetic variants modifying drug metabolism or function. NCI has funded Administrative Supplements for Minority Biospecimens/Biobanking—Geographic Management Programs dedicated to ensuring the adequate and continuous supply of high-quality human biospecimens from multi-ethnic communities for cancer research. The plan is to create regional networks focused on cancer health disparities research and care.
However, so far minority communities have not been directly involved with these regional hubs, which are in the early stages of development. Finding the targets for detection, therapy, and prevention involves the nascent fields of genomics, proteomics, and metabolomics, all of which depend on high-quality biospecimens. Even under ideal circumstances, it will likely take years of research to identify the genetic contribution for most common diseases. Genomic technology has dramatically outpaced understanding of human genetics and our ability to conduct the public education necessary to enlighten consumers of this new technology. There are significant difficulties inherent to meaningful clinical application of such information [3]. The general public has maintained hope while expressing frustration that despite very substantial investment and effort over the past 30 years, the overall survival rate of cancer patients has changed little [4]. Certainly, most ethnic and racially diverse cancer patients, who suffer excess morbidity and mortality from a variety of cancers, have doubts as to the relevance of cancer research and clinical trials to improving their well-being.
As stated by McGuire et al. in Science, “relating genotype to phenotype is the challenge for genetic medicine over the next century” [4]. Scientists argue that without a truly robust mechanism for selecting the right treatment for the right patient at the right time—the central concepts of personalized medicine—we will continue to see only incremental improvements and have little hope for substantial survival gains. Future clinical trials are now being designed with a plan to incorporate biomarker development [5]. Figure 1 shows the current GMaP program and components. Table 1 shows the potential benefits of the GMaP project to researchers and federal partners in the research enterprise.
Fig. 1.
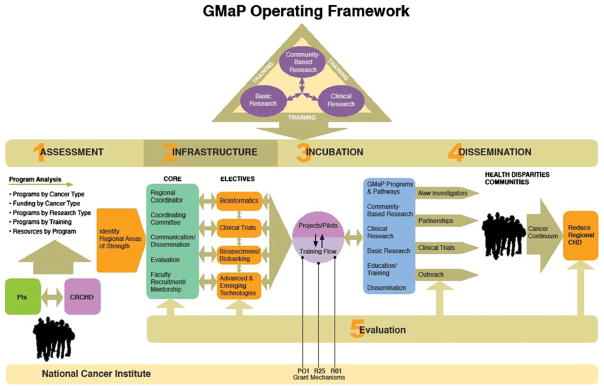
Current GMaP program and components
Table 1.
Potential benefits of the GMaP project to researchers and federal partners
| Researcher | Federal partner |
|---|---|
| Better integrated and stronger research, training, and core programs across regions | Increased leveraging of research dollars |
| Joint regional workshops to facilitate communication and disseminations | More efficient program management:
|
| Integration of GMaP/BMaP members into regional and community planning and decision-making processes for sustainability of CHD and biospecimen/biobanking efforts | Joint concept development for new funding opportunities |
| Common metrics and evaluation of CHD and biospecimen science |
CHD Cancer Health Disparities
The missing essential component is to identify community priorities, develop trust, and implement culturally acceptable interventions. Potentially, the community and community members will benefit from identifying the most effective treatments with the fewest side effects for each patient. If minority community members are not involved in this endeavor, however, health disparities will only increase, not decrease.
