Abstract
Macrophages encounter flaviviruses early after injection by arthropod vectors. Using in vivo imaging of mice inoculated with firefly luciferase-expressing single-cycle flavivirus particles (FLUC-SCFV), we examined the initial dissemination of virus particles in the presence or absence of lymph node (LN) -resident macrophages. Higher luciferase activity, indicating higher SCFV gene expression, was detected in the footpad of macrophage-depleted mice after 24 hours post infection (hpi). Moreover, FLUC-SCFV particles disseminated to the spleen within 14 hpi in macrophage-depleted, but not control mice. Although macrophages presented SCFV to naïve T cells in vitro, depletion of subcapsular sinus (SCS) macrophages did not alter the magnitude or effector function of the WNV-specific CD8+ T cell response. Together, these results indicate that SCS macrophages play a role in limiting the dissemination of SCFV early in infection but are not required for the generation of a polyfunctional WNV-specific CD8+ T cell response in the draining LN.
Keywords: West Nile virus, single-cycle flavivirus, RepliVAX, subcapsular sinus macrophages, CD8+ T cell
Introduction
West Nile virus (WNV) is a member of the Flavivirus genus of the family Flaviviridae. Flaviviruses have an 11 kb single-stranded, positive sense RNA genome with a single open reading frame encoding three structural proteins (C, prM/M and E) and seven non-structural proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B and NS5) (Lindenbach et al., 2007). WNV exists in nature in an enzootic life cycle involving transmission between mosquitoes and birds. Transmission to mammals including humans occurs by the feeding of infected mosquitoes and does not result in further transmission. Infection of humans results in disease ranging from asymptomatic to neurological including the development of meningitis, encephalitis, and occasionally mortality (Campbell et al., 2002).
Recently, we reported the development of single-cycle flaviviruses (SCFV) that can be utilized as safe and effective vaccines (Mason et al., 2006). This single-cycle technology has been used to produce a series of flavivirus vaccine candidates (named RepliVAX), that encode flavivirus genomes harboring a truncated C (trC) gene that prevents the RepliVAX genome from being packaged into infectious particles unless the C gene is supplied in trans (Ishikawa et al., 2008; Mason et al., 2006; Suzuki et al., 2009; Widman et al., 2008b). The RepliVAX WN particle can initiate a single round of infection in normal cells. These infected cells release prM/E-containing sub-viral particles (SVPs) and the nonstructural protein NS1 that have been shown to induce effective antiviral immune responses (Chung et al., 2007; Diamond et al., 2008; Roehrig et al., 2001; Widman et al., 2008a, b). RepliVAX WN-infected cells cannot produce progeny virus in vivo, thus making RepliVAX WN a safe, live-attenuated vaccine. Because the early events of infection with RepliVAX WN occur as they do following inoculation of a wild-type virus, RepliVAX WN serves as a useful model to study the early host response to flavivirus infection including initiation of the innate immune response and recognition of SCFV particle-encoded viral antigens.
Innate immunity to West Nile virus is initiated following recognition by the pattern recognition receptors TLR3 (Wang et al., 2004), TLR7 (Welte et al., 2009), RIG-I, or MDA5 (Fredericksen et al., 2008). Innate immune responses play an essential role in controlling the early stages of viral infection. Beyond the interferon (IFN)-induced cell-intrinsic responses that limit initial viral replication, innate immune cells such as neutrophils and γδ T cells are known to play a role in host protection by controlling virus infection through direct lysis of virus-infected cells or by production of cytokines (Bai et al., 2010; Wang et al., 2003). The role of natural killer (NK) cells in WNV infection is not completely understood. NK cell activating receptor NKp44 interacts directly with WNV envelope protein resulting in NK cell activation, production of IFN-γ and expression of cell-lytic properties (Hershkovitz et al., 2009). Although not required for modulating WNV infection in the spleen, a role for NK cells in controlling WNV infection in the liver is suggested by the upregulation of NK signal pathway gene expression in the liver after WNV infection (Suthar et al., 2013). Macrophage populations are present at peripheral sites as well as in draining LN and therefore may also play a direct role in limiting infection or dissemination of virus through the lymphatics during the initial stages of infection. Additionally, macrophages may play a role in activation or shaping of the adaptive immune response through presentation of viral antigen to antigen-specific cells and the secretion of proinflammatory cytokines/chemokines at the site of infection and in the draining lymph nodes. However, the role of macrophages in limiting virus dissemination and induction of WNV-specific T cell responses is not completely understood.
Activation and development of the WNV-specific CD8+ T cell response is complex and involves the interaction of many cytokines and signaling pathways including type I IFN (Pinto et al., 2011; Winkelmann et al., 2012), CD22 (Ma et al., 2013), MDA5 (Lazear et al., 2013), interferon regulatory factor-1 (Brien et al., 2011) and interferon promoter stimulator-1 (Suthar et al., 2010). Early studies demonstrated that macrophages, B cells and dendritic cells (DC) were capable of serving as antigen presenting cells for WNV-specific T cell responses (Kulkarni et al., 1991). However, more recent studies have demonstrated a dominant role for CD8α+ DC in activation of WNV-specific CD8+ T cell subsets in vivo (Hildner et al., 2008). Interestingly, subcapsular sinus (SCS) macrophages of regional lymph nodes have been shown to play a role in the development of the B cell response to vesicular stomatitis virus (Carrasco and Batista, 2007; Junt et al., 2007), in invariant NKT cell activation (Barral et al., 2010), and recently in antigen presentation and early activation of the acquired immune response (Martinez-Pomares and Gordon, 2012). While SCS macrophages are not required for a developing B cell response (Purtha et al., 2008), the role these cells play in development of CD8+ T cell responses to WNV is not fully known.
In the studies presented here, we utilized clodronate-liposome depletion of SCS macrophages from lymphoid tissue draining the site of inoculation with the SCFV particle to examine the role of these cells in controlling the initial infection and dissemination from the site of inoculation. Furthermore, we examined the role of SCS macrophages as antigen-presenting cells in the initiation of the virus-specific CD8+ T cell response. We report that depletion of SCS macrophages from the draining LN results in a diminished ability to confine the initial spread of virus at very early times post infection. Additionally, we demonstrate that SCS macrophages are not required for activation of the cell-mediated arm of the adaptive immune response in the draining LN and provide evidence for the antigen-presenting function of specific dendritic cell (DC) subsets from RepliVAX WN-infected mice. Together, these results further illuminate the role of SCS macrophages in protection against WNV during the early stages of infection.
