Significance
Nanoparticles (NPs) can serve as containers for the targeting of therapeutics to tumors. Tumors comprise many cell types including endothelial cells that form the blood vessels. Developing new strategies to target information preferentially to endothelial cells can have major implications in the development of targeted therapeutics. We have discovered that charged polymers containing aromatic sulfonate have pronounced affinity for caveolae, which are highly expressed by endothelial cells. By engineering the surface of lipid NPs to bear sulfonate-containing polymers, lipid NPs that are preferentially taken up by endothelial cells have been demonstrated.
Keywords: polyanion, aromatic polysulfonates, polystyrene sulfonate, vasculature
Abstract
Nanoparticles (NPs) constitute an important medium for the targeted delivery of cancer therapeutics. Targeting of NPs to a specific cell type is traditionally achieved through the modification of the NP surface with peptides, aptamers, or other motifs that specifically recognize a cell-surface receptor, leading to internalization of NPs via clathrin and caveolae-mediated endocytosis. We have discovered that modifying the NP surface with anionic polyelectrolytes of varying lipophilicity can regulate the uptake of lipid NPs by endothelial and epithelial cells. Furthermore, we report the finding that synthetic polyelectrolytes composed of an aromatic sulfonic acid backbone exhibit specific affinity for caveolae of endothelial cells. By exploiting the higher expression of caveolae in endothelial cells in comparison with epithelial cells, a purely physiochemical approach to the targeted uptake of lipid NPs to endothelial cells is demonstrated. The ability to confer preferential affinity for NPs to cell surface domains by varying the charge and lipophilic characteristics of an NP surface offers a general means of achieving targeted delivery without the need for receptor–ligand-type targeting strategies.
Nanoparticles (NPs) constitute an important modality for the delivery of therapeutics and imaging agents as they are capable of delivering a highly potent dose to a target site while also preserving the activity of the agent during transit in the blood stream (1). Tumors constitute a dynamic environment comprising many cell types including endothelial cells, epithelial cells, stromal cells, fibroblasts, and inflammatory cells such as macrophages. With regards to tumor delivery, the highly leaky vasculature present in a majority of epithelial-derived tumors provides an avenue for localization of therapy using NPs. However, the leaky vasculature also promotes the drainage of the therapy away from the tumor. In this context NPs that can be targeted to specific tumor cellular components are important for increasing efficacy. Typically, NPs’ guidance to, and retention at, the tumor site is achieved by modifying their surface with tumor-specific targeting motifs like antibodies or short peptides that exhibit high affinity toward tumor-specific antigens (e.g., prostate-specific antigen) or receptors (e.g., folate receptor) (2–5), or receptors associated with tumor vasculature such as endothelial growth factor receptor (6, 7). However, upon injection into the blood stream or in a local tissue environment, NP efficacy is determined in part by how they are processed by cells. Most NPs are taken up by cells through one of the classical pathways: namely, macropinocytosis, clathrin-mediated endocytosis, and caveolae-mediated endocytosis (8–11). Furthermore, arginine-rich peptides (e.g., cell-penetrating peptides), which can porate the cell membrane, can enable direct translocation of the NP into the cytosol (10, 12).
Many variables impact NP uptake into cells including size, shape, and surface charge (13–16). It is well known that positively charged NPs are well suited for endocytic processing by cells as they can interact favorably with the negatively charged phospholipid components of the cell membrane (13). The impact of NP surface chemistry on cell–NP interaction and cellular uptake is being recognized (17, 18). Nevertheless, our understanding of the role of physicochemical characteristics of NPs in cellular uptake is rather limited.
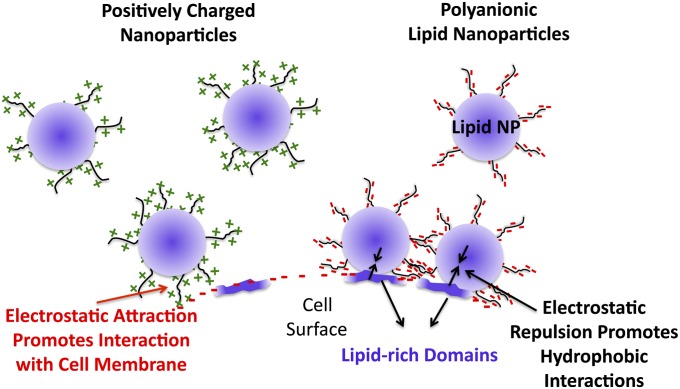
One of the challenges associated with using disease-based targets for homing of a therapeutic agent is the variability in expression of targets due to patient–patient variability and the stage of the tumor. Therefore, a highly generalized approach that can discriminate between various cell types found within a tumor environment without the need for receptor-based targeting could be very valuable. We therefore posed the question: Is it possible to target a specific cell type purely by varying the physicochemical characteristics of a nanocarrier? From a biophysical standpoint, receptors–ligand interactions can be distilled down to an interplay and balance between hydrophobic and electrostatic interactions. Based on this simple premise, we theorized that an NP system possessing two characteristics, (i) a high affinity for cell membrane lipids and (ii) a highly negatively charged surface, will diminish nonspecific charge–charge interactions between the NP and cell surface, thereby promoting lipophilic-affinity-based interactions with the cell surface, thus enabling the targeting of lipid-rich cell domains (Fig. 1).
Fig. 1.
Schematic representation of how electrostatic repulsive interactions between a hydrophobic NP and cell membrane can promote highly specific interactions with lipophilic elements on the cell surface (Right). A positively charged NP interacts with the negatively charged cell membrane, leading to uptake that is dominated by electrostatic attraction (Left).
Using lipid NPs with surfaces rich in negatively charged polyelectrolytes of different physiochemical characteristics, we have discovered that NPs with specific affinity for caveolae can be realized, and based on this specificity, NPs are preferentially taken up by endothelial cells without the need for cell-specific targeting ligands. By further understanding the relationships between NP surface physicochemical characteristics and cell-surface domains, NP systems that can inherently discriminate between healthy and diseased cells may be realized in the near future.