Community-Based Participatory Research Examples
The Walking Forward Program (WFP) is a community-based participatory research study designed to enhance American Indian (AI) enrollment on cancer treatment trials. The WFP was initiated in September 2002 as an NCI-funded study under the Cancer Disparities and Research Partnership program awarded to Rapid City Regional Hospital (RCRH)–PI D Petereit [6]. The WFP has been implemented on three AI reservations home to the Cheyenne River Sioux Tribe, Rosebud Sioux Tribe, and the Oglala Sioux Tribe, as well as the urban AI community in Rapid City, South Dakota. These reservations also include three of the five poorest counties (Todd, Shannon, and Ziebach counties) in the USA. AIs are about 8.5% of the South Dakota’s population (US Census 2000), of which about 70,000 reside in the areas served by the WFP. This project involves a genetic study to elucidate the underlying genetic basis for the higher incidence of radiation-induced toxicities anecdotally reported in some AI cancer patients undergoing radiation treatment—analysis of the ataxia telangectasia mutated (ATM) gene (ATM−) [7]. Initial planning included community and patient surveys identified barriers to cancer care in this underserved population.
The high rates of cancer mortality in the AI communities appear, in part, to arise from the lack of cancer screening, knowledge regarding screening, consequences of presentation at a late disease stage and treatment options, as well as socioeconomic factors. The WFP developed a regional research infrastructure, established trust with the AI communities, [8], and implemented a patient navigation program (Klewien B, Hamann S 2008; [8–12]). These activities, in the near term, resulted in identification of community-specific barriers to cancer care and greater patient accruals to clinical trials [13–15].
Since geographic remoteness from treatment centers and duration of a conventional course of radiation treatment present significant challenges to AI patients [16], it was hypothesized that these barriers might be lowered through various programs tailored to this population. These included: patient navigation; treatment options and clinical trials offering a shorter course of radiation via brachytherapy and/or intensity-modulated radiotherapy [17, 18]; and a comprehensive educational program encouraging screening and early detection. Treatment options that offer a shorter course of radiation would be more attractive to AIs and potentially lead to higher rates of completion of treatment and thus higher cure rates. It was also postulated that cancer education in these AI communities would eventually lead to AI cancer patients seeking treatments at early stages of the disease (stage migration). The project initiated a study of the ATM gene to determine if there is a correlation between radiation toxicities and ATM heterozygosity, which over the long term will help guide therapy and symptom management strategies.
The WFP approach to enhancing accrual of AI patients to clinical trials is summarized in Fig. 2. The increased enrollment to clinical trials is the result of the comprehensive patient navigation program that enhanced trust, increased cancer screening and education, leading to detection and immediate referral for treatment.
Fig. 2.
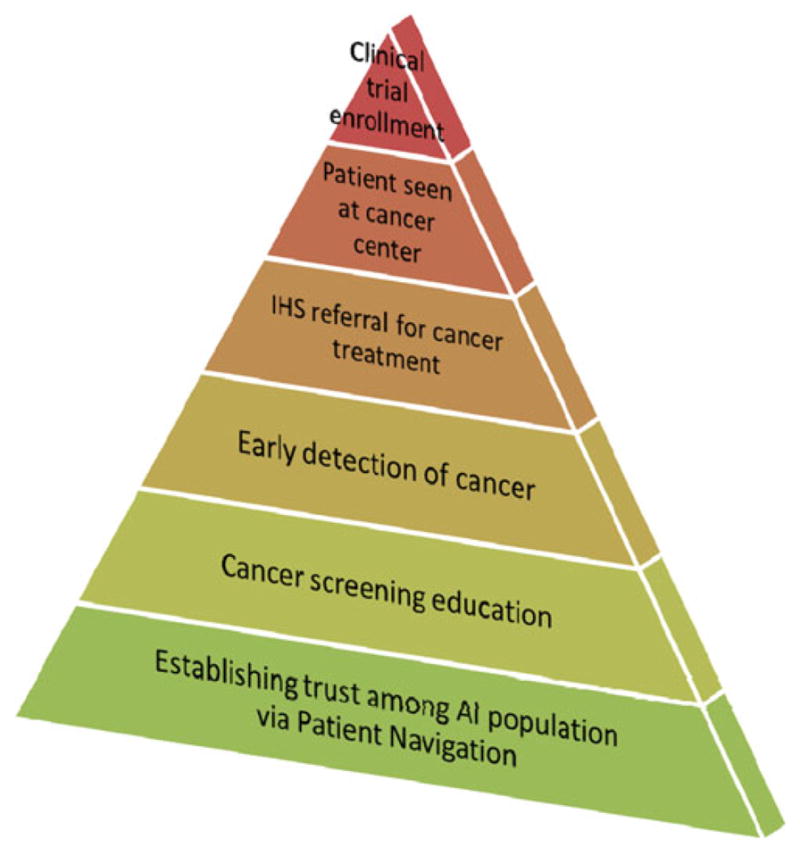
Walking Forward Program approach to enhance participation of AI patients to clinical trials
