Significance
Light and dark stimuli are separately processed by ON and OFF channels in retina and thalamus. Although most textbooks assume that ON and OFF visual responses are relatively balanced throughout the visual system, recent studies have identified a pronounced overrepresentation of OFF responses in the cerebral cortex. This recent discovery resonates with Galileo and Helmholtz’s pioneering observations that visual spatial resolution is higher for darks than lights. In this paper, we demonstrate that these two seemingly separate findings are related and caused by a pronounced difference between ON and OFF luminance response functions, which most likely originates in photoreceptors. Therefore, asymmetric ON and OFF neural responses provide the neurophysiological explanation for an almost four-century-old puzzle dating back to Galileo.
Keywords: neuronal coding, lateral geniculate nucleus, area V1, irradiation illusion, LFP
Abstract
Astronomers and physicists noticed centuries ago that visual spatial resolution is higher for dark than light stimuli, but the neuronal mechanisms for this perceptual asymmetry remain unknown. Here we demonstrate that the asymmetry is caused by a neuronal nonlinearity in the early visual pathway. We show that neurons driven by darks (OFF neurons) increase their responses roughly linearly with luminance decrements, independent of the background luminance. However, neurons driven by lights (ON neurons) saturate their responses with small increases in luminance and need bright backgrounds to approach the linearity of OFF neurons. We show that, as a consequence of this difference in linearity, receptive fields are larger in ON than OFF thalamic neurons, and cortical neurons are more strongly driven by darks than lights at low spatial frequencies. This ON/OFF asymmetry in linearity could be demonstrated in the visual cortex of cats, monkeys, and humans and in the cat visual thalamus. Furthermore, in the cat visual thalamus, we show that the neuronal nonlinearity is present at the ON receptive field center of ON-center neurons and ON receptive field surround of OFF-center neurons, suggesting an origin at the level of the photoreceptor. These results demonstrate a fundamental difference in visual processing between ON and OFF channels and reveal a competitive advantage for OFF neurons over ON neurons at low spatial frequencies, which could be important during cortical development when retinal images are blurred by immature optics in infant eyes.
Light and dark stimuli are separately processed by ON and OFF channels in the retina and visual thalamus. Surprisingly, although most textbooks assume that ON and OFF visual responses are balanced throughout the visual system, recent studies have identified a pronounced overrepresentation of the OFF visual responses in primary visual cortex (area V1) (1–3). This recent discovery resonates with pioneering studies by Galilei (4) and von Helmholtz (5) who noticed that visual spatial resolution was higher for dark than light stimuli. Galilei (4) related the difference in resolution to the observation that a light patch on a dark background appears larger than the same sized dark patch on a light background, an illusion that von Helmholtz (5) named the “irradiation illusion.” Although this illusion has been studied in the past (6, 7), its underlying neuronal mechanisms remain unknown. It has been suggested that the perceived size differences could be caused by the light scatter in the optics of the eye followed by a neuronal nonlinearity (6, 7), but there are no neuronal measurements of a nonlinearity that fits the explanation. Previous studies revealed differences in response linearity between ON and OFF retinal ganglion cells (8, 9) and horizontal cells (10). However, a main conclusion from these studies was that ON retinal ganglion cells were roughly linear and less rectified than OFF retinal ganglion cells (8, 9), which is exactly the opposite of what would be needed to explain the irradiation illusion. Moreover, it remains unclear if ON/OFF retinal differences in response linearity and response gain propagate from retina to visual cortex. To investigate the neuronal mechanisms of the irradiation illusion, we recorded neuronal activity in the visual thalamus and cortex of anesthetized cats, local field potentials in awake monkeys, and visually evoked potentials in humans. We show that OFF neurons in thalamus and cortex increase their responses roughly linearly with luminance contrast, independently of the background luminance. In contrast, ON neurons saturate their responses with small increases in luminance, and approach the linearity of the OFF neurons only on bright backgrounds that make ON responses weaker. We also show that a simple model that uses an early retinal nonlinearity can explain several seemingly unrelated ON/OFF spatial asymmetries, including the difference in spatial resolution between darks and lights, the spatial frequency dependence of OFF dominance in visual cortex, and the difference in receptive field size between ON and OFF retinal ganglion cells. Moreover, because the asymmetry between ON and OFF neurons is present both at the receptive field center and surround of thalamic neurons, our results strongly suggest that it originates at the level of photoreceptors.
Results
Irradiation Illusion.
To investigate the neuronal correlate of the illusion, we recorded extracellular activity of single neurons in the lateral geniculate nucleus (LGN) and multiunit activity in the primary visual cortex (V1) of cats. We used light patches on a dark background to map the receptive fields of ON-center cells and dark patches on a light background to map the receptive fields of OFF-center cells. We hypothesized that light patches are perceived as larger than dark patches when the stimulation conditions make ON receptive fields larger than OFF receptive fields (8, 11). Consistent with this interpretation, our measurements revealed that the average receptive field center is larger in ON than OFF cells in LGN (Fig. 1A, Upper; ONsize/OFFsize = 1.47, P < 0.001, Wilcoxon test used here and in all subsequent statistical comparisons) and a significant difference could also be demonstrated in V1 (Fig. 1A, Lower; ONsize/OFFsize = 1.29, P < 0.001). As with the irradiation illusion (5, 7), a gray background made the differences between ON and OFF receptive field sizes disappear in LGN (Fig. 1B, Upper; ONsize/OFFsize = 0.99, P = 1) and could even reverse them slightly in V1 (Fig. 1B, Lower; ONsize/OFFsize = 0.8, P = 0.01).
Fig. 1.