Results and Discussion
In the context of targeting hydrophobic domains on a cell surface, lipid-based NPs are well suited, as they exhibit thermal transitions that are compatible with the physiological environment and additionally possess a high biocompatibility and low toxicity (19). To eliminate NP size and surface charge characteristics as a variable, lipid NPs of similar size and surface charge were used in this study. All NPs were around 180 nm in size, with a polydispersity of less than 0.2, and zeta potential in the range of –30 to –55 mV (Fig. S1). The similarity in size and morphology between the various NPs was also confirmed using cryotransmission electron microscopy (Fig. S2). The NPs showed no disintegration or aggregation in culture medium at 37 °C and, more importantly, after exposure to serum showed almost identical zeta potential of around –40 mV (Fig. S3). Toxicity studies showed that the NPs were nontoxic to the cells over the time scale of the experiment (Fig. S4). Lipid NP surfaces modified with polyanions were synthesized using a modified nanoprecipitation process developed in our laboratory (20). To enable tracking of the NP during cellular uptake, a lipophilic long-chain alkyl-modified Rhodamine conjugate was incorporated into the NP during synthesis. Because the tumor environment is composed predominantly of two cell types—namely, endothelial and epithelial cells—NP uptake was investigated using primary endothelial cells and tumor-derived epithelial cells.
HUVECs Can Discriminate Between Lipid NPs Bearing Different Polyanions.
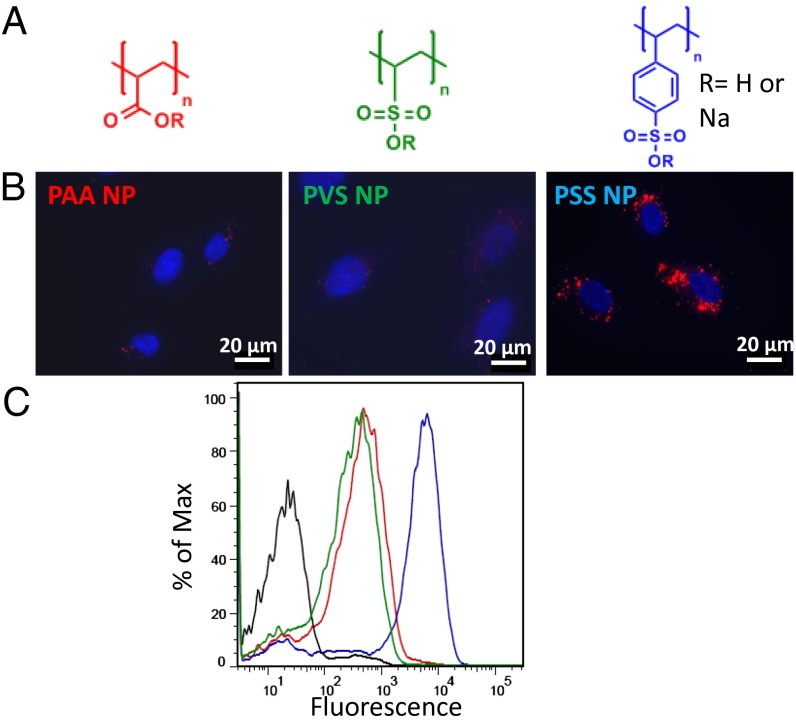
In the initial screening experiments, the uptake of NPs bearing three different polyelectrolytes, poly(styrene sulfonate) (PSS), poly(vinyl sulfonic acid) (PVS), and poly(acrylic acid) (PAA) in human umbilical vein endothelial cells (HUVECs) were investigated to gain insights into the role of polyelectrolyte charge characteristics on cellular uptake. We made a surprising finding that despite all of the three polyelectrolytes possessing negative charge, lipid NPs with PSS surface were internalized in HUVECs to a significantly greater extent than lipid NPs with PVS and PAA surface functionality and underwent perinuclear localization (Fig. 2). Upon comparison of the physiochemical characteristics of the three electrolytes, two variables emerge: (i) the ionizable group (i.e., sulfonic acid versus carboxylic acid) and (ii) the lipophilicity of the moiety bearing the charged functional group. Because at physiological pH both sulfonic acid and carboxylic acid groups are highly ionized, the lipophilicity of the polymer backbone is the key variable. One measure of the affinity of species to lipids is the octanol-water partition coefficient (LogP), which is an estimate of the solubility of a chemical moiety in lipid versus water at equilibrium. In this case, because the polyelectrolytes are homopolymers, the LogP of the monomer repeat unit is a reasonable indicator of the relative affinity of the polyelectrolytes to lipid environments. The LogP values for the PSS, PVS, and PAA repeat units were calculated to be –1.211, –3.283, and –2.227, respectively. It is clear that the styrene sulfonate repeat unit, although ionic in nature, has an order of magnitude more affinity for lipid environment in comparison with the other two polyelectrolytes. This is reasonable as the PSS molecule has an aromatic ring that in theory should confer higher lipophilicity to the polyelectrolyte backbone. Therefore, we can conclude that the dominant element in lipid NP interaction with HUVECs is the surface chemistry. Because surface chemical moieties (i.e., PSS, PVS, and PAA) are electrostatically repulsive to the cell membrane, one might expect hydrophobic interactions conferred by the surface chemistry to dominate as theorized (Fig. 1). To exclude the possibility that the observed effects do not result from differences in the protein corona around the NP, we also executed the experiments in absence of serum (Fig. S5) and observed the same results. Thus, the observed selectivity in NP uptake must originate in the differences in the affinity of the polyelectrolyte structure to lipids.
Fig. 2.
Uptake of lipid NP with various polyanionic surfaces in HUVECs. The structures of the polyelectrolytes used to screen surface chemistry effects are shown in A. Fluorescent images (B) reveal that PSS lipid NPs are strongly taken up by HUVECs and undergo perinuclear localization, which is not observed with PAA and PVS lipid NPs (DAPI nuclear stain, blue; Rhodamine-labeled lipid NPs, red). FACS histograms of HUVECs incubated with the different NPs (in black untreated cells) show that the uptake efficiency of PSS lipid NPs is an order of magnitude higher than the PAA and PVS lipid NPs (C).
Aromatic Sulfonic Acid Is a Prerequisite for Interaction with HUVECs.