Results
SCS Macrophages limit SCFV dissemination and SCFV gene expression
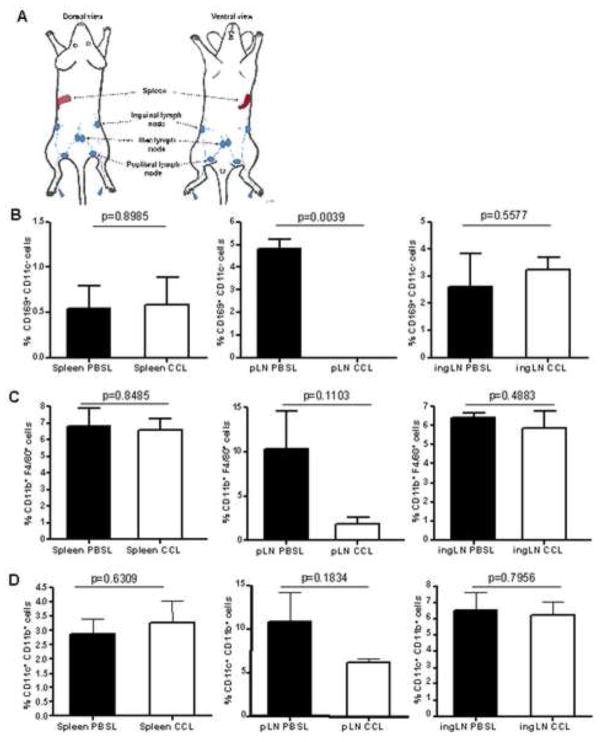
Tissue resident macrophages are present at sites of pathogen entry and play important roles in the innate immune response against many viral pathogens. To study the biological function of macrophages in the initial stages of a WNV infection, we depleted mice of macrophages in the draining lymph nodes by subcutaneous inoculation of hind footpads (FP) with clodronate-containing liposomes (CCL) (Delemarre et al., 1990) prior to FP inoculation of SCFV (Fig 1A). To confirm CCL-mediated depletion from draining lymph nodes, cells from the popliteal LN (pLN), inguinal LN (ingLN) and spleens were harvested 7 days after injection of CCL or PBS-loaded liposomes (PBSL) into the hind FP and analyzed for expression of the macrophage surface markers, integrin αM chain (CD11b) and F4/80, or the SCS macrophage-expressed protein CD169, by flow cytometry. Additionally, lymphoid cells were tested for depletion of dendritic cells by staining for expression of the integrin αX chain (CD11c). As expected, mice treated with CCL were depleted of CD169+ CD11c− cells and, to a lesser extent, F4/80+ CD11b+ cell populations in the pLN, corresponding to SCS and medullary macrophages, respectively (Fig. 1B, C). CCL treatment did not deplete macrophages from the ingLN. Additionally, consistent with reports by others (Delemarre et al., 1990; Purtha et al., 2008), FP administration of CCL did not deplete macrophage populations from the spleen. As shown in Fig. 1D, treatment of mice with CCL did not result in significant reduction of dendritic cells (CD11b+, CD11c+) from any of the lymphoid tissues tested.
FIG. 1.
Macrophage depletion after subcutaneous FP injection of CCL. Seven days post injection, cells from the spleen, pLN and ingLN were analyzed for the expression of CD11b, CD11c, F4/80 and MOMA-I (CD169) by flow cytometry. (A) Schematic showing lymphatic drainage from the footpad. The percentage of (B) CD169+ CD11c− cells (SCS macrophages), (C) CD11b+ F4/80+ cells (medullary macrophages) or CD11b+, CD11c+ (dendritic cells) from CCL- or PBSL-treated mice are shown. Error bars indicate standard deviation (3 mice per group). P value determined by Student’s t test.
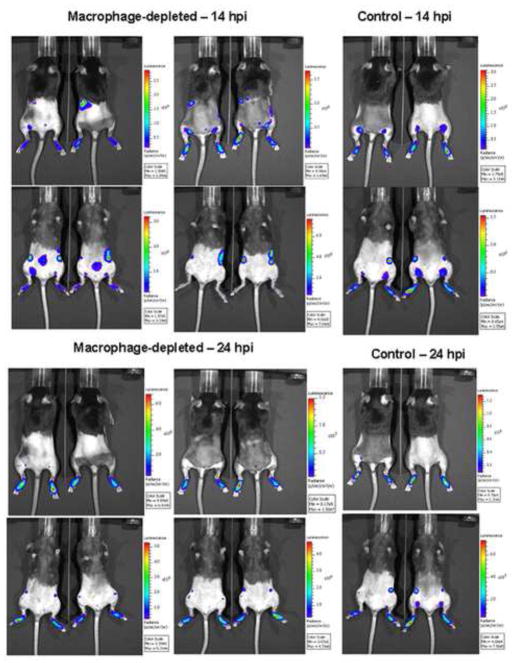
Macrophages can function in protection through direct clearance of viral particles and evidence from depletion studies by others suggests that they are essential to control systemic WNV infection and limit infiltration of virus into the central nervous system (Ben-Nathan et al., 1996). However, it is not clear from these studies if macrophages play a role in control of the initial dissemination of virus from the site of inoculation or provide protection only at later stages of infection. Assessing control of dissemination of the initial viral inoculum is complicated by the presence of continuous virus replication and new rounds of infection. Therefore, we visualized the initial dissemination of FLUC-SCFV from the FP in control-treated and macrophage-depleted mice using an in vivo imaging system (IVIS). Groups of B6 mice received PBSL or CCL, were inoculated in the hind FP with FLUC-SCFV, and imaged at intervals between 14 and 48 hours post-inoculation (hpi). SCFV-encoded FLUC gene expression was quantified at the FP site of infection, draining LN and spleen. As shown in Fig. 2, luciferase bioluminescence was detected in the FP of all CCL- and PBSL- treated mice at 14 hpi, and by 24 hpi differential expression of FLUC was detected in the FP and lymphoid tissues of macrophage-depleted and control mice.
FIG. 2.
Role of SCS macrophages in limiting the dissemination of SCFV. CCL- or PBSL-treated B6 mice were inoculated subcutaneously in both hind FP with 107 IU of FLUC-SCFV on day 7 after liposome injection. Mice were imaged at 14, 24, 36 and 48 hpi. Representative images of mice at 14 hpi and 24 hpi.
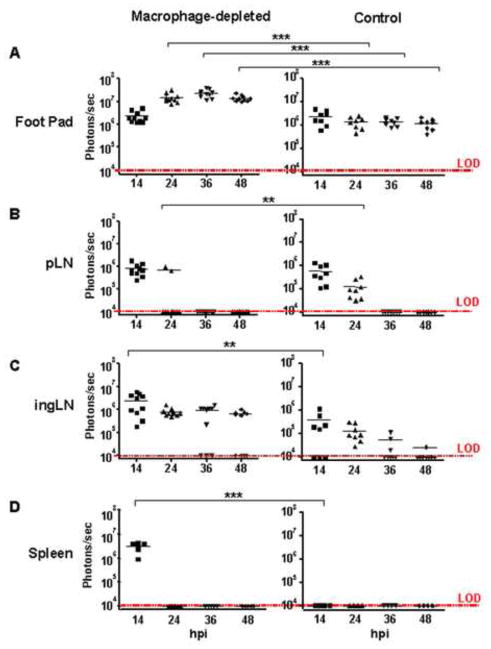
The results of IVIS analysis of FLUC-SCFV dissemination to specific tissues are shown in graphic form in Fig. 3. Expression of FLUC-SCFV genes was readily detected in the hind FP of all FLUC-SCFV-infected mice. At 14 hpi, FLUC gene expression at this site was detected at similar levels in the CCL- and PBSL-treated groups. However, expression increased approximately 10-fold in the CCL-treated mice and was significantly higher than expression in PBSL-treated mice at all time points from 24–48 hpi (p<0.001, Fig. 3A). Lymphatic drainage of the hind FP of mice involves both the pLN and ingLN (Harrell et al., 2008). The luciferase bioluminescence detected in the pLN of CCL- and PBSL-treated mice was nearly equivalent at 14 hpi and was absent in both mouse groups by 36 hpi. Interestingly, at 24 hpi FLUC gene expression was detected in all PBSL-treated mice but in only 2 of 10 pLN in macrophage-depleted mice although at a significantly higher expression level (p<0.01) (Fig. 3B). In the ingLN, luciferase bioluminescence was detected in 5 of 8 in PBSL-treated mice at 14 hpi and expression decreased thereafter such that FLUC activity was detected at low levels in only 1 of 8 ingLN at 48 hpi. By contrast, FLUC gene expression was significantly higher at 14 hpi in the ingLN of CCL-treated group (p<0.01), and remained higher at the 24, 36, and 48 hours time points (Fig. 3C). A greater frequency of CCL-treated mice expressed FLUC activity in the ingLN at both 36 and 48 hpi: 7 of 10 compared to 3 of 8 in PBSL-treated mice at 36 hpi and 6 of 10 compared to 1 of 8 in PBSL-treated mice at 48 hpi (Fig. 3C). Luciferase bioluminescence was not detected at any time point in the spleens of PBSL-treated mice although it was detected at high level in all CCL-treated mice at 14 hpi (p<0.001, Fig. 3D).
