Significance
Pathogens must express their virulence genes in the correct locales to cause disease. This task requires a pathogen’s ability to sense host signals and to transduce this information to its expression machinery. Here we establish that the facultative intracellular pathogen Salmonella enterica responds to a decrease in the levels of proline-charged tRNAPro by promoting expression of the mgtCBR virulence operon. We determine that hyperosmotic stress and proline limitation reduce proline-charged tRNAPro levels and show that the compartment harboring Salmonella is limited in proline and that Salmonella experiences high osmolarity inside macrophages. Our findings indicate that Salmonella detects host cues by the changes they produce on bacterial constituents.
Keywords: leader mRNA, transcription attenuation
Abstract
The intracellular pathogen Salmonella enterica serovar Typhimurium requires the mgtC gene to cause disease. The mgtC transcript includes a long leader region that harbors a short proline codon-rich ORF—termed mgtP—the translation of which is predicted to favor formation of one of two alternative stem-loop structures. We now report that the mgtP proline codons are critical for expression of the mgtC coding region inside host cells, for Salmonella survival inside macrophages, and for virulence in mice. We determine that the mgtP proline codons mediate the response to proline-charged tRNAPro, the levels of which decrease under proline limitation and/or hyperosmotic stress. The host compartment harboring Salmonella appears to be limited in proline because proline auxotrophs were defective for intramacrophage survival and virulence in mice. Salmonella seems to experience hyperosmotic stress during infection because osmotically regulated genes were highly induced inside phagocytic cells. Replacing mgtP proline codons with codons specifying threonine converted the mgtC leader into a threonine-responding element. Our findings indicate that an attenuation-like mechanism governs transcription elongation into the mgtCBR coding region. Moreover, they highlight how pathogens construe host signals by the effect they have on bacterial constituents.
MgtC is a virulence protein used by several unrelated intracellular pathogens to survive within acidic macrophage phagosomes and to cause a lethal infection in mice (1–5). In the bacterium Salmonella enterica serovar Typhimurium—the etiologic agent of human gastroenteritis and murine typhoid fever—MgtC inhibits Salmonella’s own F1Fo ATP synthase, thereby preventing the decrease in cytosolic pH and the excessive ATP accumulation resulting from acidification of Salmonella’s surroundings (6). MgtC’s virulence role is unique because, rather than inhibiting a host protein, it targets a bacterial complex (i.e., the F1Fo ATP synthase) that itself is necessary for virulence (7).
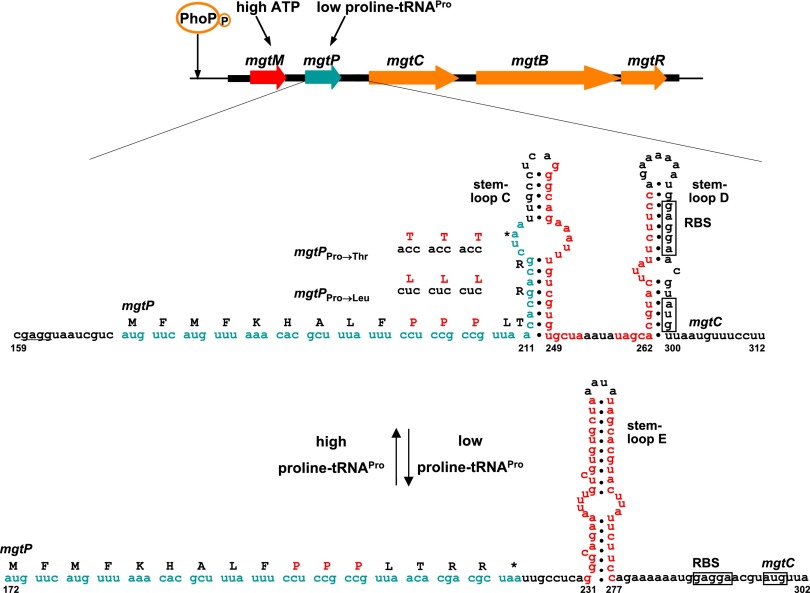
Expression of the MgtC protein is regulated at multiple levels. Transcription from the mgtC promoter is dependent on PhoP/PhoQ (8), a major virulence regulatory system highly active inside phagocytic cells (Fig. 1) (9). Transcription elongation into the mgtC coding region is, in turn, controlled by the 296-nucleotide-long leader RNA that precedes the mgtC ORF (Fig. 1). The mgtC leader RNA responds to an increase in cytosolic ATP by stimulating transcription elongation into the mgtC coding region (10, 11) (Fig. 1), an ability critical for Salmonella pathogenicity (10, 11). However, an additional signal appears to act on the mgtC leader during infection of a mammalian host because a strain with an ATP-insensitive mgtC leader RNA exhibited residual mgtC induction inside macrophages and was not as attenuated in mice as a strain deleted for the coding regions of the mgtC and mgtB genes (10). mgtB specifies a Mg2+ transporter (12) and follows mgtC in the mgtCBR operon (1), whereas mgtR specifies a peptide that promotes the degradation of the MgtC protein (13).
Fig. 1.
Regulation of the mgtCBR virulence operon by ATP and proline tRNAPro. The phosphorylated PhoP protein binds to the promoter of the mgtCBR operon and stimulates transcription initiation. The 296-long mgtCBR leader mRNA harboring two short ORFs (termed mgtM and mgtP) controls transcription elongation into the coding region in response to ATP and proline tRNAPro levels. Proline tRNAPro levels determine the coupling/uncoupling between transcription of the mgtCBR leader and translation of mgtP, allowing formation of one of two alternative stem-loop structures (stem loop D versus E), hence controlling transcription elongation into the mgtCBR coding region. The sequences of mgtP variants used in this work are indicated above the Pro codons.
The mgtC leader RNA also includes a short ORF—termed mgtP—with three consecutive proline codons, two of which are necessary to promote transcription elongation into the mgtC coding region when Salmonella experiences proline limitation or hyperosmotic stress (11) (Fig. 1). This portion of the mgtC leader appears to be part of a transcription attenuator because (i) translation of mgtP is predicted to favor formation of one of two alternative stem-loop structures, (ii) stop codon mutations early in mgtP abolish expression of the associated coding region, and (iii) expression could be restored to the mgtP stop codon mutants by additional mutations that lock the mgtC leader into one of the two alternative stem-loop structures (11). Interestingly, one of the stem-loop structures sequesters the ribosome binding sequence and the start codon of the mgtC gene, suggesting additional regulation at the level of translation (Fig. 1).