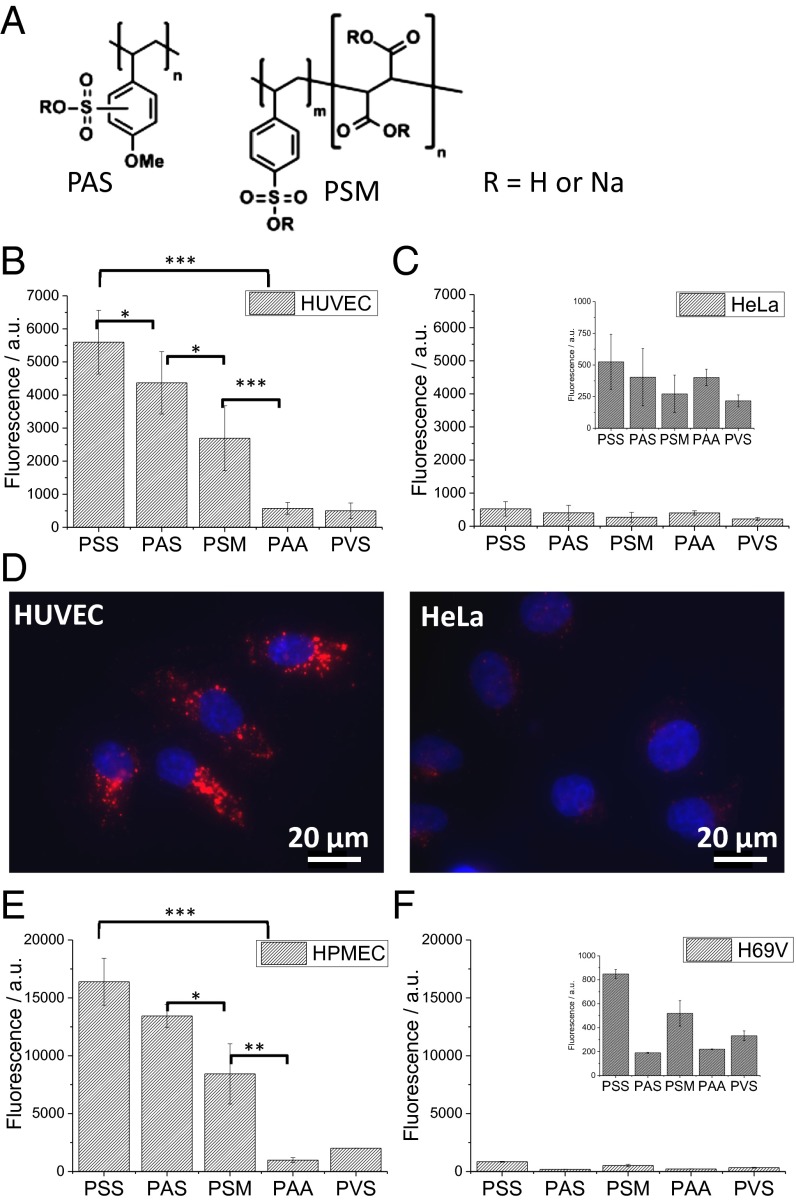
To further investigate this premise, the effect of two derivatives of PSS, poly(4-styrenesulfonic acid–comaleic acid) (PSM) and poly(anetholesulfonic acid) (PAS), on NP uptake was studied (Fig. 3A). PSM has a lower LogP (LogP = –1.997) in comparison with PSS. PAS, which differs from PSS in the presence of a methoxy substitution on the aromatic ring, has only a slightly lower LogP (LogP = –1.227) in comparison to PSS. Therefore, in theory, lipid NPs with PSM surface should demonstrate a marked decrease in uptake, and lipid NPs modified with PAS should have comparable uptake as PSS. In fact, although a reduction of over 50% in NP uptake is observed when the surface-bound species is PSM, the uptake on NP with PAS surface is similar to that of NP with PSS surface (Fig. 3B). Because in PSM the number of styrenesulfonate groups along the polymer backbone is 50% fewer in comparison with PSS backbone, the observed reduction in NP uptake can be attributed to a loss of styrenesulfonic groups along the backbone.
Fig. 3.
Role of backbone lipophilicity in the uptake of negatively charged lipid NPs in HUVECs and HeLa cells. Lipid NPs with different surface chemistries (A) were incubated with different cells and analyzed with FACS. In HUVECs (B) the uptake of lipid NPs modified with derivatives of PSS, namely, PAS and PSM, is greater than PAA and PVS, suggesting an important role for the aromatic sulfonic acid group in the interaction with HUVECs. This is confirmed by fluorescent microscopy that shows significantly higher uptake in HUVECs in comparison with HeLa cells (D). More importantly, this correlation between surface chemistry, lipophilicity, and lipid NP uptake is also observed in primary HPMECs (E). The uptake behavior of lipid NPs in HeLa (C) and H69V (F), both of which are tumor-derived epithelial cells, however, does not show the same dependency on surface chemistry (Inset, adjusted axis scale for improved clarity) (*P < 0.05, **P > 0.01, ***P < 0.001).
We then posed the following question: Can other cells also discriminate between NPs of differing surface chemistries, or is this unique to HUVECs? Using HeLa cells, a cervical-cancer–derived epithelial cell line that is commonly used to study NP uptake, we observed that the uptake behavior is different from the one observed in HUVECs (Fig. 3C). In addition to the absence of any distinct trend with respect to surface chemistry, the uptake of PSS lipid NPs is markedly diminished (Fig. 3 B–D). This observation hinted to the presence of cell-surface components that PSS lipid NP interacts with that are unique to or highly expressed in HUVECs compared with epithelial cells. More importantly, we observed that the uptake behavior of the various NPs could be extrapolated to human pulmonary microvascular endothelial cells (HPMECs) and epithelial cells derived from human lung carcinoma (H69V)—thus suggesting that the preferential uptake of PSS lipid NPs and the higher uptake rate in endothelial cells can be attributed to an elementary difference between endothelial and epithelial cells.
Caveolae Expression Is Up-Regulated in Endothelial Cells Versus Epithelial Cells.
To understand the mechanism of the observed phenomenon, we investigated the differences in endocytosis behavior between HUVECs and HeLa cells, using fluorescently labeled BSA (21) and cholera toxin B (CTB), which are known as markers for caveolae-mediated endocytosis and transferrin for clathrin-mediated endocytosis (10) (Fig. 4A).
Fig. 4.