AIs appear to be hypersensitive to the effects of ionizing radiation. In a retrospective analysis of 61 AIs undergoing definitive radiotherapy at RCRH CCI, 50% of patients experienced grade 2 toxicities (G2) and 17% experienced grade 3 toxicities (G3). The majority of these toxicities were skin reactions in patients with breast and colorectal cancer [13, 14]. This is manifested as a more severe skin reaction ranging from a mild skin erythema to a patchy moist desquamation with moderate edema eventually progressing to a confluent moist desquamation. Fear of this treatment-related side effect could potentially contribute to observed significant treatment delays in this patient population. In an analysis from RCRH, 28% had delays >6 or more days, and 15% had delays >11 days which could lead to compromised tumor control [14, 19]. Homozygosity of mutations in ATM leads to extreme hypersensitivity to radiation [15, 16], while heterozygosity may be associated with an increased risk for developing cancer and radiation-induced toxicities [17]. It was postulated that studying the ATM gene in AIs would provide a better understanding of the underlying molecular mechanism for the observed higher incidence of toxicities in this population. Establishing a predictive molecular marker could help facilitate:
Accurate counseling of radiation side effects and treatment options.
Possible modification of the radiation treatment plan to minimize likely adverse effects.
If the presence of ATM does predispose AI to a higher incidence of cancer, then issues of education, screening, and lifestyle changes would be critical.
If a higher rate of ATM heterozygosity was observed among the AI, genetic counseling would be a critical part of this study.
To date, 100 AIs (Rapid City) and 100 non-AIs (52 from Rapid City and 48 from UW–Madison) have participated in the ATM study. A total of 25 single nucleotide polymorphisms (SNPs) have been identified in 21 of 62 sequenced exons from 200 samples. Four of the variants have not been reported in any of the current ATM databases, and are thus potentially new variants. There was no statistically significant difference for total prevalence of SNPs among AI (40%) and non-AI (48%) patients (p=0.32). Five SNPs had a prevalence of >2%, of which four occurred at a rate of >5% in one or both groups. The prevalence of these could meaningfully be compared statistically in the two groups. The only statistically significant difference among the groups was the c.4138 C>T SNP seen in 8% of AI versus 0% of non-AI patients (p=0.007). However, this SNP is predicted using the Polyphen software tool not to affect protein function. The prevalence of those SNPs predicted to result in potentially deleterious missense mutations was 28% among non-AI and 18% among AI (p=0.13). Of particular interest is SNP c.5557 G>A, which had a prevalence of 25% in non-AIs versus 14% in AIs (p=0.07). Three homozygous patients were identified for this SNP, all in the non-AI group.
Analysis of toxicity data for the 200 patients enrolled in this trial reveals that 53% have developed G2 acute toxicities, and 18% G3. While the follow-up is relatively short, 16% have developed G2 late toxicities, and 2% G3. Correlating the SNP data with available patient toxicity data is underway and will reveal any important SNPs that can be predictive of radiosensitivity [20].
This program has two clear messages: (1) it is possible to do quality genetic research in an AIAN population as part of an ongoing clinical trial and (2) the success of the program revolved around building trust and providing services such as the Patient Navigator program so that the community could see direct benefit from the research being offered.
