Significance
Peptide immunotherapy (PIT) of ongoing allergy must control “memory” T helper 2 (Th2) cells. Memory T cells can be subdivided into effector memory T cells (Tem), which seem to be involved in immediate immune responses, and central memory T cells (Tcm), which are thought to provide long-term memory. We show that PIT can control allergic lung disease more effectively when the disease is driven by Tem Th2 cells, rather than by Tcm Th2 cells. PIT-treated Tcm remained more responsive to allergen, with a greater capacity to produce inflammatory Th2 cytokines in the lung. These were suppressed in PIT-treated Tem. These differences are important for clinical translation of PIT, because Tcm may be particularly dominant in some seasonal allergic conditions, such as hay fever.
Abstract
Peptide immunotherapy (PIT) offers realistic prospects for the treatment of allergic diseases, including allergic asthma. Much is understood of the behavior of naive T cells in response to PIT. However, treatment of patients with ongoing allergic disease requires detailed understanding of the responses of allergen-experienced T cells. CD62L expression by allergen-experienced T cells corresponds to effector/effector memory (CD62Llo) and central memory (CD62Lhi) subsets, which vary with allergen exposure (e.g., during, or out with, pollen season). The efficacy of PIT on different T helper 2 (Th2) cell memory populations is unknown. We developed a murine model of PIT in allergic airway inflammation (AAI) driven by adoptively transferred, traceable ovalbumin-experienced Th2 cells. PIT effectively suppressed AAI driven by unfractionated Th2 cells. Selective transfer of CD62Lhi and CD62Llo Th2 cells revealed that these two populations behaved differently from one another and from previously characterized (early deletional) responses of naive CD4+ T cells to PIT. Most notably, allergen-reactive CD62Llo Th2 cells were long-lived within the lung after PIT, before allergen challenge, in contrast to CD62Lhi Th2 cells. Despite this, PIT was most potent against CD62Llo Th2 cells in protecting from AAI, impairing their ability to produce Th2 cytokines, whereas this capacity was heightened in PIT-treated CD62Lhi Th2 cells. We conclude that Th2 cells do not undergo an early deletional form of tolerance after PIT. Moreover, memory Th2 subsets respond differently to PIT. These findings have implications for the clinical translation of PIT in different allergic scenarios.
Specific immunotherapy involves therapeutic delivery of a disease-relevant antigen to induce tolerance (particularly of CD4+ T cells) toward that antigen (1, 2). It represents a realistic and potentially disease-modifying therapeutic approach for the treatment of allergic and autoimmune diseases with strong CD4+ T-cell components to their pathogenesis, such as allergic asthma (3–5). Traditional immunotherapy, using whole-protein antigens, is associated with the risk of severe allergic reactions, particularly anaphylaxis, in patients harboring allergen-reactive IgE (6, 7). Peptide immunotherapy (PIT) obviates this risk because it uses short synthetic peptides containing known T-cell epitopes, but not conformational antibody epitopes, thereby targeting disease-driving CD4+ T cells while avoiding IgE binding (8, 9).
In animal studies, PIT can effectively reduce or prevent CD4+ T-cell–driven diseases (10–15). Encouraging findings have also been reported in allergic patients (16–20). However, reduced disease severity is not universal, and limitations in our understanding of the workings of PIT are impeding clinical translation. Mechanistic murine PIT studies have been advanced through the use of traceable populations of T-cell receptor (TCR) transgenic T cells. PIT is highly effective in silencing “naive” T cells whose first encounter with their cognate antigen is at the point of tolerogenic peptide application (21, 22). This is different from the clinical setting where established T-cell–driven pathology, by definition, presents with an increased frequency of antigen-experienced T cells (23). We, and others, have previously shown that application of tolerogenic peptide induces naive CD4+ T cells to enter a brief but abortive phase of proliferation that is followed by their wide-scale apoptotic deletion (21, 22, 24). This is most likely the result of insufficient costimulation from the antigen-presenting cell in the absence of innate immune triggers (21, 22, 24). However, several characteristics of antigen-experienced T cells hint that they may not necessarily respond to PIT in the same way. First, they have lower costimulation requirements (25, 26) that may make them less susceptible to deletion in response to costimulation deprivation in the tolerogenic setting. Antigen-experienced T cells can be phenotypically classified into effector and memory T-cell populations, the latter being subdivided into effector memory T cells (Tem) and central memory T cells (Tcm) (27, 28). Importantly, the phenotype and frequency of allergen-reactive T cells can vary, depending on the presence or absence of allergen exposure (e.g., perennial vs. seasonal allergy) (29–31). In addition, the phenotype of T cells in the end organ (e.g., the lung) may differ from those in peripheral blood (32–34). These complexities could have a major impact upon the clinical response to PIT and have not previously been addressed.
Here, we developed a model to study the effects of PIT upon Th2-polarized TCR transgenic cells driving allergic airway inflammation (AAI). PIT effectively reduced AAI, despite the allergen-experienced nature of the eliciting Th2 cells. Furthermore, PIT was most potent against AAI driven by CD62Llo Th2 cells (a phenotype associated with effector and Tem) compared with CD62Lhi Th2 cells (associated with Tcm) (27, 28). Importantly, CD62Llo and CD62Lhi Th2 cells showed markedly distinct behavior in response to PIT, both in comparison with one another and compared with the known behavior of naive T cells. Notably, a sizeable population of PIT-treated CD62Llo cells persisted long-term within the lung following PIT, in contrast to CD62Lhi cells. Whereas PIT led to diminished Th2 cytokine production at the time of airway challenge with allergen in CD62Llo cells, this effect of PIT was not seen in CD62Lhi cells, which therefore retained pathogenic activity. The composition of Th2 cell subpopulations at the time of PIT is thus an important additional consideration in respect to clinical translation.
Results
A Th2 Cell Transfer Model of Chicken Egg Ovalbumin-Driven Allergic Airway Inflammation.
We established a model of AAI driven by traceable (CD45.1+) Th2 cells, using OT-II TCR transgenic mice (35) as a source of T cells recognizing the 323–339 peptide of chicken egg ovalbumin (pOVA). In vitro activation of OT-II T cells by pOVA under Th2-polarizing conditions generated a GATA-3–expressing T-cell population that produced high levels of IL-5 and IL-13 upon restimulation with OVA, compared with naive OT-II T cells (Fig. 1A). Transfer of these Th2-polarized OT-II cells, followed by intratracheal (i.t.) challenge with OVA (Fig. 1A), induced AAI, as evidenced by cellular infiltration around airways and blood vessels and goblet cell formation (Fig. 1B). An increase in total cells and eosinophils was evident within the bronchoalveolar lavage (BAL) (Fig. 1C). These pathological changes required OVA in the i.t. challenge and could not be elicited if naive OT-II T cells were transferred (Fig. 1 B and C). Flow cytometry identified transferred CD45.1+ cells within the lung (Fig. 1D). Of note, low numbers of Th2 cells, but not naive T cells, were evident in the absence of OVA i.t. challenge, but both the number and the frequency of Th2 cells were significantly increased following OVA challenge (Fig. 1D). This model therefore allowed us to relate numbers, phenotype, and function of preformed Th2 cells in the lung to their pathogenic activity (AAI), in the presence or absence of PIT.
Fig. 1.
