Significance
One hypothetical pathway leading to oncogenic transformation involves a transient phase of tetraploidization, followed by asymmetric cell divisions, aneuploidy, and genomic instability. By means of a pharmacological screen, we identified resveratrol and salicylate as compounds that kill tetraploid cells more efficiently than their parental diploid counterparts. Resveratrol and salicylate reduced the frequency of tetraploid cells arising from primary epithelial cell cultures exposed to mitotic inhibitors. In a mouse model of intestinal oncogenesis resembling familial adenomatous polyposis both resveratrol and aspirin, the salicylate prodrug, reduced the frequency of tetraploid cells accumulating in the gut, correlating with their chemopreventive action. These findings underscore the relationship between tetraploidy and oncogenesis as they unveil the mechanisms through which aspirin can prevent the development of cancer.
Abstract
Tetraploidy constitutes a genomically metastable state that can lead to aneuploidy and genomic instability. Tetraploid cells are frequently found in preneoplastic lesions, including intestinal cancers arising due to the inactivation of the tumor suppressor adenomatous polyposis coli (APC). Using a phenotypic screen, we identified resveratrol as an agent that selectively reduces the fitness of tetraploid cells by slowing down their cell cycle progression and by stimulating the intrinsic pathway of apoptosis. Selective killing of tetraploid cells was observed for a series of additional agents that indirectly or directly stimulate AMP-activated protein kinase (AMPK) including salicylate, whose chemopreventive action has been established by epidemiological studies and clinical trials. Both resveratrol and salicylate reduced the formation of tetraploid or higher-order polyploid cells resulting from the culture of human colon carcinoma cell lines or primary mouse epithelial cells lacking tumor protein p53 (TP53, best known as p53) in the presence of antimitotic agents, as determined by cytofluorometric and videomicroscopic assays. Moreover, oral treatment with either resveratrol or aspirin, the prodrug of salicylate, repressed the accumulation of tetraploid intestinal epithelial cells in the ApcMin/+ mouse model of colon cancer. Collectively, our results suggest that the chemopreventive action of resveratrol and aspirin involves the elimination of tetraploid cancer cell precursors.
One of the initiating triggers of carcinogenesis is illicit tetraploidization, i.e., the formation of cells that encompass twice as many chromosomes as their normal, diploid counterparts (1–4). Such an augmentation in nuclear DNA content may originate from cell-to-cell fusion, endocycling, or endomitosis. Contrasting with some exceptions (such as hepatocytes, syncytiotrophoblasts, megakaryocytes, and myocytes), most cell types do not tolerate significant variations from the diploid status, meaning that tetraploid as well as higher-order polyploid cells usually activate programmed death pathways as soon as they are generated (5) or elicit immune responses resulting in their elimination (6).
A supraphysiological frequency of tetraploid cells has been detected at early stages of multiple cancer cell types (including bronchial, esophageal, gastric, mammary, colorectal, ovarian, cervical, and prostate carcinomas), often correlating with the inactivation of the tumor suppressors retinoblastoma 1 (RB1) and tumor protein p53 (TP53, best known as p53) (7). The inactivation of p53 facilitates the tetraploidization of cell lines (8–10) and primary epithelial cells from the colon and the mammary gland (11–13). Similarly, inactivation of the adenomatous polyposis coli (APC) tumor suppressor gene (whose mutations initiate a majority of colorectal cancers) results in tetraploidization both in vitro and in vivo in mouse models (14, 15).
Tetraploid cells can give rise to an aneuploid offspring through several mechanisms, namely the gradual gain or loss of chromosomes during subsequent rounds of bipolar (and aberrant) mitosis or, alternatively, the reduction of the chromosomal content during multipolar mitoses (16). Such multipolar mitoses, which result from the presence of extra centrosomes, provoke asymmetric cell divisions in which chromosomes are close-to-randomly distributed among three or more daughter cells (12, 17). Exceptionally, newly generated aneuploid cells are fitter than their tetraploid progenitors, thus progressively transforming into malignant cells (2–5, 18).
Given the importance of tetraploidization for oncogenesis, it is tempting to develop strategies for the selective eradication of such cells. Tetraploid cells are intrinsically resistant against DNA damaging agents (9), yet are more susceptible to a variety of agents including inhibitors of checkpoint kinase 1 (19), Aurora kinase B (20), and mitotic kinesins (21, 22). Nonetheless, such agents can perturb normal mitoses and mitotic checkpoints, casting doubts on their potential utility as chemopreventive agents. Driven by this consideration, we developed a screen for the identification of selective killers of tetraploid cells. This screen led to the identification of resveratrol and other AMP-activated protein kinase (AMPK) activators, including salicylate as potent antitetraploids.
Results
Selective Killing of Tetraploid Cells by Resveratrol.