We now report that the ability of the mgtC leader RNA to respond to a decrease in the levels of proline-charged tRNAPro is critical for expression of the associated coding region inside host cells, for survival inside macrophages, and for virulence in mice. By replacing the mgtP proline codons with codons specifying threonine, we rendered expression of the mgtC coding region responsive to threonine. Taken together with the finding that proline limitation and hyperosmotic stress decrease the levels of proline-charged tRNAPro, these results indicate that mgtP controls gene expression by a transcription attenuation-like mechanism. Our data suggest that a compartment harboring Salmonella is limited in proline and that Salmonella experiences hyperosmotic stress inside macrophages.
Results
Replacing mgtP’s Proline Codons by Threonine Codons Converts the mgtC Leader into a Threonine-Responding Genetic Element.
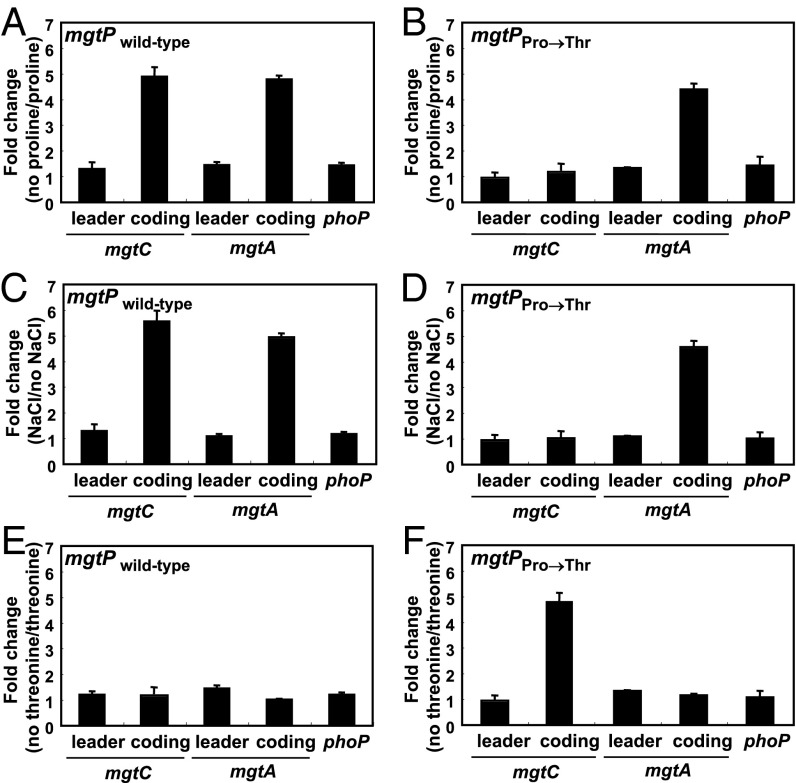
To investigate how the wild-type mgtP controls mgtC expression when Salmonella experiences proline limitation or hyperosmotic stress (11), we constructed a derivative of a proline auxotroph in which the three proline codons in the chromosomal copy of mgtP were substituted by threonine codons (Fig. 1). This derivative could no longer increase the mRNA levels of the mgtC coding region when experiencing proline limitation (Fig. 2 A and B, Fig. S1 A and B) or hyperosmotic stress (Fig. 2 C and D, Fig. S1 C and D). These results are in agreement with our previous report demonstrating that both proline limitation and hyperosmotic stress induced expression of the mgtC coding region in an organism harboring the wild-type mgtP sequence but not in one where the proline codons were substituted by codons specifying glycine (11). Control experiments revealed that substitution of the mgtP proline codons specifically affected the mRNA levels of the mgtC coding region because the levels of the mgtA coding region, which are also induced upon proline limitation (14), were similar in the mgtP+ strain and in the variant with the mgtP proline codons replaced by threonine codons (Fig. 2 A–D, Fig. S1 A–D). Likewise, the mRNA levels of the phoP gene and of the mgtC and mgtA leader regions, which are not induced by proline limitation (11, 14), were not affected by substitutions in the mgtP codons (Fig. 2 A–D, Fig. S1 A–D).
Fig. 2.
Proline limitation promotes transcription of the mgtCBR coding region in a manner dependent on the mgtP proline codons. (A and B) Fold change in the mRNA levels of the leader regions of the mgtC and mgtA transcripts and the coding regions of the mgtC, mgtA, and phoP genes produced by a proline auxotroph harboring either the wild-type mgtCBR leader (EG19886) (A) or a derivative in which the three mgtP Pro codons were substituted by Thr codons (mgtPPro→Thr; EL417) (B). Bacteria were grown in N-minimal media with 500 μM Mg2+ in the presence of 1 mM proline for 1 h and then grown for 45 min in media containing or lacking proline. The expression levels of target genes were normalized to that of 16S ribosomal RNA rrs gene. Fold change was calculated by dividing the mRNA levels of cells grown in the absence of proline by that of cells grown in the presence of proline. Shown are the means and SDs from two independent experiments. (C and D) Fold change in the mRNA levels of the leader regions of the mgtC and mgtA transcripts and the coding regions of the mgtC, mgtA, and phoP genes in strains with the wild-type mgtCBR leader (14028s) (C) or a derivative with the three mgtP Pro codons substituted by Thr codons (EL611) (D) that were subjected to hyperosmotic stress. The RNA values were normalized relative to those corresponding to the rrs gene. Bacteria were grown for 1 h in modified N-minimal medium without casamino acids and containing 500 μM Mg2+ or in media that also had 0.3 M NaCl. Shown are the means and SDs from two independent experiments. (E and F) Fold change in the mRNA levels of the mRNA regions listed in A and B produced by a threonine auxotroph harboring either the wild-type mgtCBR leader (EL601) (E) or a derivative in which the three mgtP Pro codons were substituted by Thr codons (mgtPPro→Thr; EL621) (F) under threonine limitation conditions analogous to those described above for proline limitation. Shown are the means and SDs from two independent experiments. mRNA levels relative to those of the 16S rRNA rrs gene are presented in Fig. S1.
