Abstract
Some observational studies have identified elevated uric acid concentration as a risk factor for diabetes, while others have found an inverse relationship. We examined both the association of uric acid level with incident diabetes and the change in uric acid concentration after a diabetes diagnosis. We analyzed data from the Atherosclerosis Risk in Communities (ARIC) Study and quantified the independent association between uric acid level and incident diabetes via Cox proportional hazards models. The association between duration of diabetes and change in uric acid level was examined via linear regression. Among 11,134 participants without diagnosed diabetes at baseline (1987–1989), there were 1,294 incident cases of diabetes during a median of 9 years of follow-up (1987–1998). Uric acid level was associated with diabetes even after adjustment for risk factors (per 1 mg/dL, hazard ratio = 1.18, 95% confidence interval: 1.13, 1.23), and the association remained significant after adjustment for fasting glucose and insulin levels. Among participants with diabetes (n = 1,510), every additional 5 years’ duration of diabetes was associated with a 0.10-mg/dL (95% confidence interval: 0.04, 0.15) lower uric acid level after adjustment. We conclude that uric acid concentration rises prior to diagnosis of diabetes and then declines with diabetes duration. Future studies investigating uric acid as a risk factor for cardiovascular disease should adequately account for the impact and timing of diabetes development.
Keywords: cohort studies, diabetes mellitus, uric acid
The prevalence of diabetes mellitus is increasing (1). Despite improved understanding of the pathophysiology of diabetes, identifying which diabetes patients are most likely to develop life-threatening complications is an ongoing challenge (2). In this context, uric acid concentration is emerging as a potential marker of diabetes risk. Uric acid is a product of glucose metabolism that is filtered by glomeruli and reabsorbed by the proximal tubule. Greater serum concentrations of insulin cause higher renal reabsorption of uric acid, increasing serum concentrations of uric acid (3). There is mounting observational evidence that an elevated uric acid level precedes the development of insulin resistance (4, 5) and diabetes (6–8). However, these findings conflict with studies showing that diabetes is inversely associated with uric acid level (8–14) and protective against complications of hyperuricemia (15). Furthermore, despite evidence suggesting a positive association between uric acid and cardiovascular disease (16), findings on the relationship between uric acid and cardiovascular complications in the setting of diabetes have been inconsistent (17, 18). A better understanding of the temporal relationship between uric acid level and diabetes is necessary to clarify the role of uric acid as a risk factor for both diabetes and vascular outcomes.
In this study, we examined the temporal relationship between uric acid concentration and diabetes in a large community-based cohort of middle-aged adults. Our objectives were 1) to examine uric acid concentrations in participants with varying degrees of insulin sensitivity prior to a diagnosis of diabetes and 2) to examine change in uric acid level after a diagnosis of diabetes. On the basis of prior cross-sectional studies (8–14), we hypothesized that baseline uric acid levels would be elevated in participants prior to a diagnosis of diabetes but uric acid levels would decline after a diabetes diagnosis. We also hypothesized that a longer duration of diabetes would be associated with lower uric acid concentrations.
METHODS
Study population
The Atherosclerosis Risk in Communities (ARIC) Study is a community-based prospective cohort study of 15,792 adults aged 45–64 years at baseline (1987–1989). Participants were enrolled from 4 US communities (Forsyth County, North Carolina; Jackson, Mississippi; suburban Minneapolis, Minnesota; and Washington County, Maryland) and have been followed for over 2 decades (19–21). Study participants returned for follow-up visits in 1990–1992, 1993–1995, and 1996–1998, and a fifth follow-up was recently completed (2011–2013). Physical examinations, interviews, and laboratory tests were conducted as part of each visit. In addition, participants completed annual telephone questionnaires that obtained information on new disease diagnoses (e.g., diabetes) and medication use.
We analyzed data from ARIC participants with measured uric acid concentrations who attended visit 1. We included participants who had fasted for 8 or more hours, had valid uric acid measurements, had a known diabetes case status, and were not missing data on relevant covariates. In our prospective analyses of uric acid level and incident diabetes, we excluded persons who had received a diagnosis of diabetes, defined as a self-reported physician's diagnosis of diabetes, use of glucose-lowering medications, a baseline fasting glucose level of ≥126 mg/dL, or a baseline nonfasting glucose level of ≥200 mg/dL. After exclusions, there were 11,134 participants. In a cross-sectional analysis examining the relationship between duration of diabetes and uric acid level, we limited the study population to participants who received a diagnosis of diabetes by visit 4, using the same case definition as above (n = 1,510) (see Web Figure 1, available at http://aje.oxfordjournals.org/).
Written informed consent was obtained from all participants, and the study protocol was approved by the institutional review board at each clinical site.