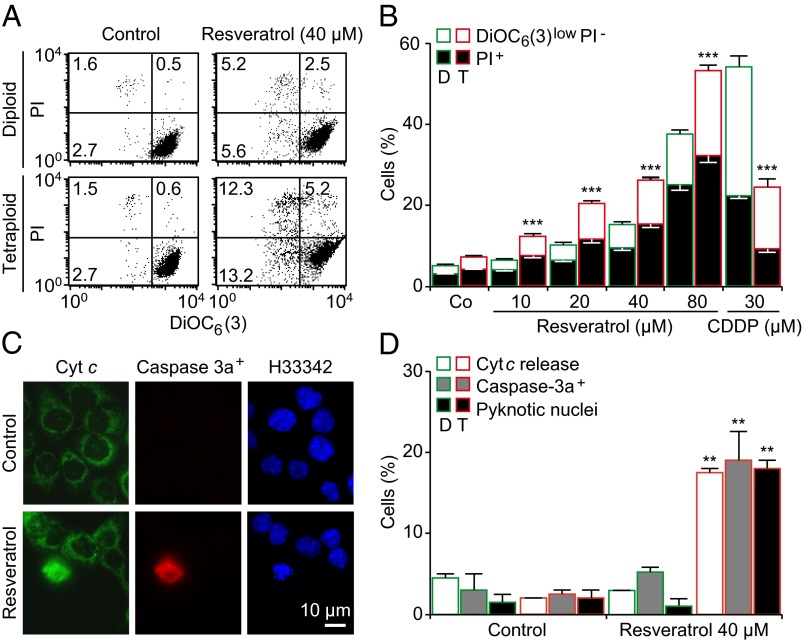
In a pharmacological screen, we identified resveratrol as an agent that kills tetraploid cells more efficiently than their precursors (Fig. S1). To confirm the impact of resveratrol on tetraploid cell survival we used several established diploid and tetraploid human colon carcinoma HCT116 clones (9). Resveratrol induced an increased frequency of cell death in tetraploid HCT116 clones, as determined by staining with the mitochondrial inner transmembrane potential (Δψm)-sensitive dye DiOC6(3) and the vital dye propidium iodide (PI) that leads to the identification of dying (DiOC6(3)low PI−) and dead (PI+) cells (Fig. 1 A and B). Similar results were obtained when apoptosis was detected by staining with FITC-labeled Annexin V, which recognizes phosphatidylserine residues present in the outer leaflet of the plasma membrane (Fig. S2A). Resveratrol was selectively cytotoxic for tetraploid human colorectal carcinoma RKO cells, mouse Lewis lung cancer (LLC) cells, and mouse embryonic fibroblasts (MEF) with respect to their diploid counterparts (Fig. S2 B–D). The close structural resveratrol analog piceatannol (which is a hydroxylated version of resveratrol) (23, 24) also selectively depleted both tetraploid HCT116 and tetraploid RKO cells (Fig. S2 E and F). In contrast, other cytotoxic agents such as the DNA damaging agent cisplatin, the flavonoid quercetin, or the herbicide paraquat eliminated HCT116 parental cells more efficiently than tetraploid ones (Fig. S3 A–C). Although resveratrol has been reported to inhibit the respiratory chain complex I (25, 26), rotenone, a specific complex I inhibitor, had no selective antitetraploid effect (Fig. S3D). The resveratrol-induced killing of tetraploid HCT116 cells was abolished by the pan-caspase inhibitor Z-VAD-fmk (Fig. S4A) and partially suppressed by the knockdown of the proapoptotic proteins from the Bcl-2 family BAX or BAK1, whereas it was increased by the knockdown of the antiapoptotic Bcl-2 family proteins BCL2, BCL2L1 (best known as BCLXL) and MCL1 (Fig. S4B). Tetraploid cell death induced by resveratrol was accompanied by classical features of apoptosis (27), including cytochrome c release from mitochondria, proteolytic maturation of caspase-3, and chromatin condensation with nuclear shrinkage (Fig. 1 C and D). Finally, videomicroscopic observation of tetraploid cells with fluorescent nuclei (due to the stable expression of H2B-GFP) exposed to resveratrol confirmed an increased induction of apoptosis, as well as a significant reduction in mitoses in resveratrol-treated tetraploid cell cultures compared with their diploid controls (Fig. S5 and Movies S1 and S2).
Fig. 1.
Validation of the selective inhibitory effect of resveratrol toward tetraploid cells. (A–D) Multiple diploid and tetraploid clones from human colon carcinoma HCT116 cells (framed in green and red, respectively) were treated with the indicated concentrations of resveratrol for 48 h before the evaluation of the cell-death–associated parameters either by cytofluorometry upon costaining with the vital dye iodure propidium (PI) and the mitochondrial membrane potential (Δψm)-sensing dye DiOC6(3) (A and B) or by fluorescence microcopy upon coimmunostaining with antibodies directed against cytochrome c (Cyt c) and activated caspase 3 (Caspase-3a) (C and D). A illustrates representative dot plots (numbers refer to the percentage of cells found in each quadrants), whereas B shows quantitative data (mean ± SEM; n = 5) from experiments performed on eight different diploid and seven tetraploid clones. Representative pictures and the quantification of cells displaying Cyt c release, caspase-3 activation (Caspase-3a+), and pyknotic nuclei (as determined by nuclear counterstaining with Hoechst 33342) are reported in C and D, respectively (mean ± SEM; n = 500 cells). In B, the fraction of dying (DiOC6(3)low PI−) and dead cells (PI+) is represented by white and black columns, respectively, and cisplatin (CDDP) was used as a negative control. **P < 0.01; ***P < 0.001 (Student t test), compared with the equally treated diploid cells. See also Figs. S2 and S3.
Selective Elimination of Tetraploid Cells by Multiple AMPK Activators.