FIG. 3.
Magnitude of SCFV gene expression in vivo in SCS macrophage-depleted and control-treated B6 mice. Images were taken at the indicated time points and bioluminescence was observed using up to 90 seconds exposures at medium binning. The total flux emanating from each region of interest (ROI) was measured for the indicated tissues from each mouse group. Each point represents the measurement of one ROI (in the cases of FP and LN, two per animal) and the line represents the average of all site per group (macrophage-depleted, n=5; control group, n=4) above the limit of detection (LOD). **p<0.01, ***p<0.001, two-way ANOVA, Bonferroni post test.
Higher serum IFN-α level in SCS macrophage-depleted mice
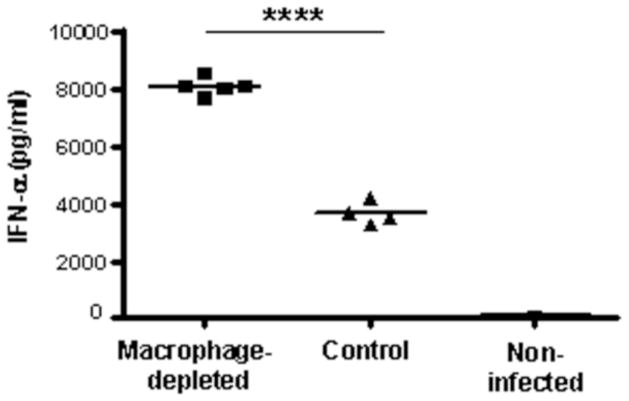
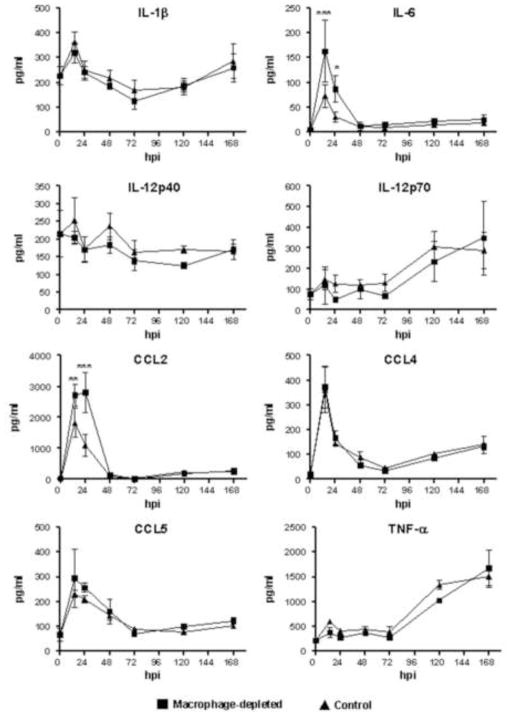
Type I IFN are produced by many cells types and have a critical function as an initial mechanism to prevent virus infection through their antiviral effects. To investigate the effects of SCS macrophage depletion at the regional site of infection on systemic production of type I IFN, we measured the amount of IFN-alpha (IFN-α) and IFN-beta (IFN-β) in the serum of mice at 24 hpi. Interestingly, the serum level of IFN-α was two-times higher in the macrophage-depleted group (p<0.0001) (Fig. 4). IFN-β was not detected in any treatment group. This result is consistent with our previous report showing that IFN-β is produced at low levels in mice inoculated with a single-cycle flavivirus (Bourne et al., 2007). Cytokines and chemokines were quantified in the sera of RepliVAX WN-inoculated mice at 14, 24, 48, 72, 120, and 168 hpi. As shown in Fig. 5, serum levels of IL-1β, IL-6, CCL2, CCL4, and CCL5 peaked at 14 hpi in both CCL- and PBSL-treated mice and declined precipitously thereafter. Serum levels of IL-12p70, and TNF-α remained at baseline levels until 72 hpi before rising through 168 hpi. Levels of IL-6 and CCL2 were significantly higher at 14 and 24 hpi in CCL-treated compared to PBSL-treated mice. However, for IL-1β, IL-12p70, TNF-α, CCL4, and CCL5 the pattern and quantity of cytokines and chemokines produced following RepliVAX WN FP inoculation were not different between CCL- and PBSL-treated mice.
FIG. 4.
Serum levels of IFN-α in SCS macrophage-depleted and control-treated B6 mice. Sera were collected at 24 hours post-infection from the mice described in Fig. 3 and the IFN-α concentration was determined by ELISA. A mock-infected CCL-treated mouse was included as an additional control. Each point represents the IFN-α levels of each mouse and the line represents the average per group of mice. ****p<0.0001, Student’s t test.
FIG. 5.
Serum levels of selected cytokines and chemokines in SCS macrophage-depleted and control-treated B6 mice. Mice were inoculated in the FP with 107 IU of RepliVAX WN and sera were collected at 14, 24, 48, 72, 120, and 168 hpi and assayed for the indicated cytokines and chemokines. The results represent the mean response of 3 mice/group (* p < 0.05, ** p < 0.01, *** p < 0.001, Two-way ANOVA).
SCS Macrophages are not required to initiate a WNV-specific CD8+ T-cell response in the draining LN
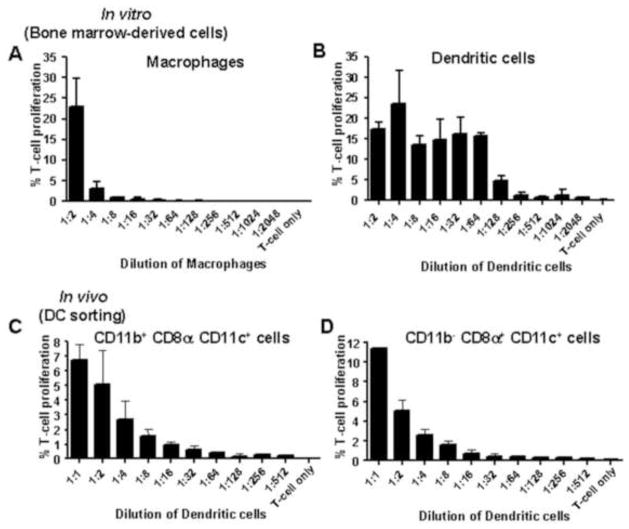
Beyond phagocytosis of viral particles and production of cytokines, macrophages can serve as potent antigen-presenting cells (APC) and may therefore play a critical role in presenting WNV antigen to and activating naive WNV-specific T cells in draining LN. To examine the requirement for SCS macrophages to initiate the WNV-specific CD8+ T cell response, mice were CCL- or PBSL-treated prior to inoculation with FLUC-SCFV. As shown in Figure 6, the number of IFN-gamma (IFN-γ)-secreting cells specific for the immunodominant CD8+ T cell epitope of the NS4B protein, NS4B2488, was not different in the spleen or draining LN between PBSL-treated and CCL-treated mice. These data suggest that SCS macrophages are not required for development of the cell-mediated response. This result is not due to an inability of macrophages to present virus-derived epitopes as bone marrow-derived macrophages (BM-MØ) infected with gB/OVA-RepliVAX WN efficiently presented virus-expressed ovalbumin SIINFEKL epitope to naïve OVA-specific OT-I T cells resulting in T cell proliferation (Fig. 7A). These results suggest that other cells, such as DC most likely served as the APC in macrophage-depleted mice. Consistent with this notion, co-culture of naïve OT-I T cells and bone marrow-derived DC (BM-DC) infected with gB/OVA-RepliVAX WN also resulted in T cell replication (Fig. 7B). Additionally, DC isolated from the spleens of gB/OVA-RepliVAX WN-infected mice were also capable of presenting antigen to naïve OT-I T cells. Two major populations of spleen DC have been described; CD11b− CD8α+CD11c+ and CD11b+ CD8α− CD11c+ (Anjuere et al., 1999). OVA-specific T cell proliferation was observed following culture with either CD11b+ CD8α− CD11c+ (Fig. 7C) or CD11b− CD8α+ CD11c+ (Fig. 7D) DC subsets isolated on day 3 post inoculation, consistent with the role of these cells as professional APC.