Uric acid
Uric acid concentration was measured from serum specimens collected during visit 1 (1987–1989) and plasma specimens collected during visit 4 (1996–1998). The visit 1 uric acid levels were determined as specimens were collected, while visit 4 samples were measured from stored samples in 2009–2011. For both visits, uric acid was measured using the uricase-peroxidase enzymatic method. The coefficient of variation measured by the laboratory at visit 1 was 7.2% (22). The manufacturer-supplied coefficient of variation for the uric acid assay used at visit 4 was 0.98% (mean = 7.3 mg/dL). A calibration study (using a common standard) was conducted using 200 specimens from both visit 1 and visit 4 in order to address potential issues related to laboratory drift and differences in specimen type. There was a significant laboratory difference in measurements conducted at the two time points. Thus, uric acid values (mg/dL) from visit 1 were adjusted by means of the following equation: calibrated uric acid = visit 1 uric acid − 0.80. Similarly, uric acid values from visit 4 were recalibrated using the following equation: calibrated uric acid = 0.97 × visit 4 uric acid − 0.04.
Incident diabetes
Our case definition of diabetes was based on a fasting glucose concentration of ≥126 mg/dL, a nonfasting glucose concentration of ≥200 mg/dL (measured at visits 2–4), use of diabetes medication, or a self-reported physician's diagnosis of diabetes. The date of diabetes onset was estimated on the basis of glucose measurements, using linear interpolation to estimate the time at which glucose level became diagnostic for diabetes (23).
Diabetes duration
Duration of diabetes was determined for all cases of diabetes identified by visit 4. For persons with prevalent diabetes who were identified as having diabetes at visit 1, diabetes duration was determined using the difference between the reported age of diabetes diagnosis and the age at visit 4. For participants diagnosed with diabetes after visit 1 and through visit 4, diabetes duration was determined using the difference between the date of diabetes diagnosis and the participant's visit 4 study participation date.
Other variables of interest
Trained study personnel conducted all measurements using standardized protocols described elsewhere (19–21). Age, sex, race, parental history of diabetes, educational attainment, alcohol drinking status, and smoking status were self-reported. Use of diuretics was based on information obtained by interviewers trained in collecting data on medication use. Body mass index (weight (kg)/height (m)2) was calculated from measured height and weight. Systolic and diastolic blood pressures were taken using a random-zero sphygmomanometer, and readings were averaged across 2 measurements. Hypertension was defined as a mean systolic blood pressure of ≥140 mm Hg, a mean diastolic blood pressure of ≥90 mm Hg, or current use of blood pressure-lowering medication. Estimated glomerular filtration rate (mL/minute per 1.73 m2) was calculated from standardized serum creatinine using the 2009 Chronic Kidney Disease Epidemiology Collaboration equation (24). Low-density lipoprotein cholesterol was estimated using the Friedewald equation (25), while high-density lipoprotein cholesterol, triglycerides (log10-transformed), and insulin were measured in serum. Serum insulin concentration was treated as a continuous variable and was included in stratified analyses as a dichotomous variable, using 15 μU/mL as a cutpoint (26). Serum glucose concentration was quantified using a hexokinase method. The homeostatic model assessment insulin resistance index (HOMA-IR) was determined by means of the formula [(fasting insulin in μU/mL) × (fasting glucose in mg/dL)]/405, and results were dichotomized using a previously determined clinical cutpoint (<2.6 vs. ≥2.6) (27).
Statistical analyses
We performed a prospective analysis of uric acid level and incident diabetes using Cox proportional hazards models, examining the association between uric acid concentration measured at visit 1 and the risk of incident visit-based diabetes ascertained between visits 1 and 4. Uric acid level was modeled as a categorical variable (quartiles) and as a continuous variable (per 1 mg/dL) in 3 models. In model 1, results were adjusted for age, sex, and race/ARIC study center; in model 2, results were adjusted for the variables in model 1 plus low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides, body mass index, estimated glomerular filtration rate, hypertension (yes or no), parental history of diabetes (yes or no), education (high school completion or more; yes or no), smoking status (never, former, or current smoker), alcohol use (never, former, or current drinker), and diuretic use (yes or no); and in model 3, results were adjusted for all of the variables in model 2 plus fasting glucose and fasting insulin levels. The above covariates were chosen on the basis of previous literature describing their relationships with uric acid and diabetes. We repeated these 2 prospective analyses across strata of glucose level (<100 mg/dL vs. 100–125 mg/dL), insulin level (<15 μU/mL vs. ≥15 μU/mL), and HOMA-IR (<2.6 vs. ≥2.6), adjusting for all of the covariates in model 2. In addition, we modeled uric acid level using restricted cubic splines with knots at the 25th, 50th, and 75th percentiles and centered at the 25th percentile to show the continuous relationship between uric acid and risk of diabetes.