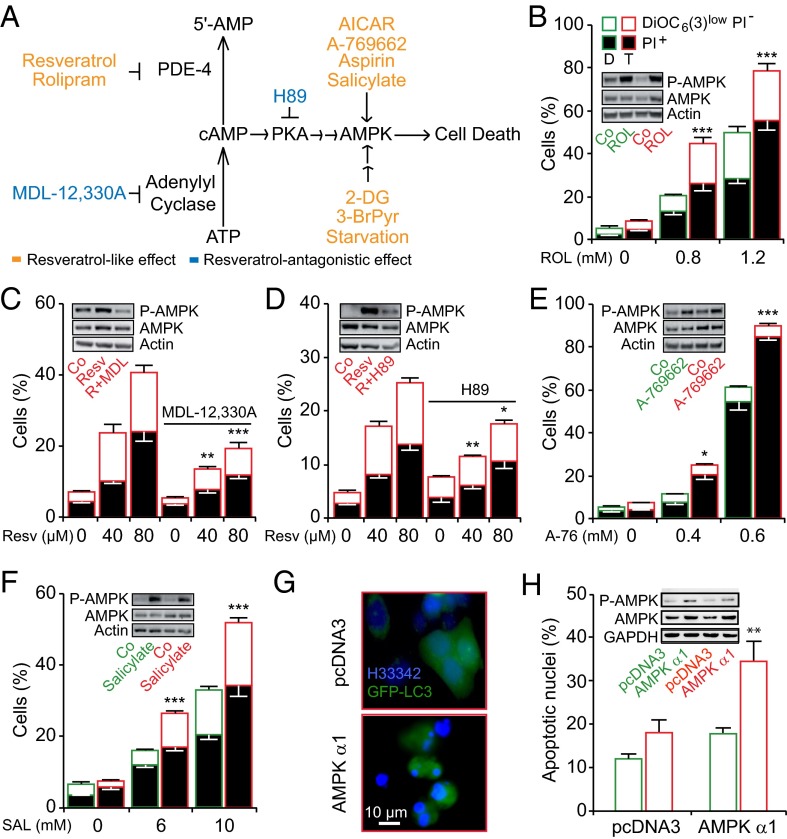
Resveratrol stimulates the activation of AMPK by inhibiting phosphodiesterase 4 (PDE-4), thereby provoking the accumulation of cAMP, which triggers cAMP-activated protein kinase A (PKA) (28) (Fig. 2A). After resveratrol treatment, AMPK displayed a similar level of activation in diploid and tetraploid cells, as indicated by its phosphorylation and that of its substrate acetyl CoA carboxylase (ACC) (Fig. S6). Inhibition of PDE-4 with rolipram (29) mimicked the effects of resveratrol with respect to selective tetraploid depletion and AMPK activation (Fig. 2B), whereas inhibitors of adenylyl cyclase (MDL-12330A) and PKA (H89) interfered with the killing of tetraploid cells by resveratrol (Fig. 2 C and D). In contrast, EX-527, a sirtuin-1-specific inhibitor that blocks resveratrol-induced autophagy (30), failed to protect tetraploid cells from resveratrol-induced cytotoxicity, indicating that neither sirtuin-1 nor autophagy are involved in this phenomenon (Fig. S7). Moreover, several AMPK-activating agents were endowed with the capacity of selectively eliminating tetraploid cells. This applies to AMPK activators such as acetyl salicylate (better known as aspirin), salicylate (31, 32) (which is more active than its precursor aspirin), 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICAR), and A-769662 (Fig. 2 E and F and Figs. S6 and S8), as well as to indirect strategies for AMPK activation, including inhibition of glycolysis with 2-deoxyglucose or 3-bromopyruvate or culture of cells in nutrient-free medium (Figs. S6 and S8). Transfection-enforced overexpression of the AMPK α1 subunit also caused AMPK activation and selective tetraploid cell killing (Fig. 2 G and H).
Fig. 2.
Activation of the energy sensor 5′AMP-activated protein kinase (AMPK) preferentially induces tetraploid cell death. (A) Proposed model of how resveratrol activates AMPK and induces tetraploid cell death (see text for more details). (B–F) Diploid and tetraploid human colon carcinoma HCT116 cells (depicted in green and red, respectively), treated for 48 h with the indicated concentrations of the phosphodiesterase 4 (PDE-4) inhibitor rolipram (B), resveratrol in combination with either the adenylate cyclase inhibitor MDL-12330A (C), or the PKA inhibitor H89 (D), the AMPK activator A-769662 (E), or salicylate (SAL) (F). Thereafter, the cells were subjected to the flow cytometry-assisted measurement of cell death parameters after DiOC6(3)/PI costaining (mean ± SEM; three independent experiments conducted with three diploid and three tetraploid clones). Alternatively, cells were transfected with GFP-LC3 plus vector (pcDNA3) or a cDNA encoding AMPK α1, followed by the quantitation of apoptotic nuclei in transfected (GFP+) cells (G and H). *P < 0.05; **P < 0.01; ***P < 0.001 (Student t test), compared with the equally treated diploid cells (B, E, F, and H) or to cells treated only with resveratrol (C and D). In B–F and H, representative immunoblots of phosphorylated AMPK (Thr172) and total AMPK for each drug are depicted as Insets. Actin or GAPDH were used as loading controls. See also Figs. S6 and S8.
Abortion of Newly Formed Tetraploid Cells by Resveratrol and Salicylate in Vitro and ex Vivo.