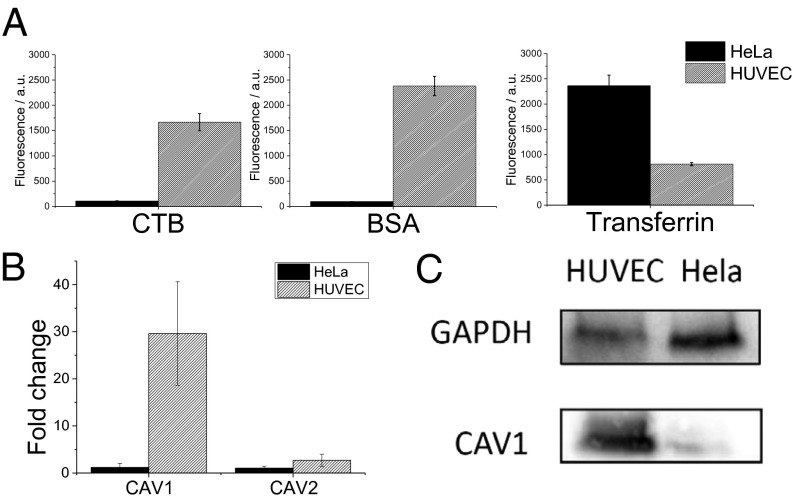
Endocytic pathways in HUVECs and HeLa cells. The dominant endocytic uptake mechanism in HUVECs and HeLa cells was probed using fluorescently labeled BSA and CTB (markers for caveolae-mediated uptake) and transferrin (marker for clathrin-mediated endocytosis) (A). Uptake was quantified using FACS analysis. Caveolae-mediated uptake is prominent in HUVECs and significantly lower in HeLa cells. In comparison, transferrin-mediated endocytosis is threefold higher in HeLa cells (A). Real-time PCR (B) and Western blot (C) analysis of HUVECs and HeLa cells show a higher CAV1 expression level in HUVECs.
Although HeLa cells internalize more transferrin than HUVECs by threefold, a much stronger distinction in the uptake behavior of BSA and CTB was observed. The uptake of CTB and BSA is 18-fold and 32-fold, respectively, higher in HUVECs in comparison with HeLa cells (Fig. 4A). Because BSA and CTB are reported to be taken up through lipid rafts or more precisely caveolae-mediated endocytosis, this suggests a possible role for caveolae in endocytic transport of lipid PSS NPs in endothelial cells. Because caveolin-1 (CAV1) and caveolin-2 (CAV2) are important components of the caveolae structure and function, we carried out gene expression analysis in HUVECs and HeLa cells and observed that CAV1 was 30-fold higher expressed in HUVECs in comparison with HeLa cells, however no significant differences in CAV2 expression in the two cells types were observed (Fig. 4B). The up-regulation of CAV1 expression was confirmed at the protein level (Fig. 4C), demonstrating that HUVECs express more caveolins than HeLa cells. It has been reported that the cellular membrane of endothelial cells is high in caveolae (22), however our findings shed unique light on some fundamental differences in the endocytic chaperons on endothelial and epithelial cells and this might have important implications in cancer therapy.
PSS Lipid NPs Colocalize with Caveolin.
Considering the fact that NP uptake is higher in HUVECs and taking into account the finding that HUVECs have higher caveolin content, we suggest that the NP uptake in endothelial cells occurs primarily via a caveolin-dependent mechanism. We first used colocalization studies, using fluorescent immunohistochemisty toward CAV1, and labeled NPs to ascertain this hypothesis. It is clear from fluorescent images that the HUVEC cell membrane is rich in caveolin (green). More importantly, the PSS lipid NPs (red) in HUVECs were always associated with CAV1 (Fig. 5A, Inset). Similar observations were made in HeLa cells as well (Fig. S6). This suggests that the PSS surface might have special affinity to the caveolae membrane domains. To solidify this conclusion, NP uptake was carried out in the presence of caveolae inhibition. The inhibition of caveolin-mediated endocytosis is usually undertaken by the administration of drugs that lead to cholesterol depletion (21). The most commonly used method to determine the uptake mechanism of NP is to use specific endocytosis inhibitors and to evaluate whether these inhibitors reduce the amount of NPs taken up (10, 23, 24). We noticed that the cholesterol depletion approach only works for cells such as HeLa cells that have moderate caveolin levels, however in cells with high caveolin levels like HUVECs the cell membrane either collapses or is disrupted during trypsinization for FACS analysis. Therefore, the uptake behavior of PSS, PAS, and PSM NPs was investigated in the presence of Filipin-III in HeLa cells. It is well established that Filipin-III binds specifically to cholesterol in the caveolae structures and in doing so disrupts the integrity of caveolae and uptake through caveolae (25, 26). We observed that in the presence of Filipin-III the uptake of all three NPs in HeLa cells was drastically diminished and importantly to the same baseline levels (Fig. S7), clearly suggesting a prominent role for caveolae in the uptake of these NPs.
Fig. 5.
(A) PSS lipid NPs colocalize with CAV1. Immunofluorescence staining for CAV1 reveals that the surface of HUVEC is rich in caveolin. It is evident that PSS lipid NPs are colocalized with CAV1, and this is clearly illustrated with arrows in the magnification of the marked regions. (B) When incubating other human and mouse cell lines (endo, endothelial cells; epi, epithelial cells) with lipid PSS and PAA NPs, it can also be seen that endothelial cells have the highest PSS lipid NP uptake and the highest NP selectivity, as shown with flow cytometry. (C) Western blotting shows that endothelial cells have a higher CAV1 content compared with epithelial cells.
To broaden the findings and to confirm that indeed endothelial cells have an enhanced PSS NP uptake and this is species independent, we compared the uptake of PSS and PAA NPs in other relevant tumor cell lines [human embryonic kidney cells (HEK293) and human breast cancer cells (MCF7A)], different types of murine cells [preosteoblasts (MC3T3), fibroblasts [National Institute of Health (NIH) 3T3], macrophages (RAW264.7), and lung microvascular endothelial cells (C57-6011)] (Fig. 5B), and bovine aortic endothelial cells (BAECs) (Fig. S8). Once again we saw an increased uptake of PSS NPs in bovine and mouse endothelial cells, indicating that the endothelial phenotype and the high CAV1 level (Fig. 5C) can be correlated to the increased uptake of PSS NPs. An enhanced NP uptake was also found in macrophages, but in contrary to endothelial cells, the uptake is less specific to PSS NPs. This is reasonable as macrophages are phagocytic cells and they are more prone to take up particulates.
