Abstract
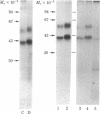
Preincubation of turkey erythrocytes with catecholamines desensitizes the beta-adrenergic receptor-adenylate cyclase complex in the plasma membranes of these cells. Photoaffinity labeling of the beta-adrenergic receptors with 125I-labeled p-azidobenzylcarazolol (125I-pABC) and subsequent analysis by NaDodSO4/polyacrylamide gel electrophoresis demonstrates an altered mobility of receptor peptides from desensitized cells compared to controls [Stadel, J.M., Nambi, P., Lavin, T.N., Heald, S.L., Caron, M.G. & Lefkowitz, R.J. (1982) J. Biol. Chem. 257, 9242-9245]. The time course of alteration in beta-adrenergic receptor mobility correlates with that for desensitization of isoproterenol-stimulated adenylate cyclase activity. The altered mobility of the receptor peptides from desensitized cells is also observed if the receptors are first purified and then photoaffinity labeled with 125I-pABC. The cyclic nucleotide analog 8-bromoadenosine 3',5'-cyclic monophosphate partially mimics catecholamines in promoting desensitization of the adenylate cyclase and modification of the receptor. Phosphorylation of the beta-adrenergic receptor in intact turkey erythrocytes was assessed by preincubating the cells with [32P]orthophosphate, desensitizing them with catecholamine, purifying the receptors, and then subjecting them to NaDodSO4/polyacrylamide gel electrophoresis. Desensitization is associated with a 2- to 3-fold increase in 32P incorporation into the receptor, which also demonstrates the characteristic alterations in mobility. These effects are blocked by the beta-adrenergic antagonist propranolol. Purified turkey erythrocyte beta-adrenergic receptors could be phosphorylated by incubation with [gamma-32P]ATP and the catalytic subunit of cAMP-dependent protein kinase. The mobility of the phosphorylated receptor peptides on NaDodSO4/polyacrylamide gel electrophoresis appears to correspond to that of the desensitized receptors. These data show that catecholamine-induced desensitization of adenylate cyclase in turkey erythrocytes correlates with a stable modification of the beta-adrenergic receptor and is associated with agonist-promoted phosphorylation of beta-receptor peptides.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alper S. L., Beam K. G., Greengard P. Hormonal control of Na+-K+ co-transport in turkey erythrocytes. Multiple site phosphorylation of goblin, a high molecular weight protein of the plasma membrane. J Biol Chem. 1980 May 25;255(10):4864–4871. [PubMed] [Google Scholar]
- Alper S. L., Palfrey H. C., DeRiemer S. A., Greengard P. Hormonal control of protein phosphorylation in turkey erythrocytes. Phosphorylation by cAMP-dependent and Ca2+-dependent protein kinases of distinct sites in goblin, a high molecular weight protein of the plasma membrane. J Biol Chem. 1980 Nov 25;255(22):11029–11039. [PubMed] [Google Scholar]
- Cohen P. The role of cyclic-AMP-dependent protein kinase in the regulation of glycogen metabolism in mammalian skeletal muscle. Curr Top Cell Regul. 1978;14:117–196. doi: 10.1016/b978-0-12-152814-0.50008-3. [DOI] [PubMed] [Google Scholar]
- Cohen S., Carpenter G., King L., Jr Epidermal growth factor-receptor-protein kinase interactions. Co-purification of receptor and epidermal growth factor-enhanced phosphorylation activity. J Biol Chem. 1980 May 25;255(10):4834–4842. [PubMed] [Google Scholar]
- Gavin J. R., 3rd, Roth J., Neville D. M., Jr, de Meyts P., Buell D. N. Insulin-dependent regulation of insulin receptor concentrations: a direct demonstration in cell culture. Proc Natl Acad Sci U S A. 1974 Jan;71(1):84–88. doi: 10.1073/pnas.71.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon A. S., Davis C. G., Milfay D., Diamond I. Phosphorylation of acetylcholine receptor by endogenous membrane protein kinase in receptor-enriched membranes of Torpedo californica. Nature. 1977 Jun 9;267(5611):539–540. doi: 10.1038/267539a0. [DOI] [PubMed] [Google Scholar]
- Greengard P. Phosphorylated proteins as physiological effectors. Science. 1978 Jan 13;199(4325):146–152. doi: 10.1126/science.22932. [DOI] [PubMed] [Google Scholar]