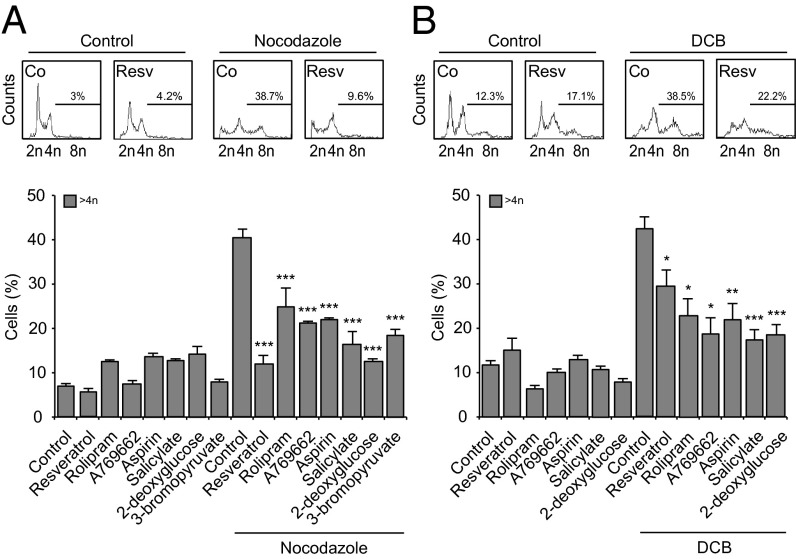
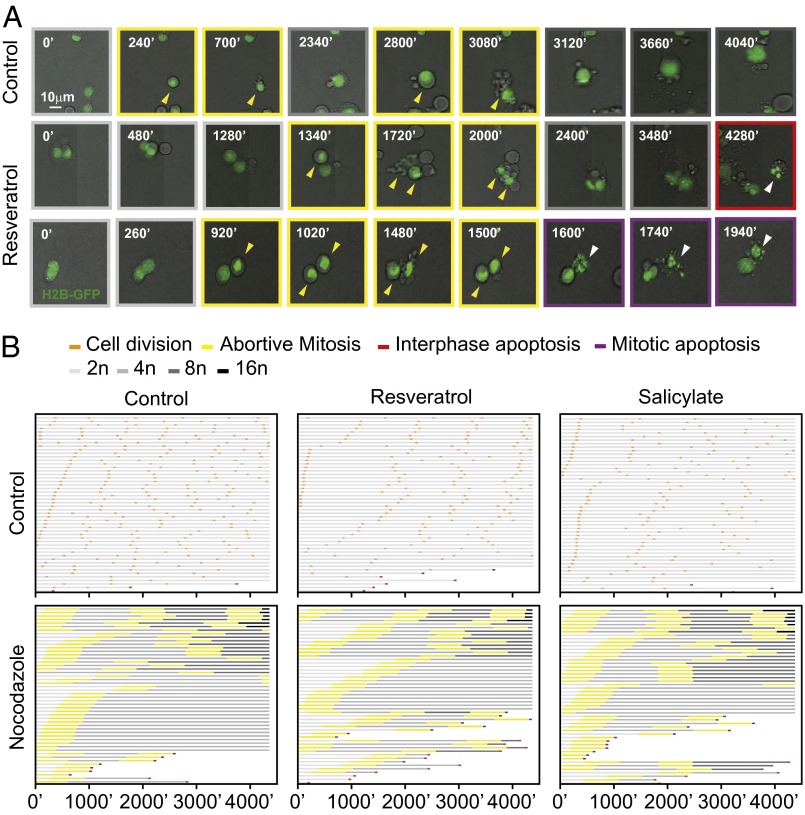
Next, we wondered whether agents with well-established chemopreventive effects such as resveratrol and salicylate would be capable of impeding the de novo formation of tetraploid cells. The microtubule inhibitor nocodazole and the actin cytoskeleton inhibitor cytochalasin D increased the frequency of tetraploid p53−/− HCT116 cells (33), and similarly depletion of Aurora kinase B (AURKB) induced tetraploidization (DNA content >4n) of WT HCT116 cells. All these tetraploidy-inducing effects were significantly reduced in the presence of resveratrol, rolipram, A769662, aspirin, salicylate, 2-deoxyglucose, or 3-bromopyruvate, which concomitantly increased the frequency of cell deaths (Fig. 3A and Fig. S9). Very similar results were obtained with primary epithelial cells from p53−/− mice, which, in contrast to their WT counterparts, are prone to in vitro tetraploidization (11–13). Again, multiple agents that directly or indirectly activate AMPK reduced the tetraploidization efficacy of the cytokinesis inhibitor dihydrocytochalasin B (Fig. 3B). Resveratrol and salicylate also significantly increased the frequency of cell deaths in nocodazole-treated p53−/− HCT116 cultures and reduced the generation of polyploid cells, as determined by videomicroscopy (Fig. 4 A and B and Movies S3–S5).
Fig. 3.
Resveratrol and AMPK activator effects during polyploidization. (A and B) p53-deficient human colon carcinoma HCT116 cells (A) and p53−/− mouse mammary gland epithelial cells (MMECs) (B) were left untreated (control) or incubated for 48 h with the indicated drugs in combination or not with the polyploidizing agents nocodazole 100 nM (A) or dihydrocytochalasin B 1 μM (B). The following concentrations were used (for HCT116 and MMECs, respectively, if different): resveratrol (40 μM), rolipram (0.8 and 1 mM), A-769662 (0.4 and 0.2 mM), aspirin (10 mM), salicylate (10 mM), 2-deoxyglucose (40 and 1 mM), and 3-bromopyruvate (80 μM). At the end of the incubation, DNA content was assessed by cytofluorometry upon Hoechst 33342 staining. Representative cell cycle profiles (Upper) and quantification of polyploid (>4n) cells (Lower) are reported. (Upper) Numbers refer to percentage of polyploid cells. The results are reported as mean ± SEM (n = 3). *P < 0.05; **P < 0.01; ***P < 0.001 (Student t test), compared with the cells exposed to nocodazole or dihydrocytochalasin B alone (Lower). See also Fig. S9.
Fig. 4.
Cell fate profiling of newly generated polyploid cells treated with resveratrol or salicylate. (A and B) p53-deficient human colon carcinoma HCT116 cells stably transfected with H2B-GFP were exposed to the indicated drugs combined or not with nocodazole to induce polyploidization. Cell fate was monitored by videomicroscopy for 72 h. Representative pictures of cells treated with nocodazole alone or in combination with resveratrol are shown in A (arrowheads indicate relevant events). In B, the cell fate profiles of 50 cells left untreated (control) or incubated with resveratrol or salicylate, in combination or not with nocodazole are reported. Light gray depicts interphase cell; orange, successful division; yellow, abortive mitosis; red, interphase apoptosis; and purple, mitotic apoptosis. Gray darkening depicts a change in nuclear size and ploidy, from diploidy to decahexaploidy.