A Th2 transfer model of OVA-driven allergic airway inflammation. (A) IL-5 and IL-13 production by naive (□) and Th2-polarized (■) CD4+ OT-II cells in response to OVA and experimental design for AAI using transfer of 4 × 106 naive or Th2-polarized OT-II cells (see Materials and Methods for dose of OVA). (B) Day 9 analysis of H&E and PAS. (C) Differential BAL cell counts. (D) The percentage of OT-II cells in the CD4+ population and the total number of OT-II cells in the left lung lobe. Data represent one of two experiments giving consistent results. n ≥ 3, **P ≤ 0.01, ***P ≤ 0.001 vs. all other groups.
PIT Suppresses AAI Driven by Preexisting Th2 Cells.
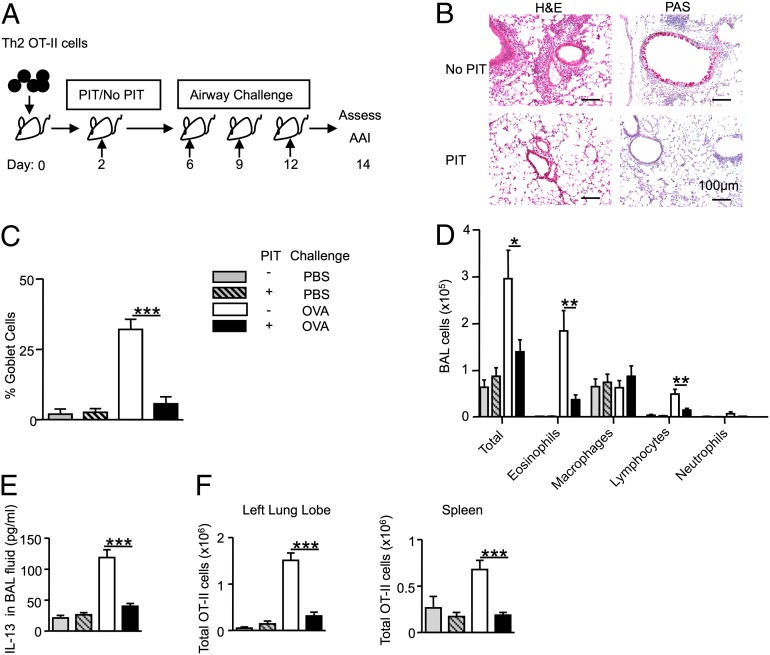
Naive OT-II cells are sensitive to PIT when pOVA is administered i.v. in PBS (21, 22, 24). We assessed therapeutic efficacy of PIT in AAI by administration of pOVA 2 d after Th2 cell transfer and 4 d before i.t. challenges (Fig. 2A). This PIT regimen led to reductions in cellular infiltration of the lungs, goblet cells in the airways, BAL eosinophilia, and IL-13 in BAL (Fig. 2 B–E). The total numbers of OT-II cells in both the lung and the spleen were increased by OVA challenges in the absence of PIT (Fig. 2F). In PIT-treated mice, however, numbers of OT-II cells following OVA i.t. challenge were not significantly different from those seen in PBS-challenged control mice (Fig. 2F). We therefore conclude that PIT is effective at diminishing the AAI-inducing function of allergen-experienced Th2 cells.
Fig. 2.
PIT suppresses AAI driven by preexisting Th2 cells. (A) Experimental design (see Materials and Methods for doses of pOVA and OVA). (B) H&E and PAS stains of lung sections. (C) Quantification of goblet cells. (D) Differential BAL cell counts. (E) Concentrations of IL-13 in BAL fluid. (F) Total OT-II numbers in left lung lobes and spleens. “No PIT”: mice received PBS alone. Data are pooled from two experiments; consistent data were found in three or more experiments. n ≥ 6, *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001.
PIT Drives Th2 Cell Accumulation in the Lung Before Allergen Challenge.
We, and others, have previously demonstrated that the effectiveness of PIT upon naive OT-II cells predominantly involves a deletional effect, which is evident 4 d after PIT (21, 22, 36). The data in Fig. 2F highlighted reduced numbers of transferred Th2 cells after airway challenge in lungs of mice protected from AAI by PIT. The simplest explanation for this was therefore that, as with naive T cells, PIT drives the rapid deletion of transferred Th2 cells. However, assessment of transferred OT-II Th2 cell numbers at the time of first i.t. challenge (4 d after PIT, experimental protocol in Fig. 3A) revealed a widespread increase in number and frequency of OT-II cells in spleen, lymph nodes, and lung (Fig. 3 B and C). Of particular note was the significant increase in the percentage of OT-II cells in the lung CD4+ population, compared with that in distal lymph nodes and spleen (Fig. 3C). Importantly, this difference was most pronounced after PIT (Fig. 3C).
Fig. 3.
PIT drives Th2 cell accumulation in the lung before allergen challenge. (A) Experimental design. (B) The total number of OT-II cells in spleen and lung. (C) The percentage of OT-II cells in the CD4+ population in spleen, para-aortic and inguinal lymph nodes (LN), and lung. No PIT: mice received PBS alone. Data are from one experiment and are representative of findings from two or more experiments giving consistent results. n = 4, **P ≤ 0.01, ***P ≤ 0.001.
Our data thus far provided an intriguing scenario in which the protective effects of PIT against Th2-driven AAI (Fig. 2 B–E) were associated with ultimately reduced numbers of OT-II cells after allergen challenge (Fig. 2F), despite a striking initial enrichment of these cells in the lungs immediately after PIT and before allergen challenge (Fig. 3C). Thus, PIT promotes accumulation of allergen-reactive T cells within the lung, but these have substantially impaired pathogenic activity in this relatively compressed model (4 d between exposure to PIT and first airway challenge).
We next sought to understand how long this population of PIT-exposed T cells would persist within the lung. Moreover, given the possible distinct roles for Tem and Tcm in maintaining memory in the presence or absence of allergen exposure, we considered it important to determine whether these two memory populations might behave differently, either (i) in their colonization of and persistence in the lung following PIT or (ii) in their functional/pathogenic impairment following PIT. Separation of putative Tem and Tcm populations was possible because, although Th2-polarized OT-II cells on the day of adoptive transfer were all CD44hi, they consistently displayed a heterogeneous CD62L profile, including both high- and low-CD62L expressors (Fig. 4A). These two profiles are associated with Tem (CD44hiCD62Llo) or Tcm (CD44hiCD62Lhi) (27). The presence of antigen-experienced adoptively transferred T cells in nonlymphoid organs is well documented (37, 38), as is the preference for CD62Llo cells to home to nonlymphoid tissues such as the lung, because of the requirement for CD62L expression to facilitate entry into lymph nodes (32, 33, 39). We therefore predicted that the cells that accumulated in the lung after PIT were derived from the CD62Llo fraction.
Fig. 4.