We used multivariable linear regression to evaluate the cross-sectional association between diabetes duration and uric acid level measured at visit 4. Diabetes duration was modeled in quartiles (0.0–4.3, 4.4–7.2, 7.3–9.0, or 9.1–65.0 years) and as a continuous variable (per 5 years). We also conducted analyses of prevalent diabetes at visit 1 with 9-year change in uric acid level (visit 4 uric acid concentration minus visit 1 uric acid concentration). In addition, we performed sensitivity analyses excluding participants who reported use of gout medication at either visit 1 or visit 4 (n = 61), participants using insulin medication at visit 4 (n = 177), and participants using any diabetes medication (n = 650). Finally, we performed a sensitivity analysis comparing 9-year change in uric acid among participants with diabetes at both visit 1 and visit 4 to 9-year change among participants without diabetes at both visit 1 and visit 4.
Schoenfeld tests were used to evaluate the proportional hazards assumption of the Cox proportional hazards models. With regard to linear models, scatterplots and locally weighted scatterplot smoothing (LOWESS) curves were used to ensure that model residuals were randomly distributed with respect to fitted values from the models.
RESULTS
Characteristics of the different study populations are reported in Table 1. The mean age was 54 years among nonprevalent cases of diabetes. The proportion male ranged between 43% and 49%. Thirty percent of participants with incident diabetes were black, as compared with 18% of noncases. Fasting glucose concentration was 108 mg/dL among incident cases and 97 mg/dL among noncases.
Table 1.
Characteristics of the Study Population by Diabetes Case Status at Visit 1, Atherosclerosis Risk in Communities Study, 1987–1989
| Diabetes Case Status |
||||||
|---|---|---|---|---|---|---|
| Noncases (n = 9,840)a |
Incident Cases (n = 1,294) |
|||||
| Mean (SD) | No. | % | Mean (SD) | No. | % | |
| Uric acid concentration, mg/dL | 5.0 (1.5) | 5.9 (1.6) | ||||
| Age, years | 53.9 (5.7) | 54.1 (5.6) | ||||
| Male sex | 4,258 | 43.3 | 634 | 49.0 | ||
| Black race | 1,798 | 18.3 | 387 | 29.9 | ||
| Fasting glucose concentration, mg/dL | 97.3 (8.4) | 108.1 (9.5) | ||||
| Fasting glucose category, mg/dL | ||||||
| <100 | 1,798 | 18.3 | 257 | 19.9 | ||
| 100–<126 | 3,614 | 36.7 | 1,037 | 80.1 | ||
| Fasting insulin concentration, μM/mL | 9.8 (7.1) | 16.5 (10.6) | ||||
| HOMA-IR | 2.4 (1.8) | 4.4 (3.0) | ||||
| Fasting cholesterol level, mg/dL | ||||||
| Low-density lipoprotein | 136.6 (38.5) | 140.2 (39.3) | ||||
| High-density lipoprotein | 53.7 (17.3) | 45.9 (13.9) | ||||
| Fasting triglyceride level, mg/dL | 103 (75–144)b | 132.5 (95–184)b | ||||
| Body mass indexc | 26.7 (4.7) | 30.5 (5.7) | ||||
| Estimated glomerular filtration rate, mL/minute per 1.73 m2 | 95.23 (14.0) | 96.0 (15.6) | ||||
| Hypertension | 2,663 | 27.1 | 597 | 46.1 | ||
| Family history of diabetes | 2,256 | 22.9 | 484 | 37.4 | ||
| Education beyond high school | 4,760 | 48.4 | 536 | 41.4 | ||
| Smoking status | ||||||
| Never smoker | 4,248 | 43.2 | 503 | 38.9 | ||
| Former smoker | 3,220 | 32.7 | 472 | 36.5 | ||
| Current smoker | 2,372 | 24.1 | 319 | 24.7 | ||
| Alcohol drinking status | ||||||
| Never drinker | 2,250 | 22.9 | 355 | 27.4 | ||
| Former drinker | 1,600 | 16.3 | 256 | 19.8 | ||
| Current drinker | 5,990 | 60.9 | 683 | 52.8 | ||
| Use of diuretic medication | 1,458 | 14.8 | 342 | 26.4 | ||
Abbreviations: HOMA-IR, homeostatic model assessment insulin resistance index; SD, standard deviation.
a Visit 1 noncases were study participants who did not report a physician's diagnosis of diabetes at visit 1, were not using diabetes medication at visit 1, did not have a serum glucose level of ≥126 mg/dL at visit 1, did not have a nonfasting glucose level of ≥200 mg/dL at visit 1, and did not go on to develop visit-based diabetes between visit 1 and visit 4.
b Median value (interquartile range).
c Weight (kg)/height (m)2.
During a median of 9 years of follow-up, there were 1,294 incident cases of diabetes from visit 1 (1987–1989) to visit 4 (1996–1998). There was a significant, graded increase in diabetes risk across baseline categories of uric acid level (models 1 and 2; P-trend < 0.001) (Table 2). This trend was attenuated after adjustment for fasting glucose and insulin levels (model 3; P-trend ≈ 0.1). Further, when modeled as a continuous variable, uric acid remained significantly associated with incident diabetes even after adjustment for fasting glucose and insulin levels (hazard ratio = 1.05, 95% confidence interval (CI): 1.00, 1.10; P = 0.03).