FIG. 6.
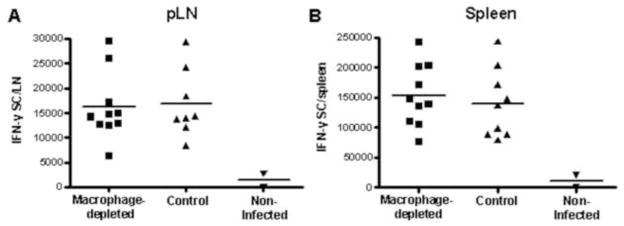
SCS macrophages are not required for the development of the WNV-specific CD8+ T cell response in the draining LN. SCS macrophage-depleted and control B6 mice were inoculated with 107 IU of FLUC-SCFV at day 7 after liposome injection. The two pLN were pooled for individual animals (A) and spleens (B) were harvested at day 7 post infection and cells were used for quantification of the number of IFN-γ-secreting cells (IFN-γ SC) specific for the WNV NS4B2488 CD8+ T cell epitope by ELISPOT. Each point represents the number of IFN-γ SC from pooled LN of individual mice and the line represents the mean per group of mice. Data are representative of two independent experiments.
FIG. 7.
Ability of macrophages and dendritic cells to stimulate CD8+ T cell proliferation. Serial dilutions of BM-MØ (A) and BM-DC (B) infected with gB/OVA-RepliVAX WN at a MOI of 100 were co-cultured with constant numbers of naïve OT-1 CD8+ T cells for 72 hours (see material and methods). Bars represent proliferation of T cells measured by CFSE signal dilution. Error bars represent standard deviation of duplicates or triplicates from each dilution. (C) and (D) B6 mice were immunized with 2×107 IU gB/OVA-RepliVAX WN. On day 3, cells from spleen were harvested and CD11b+ CD8α− CD11c+ (C) and CD11b− CD8α+ CD11c+ (D) DC subpopulations were sorted by flow cytometry and co-cultured with naïve CD8+ T cells from OT-I mice as described above (see also materials and methods). Bars represent proliferation of T cells measured by CFSE signal dilution and error bars represent standard deviation of two independent experiments.
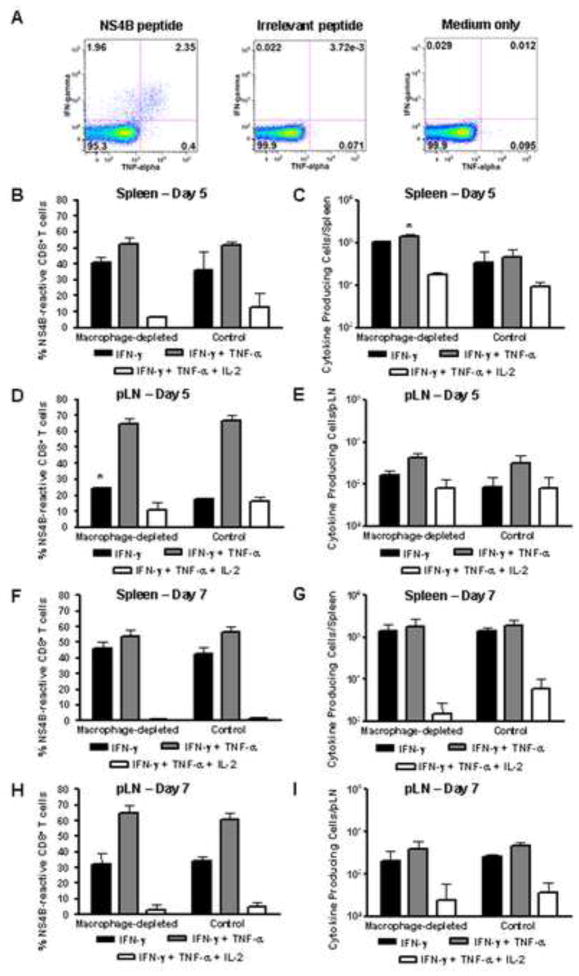
We considered the possibility that SCS macrophages played a role in determining the quality of the CD8+ T cell response. The polyfunctionality of WNV-specific effector CD8+ T cells was measured as the ability to simultaneously produce IFN-γ, TNF-α, and IL-2. As shown in Figure 8A, cytokines were produced as a result of stimulation of CD8+ T cells from RepliVAX WN-inoculated mice with the peptide representing the immunodominant epitope from the NS4B protein, but not following stimulation with an irrelevant peptide or medium only. Both the frequency and total number of CD8+ T cells producing IFN-γ only, IFN-γ + TNF-α, or IFN-γ + TNF-α + IL-2 were very similar between spleen cells (Fig. 8B, C, F, G) or pLN cells (Fig. 8D, E, H, I) from PBSL-treated and CCL-treated mice on both day 5 and day 7 post inoculation. The only exceptions were that significantly higher numbers of spleen cells producing IFN-γ + TNF-α were detected on day 5 and the frequency of pLN cells secreting only IFN-γ reached significance on day 5. The presence of these cytokine producing cells is likely responsible for the high serum concentration of TNF-α (Fig 5) and IFN-γ (data not shown) at 120 and 168 hpi. In total, these data indicate that depletion of SCS macrophages had little effect on the development of CD8+ T cell effector function in the draining LN following RepliVAX WN inoculation.
FIG. 8.
Cytokine production by WNV-specific CD8+ T lymphocytes is not affected by depletion of SCS macrophages. CCL- or PBSL treated B6 mice (n=6) were inoculated in the FP with 107 IU RepliVAX WN. Lymphocytes from spleen and pLN were harvested on day 5 (n=2) or day 7 pi (n=4), stimulated and assayed for production of cytokines as described in methods. (A) Representative staining for IFN-γ and TNF-α in splenocytes stimulated with NS4B peptide, irrelevant peptide, or medium. The mean (± SD) frequency (B, D, F, H) or total cell number (C, E, G, I) of cytokine producing CD8+ T cells from the spleen or pLN are shown (*p <0.05).
Discussion
Tissue macrophages play a key role in maintaining homeostasis through phagocytosis and removal of pathogens and cellular debris and they represent one of the first immune cells to encounter virion particles following injection of arboviruses into a mammalian host. Macrophages can be productively infected with WNV (Kyle et al., 2007; Prestwood et al., 2012) although viral replication is controlled by downstream effects of type I IFN mediated in part by innate intrinsic protective mechanisms involving IRF-1, PKR, and RNase L (Brien et al., 2011; Kong et al., 2008; Lazear et al., 2011; Samuel et al., 2006). Because of the highly phagocytic nature of tissue macrophages, it is generally assumed that these cells play a role in limiting the initial spread of virus from the site of infection. This may occur through direct phagocytosis and destruction of virion particles or through elicitation of immune reactive molecules that initiate the infiltration and activation of a variety of innate host cells. This notion has been tested in several studies of flavivirus infection by assessing virus titers in peripheral and neuronal tissues following selective depletion of macrophages from the site of infection and from draining LN. Depletion of macrophages prior to infection with WNV (Ben-Nathan et al., 1996; Purtha et al., 2008) resulted in periods of extended viremia with elevated levels of virus culminating in enhanced virus infiltration of the central nervous system and accelerated development of encephalitis. Similar findings were reported in a murine model of dengue virus infection (Fink et al., 2009). Macrophages are involved in the orchestration of early innate immune events as well as the activation and influence on the adaptive immune system and therefore these effects on flavivirus infection in macrophage-depleted animals may reflect loss of one or several critical functions. The use of single-cycle WNV particles allowed us to focus on the role of macrophages in the initial infection as well as on downstream effects on activation of the adaptive immune response. The results of the present study extend previously reported findings (Ben-Nathan et al., 1996; Purtha et al., 2008), by demonstrating that SCS macrophages act as a natural filter to limit virus dissemination early after initial infection. Depletion of SCS macrophages from pLN resulted in spread of the FLUC-SCFV to the spleen within the first 14 hours after infection. Additionally, luciferase bioluminescence was detected at increased levels at the FP in these mice suggesting that tissue macrophages eliminated FLUC-SCFV particles (or infected cells) and limited the infection at the site of inoculation. Although macrophages can produce type I IFN in response to infection with WNV SCFV particles, this early resistance most likely did not involve type I IFN as serum levels of IFN-α were approximately 2-fold higher in SCS macrophage-depleted mice. These results suggest type I IFN production was not macrophage-dependent and are consistent with production of high levels of type I IFN by alternative cell types, such as plasmacytoid DC (Swiecki and Colonna, 2010). Similarly, serum levels of IL-6 and CCL2 were also higher at early time points in macrophage-depleted animals suggesting that production of these cytokines may be driven by the higher levels of virus gene expression in CCL-treated mice. However, depletion of SCS macrophages had no discernible effect on serum levels of IL-1β, CCL4, CCL5 IL-12p70, and TNF-α following RepliVAX WN inoculation.