Chemopreventive Effects of Resveratrol and Salicylate Correlate with Elimination of Tetraploid Cells.
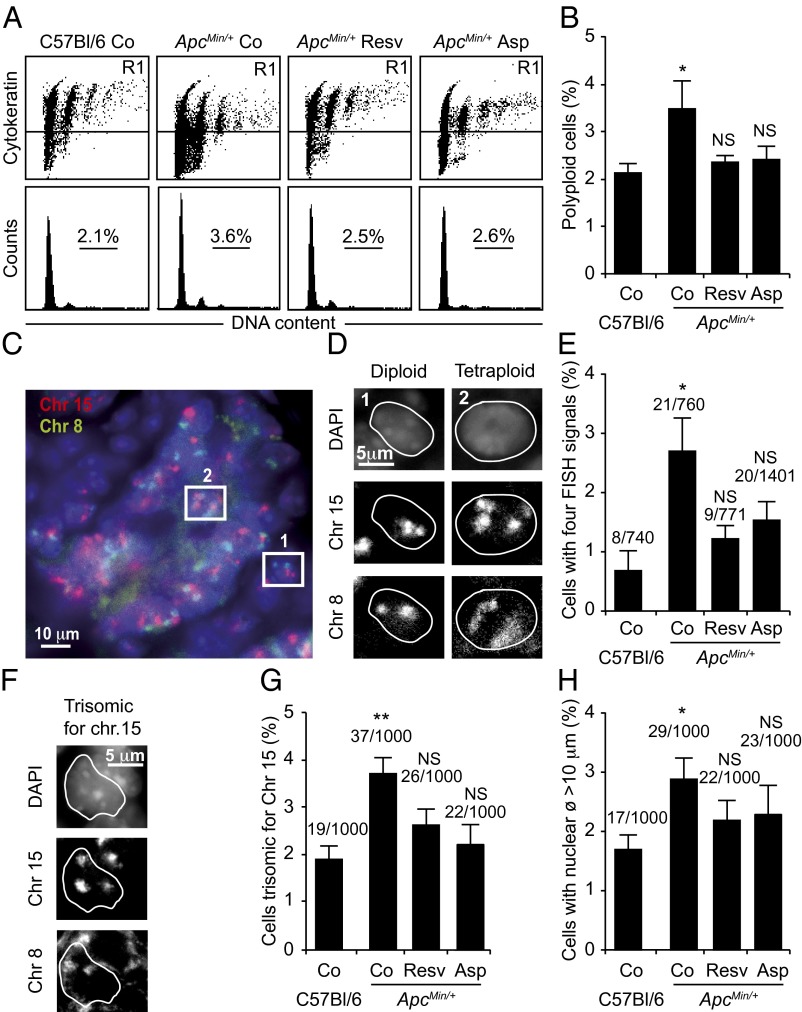
To extend the aforementioned observation to an in vivo model of chemoprevention, we took advantage of mice carrying the ApcMin mutation that develop intestinal carcinomas resembling those found in human patients with loss-of-function mutations of the tumor suppressor APC (34). In such ApcMin/+ mice, oral treatment with resveratrol or aspirin reduces the frequency of intestinal adenomas and adenocarcinomas (35–38). Intestinal epithelial cells (stained with a pancytokeratine-specific antibody) from ApcMin/+ mice contained more tetraploid cells than those from WT C57BL/6 control mice (Fig. 5A). This ApcMin/+ associated increase in the frequency of tetraploid cells was no more detectable if the mice were treated for 5 wk by gavage with resveratrol or aspirin at doses known to have chemopreventive effects (35, 36) (Fig. 5 A and B). Similar results were obtained when tetraploidy was detected by means of fluorescent in situ hybridization (FISH) with two probes recognizing two different chromosomes. Intestinal tissues from ApcMin/+mice contained a higher frequency of apparently tetraploid cells (that contained four copies of both chromosomes) and aneuploid cells (such as cells containing three copies of chromosome 15) than WT control tissues, and this effect of the ApcMin mutation was reversed by pretreatment with resveratrol or aspirin (Fig. 5 C–G). Moreover, the increase in cells with abnormally large nuclei (which frequently were tetraploid, Table S1) associated with the ApcMin/+genotype was reversed by resveratrol or aspirin (Fig. 5H).
Fig. 5.
Resveratrol decreases tetraploidy incidence in the intestine of ApcMin/+ mice. (A and B) Cell cycle analyses of cell suspensions. Six-week-old WT C57BL/6 and ApcMin/+ mice were left untreated (Co) or treated with resveratrol (Resv, 100 mg/kg) or aspirin (Asp, 25 mg/kg) for 5 wk. DNA content of cytokeratin+ intestinal crypt cells were analyzed by flow cytometry. Representative dot plots (y axis, cytokeratin; x axis, DNA content) and cell cycle profiles (y axis, cell count; x axis, DNA content) are reported in A, whereas quantification is shown in B for cytokeratin+ cells (mean ± SEM; 10 mice per group). (C–H) In situ analyses of paraffin-embedded tissues sections. Intestinal crypt cells then were assessed for their chromosome number by FISH using probes against chromosomes 8 (green) and 15 (orange). A picture of a representative cross-section from an intestinal crypt is shown in C. In D, the areas indicated by two boxes in C are magnified to provide examples of the characteristic pattern of a diploid (Left, 1) and tetraploid (Right, 2) cell. The frequency of tetraploid cells is reported in E. Representative pictures and quantification of trisomic cells for chromosome 15 are shown in F and G, respectively. Cells with a nuclear diameter >10 μm, characteristic of tetraploid cells (Table S1), are reported in H. In E, G, and H, numbers refer to the incidence of the indicated cells per total cells analyzed (mean ± SEM; 5 mice per group). NS, not significant; *P < 0.05, **P < 0.01 (Student t test), compared with WT C57BL/6 controls.