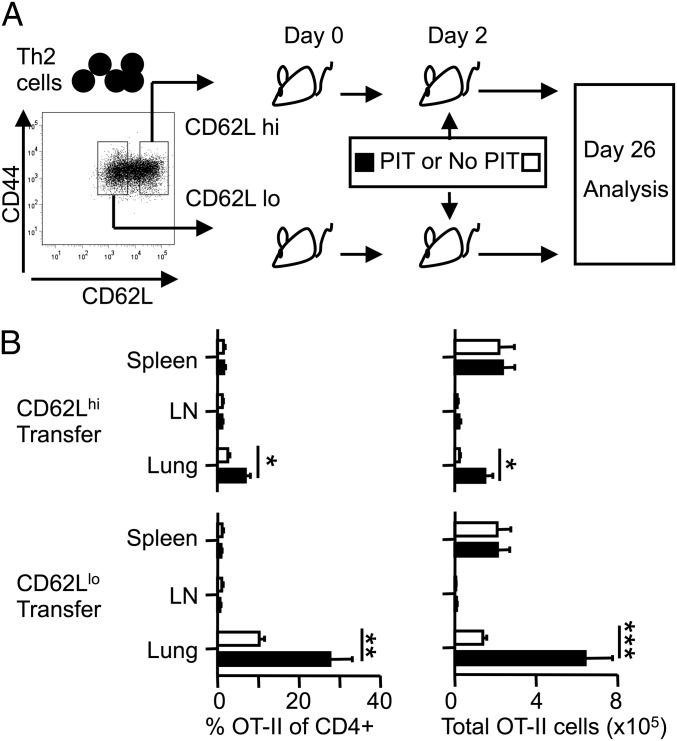
Peptide immunotherapy triggers a preferential, and long-lived, expansion of CD44hiCD62Llo Th2 cells in the lung. (A) Experimental design. Mice received 2 × 106 CD4+CD44hiCD62Llo (CD62Llo) or CD4+CD44hiCD62Lhi (CD62Lhi) Th2 cells (see Materials and Methods for doses of pOVA and OVA). (B) The percentages and total numbers of OT-II cells in spleen, inguinal and para-aortic lymph nodes (LN), and whole lung. Data are pooled from two experiments, giving consistent results. n ≥ 5, *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001.
PIT-Treated CD62Llo Th2 Cells Persist Long-Term in the Lung.
OT-II Th2 cells were FACS sorted into CD44hiCD62Llo and CD44hiCD62Lhi populations and transferred individually before PIT (Fig. 4A). To compare the longevity of each population, mice were culled 26 d after cell transfer and frequency and number of transferred OT-II cells determined. Even in the absence of allergen airway challenge, the lungs of mice that had received CD62Llo Th2 cells contained markedly elevated frequencies of transferred cells, to the extent that there were greater numbers of OT-II cells in the lung than in the spleen at 24 d after PIT. This was not the case for CD62Lhi transfer, although an OT-II accumulation in the lung was evident following PIT (Fig. 4B). There were no differences in the percentage of OT-II cells in the CD4+ population or in total OT-II cell number, in spleen or lymph nodes in any of the groups, regardless of PIT treatment (Fig. 4B). The greater accumulation of transferred CD62Llo cells in the lungs was not, therefore, because of an inherent improved viability.
We concluded that, unlike the previously reported effects of PIT upon naive OT-II cells, there was no evidence for depletion of transferred CD62Lhi or CD62Llo populations from the lymphoid organs. Moreover, the numbers of transferred cells in the lung were enriched following PIT. This effect was particularly pronounced for the transferred CD62Llo population. It therefore became even more important to determine how these differences influenced subsequent susceptibility to AAI following allergen airway challenge.
PIT-Treated CD62Llo Th2 Cells Are Least Pathogenic upon Allergen Airway Challenge.
The experimental protocol described in Fig. 4 was extended to include airway challenge with OVA, commencing at day 26 (Fig. 5A). Despite having the highest numbers of transferred Th2 cells in the lung at the time of challenge (Fig. 4B), mice that received CD62Llo cells and PIT did not develop aggravated AAI. Far from it, they showed the least severe AAI in terms of histological appearance (Fig. 5B), with comprehensive reductions in AAI parameters—namely the percentage of goblet cells, total BAL cells, BAL eosinophilia, and suppression of IL-5/IL-13 cytokine production by OT-II cells in the lungs (Fig. 5 B–D and F). In contrast, the effectiveness of PIT against CD62Lhi cells was limited, with reductions in AAI parameters restricted to IL-13 concentration in BAL and the percentage of OT-II cells producing IL-5 and/or IL-13 in the lung (Fig. 5 E and F). Although there was a tendency for CD62Lhi Th2 cells to be more pathogenic than CD62Llo Th2 cells upon allergen challenge [e.g., in terms of BAL eosinophilia (Fig. 5D)], this was not consistent across all parameters [e.g., in terms of the percentage of goblet cells (Fig. 5C)], enabling us to conclude that PIT was most potent against CD62Llo Th2 cells. There were no differences in the percentage of OT-II cells expressing Foxp3 between groups (<6% in all).
Fig. 5.
PIT-treated CD62Llo Th2 cells are least pathogenic upon allergen airway challenge. (A) Experimental design. Mice received 2 × 106 CD62Llo or CD62Lhi Th2 cells as in Fig. 4. (B and C) H&E (B) and PAS (C) staining of lung sections and the percentage of goblet cells in the airways. (D) Differential BAL cell counts. (E) Concentrations of IL-13 in BAL fluid. (F) The percentage of IL-5+ and/or IL-13+ OT-II cells in the left lung lobe after overnight culture in the presence of pOVA. (G) The percentage of OT-II cells in the CD4+ population and the total number of OT-II cells in spleen, mediastinal LN (mLN), and left lung lobe. No PIT: mice received PBS alone. Data are pooled from two experiments, giving consistent results. n ≥ 9, *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001; ns, not significant.
PIT was associated with a reduced frequency of OT-II cells following airway challenge in lung, mediastinal lymph nodes (mLN), and spleen following allergen challenge, compared with non-PIT controls, regardless of whether mice had received CD62Llo or CD62Lhi OT-II cells (Fig. 5G).
Both CD62Lhi and CD62Llo Cells Accumulate in the Lung, but PIT-Treated CD62Llo Cells Have More Stable Persistence.
To better understand the PIT-induced changes in the transferred CD62Lhi and CD62Llo cells, we compared these populations at additional time points following PIT administration and allergen challenge for their accumulation within the lung, their proliferative state (by Ki-67 staining), and their functional capacity (by intracellular cytokine staining), focusing particularly on the lung. Our prediction was that CD62Llo cells would preferentially enter the lung before PIT treatment. This proved incorrect, because similar numbers of transferred CD62Lhi cells were also evident in the lung after 2 d (the time of PIT administration) (Fig. 6). However, in PBS-treated controls, numbers and frequencies of transferred CD262Llo cells remained relatively stable in the lung from this time to the point of allergen challenge (day 26 posttransfer), whereas some attrition in the transferred CD62Lhi population could be seen. Both transferred populations showed elevated numbers and frequencies in the lung at day 6, 4 d after PIT, but the greatest increase was seen in the transferred CD62Lhi cells. Thereafter, PIT-exposed CD62Lhi cells declined in the lung, whereas PIT-exposed CD62Llo cells were maintained at elevated frequencies and increased in numbers to the point of the first airway challenge (day 26).
Fig. 6.