Lymph which drains from body tissues enters the regional LN through the afferent lymphatics and empties initially into the SCS. Numerous macrophages have been shown to line both the subcapsular and medullary sinuses of the LN. A population of SCS macrophages expressing CD169 has been shown to play a critical role in capture and removal of virus particles from the lymph and to prevent hematogenous virus spread (Junt et al., 2007). This filtration function was shown to occur as early as 2 hours post inoculation with vesicular stomatitis virus (VSV) and was required for efficient passage of virion antigen from the SCS to LN B cells located in superficial follicles (Junt et al., 2007). Subsequent studies by Purtha et al. (2008) found that SCS macrophages were not required for early B cell activation and development of the WNV-specific antibody response. In addition to the role of SCS macrophages in the development of B cell response (Carrasco and Batista, 2007; Junt et al., 2007) and iNKT cell activation (Barral et al., 2010), recent evidences also demonstrate the function of this cell population in antigen presentation and early activation of the acquired immune response (Martinez-Pomares and Gordon, 2012). The results of the present study extend these findings by examining the requirement of SCS macrophages in activation of the WNV-specific CD8+ T cell response in the draining LN. In agreement with reports by others (Kulkarni et al., 1991), both SCFV-infected macrophages and DC presented antigen to antigen-specific T cells. Importantly, our results demonstrate that SCS macrophages in the draining pLN were not required for induction of an antigen-specific CD8+ T cell response or for development of effector T cell function. This function was likely provided by DC and our results further show that both CD11b+ CD8α− CD11c+ and CD11b− CD8α+ CD11c+ DC subsets isolated from spleens of WNV SCFV particle inoculated mice were capable of presenting virus-encoded antigen to naïve T lymphocytes.
Taken together, the results of this study demonstrate that SCS macrophages play an active role in limiting dissemination of virus from the site of entry to the spleen at very early times after inoculation. Tissue macrophages act at the site of inoculation and reduce infectious load while SCS macrophages in the draining LN act to prevent spread through the lymphatics and ultimately into the spleen. Although SCFV-infected macrophages are capable of presenting antigen to and activating naïve CD8+ T cells, SCS macrophages are apparently not essential for initiating the cell-mediated immune response or for development of effector T cell function. These results extend previous results suggesting a role for macrophages in preventing entry of WNV into the central nervous system and clarify the role for this important innate immune cell in the early stages of infection.
Materials and Methods
Mice
Seven-week-old B6 mice were purchased from The Jackson Laboratories (Bar Harbor, ME). T cell receptor (TCR) transgenic OT-I mice (Falk et al.,1993) were bred onto a Thy1.1 background and maintained as a breeding colony at the AAALAC-approved Animal Resources Center at the University of Texas Medical Branch (UTMB). All animal work was approved by the Institutional Animal Care and Use Committees of with oversight of staff veterinarians.
Cells
Vero cells were maintained in Dulbecco’s Modified Eagle’s Medium (DMEM) (Cellgro, Mediatech, Manassas, VA) containing 6% fetal bovine serum (FBS) (HyClone, Thermo Scientific, Logan, UT), 100 U/mL penicillin, 100 μg/mL streptomycin (GIBCO, Invitrogen, Grand Island, NY) and 20ug/mL Gentamicin (Cellgro). BHK(VEErep/Pac-Ubi-C*) expressing the WNV C protein (Widman et al., 2008b) and BHK(VEErep/WNVC*-E/Pac) cells expressing the WNV C-prM-E proteins (Fayzulin et al., 2006) were propagated in DMEM supplemented with 10% FBS and 10 ug/ml puromycin (Cellgro) as previously described (Fayzulin et al., 2006).
Production of RepliVAX WN and related WNV-SCFV particles
RepliVAX WN and the gB/OVA-RepliVAX WN which encodes the epitopes recognized by OT-I and gBT-I CD8+ T cells (ovalbumin OVA257–264 [SIINFEKL] and HSV gB498–505 [SSIEFARL], respectively) (Hogquist et al., 1994; Mueller et al., 2002) inserted into the truncated capsid gene of WNV (Fig. S1) were produced in BHK (VEErep/Pac-Ubi-C*) cells as described previously (Widman et al., 2008b). FLUC-SCFV were produced in BHK (VEErep/C*-prM-E-Pac) cells as described previously (Gilfoy et al., 2009). Infectious titers (IU/ml) of RepliVAX WN and WNV SCFV particles were determined on Vero cells as previously described (Fayzulin et al., 2006).
Depletion of macrophages by injection of clodronate liposomes
To deplete macrophages from the regional draining LN of B6 mice, 20ul of a suspension of CCL containing 5mg/ml clodronate (a gift of Roche Diagnostics GmbH, Mannheim, Germany) or control PBSL was injected subcutaneously in each FP 7 days before SCFV inoculation. CCL and control PBSL were prepared as previously described (van Rooijen and Sanders, 1994). To confirm the depletion, cells from spleen, pLN and ingLN were stained with fluorochrome-conjugated mAb anti-CD11c (PE) (BD Biosciences, San Jose, CA), -F4/80 (APC) (AbD Serotec, Raleigh, NC), -CD11b (FITC) (BD Biosciences) or -MOMA-1 (CD169) (FITC) (AbD Serotec) and data were acquired on a BD LSRII Fortessa and analyzed using FlowJo software (Tree Star, Ashland, OR).
In vivo imaging
Mice were inoculated subcutaneously in both hind FP with FLUC-SCFV (Gilfoy et al., 2009) at a dose of 107 IU/FP 7 days after CCL or PBSL treatment. Prior to inoculation, the posterior half of all animals were shaved to facilitate the acquisition of luciferase signal. Mice were imaged at 14, 24, 36 and 48 hpi. At each time point, mice were injected intraperitoneally with a D-luciferin solution (Caliper LS, Hopkinton, MA) corresponding to the dose of 15 mg/kg body weight. After 20 min to allow D-luciferin distribution, mice were anesthetized with 90mg/kg ketamine and 8mg/kg xylazine, and real-time in vivo imaging was performed using a Xenogen IVIS 200 (Caliper LS) with exposure times ranging from 1 to 90 sec at medium binning. The images were analyzed using the Living Image 4.0 software (Caliper LS), where the total flux from each region of interested (ROI) was measured and reported as photons per second (photons/sec). The limit of detection of the luciferase signal was considered to be 104 photons/sec of each ROI analyzed.
