Abstract
Glioblastoma multiforme (GBM) is an aggressive brain tumor, fatal within 1 year from diagnosis in most patients despite intensive multimodality therapy. The migratory and microscopically invasive nature of GBM as well as its resistance to chemotherapy renders conventional therapies inadequate in its treatment. Although Mer receptor tyrosine kinase (RTK) inhibition has been shown to decrease the long-term survival and improve the chemo-sensitivity of GBM in vitro, its role in malignant cellular migration has not been previously evaluated. In this study, we report for the first time a role for Mer RTK in brain tumor migration and show that Mer inhibition profoundly impedes GBM migration and alters cellular morphology. Our data demonstrate that Mer RTK inhibition results in altered signaling through focal adhesion kinase (FAK) and RhoA GTPase and a transformation of cytoskeletal organization, suggesting both molecular and structural mechanisms for the abrogation of migration. We also describe a novel and translational method of Mer RTK inhibition using a newly developed monoclonal antibody, providing proof of principle for future evaluation of Mer-targeted translational therapies in the treatment of GBM. Previous findings implicating Mer signaling in glioblastoma survival and chemotherapy resistance coupled with our discovery of the role of Mer RTK in GBM cellular migration support the development of novel Mer-targeted therapies for this devastating disease.
Keywords: MerTK, receptor tyrosine kinase, glioblastoma multiforme, migration, focal adhesion kinase, morphology
Introduction
Glioblastoma multiforme (GBM) is a highly aggressive brain tumor affecting both adults and children. Despite advances in oncologic care, the prognosis of patients with this disease is dismal with an average overall survival of 9–15 months from diagnosis (Souhami et al., 2004; Stupp et al., 2005; Louis et al., 2007). Glioblastoma is highly migratory, microscopically invasive and consistently resistant to chemotherapy rendering conventional therapies inadequate in its treatment. The most recent clinical trial combining surgery and radiotherapy with temozolomide advanced median overall survival from 12 to only 14 months (Stupp et al., 2005, 2009). Death from GBM is most commonly from progressive or recurrent disease at the margins of the resected primary tumor bed (Hochberg and Pruitt, 1980); therefore, impeding cellular migration is essential for therapy and potential cure. Improved understanding of the molecular mechanisms of GBM migration is needed to facilitate development of new therapeutic approaches to treat this disease.
Abnormal activation of the TAM (Tyro-3, Axl and Mer) family of receptor tyrosine kinases (RTKs) is implicated in the oncogenesis of many human cancers (Graham et al., 2006; Linger et al., 2008, 2009). Upregulation of Mer and Axl RTK provides a survival advantage to tumors through anti-apoptotic signaling via the PI3K/Akt and MAPK/Erk pathways (Linger et al., 2008). Clinically, aberrant TAM RTK expression has been correlated with poor prognosis in numerous cancers including GBM, non-small cell lung cancer, breast cancer, pancreatic cancer and leukemia (Rochlitz et al., 1999; Hutterer et al., 2008; Koorstra et al., 2009; Li et al., 2009; Gjerdrum et al., 2010). Coexpression of Mer and Axl in tumors of patients with gastric carcinoma correlates with a poorer prognosis than expression of either RTK alone (Wu et al., 2002). Previous research has demonstrated that aberrant Mer expression in mouse hematopoietic cells increased spontaneous leukemia/lymphoma development in vivo, while Mer inhibition led to increased chemosensitivity of lymphoblasts in vitro and prolonged survival of mice with ALL in vivo (Keating et al., 2006; Linger et al., 2009).
Mer and Axl RTK are important to the pathogenesis of GBM specifically. Axl inhibition decreased glioblas-toma invasiveness and growth in a murine model (Vajkoczy et al., 2006). Clinically, GBM patients with high levels of Axl expression showed decreased time to recurrence/progression, from 9 to 4 months, and a trend toward decreased overall survival (Hutterer et al., 2008). The recent first report of Mer RTK involvement in GBM shows that Mer and Axl RTKs are highly expressed and often co-expressed in astrocytic cell lines and patient tumors (Keating et al., 2010), which may be important clinically given the correlation of TAM RTK coexpression and poor prognosis in gastric carcinoma. Downregulation of Mer or Axl RTK resulted in increased apoptosis, decreased short- and long-term survival, upregulation of autophagy and increased chemosensitivity of GBM in vitro (Keating et al., 2010). Here for the first time, we report a role for Mer in GBM migration. Given the role of migration in GBM tumor progression, understanding the role of Mer in tumor migration is integral to the evaluation of its validity as a therapeutic target, and may increase our understanding of the molecular mechanisms behind the therapeutic resistance of GBM.
Results
Mer RTK inhibition with constitutive shRNA leads to decreased glioblastoma migration
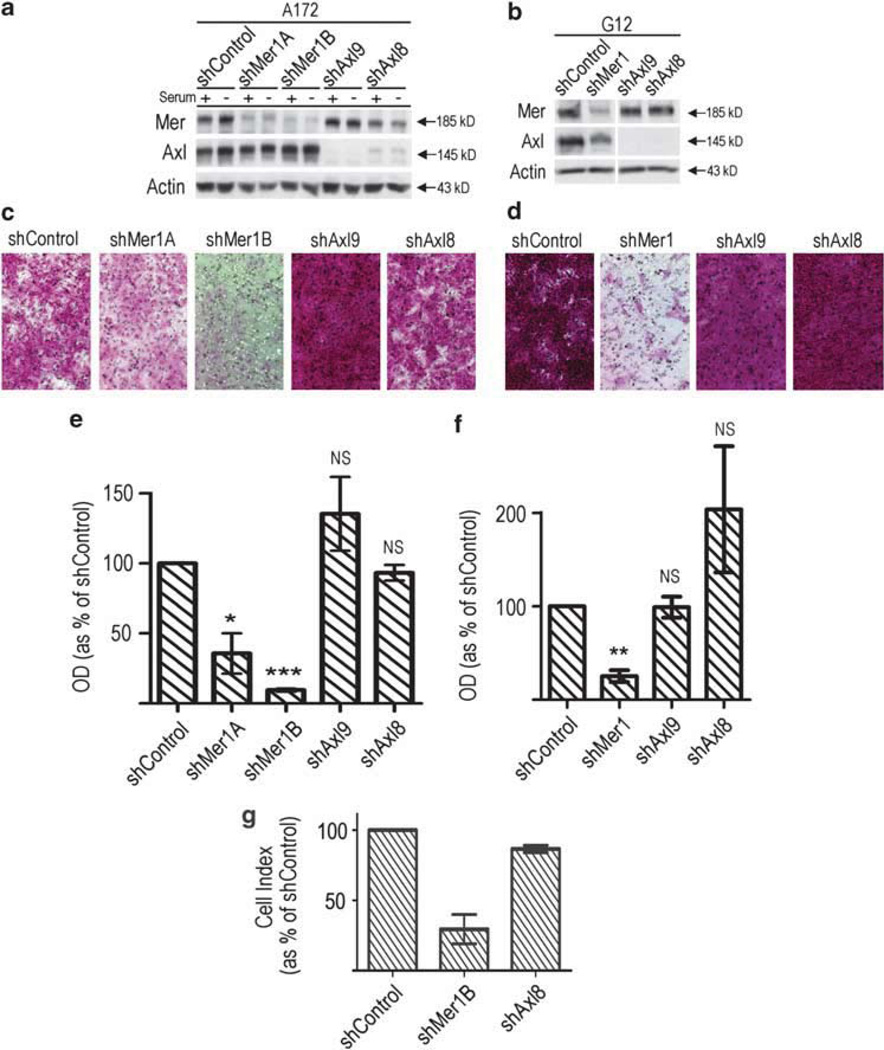
We introduced stable shRNA constructs designed to inhibit the transcription of Mer, Axl and GFP (control) into two astrocytoma cell lines, A172 and G12, as previously described (Keating et al., 2010), and utilized these clonal RTK knockdown lines to evaluate migration. Cells containing shRNA directed against Mer or Axl show effective knockdown of respective RTK protein expression, whether cultured in complete or serum-deprived media (Figures 1a and b).