Free PSS Inhibits Caveolin-Mediated Endocytosis.
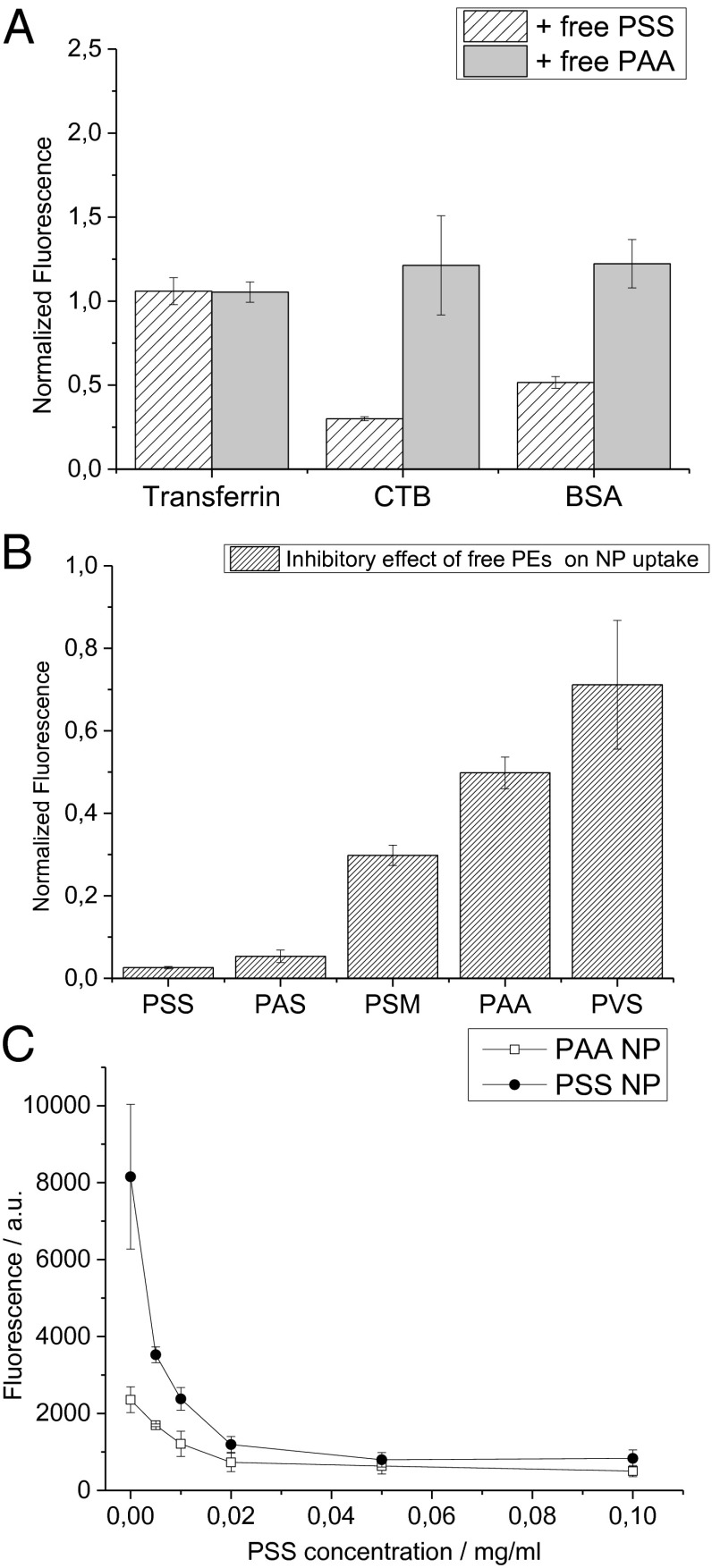
If PSS and its derivatives indeed have a special binding affinity for endothelial caveolae, then the presence of free PSS in solution will compete for the caveolin transporters and diminish the uptake of the NP via this route. The uptake of endocytosis markers by HUVECs was studied in the absence and presence of free PSS (Fig. 6). As postulated, free PSS inhibits the uptake of both BSA and CTB, which are known markers for caveolae-mediated endocytosis. However, free PSS does not have an impact on the uptake of transferrin in HUVECs. This effect can therefore be attributed to the interaction of free PSS with caveolae, thereby reducing their availability for transport. To exclude the possibility that the observed effect results from an unspecific, electrostatic interaction between negatively charged polymers and the cell membrane and to verify the crucial role of lipophilicity, competitive inhibition studies were also carried out with free PAA. Unlike PSS, PAA had no effect on the uptake of any of the endocytic markers (Fig. 6A). It is therefore reasonable to conclude that PSS has the right balance between hydrophobicity and charge that enables interaction with the lipid raft structure of the caveolae (21, 27, 28) while maintaining water solubility, and in this case additionally providing electrostatic stabilization of the lipid NPs.
Fig. 6.
Free PSS inhibits caveolin-mediated endocytosis in HUVECs. Uptake of endocytic markers BSA, CTB, and transferrin in the presence of 0.1 mg/mL of free PSS shows that only CTB and BSA uptake is inhibited by free PSS, suggesting that PSS competitively binds to caveolae (A). Lipid PSS NPs were incubated with all different polyelectrolytes (0.1 mg/mL) and analyzed using FACS. Although free PSS and its derivatives PAS and PSM diminish PSS lipid NP uptake, with the more hydrophobic PSS and PAS having the most pronounced effect, no such inhibitory trends are observed in the presence of free PAA and free PVS, suggesting that free PSS and PSS lipid NP are both competing for caveolae on the HUVEC surface (B). The ability of free PSS to inhibit the uptake of PSS and PAA NPs was studied as a function of concentration (C). The threshold concentration of free PSS for inhibition of PSS lipid NP uptake is very low, suggesting a highly competitive binding of the free PSS to the caveolae. The effect of free PSS on the inhibition of PSS lipid NPs was much more pronounced in comparison with PAA lipid NPs, which is consistent with the observed NP uptake behavior.
Free PSS Inhibits Lipid NP Uptake.