Introduction of the mgtP allele with the proline codons replaced by threonine codons conferred the ability to promote mgtC expression in response to threonine limitation upon a threonine auxotroph (Fig. 2F, Fig. S1F). This ability was specific to the associated mgtC coding region as threonine limitation had no effect on the mRNA levels of the mgtC and mgtA leader regions or the mgtA and phoP coding regions (Fig. 2F, Fig. S1F). Moreover, it required the threonine codons in the mgtP variant because the mRNA levels of the mgtC coding region did not increase upon threonine limitation in the isogenic mgtP+ strain (Fig. 2E, Fig. S1E). These results indicate that expression of the mgtC coding region responds to limitation for the particular amino acid having consecutive codons in mgtP rather than to nutrient limitation per se. Furthermore, they support the notion that the mgtP portion of the mgtC leader RNA controls transcription elongation into the mgtC coding region by a mechanism resembling transcription attenuation (15, 16).
mgtP Proline Codons Are Necessary for Normal mgtC Expression Inside Macrophages.
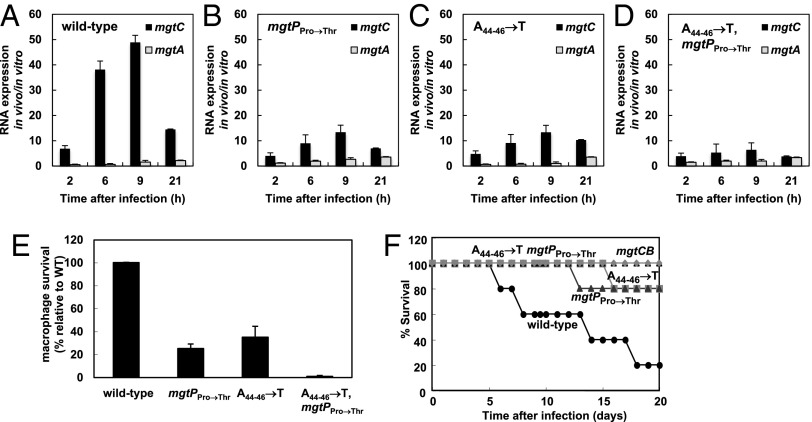
To evaluate mgtP’s role during infection, we introduced the mgtP allele with the proline codons substituted by threonine codons into a wild-type genetic background (i.e., prototroph for both proline and threonine). The resulting variant failed to fully induce mgtC expression inside the macrophage-like cell line J774 A.1, achieving mRNA levels that were only one-third to one-half of those produced by the isogenic mgtP+ strain (Fig. 3 A and B). This defect is due to the absence of codons specifying proline, as opposed to the presence of codons specifying threonine per se. This is because replacing the mgtP proline codons with codons specifying leucine prevented normal mgtC induction both in a proline auxotroph experiencing proline limitation (Fig. S2 A and B) and in an otherwise wild-type strain inside macrophages (Fig. S2 C and D). As expected, the strain harboring the mgtP allele with the proline codons substituted by leucine codons retained the ability to induce the mgtA coding region in a proline auxotroph experiencing proline limitation and had no effect on the expression of the mgtC and mgtA leader regions or the phoP coding region (Fig. S2 A and B). These data suggested that the levels of proline-charged tRNAPro decrease during Salmonella infection of a phagocytic cell.
Fig. 3.
The mgtP proline codons are necessary for mgtC expression and survival inside macrophages as well as virulence in mice. (A–D) Relative mRNA levels of the mgtC and mgtA coding regions produced by wild-type Salmonella (14028s) (A), an mgtP mutant with the Pro codons replaced by Thr codons (EL611) (B), an ATP-sensing defective leader mutant (EL341) (C), and a mutant defective for sensing both signals (EL602) (D) inside J774 A.1 macrophages at the indicated times after infection. (E) Survival inside J774 A.1 macrophages of the Salmonella strains listed in A–D at 18 h after infection. (F) Survival of C3H/HeN mice inoculated intraperitoneally with ∼103 colony-forming units of the Salmonella strains listed in A–D and of a mutant Salmonella deleted for both the mgtC and mgtB genes (EL6).
mgtP Proline Codons Are Necessary for Salmonella Survival Inside Macrophages and Virulence in Mice.
The mgtP variant with the proline codons replaced by threonine codons was defective for survival inside J774 A.1 macrophages (Fig. 3E). In addition, the mgtP variants with proline codons replaced by either threonine or leucine codons were defective for virulence in C3H/HeN mice inoculated intraperitoneally (Fig. 3F, Fig. S2E). These mgtP mutants were attenuated to a similar extent as our previously reported mutant with an mgtC leader that is not able to respond to an increase in cytosolic ATP (Fig. 3 E and F) (10, 11), and thus not as attenuated as a strain deleted for both the mgtC and mgtB coding regions (Fig. 3F).
We determined that a Salmonella strain with an mgtC leader unable to respond to ATP and bearing the mgtP allele with the proline codons replaced by threonine codons failed to promote transcription of the mgtC coding region inside macrophages (Fig. 3D), was highly compromised for survival inside macrophages (Fig. 3E), and attenuated for virulence in mice (Fig. 3F). Actually, this mutant was as defective as the strain deleted for both the mgtC and mgtB coding regions (Fig. 3F). And this was true also for a mutant unable to respond to ATP and with the mgtP allele harboring leucine codons in place of the proline codons (Fig. S2E). These data indicate that the ability to respond to an increase in cytosolic ATP and to conditions predicted to decrease the levels of proline-charged tRNAPro is critical for mgtCBR-mediated virulence.
Salmonella Experiences Hyperosmotic Stress Inside Phagocytic Cells in a Compartment That Is Low in Proline.
The data presented above raised the question: What condition(s) promotes a decrease in the levels of proline-charged tRNAPro during infection? Salmonella may reside in a compartment that is low in proline and experience a diminished ability to synthesize proline. Alternatively or in addition, Salmonella might be subjected to hyperosmotic stress, which would decrease the availability of proline to charge tRNAPro because proline can serve as an osmoprotectant (17).