Figure 1.
Inhibition of Mer RTK impedes glioblastoma cell migration. Immunoblot of Mer and Axl expression in the (a) A172 and (b) G12 glioblastoma cell lines following transduction with constitutively expressed shRNA directed against Mer (shMer1), one of two independent constructs targeting Axl (shAxl9 or shAxl8) or an shRNA targeting GFP (shControl). Two independently isolated clones are shown for the A172 shMer line (shMer 1A and shMer1B). Actin levels are shown as a protein loading control. A172 cells were cultured in complete media (+) and following 3 h serum starvation (−) to illustrate that RTK expression is comparable in all experimental conditions. (c) A172 and (d) G12 glioblastoma control (shControl), Mer-inhibited (shMer1A, shMer1B, shMer1) and Axl-inhibited (shAxl9, shAxl8) cells were plated at equal densities in the upper chamber of a transwell. Cells that successfully migrated through a polycarbonate membrane with 8 µm pores were stained and photographed. Optic density of stained migratory (e) A172 and (f) G12 control and RTK-inhibited cells was measured. Bar graph represents migration relative to normalized shControl. Mean values and standard error were derived from three independent experiments. Means were statistically compared with the shControl with a Student’s paired t-test. P-values for the (e) A172 cell line were as follows, shMer1A = 0.02 (*), shMer1B <0.001 (***), shAxl9 =non-significant (NS), shAxl8 = NS. P-values for the (f) G12 cell line were as follows, shMer1 = 0.0013 (**), shAxl9 = NS, and shAxl8 = NS. (g) A172 glioblastoma control (shControl), Mer-inhibited (shMer1B) and Axl-inhibited (shAxl8) cells were plated at equal densities in the upper chamber of a transwell. Total cumulative number of cells that successfully migrated through a polycarbonate membrane with 8 µm pores was measured every 15min and is represented by a cell index that is normalized to shControl.
Mer inhibition with shRNA led to significantly decreased glioblastoma migration in vitro (Figure 1). Glioblastoma clones with constitutive Mer inhibition of the A172 cell line (shMer1A and shMer1B) and G12 cell line (shMer1) were compared with control cells (shCon-trol) containing shRNA to a non-human protein, GFP, and clones with constitutive Axl inhibition (shAxl9, shAxl8) in transwell assays. Cells were serum deprived for 2–3h and plated in serum-free media (SF media) in uniform densities in the upper chamber of a transwell. Complete media was placed in the lower chamber to create a serum gradient for stimulation of migration. Transwell migration was analyzed by two independent methods that included staining and measurement of optic density at 21 h (Figures 1c – f), and measurement of impedance on a continuous basis throughout the experiment generating a cell index (Figure 1g). There were very consistent outcomes between the two analyses. A172 cells that were Mer inhibited migrated at 10–40% of the shControl (Figures 1c, e and g; shMer1A P = 0.02, shMer1B P <0.001) and migrated poorly throughout the entire experiment when measured continuously by impedance (data not shown). G12 cells that were Mer inhibited migrated at 20% of the shControl (Figures 1d and f; shMer1 P= 0.0013). The degree of Mer inhibition appears to affect migration in a dose-dependent manner with A172 shMer1B cells showing a greater degree of Mer inhibition and a corresponding 90% inhibition of migration as compared with the shMer1A cells that exhibit a lesser degree of knockdown and a 60% decrease in migration. The G12 shMer1 construct shows an 80% decrease in migration correlating with its intermediate degree of Mer knockdown in comparison to the two A172 clones. Interestingly, Axl inhibition with multiple different constructs resulted in similar increased levels of migration, but there were no significant differences when compared with the control in either cell line. There was no migration difference between parental A172 and their respective shControl cells, whereas parental G12 cells showed lower levels of migration compared with shControl cells consistent with their respective levels of Mer expression (data not shown).
The control and experimental cells were also compared in a chemokinesis assay. Cells were plated in a transwell assay without serum deprivation and exposed to complete media for the duration of the experiment. Mer knockdown cells again showed decreased migration in comparison to control (P = 0.0073, Supplementary Figure 1), suggesting that Mer inhibition primarily affects chemokinesis of GBM cells.
The observed differences in migration were not due to differences in viability or proliferation. Viability of the control and experimental cells was evaluated using a flow cytometry-based assay. Cells were prepared under the same conditions used for the transwell assay, with serum deprivation followed by exposure to serum. Mer and Axl knockdown cells had slightly higher rates of apoptosis than control cells following serum deprivation, but only viable cells were plated in the assay, and cells recovered completely once placed in the serum-replete experimental conditions. Final viable cell counts were equivalent among control, Mer knockdown and Axl knockdown cells, at the short experimental time-point of 21 h, indicating that decreased migration is not a result of changes in viable cell number (Supplementary Figure 2).
Mer RTK inhibition with inducible shRNA leads to decreased glioblastoma migration
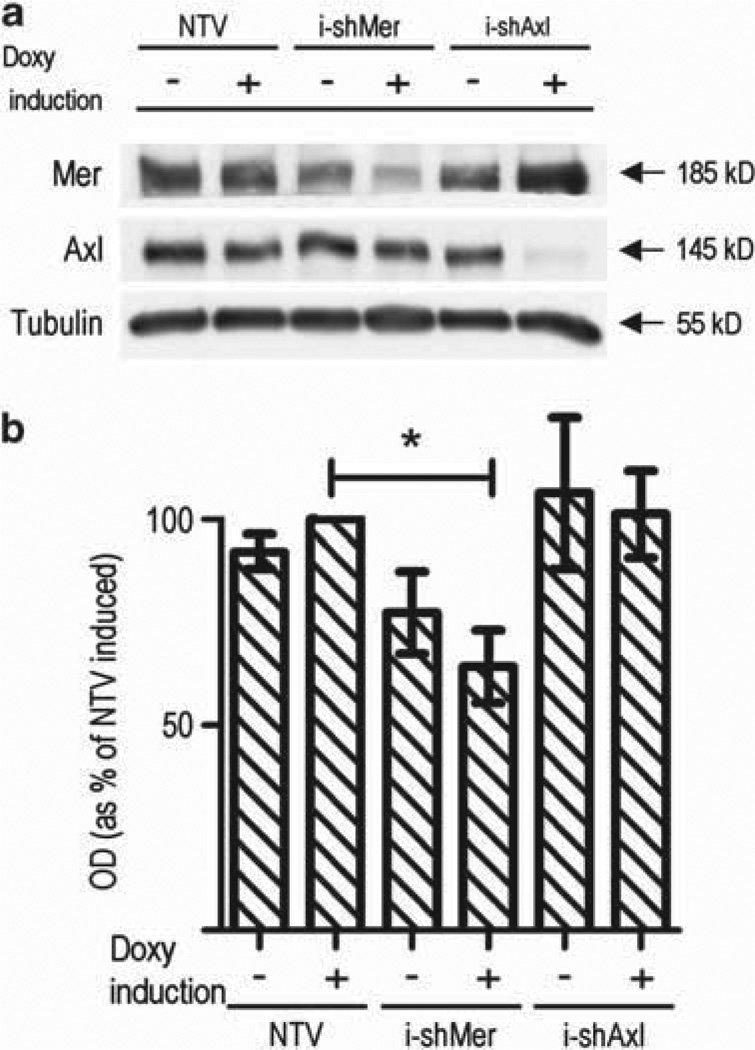
To confirm whether GBM migration was dependent upon Mer signaling, we developed additional Mer and Axl knockdown lines with novel shRNA constructs under the control of a doxycycline-inducible promoter designed to inhibit respective RTK transcription in a controlled manner as well as a unique control using a non-targeting vector (NTV) shRNA in the A172 cell line. Immunoblotting illustrated that there was a small degree of Mer knockdown before doxycycline induction ((−)i-shMer), and that the degree of Mer RTK knockdown following induction ((+)i-shMer) was less than that seen in the constitutive constructs (Figure 2a).
