Significance
Parathyroid adenomas, the main cause of primary hyperparathyroidism (PHPT), are thought to result from clonal expansion of tumor cells and to be insensitive to normal calcium feedback due to the loss of the calcium-sensing receptor (CASR). Utilizing flow cytometric analysis to isolate and individually study oxyphil cells, chief cells, and lymphocytes from resected parathyroid tumors and glands, we now report previously unrecognized heterogeneity in these tissues with respect to calcium responsiveness, CASR expression, and clonal origin of parathyroid tumors. Such heterogeneity of parathyroid adenomas likely reflects the complex etiopathogenesis and clinical heterogeneity of PHPT.
Keywords: tumor heterogeneity, endocrine neoplasia
Abstract
Parathyroid adenomas (PAs) causing primary hyperparathyroidism (PHPT) are histologically heterogeneous yet have been historically viewed as largely monotypic entities arising from clonal expansion of a single transformed progenitor. Using flow cytometric analysis of resected adenomatous parathyroid glands, we have isolated and characterized chief cells, oxyphil cells, and tumor-infiltrating lymphocytes. The parathyroid chief and oxyphil cells produce parathyroid hormone (PTH), express the calcium-sensing receptor (CASR), and mobilize intracellular calcium in response to CASR activation. Parathyroid tumor infiltrating lymphocytes are T cells by immunophenotyping. Under normocalcemic conditions, oxyphil cells produce ∼50% more PTH than do chief cells, yet display significantly greater PTH suppression and calcium flux response to elevated calcium. In contrast, CASR expression and localization are equivalent in the respective parathyroid cell populations. Analysis of tumor clonality using X-linked inactivation assays in a patient-matched series of intact tumors, preparatively isolated oxyphil and chief cells, and laser-captured microdissected PA specimens demonstrate polyclonality in 5 of 14 cases. These data demonstrate the presence of functionally distinct oxyphil and chief cells within parathyroid primary adenomas and provide evidence that primary PA can arise by both clonal and polyclonal mechanisms. The clonal differences, biochemical activity, and relative abundance of these parathyroid adenoma subpopulations likely reflect distinct mechanisms of disease in PHPT.
The parathyroid glands maintain serum calcium concentration within a narrow physiological range through regulated synthesis and secretion of parathyroid hormone (PTH) (1). Parathyroid neoplasia results in inappropriate secretion of PTH by one or more glands, leading to hypercalcemia and the disease primary hyperparathyroidism (PHPT) (2). To date, research in the field has focused on histologic and molecular profiling of parathyroid tumors and the investigation of calcium sensing in dispersed cells from parathyroid tumors and normal bovine parathyroid glands. Few studies have characterized the individual cellular constituents of human parathyroid tumors, and no publications have reported live-cell functional evaluation of the different cellular subtypes observed in parathyroid tumors.
Parathyroid adenomas, the most common cause of PHPT, are considered clonal proliferations of a transformed parathyroid cell that has acquired proliferative or survival advantage due to one of several genetic abnormalities including the PRAD1 translocation or mutations in the genes encoding menin, P53, and P27 (3-7). Regardless of type, most authors consider parathyroid tumors clonal (8, 9) although intratumoral heterogeneity has been observed and polyclonality of microdissected parathyroid adenomas has been reported (10, 11).
Although most data support mutation-driven clonal expansion of parathyroid tumors, an alternative model for the origin of parathyroid tumors is that abnormal calcium sensing by parathyroid cells leads to abnormal secretion of PTH initially, followed by proliferation of parathyroid cells in response to chronic demand for increased PTH (12, 13). An abnormal calcium–PTH set point has been well-described in aggregate dispersed cells from parathyroid adenomas, and most reports attribute the impaired set point in these cells to decreased expression of the calcium-sensing receptor (CASR) (14, 15) or more recently to altered expression of downstream molecules linked to CASR signaling, including RhoGEF and RGS5 (16, 17). In contrast to clonal expansion following genomic tumor-initiating events, attenuated calcium responsiveness could be expected to drive polyclonal proliferation in the parathyroid gland.
To investigate the composition of parathyroid tumors, we sought to characterize a series of parathyroid adenomas at the cellular and functional level. In this study, we report the isolation and characterization of chief cells, oxyphil cells, and lymphocytes present in parathyroid adenomas and histologically normal parathyroid glands from patients with PHPT. Our results show that parathyroid adenomas removed from patients with PHPT are composed of functionally and genetically distinct oxyphil and chief cells and have varying amounts of infiltrating lymphocytes. The chief and oxyphil cells within parathyroid adenomas have differing ability to respond to changes in ambient calcium and produce PTH although CASR expression is comparable between these parathyroid cell subtypes. Further, we show that a significant proportion of PHPT patients have polyclonal tumors. The relative abundance, functional behavior, and clonal origin of distinct parathyroid-cell subpopulations in parathyroid tumors likely reflect multiple alternative etiologies of PHPT.