The Havasupai Case
Biospecimen collection, annotation, and interpretation for different diseases including cancer are increasing in the hopes of gaining scientific understanding and improvement in treatments available for patients. However, the use of biospecimens is fraught with many ethical challenges.
The general American public may have limited recognition of a major suit by the Havasupai tribe against the Arizona State University Board of Regents for alleged misuse of research blood samples [21]. However, this issue is the subject of continued discussions among tribes that have collaborated in research or consider doing so in the future.
The Havasupai Tribe is made up of about 650 people who mostly live in Supai Village, about 10 miles down a scenic side canyon of the Grand Canyon. The impoverished community is accessible only by helicopter or by a trail into the Grand Canyon. Like many AIAN populations, the Havasupai tribal members had noted an increase in diabetes. The tribe earnestly requested help with this health disparity in 1989 and agreed to work with specific researchers to provide blood and DNA. They understood the samples would be used for collaborative research on diabetes only. Samples drawn from Havasupai tribal members during 1991–1994, however, were provided to other researchers without specific consent of individuals or the tribe. Specimens were used to study other issues—some of which violated tribal beliefs. When the tribe inadvertently learned that other studies had been done without their consent, they sued [22]. The original suit in Arizona state court was thrown out on a technicality, but the Arizona Court of Appeals reinstated the case. That Court of Appeals made a legal determination that the plaintiffs may have a privacy interest in their blood specimens. This was a new legal finding, that tribes may have privacy interests and can suffer “dignity harm.” A negotiated out-of-court settlement was announced publicly on April 21, 2010. The blood samples that had been the subject of the bitter lawsuit were restored to the tribe for proper burial on the floor of the Grand Canyon (http://www.azcentral.com/arizonarepublic/local/articles/2010/04/22/20100422arizona-havasupai-tribe-regents-lawsuit.html).
Traditional Institutional Review Board standards revolve around protecting individuals from harm, so this precedent may have implications for future genetic research and biospecimen collections involving tribal members [18, 23]. Truly informed consent recognizes that different people have different values and concerns. To enable each individual—and tribe—to make an informed decision, the researcher must present information that is “understandable” (45 CFR§46.116, General Requirements for Informed Consent). Researchers engaged with AIAN tribes should be aware of this case because tribes will be sensitized to research utilizing stored biospecimens. On a positive note, the Havasupai still believe that research can be valuable to their people and acknowledged ASU’s efforts to improve oversight and conduct of research. The settlement, after 6 years of acrimony, now allows the beginning of a partnership between the universities in Arizona and the tribe.
There are many implications of this court case. Havasupai tribe and members, and other AIAN tribes and members, are not unique in believing they have a privacy interest in the proper future use of their specimens given for research purposes. A recent nested research project involved members of Group Health of Seattle who had consented to store their specimens and data in NIH’s national database of Genotype and Phenotype as part of their participation in a larger study. A subgroup of 1,159 (86%) who had already signed informed consent documents to send their specimens and data were interviewed about the importance and acceptability of different methods the parent study could have used to send their specimens to a central repository. Of the 365 participants interviewed, 90% said it was important to have been asked to consent first (69% “very important,” 21% “somewhat important”); 70% said it was unacceptable to have sent their specimens to a central repository without telling them or asking for permission (54% “completely unacceptable,” 16% “somewhat unacceptable”); 67% said it was unacceptable to have only informed them by letter that their specimens and data had been sent—without consent or an “opt-out procedure” (47% “completely,” 20% “somewhat”) thus using specimens and data without at least on “opt-out” procedure is unacceptable not just to most AIAN people but also probably to most people in the USA.