Figure 2.
Inducible Mer RTK inhibition impedes migration. (a) Immunoblot of Mer and Axl expression in the A172 glioblastoma cell line following transduction with doxycycline-inducible shRNA. NTV represents the parental line following introduction of a non-targeting vector. i-shMer and i-shAxl are clones derived from the parental line following introduction of inducible shRNA constructs against Mer and Axl, respectively, shown without (−) and with (+) doxycycline induction. Tubulin is shown as a protein loading control. (b) Uninduced (−) and induced (+) control (NTV), Mer-inhibited (i-shMer) and Axl-inhibited (i-shAxl) cells were plated at equal densities in the upper chamber of a transwell. Cells that successfully migrated through a polycarbonate membrane with 8 µm pores were stained and optic density was measured. Bar graph represents migration relative to normalized induced (+) NTV. Mean values and standard error were derived from three independent experiments. Means were statistically compared with the induced (+) NTV with a Student’s t-test. P-value for induced (+) i-shMer = 0.028 (*), whereas all other statistical comparisons were non-significant.
Using these doxycycline-inducible shRNA cell lines, we confirmed that Mer inhibition significantly decreased glioblastoma migration (Figure 2b). A172 cells with induced Mer inhibition ((+)i-shMer) migrated at 65% of the control ((+)NTV, P = 0.028). Again, Axl inhibition ((+)i-shAxl) did not affect GBM migration. The uninduced shMer line ((−)i-shMer) showed decreased migration, although not to a statistically significant level, consistent with the decreased Mer expression level and a knockdown dose-dependence.
Mer RTK inhibition using monoclonal antibody inhibits GBM migration
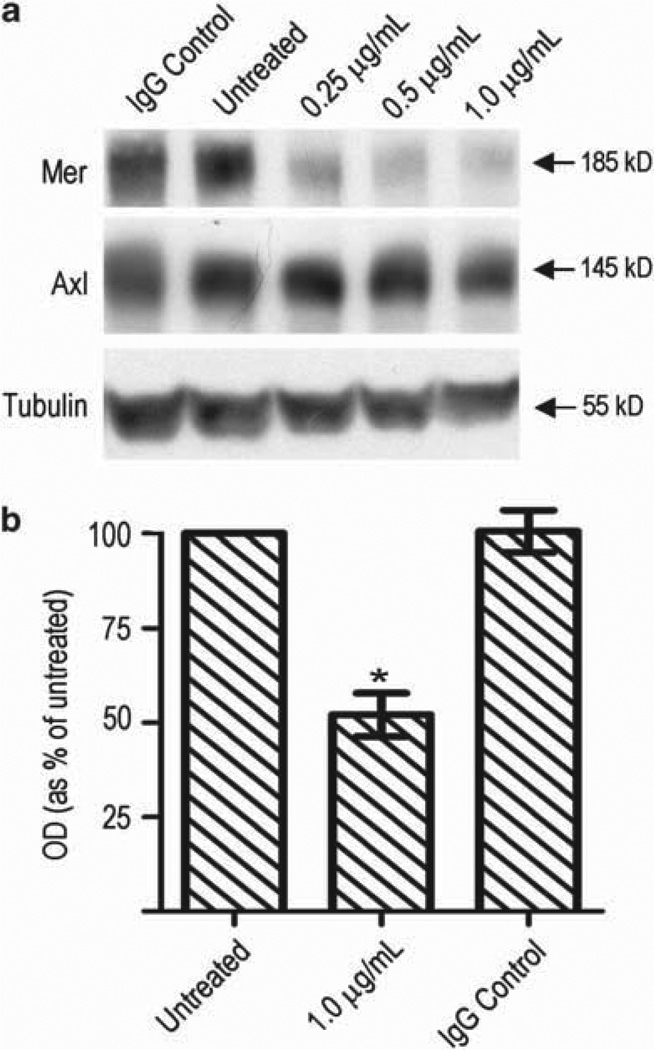
To substantiate our hypothesis that the migratory differences we observed were due to Mer RTK inhibition, we developed a novel monoclonal antibody to human Mer as an independent and translational means to inhibit Mer signaling. A172 cells were treated with monoclonal antibody and Mer expression was analyzed with immunoblot (Figure 3a). Mer knockdown occurred consistently in each independent experiment and occurred at concentrations ranging from 0.05 mg/ml to 2 mg/ml; Figure 3a displays antibody treatments from 0.25 to 1µg/ml. Mer knockdown occurred within 4 h of initial treatment and lasted up to 96 h (data not shown). The degress of Mer knockdown using monoclonal antibody therapy was less than that seen with constitutive shRNA and was comparable with knockdown achieved with inducible shRNA. Monoclonal antibody-mediated RTK inhibition was specific to Mer and was associated with no change in Axl expression.
Figure 3.
Inhibition of Mer RTK with monoclonal antibody impedes migration. (a) Immunoblot of Mer and Axl protein expression following treatment of A172 cells with anti-Mer monoclonal antibody over a range of concentrations or species-specific mouse isotype control IgG1 at 1.0 µg/ml. Tubulin is shown as loading control. Following 72h of no treatment (left), treatment with anti-Mer monoclonal antibody at 1.0 µg/ml (middle) or treatment with mouse IgG (right), cells were plated at equal densities in the upper chamber of a transwell. Cells that successfully migrated through a polycarbonate membrane with 8 mm pores were stained. (b) Optic density of stained migratory cells was measured. Bar graph represents migration relative to untreated cells. Mean values and standard error were derived from three independent experiments. Means were statistically compared with untreated cells with a Student’s paired t-test. P = 0.0141 (*).
To evaluate the effect on migration, A172 parental cells were treated with 1 µg/ml monoclonal antibody for 72 h and then plated in a transwell assay following 2–3 h of serum deprivation. Treatment with monoclonal antibody resulted in a 50% decrease in migration of glioblastoma cells (P = 0.0141) (Figure 3b).