These results support the notion that oncogenesis-associated tetraploidization is avoided by long-term treatment with resveratrol or aspirin.
Discussion
In the present work, we show that several agents that are well reputed for their chemopreventive action, namely resveratrol (and its hydroxylated metabolite piceatannol) and aspirin (and its active deacetylated derivative salicylate) are endowed with the capacity of reducing the generation, proliferation, and survival of tetraploid cells, in a variety of cellular models comprising several human and mouse cancer cell lines, as well as primary epithelial cells, in vitro, ex vivo, and in vivo. Although chemoprevention by resveratrol in humans is debated (39, 40), there is ample evidence that prolonged daily intake of aspirin has a major preventive effect on proximal colon cancer (41).
Resveratrol has multiple targets within cells (42, 43). Based on our results, it can be excluded that inhibition of respiratory chain complex I or activation of sirtuin-1 by resveratrol participates in the selective antitetraploid effect of resveratrol. Rather, it appears that an increase in intracellular cAMP levels with subsequent PKA activation (28) is involved in this effect, because pharmacological manipulations designed to lower cAMP or to suppress PKA reduced the killing of tetraploid cells by resveratrol. At least in the context of colon cancer, aspirin is generally thought to act via inhibition of prostaglandin-endoperoxide synthase 2 (PTGS2, better known as cyclooxygenase-2, COX2) (44, 45). Nonetheless, we did not observe that more selective COX2 inhibitors than aspirin would selectively kill tetraploid cells (Fig. S10). Reportedly, aspirin activates AMPK, and salicylate directly interacts with AMPK (31, 32). In line with this evidence, we found indeed that multiple ways of activating AMPK either directly (with compounds such as AICAR or A769662) or indirectly (by energy depletion due to nutrient shortage or inhibition of glycolysis) were selectively toxic for tetraploid cells. That said, resveratrol and salicylate induced a similar degree of AMPK activation in tetraploid and diploid cells. Thus, it appears that a baseline level of AMPK activation is required for cell survival and that, for yet-to-be-elucidated reasons, tetraploid cells tolerate a lower degree of AMPK activation. One of the downstream targets of AMPK is 3-hydroxy-3-methylglutaryl CoA (HMG-CoA) reductase, which undergoes an inhibitory phosphorylation. Interestingly, statins (which inhibit HMG-CoA reductase and have putative chemopreventive effects) (46) also killed tetraploid HCT116 cells more efficiently than diploid cells (Fig. S10), suggesting that this pathway may operate downstream of AMPK.
Irrespective of these speculations, our results establish a novel continuum between selective antitetraploid effects and chemoprevention. We provide evidence that several highly effective chemopreventive agents, including resveratrol and aspirin as well as their active metabolites, eliminate tetraploid cells from mixed diploid/tetraploid cultures and prevent the formation of tetraploid cells in vitro and in vivo, in a model of intestinal carcinogenesis. Based on these findings, it may be interesting to launch large high-throughput screening efforts for the identification of novel antitetraploids with putative chemopreventive properties. One of the challenges will be to identify agents without major side effects (such as bleeding induced by aspirin), including those that might arise from the perturbation of physiological polyploidization processes.
Materials and Methods
Mouse Strains and in Vivo Experiments.
Six-week-old C57BL/6 and ApcMin/+ mice (Charles River) were treated for 5 wk by oral gavage (five times a week) with resveratrol (100 mg/kg) or aspirin (25 mg/kg), both suspended in water. After the mice were killed, intestines were processed for flow cytometric determination of DNA content or in situ hybridization. Tp53−/− mice were obtained by crossing Tp53+/− C57BL/6 (CNRS UMR6218).
Mice were maintained in specific pathogen-free conditions. Animal experiments were approved by the local Ethics Committee [Comité d'éthique en expérimentation animale (CEEA) Integrated Research Cancer Institute in Villejuif (IRCIV)/Institut Gustave Roussy (IGR) n°26, registered with the French Ministry of Research], and in accordance with the Directive EU 63/2010. Experiments followed the Federation for Laboratory Animal Science Association guidelines.
FISH.
Intestines were rinsed in PBS and fixed in formalin solution (DiaPath) before paraffin embedding. Ten-micrometer slices were processed (KBI-60004 Tissue Digestion kit II; Kreatech Diagnostics), and hybridized with two FISH probes targeting chromosomes 8 or 15 [XCyting Muticolor FISH Probes (XMP) 8 green and XMP 15 orange, MetaSystems]. Images were acquired at 0.4-μm intervals in the z dimension, using an oil immersion objective (63×). Images were analyzed with the open-source software Image J. Scoring of FISH signals was performed by manual counting. Nuclear diameter was measured at the largest cross-section.
Supplementary Material
Acknowledgments
We thank Dr. Benoit Viollet (Cochin Institute) for plasmids. G.K. is supported by the Ligue contre le Cancer, Agence National de la Recherche, Association pour la recherche sur le cancer, Cancéropôle Ile-de-France, Institut National du Cancer, Fondation Bettencourt-Schueller, Fondation de France, Fondation pour la Recherche Médicale, the European Commission (ArtForce), the European Research Council (Advanced Investigator Award), the LabEx Immuno-Oncology, the Site de Recherche Intégrée sur le Cancer (SIRIC) Stratified Oncology Cell DNA Repair and Tumor Immune Elimination, the SIRIC Cancer Research and Personalized Medicine, and the Paris Alliance of Cancer Research Institutes. I.V. is supported by Associazione Italiana per la Ricerca sul Cancro.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1318440111/-/DCSupplemental.