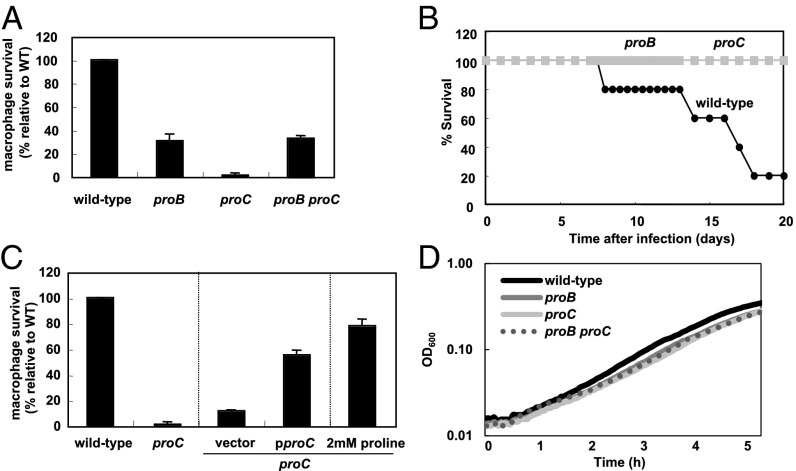
To explore the possibility of the compartment harboring Salmonella during infection being low in proline, we investigated the behavior of proline auxotrophs harboring mutations in the proB and proC genes, which specify proteins that carry out the first and third steps in proline biosynthesis, respectively (17). Both the proB and proC single mutants were defective for survival inside J774 A.1 macrophages (Fig. 4A) and for virulence in mice inoculated intraperitoneally (Fig. 4B). The virulence defect of the proC mutant is due to its inability to synthesize proline (as opposed to a potential effect of the proC mutation affecting a neighboring gene) because its macrophage survival defect could be partially corrected by a plasmid harboring the wild-type proC gene but not by the plasmid vector (Fig. 4C). Furthermore, proline addition to the tissue culture media rescued growth of the proC mutant inside macrophages (Fig. 4C).
Fig. 4.
Proline auxotrophs are defective for survival inside macrophages and virulence in mice. (A) Survival inside J774 A.1 macrophages of wild-type (14028s), proB (EG19886), proC (EL605), and proB proC (EL625) Salmonella at 18 h after infection. (B) Survival of C3H/HeN mice inoculated intraperitoneally with ∼103 colony-forming units of wild-type (14028s), proB (EG19886), or proC (EL605) Salmonella. The data are representative of two independent experiments, which gave similar results. (C) Survival inside J774 A.1 macrophages of wild-type (14028s), proC (EL605), Salmonella, and a proC mutant Salmonella harboring either the plasmid vector or the wild-type proC gene, or in tissue culture media with 2 mM proline at 18 h after infection. (D) Growth curves of wild-type (14028s), proB (EG19886), proC (EL605), and proB proC (EL625) Salmonella in N-minimal medium containing 1 mM proline. Bacteria were grown at 37 °C for 5 h in a 96-well plate with orbital shaking and absorbance measured at OD600 every 2.5 min.
We were surprised to find that the proC mutant was more attenuated than the proB mutant inside macrophages (Fig. 4A). This virulence difference could be due to the accumulation of gamma-glutamyl semialdehyde (i.e., the ProC substrate) in the proC mutant because a proB proC double mutant displayed the same attenuation as the proB single mutant (Fig. 4A). The proC single mutant grew like the proB single mutant and the proB proC double mutant in defined media containing casamino acids and 1 mM proline (Fig. 4D). The different phenotypes displayed by the proC mutant when Salmonella is inside host cells versus grown in defined media could reflect a lower accumulation of gamma-glutamyl semialdehyde in the latter growth condition due to feedback inhibition of proline on the enzyme encoded by the proB gene (18, 19).
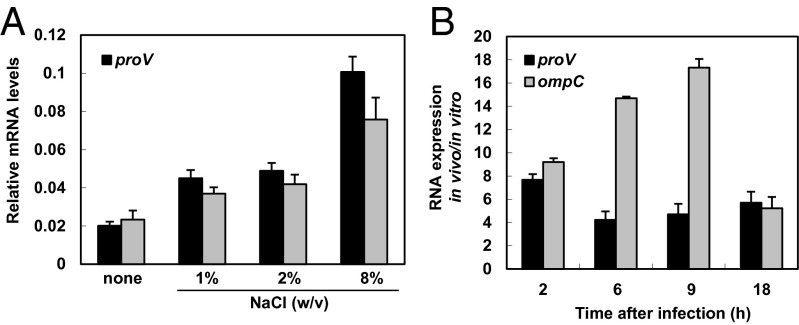
Next, we examined the mRNA levels of the hyperosmolarity-induced proV and ompC genes, which specify a component of a glycine betaine/proline uptake system and an outer membrane porin, respectively. In agreement with previous reports (20), the mRNA levels of the proV and ompC genes rose in response to increasing osmolarity (Fig. 5A). Both proV and ompC were highly induced when Salmonella was inside macrophages (Fig. 5B). Differences in induction kinetics between proV and ompC could be due to an additional signal(s) acting on proV and/or ompC. Cumulatively, these data indicate that Salmonella faces high osmolarity inside phagocytic cells, residing in a compartment of low proline.
Fig. 5.
Hyperosmotic stress-inducible proV and ompC genes are highly expressed inside macrophages. (A) Relative mRNA levels of the coding regions of the proV and ompC genes in wild-type Salmonella (14028s) under different osmotic conditions. Bacteria were grown for 1 h in modified N-minimal medium without casamino acids and containing 500 μM Mg2+ or in media that also had 1%, 2%, or 8% NaCl (wt/vol). Shown are the means and SDs from two independent experiments. (B) Relative mRNA levels of the proV and ompC coding regions of wild-type Salmonella (14028s) inside J774 A.1 macrophages at the indicated times after infection. mRNA levels are relative to those of the 16S rRNA rrs gene.
Proline Limitation and Hyperosmotic Stress Decrease the Levels of Proline-Charged tRNAPro.
Taken together with our published data (11), the results presented above suggest that mgtP-mediated transcription elongation into the mgtC coding region is controlled by an attenuation mechanism that entails formation of one of two alternative stem-loop structures. Which structure forms is determined by the coupling/uncoupling between transcription of the mgtCBR leader and translation of the mgtP ORF. Uncoupling of transcription and translation, and the resulting transcription elongation into the mgtC coding region, may occur if ribosomes stall at the mgtP proline codons due to low levels of proline-charged tRNAPro.