Because PSS is capable of competing for caveolae on the cell surface, we investigated if it can impact the uptake of the lipid NPs investigated in this study. We observed that PSS was indeed capable of significantly diminishing the uptake of PSS lipid NPs in HUVECs. Additionally, the derivatives of PSS, namely, PAS and PSM, also inhibited uptake but showed the same trend as the uptake behavior of NPs functionalized with these polyelectrolytes (Fig. 6B). More importantly, no significant changes in PSS lipid NP uptake was observed in the presence of free PAA and PVS, suggesting that the uptake of PSS lipid NPs occurs as a direct consequence of the interaction of the PSS with caveolae and not through some nonspecific mechanism. Titration of the NP uptake against increasing concentration of free PSS revealed that this effect is pronounced even at very low concentrations of free PSS (Fig. 6C).
PSS is approved by the United States Food and Drug Administration for the treatment of hyperkalemia (29, 30), which is a condition where the patient has a high endogenous level of potassium in the blood. Because PSS can sequester potassium, we investigated if potassium sequestration has a role in the inhibitory effects observed in the presence of free PSS. We carried out uptake studies of PSS lipid NPs in the presence of free PSS, where the solution was supplemented with 40 equivalents of potassium ions per sulfonic binding group in the PSS backbone, and we observed no difference in the inhibitory effect of free PSS on the uptake of PSS lipid NPs (Fig. S9). Additionally potassium did not impact the uptake of PSS lipid NPs. These observations in sum suggest that the inhibition of lipid PSS NP uptake by free PSS occurs due to the competitive binding of PSS to caveolae on the HUVEC surface and not due to depletion of ions.
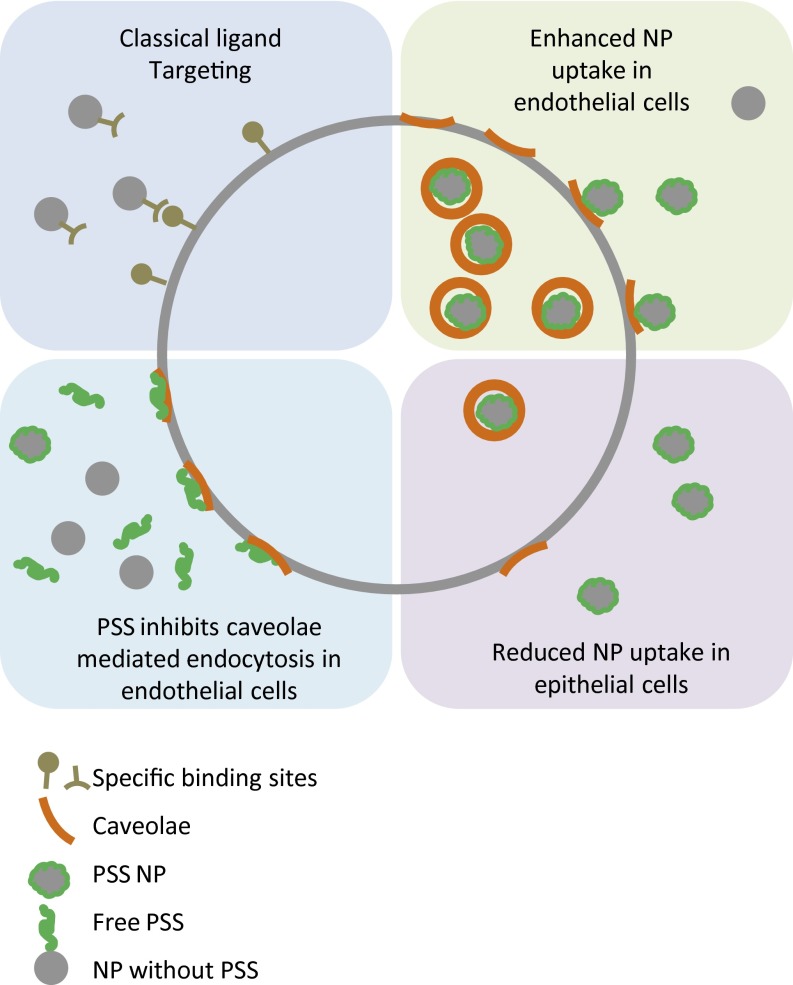
Classical NP targeting with cell-surface ligands using the lock and key principle offers specificity but is sensitive to the conformation and expression of these biological targets. The paradigm shift presented in this study (Fig. 7), which is based on exploiting physicochemical characteristics of polymers to engage specifically cell surface motifs, offers unique possibilities for the discovery of unique cell surface targets for targeted nanomedicine. In drug delivery, the targeting of caveolae in endothelial cells has two major advantages. First, this mechanism is responsible for the transport across the vascular endothelium (28, 31), therefore allowing the NPs injected in the bloodstream to reach the tissue underneath. Second, cargoes taken up by caveolin-mediated endocytosis can bypass the lysosome and therefore escape lysosomal degradation (2, 32). Our findings that synthetic polyelectrolytes (PSS and PAS) can exhibit a high specificity to a cell transporter domain that is critical in the regulation of movement of information in and out of cells provides a unique avenue for targeting and transport of payload into cells. Further studies are necessary to fully exploit the findings presented herein and achieve clinical translation.
Fig. 7.
Scheme showing the specific targeting using antibodies or other ligands (Upper Left) compared with the more general binding of PSS NPs to caveolae (Upper Right). In the latter, this can be used to deliver NP to cells that have many caveolae, whereas the cells with low caveolae level or no caveolae at all (Lower Right) are not targets for these NPs. Using the same interaction, one can also use free PSS in solution to generally inhibit the uptake via caveolin-mediated endocytosis (Lower Left).
Materials and Methods
NP Synthesis and Characterization.
All chemicals were from Sigma-Aldrich unless stated differently. The NPs were synthesized using nanoprecipitation. The organic phase (NMP/acetone mixture 80/20) containing the lipid (Softisan 100, 2.3 mg/mL) and the dye (Rhodamine B octadecyl esterperchlorate, 0.05 mg/mL) was rapidly mixed with an aqueous phase containing the polyelectrolyte (0.5 mg/mL) and BSA (0.25 mg/mL). The polyelectrolytes used are PSS [molecular weight (MW) = 70,000 g/mole], PAA (MW = 8,000 g/mole), poly(vinyl alcohol), PVS (MW = 4,000–6,000 g/mole, Polysciences, Inc.), PAS sodium salt (MW = 9,000–11,000 g/mole), and PSM (MW = 20,000 g/mole). The formation of NPs could be visually observed by the clouding of the mixture. Organic solvent and excess polyelectrolyte were removed by overnight dialysis against deionized water to obtain a 1.5 mg/mL NP suspension in water. Particle size, polydispersity index, and zeta potential were determined using light scattering (DelsaNano C, Beckman Coulter). All particles were at least synthesized in triplicate, and each batch was analyzed in triplicate.