Results
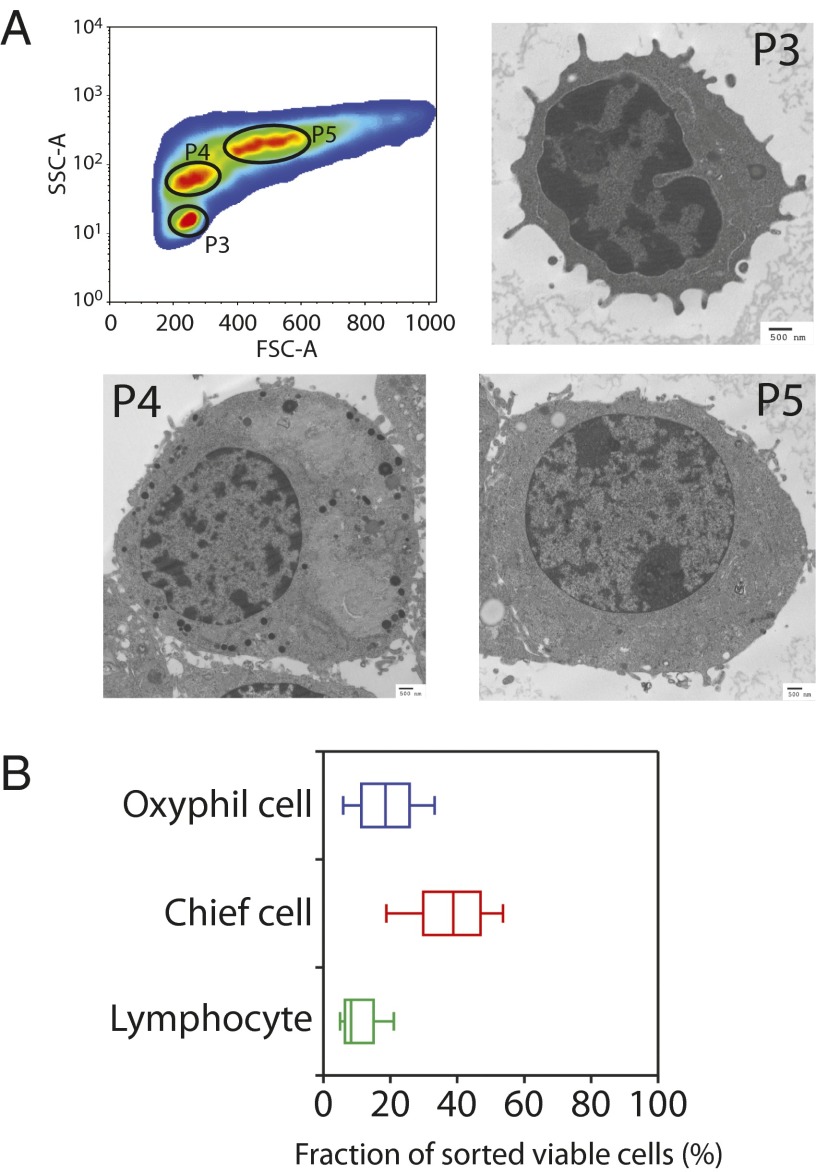
Flow cytometric analysis of dispersed parathyroid adenoma cells revealed discrete subpopulations of cells resolved on the basis of visible-light diffraction detected by the in-line axis [forward scatter (FSC-A)] and orthogonal axis [side scatter (SSC-A)] sensors. After excluding nonviable and doublet cells, three distinct populations, initially designated P3, P4, and P5, were clearly and reproducibly resolved as separate peaks in a bivariate SSC-A/FSC-A contour plot (Fig. 1A, Upper Left). Ultrastructural analysis by transmission electron microscopy of preparatively isolated P3, P4, and P5 cells showed clear morphological differences between the three populations (Fig. 1A). Based upon size and morphology, P3 cells were provisionally identified as peripheral blood lymphocytes. P4 cells displayed highly interdigitated plasma membranes, sparse mitochondria, and prominent intracellular secretory granules, all features of parathyroid chief cells (18). P5 cells were large and contained round nuclei, moderate peripheral chromatin condensation, and numerous mitochondria densely packed throughout the cytoplasm. These characteristics are consistent with the parathyroid oxyphil cell type (19). We used the same sorting strategy to examine the distribution of these cells types among cells dispersed from single adenomas from 20 patients with PHPT (Fig. 1B). In these samples, the proportion of lymphocytes present in the dispersed parathyroid adenoma samples tested ranged from 2% to 31.9%. The relative abundance of chief cells ranged from 9.4% to 57.8% of the total viable population. The relative abundance of oxyphil cells ranged from 0.9% to 59.8%. The distribution of these cells did not correlate with any feature of the disease including sex, age, preoperative calcium or PTH, gland weight, or histology.
Fig. 1.
Cellular heterogeneity in parathyroid adenomas. (A, Upper Left) Contour diagram showing the distribution of parathyroid cells (P3, P4, and P5) from dispersed parathyroid adenoma samples based upon the forward and side scatter channel outputs. Nonviable cells and aggregated cells were excluded by gating for the absence of propidium iodide uptake. In panels labeled P3, P4, and P5 is shown electron microscopy of isolated cells (P3, P4, and P5) from dispersed parathyroid adenomas. (Scale bar: 500 nm.) (B) Box and whisker plots of lymphocytes, chief cells, and oxyphil cells isolated from a total of 20 cases.
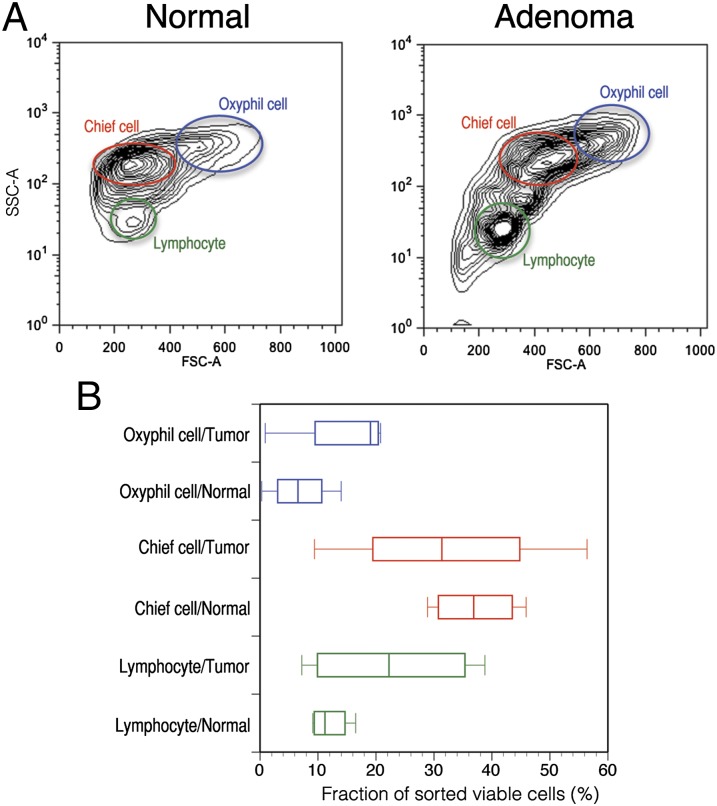
To compare the cellular composition of histologically normal and neoplastic parathyroid tissue, we examined a series of five paired tumor and histologically normal (but physiologically suppressed) parathyroid gland biopsy specimens using flow cytometry to assay for the presence of the three cell populations. The relative distribution of oxyphil cells, chief cells, and lymphocytes was remarkably consistent between normal tissues. In contrast, adenomas from different patients have highly variable distribution patterns (Fig. 2 A and B). These data indicate that oxyphil cells, chief cells, and lymphocytes are present in both normal tissue and adenomatous tissue, but the relative abundance of these subpopulations can be markedly variable between different adenomas.