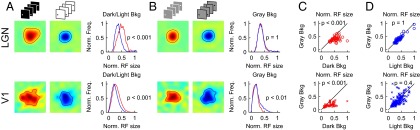
Correlate of the irradiation illusion in visual thalamus and cortex. (A) Receptive fields of ON-center LGN cells are larger than receptive fields of OFF-center LGN cells when mapped in conditions that resemble the irradiation illusion (ON, light targets on dark background; OFF, dark targets on light background) (Upper). Likewise, ON subfields of V1 neurons are larger than OFF subfields of V1 neurons (Lower). Receptive field maps (Left and Middle) are shown for ON and OFF LGN cells that are retinotopically aligned and for ON and OFF visual responses of a V1 recording site. The distributions of receptive field size (Right) have been normalized by the maximum receptive field size measured. In this and the following figures, red color represents ON and blue represents OFF. (B) As in the irradiation illusion on gray background, differences in receptive field size disappear in LGN (Upper) and slightly reverse in V1 (Lower). (C) ON receptive field sizes are larger in the dark than on gray backgrounds (Upper circles, LGN; Lower crosses, V1). (D) OFF receptive field sizes are similar in light and gray backgrounds (Upper circles, LGN; Lower crosses, V1).
Interestingly, although the receptive field sizes of ON LGN and V1 neurons were strongly affected by background illumination (Fig. 1C), the receptive field sizes of OFF neurons were similar in light and gray backgrounds (Fig. 1D). These differences in receptive field size could be demonstrated in most thalamic neurons (Fig. 1 C and D) and could be replicated across neurons of different types (SI Text).
Spatial Resolution.
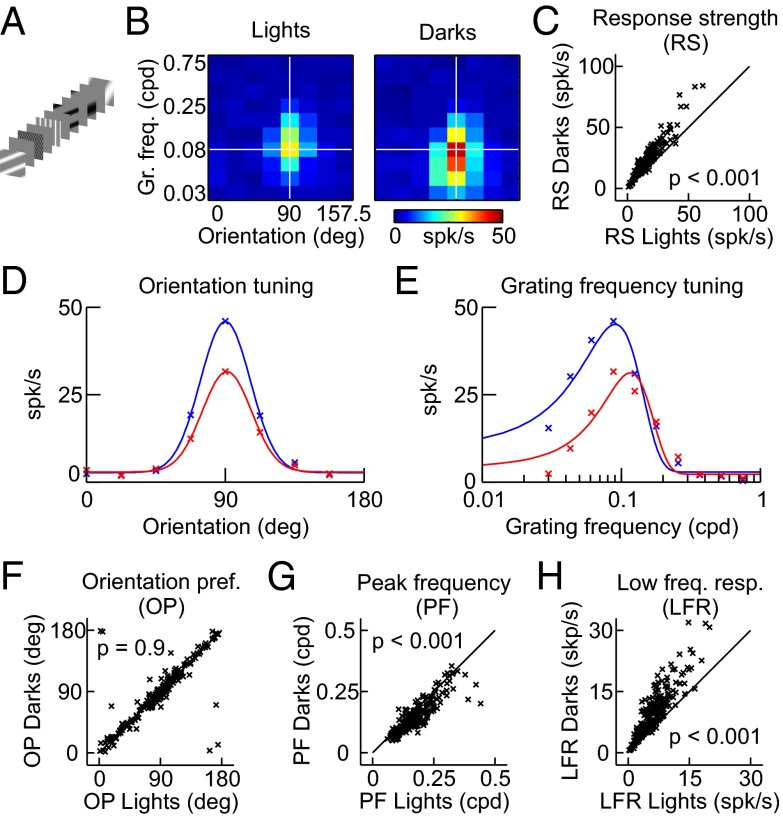
Consistent with the irradiation illusion, these measurements suggest that the spatial resolution of LGN and V1 neurons is higher for dark than light stimuli. Surprisingly, measurements with gratings in V1 seemed to indicate the opposite: The ON channel responded to higher grating frequencies than the OFF channel. Grating spatial resolution of ON and OFF visual responses in V1 was measured with flashed stationary half-wave rectified sinusoidal gratings of varying spatial frequency, orientation, and phase on a gray background (Fig. 2A). Overall, V1 responses were stronger when driven by the dark half of the rectified sinusoidal grating than by the light half (Fig. 2 B and C), confirming a cortical OFF dominance previously demonstrated in cats, primates, and humans (1–3, 12, 13). As expected, ON and OFF visual responses had similar orientation preference (Fig. 2 D and F; ON-OFF = 3°, P = 0.9); however, the orientation bandwidth was slightly broader for OFF than ON (ON/OFF = 0.9, P < 0.001). More surprisingly, ON visual responses had a higher peak grating frequency (Fig. 2 E and G; ON/OFF = 1.2, P < 0.001) and were more weakly driven by low grating frequencies than OFF visual responses (Fig. 2 E and H; ON/OFF = 0.6, P < 0.001). These ON/OFF differences in grating frequency tuning were robust and could also be demonstrated with reverse correlation methods (SI Text). Therefore, the measurements with rectified sinusoidal gratings suggest that the ON visual channel can respond to higher-frequency gratings than the OFF visual channel, just the contrary of what we concluded from measurements of receptive field size (Fig. 1). How can we reconcile these two seemingly contradictory findings?
Fig. 2.
Orientation and grating frequency tuning of lights and darks in V1. (A) Stimulus design. Light and dark half-wave rectified sinusoidal gratings were flashed for 100 ms on a gray background. Grating frequency and orientation were varied in random order. (B) Example response maps to light and dark gratings measured in a V1 recording site. Dark gratings caused stronger responses than light gratings, especially when the grating frequency was low. (C) The V1 response strength (RS) was consistently higher for dark than light gratings. (D) Orientation tuning estimated at the peak grating frequency (horizontal white lines in B). (E) Grating frequency tuning estimated at the preferred orientation (vertical white lines in B). (F) V1 orientation preference (OP) was similar when measured with dark and light gratings. (G) V1 peak grating frequency (PF) was higher when measured with light than dark gratings. (H) The V1 low frequency response (LFR) was much stronger for dark than light gratings. LFR, value of Gaussian fit at a grating frequency of 0 cpd.