The effects of PIT on transferred CD62Llo or CD62Lhi Th2 populations. Mice received 2 × 106 CD4+CD44hiCD62Llo (CD62Llo) or CD4+CD44hiCD62Lhi (CD62Lhi) Th2 cells (see Materials and Methods for doses of pOVA and OVA). (A and B) The percentage of OT-II cells in the CD4+ population (Left) and the total numbers (Right) of CD45.1+ OT-II cells in (A) lung and (B) spleen were determined by flow cytometry on the days shown on the x axis. Dotted vertical lines show days of PIT and airway challenge. Solid symbols, PIT; open symbols, no PIT. Error bars show SEM. Data were collated from seven experiments with 3–12 mice per group, per time point. Note that total numbers were not available for the lung on day 34 as only left lung lobes were prepared for flow cytometry to allow concomitant histological processing of the remaining lung tissue.
PIT Conditions for the Loss of Transferred Th2 Cells in the Lung upon Airway Challenge with Allergen.
Assessment 24 h after the second airway challenge (day 30) revealed no rise in frequency of OT-II cells in the lungs of mice that received CD62Llo cells without PIT (this was only seen on day 34, after the third airway challenge) (Fig. 6). In contrast, frequencies of OT-II cells in the lungs of mice that received CD62Lhi cells without PIT were beginning to rise after the second airway challenge. Regardless of their CD62L status, frequencies of PIT-exposed transferred cells had declined after the second airway challenge (markedly so in the case of PIT-treated CD62Llo cells). This decline in PIT-treated CD62Llo cells continued to the final time point, after the third airway challenge (day 34), but frequencies of OT-II cells in mice that received CD62Lhi cells and PIT appeared to stabilize or even increase by this point.
Proliferative Capacity Is Greater in Transferred CD62Lhi Cells than in CD62Llo Cells upon Airway Challenge.
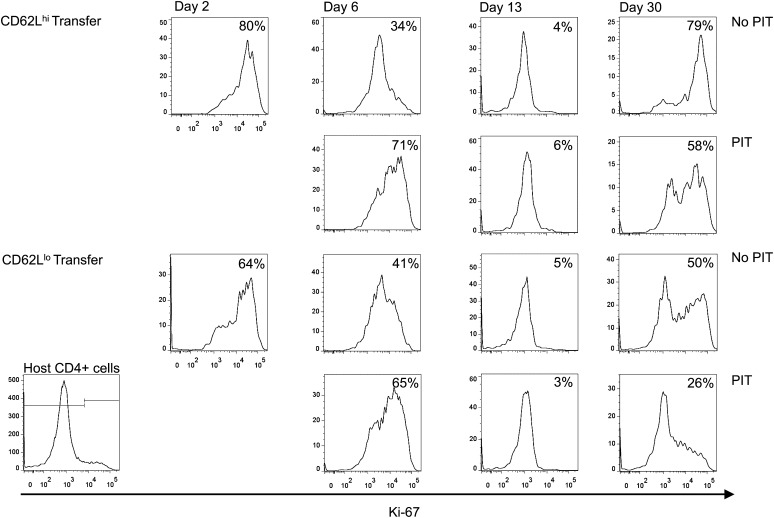
As would be expected, both transferred populations showed evidence of maintained proliferation 2 d after transfer (Fig. 7). Ki-67 staining 4 d after PIT was indicative of TCR stimulation, but by day 13 after transfer (11 d after PIT), few cells were in cell cycle, indicating that the more pronounced long-term persistence of CD62Llo cells in the lung was not due to a greater/longer proliferative capacity. Following the second airway challenge (day 30), Ki-67 staining was evident in all transferred populations, but the frequencies of Ki-67+ cells were highest in non–PIT-exposed CD62Lhi cells (Fig. 7), consistent with these cells showing the most elevated frequencies at days 30 and 34, relative to day 26 (Fig. 6). Frequencies of Ki-67+ PIT-treated cells on day 30 were below those seen for non–PIT-treated cells (Fig. 7), with the lowest frequencies seen in the PIT-treated CD62Llo population, consistent with this group having the poorest representation of transferred cells in the lung at day 34 (Figs. 5 and 6).
Fig. 7.
The effects of PIT upon proliferation of OT-II cells in the lung. Cells were transferred as described in Fig. 6. The percentage of Ki-67+ OT-II cells was assessed in lung cell suspensions at each time point. Representative plots are shown with the median percentage for the group shown in each panel. Host CD4+ cells were used to set the threshold of positive staining (Bottom Left shows an example plot). Data are from three experiments, with three to six mice per group, per time point.
PIT-Exposed CD62Lhi Cells Maintain a Heightened Capacity for Cytokine Production.
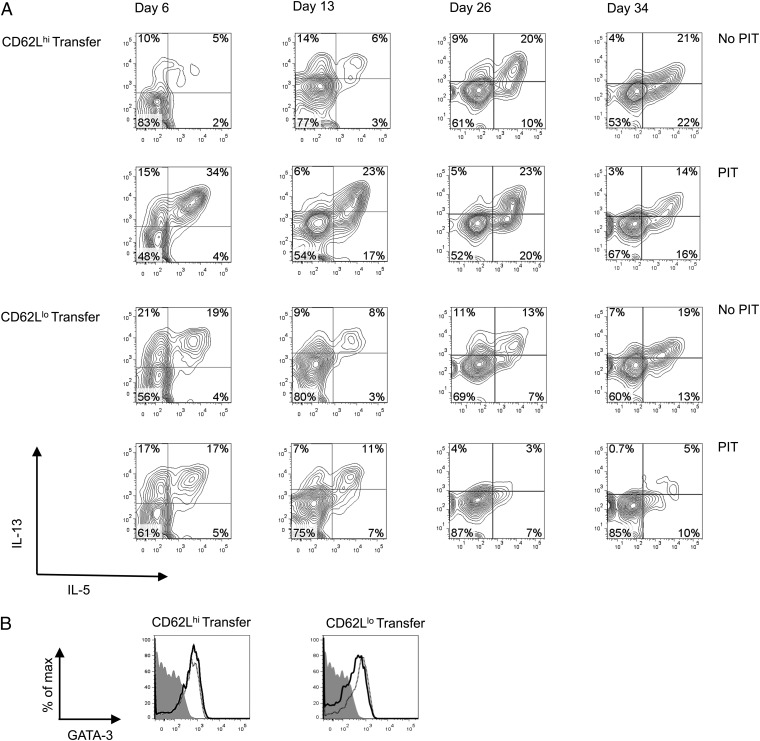
The transferred CD62Lhi population showed robust Th2 cell function in the lung after airway challenge (Fig. 5). To be of relevance to the increased pathology seen in mice receiving these cells, this feature should be evident earlier, at the time of airway challenge. We therefore assessed Th2 cytokine (IL-13 and/or IL-5) production following PIT and at the point of allergen challenge (Fig. 8A). In PBS-treated controls at day 6, cytokine production was highest among CD62Llo cells, consistent with a predicted strong effector function. In contrast, by the time of airway challenge, cytokine production was at least as evident in the CD62Lhi group as in the CD62Llo group in the absence of PIT. At days 6 and 13, PIT-exposed CD62Lhi cells showed elevated cytokine production relative to controls. This was not seen in PIT-exposed CD62Llo cells and, by the time of airway challenge, these PIT-exposed cells showed lower frequencies of cytokine-expressing cells than their control group. We did not find any evidence for loss of GATA-3 expression in the transferred CD62Llo cells (whether they had been exposed to PIT or not) that could account for their poorer production of Th2 cytokines (Fig. 8B). Importantly, at the time of first airway challenge (day 26) CD62Lhi cells showed strong production of IL-5 and IL-13 whether they had been previously exposed to PIT or not (Fig. 8A). We conclude that, whereas transferred CD62Llo cells that are exposed to PIT have diminished Th2 effector cytokine production in the lung by the time of allergen challenge, this key feature of pathogenic activity is actually prompted, and sustained, in transferred CD62Lhi cells responding to PIT.