Fig. 2.
Relative abundance and distribution of lymphocytes, chief cells, and oxyphil cells from parathyroid adenomas and matched normal parathyroid gland biopsies from patients with PHPT. (A) Representative contour plots of side scatter and forward scatter parameters from flow-cytometry analysis of parathyroid adenoma and matched normal tissue. (B) Box and whisker plots of lymphocytes, chief cells, and oxyphil cells isolated from a total of five cases.
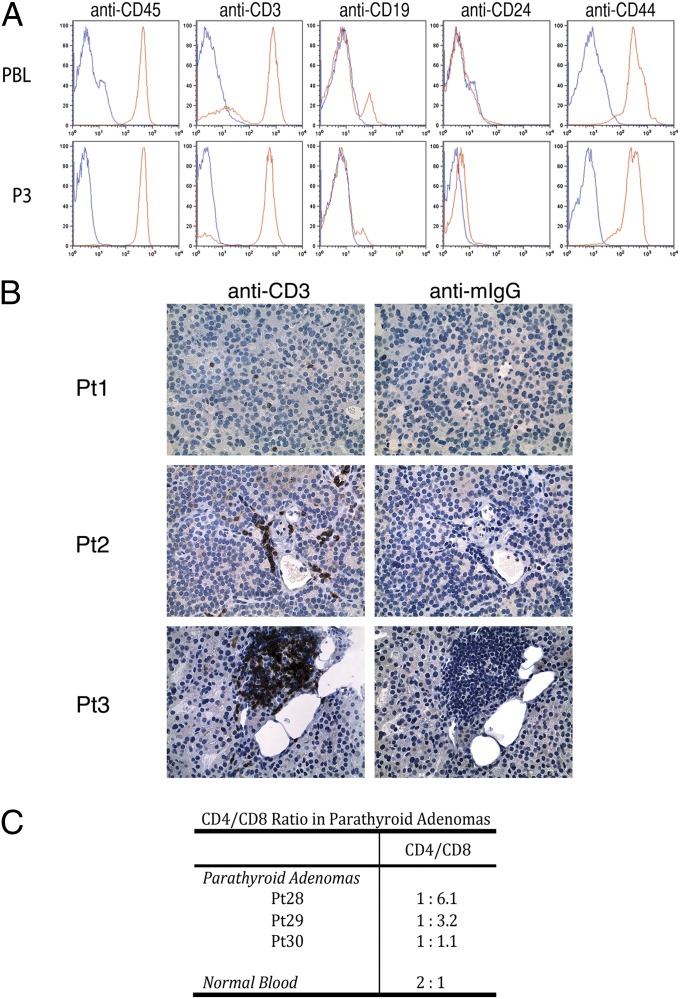
To confirm the designation of P3 cells as lymphocytes, we probed P3 cells with the leukocyte common antigen CD45 antibody using standard flow-cytometry methods. P3 cells are uniformly CD45-positive and are indistinguishable in this assay from patient-matched isolated peripheral blood lymphocytes. Subsequent cell-surface marker studies revealed that the majority of P3 cells are CD45+/CD3+/CD19−/CD24−/CD44+, an immunotype consistent with peripheral T cells (Fig. 3A). These results unambiguously confirm that P3 cells are lymphocytes. CD3 staining on parathyroid adenoma tissue sections showed three predominant patterns: widely separated single cells (Fig. 3B, Pt1), clustered cells extravasated into the parenchyma of the gland but adjacent to vascular structures (Fig. 3B, Pt2), and focal, densely packed infiltrates (Fig. 3B, Pt3). To verify that the P3 cells are tumor-infiltrating lymphocytes as opposed to contaminating peripheral blood mononuclear cells (PBMCs) adventitiously carried over from the tumor vasculature, we examined the CD4+/CD8+ ratio in the P3 population. In contrast to the 2:1 ratio of CD4+ to CD8+ cells found in the bloodstream, CD8+ effector cells are highly overrepresented in the P3 lymphocytes (Fig. 3C). Taken together with the physical distribution of lymphocytes in the parathyroid parenchyma observed by immunohistochemistry (IHC), this result confirms the identity of parathyroid adenoma P3 cells as tumor-infiltrating T cells as opposed to contaminating peripheral blood lymphocytes.
Fig. 3.
Immunophenotypic characterization of cells isolated from parathyroid adenomas. (A) Flow-cytometric analysis of cell-surface markers CD45, CD3, CD19, CD24, and CD44 on tumor-infiltrating lymphocytes (TILs) (Lower) and patient-matched PBLs (Upper). (B) Tissue sections from three independent parathyroid adenomas were probed with an anti-CD3 antibody, and reactivity was visualized by DAB staining and with hematoxylin/eosin counterstaining. (C) CD4 and CD8 cell-surface expression in P3 cells derived from three parathyroid adenomas was determined by FACS. The ratio of CD4+ to CD8+ cells is based upon quadrant gating using standard conditions.
