Inflammation is the immediate response of our tissues to clear and present danger: microbial invasion, injury, or a serious malfunction of our body’s internal workings. Whereas all cells can respond to danger, innate immunity cells have that as their mission: They deploy a vast array of receptors to detect telltale molecules from pathogens or ailing cells and secrete an equally vast number of soluble proteins to communicate among themselves and with the resident cells of the tissue. In PNAS, Lu et al. report on how myeloid cells use a key word in this cellular conversation: high-mobility group box 1 (HMGB1) (1).
HMGB1 is a component of eukaryotic chromatin, and within the nucleus, it bends DNA and facilitates its interaction with transcription factors and other molecules, including histones, to form nucleosomes (2). However, HMGB1 is also the archetypal damage-associated molecular pattern (DAMP): molecules that, when released from a cell’s interior following its untimely death, alert immune cells to danger (3). Notably, cells that die by apoptosis (and therefore kill themselves presumably after careful consideration) do not release HMGB1 to activate the immune system (4), but rather expose “eat-me” signals to request swift disposal.
Interestingly, the meaning of extracellular HMGB1 goes beyond a simple “I’m dead”: Most cells can secrete HMGB1 before they die and therefore can cry out for help before it is too late for them. In fact, cells can secrete most of the HMGB1 they contain without necessarily being committed to die and can resume their normal occupations (and replenish HMGB1 by resynthesis) once the danger is over. Examples are HMGB1 secretion by cardiomyocytes in hypoxic conditions following ischemia (5) or by neural cells following the depolarization of their plasma membrane (6). Myeloid cells secrete HMGB1 once they are activated by the presence of pathogens, by the detection of injured cells, or by cytokines, in order to rebroadcast the danger message.
Extracellular HMGB1 plays a central role in inflammation, both acute and chronic, and indeed in sepsis when innate immunity goes awry (7). HMGB1 both recruits and activates myeloid cells; however, these are separable processes, which depend on different redox states of HMGB1 and on different receptors. HMGB1 contains three cysteines (at positions 23, 45, and 106): When all are in the reduced (thiol) state, HMGB1 forms a heterocomplex with the chemokine stromal cell-derived factor (SDF-1/CXCL12) and binds to the CXCR4 receptor, acting as a potent chemoattractant for all motile cells, including innate immunity cells (8, 9). When cysteines 23 and 45 form a disulfide bond and the cysteine 106 is in the thiol state, HMGB1 binds to the TLR4 receptor, activating NF-κB to drive the transcription of genes involved in inflammation, including chemokine and cytokine genes; when one of the cysteines is terminally oxidized to a sulfonate, HMGB1 loses activity both as a chemoattractant and as an inflammatory ligand (9, 10). HMGB1 also binds to other surface molecules, including receptor for advanced glycation end products (RAGE), thrombomodulin, CD24, and TIM-3; RAGE can cooperate both with HMGB1’s chemoattractant and inflammatory activities, whereas the other molecules limit HMGB’s effects (7).