Conclusions
Cancer research priorities are currently focused on more specific biomarkers that can individualize patient treatments in the hopes of improving survival from cancer. The development of a national program which focuses on developing regional infrastructure to collect annotated biospecimens (GMaP) is going forward as a priority of the National Cancer Institute. This will require acceptance and participation from a diverse population to draw conclusions about response rates, toxicities, and outcomes for new cancer therapies. The Spirit of E.A.G.L.E.S. is a national Community Networks Program working with all AIAN populations on education, training, and research across the cancer care continuum. This and other CNPs, which advocate for a community-based participatory research model, must now engage their respective communities if these regional networks are to be successful in accessing an adequate and continuous supply of high-quality human biospecimens from racially and ethnically diverse (and presumably genetically diverse) underserved communities. Dialogue has now begun on how to achieve positive results that will help overcome cancer health disparities.
Acknowledgments
This study was supported in part by U54–CA153605 (Kaur) and RFA 1U56CA99010-01 (Petereit), and U01 114609. This was also presented in part at the 8th Triennial Changing Cancer Patterns in Native Communities in Seattle, WA on September 11–14, 2010. Changing Cancer Patterns in Native Communities National Conference.
Footnotes
Conflict of Interest The authors declare that they have no conflict of interest.
Contributor Information
Judith Salmon Kaur, Email: Kaur.judith@mayo.edu, Mayo Clinic Comprehensive Cancer Center, 200 First Street SW, Rochester, MN 55905, USA.
Daniel G. Petereit, Email: dpetereit@regionalhealth.com, Department of Radiation Oncology, John T. Vucurevich Cancer Care Institute, Rapid City Regional Hospital, Rapid City, SD 57701, USA, Department of Human Oncology, University of Wisconsin School of Medicine and Public Health, Madison, WI, USA
References
- 1.Hirschfield GM, Amos CI, Siminovitch KA. Navigating the road to personalized medicine: can we believe? CMAJ. 2010;182 (7):651–652. doi: 10.1503/cmaj.100300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. JAMA. 1998;279(1):1200–1205. doi: 10.1001/jama.279.15.1200. [DOI] [PubMed] [Google Scholar]
- 3.Roberts R. Personalized medicine: a reality whting this decade. J Cardiovasc Transl Res. 2008;1(1):11–16. doi: 10.1007/s12265-007-9001-1. [DOI] [PubMed] [Google Scholar]
- 4.McGuire AL, Cho MK, McGuire SE. Medicine: the future of personal genomics. Science. 2007;317:1687. doi: 10.1126/science.1147475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Potti A, Schilsky RL, Nevins JR. Refocusing the war on cancer: the critical role of personalized treatment. Sci Transl Med. 2010;2 (28):28cm13. doi: 10.1126/scitranslmed.3000643. [DOI] [PubMed] [Google Scholar]
- 6.Petereit DG, Rogers D, Govern F, Coleman N, Osburn CH, Howard SP, Kaur J, Burhansstipanov L, Fowler CJF, Chappell R, Mehta MP. Increasing access to clinical cancer trials and emerging technologies for minority populations: The Native American Project. Journal of Clinical Oncology. 2004;22(22):4452–4455. doi: 10.1200/JCO.2004.01.119. [DOI] [PubMed] [Google Scholar]
- 7.Petereit DG, Burhansstipanov L. Establishing trusting partnerships for successful recruitment of American Indians to clinical trials. Cancer Control. 2008;15(3):260–268. doi: 10.1177/107327480801500310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petereit DG, Molloy K, Reiner ML, Helbig P, Cina K, Miner R, Spotted Tail C, Rost C, Conroy P, Roberts CR. Establishing a patient navigator program to reduce cancer disparities in the American Indian communities of Western South Dakota: initial observations and results. Cancer Control. 