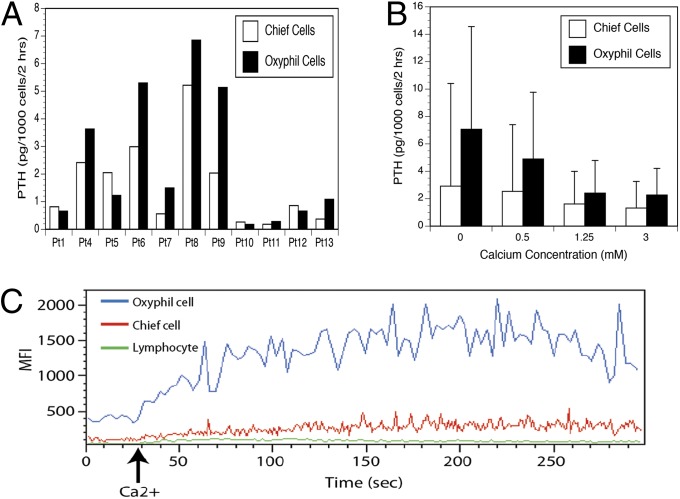
We then sought to functionally characterize isolated oxyphil and chief cells by evaluating basal PTH production and cellular response (PTH suppression and intracellular calcium flux) to exogenous calcium. Comparison of PTH production under normocalcemic conditions (1.25 mM) in 11 patient-matched pairs of oxyphil and chief cells showed significantly higher baseline PTH production in the oxyphil cells relative to the chief cells (P = 0.026 by paired t test) (Fig. 4A). The paired samples showed significant correlation to each other (r = 0.9017), indicating that adenoma PTH output is strongly influenced by patient origin as well as cell type (chief vs. oxyphil). In vitro PTH production by isolated cells correlated well with unsorted cells.
Fig. 4.
Parathyroid hormone (PTH) secretion and intracellular calcium-flux response to 3.0 mM extracellular calcium in dispersed primary cells isolated from parathyroid adenomas. (A) Basal PTH secretion over 2 h by tumor-matched chief and oxyphil cells from 11 PHPT patients under normocalcemic (1.25 mM) conditions. (B) PTH secretion over 2 h under varied calcium concentration by tumor-matched chief and oxyphil cells (n = 6). (C) Intracellular flux response among isolated lymphocytes, chief cells, and oxyphil cells. One representative experiment out of seven is shown.
We next compared PTH production over a 2-h period by preparatively isolated oxyphil and chief cells under varied calcium concentrations. Both oxyphil and chief cells secrete PTH when cultured in 0.5 mM, 1.25 mM, and 3.0 mM calcium-containing media. Regardless of calcium concentration, oxyphil cells secreted more PTH per cell than chief cells over the assay interval. At elevated calcium concentrations, PTH production was inhibited incompletely in both oxyphil and chief cells. Normalized PTH secretion profiles of oxyphil and chief cells reveal that both populations show similar PTH suppression at a 3-mM ambient calcium concentration (Fig. 4B). However, the wide SD clearly reflects heterogeneous responsiveness among the adenomas. The suppressability of PTH secretion by calcium in vitro did not correlate with severity of clinical disease (i.e., calcium and PTH levels in the blood).
We also used flow cytometry-based kinetic analysis to evaluate the relative intracellular responsiveness of the parathyroid cells to changes in extracellular calcium concentration. Stimulation with 3 mM extracellular calcium elicited a rapid and robust intracellular calcium-flux response in the dispersed parathyroid cells as detected by increased Fluo-4 AM mean fluorescent intensity. Clear differences in calcium-flux responses were revealed when we compared the relative activity of the oxyphil cells, chief cells, and lymphocytes (Fig. 4C). Oxyphil cells are the most responsive and account for the great majority of the overall calcium-flux activity in the dispersed parathyroid-cell parental population. Chief cells display a much lower response whereas lymphocytes cells are completely unresponsive. These data indicate that readily identifiable subpopulations of cells within a given parathyroid adenoma display categorically distinct quantitative responses to extracellular-calcium stimulation.
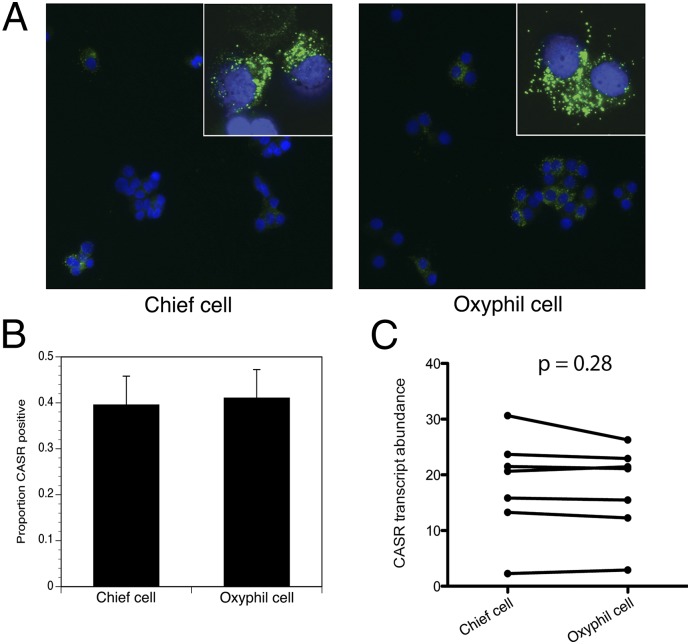
To better understand the heterogeneity of parathyroid adenomas and to explore the question of why parathyroid adenomas do not sense calcium properly and fail to regulate PTH secretion, we examined CASR expression in parathyroid adenomas from a series of patients with PHPT. First, we used immunofluorescence detection to evaluate CASR protein expression in isolated oxyphil and chief cells from a panel of primary parathyroid adenoma specimens. As shown in Fig. 5A, CASR immunoreactivity is detected on the surface of both oxyphil and chief cells. For quantitation of the relative abundance of CASR-positive cells within each population, at least 100 cells per field were counted in each of three different fields, and the proportion of CASR-positive cells was calculated as a percentage. Both cell populations were found to contain similar proportions of CASR-positive cells (39.5 ± 6.3% for chief cells and 41.0 ± 6.2% for oxyphil cells) (Fig. 5B). Second, we examined CASR transcript abundance in chief and oxyphil cells by quantitative reverse-transcriptase polymerase chain reaction (qRT-PCR). Both cell types were found to contain equivalent amounts of the CASR mRNA (Fig. 5C). Because oxyphil and chief cells differ greatly in their calcium responsiveness as evaluated in the flux assay, the presence of comparable CASR expression in both populations indicates that the degree of calcium responsiveness in parathyroid cells is not solely determined by CASR abundance.