Table 2.
Adjusted Hazard Ratios for Incident Diabetes According to Baseline Uric Acid Concentration, Atherosclerosis Risk in Communities Study, 1987–1998
| Visit-based Diabetes (During 9 Years of Follow-up) (n =11,134) | No. of Cases | Risk of Incident Diabetes |
|||||
|---|---|---|---|---|---|---|---|
| Model 1a |
Model 2b |
Model 3c |
|||||
| HR | 95% CI | HR | 95% CI | HR | 95% CI | ||
| Quartile of uric acid concentration, mg/dL | |||||||
| ≤4.9 | 165 | 1 | Reference | 1 | Reference | 1 | Reference |
| 5.0–5.8 | 218 | 1.54 | 1.25, 1.88 | 1.10 | 0.89, 1.35 | 0.91 | 0.74, 1.12 |
| 5.9–6.9 | 397 | 2.77 | 2.29, 3.35 | 1.51 | 1.24, 1.84 | 1.20 | 0.98, 1.46 |
| 7.0–15.9 | 514 | 4.33 | 3.58, 5.24 | 1.79 | 1.45, 2.21 | 1.12 | 0.90, 1.39 |
| P-trendd | <0.001 | <0.001 | 0.11 | ||||
| Uric acid concentration, per 1 mg/dL | 1,294 | 1.39 | 1.34, 1.44 | 1.18 | 1.13, 1.23 | 1.05 | 1.00, 1.10 |
| P value | <0.001 | <0.001 | 0.03 | ||||
Abbreviations: CI, confidence interval; HR, hazard ratio.
a Results were adjusted for age, sex, and race/study center.
b Results were adjusted for all variables in model 1 plus low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides, body mass index, estimated glomerular filtration rate, hypertension, parental family history of diabetes, education, smoking status, alcohol use, and diuretic use.
c Results were adjusted for all variables in model 2 plus fasting glucose and fasting insulin levels.
d P-trend was calculated using the median value of each quartile to construct an ordinal variable.
The shape of the association between uric acid and risk of diabetes is shown in Figure 1. The relationship between uric acid and diabetes risk was approximately linear, with higher uric acid concentrations being associated with greater risk of diabetes. When stratified by baseline glucose, insulin, or HOMA-IR level, we found that uric acid concentrations were more strongly associated with incident diabetes when fasting glucose was in the prediabetic range of 100–125 mg/dL, as well as when insulin concentration was less than 15 μU/mL. Uric acid level was significantly associated with incident diabetes regardless of baseline HOMA-IR (Web Table 1).
Figure 1.
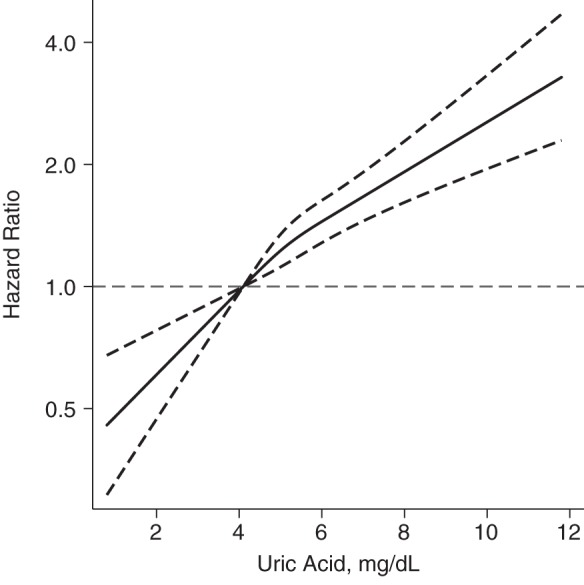
Adjusted hazard ratios (solid line) for incident diabetes between visit 1 and visit 4, according to baseline uric acid concentration (restricted cubic spline models), Atherosclerosis Risk in Communities Study, 1987–1998. Hazard ratios are shown on a natural log scale; the dashed lines represent 95% confidence intervals (horizontal dashed line, referent). The model is expressed relative to the 25th percentile with knots specified at the 25th, 50th, and 75th percentiles, after adjustment for age, sex, race/study center, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides, body mass index, estimated glomerular filtration rate, hypertension, family history of diabetes, education, smoking status, alcohol use, and diuretic use. The plot was truncated at the first and 99th percentiles.
The association between diabetes duration and visit 4 uric acid concentration, as well as 9-year change in uric acid concentration, is shown in Table 3. Persons with a diagnosis of diabetes by visit 4 had a median diabetes duration of 7.9 years. Duration of diabetes diagnosis was associated with a progressively lower concentration of uric acid before and after adjustment for covariates, including fasting glucose and insulin (P-trend values ≤ 0.01). After adjustment, for each additional 5 years with diabetes, uric acid was lower by 0.10 mg/dL (95% CI: 0.04, 0.15). With regard to 9-year change in uric acid level, the greatest reduction in uric acid was observed among participants with a diabetes duration of 7.2–9.0 years, which averaged −0.42 mg/dL (95% CI: −0.62, −0.22) after adjustment. The change in uric acid level seemed to plateau over time, since participants who had had diabetes for more than 9 years demonstrated almost no change in uric acid from visit 1 to visit 4.