Interferon and Cytokine detection
Type I interferon was quantified using commercial IFN-α and IFN-β ELISA kits (PBL Biomedical Laboratories, Piscataway, NJ), following the manufacturer’s protocol. The limit of detection for the assays was 12.5 pg/ml. Cytokine and chemokine levels in the sera of individual RepliVAX WN-inoculated mice were determined using a luminescence-based multiplex bead assay (Bio-Rad, Hercules, CA) from a panel of 23 cytokines following the manufacturer’s protocols as performed previously (Winkelmann et al., 2012).
Enzyme-linked immunospot assay (ELISPOT)
ELISPOT assays were performed as previously described (Nelson et al., 2010) using microtiter filter plates (Millipore, Billerica, MA) coated with purified anti-mouse IFN-γ monoclonal antibody (BD Pharmingen) and incubated at 4°C overnight. Serial dilutions of splenocytes and pooled pLN cells from individual CCL- and PBSL-treated mice were plated and stimulated with an immunogenic peptide representing the WNV-specific CD8+ T cell epitope (NS4B2488) (Brien et al., 2007; Purtha et al., 2007). After 40h incubation, plates were developed with biotinylated anti-mouse IFN-γ (BD Pharmingen) and streptavidin-peroxidase (Sigma-Aldrich, St. Louis, MO) and the number of spots in each well, representing the number of IFN-γ secreting cells (SC), were quantified using an ImmunoSpot reader and analyzed with ImmunoSpot software (Cellular Technology Ltd, Cleveland, OH). The total number of IFN-γ secreting cells were calculated and expressed as IFN-γ SC per spleen or pLN.
Intracellular cytokine staining
Lymphocytes from spleens and pLN from CCL- or PBSL-treated mice were harvested on day 5 or 7 after RepliVAX WN vaccination and were re-stimulated with NS4B2488 peptide and stained for IFN-γ, IL-2, and TNF-α as described previously (Winkelmann et al., 2012). Data were acquired on a BD LSRII Fortessa (BD Biosciences) and analyzed using FlowJo software (Tree Star). The total number of cells secreting IFN-γ, IFN-γ + TNF-α, or IFN-γ + TNF-α + IL-2 was derived by multiplying the % of cells secreting a particular cytokine combination by the total number of viable spleen or pLN cells.
Antigen presentation by DC subsets isolated from RepliVAX WN infected mice
Three days after intraperitoneal inoculation of B6 with 2×107 IU of gB/OVA-RepliVAX WN, splenocytes were harvested and DC were enriched by depletion of B, T and NK cells using anti-CD19, -CD90.2, -CD49 (DX5) microbeads (Miltenyi Biotec, Auburn, CA), following the manufacturer’s protocol. Enriched cells were then surface-stained with fluorochrome-conjugated mAb anti-CD8α (APC), -CD11c (PE) and -CD11b (PE-Cy7) (BD Biosciences) and sorted into CD11b+ CD11c+ and CD8α+ CD11c+ subpopulations using a BD FACS Aria. Serial 2-fold dilutions of CD11c+CD11b+ or CD11c+CD8α+ DC subpopulations were co-cultured with 105 naïve OT-I T cells selected by using the CD8+ T cell Isolation Kit II (Miltenyi Biotec) and labeled with 2μM of carboxyfluorescein succinimidyl ester (CFSE) (Molecular Probes, Invitrogen) in 96-well plates. After 72h, cells were surface-stained with fluorochrome-conjugated mAb anti-CD90.2 (PE) (BD biosciences), data were acquired on a BD LSRII Fortessa, and the proliferation of T cells measured as dilution of intracellular CFSE was determined by using FlowJo software (Tree Star).
In vitro studies using BM-DC and BM-MØ
To generate BM-DC, femurs were aseptically collected, extraneous tissues were removed and then both ends of the bone were cut to expose the lumen. Marrow cells were flushed with 4 ml of Hank’s Balanced Salt Solution (HBSS) (Sigma-Aldrich) containing 5% new born calf serum (NBCS) (GIBCO, Invitrogen) and 1% penicillin/streptomycin using a 26-gauge needle and pushed through a mesh screen to create a single-cell suspension. After washing the cells once with HBSS, red blood cells were removed using RBC lysis buffer (Sigma-Aldrich) and lymphocytes were washed 3 times with HBSS and resuspended at the concentration of 2×106 cells/ml in culture medium [RPMI-1640 (Cellgro) supplemented with 10% FBS, 1% penicillin/streptomycin and 1mM sodium pyruvate (Sigma-Aldrich)] containing 20ng/ml of recombinant mouse granulocyte-macrophage colony-stimulating factor (GM-CSF) (R&D Systems, Minneapolis, MN) and 20ng/ml recombinant mouse IL-4 (BD Pharmingen). Cells were plated in 100 mm cell culture dishes in a total 10 ml per dish and incubated for 10 days. On day 3, an additional 10 ml of medium was added to each culture dish. On days six and eight, 10 ml of the medium was removed without discarding any cells and fresh medium was added to the culture dishes.
To generate BM-MØ, bone marrow cells were resuspended at 7×105 cells/ml and plated in 100 mm non-treated culture dishes in 10 ml of culture medium containing 100ng/ml of recombinant mouse macrophage colony-stimulating factor (M-CSF) (PeproTech, Rocky Hill, NJ) and incubated at 37°C, 5% CO2 for 7 days. On day 3 an additional 5 ml of medium was added to each culture dish.
Infection of BM-DC and BM-MØ and co-culture with CD8+ T cells
BM-DC and BM-MØ were infected with gB/OVA-RepliVAX WN at a multiplicity of infection (MOI) of 100 for 2 hours at 37°C. Cells were washed 3 times with HBSS, resuspended in culture media and incubated for an additional 24 hours. Serial 2-fold dilutions of infected BM-DC or BM-MØ were co-cultured with 105 naïve OT-I x Thy1.1+ CD8+ T cells as described above. After 72h, cells were surface-stained with fluorochrome-conjugated mAb anti-CD90.1 (PE) (BD Biosciences), data were acquired on a BD LSRII Fortessa, and the proliferation of T cells was measured as dilution of intracellular CFSE analyzed using FlowJo software (Tree Star).
Statistical analyses
GraphPad Prism (GraphPad Software, San Diego, CA) was used to analyze data. Student’s t test, one-way or two-way analysis of variance (ANOVA) with the Bonferroni post-test was used where appropriate. P values less than 0.05 were considered to indicate statistical significance.
Supplementary Material
Schematic representation of the single-cycle flavivirus (SCFV) genomes. RepliVAX WN (Widman et al., 2008b) genome (A) was used for the construction of gB/OVA-RepliVAX WN (B) which contains the HSV gB498–505 (SSIEFARL) and ovalbumin OVA257–264 (SIINFEKL) peptides. (C) FLUC-SCFV genome, expressing a luciferase reporter gene (Gilfoy et al., 2009). Sequences of all constructs can be available from the authors upon request.