Fig. 5.
Expression of CASR in isolated parathyroid cells. (A) Immunofluorescence (IF) for CASR protein in chief and oxyphil cells using anti-CASR monoclonal antibody (20x). (Inset) CASR expression-magnified (63x, oil). (B) Quantitation of CASR IF-positive cells in chief and oxyphil cells from A. A total of more than 100 cells per field were counted in each of three different fields. Data are from three patients. (C) Pairwise comparison of CASR mRNA expression measured by qRT-PCR in chief and oxyphil cells from parathyroid adenomas from seven patients, normalized to an internal GAPDH control.
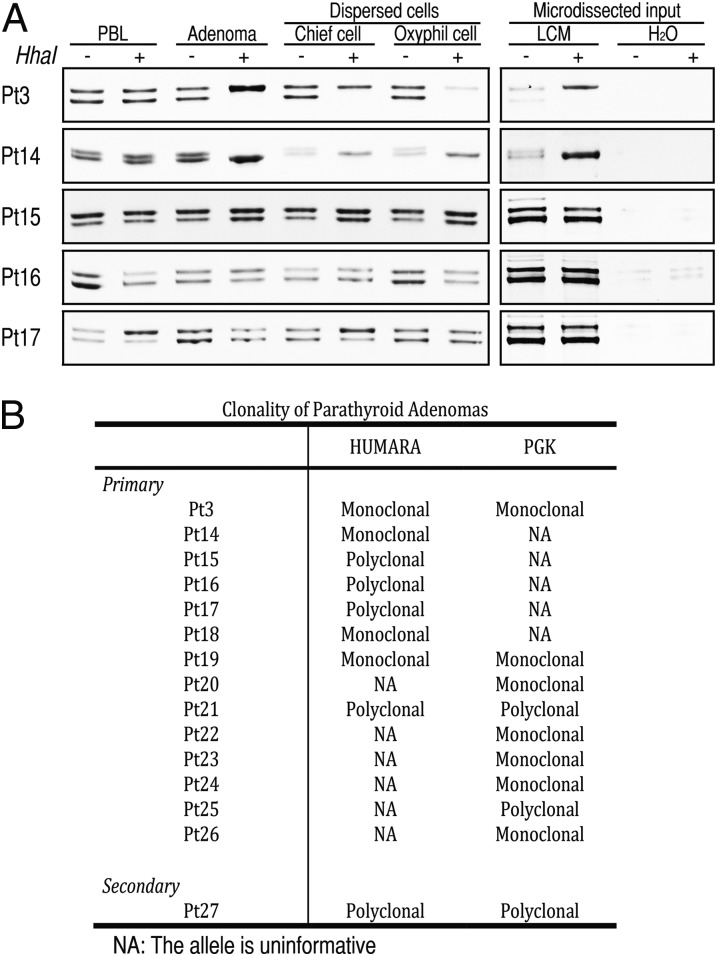
The functional and morphological heterogeneity we observed within primary parathyroid adenomas and their cell isolates raised the question of whether the originating tumors were uniformly clonal as is widely believed (8, 9). To test this concept, we probed for allele-specific X-inactivation in parathyroid adenoma cells derived from female patients at two highly polymorphic imprinted loci, the human androgen receptor (HUMARA) (20) and the phosphate glycerate kinase PGK genes (21). Five female HUMARA locus informative cases were subsequently assessed for clonality. Two of the five cases were found to be monoclonal using both whole adenoma tissue and laser-capture microdissected frozen-section specimens as input (Fig. 6A). In contrast, the remaining three cases showed a polyclonal pattern both in whole adenoma tissue and microdissected frozen-section specimens (Fig. 6A). Patient-matched peripheral blood lymphocyte genomic DNA was used in as an allelic control to demonstrate the polyclonal status of the HUMARA locus in all five of the cases tested. We next probed for the clonal status of isolated oxyphil and chief cells and found that these cells reflected the same clonality pattern observed in the originating patient-matched whole adenoma tissue (Fig. 6A). In the monoclonal cases, the oxyphil and chief cells both harbored the same maternally imprinted HUMARA allele, indicating that these populations arose from a common progenitor. Conversely, in the polyclonal specimens, both the oxyphil and chief populations were found to contain equal representations of each imprinted HUMARA allele, indicating the nonclonal provenance of both cell types. To confirm the HUMARA findings, we also performed methylation-sensitive PCR of the imprinted PGK gene (21). Of 8 HUMARA locus informative cases, 4 were monoclonal and 4 were polyclonal; of 9 PGK locus informative cases, 7 cases were monoclonal and 2 were polyclonal (Fig. 6B). Three cases were informative at both HUMARA and PGK loci, and all 3 yielded concordant results (2/3 monoclonal, 1/3 polyclonal). Altogether, 14 informative cases assayed by either method or both methods showed polyclonal origin in 5 and monoclonal origin in 9 parathyroid adenomas. A single tumor from a patient with renal failure-induced secondary HPT was investigated as control and showed polyclonal status in both HUMARA and PGK assays.
Fig. 6.
Clonality of parathyroid adenomas. (A) Clonality of parathyroid cell populations isolated from five parathyroid adenomas. HUMARA alleles were amplified using methylation-sensitive PCR in HhaI undigested (−) and digested (+) genomic DNA from peripheral blood lymphocytes (PBL), whole tumor tissue (Adenoma), preparatively sorted chief and oxyphil cells, or microdissected tumor tissue (LCM). The presence of a single band reveals a single methylated allele from a monoclonal tumor (Pt3 and -14) whereas two bands reflects random methylation of alleles in a polyclonal tumor (Pt15, -16, and -17). (B) Clonality of 14 parathyroid adenomas identified by HUMARA and PGK methods.