Fig. 8.
The effects of PIT upon Th2 cytokine capabilities of transferred CD62Llo or CD62Lhi cells in the lung. Cells were transferred as described in Fig. 6. Pooled lung samples isolated on the indicated days were cultured overnight in the presence of pOVA before intracellular staining for cytokines. (A) IL-13 and IL-5 staining of OT-II cells (gated on CD4+CD45.1+ cells) after culture. (B) GATA-3 expression was assessed by gating on OT-II cells after overnight culture as in A (day 6). Solid line, PIT treated; dotted line, no PIT; shaded histogram, isotype. Data are from three experiments with three to six mice, providing samples for each time point.
Discussion
True mechanistic understanding of the effects of PIT necessitates assessment in the context of antigen-experienced, rather than naive, T cells, because the overriding aim of allergen immunotherapy is to combat established allergic immune responses (40). Previous work providing PIT between airway challenges suggested therapeutic benefit in experimental models in which allergen-experienced T cells should predominate (15). Here we have followed the fates of defined, allergen-experienced Th2 populations to show definitively that PIT can be highly effective in controlling the pathogenic activity of such cells in AAI. We show that the effects of PIT upon Th2 cells differ substantially from the early deletional response known to occur with naive CD4+ T cells (including when we have used the same OT-II TCR transgenic cells and PIT regimen) (21). Beyond this, we show that the behavior of Th2 cells in response to PIT varies with their level of CD62L expression.
Both the CD62Llo and the CD62Lhi populations we transferred contained cells that were long-lived, indicative of memory function. Our data indicate that, although both transferred CD62Lhi and CD62Llo Th2 cells can infiltrate the lung and undergo rapid proliferation therein in response to PIT, PIT-treated CD62Llo cells have a greater capacity to persist long-term in the lung. However, by the time of airway allergen challenge, these persisting PIT-treated CD62Llo cells have a poorer proliferative capacity and poorer Th2 cytokine-producing capacity than their PIT-treated CD62Lhi counterparts. Our data indicate that, rather than driving an immediate apoptotic deletion (as has been reported for naive OT-II cells), PIT conditions the transferred Th2 cells for deletion (presumably via apoptosis) upon subsequent exposure to allergen within the lung. This was particularly the case for the transferred CD62Llo population. This, together with the emphatic PIT-induced suppression of Th2 cytokine production by CD62Llo cells at the time of allergen challenge, means that PIT is most potent against these cells. The greater proliferative activity and cytokine-producing capacity of PIT-treated CD62Lhi cells allows them to retain greater pathogenic activity. We believe the observation that this Tcm population can be triggered to gain effector function (Th2 cytokine production) in response to PIT is also a novel finding.
Tcm can make use of high CD62L expression to traffic into lymph nodes across high endothelial venules, whereas CD62Llo Tem more readily access the tissues. The paradigm is that Tem respond to antigenic challenge with rapid production of effector cytokines but only limited proliferative capacity (27, 28). Tcm show low effector cytokine production compared with Tem, but their strong proliferative capacity upon reencountering antigen generates new effector T cells (27, 28). Tcm, rather than Tem, are thought to contribute more to the memory pool in the long-term (27, 41–43). The CD62Lhi cells we transferred showed a greater ability to enter the cell cycle following allergen challenge to the lung (whether they had been exposed to PIT or not), consistent with the Tcm paradigm. However, it is somewhat surprising that, in our study, it was the transferred CD62Llo cells rather than the CD62Lhi cells that showed greater long-term persistence in the lung after exposure to PIT. However, in some instances, CD8+ Tem are capable of proliferation and the establishment of new memory cells (44). This may support the durable nature of CD62Llo cells seen here after PIT; why CD62Lhi cells do not also behave in this way remains to be determined.
In contrast to their relatively poor proliferative capacity, Tem, by definition, should possess a greater capacity to produce effector cytokines than Tcm. This was borne out by the greater ability of the transferred CD62Llo Th2 cells than their CD62Lhi counterparts to produce IL-5 and/or IL-13 in the absence of PIT at day 6. The marked difference in effector cytokine capacity in response to PIT—a gradual decline in transferred CD62Llo cells vs. a rapid and sustained gain in transferred CD62Lhi cells—is entirely consistent with the different levels of pathology seen following allergen challenge to the lung. A reasonable interpretation of these data is that CD62Llo cells have a more advanced differentiation status and, although PIT does not trigger their rapid deletion through apoptosis (this appeared to occur only at the point of later allergen challenge), PIT does impair their effector function. In contrast, and fitting the Tcm paradigm, CD62Lhi cells are less differentiated, allowing the further TCR triggering afforded by PIT to engender more pronounced effector function. The observation that by the point of allergen challenge (day 26) transferred CD62Lhi cells had gained effector function in the absence of PIT is of interest. Whether this reflects an enrichment of the minority of transferred cells capable of IL-5 and/or IL-13 production in the lung at day 6 or a gradual gain in this function driven by the lung environment cannot be discerned from these experiments, but warrants further study.
Allergen-reactive CD4+ T cells can be found in the blood of both nonallergic and allergic individuals and are predominantly a memory phenotype, but exhibit differences in frequency (often higher in allergic patients) and are Th2 skewed (23, 29, 45, 46). Lymphocytes in BAL from asthmatic patients and controls are overwhelmingly of the memory phenotype (47). The timing of allergen exposure could plausibly be anticipated to influence a patient’s allergen-reactive T-cell pool. Indeed, increased frequency of pollen-specific CD4+ T cells has been described during pollen season whereas such fluctuation does not occur for the perennial allergen house dust mite (29–31). Furthermore, a greater frequency of birch-pollen reactive CD4+ Tem has been shown in the blood of pollen allergic individuals during pollen season, compared with a greater percentage of Tcm in house dust mite allergic patients (29). Such clinical studies provide valuable information, but can be limited by analysis of peripheral blood (perhaps skewing toward Tcm rather than Tem). The existence of lung resident memory T cells (Trm) may also mean that important changes at the site of inflammation have so far been missed. There is increasing appreciation of the importance of Trm to protective immunity (48, 49) and these are predominantly Tem in both mouse and man (28, 34). Trm in human lungs are cytokine capable (34), and a contribution of such cells to allergic disease is thought likely (28, 50, 51). Our finding that, despite a large and durable accumulation of transferred CD62Llo cells in the lungs of PIT-treated mice, these cells were relatively cytokine incapable and provoked the lowest levels of AAI is therefore reassuring and emphasizes the effectiveness of PIT on these cells.