Macrophages limit dissemination of the initial virus inoculum
Macrophage depletion resulted in higher serum levels of IFN-α, IL-6, and CCL2
Macrophage depletion did not alter the function of WNV-specific CD8+ effector T cells CD8α+ and CD11b+ DC presented SCFV-encoded antigen to naïve CD8+ T cells ex vivo
Acknowledgments
We thank Fangling Xu for the construction of gB/OVA-RepliVAX WN and Summer Gorder for excellent technical assistance. This work was supported by NIH grants R21 AI077077 and U01 AI082660. ERW was supported by an SCVD Predoctoral Fellowship and by a James McLaughlin Predoctoral Fellowship for these studies. JX and AJJ were supported by James McLaughlin Predoctoral Fellowships.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anjuere F, Martin Pl, Ferrero I, Fraga ML, del Hoyo GM, Wright N, Ardavin C. Definition of dendritic cell subpopulations present in the spleen, Peyer’s Patches, lymph nodes, and skin of the mouse. Blood. 1999;93(2):590–598. [PubMed] [Google Scholar]
- Bai F, Kong KF, Dai J, Qian F, Zhang L, Brown CR, Fikrig E, Montgomery RR. A paradoxical role for neutrophils in the pathogenesis of West Nile virus. J Infect Dis. 2010;202(12):1804–1812. doi: 10.1086/657416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barral P, Polzella P, Bruckbauer A, van Rooijen N, Besra GS, Cerundolo V, Batista FD. CD169(+) macrophages present lipid antigens to mediate early activation of iNKT cells in lymph nodes. Nat Immunol. 2010;11(4):303–312. doi: 10.1038/ni.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Nathan D, Huitinga I, Lustig S, van Rooijen N, Kobiler D. West Nile virus neuroinvasion and encephalitis induced by macrophage depletion in mice. Arch Virol. 1996;141:459–469. doi: 10.1007/BF01718310. [DOI] [PubMed] [Google Scholar]
- Bourne N, Scholle F, Silva MC, Rossi SL, Dewsbury N, Judy B, de Aguiar JB, Leon MA, Estes DM, Fayzulin R, Mason PW. Early production of type I interferon during West Nile virus infection: role for lymphoid tissues in IRF3-independent interferon production. J Virol. 2007;81(17):9100–9108. doi: 10.1128/JVI.00316-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brien JD, Uhrlaub JL, Nikolich-Zugich J. Protective capacity and epitope specificity of CD8 (+) T cells responding to lethal West Nile virus infection. European JImmunol. 2007;37:1855–1863. doi: 10.1002/eji.200737196. [DOI] [PubMed] [Google Scholar]
- Brien JD, Daffis S, Lazear HM, Cho H, Suthar MS, Gale M., Jr Interferon regulatory factor-1 (IRF-1) shapes both innate and CD8+ T cell immune responses against West Nile Virus infection. PLoS Pathog. 2011;7:e1002230. doi: 10.1371/journal.ppat.1002230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell GL, Marfin AA, Lanciotti RS, Gubler DJ. West Nile virus. Lancet Infect Dis. 2002;2(9):519–529. doi: 10.1016/s1473-3099(02)00368-7. [DOI] [PubMed] [Google Scholar]
- Carrasco YR, Batista FD. B cells acquire particulate antigen in a macrophage-rich area at the boundary between the follicle and the subcapsular sinus of the lymph node. Immunity. 2007;27:160–171. doi: 10.1016/j.immuni.2007.06.007. [DOI] [PubMed] [Google Scholar]
- Chung KM, Thompson BS, Fremont DH, Diamond MS. Antibody recognition of cell surface-associated NS1 triggers Fc-γ receptor-mediated phagocytosis and clearance of West Nile virus-infected cells. J Virol. 2007;81:9551–9555. doi: 10.1128/JVI.00879-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delemarre FG, Kors N, Kraal G, van Rooijen N. Repopulation of macrophages in popliteal lymph nodes of mice after liposome-mediated depletion. J Leukoc Biol. 1990;47(3):251–257. doi: 10.1002/jlb.47.3.251. [DOI] [PubMed] [Google Scholar]
- Diamond MS, Pierson TC, Fremont DH. The structural immunology of antibody protection against West Nile virus. Immunol, Rev. 2008;225:212–225. doi: 10.1111/j.1600-065X.2008.00676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk K, Rotzschke O, Faath S, Goth S, Graef I, Shastri N, Rammensee HG. Both human and mouse cells expressing H-2Kb and ovalbumin process the same peptide, SIINFEKL. Cell Immunol. 1993;150:447–452. doi: 10.1006/cimm.1993.1212. [DOI] [PubMed] [Google Scholar]
- Fayzulin R, Scholle F, Petrakova O, Frolov I, Mason PW. Evaluation of replicative capacity and genetic stability of West Nile virus replicons using highly efficient packaging cell lines. Virology. 2006;351(1):196–209. doi: 10.1016/j.virol.2006.02.036. [DOI] [PubMed] [Google Scholar]
- Fink K, Ng C, Nkenfou C, Vasudevan SG, van Rooijen N, Schul W. Depletion of macrophages in mice results in higher dengue virus titers and highlights the role of macrophages for virus control. Eur J Immunol. 2009;39:2809–2821. doi: 10.1002/eji.200939389. [DOI] [PubMed] [Google Scholar]
- Fredericksen BL, Keller BC, Fornek J, Katze MG, Gale M., Jr Establishment and maintenance of the innate antiviral response to West Nile virus involves both RIG-I and MDA5 signaling through IPS-1. J Virol. 2008;82:609–616. doi: 10.1128/JVI.01305-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilfoy F, Fayzulin R, Mason PW. West Nile virus genome amplification requires the functional activities of the proteasome. Virology. 2009;385(1):74–84. doi: 10.1016/j.virol.2008.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrell MI, Iritani BM, Ruddell A. Lymph node mapping in the mouse. J Immunol Methods. 2008;332(1–2):170–174. doi: 10.1016/j.jim.2007.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershkovitz O, Rosental B, Rosenberg LA, Navarro-Sanchez ME, Jivov S, Zilka A, Gershoni-Yahalom O, Brient-Litzler E, Bedouelle H, Ho JW, Campbell KS, Rager-Zisman B, Despres P, Porgador A. NKp44 receptor mediates interaction of the envelope glycoproteins from the West Nile and dengue viruses with NK cells. J Immunol. 2009;183(4):2610–2621. doi: 10.4049/jimmunol.0802806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildner K, Edelson BT, Purtha WE, Diamond M, Matsushita H, Kohyama M, Calderon B, Schraml B, Unanue ER, Diamond MS, Schreiber BD, Murphy TL, Murphy KM. Batf3 deficiency reveals a critical role for CD8a+ dendritic cells in cytotoxic T cell immunity. Science. 2008;322(5904):1097–1100. doi: 10.1126/science.1164206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- Ishikawa T, Widman DG, Bourne N, Konishi E, Mason PW. Construction and evaluation of a chimeric pseudoinfectious virus vaccine to prevent Japanese encephalitis. Vaccine. 2008;26(22):2772–2781. doi: 10.1016/j.vaccine.2008.03.010. [DOI] [PubMed] [Google Scholar]
- Junt T, Moseman EA, Iannacone M, Massberg S, Lang PA, Boes M, Finnk K, Henrickson SE, Shayakhmeetov DM, Di Paolo NC, van Rooijen N, Mempel TR, Whelan SP, von Andrian UH. Subcapsular sinus macrophages in lymph nodes clear lymph-borne viruses and present them to antiviral B cells. Nature. 2007;450:110–114. doi: 10.1038/nature06287. [DOI] [PubMed] [Google Scholar]
- Kong KF, Wang X, Anderson JF, Fikrig E, Montgomery RR. West Nile virus attenuates activation of primary human macrophages. Viral Immunol. 2008;21:78–82. doi: 10.1089/vim.2007.0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni AB, Mullabacher A, Blanden RV. Functional analysis of macrophages, B cells, and splenic dendrititc cells as antigen-presenting cells in West Nile virus-specific murine T lymphocyte proliferation. Immunol Cell Biol. 1991;69:71–80. doi: 10.1038/icb.1991.12. [DOI] [PubMed] [Google Scholar]