Nonlinear Luminance/Response Function.
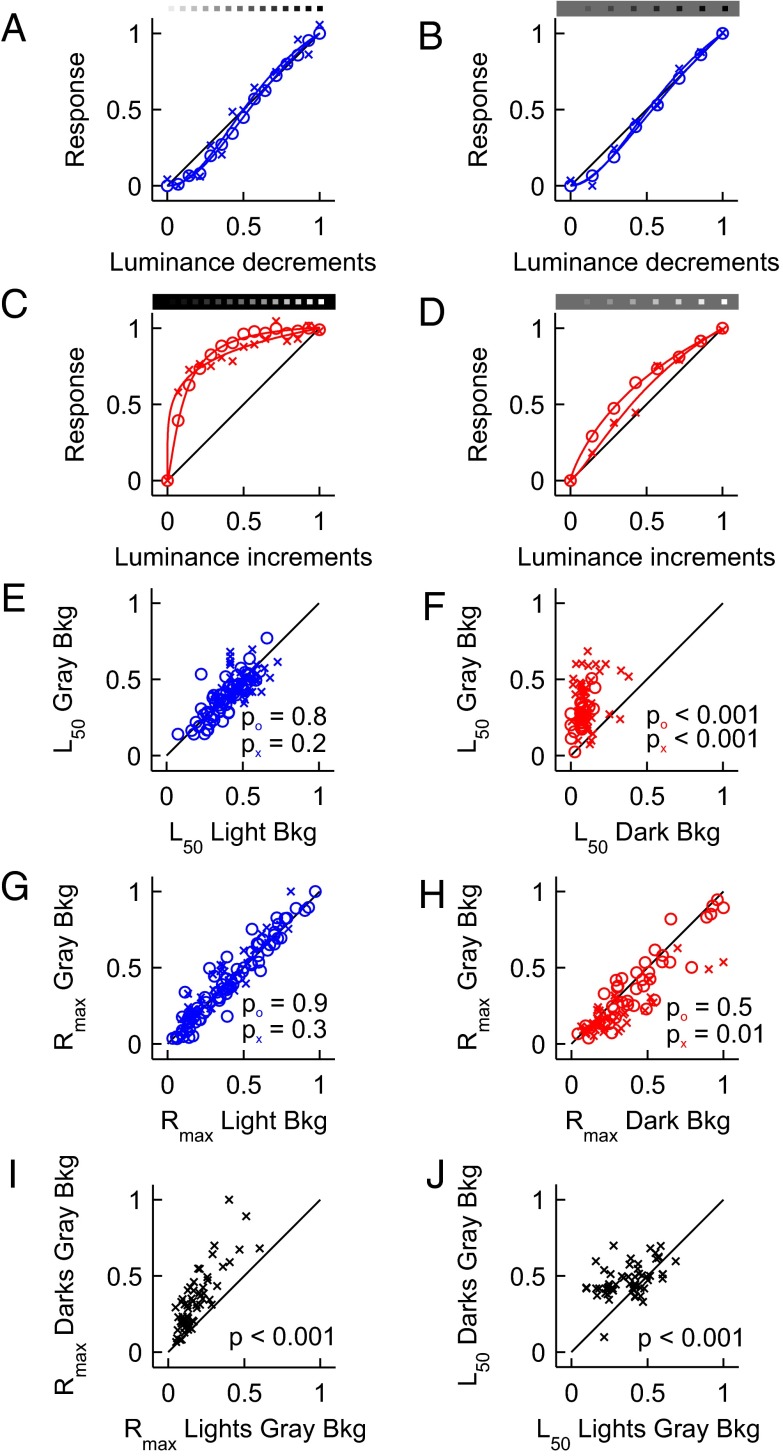
We recently proposed (7) that differences in spatial resolution between darks and lights could be caused by a compressive nonlinearity in the luminance/response function at the retina (6, 7). However, this explanation seemed unlikely because such nonlinearity would cause pronounced differences in the strength of ON and OFF visual responses throughout the visual pathway. Because these differences in response strength were not reported before in the retina/LGN of cats (14, 15) and/or V1 input layers of primates (1, 2), we decided to directly measure the luminance/response functions of LGN and V1 neurons. To our surprise, OFF visual responses increased their strength roughly linearly with luminance contrast, independently of the background luminance, in both LGN and cortex (Fig. 3 A and B; LGN, circles; V1, crosses). Conversely, ON visual responses saturated with very small increases in luminance and required gray backgrounds to approach the linearity of the OFF visual channel (Fig. 3 C and D). In both LGN and V1, OFF visual responses had similar luminance half-saturation (L50) in light and gray backgrounds (Fig. 3E; LGN OFF L50 light/gray = 0.96, P = 0.8; V1 OFF L50 light/gray = 1.03, P = 0.2). On the contrary, ON visual responses had an L50 that was three to four times higher in dark than gray backgrounds (Fig. 3F; LGN ON L50 dark/gray = 0.26, P < 0.001; V1 ON L50 dark/gray = 0.3, P < 0.001). It should be noted that the differences in linearity between darks and lights could not be explained simply by differences in the level of light adaptation because the luminance half-saturation (L50) was still higher for darks than lights on gray backgrounds (LGN OFF/ON L50 = 1.49, P < 0.0001; see below for similar results with V1 data).
Fig. 3.