Clinically, there is limited information on the activation status of T cells before, and after, immunotherapy, and there is no consensus as to the most efficacious means to deliver specific immunotherapy, including PIT, in terms of dose, route, and frequency (40, 52). In light of this study, it is interesting to speculate on how currently favored regimes for delivering allergen immunotherapy, for example multidose regimes (17, 20, 53, 54), affect the dynamics of CD62L expression of Th2 cells and hence their behavior in response to PIT. Here we used a single PIT protocol known to be effective against naive OT-II T cells and found it had limited efficacy in preventing pathogenic activity of Tcm. This therefore provides the impetus, and a tractable system, for further detailed exploration of how different effector and memory T-cell populations might best be controlled by different therapeutic regimens. This should deliver the broadest and most robust protocols for clinical translation.
Materials and Methods
Mice.
Congenically identifiable (CD45.1) OT-II transgenic mice with an I-Ab–restricted TCR reactive toward ovalbumin peptide 323–339 (pOVA) (35) were maintained at the University of Edinburgh. Sex-matched C57BL/6J mice, 6–12 wk old (Charles River), were maintained on chicken egg OVA-free diets. Experiments were conducted under a UK Home Office license and approved by the local ethics review panel.
Antigens.
OVA was obtained from Worthington Biochemical Corporation. pOVA was synthesized by Peplogic and reconstituted using sterile water (Sigma-Aldrich).
Th2 Polarization of OT-II Cells.
Cells from spleens and lymph nodes of naive OT-II mice were seeded at 4 × 106 cells/mL in RPMI 1640 (Gibco) with 2 mM l-glutamine, 100 units/mL penicillin, 100 µg/mL streptomycin (all from PAA), 50 µM 2-β-mercaptoethanol, and 5% (vol/vol) heat-inactivated FCS (Labtech) with 40 units/mL rIL-2 (Peprotech), 4 ng/mL rIL-4 (Peprotech), 5 µg/mL anti–IL-12 (BioXcell), 5 µg/mL anti–IFN-γ (BioXcell), and 10 µg/mL pOVA. CD4+ cells were positively selected after 96 h of culture, or from naive mice, using CD4 (L3T4) MACS Microbeads and an autoMACS Pro Separator (both from Miltenyi Biotec). A total of 4 × 106 cells were adoptively transferred to mice via the tail vein.
FACS Sorting of CD62Lhi and CD62Llo Th2 Cell Populations.
Th2-polarized, CD4+-purified OT-II cells stained with CD4 (Invitrogen; clone RM4.5), CD44 (eBioscience; clone IM7), and CD62L (eBioscience; clone MEL-14) were sorted (BD FACSAria II) into CD4+CD44+CD62Llo and CD4+CD44+CD62Lhi populations. A total of 2 × 106 cells of either population were transferred i.v. to recipient mice.
Assessing Th2 OT-II Cell Function.
A total of 2 × 104 per well Th2 cells [rested for 3 d in 10 units/mL of IL-2 (Peprotech)], or naive CD4+ OT-II cells, were cultured with 5 × 105 per well irradiated C57BL/6J splenocytes and OVA (Worthington Biochemical Corporation) in 96-well flat-bottom plates. Cytokines in supernatants were measured by ELISA after 72 h.
Administration of Soluble Peptides.
Two days after transfer of OT-II cells, 500 µg pOVA diluted in sterile PBS (or PBS only as a control) was given i.v. via the tail vein.
Inducing AAI.
Mice were anesthetized and 50 µg of OVA (Worthington Biochemical Corporation), or PBS as a control, was instilled into the trachea. Three i.t. challenges were given 3 d apart. Mice were culled 2 d after final i.t. instillation. In some experiments, a cohort was culled 24 h after the second i.t. instillation to assess OT-II cell location and frequency at this time.
BAL.
Lungs were lavaged with 1 mL PBS. BAL fluid was stored at −80 °C for cytokine analysis. Cytospins of BAL cells were stained with Quick-Diff (Gamidor Technical Services) and differentially counted at ×650 magnification under blinded conditions (300 cells per slide).
Histological Scoring.
Lungs were perfused with PBS to remove blood. In experiments with airway challenge, the left lung lobe was tied off for flow cytometry and removed, and the right lobe was fixed in methacarn and embedded in paraffin. Sections were stained with hematoxylin and eosin (H&E) or Periodic acid-Schiff (PAS). Slides were scored for the presence of goblet cells as previously described (55). Average percentages of goblet cells within 10 consecutive small airways (less than half the width of a field at ×200 magnification) were calculated for each slide, under experimentally blinded conditions.
Isolating Lung Cells.
Lung tissue was finely chopped and incubated in collagenase (type I-AS; Sigma-Aldrich) solution for 45 min at 37 °C. Tissue was disaggregated via a 20-gauge needle.
Flow Cytometry.
Single-cell suspensions from spleens, lymph nodes, and lungs were stained with LIVE/DEAD fixable cell stain (Life Technologies), CD45.1 (eBioscience; clone A20) CD4, CD44, and CD62L (as above). Foxp3 (eBioscience; clone FJK-16a), Ki-67 (eBioscience; clone SoIA15), and GATA-3 (BD Biosciences; clone L50-823) staining was carried out using the Foxp3/Transcription Factor staining buffer set (eBioscience). Ki67 and GATA-3 antibodies were applied for 30 min at room temperature whereas Foxp3 antibodies were applied for 30 min at 4 °C. GATA-3 was also assessed after overnight culture of lung cells in the presence of 20 µg/mL pOVA.
Intracellular Cytokine Staining.
Lung cells were pooled proportionately for each group and cultured overnight in the presence of 20 µg/mL pOVA. Intracellular cytokine staining was carried out using the BD Cytofix/Cytoperm kit (BD Biosciences), IL-5 (BD Biosciences; clone TRFK5), and IL-13 (eBioscience; clone ebio13A) antibodies.
Cytokine Detection.
Cytokines were detected by ELISA, using IL-5 (BD Biosciences; clone TRFK5) or IL-13 (eBioscience; clone ebio13A) capture antibodies and IL-5 (BD Biosciences; clone TRFK4) or IL-13 (eBioscience; clone ebio1316H) detection antibodies. Binding was visualized using Streptavidin–HRP (R+D Systems) and TMB (Invitrogen). Plates were read at 450 nm, using a BioTek Synergy HT plate reader and Gen5 software (BioTek). IL-13 in BAL fluid was detected using a FlowCytomix analyte detection system (eBioscience).
Flow Cytometric Analysis.
Data were collected using an LSR Fortessa with FACS DIVA software (BD Biosciences) and analyzed using FlowJo software (Treestar).
Statistical Analysis.
Prism 4 (Microsoft) software was used. Data were analyzed using unpaired t tests and P < 0.05 was considered significant for all tests.