References
- 1.Ganem NJ, Pellman D. Limiting the proliferation of polyploid cells. Cell. 2007;131(3):437–440. doi: 10.1016/j.cell.2007.10.024. [DOI] [PubMed] [Google Scholar]
- 2.Gordon DJ, Resio B, Pellman D. Causes and consequences of aneuploidy in cancer. Nat Rev Genet. 2012;13(3):189–203. doi: 10.1038/nrg3123. [DOI] [PubMed] [Google Scholar]
- 3.Storchova Z, Kuffer C. The consequences of tetraploidy and aneuploidy. J Cell Sci. 2008;121(Pt 23):3859–3866. doi: 10.1242/jcs.039537. [DOI] [PubMed] [Google Scholar]
- 4.Vitale I, et al. Illicit survival of cancer cells during polyploidization and depolyploidization. Cell Death Differ. 2011;18(9):1403–1413. doi: 10.1038/cdd.2010.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vitale I, Galluzzi L, Castedo M, Kroemer G. Mitotic catastrophe: A mechanism for avoiding genomic instability. Nat Rev Mol Cell Biol. 2011;12(6):385–392. doi: 10.1038/nrm3115. [DOI] [PubMed] [Google Scholar]
- 6.Senovilla L, et al. An immunosurveillance mechanism controls cancer cell ploidy. Science. 2012;337(6102):1678–1684. doi: 10.1126/science.1224922. [DOI] [PubMed] [Google Scholar]
- 7.Davoli T, de Lange T. The causes and consequences of polyploidy in normal development and cancer. Annu Rev Cell Dev Biol. 2011;27:585–610. doi: 10.1146/annurev-cellbio-092910-154234. [DOI] [PubMed] [Google Scholar]
- 8.Andreassen PR, Lohez OD, Lacroix FB, Margolis RL. Tetraploid state induces p53-dependent arrest of nontransformed mammalian cells in G1. Mol Biol Cell. 2001;12(5):1315–1328. doi: 10.1091/mbc.12.5.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Castedo M, et al. Apoptosis regulation in tetraploid cancer cells. EMBO J. 2006;25(11):2584–2595. doi: 10.1038/sj.emboj.7601127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vitale I, et al. Multipolar mitosis of tetraploid cells: Inhibition by p53 and dependency on Mos. EMBO J. 2010;29(7):1272–1284. doi: 10.1038/emboj.2010.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boilève A, et al. Immunosurveillance against tetraploidization-induced colon tumorigenesis. Cell Cycle. 2013;12(3):473–479. doi: 10.4161/cc.23369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fujiwara T, et al. Cytokinesis failure generating tetraploids promotes tumorigenesis in p53-null cells. Nature. 2005;437(7061):1043–1047. doi: 10.1038/nature04217. [DOI] [PubMed] [Google Scholar]
- 13.Senovilla L, et al. p53 represses the polyploidization of primary mammary epithelial cells by activating apoptosis. Cell Cycle. 2009;8(9):1380–1385. doi: 10.4161/cc.8.9.8305. [DOI] [PubMed] [Google Scholar]
- 14.Caldwell CM, Green RA, Kaplan KB. APC mutations lead to cytokinetic failures in vitro and tetraploid genotypes in Min mice. J Cell Biol. 2007;178(7):1109–1120. doi: 10.1083/jcb.200703186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dikovskaya D, et al. Loss of APC induces polyploidy as a result of a combination of defects in mitosis and apoptosis. J Cell Biol. 2007;176(2):183–195. doi: 10.1083/jcb.200610099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weaver BA, Silk AD, Cleveland DW. Cell biology: Nondisjunction, aneuploidy and tetraploidy. Nature. 2006;442(7104):E9–E10, discussion E10. doi: 10.1038/nature05139. [DOI] [PubMed] [Google Scholar]
- 17.Ganem NJ, Godinho SA, Pellman D. A mechanism linking extra centrosomes to chromosomal instability. Nature. 2009;460(7252):278–282. doi: 10.1038/nature08136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Silk AD, et al. Chromosome missegregation rate predicts whether aneuploidy will promote or suppress tumors. Proc Natl Acad Sci USA. 2013;110(44):E4134–E4141. doi: 10.1073/pnas.1317042110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vitale I, et al. Inhibition of Chk1 kills tetraploid tumor cells through a p53-dependent pathway. PLoS ONE. 2007;2(12):e1337. doi: 10.1371/journal.pone.0001337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marxer M, et al. Tetraploidization increases sensitivity to Aurora B kinase inhibition. Cell Cycle. 2012;11(13):2567–2577. doi: 10.4161/cc.20947. [DOI] [PubMed] [Google Scholar]
- 21.Kwon M, et al. Mechanisms to suppress multipolar divisions in cancer cells with extra centrosomes. Genes Dev. 2008;22(16):2189–2203. doi: 10.1101/gad.1700908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rello-Varona S, et al. Preferential killing of tetraploid tumor cells by targeting the mitotic kinesin Eg5. Cell Cycle. 2009;8(7):1030–1035. doi: 10.4161/cc.8.7.7950. [DOI] [PubMed] [Google Scholar]
- 23.Piotrowska H, Kucinska M, Murias M. Biological activity of piceatannol: Leaving the shadow of resveratrol. Mutat Res. 2012;750(1):60–82. doi: 10.1016/j.mrrev.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 24.Potter GA, et al. The cancer preventative agent resveratrol is converted to the anticancer agent piceatannol by the cytochrome P450 enzyme CYP1B1. Br J Cancer. 2002;86(5):774–778. doi: 10.1038/sj.bjc.6600197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moreira AC, Silva AM, Santos MS, Sardão VA. Resveratrol affects differently rat liver and brain mitochondrial bioenergetics and oxidative stress in vitro: investigation of the role of gender. Food Chem Toxicol. 2013;53:18–26. doi: 10.1016/j.fct.2012.11.031. [DOI] [PubMed] [Google Scholar]