Reintroduction of Mer RTK expression rescues GBM migration
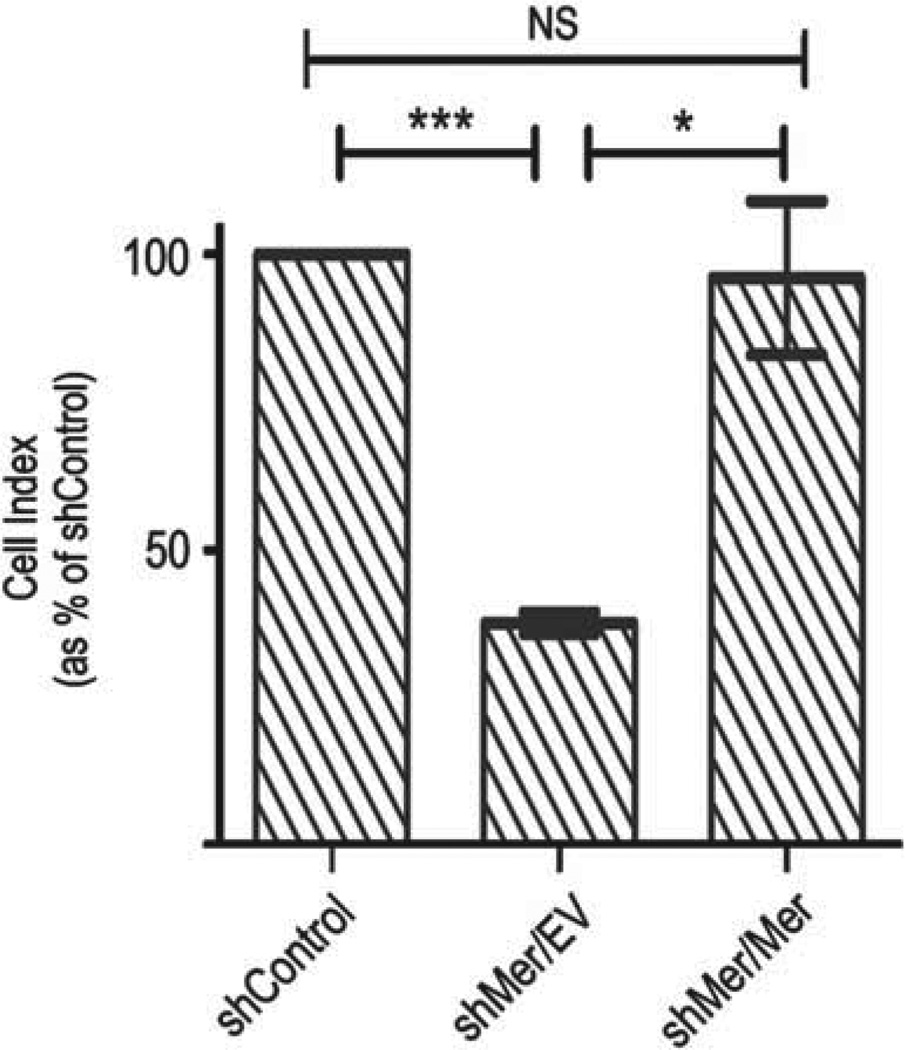
Mer RTK expression was reintroduced into the A172 constitutive shMer knockdown (shMer1B) clone with retroviral vectors containing sequences for Mer RTK driven by a cytomegaloviral promoter. This Mer gene construct lacks the 3’-untranslatated region that is recognized by the shMer, therefore Mer will be expressed in its presence (shMer/Mer cells). As a control, cells were also transduced with an empty vector (shMer/EV cells). Immunoblot of shMer/Mer lysates demonstrated that there is Mer RTK expression following Mer add-back, similar in expression level to that of the parental or shControl cells.
Following reintroduction of Mer RTK expression into Mer knockdown GBM cells there was complete recovery of cellular motility with a return of 96% of migration compared with the GFP (Figure 4).
Figure 4.
Mer add-back rescues glioblastoma migration. (a) Control (shControl), Mer knockdown (shMer/EV) and Mer add-back (shMer/Mer) cells were plated at equal densities in the upper chamber of a transwell. Cells that successfully migrated through a polycarbonate membrane with 8mm pores were measured by changes in impedance. Bar graph represents migration relative to shControl cells. Student’s t-test in comparison to shControl showed a significant difference in migration compared with shMer/EV P = 0.0008 (***), while there was no significant difference when compared with the shMer/Mer.
Mer RTK inhibition causes morphological change in glioblastoma cells
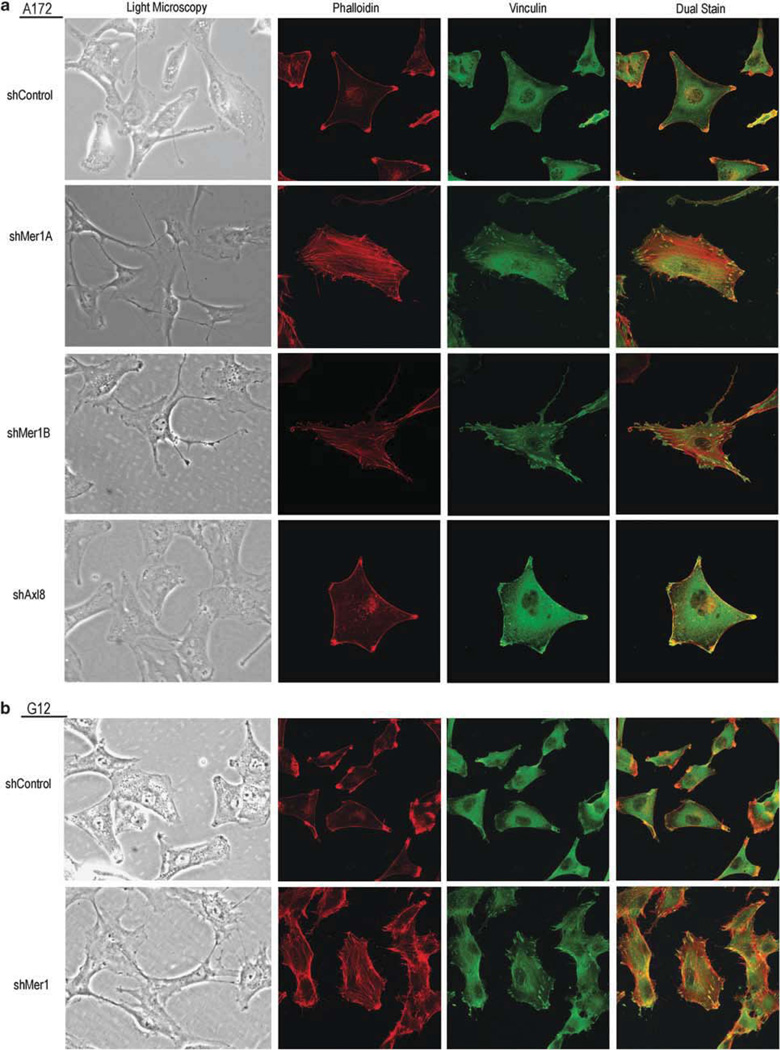
The morphology of A172 (Figure 5a) and G12 (Figure 5b) clonal shControl glioblastoma cells was evaluated and compared with Mer clonal knockdown (A172 shMer1A, A172 shMer1B, G12 Mer1) and Axl clonal knockdown (A172 shAxl8) cells using inverted light microscopy and prominent differences were noted. Control cells exhibited large cell bodies and diffuse granulation with thick axonal projections. Cells with Mer inhibition developed slender cell bodies with focal granulations and thin, tortuous, beaded axonal projections. Similar to control cells, Axl knockdown cells exhibited large cell bodies with thick axonal projections but were more polygonal in shape.
Figure 5.
Mer RTK inhibition alters glioblastoma cellular morphology. (a) A172 shControl, Mer knockdown shMer1A and shMer1B, Axl knockdown shAxl8, and (b) G12 shControl and Mer knockdown shMer1 clonal glioblastoma cells were plated at equal density in complete media, incubated overnight and visualized using light microscopy. Cells were seeded onto glass coverslips at equal densities, formalin fixed, permeabilized and treated with anti-phalloidin primary with rhodamine (red) secondary and anti-vinculin primary with FITC (green) secondary antibodies to stain actin stress fibers and highlight focal adhesions, respectively, and visualized by confocal microscopy. Photomicrographs are representative of three independent experiments.
Cells were stained for actin using rhodamine-tagged phalloidin and for the vinculin component of focal adhesions using anti-vinculin antibody (secondary antibody tagged with FITC). In both A172 (Figure 5a) and G12 (Figure 5b) shControl cells showed polarization with coordinated actin collection in membrane ruffles. Mer-inhibited cells showed diffuse and disorganized stress fiber and focal adhesion formation with considerably more abundant focal adhesions than control cells. Axl knockdown cells had a similar appearance to control cells, with organized membrane ruffles and cellular polarization.
Mer RTK inhibition alters activation and signaling of focal adhesion kinase (FAK) and RhoA
Cellular movement is a dynamic process requiring the coordinated assembly and disassembly of actin structures. Alternation between cellular morphologies requires a complex system of signaling. FAK is a protein tyrosine kinase ubiquitously expressed in the cytoplasm of cells that regulates focal adhesion complexes or the sites of cellular attachment to the extracellular matrix, the turnover of which in part allows the process of movement (Schwock et al., 2010).