Acknowledgments
This work was funded by the UK Medical Research Council, Asthma UK, the Wellcome Trust, Tenovus Scotland (K.J.M.), and the Dowager Countess Eleanor Peel Trust (K.J.M.). K.J.M. received a Medical Research Council Clinical Research Training Fellowship.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
References
- 1.Frew AJ. Allergen immunotherapy. J Allergy Clin Immunol. 2010;125(2) Suppl 2:S306–S313. doi: 10.1016/j.jaci.2009.10.064. [DOI] [PubMed] [Google Scholar]
- 2.Akdis CA. Therapies for allergic inflammation: Refining strategies to induce tolerance. Nat Med. 2012;18(5):736–749. doi: 10.1038/nm.2754. [DOI] [PubMed] [Google Scholar]
- 3.Pajno GB, Barberio G, De Luca F, Morabito L, Parmiani S. Prevention of new sensitizations in asthmatic children monosensitized to house dust mite by specific immunotherapy. A six-year follow-up study. Clin Exp Allergy. 2001;31(9):1392–1397. doi: 10.1046/j.1365-2222.2001.01161.x. [DOI] [PubMed] [Google Scholar]
- 4.Jacobsen L, Valovirta E. How strong is the evidence that immunotherapy in children prevents the progression of allergy and asthma? Curr Opin Allergy Clin Immunol. 2007;7(6):556–560. doi: 10.1097/ACI.0b013e3282f1d67e. [DOI] [PubMed] [Google Scholar]
- 5.Sabatos-Peyton CA, Verhagen J, Wraith DC. Antigen-specific immunotherapy of autoimmune and allergic diseases. Curr Opin Immunol. 2010;22(5):609–615. doi: 10.1016/j.coi.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abramson MJ, Puy RM, Weiner JM. Injection allergen immunotherapy for asthma. Cochrane Database Syst Rev. 2010;(8):CD001186. doi: 10.1002/14651858.CD001186.pub2. [DOI] [PubMed] [Google Scholar]
- 7.Borchers AT, Keen CL, Gershwin ME. Fatalities following allergen immunotherapy. Clin Rev Allergy Immunol. 2004;27(2):147–158. doi: 10.1385/CRIAI:27:2:147. [DOI] [PubMed] [Google Scholar]
- 8.Larché M, Wraith DC. Peptide-based therapeutic vaccines for allergic and autoimmune diseases. Nat Med. 2005;11(4) Suppl:S69–S76. doi: 10.1038/nm1226. [DOI] [PubMed] [Google Scholar]
- 9.Anderton SM. Peptide-based immunotherapy of autoimmunity: A path of puzzles, paradoxes and possibilities. Immunology. 2001;104(4):367–376. doi: 10.1046/j.1365-2567.2001.01324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Metzler B, Wraith DC. Inhibition of experimental autoimmune encephalomyelitis by inhalation but not oral administration of the encephalitogenic peptide: Influence of MHC binding affinity. Int Immunol. 1993;5(9):1159–1165. doi: 10.1093/intimm/5.9.1159. [DOI] [PubMed] [Google Scholar]
- 11.Burkhart C, Liu GY, Anderton SM, Metzler B, Wraith DC. Peptide-induced T cell regulation of experimental autoimmune encephalomyelitis: A role for IL-10. Int Immunol. 1999;11(10):1625–1634. doi: 10.1093/intimm/11.10.1625. [DOI] [PubMed] [Google Scholar]
- 12.Daniel D, Wegmann DR. Protection of nonobese diabetic mice from diabetes by intranasal or subcutaneous administration of insulin peptide B-(9-23) Proc Natl Acad Sci USA. 1996;93(2):956–960. doi: 10.1073/pnas.93.2.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderton SM, Wraith DC. Hierarchy in the ability of T cell epitopes to induce peripheral tolerance to antigens from myelin. Eur J Immunol. 1998;28(4):1251–1261. doi: 10.1002/(SICI)1521-4141(199804)28:04<1251::AID-IMMU1251>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 14.Hoyne GF, O’Hehir RE, Wraith DC, Thomas WR, Lamb JR. Inhibition of T cell and antibody responses to house dust mite allergen by inhalation of the dominant T cell epitope in naive and sensitized mice. J Exp Med. 1993;178(5):1783–1788. doi: 10.1084/jem.178.5.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Campbell JD, et al. Peptide immunotherapy in allergic asthma generates IL-10-dependent immunological tolerance associated with linked epitope suppression. J Exp Med. 2009;206(7):1535–1547. doi: 10.1084/jem.20082901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moldaver D, Larché M. Immunotherapy with peptides. Allergy. 2011;66(6):784–791. doi: 10.1111/j.1398-9995.2011.02610.x. [DOI] [PubMed] [Google Scholar]
- 17.Alexander C, Tarzi M, Larché M, Kay AB. The effect of Fel d 1-derived T-cell peptides on upper and lower airway outcome measurements in cat-allergic subjects. Allergy. 2005;60(10):1269–1274. doi: 10.1111/j.1398-9995.2005.00885.x. [DOI] [PubMed] [Google Scholar]
- 18.Oldfield WLG, Larché M, Kay AB. Effect of T-cell peptides derived from Fel d 1 on allergic reactions and cytokine production in patients sensitive to cats: A randomised controlled trial. Lancet. 2002;360(9326):47–53. doi: 10.1016/s0140-6736(02)09332-7. [DOI] [PubMed] [Google Scholar]
- 19.Tarzi M, et al. Induction of interleukin-10 and suppressor of cytokine signalling-3 gene expression following peptide immunotherapy. Clin Exp Allergy. 2006;36(4):465–474. doi: 10.1111/j.1365-2222.2006.02469.x. [DOI] [PubMed] [Google Scholar]
- 20.Patel D, et al. Fel d 1-derived peptide antigen desensitization shows a persistent treatment effect 1 year after the start of dosing: Arandomized, placebo-controlled study. J Allergy Clin Immunol. 2013;131(1):103–109, e1–e7. doi: 10.1016/j.jaci.2012.07.028. [DOI] [PubMed] [Google Scholar]
- 21.Hochweller K, Anderton SM. Kinetics of costimulatory molecule expression by T cells and dendritic cells during the induction of tolerance versus immunity in vivo. Eur J Immunol. 2005;35(4):1086–1096. doi: 10.1002/eji.200425891. [DOI] [PubMed] [Google Scholar]
- 22.Kearney ER, Pape KA, Loh DY, Jenkins MK. Visualization of peptide-specific T cell immunity and peripheral tolerance induction in vivo. Immunity. 1994;1(4):327–339. doi: 10.1016/1074-7613(94)90084-1. [DOI] [PubMed] [Google Scholar]
- 23.DeLong JH, et al. Ara h 1-reactive T cells in individuals with peanut allergy. J Allergy Clin Immunol. 2011;127(5):1211–1218, e3. doi: 10.1016/j.jaci.2011.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hochweller K, Sweenie CH, Anderton SM. Circumventing tolerance at the T cell or the antigen-presenting cell surface: Antibodies that ligate CD40 and OX40 have different effects. Eur J Immunol. 2006;36(2):389–396. doi: 10.1002/eji.200535506. [DOI] [PubMed] [Google Scholar]
- 25.London CA, Lodge MP, Abbas AK. Functional responses and costimulator dependence of memory CD4+ T cells. J Immunol. 2000;164(1):265–272. doi: 10.4049/jimmunol.164.1.265. [DOI] [PubMed] [Google Scholar]
- 26.Croft M, Bradley LM, Swain SL. Naive versus memory CD4 T cell response to antigen. Memory cells are less dependent on accessory cell costimulation and can respond to many antigen-presenting cell types including resting B cells. J Immunol. 1994;152(6):2675–2685. [PubMed] [Google Scholar]