Luminance/response functions of ON and OFF visual responses. (A and B) Luminance/response functions of OFF responses in LGN (circles) and V1 (crosses) are roughly linear when measured on light (A) and gray (B) backgrounds. Visual responses were fitted with a Naka-Rushton function and the half saturation (L50) and maximum response (Rmax) constants were estimated. (C) Luminance/response functions for ON responses in LGN (circles) and V1 (crosses) saturated with small luminance increments on dark backgrounds. (D) On gray backgrounds, the ON luminance/response functions became more linear; however, ON functions were still more compressive than OFF functions. (E) L50 for OFF responses was similar on gray and light backgrounds. (F) L50 for ON responses was much higher on gray than dark backgrounds. (G) Rmax for OFF responses was similar in gray and light backgrounds. (H) In LGN (circles), Rmax for ON responses was similar in dark and gray backgrounds. In V1 (crosses), Rmax for ON responses was lower in gray than dark backgrounds. (I and J) On gray backgrounds, Rmax (I) and L50 (J) in V1 were larger for OFF than ON responses. Notice that I and J compared OFF and ON responses generated by the same V1 recording site.
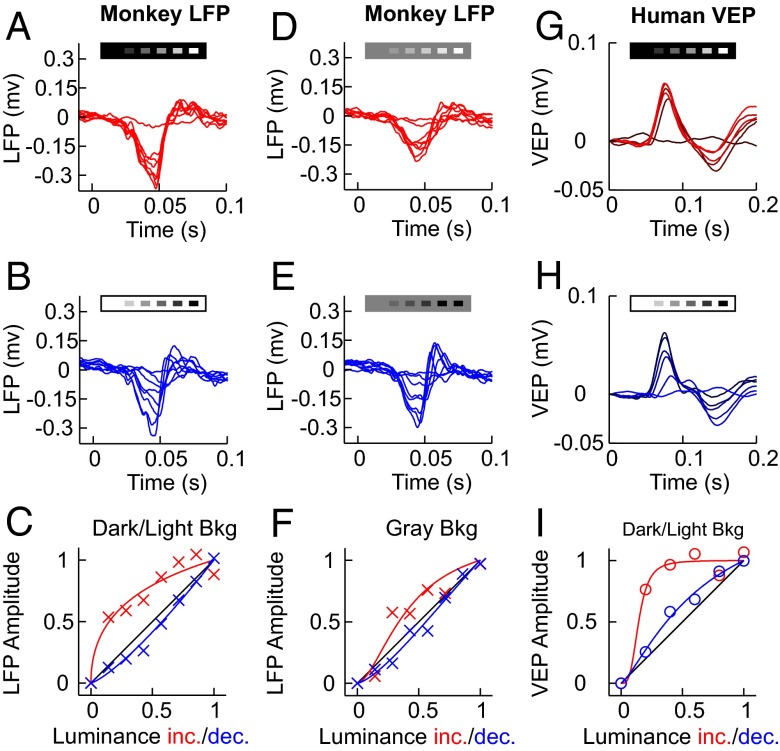
Importantly, changes in background illumination from gray to light/dark did not affect the maximum response (Rmax) of LGN neurons (Fig. 3 G and H; LGN OFF Rmax light/gray = 0.96, P = 0.9; LGN ON Rmax dark/gray = 1.13, P = 0.5) or V1 OFF responses but affected V1 ON responses (Fig. 3 G and H; V1 OFF Rmax light/gray = 0.89, P = 0.3; V1 ON Rmax light/gray = 1.45, P = 0.01). V1 neurons generated weaker ON than OFF responses on gray backgrounds (Fig. 3I; V1 Rmax OFF/ON = 2.1, P < 0.001) even if the L50 was still larger for OFF responses (Fig. 3J; V1 L50 OFF/ON = 1.25, P < 0.001). OFF responses were also slightly stronger than ON responses in LGN; however, the difference was smaller than in V1 (LGN Rmax OFF/ON = 1.3, P = 0.03). Therefore, we conclude that OFF dominance is already present in the visual thalamus but is greatly amplified in visual cortex, consistent with previous cortical measurements (1–3). Importantly, these differences in ON/OFF response linearity in the cat could be replicated in the visual cortex of the awake monkey using local field potential recordings (Fig. 4 A–F; dark/light background OFF/ON L50 = 2.5, P < 0.001; gray background OFF/ON L50 = 1.3, P < 0.01; ON L50 dark/gray = 0.47, P < 0.001; OFF L50 light/gray = 0.87, P = 0.75; n = 21) and humans using visually evoked potentials (Fig. 4 G–I, dark/light background OFF/ON L50 = 3.23, P < 0.01, n = 6). Therefore, these differences in linearity between ON and OFF responses are present in different species, are effectively transmitted across the visual pathway, and are likely to play an important role in cortical processing.
Fig. 4.
ON/OFF asymmetry in awake monkeys and human. (A and B) LFP responses in V1 of awake monkeys saturate with small luminance increments in dark backgrounds (A) but encode luminance decrements roughly linearly on light backgrounds (B). (C) LFP luminance/response functions for lights and darks. (D and E) LFP responses on a gray background still saturate with small increments in luminance (D) but encode luminance decrements roughly linearly on gray backgrounds (E). (F) LFP luminance/response function on a gray background. (G–I) Human VEPs show similar asymmetry in the integration of darks and lights. Note that we measured luminance/response functions only on dark/light backgrounds due to the low signal-to-noise level in VEP recordings. Stimuli were small squares (monkey LFP) and full-field checkerboards (human VEP). See SI Text for more details.
Origin of the Nonlinearity in the Luminance/Response Function.