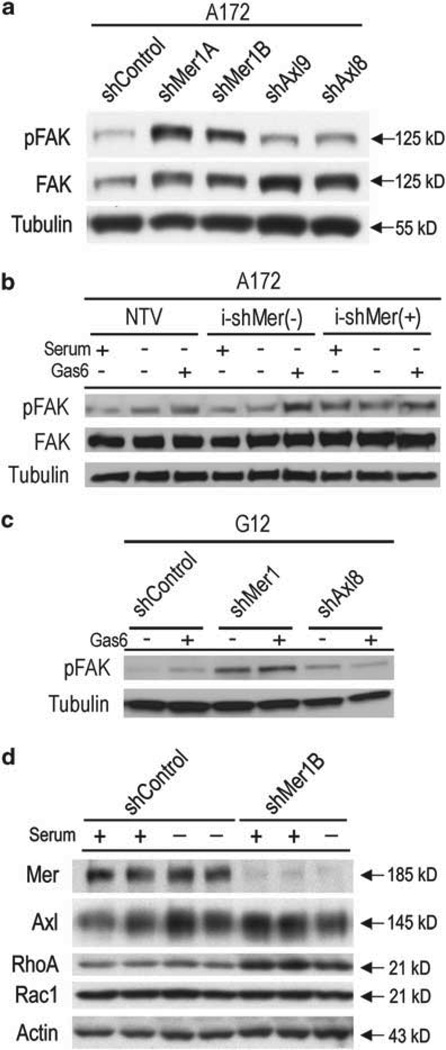
In order to assess Mer RTK involvement in FAK signaling, A172 shControl cells were compared with A172 cells with constitutive Mer inhibition (A172 shMer1A and shMer1B) or constitutive Axl inhibition (A172 shAxl9 and shAxl8). Cells were plated in equal densities, serum starved and stimulated with the activating Mer/Axl RTK ligand, growth arrest specific 6 (Gas6). Mer inhibition led to altered FAK signaling with increased phosphorylation of FAK in comparison to the control or Axl-inhibited cells (Figure 6a). To confirm this dysregulation of FAK activation, we utilized the doxycycline-inducible A172 cell lines, which consist of a NTV control, the uninduced control line (i-shMer(−)) and the line with induced Mer inhibition (i-shMer(+)). Cells were plated in equal densities, serum starved and then treated with serum replete media, left untreated (serum starved) or stimulated with Gas6. Mer inhibition again led to alteration in FAK signaling with increased phosphorylation of FAK in comparison to controls. This FAK dysregulation was present regardless of media treatment (Figure 6b) and was not observed following Axl inhibition (Supplementary Figure 3). Additionally, we tested FAK activation in the primary G12 cell line and again found that whether cells were left untreated or stimulated with Gas6, there was a large increase in FAK phosphorylation (Figure 6c) following Mer inhibition. These data suggest that intact Mer RTK signaling is necessary for appropriate levels of FAK activation, and this altered FAK signaling may be responsible in part for the aberrant migration of Mer-inhibited GBM cells.
Figure 6.
Mer RTK alters FAK activation and Rho migratory signaling. (a) Immunoblot of phospho-FAK and FAK expression in the A172 glioblastoma cell line following transduction with constitutively expressed shRNA. shControl represents the parental line following introduction of shRNA against GFP. shMer1A and shMer1B are clones derived from the parental line following introduction of an shRNA construct against Mer. shAxl9 and shAxl8are clones derived from the parental line following introduction of two independent constructs against Axl. Tubulin is shown as a protein loading control. Cells were serum starved for 2 h and stimulated with 200 nM Gas6 for 10 min. (b) Immunoblot of phospho-FAK and FAK expression in theA172 glioblastoma cell line following introduction of a doxycycline-inducible shRNA against a non-targeting vector (NTV) or Mer (i-shMer). i-shMer(−) represents the clone was not treated with doxycycline (Mer expression intact), while i-shMer(+) was doxycycline treated (Mer expression inhibited). Cells were serum starved for 2h then treated with complete media replacement (Serum +), left untreated (Serum−, Gas6−) or treated with 200 nM Gas6 for 10 min (Serum −, Gas6 +). Tubulin is shown as a protein loading control. (c) Immunoblot of phospho-FAK and FAK expression in the G12 glioblastoma primary cell line following transduction with constitutively expressed shRNA. shControl represents the parental line following introduction of shRNA against GFP. shMer1 and shAxl8 are clones derived from the parental line following introduction of an shRNA construct against Mer or Axl, respectively. Cells were serum starved for 2h then left untreated (Gas6−) or treated with 200 nM Gas6 for 10 min (Gas6 +). Tubulin is shown as a protein loading control. (d) Immunoblot of Mer, Axl, RhoA and Rac1 expression in the A172 control (shControl) and Mer knockdown (shMer1B) clones. Actin is shown as loading control. Cells are shown in the presence of complete media and after a 3 h serum deprivation. Data presented is representative of three independent experiments.
Actin assembly for cellular movement is in part controlled by the Rho family of GTPases, namely Rac1 and RhoA. We evaluated total levels of Rac and Rho in our GBM cell lines and found that in the Mer knockdown state, total levels of RhoA were elevated in both the serum replete and serum starved states (Figure 6d). There was no difference in Rac1 levels between the control and Mer knockdown glioblastoma cells.
Discussion
Our laboratory previously established that Mer inhibition results in increased chemosensitivity and decreased survival of GBM cells in vitro (Keating et al., 2010). The current study represents the first report of Mer inhibition impeding GBM migration and altering GBM morphology. In this study, we utilized multiple shRNA constructs and a novel anti-Mer monoclonal antibody to minimize off-target effects and focus specifically on Mer RTK downregulation; in all cases, we discovered that Mer inhibition of GBM cells led to decreased in vitro migration. The importance of migration to GBM survival is evidenced by the predilection of GBM to recur at the leading edge of tumor and at therapeutic margins. This characteristic suggests that research into inhibitors of cancer cell migration is critical for development of curative therapy. In this manuscript, we introduce the first translational Mer inhibitor as a potential therapeutic for GBM. Though it is unknown at this time whether a Mer-directed monoclonal antibody can reach the CNS, this data provides the proof of principle for translational agents currently in development with ability to penetrate the blood brain barrier.
We found that a threshold level of Mer is necessary to maintain normal migration of GBM cells in vitro. Cells with moderate levels of Mer inhibition showed moderate decreases in migration, while cells with profound Mer inhibition demonstrated very minimal ability to migrate. Interestingly, although Axl has been shown to affect GBM survival and chemosensitivity (Keating et al., 2010), Axl inhibition did not significantly affect glioblastoma migration when explored with multiple shRNA constructs in two different cell lines. Another study has shown that Axl inhibition can decrease GBM migration in vitro and invasiveness in vivo; however, this report did not investigate the effects of Mer expression or inhibition. Additionally the method used to inhibit Axl, introduction of a dominant negative kinase, could have inadvertently led to decreased activity of Mer RTK signaling as well (Vajkoczy et al., 2006).
We evaluated the viability and proliferation rates of Mer and Axl knockdown cells during the short duration of the migration study and showed that although Mer and Axl inhibition led to higher apoptotic rates with serum deprivation, only viable cells were plated and cell counts following exposure to a serum gradient were equal. Contrary to expectation, the control cells did not have increased proliferation relative to the Mer and Axl knockdown cells in this short-term assay. The significant difference in migration observed is far greater than would be expected due to minor variances in apoptosis occurring over the short serum-deprivation period. In addition, we have previously shown that Axl or Mer inhibition increases apoptosis and decreases survival of GBM, more significantly following Axl knockdown. As such, the lack of migratory difference following Axl inhibition serves as a relative control against viability or proliferative confounders.
These findings strongly suggest that Mer RTK signaling is necessary for normal migration of GBM cells. Our studies demonstrate that with inhibition of Mer RTK expression, we can significantly impede GBM cellular migration, and that we can fully rescue the migratory phenotype with the reintroduction of Mer expression.