Cell Culture.
All primary cells were split at a ratio of 1:4–1:8, except in the case of C57-6011, where they were split in a ratio of 1:2, and cells between passages 3–6 were used for the NP uptake studies. HUVECs were cultured in Vasculife Basal Medium (Cell Systems); HeLa, MCF7A, and H69V cells were cultured in RPMI supplemented with 10% (vol/vol) FBS and 1% Penicilin–Strepomycin–Amphotericin B mixture (Pan Biotech); and BAEC, HEK293T, MC3T3, NIH 3T3, and RAW264.7 were cultured in DMEM supplemented with 10% (vol/vol) FBS and 1% Penicilin–Strepomycin–Amphotericin B mixture (Pan Biotech). HPMECs were cultured in endothelial cell medium (ScienCell), and C57-6011 (http://cellbiologics.com/) was cultured in gelatin-coated flasks using complete mouse endothelial cell culture medium (http://cellbiologics.com/). All cells were grown in a humidified incubator at 5% CO2 and 37 °C.
Uptake Study.
Cells were seeded in 24-well plates and grown to 80% confluency. Media was aspirated and the cells were washed with PBS+. If not indicated differently, the cells were coincubated with NPs (5 µL NP/1 mL medium) in RPMI (supplemented with 1% serum). To make sure that differences observed between cell lines do not originate due to differences in media composition, we decided, for the duration of the experiment, to use RPMI for all cells. After 2 h incubation with NPs, the medium was aspirated and the cells were washed with PBS before analysis.
The markers for endocytosis, transferrin from human serum (Alexa Fluor 647 Conjugate), cholera toxin subunit B (Recombinant) (Alexa Fluor 647 Conjugate), and albumin from bovine serum (Tetramethylrhodamine Conjugate) were purchased from Invitrogen and used in the following concentrations: CTB (3.5 µg/mL), BSA (10 µg/mL), and transferrin (50 µg/mL) for 2 h incubation with cells. For experiments to test the inhibitory effect of PSS and PAA, cells were pretreated with PSS and PAA (0.1 mg/mL) for 15 min, and the NPs or markers were added in the concentration described above. The details of inhibition of caveolae-mediated uptake of NPs in HeLa cells are provided in SI Materials and Methods.
For flow cytometry, cells were trypsinized and measured in a FACS buffer [PBS+ with 10% (vol/vol) FBS]. A total of 10,000 events were recorded for each sample using a LSRFortessa FACS Analyzer (Becton Dickinson). The samples were analyzed with Flowing Software (Perttu Terho), and an average of the medians of at least three different experiments (with different sets of NPs) was calculated. The histogram in Fig. 1 was generated using FlowJo software.
For fluorescence microscopy, cells were seeded in eight-well chamber slides. After the incubation with the NPs (2h, 5 μl/ml medium), cells were washed with PBS, fixed with paraformaldehyde [4% (vol/vol)] for 15 min, and mounted onto coverslips using VECTASHIELD HardSet Mounting Medium with DAPI (Vector Laboratories). Microscope images were taken with a Zeiss cell observer Z1.
The 3-(4,5-Dimethylthiazol-2yl)2,5-diphenyl-2H-tetrazoliumbromide assay, immunofluorescence, Western blot, quantitative real-time PCR, statistics, LogP determination, and transmission electron microscopy details are provided in SI Materials and Methods. The primer sequences used in the PCR analysis are listed in Fig. S10.
Supplementary Material
Acknowledgments
The authors thank Dr. Marie Follo at the Core Facility, Department of Hematology/Oncology, University of Freiburg Medical Center, for valuable insights in FACS analysis. We thank Ralf Thomann for the transmission electron micrographs. The technical assistance of Chantal Frey, Anne Bühler, and Luisa Schuble is also acknowledged. This work was funded by the 5th INTERREG Upper Rhine Program (Project A21: NANO@MATRIX), the excellence initiative of the German Federal and State Governments (Grant EXC 294; Centre for Biological Signalling Studies, BIOSS), and the University of Freiburg.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1322356111/-/DCSupplemental.