Table 3.
Cross-Sectional Association Between Diabetes Duration and Visit 4 Uric Acid Concentration (mg/dL) or 9-year Within-Person Change in Uric Acid Concentration (n =1,510a), Atherosclerosis Risk in Communities Study, 1987–1998
| Model 1b |
Model 2c |
Model 3d |
||||
|---|---|---|---|---|---|---|
| β | 95% CI | β | 95% CI | β | 95% CI | |
| Mean uric acid concentration | ||||||
| Category of diabetes duration, years | ||||||
| 0.0–4.3 (n = 378) | 1 | Reference | 1 | Reference | 1 | Reference |
| 4.4–7.2 (n = 377) | −0.29 | −0.50, −0.08 | −0.15 | −0.34, 0.03 | −0.09 | −0.27, 0.10 |
| 7.3–9.0 (n = 467) | −0.55 | −0.75, −0.35 | −0.36 | −0.54, −0.18 | −0.16 | −0.34, 0.02 |
| 9.1–65.0 (n = 288) | −0.52 | −0.75, −0.30 | −0.49 | −0.69, −0.29 | −0.27 | −0.47, −0.06 |
| P-trende | <0.001 | <0.001 | 0.01 | |||
| Continuous diabetes duration (per 5 years) | −0.15 | −0.21, −0.09 | −0.15 | −0.20, −0.09 | −0.10 | −0.15, −0.04 |
| Mean 9-year within-person change in uric acid concentration | ||||||
| Category of diabetes duration, years | ||||||
| 0.0–4.3 (n = 378) | 1 | Reference | 1 | Reference | 1 | Reference |
| 4.4–7.2 (n = 377) | −0.42 | −0.63, −0.21 | −0.37 | −0.58, −0.17 | −0.32 | −0.52, −0.11 |
| 7.3–9.0 (n = 467) | −0.68 | −0.88, −0.48 | −0.61 | −0.81, −0.41 | −0.42 | −0.62, −0.22 |
| 9.1–65.0 (n = 288) | −0.04 | −0.27, 0.19 | −0.06 | −0.28, 0.17 | 0.16 | −0.08, 0.39 |
| P-trende | 0.32 | 0.27 | 0.30 | |||
| Mean 9-year change (per 5 years with diabetes) | −0.01 | −0.08, 0.05 | −0.03 | −0.09, 0.03 | 0.02 | −0.04, 0.08 |
Abbreviation: CI, confidence interval.
a This analysis was conducted among visit 4 participants who had a visit-based diagnosis of diabetes by visit 4.
b Results were adjusted for age, sex, and race/study center.
c Results were adjusted for model 1 variables plus low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides, body mass index, estimated glomerular filtration rate, hypertension, parental family history of diabetes, education, smoking status, alcohol use, and diuretic use.
d Results were adjusted for model 2 variables plus fasting glucose and fasting insulin levels.
e P-trend was calculated using the median value of each quartile to construct an ordinal variable.
Sensitivity analyses excluding persons using gout medication at either visit 1 or visit 4 did not meaningfully change our findings (Web Table 2). Similarly, sensitivity analyses excluding persons using insulin medication or any diabetes medication attenuated but did not significantly alter our findings (Web Tables 3 and 4). Furthermore, a sensitivity analysis comparing participants who had diabetes at both visit 1 and visit 4 with participants who did not have diabetes at either visit 1 or visit 4 confirmed our findings; that is, diabetes was associated with a greater reduction in uric acid levels over time (Web Table 5).
DISCUSSION
This study represents a comprehensive examination of the temporal relationship between uric acid concentration and diabetes in a community-based population. Previous studies have shown that a high uric acid level is associated with an increased risk of diabetes but also that, paradoxically, prevalent diabetes is associated with a lower risk of hyperuricemia (high uric acid levels). Our results help reconcile conflicting evidence in the literature and demonstrate that: 1) higher baseline concentrations of uric acid are associated with an increased risk of incident diabetes regardless of baseline insulin resistance; and 2) uric acid concentrations decline after participants report a diabetes diagnosis or develop a fasting glucose concentration above the diagnostic threshold for diabetes.
Previous observational studies have demonstrated an association between uric acid level and risk of diabetes (4–8). Our results confirm these previous findings, but we further showed that this association was present even after accounting for baseline fasting glucose and insulin concentrations. Indeed, uric acid level was significantly associated with a higher risk of diabetes among persons with a low-normal baseline insulin concentration (<15 μU/mL). It has been postulated that uric acid plays a causal role in the development of diabetes (28), but it is also possible that uric acid level is a marker for other diabetes risk factors, reflecting high concentrations of intracellular glycolytic substrates (29), dietary intake (30), or a genetic predisposition to diabetes (31). Future studies are needed to investigate whether modifying uric acid concentrations can alter diabetes risk.