Discussion
Parathyroid neoplasias result in abnormal PTH secretion from an expanded population of parathyroid cells. With few exceptions, a significant body of prior work has shown that parathyroid tumors in PHPT are clonal and that aggregate dispersed parathyroid tumor cells secrete PTH in culture but can be poorly responsive to negative feedback by calcium. In our work to identify differential gene expression and mechanisms of abnormal calcium sensing in parathyroid adenomas, we noticed substantial heterogeneity in these tumors. This finding, coupled with the observed histologic heterogeneity of parathyroid adenomas, led us to design the current study to isolate and functionally characterize individual populations of cells from parathyroid adenomas. We report that parathyroid adenomas comprise variable proportions of chief cells, oxyphil cells, and infiltrating T lymphocytes and that the two parathyroid cell types manifest distinct functional properties. Parathyroid chief cells and oxyphil cells express similar amounts of CASR, and both exhibit attenuated PTH secretory responses to changes in ambient calcium. However, the respective cell types are markedly different in their baseline PTH production and intracellular flux response to extracellular calcium challenge. As the basis for this differential calcium responsiveness is not attributable solely to CASR relative abundance, additional mechanisms, including enhanced biochemical opposition to CASR signaling, altered CASR protein trafficking, or CASR-independent calcium sensing, may prove to be important functional determinants in these cells. Further, we show that a significant proportion of parathyroid adenomas causing PHPT are polyclonal rather than monoclonal. These results indicate that parathyroid tumors causing primary hyperparathyroidism are heterogeneous at the cellular and functional level and further show that some patients’ tumors are monoclonal whereas other patients have polyclonal tumors. Expanded studies of a large number of patients with PHPT to assess frequency of these different tumor types and the clinical phenotypes associated with them will be needed to fully understand the meaning of these findings. It will be important in future studies to relate the variable biochemical behavior and clonal status of PHPT tumors to the presence of driver mutations in known parathyroid tumor suppressor genes and oncogenes such as MEN1 and CCND1 (22).
Earlier investigations using histologic methods have revealed that parathyroid adenomas comprise mainly chief cells, transitional oxyphil cells, and oxyphil cells. These observational studies were limited to analysis of fixed parathyroid tissue sections. Until the current work, the successful preparative isolation of living oxyphil and chief cells from human parathyroid adenoma tissue has not been reported. Using a flow cytometry-based approach, we analyzed 20 adenomas resected from patients with PHPT and determined the proportional representation of chief cells, oxyphil cells, and infiltrating T lymphocytes in the primary tumor. Wide variations among adenomas were observed. In contrast, the distribution of these cell types proved remarkably consistent in a series of five histologically normal parathyroid glands ipsilateral to adenomatous tissue. This result suggests that the variable composition of parathyroid adenomas relative to normal tissue could reveal a greater degree of functional heterogeneity among primary parathyroid adenomas than is currently appreciated.
To address this question, we isolated live oxyphil and chief cells from parathyroid adenomas and compared the behavior of the respective cell types in functional assays measuring calcium responsiveness and PTH secretion, two definitive performance metrics of parathyroid gland function. Our data indicate that the subpopulations of chief and oxyphil cells within a given parathyroid adenoma display clearly different quantitative responses to extracellular calcium stimulation. In all cases tested, oxyphil cells responded much more strongly than chief cells to increased ambient calcium concentration. Baseline PTH production under normocalcemic conditions (1.25 mM) in individual patient-matched pairs of chief and oxyphil cells was significantly higher in oxyphil cells relative to the chief cells. The enhanced calcium sensitivity and increased PTH production we observed in oxyphil cells relative to chief cells is consistent with a recent report that used immunohistochemistry to compare oxyphil and chief cell gene expression in hyperplastic parathyroid tissue from patients with hyperparathyroidism secondary to chronic kidney disease (23). In their study, Ritter et al. (23) demonstrated elevated expression of parathyroid-relevant genes including PTH and PTHrP in oxyphil cells relative to chief cells. In conjunction with this finding, our data support the notion that oxyphil cells are important functional participants in the calcium-sensing and secretory properties of parathyroid tissue. Although the numbers of patients studied are too small to form clear conclusions, the behavior of preparatively isolated chief and oxyphil cells appears similar to the activity of each of these cell types when analyzed as unsorted cell suspensions. Further, in vitro basal and calcium stimulated PTH production by isolated cells did not correlate with severity of patient disease, underscoring the complex physiology of calcium homeostasis in patients.
CASR down-regulation is generally believed to be the principal mechanism for the abnormal calcium-PTH set-point relationship in patients with parathyroid tumors. If this assumption is true, then CASR expression should be significantly reduced in all parathyroid tumors, and calcium responsiveness should be demonstrably dependent upon CASR expression. The data in our study challenge this prevailing model. We show that chief and oxyphil cells display comparable CASR expression at both the protein and transcript levels, yet oxyphil cells are clearly more responsive to calcium and exhibit consistently higher basal PTH secretion. These data indicate that CASR expression is not the sole determinant of PTH production and calcium responsiveness in the parathyroid gland and suggest that the elevated PTH associated with PHPT could be reflective of not only increased tumor-cell number but also the higher basal PTH secretion profile of oxyphil cells compared with chief cells.
Our study indicates that the presence of lymphocytic infiltration is a common feature in parathyroid adenomas. Using flow cytometry, we identified the parathyroid adenoma-derived P3 population as T lymphocytes expressing the leukocyte common antigen CD45 and T-cell receptor unit CD3. Additional marker studies revealed the T lymphocytes to be CD45+/CD3+/CD19−/CD24−/CD44+. The role of immune cells in the pathogenesis of human parathyroid adenoma is less clear. Immune cells can release inflammatory mediators with proangiogenic and prometastatic effects. Tumor cells can express antigens and become targets for a T cell-mediated adaptive immune response. The presence of high numbers of tumor-infiltrating lymphocytes, particularly T cells, has been found to be a major predictor of favorable clinical outcomes in several solid cancers (24–26). These findings support the hypothesis that the adaptive immune response influences the behavior of human tumors. However, lymphocytic infiltration in parathyroid adenoma is rarely reported (27), which suggests that our current findings may represent a newly recognized histologic feature. Because we found lymphocytes in both normal and adenomatous parathyroid glands, the role of these T cells is still unclear. As the ratio of effector (CD8+) to helper (CD4+) T cells in P3 lymphocytes recovered from parathyroid adenoma tissue is consistently distinct from the 1:2 ratio maintained in the bloodstream, it is clear that the P3 population represents tumor infiltrating lymphocytes (TILs) rather than adventitious contaminating cells carried over from vascular elements. It is possible that the TILs may be homing to antibodies targeting CASR, PTH, or other parathyroid antigens and that antibody-mediated inhibition of CASR or clearance of CASR-expressing or PTH-producing cells could induce compensatory hyperplasia in the gland leading to adenoma development. Alternatively, the P3 TILs could be responding to inflammatory mediators, tumor-specific antigens, or senescence-associated proteins released upon oncogene-induced senescence and may be participating in tumor-clearance activities. Further study will be necessary to verify the functions of these immune cells and to discover any potential relationships to parathyroid disease.