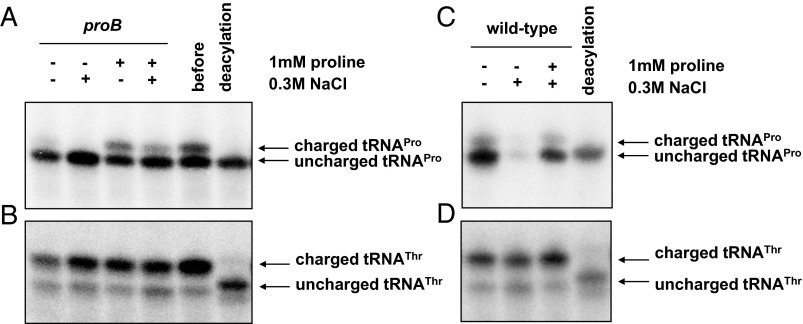
To test this model, we examined the levels of proline-charged tRNAPro in Salmonella subjected to proline limitation and/or hyperosmotic stress. The levels of proline-charged tRNA were lower in a proB mutant in media lacking proline compared with media with 1 mM proline (Fig. 6A), and the latter were reduced upon addition of 0.3 M of sodium chloride (Fig. 6A). The levels of proline-charged tRNAPro decreased also in wild-type Salmonella when incubated in 0.3 M of sodium chloride (Fig. 6C), and they were partially restored upon addition of 1 mM proline (Fig. 6C). Hyperosmotic stress and proline limitation appear to affect the levels of proline-charged tRNAPro specifically because the levels of threonine-charged tRNAThr were not altered under these conditions (Fig. 6 B and D).
Fig. 6.
Proline limitation and hyperosmotic stress decrease the levels of proline tRNAPro. (A and B) Northern blot analysis using a probe detecting tRNAPro (A) and tRNAThr (B) in proB Salmonella (EG19886) before or after switching bacteria to media containing or lacking 1 mM proline or 0.3 M NaCl. Bacteria were grown for 30 min in N-minimal media containing 500 μM Mg2+ in the presence or absence of 1 mM proline or 0.3 M NaCl. (C and D) Northern blot analysis using a probe detecting tRNAPro (C) and tRNAThr (D) in wild-type Salmonella (14028s) treated with 0.3 M NaCl or 0.3 M NaCl together with 1 mM proline. The levels of tRNAThr in B and D were used as internal controls for the levels of tRNAPro in A and C, respectively. Bacteria were grown for 1 h in N-minimal modified media containing 500 μM Mg2+ in the presence or absence of 1 mM proline or 0.3 M NaCl.
Discussion
We have identified a singular example in which proline-charged tRNAPro acts as a regulatory signal controlling virulence gene expression. Specifically, we established that Salmonella’s ability to sense a decrease in proline-charged tRNAPro levels via a proline codon-rich ORF in the leader region of the mgtCBR transcript is required for bacterial survival inside macrophages (Fig. 3) and for causing disease in mice (Fig. 3, Fig. S2). Moreover, an mgtC leader RNA that was unable to sense both proline-charged tRNAPro levels and ATP, which is detected by a different portion of the mgtCBR leader (10), failed to induce mgtC expression inside macrophages (Fig. 3D) and was as attenuated as a strain deleted for the mgtC and mgtB coding regions (Fig. 3F, Fig. S2E). These data demonstrate the critical role that both proline-charged tRNAPro and ATP play in expression of a critical virulence determinant during infection.
mgtP Is Part of a Transcriptional Attenuator.
We previously proposed that the mgtP-including portion of the mgtC leader RNA controls transcription elongation into the associated coding region by an attenuation-like mechanism (11). This proposal was based on the ability of the mgtP portion of the mgtC leader to adopt two alternative stem-loop structures (Fig. 1), on mgtP translation predicted to favor formation of one of the two structures (Fig. 1), and on the results of genetic experiments in which full mgtP translation was compromised and/or the leader RNA locked into one of the two structures (11).
Two unique sets of independent experiments now provide further support for the notion that transcription attenuation controls expression of the mgtC coding region. First, we established that an mgtP allele in which the three proline codons were substituted with threonine codons gained the ability to respond to threonine limitation (Fig. 2F). And second, we determined that proline limitation and hyperosmotic stress decrease the levels of proline-charged tRNAPro (Fig. 6).
Differential Intramacrophage Expression Behavior of Salmonella Genes Regulated by Leader Sequences Harboring Short Proline Codon-Rich ORFs.
The leader regions of the mgtA and mgtCBR transcripts harbor two different short proline codon-rich ORFs, designated mgtL and mgtP, respectively, which enable proline limitation and hyperosmotic stress to induce expression of their associated coding regions (11, 14). Given that Salmonella experiences hyperosmotic stress (Fig. 5) during infection in a compartment with proline levels so low that it cannot support growth of proline auxotrophs (Fig. 4), why is the mgtA coding region not induced inside macrophages whereas mgtC’s is?
The mgtA coding region might not be induced inside macrophages because of several nonmutually exclusive possibilities. First, mgtL’s proline codons are not consecutive, being located at the third, fifth, seventh, and ninth positions (14), whereas mgtP harbors three consecutive proline codons (Fig. 1). The presence of consecutive proline codons might enhance ribosome stalling at mgtP. In this context, it is interesting that elongation factor P is required to alleviate ribosome stalling in ORFs harboring consecutive proline codons (21–24) and that a functional elongation factor P is necessary for Salmonella virulence (25). Second, mgtP is preceded by a large RNA segment, whereas that is not the case for mgtL. And third, the levels of the mgtA transcript are affected by RNase E, which targets the mgtA leader RNA (26). The different expression behavior of the mgtC and mgtA genes makes sense because mgtC is required for Salmonella virulence, whereas mgtA is not (1).
Nutritional Environment of the Salmonella-Containing Vacuole.