- 27.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: Function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 28.Islam SA, Luster AD. T cell homing to epithelial barriers in allergic disease. Nat Med. 2012;18(5):705–715. doi: 10.1038/nm.2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wambre E, et al. Distinct characteristics of seasonal (Bet v 1) vs. perennial (Der p 1/Der p 2) allergen-specific CD4(+) T cell responses. Clin Exp Allergy. 2011;41(2):192–203. doi: 10.1111/j.1365-2222.2010.03641.x. [DOI] [PubMed] [Google Scholar]
- 30.Van Overtvelt L, et al. Assessment of Bet v 1-specific CD4+ T cell responses in allergic and nonallergic individuals using MHC class II peptide tetramers. J Immunol. 2008;180(7):4514–4522. doi: 10.4049/jimmunol.180.7.4514. [DOI] [PubMed] [Google Scholar]
- 31.Horiguchi S, et al. Seasonal changes in antigen-specific T-helper clone sizes in patients with Japanese cedar pollinosis: A 2-year study. Clin Exp Allergy. 2008;38(3):405–412. doi: 10.1111/j.1365-2222.2007.02898.x. [DOI] [PubMed] [Google Scholar]
- 32.Bingaman AW, et al. Novel phenotypes and migratory properties distinguish memory CD4 T cell subsets in lymphoid and lung tissue. Eur J Immunol. 2005;35(11):3173–3186. doi: 10.1002/eji.200526004. [DOI] [PubMed] [Google Scholar]
- 33.Harris NL, Watt V, Ronchese F, Le Gros G. Differential T cell function and fate in lymph node and nonlymphoid tissues. J Exp Med. 2002;195(3):317–326. doi: 10.1084/jem.20011558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Purwar R, et al. Resident memory T cells (T(RM)) are abundant in human lung: Diversity, function, and antigen specificity. PLoS ONE. 2011;6(1):e16245. doi: 10.1371/journal.pone.0016245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barnden MJ, Allison J, Heath WR, Carbone FR. Defective TCR expression in transgenic mice constructed using cDNA-based alpha- and beta-chain genes under the control of heterologous regulatory elements. Immunol Cell Biol. 1998;76(1):34–40. doi: 10.1046/j.1440-1711.1998.00709.x. [DOI] [PubMed] [Google Scholar]
- 36.Konkel JE, et al. PD-1 signalling in CD4(+) T cells restrains their clonal expansion to an immunogenic stimulus, but is not critically required for peptide-induced tolerance. Immunology. 2010;130(1):92–102. doi: 10.1111/j.1365-2567.2009.03216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakagome K, et al. Antigen-sensitized CD4+CD62Llow memory/effector T helper 2 cells can induce airway hyperresponsiveness in an antigen free setting. Respir Res. 2005;6:46. doi: 10.1186/1465-9921-6-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reinhardt RL, Khoruts A, Merica R, Zell T, Jenkins MK. Visualizing the generation of memory CD4 T cells in the whole body. Nature. 2001;410(6824):101–105. doi: 10.1038/35065111. [DOI] [PubMed] [Google Scholar]
- 39.Moore TV, et al. Inducible costimulator controls migration of T cells to the lungs via down-regulation of CCR7 and CD62L. Am J Respir Cell Mol Biol. 2011;45(4):843–850. doi: 10.1165/rcmb.2010-0466OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Calderón MA, et al. Allergen-specific immunotherapy for respiratory allergies: From meta-analysis to registration and beyond. J Allergy Clin Immunol. 2011;127(1):30–38. doi: 10.1016/j.jaci.2010.08.024. [DOI] [PubMed] [Google Scholar]
- 41.Fiorenza S, et al. A combination of local inflammation and central memory T cells potentiates immunotherapy in the skin. J Immunol. 2012;189(12):5622–5631. doi: 10.4049/jimmunol.1200709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Klebanoff CA, Gattinoni L, Restifo NP. Sorting through subsets: Which T-cell populations mediate highly effective adoptive immunotherapy? J Immunother. 2012;35(9):651–660. doi: 10.1097/CJI.0b013e31827806e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roberts AD, Ely KH, Woodland DL. Differential contributions of central and effector memory T cells to recall responses. J Exp Med. 2005;202(1):123–133. doi: 10.1084/jem.20050137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roberts AD, Woodland DL. Cutting edge: Effector memory CD8+ T cells play a prominent role in recall responses to secondary viral infection in the lung. J Immunol. 2004;172(11):6533–6537. doi: 10.4049/jimmunol.172.11.6533. [DOI] [PubMed] [Google Scholar]
- 45.Bateman EAL, Ardern-Jones MR, Ogg GS. Persistent central memory phenotype of circulating Fel d 1 peptide/DRB1*0101 tetramer-binding CD4+ T cells. J Allergy Clin Immunol. 2006;118(6):1350–1356. doi: 10.1016/j.jaci.2006.07.040. [DOI] [PubMed] [Google Scholar]
- 46.Kinnunen T, et al. Allergen-specific naïve and memory CD4+ T cells exhibit functional and phenotypic differences between individuals with or without allergy. Eur J Immunol. 2010;40(9):2460–2469. doi: 10.1002/eji.201040328. [DOI] [PubMed] [Google Scholar]
- 47.Robinson DS, Bentley AM, Hartnell A, Kay AB, Durham SR. Activated memory T helper cells in bronchoalveolar lavage fluid from patients with atopic asthma: Relation to asthma symptoms, lung function, and bronchial responsiveness. Thorax. 1993;48(1):26–32. doi: 10.1136/thx.48.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Teijaro JR, et al. Cutting edge: Tissue-retentive lung memory CD4 T cells mediate optimal protection to respiratory virus infection. J Immunol. 2011;187(11):5510–5514. doi: 10.4049/jimmunol.1102243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Connor LM, et al. A key role for lung-resident memory lymphocytes in protective immune responses after BCG vaccination. Eur J Immunol. 2010;40(9):2482–2492. doi: 10.1002/eji.200940279. [DOI] [PubMed] [Google Scholar]
- 50.Sheridan BS, Lefrançois L. Regional and mucosal memory T cells. Nat Immunol. 2011;12(6):485–491. doi: 10.1038/ni.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eigenmann PA. T lymphocytes in food allergy: overview of an intricate network of circulating and organ-resident cells. Pediatr Allergy Immunol. 2002;13(3):162–171. doi: 10.1034/j.1399-3038.2002.01015.x. [DOI] [PubMed] [Google Scholar]
- 52.Larché M. Update on the current status of peptide immunotherapy. J Allergy Clin Immunol. 2007;119(4):906–909. doi: 10.1016/j.jaci.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 53.Müller U, et al. Successful immunotherapy with T-cell epitope peptides of bee venom phospholipase A2 induces specific T-cell anergy in patients allergic to bee venom. J Allergy Clin Immunol. 1998;101(6 Pt 1):747–754. doi: 10.1016/S0091-6749(98)70402-6. [DOI] [PubMed] [Google Scholar]
- 54.Rotiroti G, Shamji M, Durham SR, Till SJ. Repeated low-dose intradermal allergen injection suppresses allergen-induced cutaneous late responses. J Allergy Clin Immunol. 2012;130(4):918–924, e1. doi: 10.1016/j.jaci.2012.06.052. [DOI] [PubMed] [Google Scholar]
- 55.Leech MD, Benson RA, De Vries A, Fitch PM, Howie SEM. Resolution of Der p1-induced allergic airway inflammation is dependent on CD4+CD25+Foxp3+ regulatory cells. J Immunol. 2007;179(10):7050–7058. doi: 10.4049/jimmunol.179.10.7050. [DOI] [PubMed] [Google Scholar]