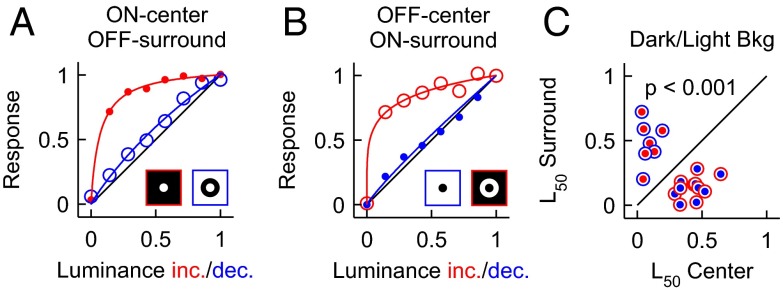
Where does the ON response nonlinearity originate in the visual pathway? Having the nonlinearity restricted to the ON receptive field center would indicate an origin in ON-center retinal cells (e.g., ganglion cells or bipolar cells). However, if the nonlinearity was also present in the ON surround of OFF-center cells, its origin could be as early as the photoreceptor itself. To distinguish between these two possibilities, we compared the center and surround luminance/response functions of ON-center and OFF-center LGN cells (Fig. 5). Our results demonstrate that the visual responses are nonlinear in both ON centers and ON surrounds (Fig. 5; ON-center L50 = 0.06, ON-surround L50 = 0.13, P = 0.234). Conversely, visual responses are similarly linear in OFF centers and OFF surrounds (Fig. 5; OFF-center L50 = 0.44, OFF-surround L50 = 0.47, P = 0.425). On average, OFF visual responses are more linear than ON visual responses in dark/light backgrounds (Fig. 5; ON-center vs. OFF-surround L50 = 0.06 vs. 0.47, P < 0.001; OFF-center vs. ON-surround L50 = 0.44 vs. 0.13, P < 0.001) and gray backgrounds (ON-center vs. OFF-surround L50 = 0.08 vs. 0.33, P = 0.03; OFF-center vs. ON-surround L50 = 0.32 vs. 0.19, P = 0.03). Moreover, the response suppression is also more saturating in ON than OFF surrounds (OFF-S50/ON-S50 = 1.5, P < 0.001; SI Text). These results indicate that the luminance/response nonlinearity is not restricted to ON-center cells and could originate as early as the photoreceptor.
Fig. 5.
Luminance/response functions of LGN receptive field center and surround. (A) Responses of an ON-center cell to luminance increments presented at the center (red dots) are more saturating than responses to luminance decrements presented only in the surround (blue circles). (B) Responses of an OFF-center cell to luminance decrements presented at the center (blue dots) are less saturating than responses to luminance increments presented in the surround (red circles). (C) L50 of center and surround of LGN cells suggest that the luminance nonlinearity is a property of the ON visual responses and not the ON-center cells. ON cells are shown by red dots with blue circles and OFF cells by blue dots with red circles. Note that both ON-center and ON-surrounds have low L50 values.
Early Retinal Nonlinearity Can Explain Spatial Asymmetries for Lights and Darks.
The luminance/response functions that we measured in the cat can fully explain the seemingly contradictory finding that the ON visual channel responds to higher-frequency gratings than the OFF visual channel, even if the average ON receptive field is larger. As demonstrated above, in the early retinal circuitry, the luminance/response function for darks (FD) on a light background is more linear (F) than the luminance/response function for lights (FL) on a dark background (Fig. 6A). Therefore, these luminance/response functions cause a greater spatial distortion (neural blur) of light stimuli (DL) than dark stimuli (DD) (Fig. 6B, Left). Notice that this neuronal blur is very different from the optical blur in that it is not linear and changes with background luminance. On binary backgrounds, the early retinal nonlinearity makes ON retinal ganglion cells responsive over a larger area of their dendritic field than OFF retinal ganglion cells and, consequently, lights are expected to look larger than darks of the same physical size (Fig. 6B, Right). On gray backgrounds, the nonlinearities being more similar, retinal ganglion cells are responsive over similar areas of the dendritic field and the irradiation illusion is expected to become weaker. Note that, although ON retinal ganglion cells have larger dendritic fields than OFF cells, the differences in dendritic field size are constant, whereas the magnitude of the illusion changes with changes in the luminance/response nonlinearity. Interestingly, the ratio of spatial distortion between dark and light stimuli, DL/DD also matches the ratio between dendritic field diameters of ON (ONdiam) and OFF (OFFdiam) retinal ganglion cells (11). That is ONdiam ∼ OFFdiam × DL/DD (Fig. 6C). Because the dendrites of retinal ganglion cells are shaped by visual experience (16) and the early retinal nonlinearity blurs more lights than darks, it could be speculated that the nonlinearity makes ON dendritic fields to grow larger than OFF dendritic fields of retinal ganglion cells during development and shape the fundamental layout of the retinal mosaics (11, 17, 18) (SI Text).
Fig. 6.
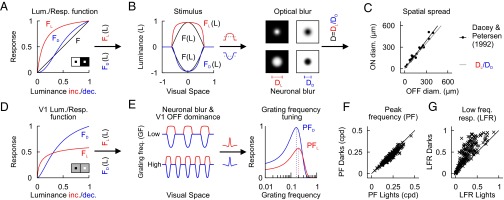
ON and OFF luminance/response functions explain difference in spatial resolution between darks and lights. (A) Luminance/response functions for lights (FL) and darks (FD) on dark/light backgrounds (stimulus shown in Inset). FD is closer to a linear function (F) than FL. (B) A stimulus projected on the retina F(L) is blurred by both the optics of the eyes (optical blur) and the luminance/response functions (neuronal blur). The light stimulus FL(L) becomes broader than the dark stimulus FD(L) and, therefore, the spatial distortion is larger for lights than darks (DL > DD). (C) The ratio DL/DD matches the ratio between the dendritic field diameters of ON (ONdiam) and OFF (OFFdiam) retinal ganglion cells (11). That is, ONdiam ∼ OFFdiam × DL/DD. (D) V1 luminance/response functions for lights and darks on gray backgrounds (stimulus shown in Inset). (E) The neuronal blur and the cortical OFF dominance cause light half-wave rectified gratings to be wider but smaller in amplitude than dark half-wave rectified gratings (Left). When convolved with the synaptic dendritic field (insets next to arrow), the grating distortions lead to differences in spatial frequency tuning. (F) The spatial distortion of the neuronal blur makes the peak frequency higher for rectified light gratings (PFL) than dark gratings (PFD). Compare with Fig. 2G. (G) The cortical OFF dominance makes the LFR stronger for dark than light gratings. Compare with Fig. 2H. For more detail, see SI Text.