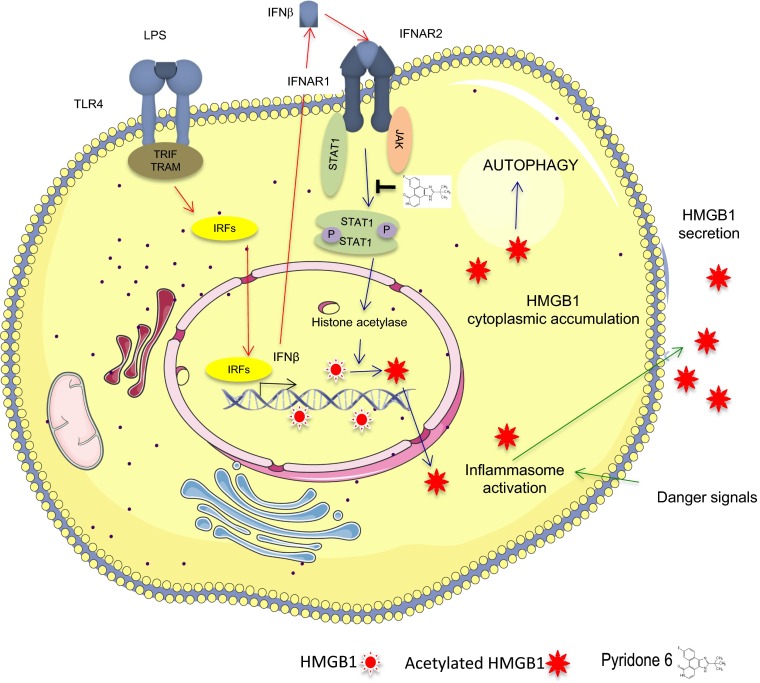
Given HMGB1’s key role in inflammation, understanding how it secreted is of central importance. HMGB1 secretion is unconventional, as may be expected for a nuclear protein that has no leader peptide to direct it to the endoplasmic reticulum and the exocytosis route. Rather, HMGB1 is endowed with nuclear localization and nuclear export signals (NLS and NES, respectively) (11), which keep it in constant flux between the nucleus and the cytoplasm. In basal conditions, almost all HMGB1 is nuclear, but removal of positive charges from its NLSs via lysine acetylation (11) or addition of negative charges via serine phosphorylation (12) shifts the balance to allow the transfer of a fraction of HMGB1 to the cytoplasm. Tracey and coworkers, who were the first to realize that HMGB1 can be secreted by macrophages (13), now implicate the JAK/STAT signaling pathway in determining the acetylation of HMGB1’s NLSs and its eventual secretion (1) (Fig. 1).
Fig. 1.
Diagrammatic representation of the pathway for HMGB1 secretion induced by LPS, as proposed by Lu et al. (1). The two subsequent steps, IFN-β secretion on LPS activation of TLR4 and HMGB1 secretion on binding of IFN-β to its own receptor, are distinguished by arrow color.
JAKs and STATs are critical for signaling by cytokine transmembrane receptors. After the receptors associate with their respective cytokine ligand, they undergo a conformational change, bringing two JAKs close enough to phosphorylate each other and the receptor. Phosphorylation of tyrosine residues in the cytoplasmic receptor tail creates docking sites for molecules of the STAT family. On binding, the STATs themselves become a JAK substrate, and phosphorylated STATs form dimers that translocate to the nucleus and bind enhancers and promoters of cytokine responsive genes (14). Receptors of type 1 and 2 IFNs (IFN-α/β and IFN-γ, respectively) depend on JAKs for their function. It had been known that HMGB1’s secretion can be induced by exposure of macrophages to IFNs and that pharmacological or genetic blockade of the JAK/STAT downstream signaling abrogates HMGB1 secretion (15, 16). Lu et al. show that pyridone 6, a pan-inhibitor of JAK1/2, blocks the LPS-induced secretion of HMGB1 from mouse macrophage-like RAW 267.4 cells, whereas it does not decrease LPS-induced secretion of TNF and other cytokines. Cytokines are secreted via the classical exocytotic pathway, whereas HMGB1’s secretion requires the acetylation of its NLSs to direct it to the cytoplasm, which is indeed the step blocked by pyridone 6. Inflammasome activation by LPS via autophosphorylation of the double-stranded RNA-dependent protein kinase (PKR) and its association to various inflammasome components are also required for HMGB1 secretion (17), but inhibition of PKR with 2-aminopurine does not influence HMGB1’s relocalization to the cytoplasm, indicating that inflammasome-dependent secretion is a separate step along the pathway to HMGB1 secretion that occurs only on the HMGB1 that has already moved to the cytoplasm.