References
- 1.Farokhzad OC, Langer R. Impact of nanotechnology on drug delivery. ACS Nano. 2009;3(1):16–20. doi: 10.1021/nn900002m. [DOI] [PubMed] [Google Scholar]
- 2.Schroeder A, et al. Treating metastatic cancer with nanotechnology. Nat Rev Cancer. 2012;12(1):39–50. doi: 10.1038/nrc3180. [DOI] [PubMed] [Google Scholar]
- 3.Yan Y, Such GK, Johnston APR, Best JP, Caruso F. Engineering particles for therapeutic delivery: Prospects and challenges. ACS Nano. 2012;6(5):3663–3669. doi: 10.1021/nn3016162. [DOI] [PubMed] [Google Scholar]
- 4.Farokhzad OC, et al. Targeted nanoparticle-aptamer bioconjugates for cancer chemotherapy in vivo. Proc Natl Acad Sci USA. 2006;103(16):6315–6320. doi: 10.1073/pnas.0601755103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heath TD, Fraley RT, Papahdjopoulos D. Antibody targeting of liposomes: Cell specificity obtained by conjugation of F(ab’)2 to vesicle surface. Science. 1980;210(4469):539–541. doi: 10.1126/science.7423203. [DOI] [PubMed] [Google Scholar]
- 6.Danhier F, et al. Targeting of tumor endothelium by RGD-grafted PLGA-nanoparticles loaded with paclitaxel. J Control Release. 2009;140(2):166–173. doi: 10.1016/j.jconrel.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 7.Neri D, Bicknell R. Tumour vascular targeting. Nat Rev Cancer. 2005;5(6):436–446. doi: 10.1038/nrc1627. [DOI] [PubMed] [Google Scholar]
- 8.Conner SD, Schmid SL. Regulated portals of entry into the cell. Nature. 2003;422(6927):37–44. doi: 10.1038/nature01451. [DOI] [PubMed] [Google Scholar]
- 9.Kumari S, Mg S, Mayor S. Endocytosis unplugged: multiple ways to enter the cell. Cell Res. 2010;20(3):256–275. doi: 10.1038/cr.2010.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duchardt F, Fotin-Mleczek M, Schwarz H, Fischer R, Brock R. A comprehensive model for the cellular uptake of cationic cell-penetrating peptides. Traffic. 2007;8(7):848–866. doi: 10.1111/j.1600-0854.2007.00572.x. [DOI] [PubMed] [Google Scholar]
- 11.Bareford LM, Swaan PW. Endocytic mechanisms for targeted drug delivery. Adv Drug Deliv Rev. 2007;59(8):748–758. doi: 10.1016/j.addr.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Torchilin VP. Cell penetrating peptide-modified pharmaceutical nanocarriers for intracellular drug and gene delivery. Biopolymers. 2008;90(5):604–610. doi: 10.1002/bip.20989. [DOI] [PubMed] [Google Scholar]
- 13.Verma A, Stellacci F. Effect of surface properties on nanoparticle-cell interactions. Small. 2010;6(1):12–21. doi: 10.1002/smll.200901158. [DOI] [PubMed] [Google Scholar]
- 14.Jiang X, et al. Specific effects of surface amines on polystyrene nanoparticles in their interactions with mesenchymal stem cells. Biomacromolecules. 2010;11(3):748–753. doi: 10.1021/bm901348z. [DOI] [PubMed] [Google Scholar]
- 15.Decuzzi P, Pasqualini R, Arap W, Ferrari M. Intravascular delivery of particulate systems: Does geometry really matter? Pharm Res. 2009;26(1):235–243. doi: 10.1007/s11095-008-9697-x. [DOI] [PubMed] [Google Scholar]
- 16.Champion JA, Mitragotri S. Role of target geometry in phagocytosis. Proc Natl Acad Sci USA. 2006;103(13):4930–4934. doi: 10.1073/pnas.0600997103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saha K, et al. Surface functionality of nanoparticles determines cellular uptake mechanisms in mammalian cells. Small. 2013;9(2):300–305. doi: 10.1002/smll.201201129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weissleder R, Kelly K, Sun EY, Shtatland T, Josephson L. Cell-specific targeting of nanoparticles by multivalent attachment of small molecules. Nat Biotechnol. 2005;23(11):1418–1423. doi: 10.1038/nbt1159. [DOI] [PubMed] [Google Scholar]
- 19.Blasi P, Giovagnoli S, Schoubben A, Ricci M, Rossi C. Solid lipid nanoparticles for targeted brain drug delivery. Adv Drug Deliv Rev. 2007;59(6):454–477. doi: 10.1016/j.addr.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 20.Schneider J, et al. Surface functionality as a means to impact polymer nanoparticle size and structure. Langmuir. 2013;29(12):4092–4095. doi: 10.1021/la304075c. [DOI] [PubMed] [Google Scholar]
- 21.Razani B, Woodman SE, Lisanti MP. Caveolae: From cell biology to animal physiology. Pharmacol Rev. 2002;54(3):431–467. doi: 10.1124/pr.54.3.431. [DOI] [PubMed] [Google Scholar]
- 22.Cohen AW, Hnasko R, Schubert W, Lisanti MP. Role of caveolae and caveolins in health and disease. Physiol Rev. 2004;84(4):1341–1379. doi: 10.1152/physrev.00046.2003. [DOI] [PubMed] [Google Scholar]
- 23.Heikkilä O, et al. Internalization of coxsackievirus A9 is mediated by beta2-microglobulin, dynamin, and Arf6 but not by caveolin-1 or clathrin. J Virol. 2010;84(7):3666–3681. doi: 10.1128/JVI.01340-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beloqui A, et al. Mechanism of transport of saquinavir-loaded nanostructured lipid carriers across the intestinal barrier. J Control Release. 2013;166(2):115–123. doi: 10.1016/j.jconrel.2012.12.021. [DOI] [PubMed] [Google Scholar]
- 25.Schnitzer JE, Oh P, Pinney E, Allard J. Filipin-sensitive caveolae-mediated transport in endothelium: Reduced transcytosis, scavenger endocytosis, and capillary permeability of select macromolecules. J Cell Biol. 1994;127(5):1217–1232. doi: 10.1083/jcb.127.5.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Torgersen ML, Skretting G, van Deurs B, Sandvig K. Internalization of cholera toxin by different endocytic mechanisms. J Cell Sci. 2001;114(Pt 20):3737–3747. doi: 10.1242/jcs.114.20.3737. [DOI] [PubMed] [Google Scholar]
- 27.Jacobson K, Mouritsen OG, Anderson RGW. Lipid rafts: At a crossroad between cell biology and physics. Nat Cell Biol. 2007;9(1):7–14. doi: 10.1038/ncb0107-7. [DOI] [PubMed] [Google Scholar]
- 28.Schnitzer JE. Caveolae: From basic trafficking mechanisms to targeting transcytosis for tissue-specific drug and gene delivery in vivo. Adv Drug Deliv Rev. 2001;49(3):265–280. doi: 10.1016/s0169-409x(01)00141-7. [DOI] [PubMed] [Google Scholar]
- 29.Watson M, Abbott KC, Yuan CM. Damned if you do, damned if you don’t: Potassium binding resins in hyperkalemia. Clin J Am Soc Nephrol. 2010;5(10):1723–1726. doi: 10.2215/CJN.03700410. [DOI] [PubMed] [Google Scholar]
- 30.Sterns RH, Rojas M, Bernstein P, Chennupati S. Ion-exchange resins for the treatment of hyperkalemia: Are they safe and effective? J Am Soc Nephrol. 2010;21(5):733–735. doi: 10.1681/ASN.2010010079. [DOI] [PubMed] [Google Scholar]
- 31.Chrastina A, Massey KA, Schnitzer JE. Overcoming in vivo barriers to targeted nanodelivery. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2011;3(4):421–437. doi: 10.1002/wnan.143. [DOI] [PubMed] [Google Scholar]
- 32.Campbell SM, Crowe SM, Mak J. Lipid rafts and HIV-1: From viral entry to assembly of progeny virions. J Clin Virol. 2001;22(3):217–227. doi: 10.1016/s1386-6532(01)00193-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.