Our results are at odds with the assumption of clonal expansion as the sole tumorigenic mechanism in parathyroid adenomas. The detection of 5 polyclonal adenomas out of 14 tested certainly suggests that monoclonal expansion and multi- or polyclonal proliferation may coexist as alternative mechanisms in the origin of primary parathyroid adenomas. Seminal work (8, 9) established that monoclonal proliferation is commonly found in primary hyperparathyroidism, yet subsequent studies using whole tissue (10, 11) have shown that polyclonal origin can occur in a significant subset of primary parathyroid adenomas. These studies were limited by the potential for contamination in samples not specifically enriched for individual cell populations. In our study, we used multiple independent methods of tissue sampling and two independent methods of clonality determination to establish definitively that both polyclonal and monoclonal mechanisms can give rise to primary parathyroid adenomas. Differences in reported frequencies of polyclonal versus monoclonal origin may be attributable to the lack of definitive pathological criteria for distinguishing adenoma from hyperplasia, sample size limitations in the number of parathyroid glands tested, as well as the application of different clonality assays with variations in methodology and the ability to exclude contaminating nonadenoma elements (11). Recently, a new model has been described in which tumor heterogeneity arises through recruitment of proximate untransformed but hyperplastic cells into aggregation chimeras induced by a monoclonal population of tumor-initiating cells (28). It is possible that a similar mechanism may contribute to the origin of the polyclonal adenomas observed in our study. Future studies will be required to analyze the genomic composition of parathyroid adenomas at single-cell resolution to determine whether polyclonal tumors are constitutively transformed or whether a monoclonal initiating component has induced hyperplastic expansion of neighboring untransformed parathyroid cells.
The current study demonstrates that flow cytometry can be used to identify, analyze, and recover functionally distinct subpopulations of live cells from human parathyroidectomy specimens. Using this approach, we show that parathyroid oxyphil and chief cells derived from parathyroid adenomas display clear differences in calcium responsiveness and PTH secretion. The differential activity and relative abundance of these distinct cell types could contribute to heterogeneity in clinical presentation, therapeutic response, and outcome in PHPT. Importantly, the demonstration of polyclonality within primary adenomas raises the possibility that such expansions may occur during an unrecognized prodrome phase analogous to the abnormal physiological contexts that drive the development of secondary parathyroid hyperplasia. Further work will be necessary to test these concepts in expanded patient cohorts to define the relationship of these findings to the clinical presentation and disease course of PHPT and to improve our understanding of the causes of primary and secondary parathyroid neoplasia.
Materials and Methods
Patients and Samples.
Informed consent was sought from patients scheduled to undergo parathyroidectomy for PHPT. Consenting patients donated resected parathyroid tissue for study (Protocol Number 00007056, approved by the Institutional Review Board of the Duke University Medical Center). All of the patients were diagnosed with sporadic, nonfamilial PHPT on the basis of standard clinical and biochemical parameters, and all had a single parathyroid tumor removed at curative surgery. Viable dispersed parathyroid cells collected from the surgical specimens were preparatively sorted by flow cytometry prior to further analysis. Cellular morphology was evaluated by electron and light microscopy. Marker gene expression was detected by immunofluorescence, immunohistochemistry, quantitative reverse-transcriptase PCR, and analytical flow cytometry. Calcium responsiveness was detected via kinetic analysis of Fluo-4 AM fluorescence intensity. Clonality status was determined by PCR assays detecting X-chromosome inactivation at the highly polymorphic HUMARA and PGK loci. A full description of the experimental procedures may be found in the SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. Sara Miller and Mr. Phillip Christopher (Duke Electron Microscopy Core Facility) for excellent technical assistance in the preparation and analysis of the EM data, Dr. Michael Cook and Ms. Lynn Martinek (Duke Flow Cytometry Shared Resource, Duke Cancer Institute) for advice and assistance, Ms. Cynthia Webb for assistance with parathyroid tissue procurement and banking, and Dr. Yasheng Gao (Duke Light Microscopy Core Facility) for assistance with the live-cell imaging studies. This work was supported by National Institutes of Health Grant R01 DK088188-03 (to J.A.O. and J.K.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1319742111/-/DCSupplemental.