Consistent with its dependence on IFN-β, HMGB1 relocalization and the acetylation of its NLSs require STAT1, and genetic ablation of STAT1 blocks HMGB1 secretion. Remarkably, acetylation of lysines is a posttranscriptional modification that requires the activation of lysine acetylases (KATs), the inhibition of histone deacetylases (HDACs), or both. Notably, all STATs likely bind CBP and p300 (14), which are ubiquitous KATs that have been shown to be capable of acetylating HMGB1 (11). Moreover, STAT1-mediated transcription requires HDACs as coactivators, even though HDAC activity is usually associated with transcriptional repression. It would be interesting to know whether STAT1 binding to DNA is required for HMGB1 acetylation and whether the same recruited coactivator complex concomitantly acetylates both HMGB1 and histones. If this is the case, HMGB1 might modulate STAT1-mediated gene expression, perhaps directly or by competing with histone tails as a substrate.
In conclusion, the present findings elucidate one step in the cascade between macrophage activation and HMGB1 secretion and convincingly implicate JAK/STAT signaling. As depicted in Fig. 1, LPS binding to Toll-like receptor (TLR)4 promotes IFN transcription, and secreted IFNs bind IFN receptors (IFNARs) and cause STAT1 phosphorylation by JAKs. Activated STAT1 promotes HMGB1 acetylation and cytoplasmic relocation. Concomitant inflammasome activation would then cause the actual secretion of HMGB1. The elucidation of this pathway may translate into therapeutic approaches: Because extracellular HMGB1 contributes decisively to sepsis (18), an inhibitor like pyridone 6 might hold a lot of promise. Several potential questions and problems remain, however. Foremost is why LPS requires JAK/STAT signaling to induce HMGB1 secretion. As first suggest by Kim et al. (16), LPS would induce IFN secretion, which in turn would cause HMGB1 secretion; this hypothesis is compatible with the finding that anti–IFN-β antibodies interfere with LPS-induced HMGB1 secretion. However, the implication is that macrophages do not secrete HMGB1 in response to LPS directly, but only indirectly. This is counterintuitive, as macrophages are well equipped to respond immediately to minute amounts of LPS, whereas the response to their own IFN production would take time; one wonders why evolution has not favored a direct response. A second question is whether JAK/STAT signaling is always necessary for HMGB1 secretion. HMGB1 secretion requires its cytoplasmic relocalization, but pathways alternative to NLS acetylation appear to exist, including calmodulin-mediated kinase (CAMK) phosphorylation of NLS serines (19). Lu et al. indeed show that HMGB1 relocalization after rapamycin-induced autophagy is not blocked by pyridone 6. Finally, and related to the second question, it is important to determine whether JAK/STAT signaling is required for HMGB1 secretion by all cells or just by macrophages; critically, the authors show that IFN-β fails to direct HMGB1 release from mouse embryonic fibroblasts. Cell type specificity is of paramount importance for therapeutic applications: Myeloid cells do not appear to contribute much to circulating levels of HMGB1 in LPS endotoxemia, because the level of HMGB1 is similar in LPS-challenged mice whether or not their myeloid cells contain HMGB1 (20). Despite all of these uncertainties, the illumination of one side of HMGB1 biology will undoubtedly help the understanding of the remaining dark sides and more generally of how our body detects and responds to its own failings (which most people would call illnesses).
Footnotes
Conflict of interest statement: M.E.B. is founder and part-owner of HMGBiotech, a biotech company that specializes in the HMGB1 field.
See companion article on page 3068.