Salmonella resides within a membrane-bound compartment inside macrophages (27). This compartment appears to be a nutrient-poor locale because Salmonella auxotrophs for purine, pyrimidine, histidine, and aromatic amino acid are avirulent (28, 29). We have now demonstrated that proline auxotrophs are attenuated for survival inside macrophages and virulence in mice (Fig. 4), indicating that this compartment is also limited for proline. It is interesting that Legionella pneumophila, which also resides within a phagosome, induces proline biosynthetic genes when inside macrophages (30). Thus, other bacterial pathogens that remain within phagosomes may also experience low proline. By contrast, high proline stimulates virulence gene expression in entomopathogenic Photorhabdus (31), indicating that different pathogens may respond to a given signal in opposite ways.
Concluding Remarks.
Our findings illustrate how a pathogen integrates signals that operate in different compartments into the decision to express a virulence determinant. For example, transcription initiation from the mgtC promoter is under direct control of the virulence regulatory protein PhoP, which is activated by its cognate sensor PhoQ when Salmonella experiences low Mg2+ (32, 33), mildly acidic pH (34), and/or antimicrobial peptides (35) in its surroundings. Because PhoP-activated promoters are highly induced inside macrophages (36), the phagosome containing Salmonella is likely to display one or more of these (and potentially other) signals that activate PhoQ in the periplasm.
The leader sequences of certain PhoP-activated transcripts control transcription elongation into the associated coding regions in response to cytosolic signals. For instance, the leader sequence of the mgtCBR transcript responds not only to ATP (10) and conditions predicted to alter the levels of proline-charged tRNAPro (11) but also to cytoplasmic Mg2+ (26, 37). This raised the possibility of the defects in mgtC expression during infection (Fig. 3 A–D and Fig. S2D), survival inside macrophages (Fig. 3E), and virulence in mice (Fig. 3F and Fig. S2E) exhibited by the mgtP mutants with substitutions in the proline codons resulting from a compromised ability of the mutant mgtC leader to respond to cytoplasmic Mg2+. However, we can rule out this possibility because the mgtP variant with the leucine codons replacing the proline codons responded to changes in Mg2+ like the isogenic mgtP+ strain (Fig. S3). Moreover, a Salmonella strain with an mgtC leader unable to respond to an increase in cytosolic ATP retained a wild-type ability to respond to a decrease in cytosolic Mg2+ (10). Furthermore, the mgtA coding region is not induced inside macrophages (10) despite harboring a long leader RNA acting as a Mg2+-sensing riboswitch (37). Cumulatively, these data argue that cytoplasmic Mg2+ is not a key signal promoting the preferential expression of the mgtC coding region inside macrophages.
In sum, that conditions decreasing the levels of proline-charged tRNAPro induce expression of a virulence operon in Salmonella reinforces the notion that bacteria that associate with a eukaryotic host may respond to host cues indirectly, by the impact such cues have on bacterial constituents. In other words, a proline-limited environment coupled with a reduced proline biosynthetic ability and/or hyperosmotic stress result in a decrease in the levels of proline-charged tRNAPro (Fig. 6), which denotes the environment experienced by Salmonella inside a macrophage. Finally, that proline-charged tRNAPro controls expression of a virulence operon by a transcription attenuation-like mechanism demonstrates that this regulatory strategy has been appropriated by Salmonella to regulate functions unrelated to nutrient biosynthesis or transport (15, 16).
Materials and Methods
Bacterial Strains, Plasmids, Oligodeoxynucleotides, and Growth Conditions.
Bacterial strains and plasmids used in this study are listed in Table S1. All Salmonella enterica serovar Typhimurium strains are derived from the wild-type strain 14028s (28) and were constructed by phage P22-mediated transductions as described (38). All DNA oligonucletides are listed in Table S2. Bacteria were grown at 37 °C in Luria–Bertani broth (LB), N-minimal media (pH 7.7) (12) supplemented with 0.1% casamino acids, 38 mM glycerol, and the indicated concentrations of MgCl2. When indicated, we used a modified N-minimal medium containing 0.2% glucose instead of 38 mM glycerol. Escherichia coli DH5α was used as the host for preparation of plasmid DNA. Ampicillin was used at 50 µg⋅ml−1, chloramphenicol at 20 µg⋅ml−1, and tetracycline at 10 μg⋅ml−1.
Effect of Proline Limitation on Gene Expression.
The proline limitation experiment was performed as described (11).
Effect of Threonine Limitation on Gene Expression.
Threonine limitation was performed as described above except that we used a threonine auxotroph and a 19-amino-acid mixture (all essential amino acids except threonine).
Effect of Hyperosmotic Stress on Gene Expression.
Experiment was performed as described (14).
Quantitative Real Time PCR.
Total RNA was isolated using RNeasy Kit (Qiagen) according to the manufacturer’s instructions. The purified RNA was quantified using a Nanodrop machine (NanoDrop Technologies). cDNA was synthesized using High Capacity RNA-to cDNA Master Mix (Applied Biosystems). The mRNA levels of the mgtC, mgtA, phoP, proV, ompC, and rrs genes were measured by quantification of cDNA using SYBR Green PCR Master Mix (Applied Biosystems) and appropriate primers (mgtC leader, 6962/6963; mgtC coding, 7530/7531; mgtA leader, 7225/7226; mgtA coding, 4308/4309; phoP coding, 4489/4490; proV, W688/W689; and ompC, W696/W697) and monitored using a Fast ABI7500 machine (Applied Biosystems). Data were normalized to the levels of 16S ribosomal RNA rrs gene amplified with primers 6970 and 6971.
Examining Survival Inside Macrophages.
Intramacrophage survival assays were conducted with the macrophage-like cell line J774 A.1 as described (1).
Mouse Virulence Assays.
Six- to eight-week-old female C3H/HeN mice were inoculated intraperitoneally with ∼103 colony-forming units. Mouse survival was followed for 21 d. Virulence assays were conducted twice with similar outcomes, and data correspond to groups of five mice. All procedures were performed according to approved protocols by the Institutional Animal Care and Use Committee from Yale University.
Measurement of the Levels of Proline-Charged tRNAPro.