References
- 1.Brown EM. Extracellular Ca2+ sensing, regulation of parathyroid cell function, and role of Ca2+ and other ions as extracellular (first) messengers. Physiol Rev. 1991;71(2):371–411. doi: 10.1152/physrev.1991.71.2.371. [DOI] [PubMed] [Google Scholar]
- 2.DeLellis RA, Mazzaglia P, Mangray S. Primary hyperparathyroidism: A current perspective. Arch Pathol Lab Med. 2008;132(8):1251–1262. doi: 10.5858/2008-132-1251-PHACP. [DOI] [PubMed] [Google Scholar]
- 3.Arnold A, et al. PRAD1 (cyclin D1): A parathyroid neoplasia gene on 11q13. Henry Ford Hosp Med J. 1992;40(3-4):177–180. [PubMed] [Google Scholar]
- 4.Carling T, et al. Parathyroid MEN1 gene mutations in relation to clinical characteristics of nonfamilial primary hyperparathyroidism. J Clin Endocrinol Metab. 1998;83(8):2960–2963. doi: 10.1210/jcem.83.8.4977. [DOI] [PubMed] [Google Scholar]
- 5.Kishikawa S, et al. Overexpression and genetic abnormality of p53 in parathyroid adenomas. Pathol Int. 1999;49(10):853–857. doi: 10.1046/j.1440-1827.1999.00961.x. [DOI] [PubMed] [Google Scholar]
- 6.Erickson LA, et al. Parathyroid hyperplasia, adenomas, and carcinomas: Differential expression of p27Kip1 protein. Am J Surg Pathol. 1999;23(3):288–295. doi: 10.1097/00000478-199903000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Cromer MK, et al. Identification of somatic mutations in parathyroid tumors using whole-exome sequencing. J Clin Endocrinol Metab. 2012;97(9):E1774–E1781. doi: 10.1210/jc.2012-1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arnold A, Staunton CE, Kim HG, Gaz RD, Kronenberg HM. Monoclonality and abnormal parathyroid hormone genes in parathyroid adenomas. N Engl J Med. 1988;318(11):658–662. doi: 10.1056/NEJM198803173181102. [DOI] [PubMed] [Google Scholar]
- 9.Noguchi S, Motomura K, Inaji H, Imaoka S, Koyama H. Clonal analysis of parathyroid adenomas by means of the polymerase chain reaction. Cancer Lett. 1994;78(1-3):93–97. doi: 10.1016/0304-3835(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 10.Fialkow PJ, Jackson CE, Block MA, Greenawald KA. Multicellular origin of parathyroid “adenomas”. N Engl J Med. 1977;297(13):696–698. doi: 10.1056/NEJM197709292971304. [DOI] [PubMed] [Google Scholar]
- 11.Sanjuan X, Bryant BR, Sobel ME, Merino MI. Clonality analysis of benign parathyroid lesions by human androgen receptor (HUMARA) gene assay. Endocr Pathol. 1998;9(1):293–300. doi: 10.1007/BF02739689. [DOI] [PubMed] [Google Scholar]
- 12.Mayer GP, Hurst JG. Sigmoidal relationship between parathyroid hormone secretion rate and plasma calcium concentration in calves. Endocrinology. 1978;102(4):1036–1042. doi: 10.1210/endo-102-4-1036. [DOI] [PubMed] [Google Scholar]
- 13.Brown EM. Four-parameter model of the sigmoidal relationship between parathyroid hormone release and extracellular calcium concentration in normal and abnormal parathyroid tissue. J Clin Endocrinol Metab. 1983;56(3):572–581. doi: 10.1210/jcem-56-3-572. [DOI] [PubMed] [Google Scholar]
- 14.Kifor O, et al. Reduced immunostaining for the extracellular Ca2+-sensing receptor in primary and uremic secondary hyperparathyroidism. J Clin Endocrinol Metab. 1996;81(4):1598–1606. doi: 10.1210/jcem.81.4.8636374. [DOI] [PubMed] [Google Scholar]
- 15.Farnebo F, Höög A, Sandelin K, Larsson C, Farnebo LO. Decreased expression of calcium-sensing receptor messenger ribonucleic acids in parathyroid adenomas. Surgery. 1998;124(6):1094–1098, discussion 1098–1099. doi: 10.1067/msy.1998.91828. [DOI] [PubMed] [Google Scholar]
- 16.Pi M, Spurney RF, Tu Q, Hinson T, Quarles LD. Calcium-sensing receptor activation of rho involves filamin and rho-guanine nucleotide exchange factor. Endocrinology. 2002;143(10):3830–3838. doi: 10.1210/en.2002-220240. [DOI] [PubMed] [Google Scholar]
- 17.Koh J, et al. Regulator of G protein signaling 5 is highly expressed in parathyroid tumors and inhibits signaling by the calcium-sensing receptor. Mol Endocrinol. 2011;25(5):867–876. doi: 10.1210/me.2010-0277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cinti S, Sbarbati A. Ultrastructure of human parathyroid cells in health and disease. Microsc Res Tech. 1995;32(2):164–179. doi: 10.1002/jemt.1070320210. [DOI] [PubMed] [Google Scholar]
- 19.McGregor DH, Lotuaco LG, Rao MS, Chu LL. Functioning oxyphil adenoma of parathyroid gland: An ultrastructural and biochemical study. Am J Pathol. 1978;92(3):691–711. [PMC free article] [PubMed] [Google Scholar]
- 20.Allen RC, Zoghbi HY, Moseley AB, Rosenblatt HM, Belmont JW. Methylation of HpaII and HhaI sites near the polymorphic CAG repeat in the human androgen-receptor gene correlates with X chromosome inactivation. Am J Hum Genet. 1992;51(6):1229–1239. [PMC free article] [PubMed] [Google Scholar]
- 21.Gilliland DG, Blanchard KL, Levy J, Perrin S, Bunn HF. Clonality in myeloproliferative disorders: Analysis by means of the polymerase chain reaction. Proc Natl Acad Sci USA. 1991;88(15):6848–6852. doi: 10.1073/pnas.88.15.6848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Costa-Guda J, Arnold A. Genetic and epigenetic changes in sporadic endocrine tumors: Parathyroid tumors. Mol Cell Endocrinol. 2013 doi: 10.1016/j.mce.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ritter CS, Haughey BH, Miller B, Brown AJ. Differential gene expression by oxyphil and chief cells of human parathyroid glands. J Clin Endocrinol Metab. 2012;97(8):E1499–E1505. doi: 10.1210/jc.2011-3366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galon J, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313(5795):1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 25.Dieu-Nosjean MC, et al. Long-term survival for patients with non-small-cell lung cancer with intratumoral lymphoid structures. J Clin Oncol. 2008;26(27):4410–4417. doi: 10.1200/JCO.2007.15.0284. [DOI] [PubMed] [Google Scholar]
- 26.Zhang L, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348(3):203–213. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 27.Lam KY, Chan AC, Lo CY. Parathyroid adenomas with pronounced lymphocytic infiltration: No evidence of autoimmune pathogenesis. Endocr Pathol. 2000;11(1):77–83. doi: 10.1385/ep:11:1:77. [DOI] [PubMed] [Google Scholar]
- 28.Thliveris AT, et al. Transformation of epithelial cells through recruitment leads to polyclonal intestinal tumors. Proc Natl Acad Sci USA. 2013;110(28):11523–11528. doi: 10.1073/pnas.1303064110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.