Cellular movement is a dynamic process requiring the coordinated assembly and disassembly of actin structures. Cells migrate by alternating between the formation of membrane ruffles, or lamellipodia, at the leading edge of a cell, and the formation of stress fibers and focal adhesions at the rear of a cell (Salhia et al., 2005). Alternation between these very different cellular morphologies requires a complex system of signaling.
FAK is a protein tyrosine kinase ubiquitously expressed in the cytoplasm of cells that regulates focal adhesion complexes or the sites of cellular attachment to the extracellular matrix (Schwock et al., 2010). Cells in motion must alternate between attachment to and detachment from the extracellular matrix. It is the turnover, or cyclic assembly and disassembly, of focal adhesions at the leading and rear ends of a cell, which in part allows the process of movement. It has been suggested that FAK signaling may lead to both the formation of focal adhesions and to the turnover of these contact points (Schlaepfer and Mitra, 2004). Alteration in FAK signaling can lead to alterations in the rate of disassembly and turnover of focal adhesions thereby affecting cellular movement (Goldberg and Kloog, 2006).
FAK also serves as a signaling hub and a regulator of the Rho-family of GTPases (Schwock et al., 2010). GBM has been shown to have elevated levels of active Ras, a signaling gateway GTPase involved in migration, cytoskeletal rearrangement and cell survival, and whose properties also include the ability to regulate the Rac and Rho GTPases (Goldberg and Kloog, 2006). Signaling via the Rho GTPases regulates cellular morphology thereby affecting cellular processes dependent on the plasticity of cellular architecture, namely cellular migration. Rac1 activation results in the formation of membrane ruffling inducing membrane protrusion, whereas RhoA activation effects the assembly of stress fibers and focal adhesions inducing contractility (Goldberg and Kloog, 2006). These proteins often act antagonistically with the activation of one leading to inhibition of the other and thereby the alternating actin organizations controlled by each protein (Ridley et al., 2003). Along with other signaling pathways, the interplay between Rac1 and RhoA signaling allows the alternating cellular phenotypes involved in cellular movement.
Any process that interferes with the balance between opposing signals can paralyze movement. It has been shown that Ras inhibition leads to decreased GBM cell migration and anchorage-independent growth in vitro via alteration in cellular morphology (Goldberg and Kloog, 2006). Treatment of GBM cell lines with a Ras inhibitor led to decrease in Rac1 and increase in RhoA levels thereby shifting the actin cytoskeleton toward formation of stress fibers and focal adhesions with resultant decrease in migration (Goldberg and Kloog, 2006). In addition to increased numbers of stress fibers and focal adhesions, treatment with the Ras inhibitor also led to decreased phosphorylation of FAK, suggesting decreased disassembly or turnover of these structures (Goldberg and Kloog, 2006). In contrast, a different study showed that FAK signaling led to increased levels of activated RhoA (Schlaepfer and Mitra, 2004). FAK-null fibroblasts have been shown to have decreased motility in response to motility stimuli due to increased formation of focal contacts associated with increased Rho activity (Schlaepfer and Mitra, 2004). In summary, the signaling behind cellular motility is highly complex and interconnected.
Our findings suggest that Mer RTK-mediated abrogation of migration and alteration of cellular morphology involves altered signaling through FAK and RhoA. In this study, we found that when Mer is inhibited, total levels of RhoA are elevated. Elevated levels of RhoA lead to stress fiber and focal adhesion formation that in turn leads to cellular attachment to the extracellular matrix. This attachment likely results in cellular fixation thereby contributing to the immotility we have observed with Mer inhibition.
In order to assess Mer RTK involvement in FAK signaling, shControl cells with maintained Mer RTK signaling were compared with GBM cells with constitutive Mer and Axl inhibition. Mer inhibition led to alteration in FAK signaling with increased phosphor-ylation of FAK in comparison to control or Axl-inhibited cells. Mer RTK has multiple physiological functions in normal cells; most relevant to this study is its role in phagocytosis. Phagocytosis is a process dependent on cellular movement, and Mer RTK is necessary for phagocytic activity with cellular engulf-ment of apoptotic cells. Animals lacking Mer expression have inability to clear apoptotic cells and develop abnormalities including retinal blindness and systemic autoimmune disease (Scott et al., 2001). It has been previously shown that in the normal process of cellular phagocytosis, Mer activation leads to increased phos-phorylation of FAK and thereby activation of Rac1 in phagocytes (Wu et al., 2005). Given the integral role of Mer RTK in phagocyte function and motility, it is not surprising that Mer inhibition would lead to decreased cellular movement as we have shown in GBM. It is interesting, however, that Mer inhibition led to increased phosphorylation of FAK in GBM where the opposite is found in phagocytes. The increased FAK activation either represents an attempted compensatory mechanism for the lack of movement in Mer knockdown GBM cells or it represents a dysregulation of migratory signaling such that excess phosphorylation of FAK leads to a shift in the balance of migratory signals needed for movement.
Our findings of differences in cellular morphology correlate with the expected cellular repercussions of the altered FAK and RhoA signaling observed with Mer RTK inhibition. Control and Axl-inhibited cells exhibit large cell bodies and diffuse granulation with thick axonal projections, while cells with Mer inhibition develop slender cell bodies with focal granulations and thin, tortuous, beaded axonal projections. Additionally, we detected that shControl and Axl knockdown cells show directed polarization with coordinated actin collection in membrane ruffles, exhibiting the cellular morphology of active movement. Both A172 and G12 glioblastoma cell lines with Mer inhibition, however, show diffuse and disorganized stress fiber and focal adhesion formation with significantly higher number of focal adhesions than control cells corresponding to the increased cellular RhoA and altered FAK signaling observed. The increase in focal adhesion formation may result in cellular fixation and thereby the decreased migration observed with Mer inhibition.
Inhibition of Mer RTK resulted in decreased survival and increased chemosensitivity of glioblastoma in vitro. This is the first study to show that its inhibition also impedes glioblastoma migration and alters cellular morphology, targeting an important molecular mechanism of GBM resistance to therapy. These data support the need for further research into Mer RTK inhibition as a novel treatment strategy for GBM.
Materials and methods
Cell lines
The A172 cell line was obtained from American Type Culture Collection (Manassas, VA, USA) and maintained per culture guidelines. The G12 cell line was derived from a GBM patient sample as previously described (Donson et al., 1999).
Production of shRNA clones
Constitutive shRNA cell lines (A172 shMer1A, shMer1B, shAxl9, shAxl8, shGFP and G12 shMer1, shAxl9, shAxl8, shGFP) were created as previously described (Keating et al., 2010). Inducible shRNA cell lines (NTV, i-shMer and i-shAxl) were created using lentiviral vectors containing doxycycline-inducible shRNA sequences targeting Mer (shMerA, oligo ID: V2THS_1645), Axl (shAxlA, oligo ID: V2THS_234991) or non-silencing control vector (NTV, #RHS4743) obtained from Open Biosystems (Huntsville, AL, USA). Stable clonal lines were developed from heterogeneous RTK knockdown populations by single-cell flow cytometry sorting and selected for inducible decrease in Mer or Axl expression. shRNA induction was done with doxycycline treatment at 1:2000 for 72h before experimentation. Mer add-back cell lines (shMer/Mer, shMer/EV) were created using retroviral vectors (pLNCX2) (Clontech, Mountain View, CA, USA) containing sequences for MerTK (pLNCX2-Mer) and GFP (pLNCX2-GFP) driven by the cytomegaloviral promoter. This MerTK lacks the 3’-untran-slatated region, which is the binding site for the shMer RNA, and therefore is resistant to Mer inhibition by shRNA. Replication incompetent viral particles were generated by transfecting GP2–293 cells (Clontech), which stably express the viral gag and pol genes, with pLNCX2, pLNCX2-Mer or pLNCX2-GFP. The retroviral expression vectors were co-transfected with VSV-G plasmid (Clontech) that expresses a cytomegaloviral-driven pantropic envelope sequence. Viral particles were transduced into shMer1B cells using Turbofect (Fermentas, Glen Burnie, MD, USA), and stably transduced cells were selected using 400 mg/ml hygromycin.