2008;15(3):254–259. doi: 10.1177/107327480801500309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guadagnolo BA, Boylan A, Sargent M, Koop D, Brunette D, Kanekar S, Short Bull V, Molloy K, Petereit DG. Patient navigation for American Indians undergoing cancer treatment: utilization and impact on care delivery in a regional health care center. Cancer. 2011;117(12):2754–2761. doi: 10.1002/cncr.25823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guadagnolo BA, Cina K, Helbig P, Molloy K, Reiner M, Cook EF, Petereit DG. Medical mistrust and less satisfaction with health care among Native Americans presenting for cancer treatment. J Health Care Poor Underserved. 2009;20(1):210–226. doi: 10.1353/hpu.0.0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kanekar S, Petereit DG. Walking forward: a program designed to lower cancer mortality rates among American Indians in western South Dakota. S D Med. 2009;62(4):151–157. [PMC free article] [PubMed] [Google Scholar]
- 12.Guadagnolo BA, Cina K, Helbig P, Molloy K, Reiner M, Cook EF, Petereit DG. Assessing cancer stage and screening disparities among Native American cancer patients. When free primary care is not enough. Public Health Rep. 2009;124:79–89. doi: 10.1177/003335490912400111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sparks SMA, Koscik R, Kanekar S, et al. Self-reported barriers to accessing health care among Native Americans in South Dakota. Journal of Healthcare to the Poor and the Underserved 2010 [Google Scholar]
- 14.Guadagnolo BA, Cina K, Helbig P, et al. Assessing cancer stage and screening disparities among Native American cancer patients: when free primary care is not enough. Public Health Rep. 2009;124:79–89. doi: 10.1177/003335490912400111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guadagnolo B, Petereit D, Helbig P, Koop D, Kussman P, Fox E, Patnaik A. Involving American Indians and medically underserved populations in cancer clinical trials. Clin Trials. 2009;6 (6):610–617. doi: 10.1177/1740774509348526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petereit DG, Rogers D, Helbig P, et al. Geographic distance from the cancer center may be a treatment barrier for American Indians undergoing radiotherapy. Intercultural Cancer Council 9th Biennial Symposium on Minorities, the Medically Underserved, and Cancer; Washington, DC. 2004. [Google Scholar]
- 17.Ritter MA, Forman JD, Kupelian P, Petereit DG, Lawton CA, Chappell RJ, Tomé WA. A phase I/II trial of increasingly hypofractionated radiation therapy for prostate cancer. American Society of Therapeutic Radiation Oncology 2009 Annual Meeting.2009. [Google Scholar]
- 18.Swartz MJ, Petereit DG. The role of radiation therapy in the management of lung, prostate and colorectal cancer in South Dakota Medicine. S D Med. 2010;2010:60–66. Special issue. [PubMed] [Google Scholar]
- 19.Petereit DG, Sarkaria JN, Hartmann TJ, Kinsella TJ, Chappell R, Thomadsen BR, Stitt JA, Buchler DA. The adverse effect of treatment prolongation in cervical carcinoma. Int J Radiat Oncol Biol Phys. 1995;32:1301–1307. doi: 10.1016/0360-3016(94)00635-X. [DOI] [PubMed] [Google Scholar]
- 20.Petereit DG, Moser A, Hahn J, Boyland A, Kanekar S, Ritter M, Bentzen SM, Koop D, Kaur J, Mehta M. Ataxia telangiectasia mutated (ATM) gene variants in American Indians. Int J Radiat Oncol Biol Phys. 2010;78(3):S90. [Google Scholar]
- 21.Harmon A. New York Times. New York: 2010. Apr 21, Indian tribe wins fight to limit research of its DNA. 2010. [Google Scholar]
- 22.Hart S, Sobraske K. Investigative report concerning the medical genetics project at Havasupai. 2003 Dec 23; 2003; available at the Arizona State University Law Library and www.geneticpiracy.com/Documents/HartReport.pdf.
- 23.Burke TW, Hoskins WJ, Heller PB, Bibro MC, Weiser EB, Park RC. Prognostic factors associated with radical hysterectomy failure. Gynecol Oncol. 1987;26:153–159. doi: 10.1016/0090-8258(87)90268-x. [DOI] [PubMed] [Google Scholar]