We measured levels of proline-charged tRNAPro or threonine-charged tRNAThr as described (39) with slight modifications. Wild-type and proline auxotrophic (EG19886) Salmonella were grown as described (11, 14). We mixed 5 mL of cultures with 5 mL of 10% (wt/vol) trichloroacetic acid and kept it on ice. All subsequent steps were carried out at 0–4 °C. Cells were pelleted and resuspended in 0.3 mL of buffer A (0.3 M sodium acetate pH 4.5, 10 mM EDTA). tRNAs were extracted with 0.3 mL of phenol equilibrated with buffer A by vortexing vigorously (150 s with intervals) and centrifuged for 15 min at 20,817 × g. The aqueous phase was transferred to new tube containing 0.3 mL of phenol and repeated as above. The nucleic acids were precipitated with ethanol at –18 °C for 15 min followed by centrifugation for 30 min at 20,817 × g. The pellet was dissolved in 60 μL of buffer A, and the nucleic acids were precipitated as above. The pellet was resuspended in 20 μL of buffer B (10 mM sodium acetate pH 4.5, 1 mM EDTA).
Before gel electrophoresis, 4 μL of tRNA was deacylated by treating with 0.1 M Tris⋅HCl (pH 9.0) in a total volume of 50 μL for 1 h at 37 °C. Then, the tRNA was neutralized by adding 50 μL of buffer A, and precipitated as above and dissolved in 4 μL of buffer B. We mixed 4 μL of either untreated or deacylated sample with 6 μL of loading buffer [0.1 M sodium acetate pH 5.0, 8 M urea, 0.05% (wt/vol) bromophenol blue, and 0.05% (wt/vol) xylene cyanol], and electrophoresed it through a 6.5% polyacrylamide (acrylamide to bisacrylamide, 19:1) gel containing 0.1 M sodium acetate (pH 5.0) and 8 M urea. The electrophoresis was carried out 500 V at 4 °C until the bromophenol blue reached the bottom of the gel. The area of the gel between the two dyes was electroblotted onto a positively charged membrane. The tRNA was crosslinked to the membrane by 0.12 J of UV light. The membranes were prehybridized at 42 °C for 1 h in 12 mL of ULTRAhyb-oligo buffer (Invitrogen). We used radiolabeled W718 and W722 primers to detect tRNAPro and tRNAThr, respectively. Hybridization was at 42 °C overnight and the membranes were washed in wash buffer (0.3 M NaCl, 30 mM sodium citrate, 0.5% SDS) for 30 min at 42 °C twice. The membranes were analyzed by a phosphor imager scanner.
Supplementary Material
Acknowledgments
We thank Dongwoo Shin for allowing E.-J.L. to conduct some of the experiments in his laboratory. This work was supported, in part, by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, Information and Computer Technology, and Future Planning (NRF-2013R1A1A1004929) (to E.-J.L.) and by Grant AI49561 from the National Institutes of Health (to E.A.G.), who is an investigator of the Howard Hughes Medical Institute.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1316209111/-/DCSupplemental.
References
- 1.Blanc-Potard AB, Groisman EA. The Salmonella selC locus contains a pathogenicity island mediating intramacrophage survival. EMBO J. 1997;16(17):5376–5385. doi: 10.1093/emboj/16.17.5376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buchmeier N, et al. A parallel intraphagosomal survival strategy shared by mycobacterium tuberculosis and Salmonella enterica. Mol Microbiol. 2000;35(6):1375–1382. doi: 10.1046/j.1365-2958.2000.01797.x. [DOI] [PubMed] [Google Scholar]
- 3.Grabenstein JP, Fukuto HS, Palmer LE, Bliska JB. Characterization of phagosome trafficking and identification of PhoP-regulated genes important for survival of Yersinia pestis in macrophages. Infect Immun. 2006;74(7):3727–3741. doi: 10.1128/IAI.00255-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lavigne JP, O’callaghan D, Blanc-Potard AB. Requirement of MgtC for Brucella suis intramacrophage growth: A potential mechanism shared by Salmonella enterica and Mycobacterium tuberculosis for adaptation to a low-Mg2+ environment. Infect Immun. 2005;73(5):3160–3163. doi: 10.1128/IAI.73.5.3160-3163.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maloney KE, Valvano MA. The mgtC gene of Burkholderia cenocepacia is required for growth under magnesium limitation conditions and intracellular survival in macrophages. Infect Immun. 2006;74(10):5477–5486. doi: 10.1128/IAI.00798-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee EJ, Pontes MH, Groisman EA. A bacterial virulence protein promotes pathogenicity by inhibiting the bacterium’s own F1Fo ATP synthase. Cell. 2013;154(1):146–156. doi: 10.1016/j.cell.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turner AK, et al. Contribution of proton-translocating proteins to the virulence of Salmonella enterica serovars Typhimurium, Gallinarum, and Dublin in chickens and mice. Infect Immun. 2003;71(6):3392–3401. doi: 10.1128/IAI.71.6.3392-3401.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soncini FC, García Véscovi E, Solomon F, Groisman EA. Molecular basis of the magnesium deprivation response in Salmonella typhimurium: Identification of PhoP-regulated genes. J Bacteriol. 1996;178(17):5092–5099. doi: 10.1128/jb.178.17.5092-5099.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Groisman EA. The pleiotropic two-component regulatory system PhoP-PhoQ. J Bacteriol. 2001;183(6):1835–1842. doi: 10.1128/JB.183.6.1835-1842.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee EJ, Groisman EA. Control of a Salmonella virulence locus by an ATP-sensing leader messenger RNA. Nature. 2012;486(7402):271–275. doi: 10.1038/nature11090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee EJ, Groisman EA. Tandem attenuators control expression of the Salmonella mgtCBR virulence operon. Mol Microbiol. 2012;86(1):212–224. doi: 10.1111/j.1365-2958.2012.08188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Snavely MD, Miller CG, Maguire ME. The mgtB Mg2+ transport locus of Salmonella typhimurium encodes a P-type ATPase. J Biol Chem. 1991;266(2):815–823. [PubMed] [Google Scholar]