The differences in V1 grating frequency tuning for lights and darks can also be explained by an early retinal nonlinearity combined with the amplified responses to darks in visual cortex (Fig. 6D). The early retinal nonlinearity broadens more the light-half than the dark-half of the rectified grating and the cortical OFF dominance makes the responses to the dark-half stronger (Fig. 6E, Left). Consequently, the neuronal blur makes the peak grating frequency higher for the light-half than the dark-half of the grating (PFL > PFD; Fig. 6E, Right) while preserving a pronounced OFF dominance at low grating frequencies. We simulated multiple V1 grating frequency tunings as Gaussians with tuning widths and peak frequencies randomly selected from a normal distribution of values matching the experimental measurements in V1. Each randomly selected Gaussian function was then passed through a luminance/response function also randomly selected from the V1 experimental measurements. These simulations were repeated 290 times to cover the range of peak frequencies, bandwidth frequencies, and luminance/response functions measured (58 luminance-response functions, 5 Gaussians per function with different peak and bandwidth frequency). This simple simulation reproduced the differences in V1 grating resolution for darks and lights illustrated in Fig. 2, including the higher ON peak frequency (cf. Figs. 6F and 2G) and the stronger OFF responses to low grating frequencies (cf. Figs. 6G and 2H). Thus, the simulation reproduces the differences in spatial resolution between darks and lights measured with spots and gratings (Fig. 6 B–D) and offers a simple neural mechanism for the irradiation illusion (see SI Text for formalized model describing how an early retinal nonlinearity can cause dark/light spatial asymmetries).
Discussion
Our study reveals asymmetries between ON and OFF pathways that have important consequences for visual perception. We demonstrate that the visual thalamus and cortex respond more linearly to dark than light stimuli and that this asymmetry explains the higher spatial resolution for darks reported by Galilei (4). Furthermore, we show that the background luminance affects more ON than OFF channels in their response magnitude, response linearity, and receptive field size. Finally, we show that changes in the ON channel with background luminance can explain the dynamics of the irradiation illusion: The illusion is perceived stronger on dark/light backgrounds than on gray backgrounds (7).
Because ON cells have larger receptive fields than OFF cells, it is generally assumed that the ON channel responds to lower spatial frequencies than the OFF channel. Surprisingly, our results demonstrate the opposite: The ON channel responds to higher peak grating frequencies than the OFF channel and, at the same time, OFF responses are stronger than ON responses at low spatial frequencies. We explain this paradox with the measured ON compressive nonlinearity and thereby provide a simple—yet powerful—framework for explaining the observed differences in spatial processing of ON and OFF responses.
Another important conclusion from our work is that the compressive nonlinearity is not unique of ON center retinal ganglion cells. By contrast, it is found in both ON- and OFF-center cells (surround stimulation in OFF cells and center stimulation in ON cells). This is an important finding that strongly suggests that the compressive nonlinearity is not a distinctive feature of ON retinal ganglion cells but of responses to lights and that it could originate as early as the photoreceptors (19–24). This conclusion is supported by our measurements in thalamic neurons and the distorted responses to sinusoidal drifting gratings of retinal horizontal cells (10).
Our results are also consistent with previous studies that reported lower contrast response thresholds for ON than OFF center retinal ganglion cells (8, 9, 25) but inconsistent with the notion that ON neurons encode visual responses more linearly than OFF neurons (8, 9). We conclude that the perception of darks and lights is influenced by both the saturating nonlinearity that we describe in ON cells and the rectifying nonlinearity previously demonstrated in OFF cells (8, 9). However, we notice that the ON saturating nonlinearity seems to dominate our perception of darks and lights, as it can be simply demonstrated by presenting a static sine grating on a monitor that has been gamma calibrated. The grating will appear greatly distorted with the white parts looking wider than the dark parts, exactly what it would be expected from a strong saturating nonlinearity in the ON pathway.
Interestingly, ON and OFF olfactory receptor neurons show similar differences in response nonlinearity, with the OFF receptors generating stronger and less saturating responses than ON receptors (26). Therefore, taken together, these results suggest that the differences in response linearity between ON and OFF channels may be a general principle of sensory systems signaling decrements and increments in a physical quantity, be it light in vision or the concentration of a chemical in olfaction.
Optical quality and visual spatial resolution have been traditionally measured with sinusoidal gratings that alternate light increments and decrements of equal magnitude around a mean level. In fact, the modulation transfer function is probably the most common measure of how faithfully a lens, a human observer or a visual neuron reproduces the spatial frequency of a grating (27–29). Binary stimuli (light and dark luminance values) are also widely used to estimate spatiotemporal receptive fields (30–33). However, both modulation transfer functions and linear receptive field estimates assume that darks and lights drive neuronal responses with equal strength and neuronal populations with similar spatial resolution, two assumptions that are not supported by our results. In addition to the consequences of our results for visual processing and perception, the pronounced ON-OFF asymmetry that we describe could play an important role in visual development. In the first few weeks after birth, the optical components of the eye are immature and the natural images projected on the retinas are blurred and dominated by low spatial frequencies (34). Because our results show that low spatial frequencies drive OFF visual responses more effectively than ON visual responses, the OFF channel could have a competitive advantage over the ON channel if neuronal activity plays a role in the wiring of visual cortex (35). Also, because the early compressive nonlinearity causes more spatial distortion in the ON than OFF channel, differences in dendritic field size between ON and OFF retinal ganglion cells (11) could also be driven by the early nonlinearity. Finally, we provide one more example of how sensory processing evolves to match the natural environment. Natural images have more darks than lights (36) and, as shown here, the neuronal machinery in the visual pathway allocates a greater linear range to measure variations in darkness than lightness.
Materials and Methods
Surgery and Preparation.