- Hoffman B. B., Mullikin-Kilpatrick D., Lefkowitz R. J. Desensitization of beta-adrenergic stimulated adenylate cyclase in turkey erythrocytes. J Cyclic Nucleotide Res. 1979 Oct;5(5):355–366. [PubMed] [Google Scholar]
- Hudson T. H., Johnson G. L. Functional alterations in components of pigeon erythrocyte adenylate cyclase following desensitization to isoproterenol. Mol Pharmacol. 1981 Nov;20(3):694–703. [PubMed] [Google Scholar]
- Kasuga M., Karlsson F. A., Kahn C. R. Insulin stimulates the phosphorylation of the 95,000-dalton subunit of its own receptor. Science. 1982 Jan 8;215(4529):185–187. doi: 10.1126/science.7031900. [DOI] [PubMed] [Google Scholar]
- Kent R. S., De Lean A., Lefkowitz R. J. A quantitative analysis of beta-adrenergic receptor interactions: resolution of high and low affinity states of the receptor by computer modeling of ligand binding data. Mol Pharmacol. 1980 Jan;17(1):14–23. [PubMed] [Google Scholar]
- Krebs E. G., Beavo J. A. Phosphorylation-dephosphorylation of enzymes. Annu Rev Biochem. 1979;48:923–959. doi: 10.1146/annurev.bi.48.070179.004423. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mickey J. V., Tate R., Mullikin D., Lefkowitz R. J. Regulation of adenylate cyclase-coupled beta adrenergic receptor binding sites by beta adrenergic catecholamines in vitro. Mol Pharmacol. 1976 May;12(3):409–419. [PubMed] [Google Scholar]
- Shear M., Insel P. A., Melmon K. L., Coffino P. Agonist-specific refractoriness induced by isoproterenol. Studies with mutant cells. J Biol Chem. 1976 Dec 10;251(23):7572–7576. [PubMed] [Google Scholar]
- Shih T. Y., Weeks M. O., Young H. A., Scholnick E. M. Identification of a sarcoma virus-coded phosphoprotein in nonproducer cells transformed by Kirsten or Harvey murine sarcoma virus. Virology. 1979 Jul 15;96(1):64–79. doi: 10.1016/0042-6822(79)90173-9. [DOI] [PubMed] [Google Scholar]
- Shorr R. G., Strohsacker M. W., Lavin T. N., Lefkowitz R. J., Caron M. G. The beta 1-adrenergic receptor of the turkey erythrocyte. Molecular heterogeneity revealed by purification and photoaffinity labeling. J Biol Chem. 1982 Oct 25;257(20):12341–12350. [PubMed] [Google Scholar]
- Simpson I. A., Pfeuffer T. Functional desensitisation of beta-adrenergic receptors of avian erythrocytes by catecholamines and adenosine 3',5'-phosphate. Eur J Biochem. 1980 Oct;111(1):111–116. doi: 10.1111/j.1432-1033.1980.tb06081.x. [DOI] [PubMed] [Google Scholar]
- Stadel J. M., De Lean A., Mullikin-Kilpatrick D., Sawyer D. D., Lefkowitz R. J. Catecholamine-induced desensitization in turkey erythrocytes: cAMP mediated impairment of high affinity agonist binding without alteration in receptor number. J Cyclic Nucleotide Res. 1981;7(1):37–47. [PubMed] [Google Scholar]
- Stadel J. M., DeLean A., Lefkowitz R. J. A high affinity agonist . beta-adrenergic receptor complex is an intermediate for catecholamine stimulation of adenylate cyclase in turkey and frog erythrocyte membranes. J Biol Chem. 1980 Feb 25;255(4):1436–1441. [PubMed] [Google Scholar]
- Stadel J. M., Nambi P., Lavin T. N., Heald S. L., Caron M. G., Lefkowitz R. J. Catecholamine-induced desensitization of turkey erythrocyte adenylate cyclase. Structural alterations in the beta-adrenergic receptor revealed by photoaffinity labeling. J Biol Chem. 1982 Aug 25;257(16):9242–9245. [PubMed] [Google Scholar]
- Su Y. F., Cubeddu L., Perkins J. P. Regulation of adenosine 3':5'-monophosphate content of human astrocytoma cells: desensitization to catecholamines and prostaglandins. J Cyclic Nucleotide Res. 1976 Jul-Aug;2(4):257–270. [PubMed] [Google Scholar]
- Su Y. F., Harden T. K., Perkins J. P. Catecholamine-specific desensitization of adenylate cyclase. Evidence for a multistep process. J Biol Chem. 1980 Aug 10;255(15):7410–7419. [PubMed] [Google Scholar]
- Terasaki W. L., Brooker G., de Vellis J., Inglish D., Hsu C. Y., Moylan R. D. Involvement of cyclic amp and protein synthesis in catecholamine refractoriness. Adv Cyclic Nucleotide Res. 1978;9:33–52. [PubMed] [Google Scholar]
- Zoller M. J., Kerlavage A. R., Taylor S. S. Structural comparisons of cAMP-dependent protein kinases I and II from porcine skeletal muscle. J Biol Chem. 1979 Apr 10;254(7):2408–2412. [PubMed] [Google Scholar]