- 13.Alix E, Blanc-Potard AB. Peptide-assisted degradation of the Salmonella MgtC virulence factor. EMBO J. 2008;27(3):546–557. doi: 10.1038/sj.emboj.7601983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park SY, Cromie MJ, Lee EJ, Groisman EA. A bacterial mRNA leader that employs different mechanisms to sense disparate intracellular signals. Cell. 2010;142(5):737–748. doi: 10.1016/j.cell.2010.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henkin TM, Yanofsky C. Regulation by transcription attenuation in bacteria: How RNA provides instructions for transcription termination/antitermination decisions. Bioessays. 2002;24(8):700–707. doi: 10.1002/bies.10125. [DOI] [PubMed] [Google Scholar]
- 16.Merino E, Yanofsky C. Transcription attenuation: A highly conserved regulatory strategy used by bacteria. Trends Genet. 2005;21(5):260–264. doi: 10.1016/j.tig.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 17.Csonka LN, Leisinger T. Biosynthesis of proline. In: Kaper J, editor. Escherichia coli and Salmonella. Cellular and Molecular Biology. Washinton, DC: ASM Press; 2007. [Google Scholar]
- 18.Baich A. Proline synthesis in Escherichia coli. A proline-inhibitable glutamic acid kinase. Biochim Biophys Acta. 1969;192(3):462–467. doi: 10.1016/0304-4165(69)90395-x. [DOI] [PubMed] [Google Scholar]
- 19.Strecker HJ. The interconversion of glutamic acid and proline. I. The formation of delta1-pyrroline-5-carboxylic acid from glutamic acid in Escherichia coli. J Biol Chem. 1957;225(2):825–834. [PubMed] [Google Scholar]
- 20.Balaji B, O’Connor K, Lucas JR, Anderson JM, Csonka LN. Timing of induction of osmotically controlled genes in Salmonella enterica Serovar Typhimurium, determined with quantitative real-time reverse transcription-PCR. Appl Environ Microbiol. 2005;71(12):8273–8283. doi: 10.1128/AEM.71.12.8273-8283.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doerfel LK, et al. EF-P is essential for rapid synthesis of proteins containing consecutive proline residues. Science. 2013;339(6115):85–88. doi: 10.1126/science.1229017. [DOI] [PubMed] [Google Scholar]
- 22.Tanner DR, Cariello DA, Woolstenhulme CJ, Broadbent MA, Buskirk AR. Genetic identification of nascent peptides that induce ribosome stalling. J Biol Chem. 2009;284(50):34809–34818. doi: 10.1074/jbc.M109.039040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ude S, et al. Translation elongation factor EF-P alleviates ribosome stalling at polyproline stretches. Science. 2013;339(6115):82–85. doi: 10.1126/science.1228985. [DOI] [PubMed] [Google Scholar]
- 24.Woolstenhulme CJ, et al. Nascent peptides that block protein synthesis in bacteria. Proc Natl Acad Sci USA. 2013;110(10):E878–E887. doi: 10.1073/pnas.1219536110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Navarre WW, et al. PoxA, yjeK, and elongation factor P coordinately modulate virulence and drug resistance in Salmonella enterica. Mol Cell. 2010;39(2):209–221. doi: 10.1016/j.molcel.2010.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spinelli SV, Pontel LB, García Véscovi E, Soncini FC. Regulation of magnesium homeostasis in Salmonella: Mg(2+) targets the mgtA transcript for degradation by RNase E. FEMS Microbiol Lett. 2008;280(2):226–234. doi: 10.1111/j.1574-6968.2008.01065.x. [DOI] [PubMed] [Google Scholar]
- 27.García-del Portillo F. Salmonella intracellular proliferation: Where, when and how? Microbes Infect. 2001;3(14-15):1305–1311. doi: 10.1016/s1286-4579(01)01491-5. [DOI] [PubMed] [Google Scholar]
- 28.Fields PI, Swanson RV, Haidaris CG, Heffron F. Mutants of Salmonella typhimurium that cannot survive within the macrophage are avirulent. Proc Natl Acad Sci USA. 1986;83(14):5189–5193. doi: 10.1073/pnas.83.14.5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steeb B, et al. Parallel exploitation of diverse host nutrients enhances Salmonella virulence. PLoS Pathog. 2013;9(4):e1003301. doi: 10.1371/journal.ppat.1003301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Faucher SP, Mueller CA, Shuman HA. Legionella Pneumophila transcriptome during intracellular multiplication in human macrophages. Front Microbiol. 2011;2:60. doi: 10.3389/fmicb.2011.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crawford JM, Kontnik R, Clardy J. Regulating alternative lifestyles in entomopathogenic bacteria. Curr Biol. 2010;20(1):69–74. doi: 10.1016/j.cub.2009.10.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.García Véscovi E, Soncini FC, Groisman EA. Mg2+ as an extracellular signal: Environmental regulation of Salmonella virulence. Cell. 1996;84(1):165–174. doi: 10.1016/s0092-8674(00)81003-x. [DOI] [PubMed] [Google Scholar]
- 33.Véscovi EG, Ayala YM, Di Cera E, Groisman EA. Characterization of the bacterial sensor protein PhoQ. Evidence for distinct binding sites for Mg2+ and Ca2+ J Biol Chem. 1997;272(3):1440–1443. doi: 10.1074/jbc.272.3.1440. [DOI] [PubMed] [Google Scholar]
- 34.Prost LR, et al. Activation of the bacterial sensor kinase PhoQ by acidic pH. Mol Cell. 2007;26(2):165–174. doi: 10.1016/j.molcel.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 35.Bader MW, et al. Recognition of antimicrobial peptides by a bacterial sensor kinase. Cell. 2005;122(3):461–472. doi: 10.1016/j.cell.2005.05.030. [DOI] [PubMed] [Google Scholar]
- 36.Heithoff DM, et al. Coordinate intracellular expression of Salmonella genes induced during infection. J Bacteriol. 1999;181(3):799–807. doi: 10.1128/jb.181.3.799-807.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cromie MJ, Shi Y, Latifi T, Groisman EA. An RNA sensor for intracellular Mg(2+) Cell. 2006;125(1):71–84. doi: 10.1016/j.cell.2006.01.043. [DOI] [PubMed] [Google Scholar]
- 38.Davis RW, Bolstein D, Roth JR. Advanced Bacterial Genetics. Cold Spring Harbor, NY: Cold Spring Harbor Lab Press; 1980. [Google Scholar]
- 39.Varshney U, Lee CP, RajBhandary UL. Direct analysis of aminoacylation levels of tRNAs in vivo. Application to studying recognition of Escherichia coli initiator tRNA mutants by glutaminyl-tRNA synthetase. J Biol Chem. 1991;266(36):24712–24718. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.