Details of the surgical procedures have been described previously (3, 37). All procedures were performed in accordance to the guidelines of the US Department of Agriculture and approved by the Institutional Animal Care and Use Committee at the State University of New York, State College of Optometry.
Electrophysiological Recordings and Data Acquisition.
Cat.
A matrix of seven independently moveable electrodes arranged circularly (Thomas Recording) was used to simultaneously record from multiple geniculate cells with spatially overlapping receptive fields in the A layers of LGN (visual eccentricity 5–20°). Cortical multiunit activity was recorded using a 32-channel multielectrode array (Neuronexus) that was tangentially introduced into the primary visual cortex. The signals from the recording electrodes were amplified, filtered, and collected by a computer running Rasputin (Plexon), as previously described (3). Spike waveforms from each geniculate cell were initially identified during the experiment and later carefully verified off-line with spike-sorting software (Plexon). Cortical multiunit activity was not sorted. Most cells/sites in this study were recorded within 10° of the area centralis.
Awake primate.
Local field potential (LFP) activity in V1 was recorded with chronically implanted ultrathin electrodes (impedance: 1–3 MOhms), independently moveable with individual microdrives (38, 39). The signals were amplified (×5,000), low-pass filtered (low-pass cutoff = 200 Hz), and sampled at a frequency of 5,000 Hz.
Human.
Visual evoked potential (VEP) (13) activity was recorded with gold electrodes from Grass Technologies (electrode impedance = 2∼5 kOhm). The electrodes were positioned according to the “10-20 International System,” i.e., the signal electrode was positioned 3–4 cm above the inion, the negative location on the right earlobe, and the ground on the forehead. The signal was amplified (×5,000) and filtered (band pass = 0.01–1kHz) using an amplifier from Grass Technologies (Model 15LT) and collected by a computer running Rasputin (Plexon). Eye position was continuously measured using an EyeLink system (EyeLink 2000). Stimuli were presented in blocks of ∼5-s duration in which we asked the subjects to maintain fixation without blinking with their eyes. In case eye blinks occurred or fixation was lost during a block, stimulation was stopped and the same block was restarted after a short break. This careful stimulus design resulted in very stable VEP signals during the valid blocks (SI Materials and Methods). The study was approved by the institutional review board at the State University of New York, State College of Optometry.
Supplementary Material
Acknowledgments
We are grateful to Rebecca Lee, Preethi Thiagarajan, Naveen Yadav, and Kenneth Ciuffreda for their help with the human recordings. This work was supported by National Institutes of Health Grants EY02067901 and EY05253 (to J.M.A.), EY007556 and EY0133312 (to Q.Z.); and a Deutsche Forschungsgemeinschaft research fellowship (to J.K.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1310442111/-/DCSupplemental.
References
- 1.Xing D, Yeh C-I, Shapley RM. Generation of black-dominant responses in V1 cortex. J Neurosci. 2010;30(40):13504–13512. doi: 10.1523/JNEUROSCI.2473-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yeh CI, Xing D, Shapley RM. “Black” responses dominate macaque primary visual cortex v1. J Neurosci. 2009;29(38):11753–11760. doi: 10.1523/JNEUROSCI.1991-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jin JZ, et al. On and off domains of geniculate afferents in cat primary visual cortex. Nat Neurosci. 2008;11(1):88–94. doi: 10.1038/nn2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galilei G. Dialogo Sopra i Due Massimi Sistemi del Mondo. Florence, Italy: Battista Landini; 1632. [Google Scholar]
- 5.von Helmholtz H. Handbuch der physiologischen optik. In: Karsten G, editor. Allgemeine Encyklopadie der Physik Vol 2. Leipzig, Germany: Voss; 1867. pp. 186–193. [Google Scholar]
- 6.Westheimer G. Illusions in the spatial sense of the eye: Geometrical-optical illusions and the neural representation of space. Vision Res. 2008;48(20):2128–2142. doi: 10.1016/j.visres.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 7.Komban SJ, Alonso J-M, Zaidi Q. Darks are processed faster than lights. J Neurosci. 2011;31(23):8654–8658. doi: 10.1523/JNEUROSCI.0504-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chichilnisky EJ, Kalmar RS. Functional asymmetries in ON and OFF ganglion cells of primate retina. J Neurosci. 2002;22(7):2737–2747. doi: 10.1523/JNEUROSCI.22-07-02737.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zaghloul KA, Boahen K, Demb JB. Different circuits for ON and OFF retinal ganglion cells cause different contrast sensitivities. J Neurosci. 2003;23(7):2645–2654. doi: 10.1523/JNEUROSCI.23-07-02645.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee BB, Dacey DM, Smith VC, Pokorny J. Dynamics of sensitivity regulation in primate outer retina: The horizontal cell network. J Vis. 2003;3(7):513–526. doi: 10.1167/3.7.5. [DOI] [PubMed] [Google Scholar]
- 11.Dacey DM, Petersen MR. Dendritic field size and morphology of midget and parasol ganglion cells of the human retina. Proc Natl Acad Sci USA. 1992;89(20):9666–9670. doi: 10.1073/pnas.89.20.9666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chubb C, Nam JH. Variance of high contrast textures is sensed using negative half-wave rectification. Vision Res. 2000;40(13):1677–1694. doi: 10.1016/s0042-6989(00)00007-9. [DOI] [PubMed] [Google Scholar]
- 13.Zemon V, Gordon J, Welch J. Asymmetries in ON and OFF visual pathways of humans revealed using contrast-evoked cortical potentials. Vis Neurosci. 1988;1(1):145–150. doi: 10.1017/s0952523800001085. [DOI] [PubMed] [Google Scholar]