We also found that while elevated uric acid concentrations were associated with increased risk of diabetes, after diagnosis of diabetes, uric acid concentrations declined. The decline in uric acid level was strongly associated with duration of diabetes. These findings are consistent with other cross-sectional and prospective studies that have shown lower uric acid levels in persons with a history of diabetes (8, 9). It is believed that elevations in serum glucose (32–34) or insulin (35) promote excretion of uric acid. However, we observed that the association between diabetes and lower uric acid level was independent of estimated glomerular filtration rate, fasting glucose, and insulin. It is possible that lifestyle changes or medication use subsequent to a diabetes diagnosis alters uric acid production; however, ongoing alterations in metabolism due to the chronic effects of diabetes cannot be ruled out.
The decline in uric acid concentration after a diabetes diagnosis may help explain the inconsistent relationship between uric acid and cardiovascular complications in the setting of diabetes. There has historically been much debate over the relationship between asymptomatic hyperuricemia and fatal and nonfatal cardiovascular events (36). Evidence for uric acid as a cardiovascular disease risk factor from epidemiologic studies has been conflicting (37). It is possible that the inclusion of persons with diabetes (subclinical or diagnosed) in previous studies may have masked a true association between uric acid and cardiovascular disease risk, since diabetes may reduce uric acid level independently of cardiovascular disease risk. For example, one recent study of uric acid in a diabetic population found no association with cardiovascular mortality (38). Nonetheless, additional studies are necessary to confirm these findings.
There are several limitations to this study that warrant discussion. First, our prospective analyses relied on single uric acid measurements. Second, the cross-sectional duration analysis was potentially subject to survival bias in that participants needed to survive to visit 4 to be included in the analysis. Finally, as in any observational study, residual confounding remains a possibility. Despite these limitations, this study had a number of strengths, including the large sample size, the long follow-up period, standardized measurements of diabetes risk factors, and repeat measurements of uric acid taken 9 years apart.
In conclusion, high uric acid concentrations were associated with risk of diabetes, even among participants with normal insulin sensitivity at baseline. However, prevalent diabetes was associated with a decline in uric acid concentrations over time, with the biggest declines being observed in participants with the longest duration of diabetes. These findings may have implications for studies evaluating the relationship between uric acid and complications of diabetes, including cardiovascular disease outcomes. Future studies investigating uric acid level as a risk factor for cardiovascular disease should adequately account for the impact and timing of diabetes development.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Department of Epidemiology, Bloomberg School of Public Health, Johns Hopkins University, Baltimore, Maryland (Stephen P. Juraschek, Mara McAdams-Demarco, Edgar R. Miller, Allan C. Gelber, J. Hunter Young, Josef Coresh, Elizabeth Selvin); Welch Center for Prevention, Epidemiology and Clinical Research, School of Medicine and Bloomberg School of Public Health, Johns Hopkins University, Baltimore, Maryland (Stephen P. Juraschek, Mara McAdams-Demarco, Edgar R. Miller, Allan C. Gelber, J. Hunter Young, Josef Coresh, Elizabeth Selvin); Department of Medicine, School of Medicine, Johns Hopkins University, Baltimore, Maryland (Stephen P. Juraschek, Mara McAdams-Demarco, Edgar R. Miller, Allan C. Gelber, J. Hunter Young, Josef Coresh, Elizabeth Selvin); Food and Drug Administration, Silver Spring, Maryland (Janet W. Maynard); and Division of Epidemiology and Community Health, School of Public Health, University of Minnesota, Minneapolis, Minnesota (James S. Pankow).
The Atherosclerosis Risk in Communities (ARIC) Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). S.P.J. was supported by Cardiovascular Epidemiology Training Grant T32HL007024 from the National Heart, Lung, and Blood Institute.
We thank the staff of the ARIC Study for their important contributions.
No official support for or endorsement of these findings by the Food and Drug Administration is intended or should be inferred.
Conflict of interest: none declared.