References
- 1.Lu B, et al. JAK/STAT1 signaling promotes HMGB1 hyperacetylation and nuclear translocation. Proc Natl Acad Sci USA. 2014;111:3068–3073. doi: 10.1073/pnas.1316925111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Celona B, et al. Substantial histone reduction modulates genomewide nucleosomal occupancy and global transcriptional output. PLoS Biol. 2011;9(6):e1001086. doi: 10.1371/journal.pbio.1001086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bianchi ME. DAMPs, PAMPs and alarmins: All we need to know about danger. J Leukoc Biol. 2007;81(1):1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- 4.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418(6894):191–195. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 5.Andrassy M, et al. High-mobility group box-1 in ischemia-reperfusion injury of the heart. Circulation. 2008;117(25):3216–3226. doi: 10.1161/CIRCULATIONAHA.108.769331. [DOI] [PubMed] [Google Scholar]
- 6.Karatas H, et al. Spreading depression triggers headache by activating neuronal Panx1 channels. Science. 2013;339(6123):1092–1095. doi: 10.1126/science.1231897. [DOI] [PubMed] [Google Scholar]
- 7.Andersson U, Tracey KJ. HMGB1 is a therapeutic target for sterile inflammation and infection. Annu Rev Immunol. 2011;29:139–162. doi: 10.1146/annurev-immunol-030409-101323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schiraldi M, et al. HMGB1 promotes recruitment of inflammatory cells to damaged tissues by forming a complex with CXCL12 and signaling via CXCR4. J Exp Med. 2012;209(3):551–563. doi: 10.1084/jem.20111739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Venereau E, et al. Mutually exclusive redox forms of HMGB1 promote cell recruitment or proinflammatory cytokine release. J Exp Med. 2012;209(9):1519–1528. doi: 10.1084/jem.20120189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang H, et al. Redox modification of cysteine residues regulates the cytokine activity of high mobility group box-1 (HMGB1) Mol Med. 2012;18:250–259. doi: 10.2119/molmed.2011.00389. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Bonaldi T, et al. Monocytic cells hyperacetylate chromatin protein HMGB1 to redirect it towards secretion. EMBO J. 2003;22(20):5551–5560. doi: 10.1093/emboj/cdg516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oh YJ, et al. HMGB1 is phosphorylated by classical protein kinase C and is secreted by a calcium-dependent mechanism. J Immunol. 2009;182(9):5800–5809. doi: 10.4049/jimmunol.0801873. [DOI] [PubMed] [Google Scholar]
- 13.Wang H, et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285(5425):248–251. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- 14.Levy DE, Darnell JE., Jr Stats: Transcriptional control and biological impact. Nat Rev Mol Cell Biol. 2002;3(9):651–662. doi: 10.1038/nrm909. [DOI] [PubMed] [Google Scholar]
- 15.Pan PH, Cardinal J, Li ML, Hu CP, Tsung A. Interferon regulatory factor-1 mediates the release of high mobility group box-1 in endotoxemia in mice. Chin Med J (Engl) 2013;126(5):918–924. [PubMed] [Google Scholar]
- 16.Kim JH, et al. Bacterial endotoxin induces the release of high mobility group box 1 via the IFN-beta signaling pathway. J Immunol. 2009;182(4):2458–2466. doi: 10.4049/jimmunol.0801364. [DOI] [PubMed] [Google Scholar]
- 17.Lu B, et al. Novel role of PKR in inflammasome activation and HMGB1 release. Nature. 2012;488(7413):670–674. doi: 10.1038/nature11290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang H, et al. Reversing established sepsis with antagonists of endogenous high-mobility group box 1. Proc Natl Acad Sci USA. 2004;101(1):296–301. doi: 10.1073/pnas.2434651100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang X, et al. Calcium/calmodulin-dependent protein kinase (CaMK) IV mediates nucleocytoplasmic shuttling and release of HMGB1 during lipopolysaccharide stimulation of macrophages. J Immunol. 2008;181(7):5015–5023. doi: 10.4049/jimmunol.181.7.5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yanai H, et al. Conditional ablation of HMGB1 in mice reveals its protective function against endotoxemia and bacterial infection. Proc Natl Acad Sci USA. 2013;110(51):20699–20704. doi: 10.1073/pnas.1320808110. [DOI] [PMC free article] [PubMed] [Google Scholar]