- 14.Saul AB, Humphrey AL. Spatial and temporal response properties of lagged and nonlagged cells in cat lateral geniculate nucleus. J Neurophysiol. 1990;64(1):206–224. doi: 10.1152/jn.1990.64.1.206. [DOI] [PubMed] [Google Scholar]
- 15.Victor JD, Shapley RM. Receptive field mechanisms of cat X and Y retinal ganglion cells. J Gen Physiol. 1979;74(2):275–298. doi: 10.1085/jgp.74.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tian N, Copenhagen DR. Visual stimulation is required for refinement of ON and OFF pathways in postnatal retina. Neuron. 2003;39(1):85–96. doi: 10.1016/s0896-6273(03)00389-1. [DOI] [PubMed] [Google Scholar]
- 17.Dacey DM. The mosaic of midget ganglion cells in the human retina. J Neurosci. 1993;13(12):5334–5355. doi: 10.1523/JNEUROSCI.13-12-05334.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Devries SH, Baylor DA. Mosaic arrangement of ganglion cell receptive fields in rabbit retina. J Neurophysiol. 1997;78(4):2048–2060. doi: 10.1152/jn.1997.78.4.2048. [DOI] [PubMed] [Google Scholar]
- 19.Schnapf JL, Nunn BJ, Meister M, Baylor DA. Visual transduction in cones of the monkey Macaca fascicularis. J Physiol. 1990;427:681–713. doi: 10.1113/jphysiol.1990.sp018193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Normann RA, Perlman I. The effects of background illumination on the photoresponses of red and green cones. J Physiol. 1979;286:491–507. doi: 10.1113/jphysiol.1979.sp012633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baylor DA, Hodgkin AL, Lamb TD. The electrical response of turtle cones to flashes and steps of light. J Physiol. 1974;242(3):685–727. doi: 10.1113/jphysiol.1974.sp010731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baylor DA, Hodgkin AL. Detection and resolution of visual stimuli by turtle photoreceptors. J Physiol. 1973;234(1):163–198. doi: 10.1113/jphysiol.1973.sp010340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Juusola M, Hardie RC. Light adaptation in Drosophila photoreceptors: II. Rising temperature increases the bandwidth of reliable signaling. J Gen Physiol. 2001;117(1):27–42. doi: 10.1085/jgp.117.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Juusola M, Kouvalainen E, Järvilehto M, Weckström M. Contrast gain, signal-to-noise ratio, and linearity in light-adapted blowfly photoreceptors. J Gen Physiol. 1994;104(3):593–621. doi: 10.1085/jgp.104.3.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Hateren H. A cellular and molecular model of response kinetics and adaptation in primate cones and horizontal cells. J Vis. 2005;5(4):331–347. doi: 10.1167/5.4.5. [DOI] [PubMed] [Google Scholar]
- 26.Burgstaller M, Tichy H. Functional asymmetries in cockroach ON and OFF olfactory receptor neurons. J Neurophysiol. 2011;105(2):834–845. doi: 10.1152/jn.00785.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bredfeldt CE, Ringach DL. Dynamics of spatial frequency tuning in macaque V1. J Neurosci. 2002;22(5):1976–1984. doi: 10.1523/JNEUROSCI.22-05-01976.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu ZL, Sperling G. Black-white asymmetry in visual perception. J Vis. 2012;12(10):8. doi: 10.1167/12.10.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peli E, Arend L, Labianca AT. Contrast perception across changes in luminance and spatial frequency. J Opt Soc Am A Opt Image Sci Vis. 1996;13(10):1953–1959. doi: 10.1364/josaa.13.001953. [DOI] [PubMed] [Google Scholar]
- 30.Jin J, Wang Y, Lashgari R, Swadlow HA, Alonso J-M. Faster thalamocortical processing for dark than light visual targets. J Neurosci. 2011;31(48):17471–17479. doi: 10.1523/JNEUROSCI.2456-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jones JP, Palmer LA. The two-dimensional spatial structure of simple receptive fields in cat striate cortex. J Neurophysiol. 1987;58(6):1187–1211. doi: 10.1152/jn.1987.58.6.1187. [DOI] [PubMed] [Google Scholar]
- 32.Pandarinath C, Victor JD, Nirenberg S. Symmetry breakdown in the ON and OFF pathways of the retina at night: Functional implications. J Neurosci. 2010;30(30):10006–10014. doi: 10.1523/JNEUROSCI.5616-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reid RC, Victor JD, Shapley RM. The use of m-sequences in the analysis of visual neurons: Linear receptive field properties. Vis Neurosci. 1997;14(6):1015–1027. doi: 10.1017/s0952523800011743. [DOI] [PubMed] [Google Scholar]
- 34.Norcia AM, Tyler CW, Hamer RD. Development of contrast sensitivity in the human infant. Vision Res. 1990;30(10):1475–1486. doi: 10.1016/0042-6989(90)90028-j. [DOI] [PubMed] [Google Scholar]
- 35.Crair MC, Gillespie DC, Stryker MP. The role of visual experience in the development of columns in cat visual cortex. Science. 1998;279(5350):566–570. doi: 10.1126/science.279.5350.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ratliff CP, Borghuis BG, Kao Y-H, Sterling P, Balasubramanian V. Retina is structured to process an excess of darkness in natural scenes. Proc Natl Acad Sci USA. 2010;107(40):17368–17373. doi: 10.1073/pnas.1005846107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lashgari R, et al. Response properties of local field potentials and neighboring single neurons in awake primary visual cortex. J Neurosci. 2012;32(33):11396–11413. doi: 10.1523/JNEUROSCI.0429-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen Y, et al. Task difficulty modulates the activity of specific neuronal populations in primary visual cortex. Nat Neurosci. 2008;11(8):974–982. doi: 10.1038/nn.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Swadlow HA, Bereshpolova Y, Bezdudnaya T, Cano M, Stoelzel CR. A multi-channel, implantable microdrive system for use with sharp, ultra-fine “Reitboeck” microelectrodes. J Neurophysiol. 2005;93(5):2959–2965. doi: 10.1152/jn.01141.2004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.