REFERENCES
- 1.Cowie CC, Rust KF, Byrd-Holt DD, et al. Prevalence of diabetes and impaired fasting glucose in adults in the U.S. population: National Health and Nutrition Examination Survey 1999–2002. Diabetes Care. 2006;29(6):1263–1268. doi: 10.2337/dc06-0062. [DOI] [PubMed] [Google Scholar]
- 2.Van Dieren S, Beulens JWJ, Kengne AP, et al. Prediction models for the risk of cardiovascular disease in patients with type 2 diabetes: a systematic review. Heart. 2012;98(5):360–369. doi: 10.1136/heartjnl-2011-300734. [DOI] [PubMed] [Google Scholar]
- 3.Muscelli E, Natali A, Bianchi S, et al. Effect of insulin on renal sodium and uric acid handling in essential hypertension. Am J Hypertens. 1996;9(8):746–752. doi: 10.1016/0895-7061(96)00098-2. [DOI] [PubMed] [Google Scholar]
- 4.Krishnan E, Pandya BJ, Chung L, et al. Hyperuricemia in young adults and risk of insulin resistance, prediabetes, and diabetes: a 15-year follow-up study. Am J Epidemiol. 2012;176(2):108–116. doi: 10.1093/aje/kws002. [DOI] [PubMed] [Google Scholar]
- 5.Niskanen L, Laaksonen DE, Lindström J, et al. Serum uric acid as a harbinger of metabolic outcome in subjects with impaired glucose tolerance: the Finnish Diabetes Prevention Study. Diabetes Care. 2006;29(3):709–711. doi: 10.2337/diacare.29.03.06.dc05-1465. [DOI] [PubMed] [Google Scholar]
- 6.Kodama S, Saito K, Yachi Y, et al. Association between serum uric acid and development of type 2 diabetes. Diabetes Care. 2009;32(9):1737–1742. doi: 10.2337/dc09-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Viazzi F, Leoncini G, Vercelli M, et al. Serum uric acid levels predict new-onset type 2 diabetes in hospitalized patients with primary hypertension: the MAGIC study. Diabetes Care. 2011;34(1):126–128. doi: 10.2337/dc10-0918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herman JB, Goldbourt U. Uric acid and diabetes: observations in a population study. Lancet. 1982;320(8292):240–243. doi: 10.1016/s0140-6736(82)90324-5. [DOI] [PubMed] [Google Scholar]
- 9.Yano K, Rhoads GG, Kagan A. Epidemiology of serum uric acid among 8000 Japanese-American men in Hawaii. J Chronic Dis. 1977;30(3):171–184. doi: 10.1016/0021-9681(77)90083-2. [DOI] [PubMed] [Google Scholar]
- 10.Ryu S, Chang Y, Zhang Y, et al. A cohort study of hyperuricemia in middle-aged South Korean men. Am J Epidemiol. 2012;175(2):133–143. doi: 10.1093/aje/kwr291. [DOI] [PubMed] [Google Scholar]
- 11.Choi HK, Ford ES. Haemoglobin A1c, fasting glucose, serum C-peptide and insulin resistance in relation to serum uric acid levels—the Third National Health and Nutrition Examination Survey. Rheumatology (Oxford) 2008;47(5):713–717. doi: 10.1093/rheumatology/ken066. [DOI] [PubMed] [Google Scholar]
- 12.Whitehead TP, Jungner I, Robinson D, et al. Serum urate, serum glucose and diabetes. Ann Clin Biochem. 1992;29(2):159–161. doi: 10.1177/000456329202900206. [DOI] [PubMed] [Google Scholar]
- 13.Cook DG, Shaper AG, Thelle DS, et al. Serum uric acid, serum glucose and diabetes: relationships in a population study. Postgrad Med J. 1986;62(733):1001–1006. doi: 10.1136/pgmj.62.733.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tuomilehto J, Zimmet P, Wolf E, et al. Plasma uric acid level and its association with diabetes mellitus and some biologic parameters in a biracial population of Fiji. Am J Epidemiol. 1988;127(2):321–336. doi: 10.1093/oxfordjournals.aje.a114807. [DOI] [PubMed] [Google Scholar]
- 15.Rodríguez G, Soriano LC, Choi HK. Impact of diabetes against the future risk of developing gout. Ann Rheum Dis. 2010;69(12):2090–2094. doi: 10.1136/ard.2010.130013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Niskanen LK, Laaksonen DE, Nyyssönen K, et al. Uric acid level as a risk factor for cardiovascular and all-cause mortality in middle-aged men: a prospective cohort study. Arch Intern Med. 2004;164(14):1546–1551. doi: 10.1001/archinte.164.14.1546. [DOI] [PubMed] [Google Scholar]
- 17.Brand FN, McGee DL, Kannel WB, et al. Hyperuricemia as a risk factor of coronary heart disease: The Framingham Study. Am J Epidemiol. 1985;121(1):11–18. doi: 10.1093/oxfordjournals.aje.a113972. [DOI] [PubMed] [Google Scholar]
- 18.Culleton BF, Larson MG, Kannel WB, et al. Serum uric acid and risk for cardiovascular disease and death: the Framingham Heart Study. Ann Intern Med. 1999;131(1):7–13. doi: 10.7326/0003-4819-131-1-199907060-00003. [DOI] [PubMed] [Google Scholar]