- Kyle JL, Beatty PR, Harris E. Dengue virus infects macrophages and dendritic cells in a mouse model of infection. J Infect Dis. 2007;195(12):1808–1817. doi: 10.1086/518007. [DOI] [PubMed] [Google Scholar]
- Lazear HM, Pinto AK, Vogt MR, Gale M, Jr, Diamond MS. Beta interferon controls West Nile virus infection and pathogenesis in mice. J Virol. 2011;85:7186–7194. doi: 10.1128/JVI.00396-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazear HM, Pinto AK, Ramos HJ, Vick SC, Shrestha B, Suthar MS, Gale M, Jr, Diamond MS. Pattern recognition receptor MDA5 modulates CD8+ T cell-dependent clearance of West Nile virus from the central nervous system. J Virol. 2013;87(21):11401–11415. doi: 10.1128/JVI.01403-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenbach BD, Theil H-J, Rice CM. Flaviviridae: The Viruses and Their Replication. In: Knipe DM, Howley PM, editors. Fields Virology. Vol. 1. Lippincott Williams & Wilkins; Philadelphia: 2007. pp. 1101–1152. 2 vols. [Google Scholar]
- Ma DY, Suthar MS, Kasahara S, Gale M, Jr, Clark EA. CD22 is required for protection against West Nile virus infection. J VIrol. 2013;87(6):3361–3375. doi: 10.1128/JVI.02368-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Pomares L, Gordon S. CD169+ macrophages at the crossroads of antigen presentation. Trends Immunol. 2012;33(2):66–70. doi: 10.1016/j.it.2011.11.001. [DOI] [PubMed] [Google Scholar]
- Mason PW, Shustov AV, Frolov I. Production and characterization of vaccines based on flaviviruses defective in replication. Virology. 2006;351(2):432–443. doi: 10.1016/j.virol.2006.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller SN, Heath W, McLain JD, Carbone FR, Jones CM. Characterization of two TCR transgenic mouse lines specific for herpes simplex virus. Immunol Cell Biol. 2002;80(2):156–163. doi: 10.1046/j.1440-1711.2002.01071.x. [DOI] [PubMed] [Google Scholar]
- Nelson MH, Winkelmann E, Ma Y, Xia J, Mason PW, Bourne N, Milligan GN. Immunogenicity of RepliVAX WN, a novel single-cycle West Nile virus vaccine. Vaccine. 2010;29(2):174–182. doi: 10.1016/j.vaccine.2010.10.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto AK, Daffis S, Brien JD, Gainey MD, Yokayama WM, Sheehan KC, Murphy KM, Schreiber RD, Diamond MS. A temporal role of type I interferon signaling in CD8+ T cell maturation during acute West Nile virus infection. PLos Pathog. 2011;7(12):e1002407. doi: 10.1371/journal.ppat.1002407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prestwood TR, May MM, Plummer EM, Morar MM, Yauch LE, Shresta S. Trafficking and replication patterns reveal splenic macrophages as major targets of dengue virus in mice. J Virol. 2012;86:12138–12147. doi: 10.1128/JVI.00375-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purtha WE, Myers N, Mitaksov V, Sitati E, Connolly J, Fremont DH, Hansen TH, Diamond MS. Antigen-specific cytotoxic T lymphocytes protect against lethal West Nile virus encephalitis. EurJ Immunol. 2007;37(7):1845–1854. doi: 10.1002/eji.200737192. [DOI] [PubMed] [Google Scholar]
- Purtha WE, Chachu KA, Virgin HW, IV, Diamond MS. Early B-cell activation after West Nile virus infection requires alpha/beta interferon but not antigen receptor signaling. J Virol. 2008;82:10964–10974. doi: 10.1128/JVI.01646-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roehrig JT, Staudinger LA, Hunt AR, Mathews JH, Blair CD. Antibody prophylaxis and therapy for flavivirus encephalitis infections. Ann N Y Acad Sci. 2001;951:286–297. doi: 10.1111/j.1749-6632.2001.tb02704.x. [DOI] [PubMed] [Google Scholar]
- Samuel MA, Whitby K, Keller BC, Marri A, Barchet W, Williams BRG, Silverman RH, Gale M, Jr, Diamond MS. PKR and RNase L contribute to protection against lethal west nile virus infection by controlling early viral spread in the periphery and replication in neurons. J Virol. 2006;80:7009–7019. doi: 10.1128/JVI.00489-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suthar MS, Ma DY, Thomas S, Lund JM, Zhang N, Daffis S, Rudensky AY, Bevan MJ, Clark EA, Kaja M-K, Diamond MS, Gale M., Jr IPS-1 is essential for the control of West Nile virus infection and immunity. PLoS Pathog. 2010;6(2):e1000757. doi: 10.1371/journal.ppat.1000757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suthar MS, Brassil MM, Blahnik B, McMillan A, Ramos HJ, Proll SC, Belisle SE, Katze MG, Gale M., Jr A systems biology approach reveals that tissue tropism to West Nile virus is regulated by antiviral genes and innate immune cellular processes. PLoS Pathog. 2013;9(2):e1003168. doi: 10.1371/journal.ppat.1003168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki R, Winkelmann ER, Mason PW. Construction and characterization of a single-cycle chimeric flavivirus vaccine candidate that protects mice against lethal challenge with dengue virus type 2. J Virol. 2009;83(4):1870–1880. doi: 10.1128/JVI.01891-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiecki M, Colonna M. Unraveling the functions of plasmacytoid dendritic cells during viral infections, autoimmunity, and tolerance. Immunol Rev. 2010;234(1):142–162. doi: 10.1111/j.0105-2896.2009.00881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rooijen N, Sanders A. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J Immunol Methods. 1994;174:83–93. doi: 10.1016/0022-1759(94)90012-4. [DOI] [PubMed] [Google Scholar]
- Wang T, Scully E, Yin Z, Kim JH, Wang S, Yan J, Mamula M, Anderson JF, Craft J, Fikrig E. IFN-gamma-producing gamma delta T cells help control murine West Nile virus infection. J Immunol. 2003;171:2524–2531. doi: 10.4049/jimmunol.171.5.2524. [DOI] [PubMed] [Google Scholar]
- Wang T, Town T, Alexopoulou L, Anderson JF, Fikrig E, Flavell RA. Toll-like receptor 3 mediates West Nile virus entry into the brain causing lethal encephalitis. Nat Med. 2004;10(12):1366–1373. doi: 10.1038/nm1140. [DOI] [PubMed] [Google Scholar]
- Welte T, Regan K, Fang H, Machain-Williams C, Zheng X, Mendell N, Chang GJ, Wu P, Blair CD, Wang T. Toll-like receptor 7-induced immune response to cutaneous West Nile virus infection. J Gen Virol. 2009;90(11):2660–2668. doi: 10.1099/vir.0.011783-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widman DG, Frolov I, Mason PW. Third-generation flavivirus vaccines based on single-cycle, encapsidation-defective viruses. Adv Virus Res. 2008a;72:77–126. doi: 10.1016/S0065-3527(08)00402-8. [DOI] [PubMed] [Google Scholar]
- Widman DG, Ishikawa T, Fayzulin R, Bourne N, Mason PW. Construction and characterization of a second-generation pseudoinfectious West Nile virus vaccine propagated using a new cultivation system. Vaccine. 2008b;26(22):2762–2771. doi: 10.1016/j.vaccine.2008.03.009. [DOI] [PubMed] [Google Scholar]
- Winkelmann ER, Widman DG, Xia J, Ishikawa T, Miller-Kittrell M, Nelson MH, Bourne N, Scholle F, Mason PW, Milligan GN. Intrinsic adjuvanting of a novel single-cycle flavivirus vaccine in the absence of tyhpe I interferon receptor signaling. Vaccine. 2012;30(8):1465–1475. doi: 10.1016/j.vaccine.2011.12.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Schematic representation of the single-cycle flavivirus (SCFV) genomes. RepliVAX WN (Widman et al., 2008b) genome (A) was used for the construction of gB/OVA-RepliVAX WN (B) which contains the HSV gB498–505 (SSIEFARL) and ovalbumin OVA257–264 (SIINFEKL) peptides. (C) FLUC-SCFV genome, expressing a luciferase reporter gene (Gilfoy et al., 2009). Sequences of all constructs can be available from the authors upon request.