Monoclonal antibody development and treatment
Balb/C mice were immunized with recombinant human Mer extracellular domain/Fc chimera produced in CHO cells. B cells from high anti-Mer/Fc titer mice were fused to FoxNY mouse myeloma cells. Hybridoma clones secreting antibodies positive for interaction with Mer/Fc and negative for human-Fc interaction by enzyme-linked immunosorbent assay were selected for screening. Flow cytometry was used to define clones secreting antibodies with strong binding to cells with known Mer expression and lack of interaction with Mer non-expressing cells. Mer binding specificity was confirmed by loss of cell antibody labeling following shRNA-mediated Mer knockdown. Two hybridomas were expanded, and secreted antibody was purified by Protein A Sepharose chromatography. Parental A172 cells were treated with monoclonal antibody at the concentrations noted or with isotype control mouse IgG1 at 1.0 mg/ml (R&D Systems, Minneapolis, MN, USA) for 72h before migratory experimentation set-up.
Immunoblotting of Mer/Axl RTK and downstream migratory signals
Whole cell protein lysates were prepared as previously described (Keating et al., 2010), resolved by SDS–polyacryla-mide gel electrophoresis and transferred onto polyvinylidene difluoride membranes. Immunoblots were probed with the following antibodies at concentrations recommended by the manufacturers’ protocols: human Mer 348 (Caveo Therapeutics, Aurora, CO, USA); human Axl (R&D Systems); phospho-FAK (Tyr397), FAK and tubulin rabbit monoclonal (Cell Signaling Technology, Danvers, MA, USA); RhoAand actin goat polyclonal (Santa Cruz Biotechnology, Santa Cruz, CA, USA); RAC1 (Millipore, Billerica, MA, USA). Proteins were detected with horseradish peroxidase chemiluminescence (PerkinElmer, Waltham, MA, USA). Immunoblots are representative of a minimum of three independent experiments.
Preparation of cells for migration and viability assays
Cells were plated in tissue culture plates and grown to 60–90% confluence. Cells were lifted using EDTA into complete media, centrifuged (1200 rpm for 5min), supernatants were removed and cells were resuspended in SF media or SF media with doxycycline (inducible lines). Cells were again centrifuged, supernatant was removed and cells were resuspended in SF media, SF media with antibody (antibody-treated cells) or SF media with doxycycline (inducible lines). Cells were serum deprived for 2–3 h at room temperature in a conical tube during which time they were resuspended every 30–60 min.
Functional assays of migration
A cell suspension containing 1.0–1.3 million viable A172 cells/ml or 1.3–1.5 million viable G12 cells/ml was prepared as described and then plated in transwells containing an inter-chamber polycarbonate membrane with 8 µm pores (Cytose-lect Migration Assay, Cell Biolabs Inc., San Diego, CA, USA). Each transwell was placed into 500 µl of complete media with antibody or doxycycline as appropriate. 300 µl of cell suspension was gently dripped into the inside of each insert. Transwells were incubated at 37 °C and 5% CO2 for 20–22 h (A172) or 48 h (G12). Following incubation, non-migratory cells remaining in the upper chamber were wiped off and migratory cells in the lower chamber were stained per product specifications, photographed using an Olympus CKX41 Inverted Microscope (Olympus America, Center Valley, PA, USA) and images were collected using Q Capture Pro Software (Q Imaging, Surrey, BC, Canada). Cell dye was extracted using the proprietary Extraction Solution and the optic density (560 nm) was measured.
xCELLigence migration assay
Migration was assessed using the xCELLigence Real-Time Cell Analysis (RTCA) system (Roche Diagnostics, Indianapolis, IN, USA, in partnership with ACEA Biosciences Inc., San Diego, CA, USA) with Cell Invasion and Migration Plates 16 (CIM-plates-16) (Roche Diagnostics). This analysis utilizes transwells in which the bottom surface of the 8 µm filter is lined with electrodes. Following cellular migration and adherence to the bottom surface of the filter there is an increase in measured impedance (Z) across these electrodes that generates a cell index that correlates to total cell number (Chung et al., 2011; Steinle et al., 2011). The upper and lower chambers of the transwells were loaded with 30 µl of SF Dulbecco’s modified Eagle’s medium and 162 µl of Dulbecco’s modified Eagle’s medium containing 10% FBS, respectively, and the assembled plate allowed to equilibrate at 37 °C in 5% CO2 for 1 h. Cell suspensions containing 7.5 × 105 viable cells/ml were prepared as described in SF Dulbecco’s modified Eagle’s medium. Background impedance was measured and 100 µl of cell suspension was added to the upper chamber of each well. Migration was quantified and recorded every 15 min for 20 h.
Viability assessment
Cells were plated at 390 000 cells per well based on the viable cell count following serum deprivation (the equivalent of the above described 300 µl aliquot of a 1.3 million/ml cell solution) into 12 well plates in duplicate in complete media. Cells were incubated for 20–21 h, rinsed with PBS and lifted with trypsin. Cell suspensions were centrifuged, supernatants were removed and cells were resuspended. Viable cells were counted using ViaCount. Gating was performed to account for viable, early apoptotic and late apoptotic/dead cell populations. All data described is representative of a minimum of three independent experiments.
Migratory signaling analyses
Cells were plated at equal density in tissue culture plates in complete media and allowed to adhere overnight. Complete media was removed, cells were washed with PBS and cells were incubated in SF media for 2h. Total RhoA and Rac1 levels were analyzed by immunoblot of lysates from this timepoint, while P-FAK and FAK analysis was performed following these same conditions, treatment with serum replete media (10% FBS) or Gas6 at 200nM for 10min as previously described.
Light microscopy
Cells were plated into six-well plates at densities of 40000–60000 cells per well and allowed to incubate overnight in complete media. Cells were photographed using an Olympus CKX41 Inverted Microscope and images were collected using Q Capture Pro Software.
Phalloidin/vinculin staining and confocal microscopy
Cells were seeded onto glass coverslips in 12-well plates at densities of 20000–60000 cells per well and allowed to incubate overnight in complete media. Cells were fixed with 10% formalin for 10min, permeabilized with 0.1% Triton X-100 (Sigma Chemical Co., St Louis, MO, USA) in PBS for 5min and blocked with 1 × (0.4µg/ml) human IgG1/PBS (Sigma-Aldrich, St Louis, MO, USA) for 30 min. Coverslips were incubated for 1 h with mouse monoclonal Anti-Vinculin Antibody (Sigma-Aldrich) diluted 1:100 in 1 × hIgG/PBS, washed and incubated for 30 min with Donkey Anti-Mouse-FITC (1:100, Jackson ImmunoResearch, West Grove, PA, USA) and with Rhodamine Phalloidin (1:40, Molecular Probes, Eugene, OR, USA) in 1 × hIgG/PBS. After washing, coverslips were mounted with ProLong Gold Antifade Reagent (Molecular Probes) and imaged using an Olympus FluoView FV-1000 FCS/RICS inverted confocal laser scanning microscope and Olympus Fluoview Ver. 2.0b Viewer software.
Statistical analyses
Averages were compared using a Student’s paired t-test and analyzed with Prism software (v.5. 03, GraphPad Software).
Supplementary Material
Acknowledgements
We thank Rachel Linger, PhD for her expertise and insight into this project. We thank Jennifer Schlegel for production of the Mer add-back vector, the Advanced Light Microscopy Core Facility at the University of Colorado School of Medicine for their technical support in microscopy, and the University of Colorado Cancer Center Tissue Culture Core for their technical support in monoclonal antibody development. DKG is supported by NIH R01 1CA137078. AKK is generously supported by the St Baldrick’s Foundation and the NIH K12 HD068372Child Health Research Career Development Award.