- 26.Zini R, Morin C, Bertelli A, Bertelli AA, Tillement JP. Effects of resveratrol on the rat brain respiratory chain. Drugs Exp Clin Res. 1999;25(2-3):87–97. [PubMed] [Google Scholar]
- 27.Galluzzi L, et al. Molecular definitions of cell death subroutines: recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ. 2012;19(1):107–120. doi: 10.1038/cdd.2011.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park SJ, et al. Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting cAMP phosphodiesterases. Cell. 2012;148(3):421–433. doi: 10.1016/j.cell.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dyke HJ, Montana JG. Update on the therapeutic potential of PDE4 inhibitors. Expert Opin Investig Drugs. 2002;11(1):1–13. doi: 10.1517/13543784.11.1.1. [DOI] [PubMed] [Google Scholar]
- 30.Morselli E, et al. Caloric restriction and resveratrol promote longevity through the Sirtuin-1-dependent induction of autophagy. Cell Death Dis. 2010;1:e10. doi: 10.1038/cddis.2009.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Din FV, et al. Aspirin inhibits mTOR signaling, activates AMP-activated protein kinase, and induces autophagy in colorectal cancer cells. Gastroenterology. 2012;142(7) doi: 10.1053/j.gastro.2012.02.050. 1504, e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hawley SA, et al. The ancient drug salicylate directly activates AMP-activated protein kinase. Science. 2012;336(6083):918–922. doi: 10.1126/science.1215327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bunz F, et al. Disruption of p53 in human cancer cells alters the responses to therapeutic agents. J Clin Invest. 1999;104(3):263–269. doi: 10.1172/JCI6863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Su LK, et al. Multiple intestinal neoplasia caused by a mutation in the murine homolog of the APC gene. Science. 1992;256(5057):668–670. doi: 10.1126/science.1350108. [DOI] [PubMed] [Google Scholar]
- 35.Mahmoud NN, et al. Aspirin prevents tumors in a murine model of familial adenomatous polyposis. Surgery. 1998;124(2):225–231. [PubMed] [Google Scholar]
- 36.Sale S, et al. Comparison of the effects of the chemopreventive agent resveratrol and its synthetic analog trans 3,4,5,4′-tetramethoxystilbene (DMU-212) on adenoma development in the Apc(Min+) mouse and cyclooxygenase-2 in human-derived colon cancer cells. Int J Cancer. 2005;115(2):194–201. doi: 10.1002/ijc.20884. [DOI] [PubMed] [Google Scholar]
- 37.Sansom OJ, Stark LA, Dunlop MG, Clarke AR. Suppression of intestinal and mammary neoplasia by lifetime administration of aspirin in Apc(Min/+) and Apc(Min/+), Msh2(-/-) mice. Cancer Res. 2001;61(19):7060–7064. [PubMed] [Google Scholar]
- 38.Schneider Y, et al. Resveratrol inhibits intestinal tumorigenesis and modulates host-defense-related gene expression in an animal model of human familial adenomatous polyposis. Nutr Cancer. 2001;39(1):102–107. doi: 10.1207/S15327914nc391_14. [DOI] [PubMed] [Google Scholar]
- 39.Chung JH, Manganiello V, Dyck JR. Resveratrol as a calorie restriction mimetic: Therapeutic implications. Trends Cell Biol. 2012;22(10):546–554. doi: 10.1016/j.tcb.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patel KR, et al. Clinical trials of resveratrol. Ann N Y Acad Sci. 2011;1215:161–169. doi: 10.1111/j.1749-6632.2010.05853.x. [DOI] [PubMed] [Google Scholar]
- 41.Rothwell PM, et al. Effect of daily aspirin on long-term risk of death due to cancer: Analysis of individual patient data from randomised trials. Lancet. 2011;377(9759):31–41. doi: 10.1016/S0140-6736(10)62110-1. [DOI] [PubMed] [Google Scholar]
- 42.Athar M, Back JH, Kopelovich L, Bickers DR, Kim AL. Multiple molecular targets of resveratrol: Anti-carcinogenic mechanisms. Arch Biochem Biophys. 2009;486(2):95–102. doi: 10.1016/j.abb.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baur JA, Sinclair DA. Therapeutic potential of resveratrol: The in vivo evidence. Nat Rev Drug Discov. 2006;5(6):493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- 44.Chan AT, Ogino S, Fuchs CS. Aspirin and the risk of colorectal cancer in relation to the expression of COX-2. N Engl J Med. 2007;356(21):2131–2142. doi: 10.1056/NEJMoa067208. [DOI] [PubMed] [Google Scholar]
- 45.Liao X, et al. Aspirin use, tumor PIK3CA mutation, and colorectal-cancer survival. N Engl J Med. 2012;367(17):1596–1606. doi: 10.1056/NEJMoa1207756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nielsen SF, Nordestgaard BG, Bojesen SE. Statin use and reduced cancer-related mortality. N Engl J Med. 2012;367(19):1792–1802. doi: 10.1056/NEJMoa1201735. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.