- 19.The ARIC Investigators. The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol. 1989;129(4):687–702. [PubMed] [Google Scholar]
- 20.Jackson R, Chambless LE, Yang K, et al. Differences between respondents and nonrespondents in a multicenter community-based study vary by gender and ethnicity. The Atherosclerosis Risk in Communities (ARIC) Study Investigators. J Clin Epidemiol. 1996;49(12):1441–1446. doi: 10.1016/0895-4356(95)00047-x. [DOI] [PubMed] [Google Scholar]
- 21.White AD, Folsom AR, Chambless LE, et al. Community surveillance of coronary heart disease in the Atherosclerosis Risk in Communities (ARIC) Study: methods and initial two years’ experience. J Clin Epidemiol. 1996;49(2):223–233. doi: 10.1016/0895-4356(95)00041-0. [DOI] [PubMed] [Google Scholar]
- 22.Tamariz L, Agarwal S, Soliman EZ, et al. Association of serum uric acid with incident atrial fibrillation (from the Atherosclerosis Risk in Communities [ARIC] study) Am J Cardiol. 2011;108(9):1272–1276. doi: 10.1016/j.amjcard.2011.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duncan BB, Schmidt MI, Pankow JS, et al. Low-grade systemic inflammation and the development of type 2 diabetes: the Atherosclerosis Risk in Communities Study. Diabetes. 2003;52(7):1799–1805. doi: 10.2337/diabetes.52.7.1799. [DOI] [PubMed] [Google Scholar]
- 24.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 26.McLaughlin T, Abbasi F, Cheal K, et al. Use of metabolic markers to identify overweight individuals who are insulin resistant. Ann Intern Med. 2003;139(10):802–809. doi: 10.7326/0003-4819-139-10-200311180-00007. [DOI] [PubMed] [Google Scholar]
- 27.Ascaso JF, Pardo S, Real JT, et al. Diagnosing insulin resistance by simple quantitative methods in subjects with normal glucose metabolism. Diabetes Care. 2003;26(12):3320–3325. doi: 10.2337/diacare.26.12.3320. [DOI] [PubMed] [Google Scholar]
- 28.Nakagawa T, Hu H, Zharikov S, et al. A causal role for uric acid in fructose-induced metabolic syndrome. Am J Physiol Renal Physiol. 2006;290(3):F625–F631. doi: 10.1152/ajprenal.00140.2005. [DOI] [PubMed] [Google Scholar]
- 29.Brecher AS, Lehti MD. A hypothesis linking hypoglycemia, hyperuricemia, lactic acidemia, and reduced gluconeogenesis in alcoholics to inactivation of glucose-6-phosphatase activity by acetaldehyde. Alcohol. 1996;13(6):553–557. doi: 10.1016/s0741-8329(96)00067-5. [DOI] [PubMed] [Google Scholar]
- 30.Choi JWJ, Ford ES, Gao X, et al. Sugar-sweetened soft drinks, diet soft drinks, and serum uric acid level: the Third National Health and Nutrition Examination Survey. Arthritis Rheum. 2008;59(1):109–116. doi: 10.1002/art.23245. [DOI] [PubMed] [Google Scholar]
- 31.Yang Q, Köttgen A, Dehghan A, et al. Multiple genetic loci influence serum urate levels and their relationship with gout and cardiovascular disease risk factors. Circ Cardiovasc Genet. 2010;3(6):523–530. doi: 10.1161/CIRCGENETICS.109.934455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boner G, Rieselbach RE. The effect of glucose upon reabsorptive transport of urate by the kidney. Adv Exp Med Biol. 1974;41:781–787. doi: 10.1007/978-1-4757-1433-3_55. [DOI] [PubMed] [Google Scholar]
- 33.Christensen PJ, Steenstrup OR. Uric acid excretion with increasing plasma glucose concentration (pregnant and non-pregnant cases) Scand J Clin Lab Invest. 1958;10(2):182–185. [PubMed] [Google Scholar]
- 34.Herman JB, Keynan A. Hyperglycemia and uric acid. Isr J Med Sci. 1969;5(5):1048–1052. [PubMed] [Google Scholar]
- 35.Facchini F, Chen YD, Hollenbeck CB, et al. Relationship between resistance to insulin-mediated glucose uptake, urinary uric acid clearance, and plasma uric acid concentration. JAMA. 1991;266(21):3008–3011. [PubMed] [Google Scholar]
- 36.Baker JF, Krishnan E, Chen L, et al. Serum uric acid and cardiovascular disease: recent developments, and where do they leave us? Am J Med. 2005;118(8):816–826. doi: 10.1016/j.amjmed.2005.03.043. [DOI] [PubMed] [Google Scholar]
- 37.Kim SY, Guevara JP, Kim KM, et al. Hyperuricemia and coronary heart disease: a systematic review and meta-analysis. Arthritis Care Res (Hoboken) 2010;62(2):170–180. doi: 10.1002/acr.20065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Panero F, Gruden G, Perotto M, et al. Uric acid is not an independent predictor of cardiovascular mortality in type 2 diabetes: a population-based study. Atherosclerosis. 2012;221(1):183–188. doi: 10.1016/j.atherosclerosis.2011.11.042. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