Footnotes
Conflict of interest
The authors declare no conflicts of interest.
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc)
References
- Chung H, Suh EK, Han IO, Oh ES. Keratinocyte-derived laminin-332 promotes adhesion and migration in melanocytes and melanoma. J Biol Chem. 2011;286:13438–13447. doi: 10.1074/jbc.M110.166751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donson AM, Weil MD, Foreman NK. Tamoxifen radiosensitization in human glioblastoma cell lines. J Neurosurg. 1999;90:533–536. doi: 10.3171/jns.1999.90.3.0533. [DOI] [PubMed] [Google Scholar]
- Gjerdrum C, Tiron C, Hoiby T, Stefansson I, Haugen H, Sandal T, et al. Axl is an essential epithelial-to-mesenchymal transition-induced regulator of breast cancer metastasis and patient survival. Proc Natl Acad Sci USA. 2010;107:1124–1129. doi: 10.1073/pnas.0909333107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg L, Kloog Y. A Ras inhibitor tilts the balance between Rac and Rho and blocks phosphatidylinositol 3-kinase-dependent glioblastoma cell migration. Cancer Res. 2006;66:11709–11717. doi: 10.1158/0008-5472.CAN-06-1878. [DOI] [PubMed] [Google Scholar]
- Graham DK, Salzberg DB, Kurtzberg J, Sather S, Matsushima GK, Keating AK, et al. Ectopic expression of the proto-oncogene Mer in pediatric T-cell acute lymphoblastic leukemia. Clin Cancer Res. 2006;12:2662–2669. doi: 10.1158/1078-0432.CCR-05-2208. [DOI] [PubMed] [Google Scholar]
- Hochberg FH, Pruitt A. Assumptions in the radiotherapy of glioblastoma. Neurology. 1980;30:907–911. doi: 10.1212/wnl.30.9.907. [DOI] [PubMed] [Google Scholar]
- Hutterer M, Knyazev P, Abate A, Reschke M, Maier H, Stefanova N, et al. Axl and growth arrest-specific gene 6 are frequently overexpressed in human gliomas and predict poor prognosis in patients with glioblastoma multiforme. Clin Cancer Res. 2008;14:130–138. doi: 10.1158/1078-0432.CCR-07-0862. [DOI] [PubMed] [Google Scholar]
- Keating AK, Kim GK, Jones AE, Donson AM, Ware K, Mulcahy JM, et al. Inhibition of Mer and Axl receptor tyrosine kinases in astrocytoma cells leads to increased apoptosis and improved chemosensitivity. Mol Cancer Ther. 2010;9:1298–1307. doi: 10.1158/1535-7163.MCT-09-0707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating AK, Salzberg DB, Sather S, Liang X, Nickoloff S, Anwar A, et al. Lymphoblastic leukemia/lymphoma in mice over-expressing the Mer (MerTK) receptor tyrosine kinase. Oncogene. 2006;25:6092–6100. doi: 10.1038/sj.onc.1209633. [DOI] [PubMed] [Google Scholar]
- Koorstra JB, Karikari CA, Feldmann G, Bisht S, Rojas PL, Offerhaus GJ, et al. The Axl receptor tyrosine kinase confers an adverse prognostic influence in pancreatic cancer and represents a new therapeutic target. Cancer Biol Ther. 2009;8:618–626. doi: 10.4161/cbt.8.7.7923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Ye X, Tan C, Hongo JA, Zha J, Liu J, et al. Axl as a potential therapeutic target in cancer: role of Axl in tumor growth, metastasis and angiogenesis. Oncogene. 2009;28:3442–3455. doi: 10.1038/onc.2009.212. [DOI] [PubMed] [Google Scholar]
- Linger RM, DeRyckere D, Brandao L, Sawczyn KK, Jacobsen KM, Liang X, et al. Mer receptor tyrosine kinase is a novel therapeutic target in pediatric B-cell acute lymphoblastic leukemia. Blood. 2009;114:2678–2687. doi: 10.1182/blood-2009-03-209247. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Linger RM, Keating AK, Earp HS, Graham DK. TAM receptor tyrosine kinases: biologic functions, signaling, and potential therapeutic targeting in human cancer. Adv Cancer Res. 2008;100:35–83. doi: 10.1016/S0065-230X(08)00002-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, et al. Cell migration: integrating signals from front to back. Science. 2003;302:1704–1709. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- Rochlitz C, Lohri A, Bacchi M, Schmidt M, Nagel S, Fopp M, et al. Axl expression is associated with adverse prognosis and with expression of Bcl-2 and CD34 in de novo acute myeloid leukemia (AML): results from a multicenter trial of the Swiss Group for Clinical Cancer Research (SAKK) Leukemia. 1999;13:1352–1358. doi: 10.1038/sj.leu.2401484. [DOI] [PubMed] [Google Scholar]
- Salhia B, Rutten F, Nakada M, Beaudry C, Berens M, Kwan A, et al. Inhibition of Rho-kinase affects astrocytoma morphology, motility, and invasion through activation of Rac1. Cancer Res. 2005;65:8792–8800. doi: 10.1158/0008-5472.CAN-05-0160. [DOI] [PubMed] [Google Scholar]
- Schlaepfer DD, Mitra SK. Multiple connections link FAK to cell motility and invasion. Curr Opin Genet Dev. 2004;14:92–101. doi: 10.1016/j.gde.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Schwock J, Dhani N, Hedley DW. Targeting focal adhesion kinase signaling in tumor growth and metastasis. Expert Opin Ther Targets. 2010;14:77–94. doi: 10.1517/14728220903460340. [DOI] [PubMed] [Google Scholar]
- Scott RS, McMahon EJ, Pop SM, Reap EA, Caricchio R, Cohen PL, et al. Phagocytosis and clearance of apoptotic cells is mediated by MER. Nature. 2001;411:207–211. doi: 10.1038/35075603. [DOI] [PubMed] [Google Scholar]
- Souhami L, Seiferheld W, Brachman D, Podgorsak EB, Werner-Wasik M, Lustig R, et al. Randomized comparison of stereotactic radiosurgery followed by conventional radiotherapy with carmustine to conventional radiotherapy with carmustine for patients with glioblastoma multiforme: report of Radiation Therapy Oncology Group 93-05 protocol. Int J Radiat Oncol Biol Phys. 2004;60:853–860. doi: 10.1016/j.ijrobp.2004.04.011. [DOI] [PubMed] [Google Scholar]
- Steinle M, Palme D, Misovic M, Rudner J, Dittmann K, Lukowski R, et al. Ionizing radiation induces migration of glioblastoma cells by activating BK K(+) channels. Radiother Oncol. 2011;101:122–126. doi: 10.1016/j.radonc.2011.05.069. [DOI] [PubMed] [Google Scholar]
- Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- Vajkoczy P, Knyazev P, Kunkel A, Capelle HH, Behrndt S, von Tengg-Kobligk H, et al. Dominant-negative inhibition of the Axl receptor tyrosine kinase suppresses brain tumor cell growth and invasion and prolongs survival. Proc Natl Acad Sci USA. 2006;103:5799–5804. doi: 10.1073/pnas.0510923103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CW, Li AF, Chi CW, Lai CH, Huang CL, Lo SS, et al. Clinical significance of AXL kinase family in gastric cancer. Anticancer Res. 2002;22:1071–1078. [PubMed] [Google Scholar]
- Wu Y, Singh S, Georgescu MM, Birge RB. A role for Mer tyrosine kinase in alphavbeta5 integrin-mediated phagocytosis of apoptotic cells. J Cell Sci. 2005;118:539–553. doi: 10.1242/jcs.01632. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.